Published online Jul 27, 2025. doi: 10.4254/wjh.v17.i7.106253
Revised: March 26, 2025
Accepted: June 24, 2025
Published online: July 27, 2025
Processing time: 155 Days and 19.7 Hours
Hepatic manifestations in chronic lymphocytic leukemia (CLL) are common: Elevation of liver enzymes frequently occurs, and differential diagnosis is often challenging. Liver infiltration by leukemic cells, primary and secondary hepatic malignancies, drug-induced hepatotoxicity, immunological disorders, and infections have been reported. Nevertheless, syncytial giant cell hepatitis (GCH) as a manifestation of autoimmune hepatitis in patients with CLL is an extremely rare condition, currently reported only in anecdotal cases.
Here, we report the case of a 62-year-old Caucasian woman affected by CLL, who developed GCH with peculiar histopathological features. The patient was evaluated for abnormal liver test results. Liver histology revealed significant inflammatory lymphomononuclear infiltrates with a plasma cell component, widespread syncytial changes in the hepatocytes with gigantocellular features, hepatocyte rosettes, and the typical feature of emperipolesis, consistent with a diagnosis of GCH. The patient was treated with corticosteroids and mycophenolate mofetil, resulting in a complete biochemical response.
Early histological diagnosis of GCH is crucial in patients with CLL, with mycophenolate mofetil representing a promising treatment option.
Core Tip: The association between chronic lymphocytic leukemia (CLL) and syncytial giant cell hepatitis (GCH) is rare, with only a few cases reported in the literature. For this reason, early diagnosis is essential to prevent liver fibrosis and distinguish GCH from other causes of liver disorders. Active immunosuppressive therapy may be beneficial for patients with GCH, with mycophenolate mofetil emerging as a promising therapeutic option. Several studies have suggested a modified expression of toll-like receptors (TLRs) in CLL, potentially contributing to autoimmune complications. Further studies are needed to evaluate the role of TLR4 in autoimmune hepatitis development in patients with CLL.
- Citation: Giacomelli M, Carotti S, Vozella F, Pagliei F, Taffon C, Baiocchini A, Gambaro FL, Picardi A, Vespasiani-Gentilucci U, Galati G. Autoimmune hepatitis with syncytial giant cells in chronic lymphocytic leukemia: A case report and literature review. World J Hepatol 2025; 17(7): 106253
- URL: https://www.wjgnet.com/1948-5182/full/v17/i7/106253.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i7.106253
Chronic lymphocytic leukemia (CLL) is the most common leukemia in the adult population[1]. It is a monoclonal disorder characterized by the progressive proliferation of mature, functionally incompetent lymphocytes. This type of cancer is characterized by the progressive accumulation of monoclonal B lymphocytes in the peripheral blood, bone marrow, and lymphoid tissues. The diagnosis of the disease requires the presence of ≥ 5000 monoclonal B lymphocytes/μL in the peripheral blood for a minimum duration of 3 months[2]. CLL may have a heterogeneous clinical course, ranging from indolent to aggressive forms, and is dominated by clinical events associated with immune dysfunction. Both cellular and humoral immunity are impaired, with qualitative and quantitative defects in T cells, B cells, natural killer cells, neutrophils, and the monocyte-macrophage lineage[3]. This immune dysregulation leads to an increased susceptibility to autoimmunity and opportunistic infections. The most common autoimmune hematological disorder associated with CLL is autoimmune hemolytic anemia[4]. Among non-hematological autoimmune disorders, the most frequently observed are cases of rheumatoid arthritis, Hashimoto’s thyroiditis, and vasculitis. Interestingly, non-hematological autoimmune complications are commonly observed in patients with CLL with early-stage disease[5]. Hepatic manifestations in CLL are common: Elevation of liver enzymes frequently occurs, and differential diagnosis is often challenging. Liver infiltration by leukemic cells, primary and secondary hepatic malignancies, drug-induced hepatotoxicity, immunological disorders, and infections have been reported[6]. Nevertheless, syncytial giant cell hepatitis (GCH) is a rare manifestation of autoimmune hepatitis (AIH) in CLL. This histopathological entity is infrequently diagnosed in adults and is characterized by the presence of multinucleated liver cells[7]. In adults, GCH can rapidly progress to cirrhosis. The pathogenesis is not yet fully understood, although substantial evidence suggests that this disorder results from hepatocyte nuclear proliferation without associated cell division[8]. GCH has been associated with several other causes, including exposure to drugs (methotrexate, 6-mercaptopurine, amitriptyline), viral infections (particularly due to paramyxovirus), toxins, and herbal products[9,10]. Nevertheless, GCH as a manifestation of AIH in patients with CLL is an extremely rare condition, currently reported only in anecdotal cases[11-13].
Here, we describe a case of a 62-year-old Caucasian woman affected by CLL, who developed GCH with peculiar histopathological and clinical features.
Abnormal liver test results in a patient affected by CLL.
A 62-year-old woman was diagnosed with CLL in 2018. At the time of diagnosis (October 2018), the patient did not present with systemic symptoms, and lymphocytosis was noted on the blood count (lymphocyte count 7.24 × 10³/µL) in the absence of anemia or thrombocytopenia. A peripheral blood smear confirmed lymphocytosis (62%), and peripheral blood flow cytometry showed 50% of the lymphoid B population with cluster of differentiation (CD) markers CD5+, CD23+, CD79b+, and clonal restriction of the lambda type. The bone marrow biopsy revealed immunomorphological features consistent with a diagnosis of CLL/small lymphocytic lymphoma according to the 2017 World Health Or
| March 2023 | July 2023 | October 2023 | |
| WBCs (× 10³/µL) | 65 | 82 | 82 |
| Lymphocytes (× 10³/µL) | 58 | 73 | 74 |
| Hb (g/L) | 151 | 145 | 149 |
| PLTs (× 10³/µL) | 157 | 156 | 167 |
| AST (U/L) | 143 | 131 | 179 |
| ALT (U/L) | 429 | 254 | 449 |
| GGT (U/L) | 21 | 22 | 27 |
| ALP (U/L) | 95 | 123 | 168 |
| Total bilirubin (mg/dL) | 1 | 1.1 | 1.2 |
| Serum total proteins (g/L) | 78 | 78 | 79 |
| Serum gamma globulins (g/L) | 19.8 | 20 | 22.1 |
The patient, with class 1 obesity (body mass index 33.7 kg/m²) and impaired fasting glucose, was affected by monoclonal gammopathy of undetermined significance and Hashimoto's thyroiditis. Additionally, the patient underwent an appendectomy for acute appendicitis at a young age.
The family history was negative.
Physical examination revealed painless lymphadenopathy, 2-3 cm in diameter, in the axillary and laterocervical regions. Abdominal examination revealed a non-tender liver with a smooth surface. No other classic clinical signs or stigmata of chronic liver disease were evident. The spleen was palpated approximately 2 cm below the costal margin.
Because clinical suspicion of AIH was high, autoimmune screening (anti-nuclear, anti-mitochondrial, anti-smooth muscle, and anti-liver-kidney microsomal antibodies) was performed, revealing positivity for antinuclear antibodies (nucleolar pattern with a titer of 1:640 and homogeneous pattern with a titer of 1:320). Blood tests also revealed increased immunoglobulin G levels (IgG 22 g/L; normal range 5.5 to 16.3 g/L). In the extended laboratory work-up, other etiologies of liver disease were excluded (transferrin, ferritin, ceruloplasmin, and alpha-1 antitrypsin were within the normal range).
Abdominal ultrasound showed splenomegaly (longitudinal diameter of the spleen, 14 cm), mild fatty liver (increased echogenicity of the liver parenchyma compared with the renal cortex), and gallbladder microlithiasis. No echographic signs of chronic liver disease were detected. In addition, abdominal ultrasound examination was negative for focal liver lesions. Vibration-controlled transient elastography was not performed due to the potential risk of false positivity in the presence of significant transaminitis.
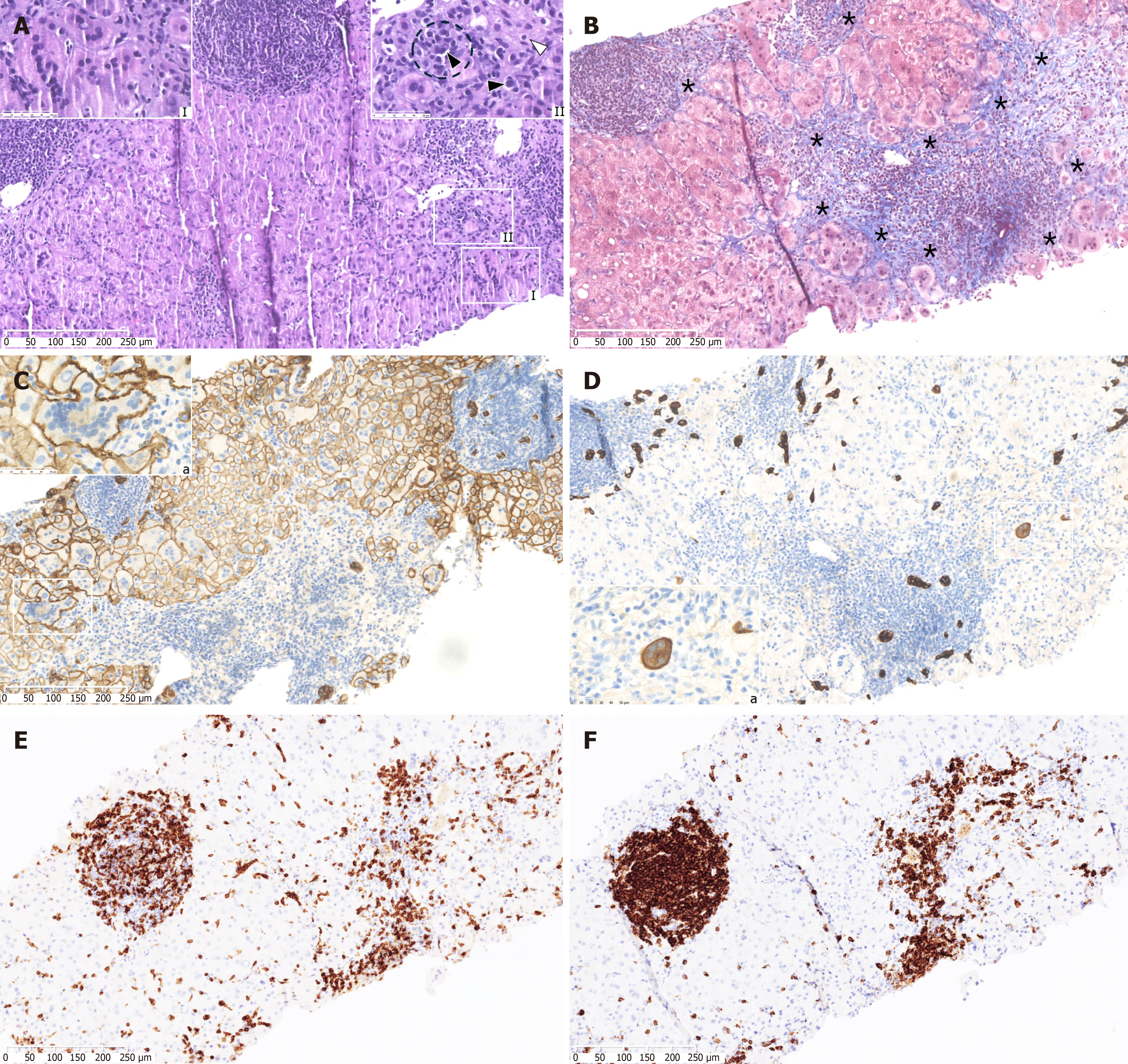
Diagnosis of AIH requires liver histopathological data, according to the criteria proposed by the International Autoimmune Hepatitis Group Scoring System[14,15]. For this reason, a liver biopsy was performed to establish the definitive diagnosis. The liver biopsy revealed significant inflammatory lymphomononuclear infiltrates with a plasma cell component, widespread syncytial changes in the hepatocytes with gigantocellular features, hepatocyte rosettes (small clusters of hepatocytes arranged around a small central lumen), and the typical feature of emperipolesis, indicating a diagnosis of GCH and excluding liver infiltration by CLL cells (Figure 1).
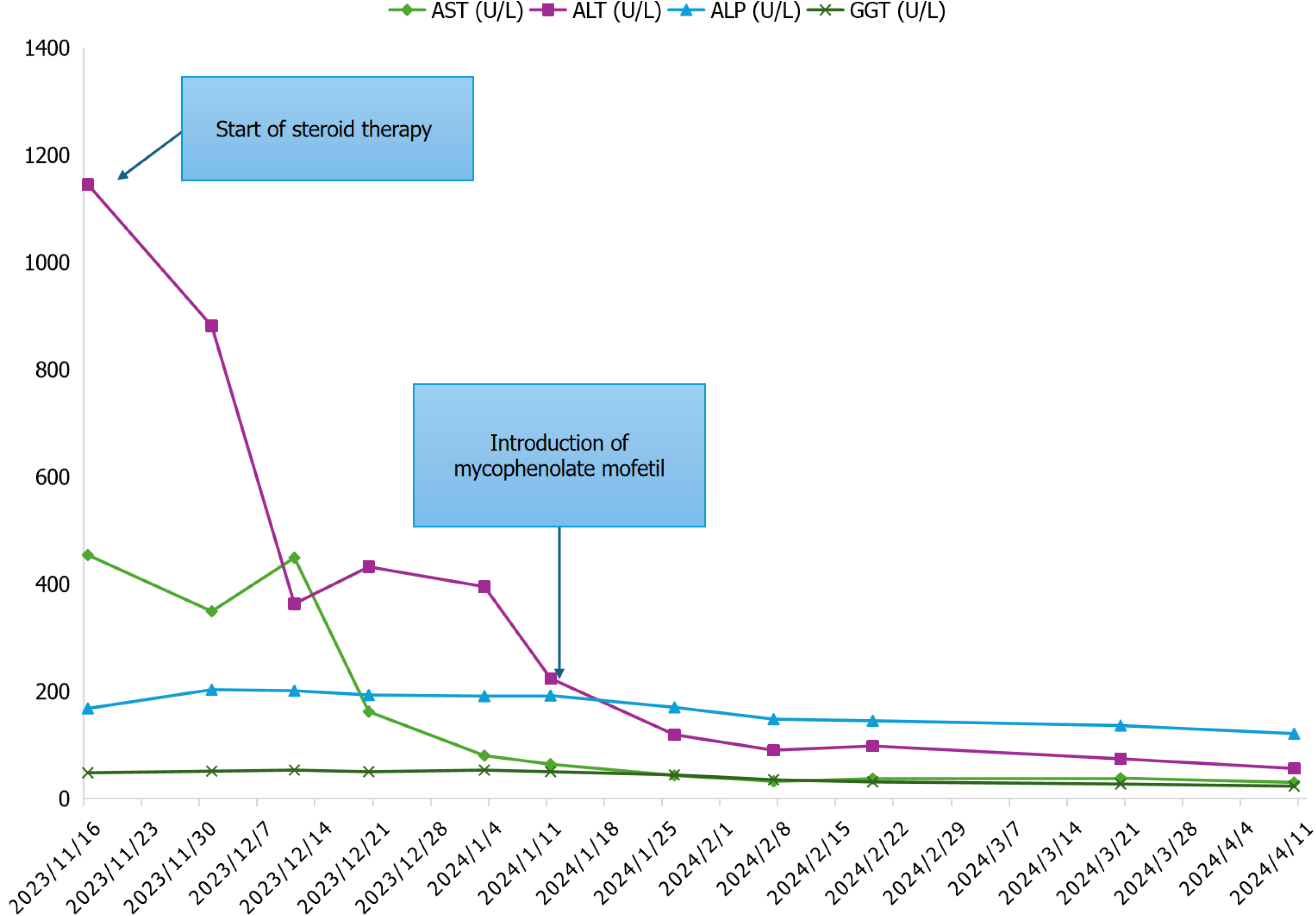
The patient began therapy with prednisone at a dose of 50 mg daily (approximately 0.5 mg per kilogram of body weight per day). An adjuvant program of regular weight-bearing exercise, a Mediterranean and low-fat diet, and vitamin D supplementation was recommended to improve the glycemic profile and protect against progressive corticosteroid-induced osteopenia.
Liver tests initially showed slight improvement following therapy with corticosteroids (Figure 2). For steroid-induced hyperglycemia, treatment with metformin and insulin glargine was initiated on the recommendation of the diabetologist. In January 2024, mycophenolate mofetil (MMF) was added (500 mg twice daily) as a steroid-sparing strategy due to the risk of hematological toxicity and therapy-related acute myeloid leukemia associated with azathioprine[16]. In addition, several trials have suggested superior tolerability of MMF compared to azathioprine and have recommended MMF as first-line induction therapy for treatment-naive autoimmune hepatitis[17]. MMF was then administered at a dose of 1500 mg daily as the dose of prednisone was tapered, with progressive improvement of liver enzymes. Blood leukocyte and platelet counts were closely monitored throughout the induction and maintenance phases due to the risk of MMF-induced myelosuppression. The patient has been followed for > 12 months since her initial presentation and is currently stable at home. She has since received maintenance therapy with MMF and a low dose of prednisone, resulting in a complete biochemical response (CBR), as evidenced by the normalization of serum AST, alanine ALT, bilirubin, and gamma globulin or IgG levels within the upper limit of normal (ULN).
GCH is an extremely rare condition that is usually associated with drugs, viral infections, and autoimmune disorders. This entity represents a nonspecific tissue reaction to various endogenous and exogenous stimuli. Few cases of GCH associated with CLL have been reported in the literature[11-13]. In the first case, Fimmel et al[13] described a 69-year-old man with GCH and CLL, investigating the possible paramyxoviral etiology of GCH. The patient's hepatitis resolved spontaneously without intervention. However, the patient progressively developed liver cirrhosis. A second case, reported by Alexopoulou et al[12], described a patient with CLL and GCH who presented with subacute liver failure. The patient was treated with steroid therapy and acyclovir for suspected AIH and reactivation of Epstein-Barr virus in the context of his hematological cancer. Post-mortem evaluation revealed liver biopsy findings consistent with giant cell hepatitis, and the examination of liver tissue showed paramyxo-like viral particles in the cytoplasm of the affected hepatocytes. However, these previous cases described an association between GCH in the setting of CLL and the presence of an unknown virus. The case reported by Gupta et al[11] is the only one that demonstrates a link between GCH and CLL in the absence of a viral etiology. Our case also demonstrates that CLL triggered GCH without the confounding presence of a virus. A cytopathic effect leading to giant cell formation has been described in association with hepatitis A, B, and C, Epstein-Barr virus, paramyxoviruses, and human immunodeficiency virus infections. However, based on the negative virological results, viral-induced GCH should be excluded. Furthermore, in our case, liver infiltration by CLL was histologically excluded. Specifically, the biopsy does not show the characteristic monotonous portal lymphocytic infiltrates composed of small, round lymphoid cells predominantly of B-cell origin (positive for CD20, CD5, and CD23). Instead, a mixed lymphoid infiltrate, consisting of CD3-positive T cells, was identified. Furthermore, there is no significant sinusoidal infiltration, nor is there evidence of atypical mitotic figures, prominent nucleoli, or hemophagocytosis. Immunohistochemical staining for CLL markers, such as lymphoid enhancer-binding factor 1 in putative B cells, was inconclusive, further supporting the exclusion of hepatic involvement by CLL in this case. In addition, our patient had not taken any new drugs or herbal products for several months before the onset of GCH, thus drug-induced hepatotoxicity should be excluded.
Histological findings were indicative of chronic AIH, characterized by lymphoplasmacytic portal infiltrates with interface and lobular hepatitis, scattered emperipolesis, spotty and confluent necrosis, and severe fibrosis with septa formation[18,14]. Emperipolesis, characterized by the internalization of lymphocytes within hepatocytes, has traditionally been regarded as a key histological feature for the diagnosis of AIH. Although emperipolesis has been commonly documented in AIH, its specificity and sensitivity have recently been questioned, as it can also occur in other hepatic conditions, thereby diminishing its diagnostic reliability as an isolated finding[19]. Nevertheless, emperipolesis remains a crucial histological finding that, when considered alongside clinical, serological, and other pathological features, continues to support the diagnosis of AIH, reflecting underlying immune-mediated hepatic injury[19,20]. Thus, emperipolesis remains clinically significant in diagnostic liver pathology, provided it is interpreted within the appropriate clinicopathological context. In our liver biopsy, inflammatory aggregates composed of lymphomononuclear cells exhibited expression of both T-cell and B-cell markers, CD3 and CD20, respectively, with features excluding involvement of CLL in the liver[21]. Diffuse presence of multinucleated hepatocytes, characteristic of GCH, was observed, suggesting an uncommon morphological change in AIH that is rare in adults[7]. The presence of GCH is often associated with advanced fibrosis and liver dysfunction, necessitating prompt therapeutic intervention to normalize liver function tests. Ductular reaction accompanied by expansion of hepatobiliary cells was observed, consistent with severe fibrosis[22]. Notably, some hepatocyte giant cells expressed cytokeratin 7, indicating hepatobiliary differentiation. Previous research has linked the expansion of hepatobiliary cells in ductular reaction with liver fibrosis and Toll-like receptor 4 (TLR4) expression by biliary cells in advanced fibrotic stages[23]. Several studies have suggested a modified expression of TLR in CLL, potentially contributing to autoimmune complications in these patients. Specifically, the altered expression of TLR4 has been implicated in infections, autoimmunity, and disease progression in CLL[24]. TLR4 is primarily involved in the recognition of bacterial lipopolysaccharides and the initiation of immune responses; however, reduced TLR4 expression has been reported in leukemic B cells in patients with CLL[24]. This impairment correlates with increased disease progression and the development of autoimmune complications, suggesting defective innate immune regulation and a reduced ability to suppress autoreactive immune responses[24-26]. Conversely, hepatic upregulation of TLR4, as previously demonstrated, has been strongly associated with liver fibrosis[23]. Thus, in the context of liver inflammation driven by CLL-related autoimmune phenomena, preserved or ‘surviving’ hepatic TLR4 expression may contribute to a pro-inflammatory environment, potentially exacerbating the progression of hepatic fibrosis. Therefore, this differential regulation of TLR4 between leukemic B cells and hepatic tissues could represent a critical factor in the interplay between autoimmunity and fibrosis, highlighting a complex scenario in which TLR4 dysregulation may promote both autoimmune and fibrotic processes associated with CLL. In fact, several studies have suggested that during non-AIH-related liver inflammation, TLR2/4 ligands may stimulate the production of factors that initiate an autoimmune response, thereby contributing to the onset of autoimmune hepatitis[27]. Moreover, some studies indicate that activation of the TLR4-related signaling pathway plays a primary role in promoting disease progression in liver diseases[28]. Among the various signaling pathways, the TLR4/myeloid differentiation factor 88/nuclear factor kappa B axis is the most critical. Consequently, the development of targeted therapies that inhibit the activity of this signaling axis has become a major focus of research for the treatment of liver diseases[28].
Corticosteroids and azathioprine are recommended as first-line treatments for AIH. Combination therapy is preferred because lower doses of steroids can be administered when combined with azathioprine, thereby reducing the frequency of steroid-related adverse events[29]. The aims of such treatment are to achieve normalization of the patient’s serum AST, ALT, and IgG levels to within the ULN, also known as a CBR, and histological remission of the disease, and eventually, to prevent fibrosis progression and the development of end-stage liver disease[30]. An incomplete response or intolerance to these drugs is considered an indication for a second-line regimen, particularly with MMF[31]. Several studies have shown that MMF could be an alternative first-line treatment option to induce and maintain CBR, with a rapid steroid-sparing effect and few side effects[32-34]. A recent open-label, multicenter, randomized, controlled trial by Snijders et al[17] demonstrated that MMF combined with prednisolone resulted in a significantly higher rate of biochemical remission at 24 weeks compared to azathioprine combined with prednisolone in patients with treatment-naive AIH. Furthermore, the use of azathioprine was associated with a higher incidence of adverse events resulting in treatment discontinuation, indicating superior tolerability of MMF[35]. Moreover, the use of MMF enables the prompt discontinuation of glucocorticoids[35].
The treatment for GCH is not entirely clear, although corticosteroids and low-dose immunosuppressive therapy have been used with limited success[36]. Orthotopic liver transplantation has been reported as the only established curative option. In this case of GCH, we did not achieve CBR with corticosteroids after 4-8 weeks. For this reason, we added MMF for further immunosuppression, resulting in a CBR. In agreement with the previous literature, our patient developed GCH when her hematological disease was at an early stage. It is well established that CLL itself predisposes patients to immune dysregulation. Indeed, autoimmune phenomena occur in 10% to 25% of patients during the disease course[3]. GCH may be another non-hematological autoimmune complication of CLL, often misdiagnosed in the absence of a correct diagnostic work-up and liver biopsy.
The present case highlights the importance of timely recognition of this uncommon condition to prevent disease progression, and the critical role of liver biopsy in distinguishing GCH from other liver disorders. Further studies are required to assess the role of TLR4 in the development of AIH in patients with CLL. The occurrence of GCH in the context of CLL is intriguing and warrants further investigation. Moreover, the optimal treatment for GCH remains uncertain, although corticosteroids and low-dose immunosuppressive therapy have been used with limited efficacy. This case supports the potential of MMF as a viable treatment option for GCH.
| 1. | Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol. 2021;96:1679-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 221] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 2. | Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating M, Montserrat E, Chiorazzi N, Stilgenbauer S, Rai KR, Byrd JC, Eichhorst B, O'Brien S, Robak T, Seymour JF, Kipps TJ. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131:2745-2760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 1116] [Article Influence: 159.4] [Reference Citation Analysis (0)] |
| 3. | Dearden C. Disease-specific complications of chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2008;450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Vitale C, Montalbano MC, Salvetti C, Boccellato E, Griggio V, Boccadoro M, Coscia M. Autoimmune Complications in Chronic Lymphocytic Leukemia in the Era of Targeted Drugs. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Barcellini W, Capalbo S, Agostinelli RM, Mauro FR, Ambrosetti A, Calori R, Cortelezzi A, Laurenti L, Pogliani EM, Pedotti P, Liso V, Mandelli F, Zanella A. Relationship between Autoimmune Phenomena and Disease Stage and Therapy in B-Cell Chronic Lymphocytic Leukaemia. Blood. 2006;108:4929-4929. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Kreiniz N, Beyar Katz O, Polliack A, Tadmor T. The Clinical Spectrum of Hepatic Manifestations in Chronic Lymphocytic Leukemia. Clin Lymphoma Myeloma Leuk. 2017;17:863-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Bihari C, Rastogi A, Sarin SK. Postinfantile giant cell hepatitis: an etiological and prognostic perspective. Hepat Res Treat. 2013;2013:601290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Fang JW, González-Peralta RP, Chong SK, Lau GM, Lau GM, Lau JY. Hepatic expression of cell proliferation markers and growth factors in giant cell hepatitis: implications for the pathogenetic mechanisms involved. J Pediatr Gastroenterol Nutr. 2011;52:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Ben-Ari Z, Broida E, Monselise Y, Kazatsker A, Baruch J, Pappo O, Skappa E, Tur-Kaspa R. Syncytial giant-cell hepatitis due to autoimmune hepatitis type II (LKM1+) presenting as subfulminant hepatitis. Am J Gastroenterol. 2000;95:799-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Torbenson M, Hart J, Westerhoff M, Azzam RK, Elgendi A, Mziray-Andrew HC, Kim GE, Scheimann A. Neonatal giant cell hepatitis: histological and etiological findings. Am J Surg Pathol. 2010;34:1498-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Gupta N, Njei B. Syncytial giant cell hepatitis in a patient with chronic lymphocytic leukemia. J Dig Dis. 2015;16:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Alexopoulou A, Deutsch M, Ageletopoulou J, Delladetsima JK, Marinos E, Kapranos N, Dourakis SP. A fatal case of postinfantile giant cell hepatitis in a patient with chronic lymphocytic leukaemia. Eur J Gastroenterol Hepatol. 2003;15:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Fimmel CJ, Guo L, Compans RW, Brunt EM, Hickman S, Perrillo RR, Mason AL. A case of syncytial giant cell hepatitis with features of a paramyxoviral infection. Am J Gastroenterol. 1998;93:1931-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, Bianchi FB, Shibata M, Schramm C, Eisenmann de Torres B, Galle PR, McFarlane I, Dienes HP, Lohse AW; International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1253] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 15. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 848] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 16. | Nouha T, Asma M, Nadia B, Emna BM, Yosra Z, Yosra S. Acute myeloid leukemia after 10 years of azathioprine treatment for Crohn's disease. Int J Clin Pharmacol Ther. 2023;61:520-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Snijders RJALM, Stoelinga AEC, Gevers TJG, Pape S, Biewenga M, Tushuizen ME, Verdonk RC, de Jonge HJM, Vrolijk JM, Bakker SF, Vanwolleghem T, de Boer YS, Baven Pronk MAMC, Beuers U, van der Meer AJ, Gerven NMFV, Sijtsma MGM, van Eijck BC, van IJzendoorn MC, van Herwaarden M, van den Brand FF, Korkmaz KS, van den Berg AP, Guichelaar MMJ, Levens AD, van Hoek B, Drenth JPH; Dutch Autoimmune Hepatitis Working Group. An open-label randomised-controlled trial of azathioprine vs. mycophenolate mofetil for the induction of remission in treatment-naive autoimmune hepatitis. J Hepatol. 2024;80:576-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 18. | Balitzer D, Shafizadeh N, Peters MG, Ferrell LD, Alshak N, Kakar S. Autoimmune hepatitis: review of histologic features included in the simplified criteria proposed by the international autoimmune hepatitis group and proposal for new histologic criteria. Mod Pathol. 2017;30:773-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Zhang X, Jain D. The many faces and pathologic diagnostic challenges of autoimmune hepatitis. Hum Pathol. 2023;132:114-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Bozward AG, Davies SP, Morris SM, Kayani K, Oo YH. Cellular interactions in self-directed immune-mediated liver diseases. J Hepatol. 2025;82:1110-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Choi WT, Gill RM. Hepatic Lymphoma Diagnosis. Surg Pathol Clin. 2018;11:389-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology. 2014;146:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 228] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 23. | Vespasiani-Gentilucci U, Carotti S, Perrone G, Mazzarelli C, Galati G, Onetti-Muda A, Picardi A, Morini S. Hepatic toll-like receptor 4 expression is associated with portal inflammation and fibrosis in patients with NAFLD. Liver Int. 2015;35:569-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Barcellini W, Imperiali FG, Zaninoni A, Reda G, Consonni D, Fattizzo B, Lonati S, Nobili L, Zanella A, Cortelezzi A. Toll-like receptor 4 and 9 expression in B-chronic lymphocytic leukemia: relationship with infections, autoimmunity and disease progression. Leuk Lymphoma. 2014;55:1768-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Rybka J, Butrym A, Wróbel T, Jaźwiec B, Bogucka-Fedorczuk A, Poręba R, Kuliczkowski K. The Expression of Toll-Like Receptors in Patients with B-Cell Chronic Lymphocytic Leukemia. Arch Immunol Ther Exp (Warsz). 2016;64:147-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Rozková D, Novotná L, Pytlík R, Hochová I, Kozák T, Bartůnková J, Spísek R. Toll-like receptors on B-CLL cells: expression and functional consequences of their stimulation. Int J Cancer. 2010;126:1132-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Chi G, Feng XX, Ru YX, Xiong T, Gao Y, Wang H, Luo ZL, Mo R, Guo F, He YP, Zhang GM, Tian DA, Feng ZH. TLR2/4 ligand-amplified liver inflammation promotes initiation of autoimmune hepatitis due to sustained IL-6/IL-12/IL-4/IL-25 expression. Mol Immunol. 2018;99:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Tang YL, Zhu L, Tao Y, Lu W, Cheng H. Role of targeting TLR4 signaling axis in liver-related diseases. Pathol Res Pract. 2023;244:154410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 29. | Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis: Current Status and Future Directions. Gut Liver. 2016;10:177-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 30. | Dalekos GN, Arvaniti P, Gatselis NK, Samakidou A, Gabeta S, Rigopoulou E, Koukoulis GK, Zachou K. First Results From a Propensity Matching Trial of Mycophenolate Mofetil vs. Azathioprine in Treatment-Naive AIH Patients. Front Immunol. 2021;12:798602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Komori A. Recent updates on the management of autoimmune hepatitis. Clin Mol Hepatol. 2021;27:58-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 32. | Zachou K, Gatselis N, Papadamou G, Rigopoulou EI, Dalekos GN. Mycophenolate for the treatment of autoimmune hepatitis: prospective assessment of its efficacy and safety for induction and maintenance of remission in a large cohort of treatment-naïve patients. J Hepatol. 2011;55:636-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 33. | Zachou K, Gatselis NK, Arvaniti P, Gabeta S, Rigopoulou EI, Koukoulis GK, Dalekos GN. A real-world study focused on the long-term efficacy of mycophenolate mofetil as first-line treatment of autoimmune hepatitis. Aliment Pharmacol Ther. 2016;43:1035-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 34. | Yu ZJ, Zhang LL, Huang TT, Zhu JS, He ZB. Comparison of mycophenolate mofetil with standard treatment for autoimmune hepatitis: a meta-analysis. Eur J Gastroenterol Hepatol. 2019;31:873-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Dalekos GN, Arvaniti P, Gatselis NK, Gabeta S, Samakidou A, Giannoulis G, Rigopoulou E, Koukoulis GK, Zachou K. Long-term results of mycophenolate mofetil vs. azathioprine use in individuals with autoimmune hepatitis. JHEP Rep. 2022;4:100601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 36. | Tajiri K, Shimizu Y, Tokimitsu Y, Tsuneyama K, Sugiyama T. An elderly man with syncytial giant cell hepatitis successfully treated by immunosuppressants. Intern Med. 2012;51:2141-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |