Published online Apr 27, 2025. doi: 10.4254/wjh.v17.i4.92426
Revised: June 30, 2024
Accepted: July 9, 2024
Published online: April 27, 2025
Processing time: 455 Days and 18.9 Hours
Genetic disorders affecting hepatobiliary transporters can be triggered by various factors, resulting in marked cholestasis.
We report two patients who experienced a severe episode of intrahepatic cholestasis triggered by an acute hepatitis E virus infection. Following an extensive clinical examination that ruled out common causes of cholestatic liver damage, we conducted next-generation sequencing to determine the genetic profiles of the patients. The analysis revealed several known and unknown variants in genes associated with hepatobiliary transporters and bile salt regulation, including ATP8B1, ABCB11, ABCB4, MYO5B, and FXR. For a comprehensive understanding of the pathophysiology, we performed ClinVar analysis and utilized PolyPhen for bioinformatic prediction of functional impact. Both patients exhibited rapid symptom improvement and a decrease in hyperbilirubinemia when treated with either rifampicin or bezafibrate.
Our findings introduce hepatitis E viral infection as a novel trigger for intrahepatic cholestasis, and we categorize the significance of the various genetic variants based on the current state of research.
Core Tip: This case report highlights the role of genetic variations in hepatobiliary transporters in predisposing individuals to intrahepatic cholestasis and identifies hepatitis E virus (HEV) as a potential trigger. We present two cases of HEV-triggered intrahepatic cholestasis in patients with polymorphisms in ATP8B1 and other transporter-related genes. These findings suggest that these genetic variants may act as predisposing factors for cholestatic episodes, underscoring the need for further research to confirm these results in larger patient cohorts and through functional studies.
- Citation: Drexler S, Haedge F, Weber SN, Krawczyk M, Matter MS, Geppert CI, Weber A, Stieger B, Trautwein C, Kremer AE. Hepatitis E virus infection-triggered intrahepatic cholestasis: A case report. World J Hepatol 2025; 17(4): 92426
- URL: https://www.wjgnet.com/1948-5182/full/v17/i4/92426.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i4.92426
Genetic variations in hepatobiliary transporters may predispose individuals to intrahepatic cholestasis, potentially leading to conditions such as benign recurrent intrahepatic cholestasis (BRIC) or progressive familial intrahepatic cholestasis (PFIC). BRIC is a heterogeneous group of rare cholestasis syndromes, which is caused by various triggers in susceptible patients carrying polymorphic variants of genes essential for bile formation[1,2]. The first episode usually occurs in early adulthood and is triggered by various factors, such as viral infections or, less frequently, certain drugs[3]. Affected individuals suffer from pronounced cholestasis with jaundice as a consequence. The most commonly affected genes are ATP8B1, ABCB11, and ABCB4, resulting in impaired function of the aminophospholipid translocase FIC1 and the transporters BSEP and MDR3[4-6].
Mutations in the same transporters can also lead to the more severe phenotype of PFIC, which typically manifests in childhood and causes profound cholestatic liver damage[6]. Patients with PFIC due to mutations in FIC1 or BSEP often show low gamma-glutamyltransferase (GGT) serum levels, whereas impaired function of MDR3 typically presents with elevated GGT levels[6].
While more than six subtypes of PFIC have been described, only mutations in ATPB8 (BRIC type 1) and ABCB11 (BRIC type 2) have been associated with BRIC[3,6]. The resulting clinical phenotype varies depending on the underlying polymorphisms and mutations as well as whether these alterations are heterozygous or homozygous. While certain pathogenic mutations can be related to certain severe clinical phenotypes, such genotype-phenotype correlations are less well-defined in milder cases, where the relationship between genetic variants and clinical manifestation remains ambiguous.
There are numerous triggers for intrahepatic cholestasis, and one potential cause is an infection with the hepatitis E virus (HEV). This zoonotic infection is known to elicit jaundice and nonspecific symptoms such as fatigue, nausea, and itching. Besides elevated liver enzymes, the presence of positive IgM antibodies, quickly followed by IgG antibodies, confirms the diagnosis. Nucleic acid testing for HEV RNA in blood and stool exhibits the highest sensitivity, detecting viremia 3–6 weeks after infection[7].
Herein, we describe two phenotypes of clinical intrahepatic cholestasis triggered by HEV infection in patients with various homozygous and heterozygous variants.
We report on two male patients, aged 39 and 58 years, who presented to our clinics in Erlangen and Aachen, Germany. Both had a normal body weight and initially presented to their general practitioner or to a peripheral hospital with fever, fatigue, jaundice, nausea, and loss of appetite.
Symptoms had started 2–4 weeks before referral to the respective university hospital.
While Patient 1 had no medical history, Patient 2 had already experienced an episode of jaundice in his early twenties.
Neither patient had a family history of liver disease or jaundice, and both denied alcohol consumption or illicit drug use.
Both patients exhibited pronounced jaundice along with pruritus. There were no signs of decompensation, such as ascites, encephalopathy, or gastrointestinal bleeding.
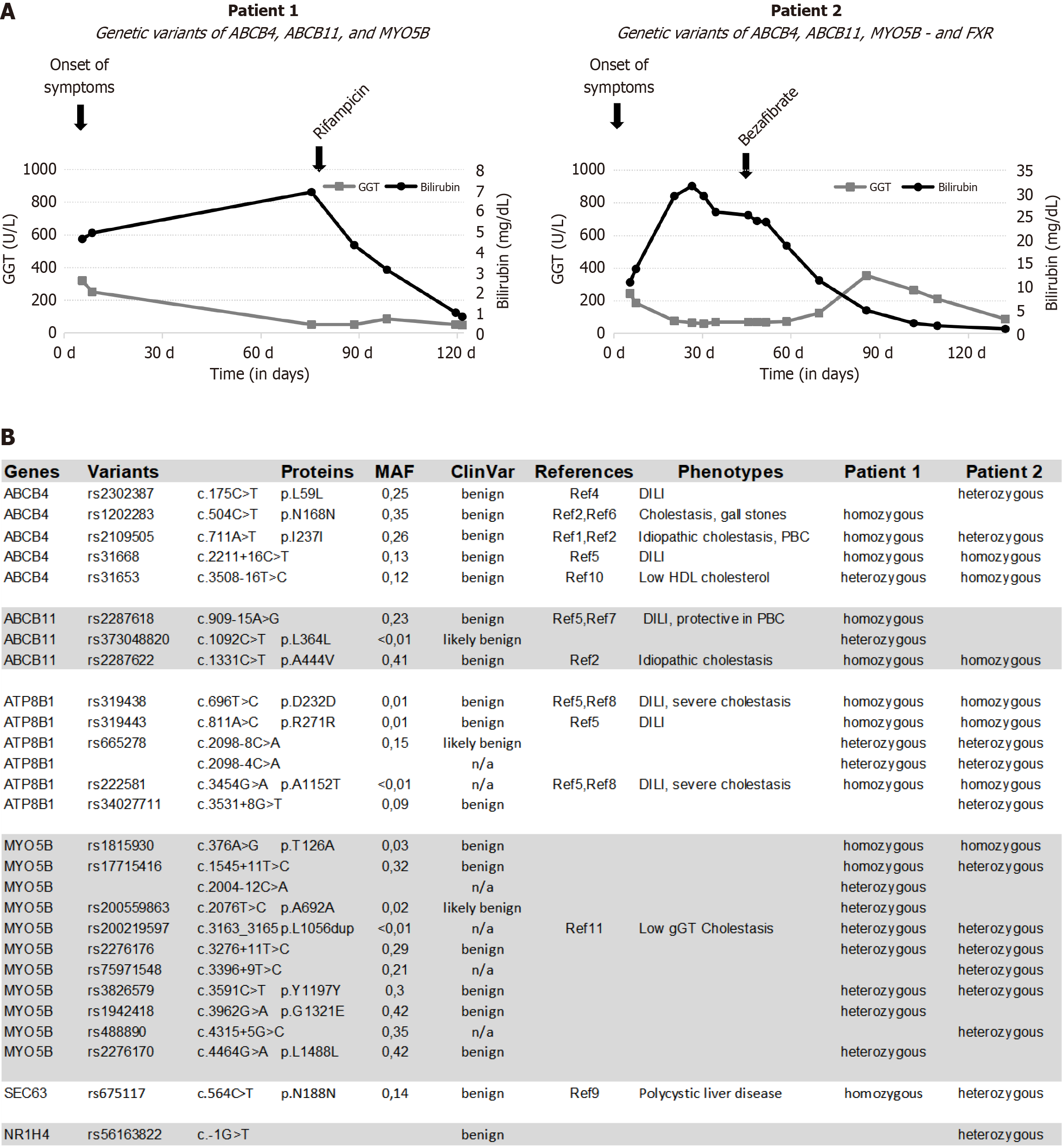
Both patients initially exhibited markedly elevated transaminases and significant hyperbilirubinemia but only slightly elevated gamma-glutamyltransferase (Figure 1A). Diagnostic assessments revealed evidence of acute hepatitis E, supported by the presence of positive anti-HEV-IgM in both patients. Additionally, Patient 1 exhibited positive nucleic acid amplification testing for HEV RNA detection in the blood.
Abdominal ultrasonography in both patients revealed a liver of normal size with a regular homogeneous parenchymal structure and no focal lesions. Magnetic resonance cholangiography in Patient 1 demonstrated a normal intrahepatic and extrahepatic bile duct system.
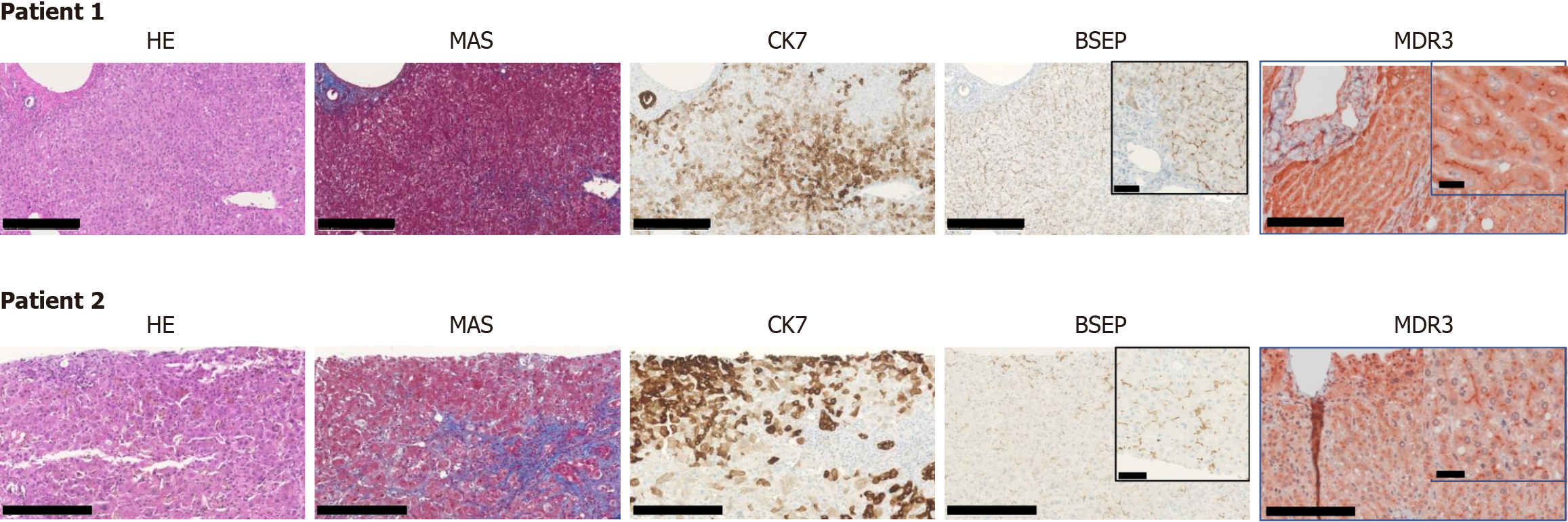
Due to progressive hyperbilirubinemia with marked pruritus and loss of appetite associated with weight loss, both patients were referred to tertiary care centers at university hospitals. Investigations excluded obstructive cholestasis, autoimmune hepatitis, primary biliary cholangitis or primary sclerosing cholangitis, alpha-1-antitrypsin deficiency, hemochromatosis, Wilson's disease, and other acute viral infections. Histology of liver biopsies demonstrated panacinar cholestasis with mild fibrosis. BSEP expression was preserved in both cases. More than 4 weeks after the onset of symptoms, neither the HEV ORF2 (capsid) protein in liver tissue[8,9] nor HEV-RNA in the blood could be detected, providing no evidence of chronic HEV infection.
Genomic DNA was isolated using standard procedures applying membrane-based QIAamp DNA extraction protocol (Qiagen, Hilden, Germany). For library preparation, the Illumina DNA prep with Enrichment (formerly Nextera Flex for Enrichment) was used according to the manufacturer’s instructions (Illumina San Diego, USA). The custom panel designed in cooperation with Illumina included the exons of ABCB4, ABCB11, ABCC2, ALG8, ATP8B1, GANAB, MYO5B, PRKCSH, PRSS1, SEC61B, and SEC63. Direct paired-end NGS sequencing (2 × 150 bp) was performed on an Illumina MiniSeq platform. Results were analyzed using the JSI SeqNext software (JSI, Ettenheim, Germany), and the detected genetic variants were further checked using the gnomAD (gnomad.broadinstitute.org)
The predisposing cholestatic variant NR1H4 (FXR) c.-1G>T (rs56163822, C__25598386_10, Thermo Fisher Scientific) was genotyped using allelic discrimination on a TaqMan 7500 Fast PCR System. Quality control was maintained by including both negative controls and DNA samples with established genotypes as internal standards. Fluorescence signals were analyzed using allelic discrimination software 7500 v.2.3.
Subsequently, we performed genotyping for various genes as well as panel sequencing from the blood (Figure 1B). In both patients, genetic variants in ATP8B1, ABCB4, ABCB11, and MYO5B could be identified, and Patient 2 also had a heterogenous pathogenic mutation in NR1H4.
Based on the clinical presentation, laboratory findings, and exclusion of other causes, a diagnosis of HEV-triggered intrahepatic cholestasis was established.
Patient 1 was subsequently treated with rifampicin 150 mg and Patient 2, with bezafibrate 400 mg until normalization of bilirubin.
Both patients showed a rapid improvement, with a marked decline in hyperbilirubinemia and resolution of pruritus and other symptoms such as loss of appetite. During follow-up examinations, both patients remained asymptomatic, with normalization of bilirubin levels for more than a year (Figures 1 and 2).
In the search for possible susceptibility loci for cholestatic liver disease, several genes (ABCB4, ABCB11, ATP8B1, ALG8, GNAB, MYO5B, PRKCSH, SEC61B, and SEC63) of the patients were analyzed by next-generation sequencing (NGS; Figure 1B). We identified both known and novel homozygous and heterozygous variants. To enhance our understanding of the pathophysiology, we conducted a ClinVar analysis and performed a bioinformatic prediction using PolyPhen (Figure 1B), aligning the results with the current literature. All variants were classified as either benign or likely benign, with some lacking data. A comprehensive summary of the literature pertaining to the identified variants is presented in the Supplementary material. The phenotypes of liver injury associated with these variants vary widely, and a definitive correlation between the genotypes and the cholestatic episodes cannot be established at this time.
Nonetheless, we posit that ATP8B1 is a promising candidate gene contributing to cholestatic susceptibility in our patients. The rare variants rs319438 and rs222581 have previously been associated with intrahepatic cholestasis[5,8]. Supporting a potential role of ATP8B1 in Patient 2, it has been reported that mutations in ATP8B1 lead to decreased FXR1 activity[10]. This patient had a variant form of NR1H4 (Figure 1B). The farnesoid X receptor plays a central role in the regulation of bile acid metabolism, and clinically relevant severe mutations are now classified as PFIC type 5[11].
Moreover, the significantly more pronounced hyperbilirubinemia in Patient 2 compared to Patient 1 (Figure 1A) aligned with previous findings associating rs319438 and rs222581 of ATP8B1 with severe cholestatic liver disease[12,13]. Although a considerable number of other variants (Figure 1B) were found in our patients, we do not believe that they are a major cause of the cholestatic episodes. Functional variants of ABCB4 typically lead to elevated GGT levels[14], which were not observed in our patients. Variants in MYO5B typically lead to altered canalicular morphology and abnormal BSEP immunohistochemical staining, both of which were not observed in our patients. Based on the current literature, our sequencing information, and the immunohistochemical staining results, the two variants of ATP8B1 appear to be the most likely genetic factors predisposing these patients to HEV-triggered intrahepatic cholestasis.
Treatment in the present cases was based on the pregnane X receptor agonist rifampicin and the peroxisome proliferator-activated receptor agonist bezafibrate. Rifampicin upregulates the expression of detoxification enzymes as well as hepatocellular export pumps including the apical transporter MRP2 and basolateral OSTb in hepatocytes[15,16]. Bezafibrate, through the activation of peroxisome proliferator-activated receptors, modulates lipid metabolism and anti-inflammatory responses[17]. The induction of these genes increases the elimination of bilirubin conjugates and bile salts and represents a therapeutic strategy in intrahepatic cholestasis. Furthermore, both drugs alleviate pruritus and are included in current treatment guidelines for cholestatic pruritus. Although their combined use may theoretically offer synergistic benefits, we advise caution due to the potential risk of hepatotoxicity with either agent. In contrast, ursodeoxycholic acid can be safely added as a baseline therapy for intrahepatic cholestasis.
After excluding common causes of cholestatic liver damage in the clinical workup, hereditary causes of cholestasis including disorders of hepatobiliary transporters represent an important differential diagnosis. In addition to known triggers, our case reports unravel HEV infection as a novel trigger for intrahepatic cholestasis. Based on our sequencing data, the ATP8B1 variants identified in both patients are likely susceptibility factors for this cholestatic phenotype. However, to confirm the pathogenic relevance of these variants, further evidence is required—either through the identification of additional patients carrying these genetic alterations or through functional studies demonstrating their biological impact.
| 1. | Summerskill WH, Walshe JM. Benign recurrent intrahepatic "obstructive" jaundice. Lancet. 1959;2:686-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 100] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Sticova E, Jirsa M, Pawłowska J. New Insights in Genetic Cholestasis: From Molecular Mechanisms to Clinical Implications. Can J Gastroenterol Hepatol. 2018;2018:2313675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Halawi A, Ibrahim N, Bitar R. Triggers of benign recurrent intrahepatic cholestasis and its pathophysiology: a review of literature. Acta Gastroenterol Belg. 2021;84:477-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Dröge C, Bonus M, Baumann U, Klindt C, Lainka E, Kathemann S, Brinkert F, Grabhorn E, Pfister ED, Wenning D, Fichtner A, Gotthardt DN, Weiss KH, McKiernan P, Puri RD, Verma IC, Kluge S, Gohlke H, Schmitt L, Kubitz R, Häussinger D, Keitel V. Sequencing of FIC1, BSEP and MDR3 in a large cohort of patients with cholestasis revealed a high number of different genetic variants. J Hepatol. 2017;67:1253-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Jüngst C, Justinger C, Fischer J, Berg T, Lammert F. Common ABCB4 and ABCB11 Genotypes Are Associated with Idiopathic Chronic Cholestasis in Adults. Dig Dis. 2022;40:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Baker A, Kerkar N, Todorova L, Kamath BM, Houwen RHJ. Systematic review of progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. 2019;43:20-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol. 2018;68:1256-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 442] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 8. | Lenggenhager D, Gouttenoire J, Malehmir M, Bawohl M, Honcharova-Biletska H, Kreutzer S, Semela D, Neuweiler J, Hürlimann S, Aepli P, Fraga M, Sahli R, Terracciano L, Rubbia-Brandt L, Müllhaupt B, Sempoux C, Moradpour D, Weber A. Visualization of hepatitis E virus RNA and proteins in the human liver. J Hepatol. 2017;67:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Lenggenhager D, Pawel S, Honcharova-Biletska H, Evert K, Wenzel JJ, Montani M, Furrer E, Fraga M, Moradpour D, Sempoux C, Weber A. The histologic presentation of hepatitis E reflects patients' immune status and pre-existing liver condition. Mod Pathol. 2021;34:233-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Chen F, Ananthanarayanan M, Emre S, Neimark E, Bull LN, Knisely AS, Strautnieks SS, Thompson RJ, Magid MS, Gordon R, Balasubramanian N, Suchy FJ, Shneider BL. Progressive familial intrahepatic cholestasis, type 1, is associated with decreased farnesoid X receptor activity. Gastroenterology. 2004;126:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Pfister ED, Dröge C, Liebe R, Stalke A, Buhl N, Ballauff A, Cantz T, Bueltmann E, Stindt J, Luedde T, Baumann U, Keitel V. Extrahepatic manifestations of progressive familial intrahepatic cholestasis syndromes: Presentation of a case series and literature review. Liver Int. 2022;42:1084-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Schreiner P, Stieger B, McLin V, Rougemont AL, Keitel V, Dröge C, Müllhaupt B. A rare cause of a cholestatic jaundice in a North African teenager. Liver Int. 2019;39:2036-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Blackmore L, Knisely AS, Hartley JL, McKay K, Gissen P, Marcus R, Shawcross DL. Polymorphisms in ABCB11 and ATP8B1 Associated with Development of Severe Intrahepatic Cholestasis in Hodgkin's Lymphoma. J Clin Exp Hepatol. 2013;3:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Stättermayer AF, Halilbasic E, Wrba F, Ferenci P, Trauner M. Variants in ABCB4 (MDR3) across the spectrum of cholestatic liver diseases in adults. J Hepatol. 2020;73:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 15. | Tandon P, Rowe BH, Vandermeer B, Bain VG. The efficacy and safety of bile Acid binding agents, opioid antagonists, or rifampin in the treatment of cholestasis-associated pruritus. Am J Gastroenterol. 2007;102:1528-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | van Dijk R, Kremer AE, Smit W, van den Elzen B, van Gulik T, Gouma D, Lameris JS, Bikker H, Enemuo V, Stokkers PC, Feist M, Bosma P, Jansen PL, Beuers U. Characterization and treatment of persistent hepatocellular secretory failure. Liver Int. 2015;35:1478-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | de Vries E, Bolier R, Goet J, Parés A, Verbeek J, de Vree M, Drenth J, van Erpecum K, van Nieuwkerk K, van der Heide F, Mostafavi N, Helder J, Ponsioen C, Oude Elferink R, van Buuren H, Beuers U; Netherlands Association for the Study of the Liver-Cholestasis Working Group. Fibrates for Itch (FITCH) in Fibrosing Cholangiopathies: A Double-Blind, Randomized, Placebo-Controlled Trial. Gastroenterology. 2021;160:734-743.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |