Published online Jul 27, 2025. doi: 10.4254/wjh.v17.i7.106675
Revised: May 1, 2025
Accepted: June 25, 2025
Published online: July 27, 2025
Processing time: 143 Days and 13.5 Hours
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most widespread chronic liver disease signified by serious life-threatening conditions. The prevalence of MASLD increases along the growing prevalence in obesity and metabolic syndrome. To minimize costs and complications, non-invasive dia
Core Tip: Clinical diagnostics of metabolic dysfunction-associated steatotic liver disease (MASLD) relies on non-invasive diagnostic tools, such as transient elastography (TE)/FibroScan®. TE measures liver stiffness, an important clinical marker of liver fibrosis and cirrhosis. However, TE measurements have limited accuracy in obese patients. This study reviews the limitations of TE-based diagnostics and discusses the combined scoring algorithm. Data review indicates that in patients with MASLD reliable diagnostic accuracy can be achieved by combining FibroScan® measurements with various blood test-based scores, including agile and fibrosis score indicators.
- Citation: Sukocheva O, Ow TW, Harding D, Le Mire M, Tse E. Liver stiffness measurements in patients with metabolic dysfunction-associated steatotic liver disease: Updates on the method effectiveness and perspectives. World J Hepatol 2025; 17(7): 106675
- URL: https://www.wjgnet.com/1948-5182/full/v17/i7/106675.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i7.106675
Representing substantial health system burden, chronic liver disease (CLD) is associated with significant morbidities and mortality[1]. CLD is manifested by a progressive accumulation of fibrous tissue and deterioration of liver functions, leading to fibrosis and cirrhosis[1-3]. Alcoholic liver disease, viral hepatitis [hepatitis B virus (HBV) and hepatitis C virus (HCV)], non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis [currently renamed as metabolic dysfunction-associated steatotic liver disease (MASLD)][4,5], and hemochromatosis[6] signify the complexity of CLD. The severe clinical form of MASLD is named metabolic dysfunction-associated steatohepatitis (MASH) (new terminology)[6,7]. New names MASLD and MASH provide a greater emphasis on the function of metabolic factors (such as obesity and diabetes) in the pathology of fatty liver. CLD progression towards decompensated cirrhosis is often accompanied by jaundice, ascites, hepatic encephalopathy, hepatorenal syndrome, or variceal haemorrhage (bleeding)[1,7-9]. Furthermore, cirrhosis may lead to development of hepatocellular carcinoma (HCC)[10].
Early detection and monitoring of advanced fibrosis and cirrhosis are critical for prevention of CLD complications and reduction of mortality. Prior to non-invasive testing, a liver biopsy is used to differentiate cirrhosis from lower fibrosis grades. Liver biopsy is an invasive and expensive procedure with minor risk of sampling error, interobserver variability, complications, and will not meet the diagnostic demands of highly prevalent liver disease. However, liver biopsy is considered as a standard criterion for advanced fibrosis assessment[11], although it was associated with the surgical complications. Cost-comparing study indicated reliability and advantages of non-invasive methods for assessment of liver fibrosis[12]. The alternative diagnostics relies on specific blood markers and radiologic investigations, including transient elastography (TE) or FibroScan®. TE is a non-invasive, reliable, simple, and less costly method[13,14]. Other types of liver stiffness (LS) measurements include point shear wave elastography (pSWE) and two-dimensional (2D)-shear wave elastography (SWE) which combine imaging with ultrasound techniques[14,15]. The pSWE and 2D-SWE methods were developed and introduced after FibroScan® and remain less validated method compared to TE. Magnetic resonance elastography (MRE) has been used for accurate assessment of liver fibrosis using specific tissue mechanical parameters[16], although MRE limitations has been noted[17]. MRE is not reviewed in this study as it is costly and not widely available technique[17,18].
FibroScan® device is often used to determine liver steatosis according to controlled attenuation parameter (CAP) which correctly indicates the level of liver fat in vast majority of patients with CLD[13,14]. TE is the most used test for the assessment of liver fibrosis[13,15,18]. However, LS diagnostic accuracy remains to be confirmed in patients with MASLD. The incidence of MASLD is growing, representing serious health and financial challenges worldwide[19]. The application of TE/FibroScan® for assessment of fibrosis in obese patients and/or patients with diagnosed metabolic syndrome is currently being in the clinical spotlight and warrants further investigations. This study aims to discuss benefits and limitations of TE/FibroScan® assessment of the liver steatosis in patients with MASLD. TE technique and impediments associated with the presence of fat and pro-inflammatory conditions in CLD patients will be examined. To define potential areas for future improvement of CLD diagnostics, we critically evaluated perspectives of TE use as combined approach with other clinical methods of hepatological assessment.
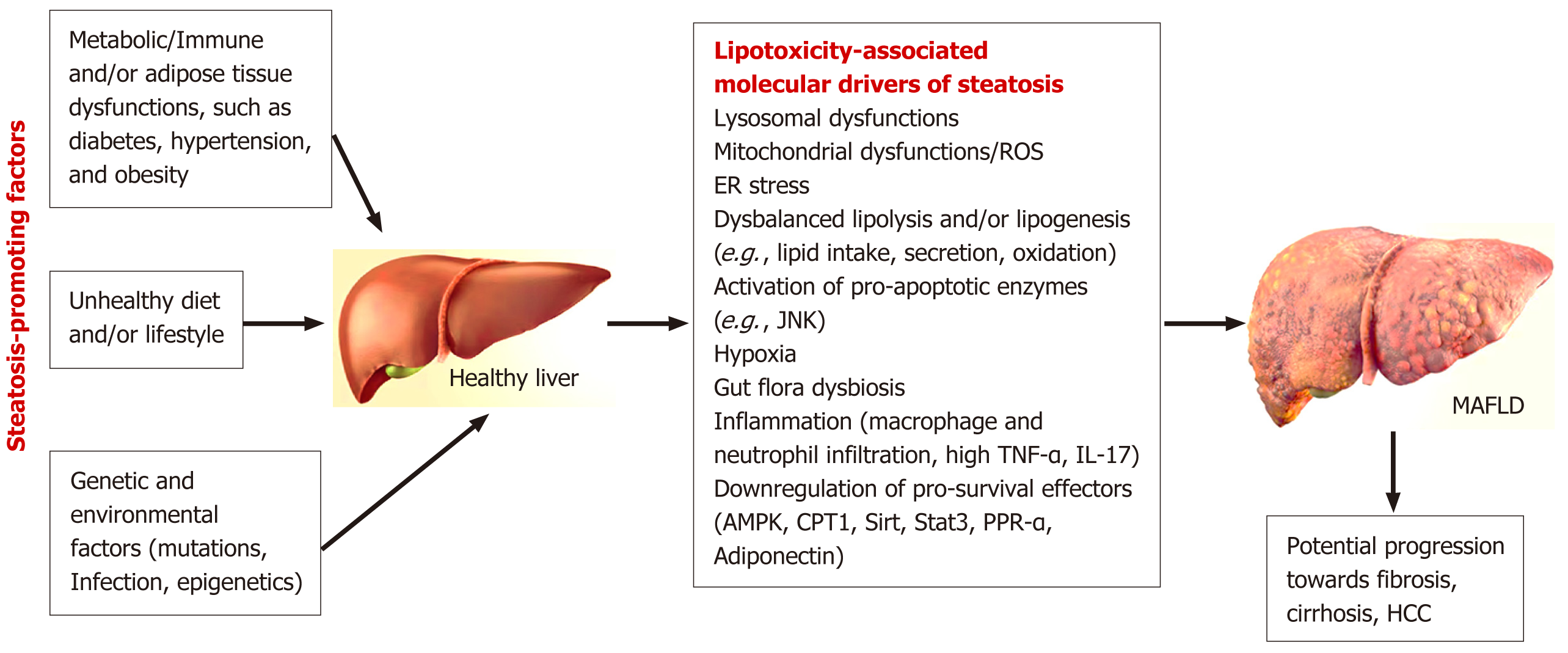
MASLD prevalence has increased dramatically over the last few decades[20]. Extensive data analysis, involving 8515431 patients (22 countries), demonstrated the global prevalence of MASLD in 2016[21]. Currently, the incidence of MASLD (hepatic steatosis) has reached the pandemic (30%) threshold in some countries and continues to rise[19]. Socioeconomic and genetic factors may contribute the geographic differences in the prevalence of MASLD. Several regions demonstrated the higher prevalence of MASLD (Latin America, the Middle East, North Africa, and South Asia), while Western Europe and the Asia-Pacific countries have shown the lower prevalence rate for this CLD[22]. Obesity, dyslipidemia, aging, and type 2 diabetes mellitus (T2DM) are top contributing risk factors for MASLD (Figure 1)[23,24]. Hepatic steatosis was shown to trigger the development HCC, accenting the key role of early diagnostic for MASLD management[4,5,25].
Although often linked to obesity, MASLD is currently used as the terminology for patients with hepatic steatosis who are not-necessarily obese (defined mostly by waist circumference), but represented with two or more metabolic abn
A longitudinal study which used liver biopsy indicators (a more expensive approach) reported that one third of MASLD patients progressed to the worse fibrosis stage within a 3-year interval[32], indicating a need for continuous screening. Less-costly TE assessment demonstrated high accuracy in initial assessments of liver fibrosis MASLD patients, confirming the suitability of this method for MASLD monitoring[15,33]. Changes towards higher LS (defined by sequential TE measurements) were also associated with greater risk of liver-related mortality in MASLD patients[34]. Many studies confirmed that TE-based sequential assessment of fibrosis can be used as a reliable predictor of MASLD-linked complications and mortality[35-37].
Adapted for steatosis quantification, CAP [measured in decibels per meter (dB/m)] is calculated using TE radiofrequency data. However, in MASLD patients with body mass index > 30 kg/m2, older patients with ascites, diabetes, or hypertension, several studies have shown a false increase in fibrosis due to higher steatosis-induced stiffness[38]. High BMI was shown to influence CAP numbers[39,40]. In obese MASLD patients, the correct TE data acquisition is impacted by the presence of fat tissue, inflammation, and other factors (see subsection 5)[38,41,42]. Therefore, for fibrosis asse
Notably, nearly half of all MASLD patients (obese) requires additional TE method adjustments and multistep measures for fibrosis assessment[43]. Accumulated data indicated that about 45% of MASLD patients have “lean” (normal, non-obese), BMI (BMI < 23 kg/m2: Below overweight or obesity threshold) and closely associated with T2DM, or other metabolic abnormalities[39,44,45]. Currently, there is limited research on non-obese MASLD, and its pathogenesis and treatment methods remain unclear. It has been reported that non-obese type MASLD patients are at a higher risk of progressing to advanced liver fibrosis[46]. This observation warrants further investigations. It has been reported recently that in non-obese MASLD patients, there are elevated levels of urea nitrogen, uric acid, triglycerides, and fasting blood glucose (compared to normal controls)[47]. Alternatively, HDL and apolipoprotein A1 were downregulated[47].
Interestingly, the products of fat metabolism, free fatty acids (FFA) (such as palmitic acid, oleic acid, linoleic acid, and arachidonic acid) were also significantly elevated in non-obese MASLD patients[47,48]. Disrupted balance between specific circulating and intracellular FFAs may lead to the accumulation of fat not only in liver (lipotoxicity), but also in cardiovascular system[49]. High FFA indicates liver cell damage. It remains to be investigated how increased FFA levels may impact the TE/FibroScan® data acquisition and whether TE maybe used to assess oxidative liver damage and inflammation. Nevertheless, non-obese MASLD patients may directly benefit from TE-based monitoring. Less expensive and easy-accessible TE diagnostics in non-obese MASLD patients is not impacted by the presence of fat tissue, although pro-inflammatory conditions may (potentially) influence TE measurements[50]. The pathogenesis of liver steatosis in non-obese MASLD patients is most likely multifactorial and may be provoked by several metabolic dysfunctions, including T2DM.
The relationship between T2DM and MASLD are complex and not completely understood[39]. Higher glucose level in circulation was associated with increased LS[51]. It is well known that one of the main liver functions is to balance and preserve normal blood glucose[52]. Active glucose metabolism, glucose storage, and endogenous glucose production from glycogen (gluconeogenesis) were observed in liver and muscles during normal ontogenesis[53]. Many studies indicated that the prevalence of liver steatosis is very high among patients with T2DM[39,54]. In patients with advanced liver fibrosis, liver cells are no longer efficient in glucose metabolism, leading to increased blood glucose (the main T2DM marker)[55]. Notably, less insulin is absorbed by hepatocytes from the circulation (chronic hyperinsulinemia, insulin resistance) in MASLD patients. Therefore, T2DM conditions could be both sequential cause (a trigger) of the fatty liver disease or MASLD-induced complication. Epidemiological data confirmed that MASLD patients are more likely to progress towards T2DM[56].
Persistent immune disturbances can also lead to aberrant liver cell metabolism, resulting in the accumulation of lipids (FFA and cholesterol) in liver cells. Excessive lipid accumulation in the liver (lipotoxicity) is marked by mitochondrial dysfunction, increased reactive oxygen species levels, and chronic inflammation[57], which, in turn, contributes pro
Distinct immune microenvironment of MASLD was shown to accompany the progression of liver steatosis and fibrosis (Figure 1)[62-64]. Macrophage-derived pro-inflammatory cytokines (such as tumor necrosis factor-alpha and interleukin-1β) were shown to promote lipid deposition in hepatocytes[58,65]. Infection or diet/lifestyle—triggered dysregulation in lipid metabolism stimulated accumulation of FFAs and modified immune cell landscape[66]. The observed changes included exhaustion or decline of Kupffer cell (KC) (liver resident macrophages) functions[66]. KCs are not only key regulators of inflammation in liver during normal ontogenesis, but also contribute fibrotic signalling cascades in MASLD[44,67,68]. Aside from KCs, atypical T cell activation and exhaustion profiles were reported in MASLD patient and rodent models[69,70]. The detected T cells failed to demonstrate the immune surveillance functions, although liver tissue damage was observed[70,71]. The diverse characteristics of liver microenvironment in patients with steatosis/fibrosis suggest the high level of heterogeneity of MASLD.
Considering the spectrum of contributing pathologies and various stages of steatosis and cirrhosis, MAFLD is highly heterogenous disease. The mechanisms for MAFLD development, progression stages, and response to therapy are also likely to be different[53,55-57]. Therefore, it may require complex approach in diagnostics and monitoring. That is why combined approach is more likely to be the most effective (discussed in sub-section 6 of this review) and may cover the larger spectrum of conditions involved.
Increased collagen content is the major indicator of growing tissue stiffness which can be manifested as fibrosis [a deposition of fibrous (dense) material]. Elastography (ultrasound-based approach) techniques is used to measure LS and estimate the level of fibrosis in liver tissue[57,72]. FibroScan® device (Echosens, Paris, France) is commonly used for TE assessment. TE/FibroScan® method works on two physical principles: (1) Strain displacement; and (2) Shear wave imaging and quantification. The latter includes pSWE. It measures the velocity of low frequency (50 Hz) elastic shear waves propagating through the liver. The stiffer the tissue, the faster the elastic shear wave propagates. TE can generate quantitative data presented as elasticity (kPa)[73].
The successful testing of FibroScan® for assessment of LS was reported since 2003[74-76]. TE helps to differentiate advanced steatohepatitis and hepatic fibrosis from simple (lower grade) steatosis. Derived in association with LS, CAP is a reliable marker for quantification of liver fat. CAP demonstrated promising area under the receiver operating characteristic curve (AUROC) > 0.80 and high sensitivity (80%) and specificity (80%)[77,78]. However, fibrosis (collagen fiber deposition) develops gradually, not linearly[38,39]. At the earlier stages, fibers are deposited with a slower increment. Alternatively, during advanced CLD, deposition of fibers happens with higher speed (exponentially). Therefore, the accuracy of TE measurements and reference standards can be negatively impacted by these non-linear differences and random accumulation of collagen[74]. Consequently, TE assessment is combined with other scores to improve the diagnostic accuracy[79-81].
Other non-invasive tests, including the Fibrosis-4 index (FIB-4)[79] and the Enhanced Liver Fibrosis test (ELF™)[80], are often used together to increase the power of assessment[81]. When used alone, specificity and sensitivity of FIB-4 or ELF™ may fluctuate according to the set of cut-offs values[82]. In MASLD patients, FIB-4 with ELF™ before FibroScan® asse
Two strongest TE advantages include the acquisition of continuous (quantitative) data and low-cost operation (easy in operation ultrasound-based technique). Comparing to TE, liver biopsy is more expensive procedure which is used to establish an important immunohistochemistry (IHC)-based (semiquantitative) clinical disease staging. A side from pathological characteristics, IHC/liver biopsy diagnostics is prone to biases associated with the location and quantity of fibrosis, because diseased tissue may not be evenly distributed in the liver. However, LS assessment by TE is also influenced (confounded) by tissue pathophysiology, such as portal hypertension, biliary obstruction, local inflammation, and passive venous congestion (thrombosis). Furthermore, LS assessment is recommended to be done in the fasting state, as liver physical condition is affected by postprandial status (Figure 2)[87]. Therefore, the diagnostic accuracy can be reached in some cases only after careful consideration of all measured characteristics, including liver imaging, liver function tests, and other available pathology markers. The role of coexisting confounders (other diseases, including metabolic dysfunctions) should be carefully evaluated. The impact of various pathologies on TE-based (and not only) assessment of liver fibrosis remains largely under-investigated.
To resolve complications associated with anatomical differences and body types, the FibroScan® devices are fitted with several different transducers (S, M, and XL). To acquire reliable data in overweight patients, it is required to use the XL transducer[88], although it may not be able to solve all diagnostic problems. To further increase the method sensitivity, CAP is combined with blood test data (such as serum transaminases) to generate a FibroScan®-AST (FAST) score (Figure 2)[76,83,89]. According to the meta-analysis, FibroScan® method allows to detect F3+ stage fibrosis with high sensitivity (85%) and specificity (82%). The accuracy of this method in detection of cirrhosis is even higher (sensitivity and specificity = 92%)[90]. However, the accuracy gets lower in patients with BMI > 35 kg/m2, representing a serious impediment for proper diagnostics[91-93]. In patients with morbid obesity (bariatric surgery cohort), one recent observational study reported that LS/CAP measurements using FibroScan® (XL probe) were technically successful in only 59% of cases[94], indicating only moderate achievements with this technique. Notable, data collected in bariatric cohorts is controversial, as another study with 190 patients (BMI: 40.2 kg/m2 ± 6.6 kg/m2) reported better FibroScan®/CAP achievements with technically reliable measurements in 88% of patients[95]. Excellent data were reported in smaller cohort with 126 obese patients (BMI ≥ 30 kg/m2) in Turkey (97% of successful measurements)[96]. The study with Brazilian MASLD cohort (287 patients, BMI ≥ 32 kg/m2), which used combined FAST score, also indicated reliable diagnostic outcome[97]. FAST score was also evaluated retrospectively and indicated similar (reliable high sensitivity) findings in Japanese cohort[98]. All recent studies indicated that unsuccessful FibroScan® measurements in MASLD cohorts were associated with increased body weight and high BMI (> 40 kg/m2)[94,95,97].
TE/imaging of liver fibrosis can be acquired using not only ultrasound, but also magnetic resonance (MR) techniques. MR-based elastography is a very reliable, but expensive method which was reviewed recently[16]. MR technique was successfully used to distinguish MASLD from MASH[17,18,99]. However, lower cost and the easiness of the ultrasound TE device application led to the preferential use of this method in clinics. Ultrasound-based TE is represented by several approaches, such as pSWE and 2D-SWE, which can be measured using devices from various manufacturers. All of them seem to produce similar outcomes[86,89]. For instance, a recent study compared six different ultrasound-adopting TE systems[100]. Reliable correlation coefficients and similar diagnostic conclusions were reported in human patients, suggesting a good agreement across the compared systems[100]. However, the quantitative TE measures somewhat different characteristic of liver pathology which cannot be easily linked to IHC staging, indicating a need for large matching IHC-TE investigations[18,101].
The difference in measured parameters between TE and IHC-based diagnostics leads to a limited usability of TE to predict histopathological stages of CLD, although the problem is not associated with the TE accuracy. Ultrasound-based techniques reflect exactly the tissue structure and may be impacted mostly by the level of operator experience, which is minimized during training. The difficulties with liver tissue visualization and confounding anatomical structures were mostly resolved[16]. However, the calculated fibrosis staging is partially impacted by low sampling volume for standards, the high level of natural tissue variability, and insufficient correlation of liver histopathology with CLD severity/outcome. These impediments may complicate TE clinical interpretation in some CLD patients.
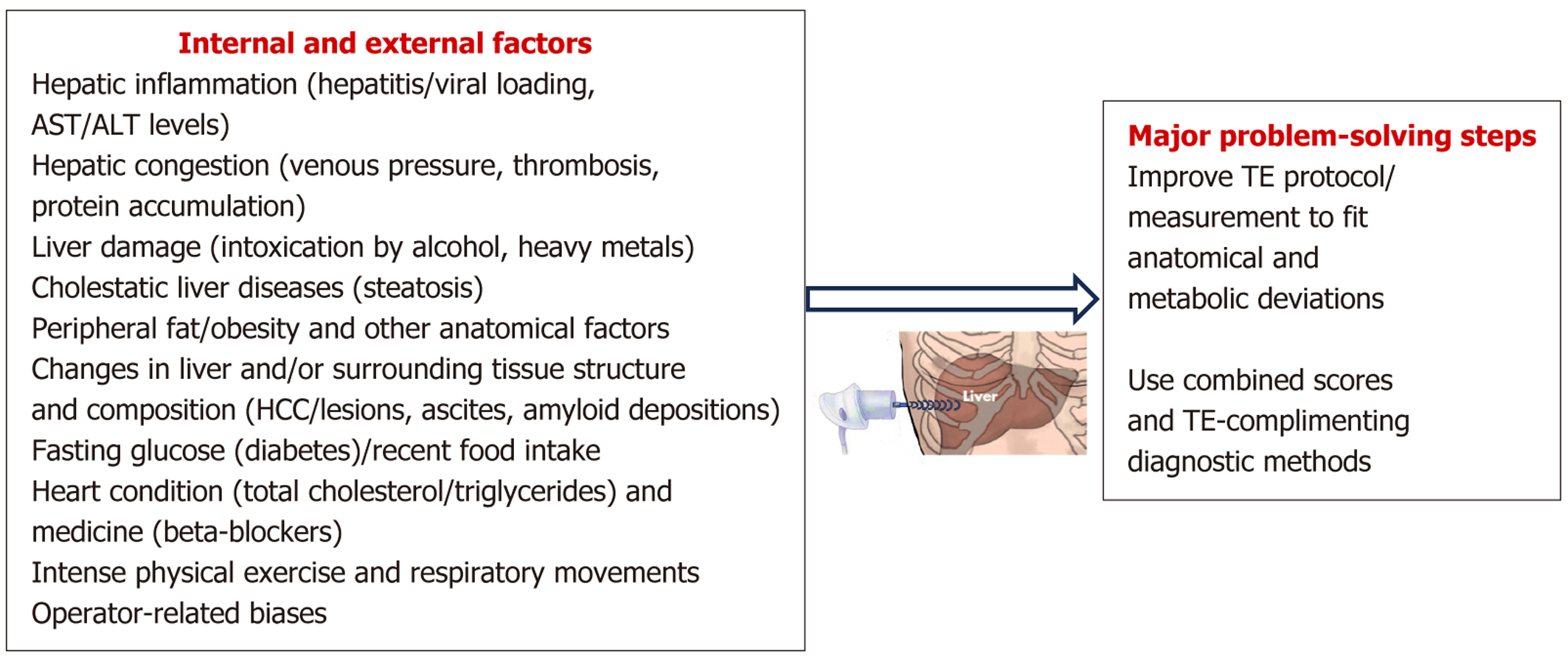
Physical characteristics of liver tissue influence TE data and interpretations. The velocity of shear wave propagation is defined not only by the severity of fibrosis, but also by several other factors. For the ideal propagation of the signal, the medium should be homogeneous, has uniform density and high level of elasticity. Obvious absence of these conditions in the human liver makes TE measurements prone to errors. Therefore, increased viscosity and tissue density impact TE imaging. Increased viscosity was observed in patients with biliary obstruction, acute hepatitis[102], and congested liver[103]. Higher tissue density was reported in patients with inflamed liver tissue and HCC (including liver tissue necrosis)[104], MASLD[105], cholestasis[106], and hepatic storage disease[107]. To disentangle LS measurement problem, TE guidelines have been developed by American Gastroenterological Association (AGA)[76] and Baveno VI groups[108]. The guidelines suggested LS cut-off points which were designed not only to help with cirrhosis assessment, but also to estimate the need for endoscopy, while excluding the compensated advanced CLD (cACLD). Extending the Baveno VI group-based guidelines, the World Federation of Ultrasound in Medicine and Biology introduced the “Rule of Five” protocol[109]. All limitations are described in Table 1 below[13,15,16,19,34,37,38,74,87,95,101,110-133].
| Ref. | TE accuracy and controlled attenuation parameter-impacting factors | Problem solving recommendations |
| Coco et al[110], Arena et al[111], Sasso et al[112], Kumar et al[113], Jung et al[114], Mi et al[115] | Hepatic inflammation (including viral loading), liver condition in general (aspartate aminotransferase, alanine aminotransferase levels and activities) | To recognize inflammation, it was recommended to apply damping ratio and shear loss modulus. Apply dispersion slope, attenuation coefficient, and shear wave speed imaging markers. Combined/multivariate tests (blood analysis, liver biopsy) |
| Sagir et al[116] Millonig et al[117], Ozturk et al[118], Trifanov et al[119], Huang et al[120] | Hepatic congestion (venous pressure, portal vein thrombosis, bilirubin, protein accumulation) and liver damage (including intoxication by alcohol, heavy metals etc.) | CVD-related tests, administer diuretics, use histopathology as reference standard, combined/multivariate tests (blood analysis, liver biopsy) are yet to be tested and characterized |
| Petta et al[34], Younossi et al[37], Yin et al[87], Yang et al[95], Cassinotto et al[101], Millonig et al[121], Sharma et al[122], Ali et al[123] | Cholestatic liver diseases (steatosis) | TE probe should be selected according to BMI (less failure rate with XL probe in high BMI patients), proper cut-off values should be defined, incorporation of hemoglobin A1c and alkaline phosphatase with liver-stiffness measurement improves accuracy in detecting significant fibrosis, better IHC matching (or different, such as blood marker-based) should be designed |
| Huang et al[19] | Focal liver lesions/HCC | N/A, HCC protocol should be followed |
| Blank et al[124], Nogami et al[125] | Distance between skin and liver; peripheral or abdominal fat (obesity/high BMI) | Automatic selection of the probe, the choice of XL or M probe should be standardized using novel SmartExam computational method |
| Lin et al[126] | Ascites | N/A, a proper disease-related protocol should be activated |
| Ozturk et al[16] | Anatomical factors/musculoskeletal deformity, age/sex | The FibroScan® system uses A and transmission metasurface mode maps which guide the operators to find the ideal location in the liver tissue. Regular calibration is reqiured, correct choice of proper controls |
| Loustaud-Ratti et al[127], Lanzi et al[128] | Amyloid deposition in liver | Reversing the ligation of the bile duct |
| Mederacke et al[129], Arena et al[130] | Fasting glucose (diabetes)/recent food ingestion | At least 4 hours fasting before procedure |
| Barr et al[15], Pennisi et al[131], Boeckmans et al[132] | Heart condition, beta-blockers, total cholesterol/triglycerides, intense physical exercise | It is recommended to apply heart disease case-finding strategies, CVD-related tests, proper cut-off values should be defined for this category of patients |
| Lemmer et al[13], Barr et al[15], Boursier et al[38] | Respiratory movements | Patients are asked to hold the breath and minimize movements. Measurements are repeated up to 3 and more times |
| Bassal et al[74], Hudson et al[133] | Operator-related biases | Regular operator training, several repeated readings per a patient to achieve the average reading value and minimize the measurement variability |
Later these guidelines were re-visited; and the range was tighter adjusted. The new rules were introduced by Society of Radiologists in Ultrasound in 2020. Accordingly, the current framework for TE data assessment is as follows: (1) Normal: LS ≤ 5 kPa; (2) Not cACLD: LS < 9 kPa (given the absence of other CLD clinical signs); (3) Suggestive cACLD: 9 kPa > LS < 13 kPa (further testing is required); (4) Likely cACLD: LS > 13 kPa; and (5) Significant portal hypertension: LS > 25 kPa[14,15]. These (current) rules are designed to guide the clinical decision, helping to avoid unnecessary and expensive endoscopic investigations. However, IHC/histopathology remains the main evidence for final diagnosis in many advanced CLD cases.
All ultrasound clinical techniques are biased by the level of operator training and experience. The operator-related biases were demonstrated in Hudson et al[133] study which estimated the accuracy of TE measurements in the same liver segments by different professionals. A decade ago, to reach the required quality of the image and measurement, it was necessary to repeat the probe reading (interquartile range of the median value < 30%) ten times in 1 patient[133,134]. This requirement significantly extended the time of assessment per a patient (although, commonly, only 3 repeats are required). Aside from operator-related problems, various patient-associated factors may impact TE imaging. The reflection of the sound (reverberation) from the internal tissue elements was associated with appearance of natural artifacts. For instance, limited penetration of the sound and resulting faulty images were linked to interaction of the sound with ribs (ribs shadow), vessel structure/content-related artifacts, the liver capsule, high body wall thickness (fat deposition), and patient’s motion[134]. To standardize the image acquisition and alleviate the variability, the imaging protocol was amended by Quantitative Imaging Biomarkers Alliance[134,135]. TE ultrasound systems should be checked for accuracy and calibrated periodically.
Considering the patient structure and composition variability, the main limitation of TE technique was associated with the increased inaccuracy of data in patients with high BMI (overweight/obese and metabolic syndrome diagnosed cases)[123,135]. In obese patients, the formation of thickened subcutaneous fat tissue leads to the sound attenuation, limited wave induction, and reduced or obstructed detection of sound/wave propagation. This is also results in the failure to define shear wave speed or estimated Young’s modulus values for specific image pixels (inadequate TE color filling)[16]. Sometimes, focal steatosis can be mistakenly identified as solid lesions, while other pathologies can mimic MASLD. For instance, localized accumulation of fat in liver can be often associated with vascular anomalies, use of specific steatogenic drugs, inherited metabolic disorders, or HCC[7,91,136].
Nevertheless, very high reliable AUROC values (used to distinguish between disease and non-disease) were reported for TE application in MASLD patients[137-139]. For instance, in patients with advanced fibrosis (F3-F4) (452 patients, MASLD was confirmed by biopsy/IHC), AUROC value for TE was 0.83[34,38,79]. Good LS accuracy for diagnosis of steatosis grades 2 and 3 was reported by Eddowes et al[137] (AUROC of 0.80). Despite this, recent meta-analyses demonstrated that higher grades of liver steatosis (MASLD/MASH/steatosis stages with liver inflammation) could not be differentiated properly by TE in patients with morbid obesity[139]. IHC-based steatosis grades did not always match well with CAP data[113,116,125]. For comparison, effective TE diagnostics of steatosis was reported in patients with viral hepatitis[50,129,130,136], but not in patients with MASLD[121,140]. Therefore, different CAP cutoffs were introduced for patients with high BMI, diabetes, high liver enzymes (AST), and sex (steroid hormones level). It has been suggested that during potential MASLD screening in obese patients, CAP values should be studied and adjusted/compared using several reference methods (not only the traditional IHC reference standard)[16,118,140]. TE adjustment (calibration and addition of other scores) may be required to achieve higher TE sensitivity in all patients with various metabolic pathologies[38,126,127,140].
TE/FibroScan® was designed to assess liver elasticity and possible development of liver fibrosis. This non-invasive assessment of liver steatosis and fibrosis is important for risk-stratification in patients with MASLD. Moreover, it is very useful to monitor disease trajectory and provide re-evaluation of liver conditions over time, particularly after therapies. Therefore, non-invasive TE assessment guidelines were set to the highest standards by AGA and other professional organizations, which currently develop and adjust the protocol for TE diagnostic procedures and disease monitoring steps[76,135]. However, MASLD encompasses a spectrum of metabolic pathologies which require a multidisciplinary diagnostic approach. Consequently, multicomponent investigation (diagnostics) method should be adapted for liver assessment in patients with MASLD[47,91,141]. Sequential combination of tests (multistep approach) allows to increase the predictive values for TE[141-143].
Currently available non-invasive tests, including TE/FibroScan® demonstrated suboptimal values for sensitivity and specificity in obese MASLD patients in many studies[126,135,141,144], confirming a need for sequential combinations of assessments. Currently available serum biomarkers for recognition of low-grade steatosis from advanced disease (MASH), such as ALT-levels and cytokeratin-18 fragments, were also reported as suboptimal[37]. However, better sensitivity and specificity was reported for sequential combined tests. For instance, the Bordeaux algorithm suggested a combination of tests which includes AST-to-platelet ratio index (APRI) and FibroTest (FibroScan®) plan[135,138,144,145]. The FAST score was validated globally and demonstrated a very good functional reliability (AUROC ranging from 0.74 to 0.95 in different cohorts)[76,139,140]. Another successful diagnostic approach recommended to calculate FIB-4 and APRI for the initial assessment, exclude the patients with unlikely fibrosis, and focus on those patients who has FIB-4 > 1.5. Those patients should be monitored using TE/FibroScan® and/or other relevant methods[50]. The time of initial assessment is the critical point when clinicians can identify a patient at the increased risk and define the appropriate pathway for the disease monitoring and treatment, minimizing the potential risk of disease-related complications.
Modified APRI was introduced recently as a better diagnostic parameter, named NAFLD fibrosis index (NFI), for assessment of fibrosis in MASLD patients[118,146]. NFI formula combines 8 indicators, including age, platelet count, postprandial plasma glucose level, conjugated bilirubin, ALT, AST, total iron binding capacity, and the level of parathyroid hormone[138,141]. Interestingly, for the identification of advanced fibrosis NFI demonstrated improved diagnostic accuracy, compared to various clinical scoring formulas (FIB-4, APRI, and BMI-AST-to-ALT ratio-diabetes mellitus). However, the NFI performance characteristics did not exceed scoring effectiveness of FibroScan®[138]. Another reliable method to increase diagnostic accuracy is to combine TE with several serum-based indicators/scores (such as hemoglobin A1c and alkaline phosphatase values)[123]. Blood serum-based and clinical characteristics score was constructed recently using the triglyceride-glucose index[147]. The index was effectively used to identify MASLD[147], but it should be combined with TE to score fibrosis. However, sequential TE-based monitoring of therapeutic inter
Recently, a novel algorithm for assessment and monitoring of patients with MASLD has been suggested[143]. Using retrospective data analysis, it was demonstrated that FibroScan® provides additional information and complement the FIB-4 scores. Best practice advice for patients with MASLD and advanced cirrhosis/fibrosis indicated that serial FibroScan® assessments combined with blood tests should be implemented[143]. The study suggested that a FIB-4 < 1.3 should be used as a negative indicator for hepatic fibrosis, while FIB-4 > 1.3 should be combined with 2 or more non-invasive assessments (serum biomarkers and imaging-based scores) for further disease staging and risk stratification. To optimize prediction of the disease grade and identify patients with advanced fibrosis, the study suggested using clinical data (such as physical examination, endoscopic, and biochemical reports) and adapt periodical LS monitoring using TE/FibroScan® method[143].
Other promising scoring tests for MASLD include agile 3+ and agile 4 which were effective in predicting HCC, HCC/cirrhosis-related death, various hepatic decompensation (ascites, variceal hemorrhage, hepatic encephalopathy), or progression towards liver transplantation[126]. Agile scoring was tested in a large cohort study which included 16603 patients with MASLD in several countries (the United States, Europe, and Asia)[126]. Furthermore, 'liquid' biomarkers were recently reported to be successful for diagnostics of liver fibrogenesis. The test is based on the scoring of collagen-derived markers [N-terminal propeptide of collagen (PRO)-C3 or PRO-C6]. The method was defined as 'multi-omics' technology associated with testing of proteomics and microRNAs levels. The “liquid” biopsy test aims to estimate intrahepatic disease level and can be compared to the liver biopsy/IHC. For comparison, FibroScan® and other ultrasound-based methods (such as MR-based elastography and proton density fat fractioning), were defined as “dry” testing methods and demonstrated very high level of accuracy in the identification of patients at the highest risk of steatosis and fibrosis[148]. However, proper clinical testing is warranted to assess and compare the effectiveness of novel tests alone and in combinations with FibroScan®. To improve MASLD management, the complimentary use of “liquid” and “dry” biomarkers should be adjusted according to the clinical indications.
Considering the complexity of confounding factors and wide spectrum of MASLD, a prospective artificial intelligence assistance should be sought for the choice of proper protocols, risk stratification, and assessment of prognosis and treatment choices.
| 1. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2296] [Article Influence: 382.7] [Reference Citation Analysis (0)] |
| 2. | Mouralidarane A, Lin CI, Suleyman N, Soeda J, Oben JA. Practical management of the increasing burden of non-alcoholic fatty liver disease. Frontline Gastroenterol. 2010;1:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1315] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 4. | Marakovits C, Francis H. Unraveling the complexities of fibrosis and ductular reaction in liver disease: pathogenesis, mechanisms, and therapeutic insights. Am J Physiol Cell Physiol. 2024;326:C698-C706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Habibullah M, Jemmieh K, Ouda A, Haider MZ, Malki MI, Elzouki AN. Metabolic-associated fatty liver disease: a selective review of pathogenesis, diagnostic approaches, and therapeutic strategies. Front Med (Lausanne). 2024;11:1291501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 6. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2836] [Article Influence: 567.2] [Reference Citation Analysis (1)] |
| 7. | Satapathy SK, Sanyal AJ. Epidemiology and Natural History of Nonalcoholic Fatty Liver Disease. Semin Liver Dis. 2015;35:221-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 8. | Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician. 2006;74:756-762. [PubMed] |
| 9. | GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1158] [Cited by in RCA: 994] [Article Influence: 198.8] [Reference Citation Analysis (4)] |
| 10. | Kanda T, Goto T, Hirotsu Y, Moriyama M, Omata M. Molecular Mechanisms Driving Progression of Liver Cirrhosis towards Hepatocellular Carcinoma in Chronic Hepatitis B and C Infections: A Review. Int J Mol Sci. 2019;20:1358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 11. | Grant A, Neuberger J. Guidelines on the use of liver biopsy in clinical practice. British Society of Gastroenterology. Gut. 1999;45 Suppl 4:IV1-IV11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 186] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Srivastava A, Jong S, Gola A, Gailer R, Morgan S, Sennett K, Tanwar S, Pizzo E, O'Beirne J, Tsochatzis E, Parkes J, Rosenberg W. Cost-comparison analysis of FIB-4, ELF and fibroscan in community pathways for non-alcoholic fatty liver disease. BMC Gastroenterol. 2019;19:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 13. | Lemmer P, Rohr LC, Henning M, Bulut K, Manka P, Canbay A, Sowa JP. Liver Stiffness Determined by Transient Elastography Is a Simple and Highly Accurate Predictor for Presence of Liver Cirrhosis in Clinical Routine. Dig Dis. 2024;42:265-275. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Barr RG. Shear wave liver elastography. Abdom Radiol (NY). 2018;43:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 15. | Barr RG, Wilson SR, Rubens D, Garcia-Tsao G, Ferraioli G. Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology. 2020;296:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (1)] |
| 16. | Ozturk A, Olson MC, Samir AE, Venkatesh SK. Liver fibrosis assessment: MR and US elastography. Abdom Radiol (NY). 2022;47:3037-3050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 17. | Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, Yoneda M, Taguri M, Hyogo H, Sumida Y, Ono M, Eguchi Y, Inoue T, Yamanaka T, Wada K, Saito S, Nakajima A. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology. 2016;150:626-637.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 597] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 18. | Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, Sy E, Savides MT, Alquiraish MH, Valasek MA, Rizo E, Richards L, Brenner D, Sirlin CB, Loomba R. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:598-607.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 543] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 19. | Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1212] [Article Influence: 303.0] [Reference Citation Analysis (0)] |
| 20. | He QJ, Li YF, Zhao LT, Lin CT, Yu CY, Wang D. Recent advances in age-related metabolic dysfunction-associated steatotic liver disease. World J Gastroenterol. 2024;30:652-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 21. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7541] [Article Influence: 837.9] [Reference Citation Analysis (0)] |
| 22. | Feng G, Targher G, Byrne CD, Yilmaz Y, Wai-Sun Wong V, Adithya Lesmana CR, Adams LA, Boursier J, Papatheodoridis G, El-Kassas M, Méndez-Sánchez N, Sookoian S, Castera L, Chan WK, Ye F, Treeprasertsuk S, Cortez-Pinto H, Yu HH, Kim W, Romero-Gómez M, Nakajima A, Win KM, Kim SU, Holleboom AG, Sebastiani G, Ocama P, Ryan JD, Lupșor-Platon M, Ghazinyan H, Al-Mahtab M, Hamid S, Perera N, Alswat KA, Pan Q, Long MT, Isakov V, Mi M, Arrese M, Sanyal AJ, Sarin SK, Leite NC, Valenti L, Newsome PN, Hagström H, Petta S, Yki-Järvinen H, Schattenberg JM, Castellanos Fernández MI, Leclercq IA, Aghayeva G, Elzouki AN, Tumi A, Sharara AI, Labidi A, Sanai FM, Matar K, Al-Mattooq M, Akroush MW, Benazzouz M, Debzi N, Alkhatry M, Barakat S, Al-Busafi SA, Rwegasha J, Yang W, Adwoa A, Opio CK, Sotoudeheian M, Wong YJ, George J, Zheng MH. Global burden of metabolic dysfunction-associated steatotic liver disease, 2010 to 2021. JHEP Rep. 2025;7:101271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 23. | Bedossa P. Pathology of non-alcoholic fatty liver disease. Liver Int. 2017;37 Suppl 1:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 238] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 24. | Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1028] [Cited by in RCA: 1710] [Article Influence: 244.3] [Reference Citation Analysis (0)] |
| 25. | Nakamura T, Masuda A, Nakano D, Amano K, Sano T, Nakano M, Kawaguchi T. Pathogenic Mechanisms of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)-Associated Hepatocellular Carcinoma. Cells. 2025;14:428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Tang C, Peng D, Zong K, Wu Z, Gong M, Li H, Huang Z, Li S. Association between the lymphocyte-to-high-density lipoprotein ratio and metabolic dysfunction-associated steatotic liver disease among US adults: a cross-sectional study from NHANES 2017 to 2020. BMC Gastroenterol. 2024;24:470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | De A, Bhagat N, Mehta M, Taneja S, Duseja A. Metabolic dysfunction-associated steatotic liver disease (MASLD) definition is better than MAFLD criteria for lean patients with NAFLD. J Hepatol. 2024;80:e61-e62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 71] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 28. | Boccatonda A, Andreetto L, D'Ardes D, Cocco G, Rossi I, Vicari S, Schiavone C, Cipollone F, Guagnano MT. From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications. Biomedicines. 2023;11:883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 29. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8239] [Article Influence: 412.0] [Reference Citation Analysis (5)] |
| 30. | Kwok R, Tse YK, Wong GL, Ha Y, Lee AU, Ngu MC, Chan HL, Wong VW. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease--the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2014;39:254-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 293] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 31. | Petta S, Wai-Sun Wong V, Bugianesi E, Fracanzani AL, Cammà C, Hiriart JB, Lai-Hung Wong G, Vergniol J, Wing-Hung Chan A, Giannetti A, Merrouche W, Lik-Yuen Chan H, Le-Bail B, Lombardi R, Guastella S, Craxì A, de Ledinghen V. Impact of Obesity and Alanine Aminotransferase Levels on the Diagnostic Accuracy for Advanced Liver Fibrosis of Noninvasive Tools in Patients With Nonalcoholic Fatty Liver Disease. Am J Gastroenterol. 2019;114:916-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 32. | Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, Chim AM, Yu J, Sung JJ, Chan HL. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 491] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 33. | Stefanescu H, Procopet B. Noninvasive assessment of portal hypertension in cirrhosis: liver stiffness and beyond. World J Gastroenterol. 2014;20:16811-16819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Petta S, Sebastiani G, Viganò M, Ampuero J, Wai-Sun Wong V, Boursier J, Berzigotti A, Bugianesi E, Fracanzani AL, Cammà C, Enea M, Grottes MD, Di Marco V, Younes R, Keyrouz A, Mazzola S, Mendoza Y, Pennisi G, Romero-Gomez M, Craxì A, de Ledinghen V. Monitoring Occurrence of Liver-Related Events and Survival by Transient Elastography in Patients With Nonalcoholic Fatty Liver Disease and Compensated Advanced Chronic Liver Disease. Clin Gastroenterol Hepatol. 2021;19:806-815.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 35. | Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, Ishigami M, Toyoda H, Wai-Sun Wong V, Peleg N, Shlomai A, Sebastiani G, Seko Y, Bhala N, Younossi ZM, Anstee QM, McPherson S, Newsome PN. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;158:1611-1625.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 739] [Article Influence: 147.8] [Reference Citation Analysis (0)] |
| 36. | Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377:2063-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 919] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 37. | Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L, Negro F, Caldwell SH, Ratziu V, Corey KE, Friedman SL, Abdelmalek MF, Harrison SA, Sanyal AJ, Lavine JE, Mathurin P, Charlton MR, Goodman ZD, Chalasani NP, Kowdley KV, George J, Lindor K. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology. 2018;68:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 316] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 38. | Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, Fouchard-Hubert I, Gallois Y, Oberti F, Bertrais S, Calès P; Multicentric Group from ANRS/HC/EP23 FIBROSTAR Studies. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 496] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 39. | Lai LL, Wan Yusoff WNI, Vethakkan SR, Nik Mustapha NR, Mahadeva S, Chan WK. Screening for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus using transient elastography. J Gastroenterol Hepatol. 2019;34:1396-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2014;29:1470-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 41. | McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, Oliveira CP, Francque S, Van Gaal L, Schattenberg JM, Tiniakos D, Burt A, Bugianesi E, Ratziu V, Day CP, Anstee QM. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol. 2017;112:740-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 644] [Cited by in RCA: 651] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 42. | Petta S, Wong VW, Cammà C, Hiriart JB, Wong GL, Marra F, Vergniol J, Chan AW, Di Marco V, Merrouche W, Chan HL, Barbara M, Le-Bail B, Arena U, Craxì A, de Ledinghen V. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology. 2017;65:1145-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 43. | Wang AY, Dhaliwal J, Mouzaki M. Lean non-alcoholic fatty liver disease. Clin Nutr. 2019;38:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 44. | Kim D, Kim WR. Nonobese Fatty Liver Disease. Clin Gastroenterol Hepatol. 2017;15:474-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 260] [Article Influence: 32.5] [Reference Citation Analysis (1)] |
| 45. | Leung JC, Loong TC, Wei JL, Wong GL, Chan AW, Choi PC, Shu SS, Chim AM, Chan HL, Wong VW. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 275] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 46. | Sookoian S, Pirola CJ. Systematic review with meta-analysis: risk factors for non-alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment Pharmacol Ther. 2017;46:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 47. | Wang W, Ren J, Zhou W, Huang J, Wu G, Yang F, Yuan S, Fang J, Liu J, Jin Y, Qi H, Miao Y, Le Y, Ge C, Qiu X, Wang J, Huang P, Liu Z, Wang S. Lean non-alcoholic fatty liver disease (Lean-NAFLD) and the development of metabolic syndrome: a retrospective study. Sci Rep. 2022;12:10977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 48. | Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, Contos MJ, Sterling RK, Fuchs M, Zhou H, Watkins SM, Sanyal AJ. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 521] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 49. | Pavithra N, Bannikoppa PS, Uthappa S, Kurpad AV, Mani I. Plasma Fatty Acid Composition and Estimated Desaturase Activities Reflect Dietary Patterns in Subjects with Metabolic Syndrome. Indian J Clin Biochem. 2018;33:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Trelsgård AM, Mulabecirovic A, Leiva RA, Nordaas IK, Mjelle AB, Gilja OH, Havre RF. Multiparametric liver assessment in patients successfully treated for hepatitis C: a 4-year follow-up. Scand J Gastroenterol. 2024;59:1184-1191. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 51. | Andreozzi F, Mancuso E, Mazza E, Mannino GC, Fiorentino TV, Arturi F, Succurro E, Perticone M, Sciacqua A, Montalcini T, Pujia A, Sesti G. One-hour post-load glucose levels are associated with hepatic steatosis assessed by transient elastography. Diabetes Obes Metab. 2024;26:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 52. | Nishida T. Diagnosis and Clinical Implications of Diabetes in Liver Cirrhosis: A Focus on the Oral Glucose Tolerance Test. J Endocr Soc. 2017;1:886-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Watt MJ, Miotto PM, De Nardo W, Montgomery MK. The Liver as an Endocrine Organ-Linking NAFLD and Insulin Resistance. Endocr Rev. 2019;40:1367-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 416] [Article Influence: 69.3] [Reference Citation Analysis (2)] |
| 54. | Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 701] [Article Influence: 38.9] [Reference Citation Analysis (1)] |
| 55. | Kosmalski M, Ziółkowska S, Czarny P, Szemraj J, Pietras T. The Coexistence of Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. J Clin Med. 2022;11:1375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 56. | Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care. 2018;41:372-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 429] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 57. | Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 2920] [Article Influence: 417.1] [Reference Citation Analysis (1)] |
| 58. | Subramanian P, Chavakis T. The complex function of macrophages and their subpopulations in metabolic injury associated fatty liver disease. J Physiol. 2023;601:1159-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 59. | Hu D, Wang P, Wang X, Hu X, Huang D, Yan W, Xi D, Han M, Ning Q, Wang H. Disease severity and antiviral response in patients with chronic hepatitis B with non-obese NAFLD. J Formos Med Assoc. 2024;123:773-780. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 60. | Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A, Moscarella S, Belli G, Zignego AL, Marra F, Laffi G, Pinzani M. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 527] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 61. | Reiberger T, Ferlitsch A, Payer BA, Pinter M, Homoncik M, Peck-Radosavljevic M; Vienna Hepatic Hemodynamic Lab. Non-selective β-blockers improve the correlation of liver stiffness and portal pressure in advanced cirrhosis. J Gastroenterol. 2012;47:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 62. | Mladenić K, Lenartić M, Marinović S, Polić B, Wensveen FM. The "Domino effect" in MASLD: The inflammatory cascade of steatohepatitis. Eur J Immunol. 2024;54:e2149641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 63. | Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor V, ElGindi M, Han M, Thornton AM, Zhang H, Egger M, Luo J, Felsher DW, McVicar DW, Weber A, Heikenwalder M, Greten TF. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 589] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 64. | Rakhra K, Bachireddy P, Zabuawala T, Zeiser R, Xu L, Kopelman A, Fan AC, Yang Q, Braunstein L, Crosby E, Ryeom S, Felsher DW. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell. 2010;18:485-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 294] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 65. | Chung KW, Lee EK, Kim DH, An HJ, Kim ND, Im DS, Lee J, Yu BP, Chung HY. Age-related sensitivity to endotoxin-induced liver inflammation: Implication of inflammasome/IL-1β for steatohepatitis. Aging Cell. 2015;14:524-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Thibaut R, Gage MC, Pineda-Torra I, Chabrier G, Venteclef N, Alzaid F. Liver macrophages and inflammation in physiology and physiopathology of non-alcoholic fatty liver disease. FEBS J. 2022;289:3024-3057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 67. | Kim SY, Jeong JM, Kim SJ, Seo W, Kim MH, Choi WM, Yoo W, Lee JH, Shim YR, Yi HS, Lee YS, Eun HS, Lee BS, Chun K, Kang SJ, Kim SC, Gao B, Kunos G, Kim HM, Jeong WI. Pro-inflammatory hepatic macrophages generate ROS through NADPH oxidase 2 via endocytosis of monomeric TLR4-MD2 complex. Nat Commun. 2017;8:2247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 68. | Govaere O, Petersen SK, Martinez-Lopez N, Wouters J, Van Haele M, Mancina RM, Jamialahmadi O, Bilkei-Gorzo O, Lassen PB, Darlay R, Peltier J, Palmer JM, Younes R, Tiniakos D, Aithal GP, Allison M, Vacca M, Göransson M, Berlinguer-Palmini R, Clark JE, Drinnan MJ, Yki-Järvinen H, Dufour JF, Ekstedt M, Francque S, Petta S, Bugianesi E, Schattenberg JM, Day CP, Cordell HJ, Topal B, Clément K, Romeo S, Ratziu V, Roskams T, Daly AK, Anstee QM, Trost M, Härtlova A. Macrophage scavenger receptor 1 mediates lipid-induced inflammation in non-alcoholic fatty liver disease. J Hepatol. 2022;76:1001-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 69. | Sawada K, Ohtake T, Hasebe T, Abe M, Tanaka H, Ikuta K, Suzuki Y, Fujiya M, Hasebe C, Kohgo Y. Augmented hepatic Toll-like receptors by fatty acids trigger the pro-inflammatory state of non-alcoholic fatty liver disease in mice. Hepatol Res. 2014;44:920-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Dudek M, Pfister D, Donakonda S, Filpe P, Schneider A, Laschinger M, Hartmann D, Hüser N, Meiser P, Bayerl F, Inverso D, Wigger J, Sebode M, Öllinger R, Rad R, Hegenbarth S, Anton M, Guillot A, Bowman A, Heide D, Müller F, Ramadori P, Leone V, Garcia-Caceres C, Gruber T, Seifert G, Kabat AM, Mallm JP, Reider S, Effenberger M, Roth S, Billeter AT, Müller-Stich B, Pearce EJ, Koch-Nolte F, Käser R, Tilg H, Thimme R, Boettler T, Tacke F, Dufour JF, Haller D, Murray PJ, Heeren R, Zehn D, Böttcher JP, Heikenwälder M, Knolle PA. Auto-aggressive CXCR6(+) CD8 T cells cause liver immune pathology in NASH. Nature. 2021;592:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 316] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 71. | Hung HC, Tsai SF, Chou HW, Tsai MJ, Hsu PL, Kuo YM. Dietary fatty acids differentially affect secretion of pro-inflammatory cytokines in human THP-1 monocytes. Sci Rep. 2023;13:5511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 72. | Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 1168] [Article Influence: 584.0] [Reference Citation Analysis (1)] |
| 73. | Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics. 2017;7:1303-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 1098] [Article Influence: 137.3] [Reference Citation Analysis (0)] |
| 74. | Bassal T, Basheer M, Boulos M, Assy N. Nonalcoholic Fatty Liver Disease-A Concise Review of Noninvasive Tests and Biomarkers. Metabolites. 2022;12:1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, Yilmaz Y, Czernichow S, Zheng MH, Wong VW, Allison M, Tsochatzis E, Anstee QM, Sheridan DA, Eddowes PJ, Guha IN, Cobbold JF, Paradis V, Bedossa P, Miette V, Fournier-Poizat C, Sandrin L, Harrison SA. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 550] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 76. | Lim JK, Flamm SL, Singh S, Falck-Ytter YT; Clinical Guidelines Committee of the American Gastroenterological Association. American Gastroenterological Association Institute Guideline on the Role of Elastography in the Evaluation of Liver Fibrosis. Gastroenterology. 2017;152:1536-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 77. | Shi J, Sun Q, Wang Y, Jing X, Ding J, Yuan Q, Ren C, Shan S, Wang Y, Du Z. Comparison of microwave ablation and surgical resection for treatment of hepatocellular carcinomas conforming to Milan criteria. J Gastroenterol Hepatol. 2014;29:1500-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 78. | de Lédinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, Merrouche W, Foucher J, Brigitte le B. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 280] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 79. | Vali Y, Lee J, Boursier J, Spijker R, Löffler J, Verheij J, Brosnan MJ, Böcskei Z, Anstee QM, Bossuyt PM, Zafarmand MH; LITMUS systematic review team(†). Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: A systematic review and meta-analysis. J Hepatol. 2020;73:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 80. | Day JW, Rosenberg WM. The enhanced liver fibrosis (ELF) test in diagnosis and management of liver fibrosis. Br J Hosp Med (Lond). 2018;79:694-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Kjaergaard M, Lindvig KP, Thorhauge KH, Andersen P, Hansen JK, Kastrup N, Jensen JM, Hansen CD, Johansen S, Israelsen M, Torp N, Trelle MB, Shan S, Detlefsen S, Antonsen S, Andersen JE, Graupera I, Ginés P, Thiele M, Krag A. Using the ELF test, FIB-4 and NAFLD fibrosis score to screen the population for liver disease. J Hepatol. 2023;79:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 105] [Reference Citation Analysis (0)] |
| 82. | Mózes FE, Lee JA, Selvaraj EA, Jayaswal ANA, Trauner M, Boursier J, Fournier C, Staufer K, Stauber RE, Bugianesi E, Younes R, Gaia S, Lupșor-Platon M, Petta S, Shima T, Okanoue T, Mahadeva S, Chan WK, Eddowes PJ, Hirschfield GM, Newsome PN, Wong VW, de Ledinghen V, Fan J, Shen F, Cobbold JF, Sumida Y, Okajima A, Schattenberg JM, Labenz C, Kim W, Lee MS, Wiegand J, Karlas T, Yılmaz Y, Aithal GP, Palaniyappan N, Cassinotto C, Aggarwal S, Garg H, Ooi GJ, Nakajima A, Yoneda M, Ziol M, Barget N, Geier A, Tuthill T, Brosnan MJ, Anstee QM, Neubauer S, Harrison SA, Bossuyt PM, Pavlides M; LITMUS Investigators. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut. 2022;71:1006-1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 308] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 83. | McPherson S, Armstrong MJ, Cobbold JF, Corless L, Anstee QM, Aspinall RJ, Barclay ST, Brennan PN, Cacciottolo TM, Goldin RD, Hallsworth K, Hebditch V, Jack K, Jarvis H, Johnson J, Li W, Mansour D, McCallum M, Mukhopadhya A, Parker R, Ross V, Rowe IA, Srivastava A, Thiagarajan P, Thompson AI, Tomlinson J, Tsochatzis EA, Yeoman A, Alazawi W. Quality standards for the management of non-alcoholic fatty liver disease (NAFLD): consensus recommendations from the British Association for the Study of the Liver and British Society of Gastroenterology NAFLD Special Interest Group. Lancet Gastroenterol Hepatol. 2022;7:755-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 84. | Canivet CM, Costentin C, Irvine KM, Delamarre A, Lannes A, Sturm N, Oberti F, Patel PJ, Decaens T, Irles-Depé M, Fouchard I, Hermabessière P, Roux M, Barthelon J, Calès P, Powell EE, de Ledinghen V, Boursier J. Validation of the new 2021 EASL algorithm for the noninvasive diagnosis of advanced fibrosis in NAFLD. Hepatology. 2023;77:920-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 85. | Graupera I, Thiele M, Serra-Burriel M, Caballeria L, Roulot D, Wong GL, Fabrellas N, Guha IN, Arslanow A, Expósito C, Hernández R, Aithal GP, Galle PR, Pera G, Wong VW, Lammert F, Ginès P, Castera L, Krag A; Investigators of the LiverScreen Consortium. Low Accuracy of FIB-4 and NAFLD Fibrosis Scores for Screening for Liver Fibrosis in the Population. Clin Gastroenterol Hepatol. 2022;20:2567-2576.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 153] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 86. | Ferraioli G, De Silvestri A, Lissandrin R, Maiocchi L, Tinelli C, Filice C, Barr RG. Evaluation of Inter-System Variability in Liver Stiffness Measurements. Ultraschall Med. 2019;40:64-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 87. | Yin M, Talwalkar JA, Glaser KJ, Venkatesh SK, Chen J, Manduca A, Ehman RL. Dynamic postprandial hepatic stiffness augmentation assessed with MR elastography in patients with chronic liver disease. AJR Am J Roentgenol. 2011;197:64-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 88. | Berger A, Shili S, Zuberbuhler F, Hiriart JB, Lannes A, Chermak F, Hunault G, Foucher J, Oberti F, Fouchard-Hubert I, Cales P, de Ledinghen V, Boursier J. Liver Stiffness Measurement With FibroScan: Use the Right Probe in the Right Conditions! Clin Transl Gastroenterol. 2019;10:e00023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 89. | Ferraioli G, Barr RG. Recent advances in noninvasive assessment of liver steatosis. Pol Arch Intern Med. 2024;134:16703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 90. | Myers RP, Pomier-Layrargues G, Kirsch R, Pollett A, Duarte-Rojo A, Wong D, Beaton M, Levstik M, Crotty P, Elkashab M. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012;55:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 380] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 91. | Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D'Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Gilja OH, Havre RF, Jenssen C, Klauser AS, Ohlinger R, Saftoiu A, Schaefer F, Sporea I, Piscaglia F. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 772] [Article Influence: 64.3] [Reference Citation Analysis (1)] |
| 92. | Giuffrè M, Giuricin M, Bonazza D, Rosso N, Giraudi PJ, Masutti F, Palmucci S, Basile A, Zanconati F, de Manzini N, Tiribelli C, Palmisano S, Crocè LS. Optimization of Point-Shear Wave Elastography by Skin-to-Liver Distance to Assess Liver Fibrosis in Patients Undergoing Bariatric Surgery. Diagnostics (Basel). 2020;10:795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 93. | Nadebaum DP, Nicoll AJ, Sood S, Gorelik A, Gibson RN. Variability of Liver Shear Wave Measurements Using a New Ultrasound Elastographic Technique. J Ultrasound Med. 2018;37:647-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 94. | Wan T, Köhn N, Kröll D, Berzigotti A. Applicability and Results of Liver Stiffness Measurement and Controlled Attenuation Parameter Using XL Probe for Metabolic-Associated Fatty Liver Disease in Candidates to Bariatric Surgery. A Single-Center Observational Study. Obes Surg. 2021;31:702-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 95. | Yang A, Nguyen M, Ju I, Brancatisano A, Ryan B, van der Poorten D. Utility of Fibroscan XL to assess the severity of non-alcoholic fatty liver disease in patients undergoing bariatric surgery. Sci Rep. 2021;11:14006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 96. | Avcu A, Kaya E, Yilmaz Y. Feasibility of Fibroscan in Assessment of Hepatic Steatosis and Fibrosis in Obese Patients: Report From a General Internal Medicine Clinic. Turk J Gastroenterol. 2021;32:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 97. | Cardoso AC, Tovo CV, Leite NC, El Bacha IA, Calçado FL, Coral GP, Sammarco GN, Cravo C, Carvalho Filho RJ, de Mello Perez R, Luiz RR, Parise ER, Villela-Nogueira CA. Validation and Performance of FibroScan®-AST (FAST) Score on a Brazilian Population with Nonalcoholic Fatty Liver Disease. Dig Dis Sci. 2022;67:5272-5279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Hirooka M, Koizumi Y, Yano R, Sunago K, Watanabe T, Yoshida O, Tokumoto Y, Abe M, Hiasa Y. Validation of the FibroScan-aspartate aminotransferase score by vibration-controlled transient and B-mode ultrasound elastography. Hepatol Res. 2021;51:652-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Ozturk A, Grajo JR, Dhyani M, Anthony BW, Samir AE. Principles of ultrasound elastography. Abdom Radiol (NY). 2018;43:773-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 100. | Gilligan LA, Trout AT, Bennett P, Dillman JR. Repeatability and Agreement of Shear Wave Speed Measurements in Phantoms and Human Livers Across 6 Ultrasound 2-Dimensional Shear Wave Elastography Systems. Invest Radiol. 2020;55:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 101. | Cassinotto C, Boursier J, de Lédinghen V, Lebigot J, Lapuyade B, Cales P, Hiriart JB, Michalak S, Bail BL, Cartier V, Mouries A, Oberti F, Fouchard-Hubert I, Vergniol J, Aubé C. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 390] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 102. | Xu XY, Wang WS, Zhang QM, Li JL, Sun JB, Qin TT, Liu HB. Performance of common imaging techniques vs serum biomarkers in assessing fibrosis in patients with chronic hepatitis B: A systematic review and meta-analysis. World J Clin Cases. 2019;7:2022-2037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 103. | Wang HW, Shi HN, Cheng J, Xie F, Luo YK, Tang J. Real-time shear wave elastography (SWE) assessment of short- and long-term treatment outcome in Budd-Chiari syndrome: A pilot study. PLoS One. 2018;13:e0197550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 104. | Ichikawa K, Narita Y, Ota Y, Komatsu N, Koike M. Transient elastography-derived liver stiffness measurements were found to be useful for predicting liver infiltration in a case of mature T-cell neoplasm involving liver dysfunction. Int J Clin Exp Pathol. 2015;8:4220-4226. [PubMed] |
| 105. | Naganuma H, Ishida H. Factors other than fibrosis that increase measured shear wave velocity. World J Gastroenterol. 2022;28:6512-6521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 106. | Kubo K, Kawakami H, Kuwatani M, Nishida M, Kawakubo K, Kawahata S, Taya Y, Kubota Y, Amano T, Shirato H, Sakamoto N. Liver elasticity measurement before and after biliary drainage in patients with obstructive jaundice: a prospective cohort studya prospective cohort study. BMC Gastroenterol. 2016;16:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 107. | Wang J, Hu M, Zhu Q, Sun L. Liver stiffness assessed by real-time two-dimensional shear wave elastography predicts hypersplenism in patients with Wilson's disease: a prospective study. BMC Med Imaging. 2022;22:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 108. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2294] [Article Influence: 229.4] [Reference Citation Analysis (3)] |
| 109. | Ferraioli G, Wong VW, Castera L, Berzigotti A, Sporea I, Dietrich CF, Choi BI, Wilson SR, Kudo M, Barr RG. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med Biol. 2018;44:2419-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 372] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 110. | Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 509] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 111. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G, Marra F, Pinzani M. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 574] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 112. | Sasso M, Tengher-Barna I, Ziol M, Miette V, Fournier C, Sandrin L, Poupon R, Cardoso AC, Marcellin P, Douvin C, de Ledinghen V, Trinchet JC, Beaugrand M. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan(®): validation in chronic hepatitis C. J Viral Hepat. 2012;19:244-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 113. | Kumar M, Rastogi A, Singh T, Behari C, Gupta E, Garg H, Kumar R, Bhatia V, Sarin SK. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis: does etiology affect performance? J Gastroenterol Hepatol. 2013;28:1194-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 114. | Jung KS, Kim BK, Kim SU, Chon YE, Chun KH, Kim SB, Lee SH, Ahn SS, Park JY, Kim DY, Ahn SH, Park YN, Han KH. Factors affecting the accuracy of controlled attenuation parameter (CAP) in assessing hepatic steatosis in patients with chronic liver disease. PLoS One. 2014;9:e98689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 115. | Mi YQ, Shi QY, Xu L, Shi RF, Liu YG, Li P, Shen F, Lu W, Fan JG. Controlled attenuation parameter for noninvasive assessment of hepatic steatosis using Fibroscan®: validation in chronic hepatitis B. Dig Dis Sci. 2015;60:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 116. | Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 386] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 117. | Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, Stiefel P, Pöschl G, Büchler MW, Seitz HK, Mueller S. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 403] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 118. | Ozturk A, Mohammadi R, Pierce TT, Kamarthi S, Dhyani M, Grajo JR, Corey KE, Chung RT, Bhan AK, Chhatwal J, Samir AE. Diagnostic Accuracy of Shear Wave Elastography as a Non-invasive Biomarker of High-Risk Non-alcoholic Steatohepatitis in Patients with Non-alcoholic Fatty Liver Disease. Ultrasound Med Biol. 2020;46:972-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 119. | Trifanov DS, Dhyani M, Bledsoe JR, Misdraji J, Bhan AK, Chung RT, Samir AE. Amyloidosis of the liver on shear wave elastography: case report and review of literature. Abdom Imaging. 2015;40:3078-3083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 120. | Huang R, Gao ZH, Tang A, Sebastiani G, Deschenes M. Transient elastography is an unreliable marker of liver fibrosis in patients with portal vein thrombosis. Hepatology. 2018;68:783-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 121. | Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 463] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 122. | Sharma D, Choudhary NS, Dhampalwar S, Saraf N, Duseja A, Gautam D, Soin AS, Sud R. Liver Stiffness Values in Persons with Normal Histology. J Clin Exp Hepatol. 2023;13:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 123. | Ali AH, Al Juboori A, Petroski GF, Diaz-Arias AA, Syed-Abdul MM, Wheeler AA, Ganga RR, Pitt JB, Spencer NM, Hammoud GM, Rector RS, Parks EJ, Ibdah JA. The Utility and Diagnostic Accuracy of Transient Elastography in Adults with Morbid Obesity: A Prospective Study. J Clin Med. 2022;11:1201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 124. | Blank V, Petroff D, Wiegand J, Karlas T. M probe comes first: Impact of initial probe choice on diagnostic performance of vibration controlled transient elastography. Dig Liver Dis. 2022;54:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 125. | Nogami A, Iwaki M, Kobayashi T, Honda Y, Ogawa Y, Imajo K, Higurashi T, Hosono K, Kirikoshi H, Saito S, Nakajima A, Yoneda M. Real-world assessment of SmartExam, a novel FibroScan computational method: A retrospective single-center cohort study. J Gastroenterol Hepatol. 2023;38:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 126. | Lin H, Lee HW, Yip TC, Tsochatzis E, Petta S, Bugianesi E, Yoneda M, Zheng MH, Hagström H, Boursier J, Calleja JL, Goh GB, Chan WK, Gallego-Durán R, Sanyal AJ, de Lédinghen V, Newsome PN, Fan JG, Castéra L, Lai M, Harrison SA, Fournier-Poizat C, Wong GL, Pennisi G, Armandi A, Nakajima A, Liu WY, Shang Y, de Saint-Loup M, Llop E, Teh KK, Lara-Romero C, Asgharpour A, Mahgoub S, Chan MS, Canivet CM, Romero-Gomez M, Kim SU, Wong VW; VCTE-Prognosis Study Group. Vibration-Controlled Transient Elastography Scores to Predict Liver-Related Events in Steatotic Liver Disease. JAMA. 2024;331:1287-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 127. | Loustaud-Ratti VR, Cypierre A, Rousseau A, Yagoubi F, Abraham J, Fauchais AL, Carrier P, Lefebvre A, Bordessoule D, Vidal E, Sautereau D, Jaccard A. Non-invasive detection of hepatic amyloidosis: FibroScan, a new tool. Amyloid. 2011;18:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 128. | Lanzi A, Gianstefani A, Mirarchi MG, Pini P, Conti F, Bolondi L. Liver AL amyloidosis as a possible cause of high liver stiffness values. Eur J Gastroenterol Hepatol. 2010;22:895-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 129. | Mederacke I, Wursthorn K, Kirschner J, Rifai K, Manns MP, Wedemeyer H, Bahr MJ. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int. 2009;29:1500-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 130. | Arena U, Lupsor Platon M, Stasi C, Moscarella S, Assarat A, Bedogni G, Piazzolla V, Badea R, Laffi G, Marra F, Mangia A, Pinzani M. Liver stiffness is influenced by a standardized meal in patients with chronic hepatitis C virus at different stages of fibrotic evolution. Hepatology. 2013;58:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 131. | Pennisi G, Di Marco V, Buscemi C, Mazzola G, Randazzo C, Spatola F, Craxì A, Buscemi S, Petta S. Interplay between non-alcoholic fatty liver disease and cardiovascular risk in an asymptomatic general population. J Gastroenterol Hepatol. 2021;36:2389-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 132. | Boeckmans J, Sandrin L, Knackstedt C, Schattenberg JM. Liver stiffness as a cornerstone in heart disease risk assessment. Liver Int. 2024;44:344-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 133. | Hudson JM, Milot L, Parry C, Williams R, Burns PN. Inter- and intra-operator reliability and repeatability of shear wave elastography in the liver: a study in healthy volunteers. Ultrasound Med Biol. 2013;39:950-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 134. | Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1071] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 135. | Palmeri ML, Milkowski A, Barr R, Carson P, Couade M, Chen J, Chen S, Dhyani M, Ehman R, Garra B, Gee A, Guenette G, Hah Z, Lynch T, Macdonald M, Managuli R, Miette V, Nightingale KR, Obuchowski N, Rouze NC, Morris DC, Fielding S, Deng Y, Chan D, Choudhury K, Yang S, Samir AE, Shamdasani V, Urban M, Wear K, Xie H, Ozturk A, Qiang B, Song P, McAleavey S, Rosenzweig S, Wang M, Okamura Y, McLaughlin G, Chen Y, Napolitano D, Carlson L, Erpelding T, Hall TJ. Radiological Society of North America/Quantitative Imaging Biomarker Alliance Shear Wave Speed Bias Quantification in Elastic and Viscoelastic Phantoms. J Ultrasound Med. 2021;40:569-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 136. | Oeda S, Tanaka K, Oshima A, Matsumoto Y, Sueoka E, Takahashi H. Diagnostic Accuracy of FibroScan and Factors Affecting Measurements. Diagnostics (Basel). 2020;10:940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 137. | Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Guha IN, Cobbold JF, Deeks JJ, Paradis V, Bedossa P, Newsome PN. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1717-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 970] [Article Influence: 161.7] [Reference Citation Analysis (0)] |
| 138. | Yang X, Xia M, Chang X, Zhu X, Sun X, Yang Y, Wang L, Liu Q, Zhang Y, Xu Y, Lin H, Liu L, Yao X, Hu X, Gao J, Yan H, Gao X, Bian H. A novel model for detecting advanced fibrosis in patients with nonalcoholic fatty liver disease. Diabetes Metab Res Rev. 2022;38:e3570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 139. | Petroff D, Blank V, Newsome PN, Shalimar, Voican CS, Thiele M, de Lédinghen V, Baumeler S, Chan WK, Perlemuter G, Cardoso AC, Aggarwal S, Sasso M, Eddowes PJ, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Cobbold JF, Naveau S, Lupsor-Platon M, Mueller S, Krag A, Irles-Depe M, Semela D, Wong GL, Wong VW, Villela-Nogueira CA, Garg H, Chazouillères O, Wiegand J, Karlas T. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:185-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 168] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 140. | Troelstra MA, Witjes JJ, van Dijk AM, Mak AL, Gurney-Champion O, Runge JH, Zwirs D, Stols-Gonçalves D, Zwinderman AH, Ten Wolde M, Monajemi H, Ramsoekh S, Sinkus R, van Delden OM, Beuers UH, Verheij J, Nieuwdorp M, Nederveen AJ, Holleboom AG. Assessment of Imaging Modalities Against Liver Biopsy in Nonalcoholic Fatty Liver Disease: The Amsterdam NAFLD-NASH Cohort. J Magn Reson Imaging. 2021;54:1937-1949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 141. | Taru MG, Neamti L, Taru V, Procopciuc LM, Procopet B, Lupsor-Platon M. How to Identify Advanced Fibrosis in Adult Patients with Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH) Using Ultrasound Elastography-A Review of the Literature and Proposed Multistep Approach. Diagnostics (Basel). 2023;13:788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 142. | Lai JC, Liang LY, Wong GL. Noninvasive tests for liver fibrosis in 2024: are there different scales for different diseases? Gastroenterol Rep (Oxf). 2024;12:goae024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 143. | Wattacheril JJ, Abdelmalek MF, Lim JK, Sanyal AJ. AGA Clinical Practice Update on the Role of Noninvasive Biomarkers in the Evaluation and Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2023;165:1080-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 144. | Miranda J, Key Wakate Teruya A, Leão Filho H, Lahan-Martins D, Tamura Sttefano Guimarães C, de Paula Reis Guimarães V, Ide Yamauchi F, Blasbalg R, Velloni FG. Diffuse and focal liver fat: advanced imaging techniques and diagnostic insights. Abdom Radiol (NY). 2024;49:4437-4462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 145. | Kogachi S, Noureddin M. Noninvasive Evaluation for Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Clin Ther. 2021;43:455-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 146. | Huang C, Seah JJ, Tan CK, Kam JW, Tan J, Teo EK, Kwek A, Wong YJ, Tan M, Ang TL, Kumar R. Modified AST to platelet ratio index improves APRI and better predicts advanced fibrosis and liver cirrhosis in patients with non-alcoholic fatty liver disease. Clin Res Hepatol Gastroenterol. 2021;45:101528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 147. | Yu R, Xie W, Peng H, Lu L, Yin S, Xu S, Hu Z, Peng XE. Diagnostic value of triglyceride-glucose index and related parameters in metabolism-associated fatty liver disease in a Chinese population: a cross-sectional study. BMJ Open. 2023;13:e075413. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 148. | Armandi A, Michel M, Gjini K, Emrich T, Bugianesi E, Schattenberg JM. Emerging concepts in the detection of liver fibrosis in non-alcoholic fatty liver disease. Expert Rev Mol Diagn. 2023;23:771-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |