Published online Jul 27, 2025. doi: 10.4254/wjh.v17.i7.106291
Revised: April 10, 2025
Accepted: June 10, 2025
Published online: July 27, 2025
Processing time: 154 Days and 16.6 Hours
Autonomic dysfunction (AD) is frequently observed in cirrhotic patients and is associated with poor clinical outcomes and prognoses. Heart rate variability (HRV), a noninvasive tool for assessing autonomic nervous system balance, has been extensively studied in a variety of conditions, including chronic liver disease (CLD); however, no recent reviews have focused on its role in CLD. This article examines the mechanisms of AD in CLD and the foundation for HRV assessment, highlighting its diagnostic, prognostic, and therapeutic applications in CLD, including liver transplantation (LT). Changes in HRV, particularly in patients with cirrhotic complications, and its prognostic significance throughout the na
Core Tip: Autonomic dysfunction (AD) is common in cirrhotic patients and is linked to poor outcomes. Heart rate variability (HRV), a noninvasive measure of autonomic balance, could be useful in managing chronic liver disease (CLD) patients. This article explores AD mechanisms, HRV assessment, and its diagnostic, prognostic, and therapeutic relevance in CLD, including liver transplantation.
- Citation: Bustos N, Giubergia F, Mora C, Lara C, Urzúa Á, Cattaneo M, Poniachik J, Vera DB, Gajardo AI. Heart rate variability in the clinical assessment of patients with chronic liver disease. World J Hepatol 2025; 17(7): 106291
- URL: https://www.wjgnet.com/1948-5182/full/v17/i7/106291.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i7.106291
Most of the health-related problems in chronic liver disease (CLD) patients result from the complications of portal hypertension (PHT) indicating decompensated cirrhosis, or from the development of hepatocellular carcinoma (HCC)[1].
Autonomic dysfunction (AD), common in cirrhotic patients, is associated with PHT. Investigations of heart rate variability (HRV), a noninvasive measure of autonomic nervous system (ANS) balance, have provided evidence of this association. Several studies have shown that HRV, associated with the presence of AD and inversely correlated with the severity of the disease, is diminished in patients with CLD. Contrariwise, HRV improves with some therapies for cirrhosis and PHT. However, there is no up-to-date information about the clinical utility of HRV in CLD.
This article aimed to review the ways in which HRV could be used as a simple tool for evaluating patients with CLD and PHT-related complications and to summarize the utility and limitations of HRV in the clinical assessment of CLD patients.
PHT is key in CLD pathophysiology, as it is associated with the extent of disease decompensation and severity[2]. The development of PHT involves a structural and a dynamic component, the latter accounting for approximately 30% of the increased portal pressure[3]. The presence of hepatocellular nodules (such as regenerative nodules and dysplastic or neoplastic nodules) increases hepatic resistance through a structural mechanism. Still, portal pressure also increases due to the increased hepatic production of endothelin and thromboxane, leading to smooth muscle cell and hepatic sinusoid contraction, which is recognized as the dynamic component of the increased hepatic resistance[3-5].
As portal pressure increases, the synthesis of endothelial vasodilator factors such as nitric oxide, carbon monoxide, adrenomedullin, and prostacyclins also increases. These vasodilator factors induce splanchnic and systemic vasodilation, lowering peripheral vascular resistance and blood pressure[6]. In response to low blood pressure, the renin-angiotensin-aldosterone system (RAAS), adenohypophysis-vasopressin system, and sympathetic nervous system (SNS) become activated[7]. Whereas the first two axes induce a reduction in natriuresis to increase the effective circulating volume, the SNS increases the HR, myocardial contractility, and left ventricle ejection fraction (LVEF), thereby increasing the cardiac output (CO) and generating a hyperdynamic circulatory state[5].
In advanced CLD with cirrhosis, this hyperdynamic circulation is decompensated. Both liver insufficiency and portal-systemic shunts increase the levels of vasodilator factors and decrease the density and reactivity of beta-adrenergic receptors to vasoconstriction. Moreover, the increased plasma concentration of angiotensin II leads to dysfunction of the parasympathetic nervous system (PSNS) and, consequently, an imbalance of the ANS. If the hyperdynamic circulation persists, downregulation of beta-adrenergic receptors occurs, impairing vasoconstriction and generating oxidative stress, myocardial damage, and endothelial damage. Finally, despite the increased HR and RAAS activation, CO decreases at this stage, a condition known as cirrhotic cardiomyopathy[8].
For further details on the pathophysiological mechanisms of AD in CLD, please consult the following references[9,10].
AD is a common finding in cirrhotic patients, with a prevalence of 30% to 67%[11,12]. Patients with CLD frequently have AD and ANS imbalance, even in the early stages of the disease[11-14]. The clinical manifestations of AD include orthostatic hypotension, gastrointestinal discomfort, bladder dysfunction, erectile dysfunction, cardiovascular complications, and cognitive decline. Patients with AD have a hyporeactive circulation that responds inappropriately to major events such as variceal bleeding and sepsis[15]. As AD has a non-specific clinic presentation, its diagnosis can easily be missed.
Although AD is strictly related to neurohumoral activation, its pathogenesis in CLD has not been completely elucidated. Many factors have been suspected to cause AD in CLD, including decreased baroreceptor sensitivity to hypotension, direct axonal damage by alcohol in alcoholic cirrhosis, altered lipid metabolism, vitamin E deficiency, immune mechanisms, and toxic metabolites[15]. However, AD is closely related to hyperdynamic circulation and PHT due to cirrhosis per se rather than the etiology of cirrhosis[5]. Therefore, AD manifestations, such as HRV, would not be related to the cause of the cirrhosis.
The relevance of AD in CLD lies in its prognostic role: It worsens with an increase in the severity of CLD, enhancing the risk of mortality in such patients[12]. Almost 30 years ago, Hendrickse et al[16] reported a mortality rate of 30% in patients with cirrhosis and AD but only 6% in cirrhotic patients without AD. The ways in which ANS imbalance influences CLD are discussed below.
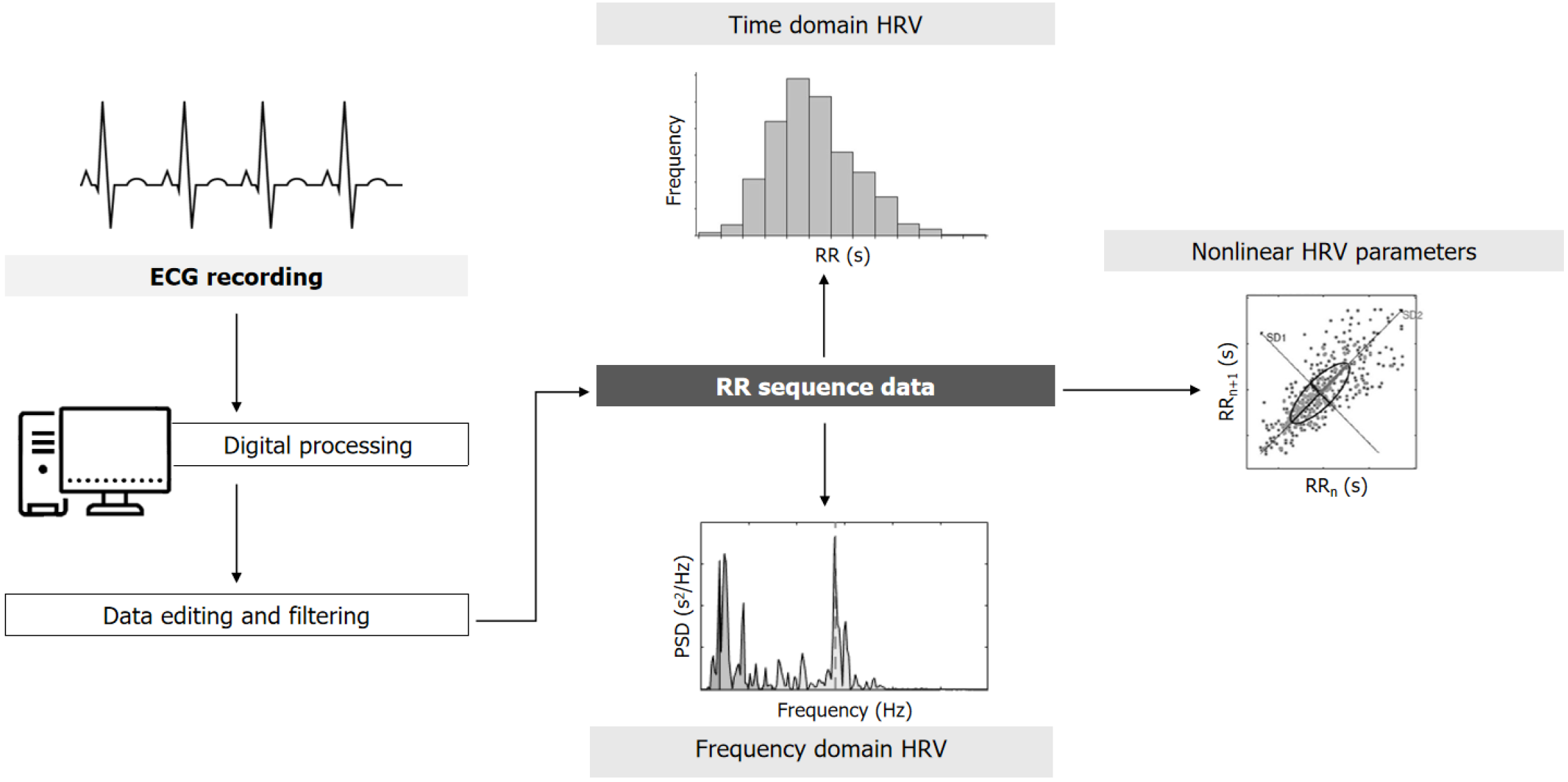
HRV is broadly used to assess ANS function in different settings, representing the dynamic balance between the SNS and PSNS of the cardiovascular system, which is reflected as variance in time between the consecutive heartbeats (i.e., the inter-beat intervals)[17,18]. These fluctuations can be evaluated using an electrocardiographic register by analyzing the RR interval (Figure 1). After appropriate analysis of the inter-beat intervals, different HRV parameters are generated in the time (SDNN, RMSSD, and pNN50) and frequency domains [high-frequency (HF), low-frequency (LH), and the LF/HF ratio][19]. In recent years, nonlinear markers of HRV have been developed based on chaos theory, which allow assessment of the regularity and complexity of ANS balance. The most common HRV parameters and their interpretations with respect to ANS balance are explained in Table 1.
| Variable | Description | ANS interpretation |
| Time domain measurements | ||
| SDNN (ms) | The standard deviation of all the NN intervals | SNS and PSNS |
| RMSSD (ms) | The square root of the mean of the sum of the squares of differences between the adjacent NN intervals | PSNS |
| NN50 | The number of adjacent NN intervals that differ by more than 50 ms | PSNS |
| pNN50 | The NN50 count divided by the total number of all NN intervals | PSNS |
| Frequency domain measurements | ||
| LF (ms2) | Power in the low frequency range (0.04–0.15 Hz) | SNS and PSNS |
| HF (ms2) | Power in the high frequency range (0.15–0.4 Hz) | PSNS |
| LF/HF (nu) | Ratio LF/HF | SNS and PSNS |
| Nonlinear measurements | ||
| SD1/SD2 | Quotient between standard deviation 1 and 2 from the Poincaré plot | SNS and PSNS |
| ApEn | Approximate entropy, measures signal complexity and regularity | SNS and PSNS |
| DFA | Detrended fluctuation analysis from RRnvs RRn+1 correlations; short (α1) and long (α2) fluctuations | SNS and PSNS |
In clinical practice, most HRV indexes can be easily obtained. The appropriate electrocardiogram (EKG) signal for calculating the time and frequency domain parameters of HRV can be obtained via 24-hour Holter monitoring. Thus, the same medical test for evaluating cardiac rhythm alterations (ambulatory EKG monitoring) allows physicians to measure HRV. In fact, the standard Holter monitoring report usually includes the results of HRV analysis[20]. The sensitivity and accuracy of HRV are higher than those of other tests evaluating autonomic function[5], allowing a better assessment of the functioning of the PSNS[21].
Given the relation of HRV with SNS activity[15], diminished HRV shows a dominant sympathetic tone due to either SNS overflow or lower PSNS activity[17]. Traditionally, HF, PNN50, and RMSSD have been associated with PSNS activity, and LF or SDNN with SNS activity[22]. However, recent evidence shows that it is incorrect to completely and exclusively associate the function of a specific ANS branch with a specific HRV parameter[23]. Related hypotheses have been refuted, e.g., that sympathovagal balance can be quantified with the LH/HF index[24]. Nevertheless, there is strong evidence that overall reduced HRV is a marker of poor health. AD, as evidenced by reduced HRV, has been associated with worse overall outcomes in patients with a variety of diseases, such as acute myocardial infarction[19,25], unstable angina[26], arterial hypertension[27], diabetes mellitus[28], dyslipidemia[29], atherosclerosis[30] and sepsis[31], among others[19,32].
Since 1990, several studies comparing CLD patients and healthy subjects have demonstrated that HRV is diminished in patients with CLD[32–34]. This decrease in HRV is more prominent in patients with evidence of PHT, such as those with hepatic encephalopathy (HE)[35], ascites[36], or portal vein thrombosis[37]. For example, it has been reported that patients with cirrhosis, especially those with Child class C disease, had a higher mean HR and lower HRV values than the corresponding values in healthy controls[35]. Similar results were found in studies comparing HRV values in patients with primary biliary cholangitis and healthy subjects[38]. Furthermore, a cohort study of healthy subjects with a median follow-up of 4.2 years showed that HRV as assessed by SDNN and RMSSD was inversely associated with the risk of incident metabolic dysfunction-associated liver disease and fibrosis[39].
Several studies have shown that lower HRV values are associated with increased CLD severity when evaluated using the Child-Pugh score[12,16,32,36,40,41]. In particular, those patients with serum hypoalbuminemia (a component of the Child-Pugh score) present significantly lower HRV values in some time and frequency domains[42]. In a study that mainly included Child-Pugh B and C cirrhotic patients, those who died during a 2-year follow-up had lower HRV than did the survivors[32]. In recent studies, HRV has been identified as a predictor of mortality in patients with cirrhosis, exhibiting an effect that is independent of the model for end-stage liver disease (MELD) score[40,43]. Furthermore, the association of reduced HRV with malnutrition in CLD increases the cardiovascular risk and is associated with a poor prognosis before and after liver transplantation (LT)[44]. Moreover, it has been reported that there is a correlation between SDNN, prothrombin activity, and serum albumin concentration, such that as AD increases, prothrombin activity and albumin levels decrease[33]. It has also been demonstrated that the SDNN parameter is reduced in cirrhotic patients as they progress toward decompensation and is further decreased in patients with acute-on-chronic liver failure, potentially serving as a predictor of 90-day mortality[45].
Reduced HRV has been associated with a higher risk of complications in patients with CLD. Mani et al[35] reported that the values of SDNN and LF decreased in parallel with worsening encephalopathy in patients with CLD. Two explanations have been proposed for this association: (1) The presence of autonomic neuropathy, particularly of the vagal branch, could delay the intestinal transit, leading to bacterial overgrowth and translocation with consecutive endo
In addition to encephalopathy, reduced HRV, as evidenced by a low SDNN, has been associated with the presence of ascites, regardless of the etiology of CLD[33]. Furthermore, a reduced SDNN was related to the presence of esophagogastric varices and ascites, suggesting that HRV is correlated with the degree of PHT[46]. In the same observational study, an HRV parameter termed SDANN showed acceptable sensitivity and specificity in estimating recent variceal bleeding[46]. Also, a prospective study demonstrated that HRV parameters were independent predictors of cardiac dysfunction and mortality in cirrhosis[47]. No data are available regarding the relationship between HRV and variceal bleeding risk, hepatorenal syndrome, spontaneous bacterial peritonitis, or the pulmonary complications of cirrhosis.
Furthermore, complications of cirrhosis develop when clinically significant PHT, defined as a hepatic venous pressure gradient (HVPG) > 10 mmHg, develops[48]. AD worsens as PHT increases[49], and HRV correlates negatively with HVPG; thus, HRV decreases as HVPG increases[50,51]. Although this indirect relation between HRV and HVPG theore
Regarding HCC, it is unknown whether cirrhotic patients with reduced HRV have an increased risk of HCC. However, a small study from Taiwan suggested that HRV could be a prognostic factor in patients with HCC[52].
In patients with severe CLD, the presence of ANS dysfunction is associated with a high mortality rate[10]. Follow-up studies of patients with CLD showed that HRV values were lower in non-survivors than in survivors during the same period of time[32,43].
Scientific studies have also established reduction in HRV as a major independent risk factor of mortality in cirrhotic patients by using both linear[16] and nonlinear HRV indices[40]. Recently, Bhogal et al[43] reported an HRV reduction in the time domain measurements, in particular noting a predictive mortality value that is independent of well-established factors, such as age, sex, use of beta-blockers, and etiology of cirrhosis[43]. At the same time, SDNN was found to be a reliable predictor of mortality regardless of cirrhosis severity scales such as MELD and Child–Pugh SD2[35] and ap
Finally, several authors have proposed that ANS dysfunction evaluated using HRV should be considered for the long-term clinical monitoring of patients with CLD. Because of its prognostic value, it may represent an important parameter for prioritizing patients with advanced disease awaiting LT[12,43]. In fact, in pediatric patients with acute liver failure, reduced HRV was significantly associated with the presence of HE and with poor outcomes (death or listing for LT)[53].
LT is the best, indeed definitive treatment for advanced decompensated cirrhosis regardless of its etiology. The procedure for LT is closely related to ANS balance because it has a significant impact on the systemic hemodynamics in cirrhotic patients. During surgery, the great abdominal and thoracic vessels are clamped to allow removal of the native liver. After the donor liver is correctly positioned and the connection between the vessels is reestablished, there is a sudden increase in the cardiac preload, which represents a high hemodynamic stress. This hemodynamic instability is amplified in the presence of AD, where the ability to regulate pressure fluctuations during LT is blunted[54].
Sympathovagal imbalance has been proposed as a risk marker for hypotension in patients with post-reperfusion syndrome of the liver[55]. Additionally, significant cardiac and cerebral circulatory complications in cirrhotic patients with AD have been reported to be associated with early death after LT[56]. However, there is little information regarding hemodynamic changes during LT in patients with AD or their long-term implications; therefore, further studies must be conducted in this area.
The hyperdynamic circulatory state can resolve after LT, along with the restoration of portal pressure and ANS balance. In fact, some post-LT studies reported regression of AD in 63% to 70% of patients[57,58], supporting the hypothesis that AD is mainly due to the circulatory and neurohumoral changes in cirrhosis rather than the result of structural nerve damage. The potential reversibility of AD after transplantation points toward HRV as a potential evaluation tool. Although cirrhotic patients awaiting LT have lower HRV than healthy subjects, some HRV parameters nevertheless normalize after LT surgery[59]. Also, deceleration capacity, another HRV index, has been shown to be one of the best predictors of 1-year mortality after LT in combination with the MELD score and heart rate complexity[60].
Prolonged QT interval (QT), which is negatively correlated with HRV[50], is a common electrocardiographic finding in cirrhotic patients. A QT segment reduction has been associated with improved outcomes in cirrhotic patients undergoing LT surgery[61]. Remarkably, it was observed that patients with prolonged QT before LT surgery showed normal QTc values after transplantation[5,62]. On the other hand, the cardiotoxic immunosuppressant tacrolimus is commonly used in patients after LT. Compared to patients undergoing treatment with a non-cardiotoxic drug, tacrolimus leads to alte
As most studies are retrospective and have intermediate-term follow-up data, it is not yet possible to state conclusively that AD constitutes a significant risk factor for increased mortality and morbidity during and after LT. Further studies analyzing the changes in HRV before and after transplantation in patients with CLD are required to support the existing evidence and validate HRV as a useful tool in assessing CLD as well as for stratifying patients awaiting LT.
HRV measurement, a noninvasive test that lasts less than 5 minutes and that can even be performed with wearable devices, could be a cost-effective and fast way to measure ANS balance in the setting of LT[64,65]. In contrast, the MELD score requires raking blood samples and waiting for the results, which is costlier and longer, presents interlaboratory parameter variability, and yields inaccurate mortality predictions in some situations[66]. Despite the growing literature on HRV, it must be analyzed carefully because standardized protocols, validity criteria, and widely validated normal values are still lacking[65]. Nevertheless, HRV could become a complement to MELD scores in LT, thus aiding in the development and clinical evolution of patients with CLD, including LT.
In patients with CLD, the use of drugs modifying the autonomic balance is a common practice. The prime example is the administration of beta-blockers for the prophylaxis of variceal bleeding[67]. In this regard, HRV could be useful in fine-tuning the drug dose and reducing adverse drug reactions.
The use of beta-blockers increases HRV values in patients with coronary disease[68] and cardiac failure[69], as well as in healthy people[70]. Cirrhotic patients treated with propranolol showed a significant reduction in the QTc, which is associated with reduced AD and mortality[71]. This is consistent with improvement in the HRV parameters related to vagal function: RMSSD, HF, and sample entropy[72]. Contrariwise, it has been reported that patients without esophageal varices but with clinically significant PHT could benefit from treatment with beta-blockers[73]. As PHT correlates with HRV, it may be a useful noninvasive tool that should be assessed in future studies as a marker of clinically significant PHT.
There is lack of evidence regarding the effects of other drugs commonly used in CLD on HRV. However, a study involving patients with compensated cardiac failure linked furosemide use to decreased RMSSD[74]. In the same manner, the use of spironolactone in patients with cardiac failure has been linked to a decrease in the LF/HF ratio[75]. There are no data available on the effect of either drug on HRV in cirrhotic patients.
Although HRV is a good clinical indicator of ANS function, it has many limitations with respect to its measurement and interpretation. For example, it is influenced by the position of patients at the time of measurement and their respiratory pattern, requiring cooperation from the patient and making the test less reproducible and harder to perform in patients with HE[15,19]. Also, it is important to note that most evidence on HRV in CLD comes from observational studies, and therefore the presence of confounding by medications and comorbidities that influence HRV is possible. Since type 2 diabetes mellitus is associated with CLD and also causes autonomic cardiomyopathy that in turns reduces HRV, further studies that specifically analyze HRV measurement in these conditions are needed[76,77]. Also, future studies on HRV and CLD should consider a prospective design and proper control of bias and confounding to yield better-quality evi
Regarding HRV interpretation, there is no clear consensus on the value limits to distinguish normal and abnormal values, making the measurement less objective and limiting its use in clinical practice[19]. In addition, the evaluation of HRV in each patient requires a post-measurement edition of artifacts and signal processing, implying the need for more time and specialized personnel. However, many wearable devices show HRV in real-time, reducing the barriers to implementing HRV in CLD patient evaluation.
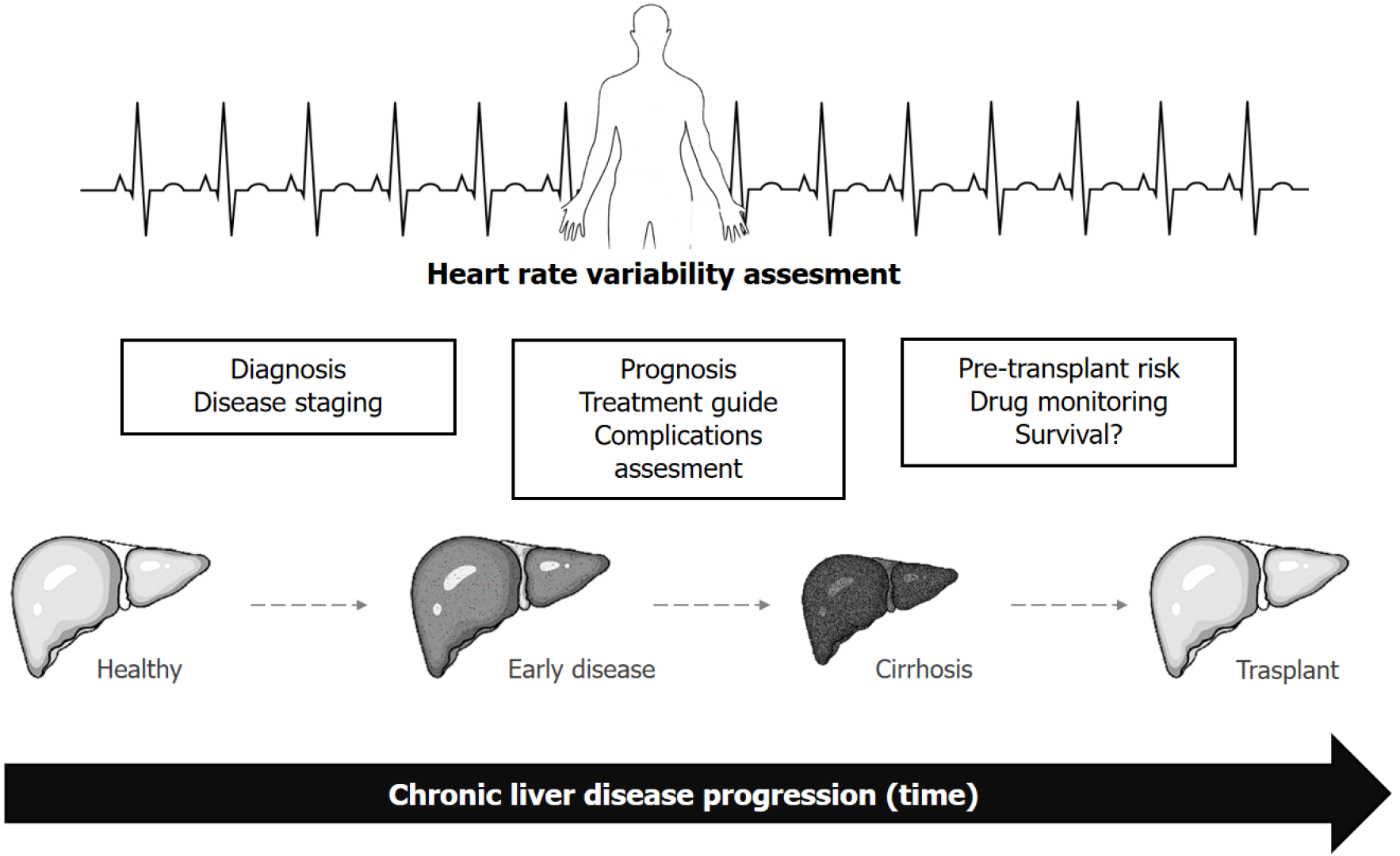
Despite the importance of ANS balance in the natural history of CLD (Table 2), to date, there are neither any recommendations regarding the evaluation of the ANS in these patients nor any clinical guidance on AD in CLD. Symptoms secondary to AD should be detected and managed because it is necessary to establish the criteria for the diagnosis, management, and treatment of this condition, which could improve the prognosis and quality of life of these patients. Thus, the physician must not overlook clinical manifestations of AD, especially in patients with advanced CLD. The study of HRV could offer some advantages in furthering our understanding of this clinical entity.
| Potential clinical utility in CLD | Main HRV findings |
| Diagnosis | ↓ HRV in CLD patients compared with healthy subjects. HRV is a risk factor for MASLD development |
| Disease staging | ↓ HRV values are associated with greater severity when evaluated using the Child-Pugh score. Prothrombin activity and plasma albumin are correlated with SDNN |
| Assessment of associated complications | ↓ HRV is associated with encephalopathy, ascites, esophageal varices, and cardiac dysfunction, and it could be a prognostic marker in hepatocellular carcinoma |
| Mortality risk | ↓ HRV increases mortality risk in cirrhotic patients, independently of MELD, Child-Pugh score, and other risk factors |
| Management in LT | Some HRV parameters normalize after LT. In combination with MELD score, HRV predicts 1-year survival after LT |
| Follow-up of medical treatment | The use of beta-blockers ↑ HRV in patients with CLD |
However, as HRV is a widely known and validated tool, serial HRV measurements should be tested in more clinical scenarios. The utility of HRV in the screening, diagnosis, and clinical evaluation of CLD complications must be evaluated in larger prospective studies (Figure 2). Given the lack of consensus on the diagnosis of AD in CLD, the validation and study of specific cut-off points for the HRV parameters should be encouraged. Also, larger-scale cohort and randomized clinical trials, with better control of bias and confounding, are needed to better understand HRV in CLD natural history and clinical management. On the other hand, consensus guidelines and reference values for HRV measurement are also needed, given the variety of methods for measuring HRV (from 24-hour registers to ultra-short-term, i.e., lower than 5 min)[65]. Such improvements in HRV assessment protocols would allow better recognition of its advantages and make HRV results more reproducible across operators according to the clinical context.
For clinical follow-up, HRV could be useful in the future in the continuous monitoring of patients with CLD from early stages (Figure 2). With respect to the therapeutic management of patients with CLD, HRV could be useful in dose titration and the therapeutic adjustment of beta-blockers and other drugs; however, prospective studies are necessary to support this strategy. Conversely, while the use of angiotensin-converting enzyme inhibitors[5] and beta-blockers has been studied in cirrhotic patients with AD, further evidence is needed. Finally, further large-scale studies with a higher level of scientific evidence are needed to establish the usefulness of HRV in risk assessment, monitoring, and therapeutic management of LT.
HRV is a well-validated tool in the assessment of ANS. In CLD, this noninvasive test could be useful in the diagnosis and assessment of the severity, complications, therapy, and prognosis of the disease (Figure 2 and Table 2); however, further studies are needed to validate its clinical utility. Thus, despite its limitations, HRV could become an important tool in the integral management of patients with CLD.
| 1. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2296] [Article Influence: 382.7] [Reference Citation Analysis (0)] |
| 2. | Sauerbruch T, Schierwagen R, Trebicka J. Managing portal hypertension in patients with liver cirrhosis. F1000Res. 2018;7:F1000 Faculty Rev-F1000 Faculty 533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Maiwall R, Maras JS, Kumar S, Sarin SK. Reply to: "Ferritin in decompensated cirrhosis: Iron or inflammation?". J Hepatol. 2015;62:500-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Mizuno K, Ueno Y. Autonomic Nervous System and the Liver. Hepatol Res. 2017;47:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Lheureux O, Trepo E, Hites M, Cotton F, Wolff F, Surin R, Creteur J, Vincent JL, Gustot T, Jacobs F, Taccone FS. Serum β-lactam concentrations in critically ill patients with cirrhosis: a matched case-control study. Liver Int. 2016;36:1002-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Colle I, Geerts AM, Van Steenkiste C, Van Vlierberghe H. Hemodynamic changes in splanchnic blood vessels in portal hypertension. Anat Rec (Hoboken). 2008;291:699-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | McGrath MS, Wentworth BJ. The Renin-Angiotensin System in Liver Disease. Int J Mol Sci. 2024;25:5807. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Chen K, Ma B, Peppelenbosch MP, Pan Q. Cytoplasmic rods and rings in mycophenolic acid treatment. Liver Int. 2017;37:1742-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Frith J, Newton JL. Autonomic dysfunction in chronic liver disease. Liver Int. 2009;29:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Hendrickse MT, Triger DR. Autonomic dysfunction in chronic liver disease. Clin Auton Res. 1993;3:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Oliver MI, Miralles R, Rubíes-Prat J, Navarro X, Espadaler JM, Solá R, Andreu M. Autonomic dysfunction in patients with non-alcoholic chronic liver disease. J Hepatol. 1997;26:1242-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Fleckenstein JF, Frank Sm, Thuluvath PJ. Presence of autonomic neuropathy is a poor prognostic indicator in patients with advanced liver disease. Hepatology. 1996;23:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Iga A, Nomura M, Sawada Y, Ito S, Nakaya Y. Autonomic nervous dysfunction in patients with liver cirrhosis using 123I-metaiodobenzylguanidine myocardial scintigraphy and spectrum analysis of heart-rate variability. J Gastroenterol Hepatol. 2003;18:651-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Yamagata M, Masaki T, Okudaira T, Imai Y, Shiina S, Shiratori Y, Omata M. Small hyperechoic nodules in chronic liver diseases include hepatocellular carcinomas with low cyclin D1 and Ki-67 expression. Hepatology. 1999;29:1722-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Dümcke CW, Møller S. Autonomic dysfunction in cirrhosis and portal hypertension. Scand J Clin Lab Invest. 2008;68:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Hendrickse MT, Thuluvath PJ, Triger DR. Natural history of autonomic neuropathy in chronic liver disease. Lancet. 1992;339:1462-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9482] [Cited by in RCA: 9032] [Article Influence: 311.4] [Reference Citation Analysis (0)] |
| 18. | Behfarnia P, Birang R, Andalib AR, Asadi S. Comparative Evaluation of IFNγ, IL4 and IL17 Cytokines in Healthy Gingiva and Moderate to Advanced Chronic Periodontitis. Dent Res J (Isfahan). 2010;7:45-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 455] [Cited by in RCA: 418] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 19. | Xhyheri B, Manfrini O, Mazzolini M, Pizzi C, Bugiardini R. Heart rate variability today. Prog Cardiovasc Dis. 2012;55:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 281] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 20. | Chorin E, Hochstadt A, Viskin S, Rozovski U, Havakuk O, Baranchuk A, Enriquez A, Strasberg B, Guevara-Valdivia ME, Márquez MF, González-Pacheco H, Hasdemir C, Rosso R. Female gender as independent risk factor of torsades de pointes during acquired atrioventricular block. Heart Rhythm. 2017;14:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Kamath MV, Fallen EL. Power spectral analysis of heart rate variability: a noninvasive signature of cardiac autonomic function. Crit Rev Biomed Eng. 1993;21:245-311. [PubMed] |
| 22. | Singh N, Moneghetti KJ, Christle JW, Hadley D, Froelicher V, Plews D. Heart Rate Variability: An Old Metric with New Meaning in the Era of Using mHealth technologies for Health and Exercise Training Guidance. Part Two: Prognosis and Training. Arrhythm Electrophysiol Rev. 2018;7:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 23. | Goldstein DS, Bentho O, Park MY, Sharabi Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol. 2011;96:1255-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 587] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 24. | Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol. 2013;4:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 554] [Cited by in RCA: 794] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 25. | Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2735] [Cited by in RCA: 2593] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 26. | Manfrini O, Pizzi C, Trerè D, Fontana F, Bugiardini R. Parasympathetic failure and risk of subsequent coronary events in unstable angina and non-ST-segment elevation myocardial infarction. Eur Heart J. 2003;24:1560-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Singh JP, Larson MG, Tsuji H, Evans JC, O'Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension. 1998;32:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 349] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 28. | Singh JP, Larson MG, O'Donnell CJ, Wilson PF, Tsuji H, Lloyd-Jones DM, Levy D. Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study). Am J Cardiol. 2000;86:309-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 292] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 29. | Karason K, Mølgaard H, Wikstrand J, Sjöström L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol. 1999;83:1242-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 252] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 30. | Huikuri HV, Jokinen V, Syvänne M, Nieminen MS, Airaksinen KE, Ikäheimo MJ, Koistinen JM, Kauma H, Kesäniemi AY, Majahalme S, Niemelä KO, Frick MH. Heart rate variability and progression of coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19:1979-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 195] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | de Castilho FM, Ribeiro ALP, da Silva JLP, Nobre V, de Sousa MR. Heart rate variability as predictor of mortality in sepsis: A prospective cohort study. PLoS One. 2017;12:e0180060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Ates F, Topal E, Kosar F, Karincaoglu M, Yildirim B, Aksoy Y, Aladag M, Harputluoglu MM, Demirel U, Alan H, Hilmioglu F. The relationship of heart rate variability with severity and prognosis of cirrhosis. Dig Dis Sci. 2006;51:1614-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Coelho L, Saraiva S, Guimaräes H, Freitas D, Providência LA. Autonomic function in chronic liver disease assessed by Heart Rate Variability Study. Rev Port Cardiol. 2001;20:25-36. [PubMed] |
| 34. | Fleisher LA, Fleckenstein JF, Frank SM, Thuluvath PJ. Heart rate variability as a predictor of autonomic dysfunction in patients awaiting liver transplantation. Dig Dis Sci. 2000;45:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Mani AR, Montagnese S, Jackson CD, Jenkins CW, Head IM, Stephens RC, Moore KP, Morgan MY. Decreased heart rate variability in patients with cirrhosis relates to the presence and degree of hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2009;296:G330-G338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Lazzeri C, La Villa G, Laffi G, Vecchiarino S, Gambilonghi F, Gentilini P, Franchi F. Autonomic regulation of heart rate and QT interval in nonalcoholic cirrhosis with ascites. Digestion. 1997;58:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Miyajima H, Nomura M, Muguruma N, Okahisa T, Shibata H, Okamura S, Honda H, Shimizu I, Harada M, Saito K, Nakaya Y, Ito S. Relationship among gastric motility, autonomic activity, and portal hemodynamics in patients with liver cirrhosis. J Gastroenterol Hepatol. 2001;16:647-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Nakajima H, Takagi H, Horiguchi N, Toyoda M, Kanda D, Otsuka T, Emoto Y, Emoto M, Mori M. Lack of macrophage migration inhibitory factor protects mice against concanavalin A-induced liver injury. Liver Int. 2006;26:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Choi IY, Chang Y, Kang G, Jung HS, Shin H, Wild SH, Byrne CD, Ryu S. Low heart rate variability from 10-s electrocardiograms is associated with development of non-alcoholic fatty liver disease. Sci Rep. 2022;12:1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Oyelade T, Canciani G, Carbone G, Alqahtani JS, Moore K, Mani AR. Heart rate variability in patients with cirrhosis: a systematic review and meta-analysis. Physiol Meas. 2021;42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Yadav A, Nawal CL, Singh A, Rankawat G. A Comparative Analysis of Heart Rate Variability Indices in Patients with Liver Cirrhosis. J Assoc Physicians India. 2024;72:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 42. | Park EJ, Yoo SD. Nutritional Biomarkers and Heart Rate Variability in Patients with Subacute Stroke. Nutrients. 2022;14:5320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 43. | Bhogal AS, De Rui M, Pavanello D, El-Azizi I, Rowshan S, Amodio P, Montagnese S, Mani AR. Which heart rate variability index is an independent predictor of mortality in cirrhosis? Dig Liver Dis. 2019;51:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | de Lima DC, Ribeiro HS, Cristina R, Oliveira M, Generoso Sde V, Lima AS, Correia MI. Functional status and heart rate variability in end-stage liver disease patients: association with nutritional status. Nutrition. 2015;31:971-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Jansen C, Chatterjee DA, Thomsen KL, Al-Kassou B, Sawhney R, Jones H, Gallego-Leon A, Lehmann J, Pohlmann A, Nickenig G, Strassburg CP, Andrié R, Jalan R, Linhart M, Trebicka J, Mookerjee RP. Significant reduction in heart rate variability is a feature of acute decompensation of cirrhosis and predicts 90-day mortality. Aliment Pharmacol Ther. 2019;50:568-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (2)] |
| 46. | Miceli G, Calvaruso V, Casuccio A, Pennisi G, Licata M, Pintus C, Basso MG, Velardo M, Daidone M, Amodio E, Petta S, Simone F, Cabibbo G, Di Raimondo D, Craxì A, Pinto A, Tuttolomondo A. Heart rate variability is associated with disease severity and portal hypertension in cirrhosis. Hepatol Commun. 2023;7:e0050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Pimentel CFMG, Salvadori R, Feldner ACCA, Aguiar MO, Gonzalez AM, Branco GR, Superbia M, Lai M, Mota DO, Ferraz MLCG, Mathias W, Kondo M. Autonomic dysfunction is common in liver cirrhosis and is associated with cardiac dysfunction and mortality: prospective observational study. Sao Paulo Med J. 2022;140:71-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 48. | Kumar A, Sharma P, Sarin SK. Hepatic venous pressure gradient measurement: time to learn! Indian J Gastroenterol. 2008;27:74-80. [PubMed] |
| 49. | Bernardi M, Fornalè L, Di Marco C, Trevisani F, Baraldini M, Gasbarrini A, De Collibus C, Zacà F, Ligabue A, Colantoni A. Hyperdynamic circulation of advanced cirrhosis: a re-appraisal based on posture-induced changes in hemodynamics. J Hepatol. 1995;22:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Satoh M, Minami Y, Takahashi Y, Tabuchi T, Itoh T, Nakamura M. Effect of intensive lipid-lowering therapy on telomere erosion in endothelial progenitor cells obtained from patients with coronary artery disease. Clin Sci (Lond). 2009;116:827-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Genovesi S, Prata Pizzala DM, Pozzi M, Ratti L, Milanese M, Vincenti A, Stella A, Mancia G. Baroreceptor sensitivity and baroreceptor effectiveness index in cirrhosis: the relevance of hepatic venous pressure gradient. Liver Int. 2010;30:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Davison SN, Jhangri GS. Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J Pain Symptom Manage. 2010;39:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 53. | Pendergrast TR, Chapin CA, Kriegermeier AA, Pardo AC, Bass LM, Sanchez-Pinto LN. Heart rate variability is associated with encephalopathy and outcomes in pediatric acute liver failure. Pediatr Res. 2023;93:1348-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 54. | Raval Z, Harinstein ME, Skaro AI, Erdogan A, DeWolf AM, Shah SJ, Fix OK, Kay N, Abecassis MI, Gheorghiade M, Flaherty JD. Cardiovascular risk assessment of the liver transplant candidate. J Am Coll Cardiol. 2011;58:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 55. | Kim YK, Lee K, Hwang GS, Cohen RJ. Sympathetic withdrawal is associated with hypotension after hepatic reperfusion. Clin Auton Res. 2013;23:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Marconi L, Figueiredo A, Campos L, Nunes P, Roseiro A, Parada B, Mota A. Renal transplantation with donors older than 70 years: does age matter? Transplant Proc. 2013;45:1251-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Pérez-Peña J, Rincón D, Bañares R, Olmedilla L, Garutti I, Arnal D, Calleja J, Clemente G. Autonomic neuropathy is associated with hemodynamic instability during human liver transplantation. Transplant Proc. 2003;35:1866-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Olthoff KM, Reddy KR. First, do no harm: The question of liver biopsy in living liver donors. Liver Transpl. 2008;14:420-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 59. | Baratta L, Tubani L, Merli M, Labbadia F, Facchini D, De Marco R, Rossi M, Attili AF, Berloco P, Ginanni Corradini S. Long-term effect of liver transplantation on cirrhotic autonomic cardiac dysfunction. Dig Liver Dis. 2010;42:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Clarke JD, Novak P, Lake AD, Hardwick RN, Cherrington NJ. Impaired N-linked glycosylation of uptake and efflux transporters in human non-alcoholic fatty liver disease. Liver Int. 2017;37:1074-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 61. | Safadi A, Homsi M, Maskoun W, Lane KA, Singh I, Sawada SG, Mahenthiran J. Perioperative risk predictors of cardiac outcomes in patients undergoing liver transplantation surgery. Circulation. 2009;120:1189-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 62. | Van der Linden P, Le Moine O, Ghysels M, Ortinez M, Devière J. Pulmonary hypertension after transjugular intrahepatic portosystemic shunt: effects on right ventricular function. Hepatology. 1996;23:982-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Therapondos G, Flapan AD, Dollinger MM, Garden OJ, Plevris JN, Hayes PC. Cardiac function after orthotopic liver transplantation and the effects of immunosuppression: a prospective randomized trial comparing cyclosporin (Neoral) and tacrolimus. Liver Transpl. 2002;8:690-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Olivieri F, Biscetti L, Pimpini L, Pelliccioni G, Sabbatinelli J, Giunta S. Heart rate variability and autonomic nervous system imbalance: Potential biomarkers and detectable hallmarks of aging and inflammaging. Ageing Res Rev. 2024;101:102521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 65. | Shaffer F, Ginsberg JP. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health. 2017;5:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1840] [Cited by in RCA: 3143] [Article Influence: 392.9] [Reference Citation Analysis (0)] |
| 66. | Bernardi M, Gitto S, Biselli M. The MELD score in patients awaiting liver transplant: strengths and weaknesses. J Hepatol. 2011;54:1297-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 67. | Biecker E. Portal hypertension and gastrointestinal bleeding: diagnosis, prevention and management. World J Gastroenterol. 2013;19:5035-5050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 68. | Nolan RP, Jong P, Barry-Bianchi SM, Tanaka TH, Floras JS. Effects of drug, biobehavioral and exercise therapies on heart rate variability in coronary artery disease: a systematic review. Eur J Cardiovasc Prev Rehabil. 2008;15:386-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 69. | Pousset F, Copie X, Lechat P, Jaillon P, Boissel JP, Hetzel M, Fillette F, Remme W, Guize L, Le Heuzey JY. Effects of bisoprolol on heart rate variability in heart failure. Am J Cardiol. 1996;77:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 70. | Cook JR, Bigger JT Jr, Kleiger RE, Fleiss JL, Steinman RC, Rolnitzky LM. Effect of atenolol and diltiazem on heart period variability in normal persons. J Am Coll Cardiol. 1991;17:480-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 181] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 71. | Louvet A, Diaz E, Dharancy S, Coevoet H, Texier F, Thévenot T, Deltenre P, Canva V, Plane C, Mathurin P. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol. 2008;48:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 72. | Kim YK, Hwang GS, Shin WJ, Bang JY, Cho SK, Han SM. Effect of propranolol on the relationship between QT interval and vagal modulation of heart rate variability in cirrhotic patients awaiting liver transplantation. Transplant Proc. 2011;43:1654-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 73. | Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, Bañares R, Morillas RM, Poca M, Peñas B, Augustin S, Abraldes JG, Alvarado E, Torres F, Bosch J. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 451] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 74. | Tomiyama H, Nakayama T, Watanabe G, Shiojima K, Sakuma Y, Yamamoto A, Imai Y, Yoshida H, Doba N. Effects of short-acting and long-acting loop diuretics on heart rate variability in patients with chronic compensated congestive heart failure. Am Heart J. 1999;137:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 75. | Davies JI, Witham MD, Struthers AD. Autonomic effects of spironolactone and MR blockers in heart failure. Heart Fail Rev. 2005;10:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 76. | Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1500] [Article Influence: 250.0] [Reference Citation Analysis (0)] |
| 77. | Agashe S, Petak S. Cardiac Autonomic Neuropathy in Diabetes Mellitus. Methodist Debakey Cardiovasc J. 2018;14:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |