Published online Apr 27, 2025. doi: 10.4254/wjh.v17.i4.105797
Revised: March 21, 2025
Accepted: April 9, 2025
Published online: April 27, 2025
Processing time: 77 Days and 5 Hours
Chronic hepatitis B virus (HBV) infection affects approximately 254 million individuals globally, contributing to significant morbidity and mortality due to HBV-related liver failure and cirrhosis, which result in millions of fatalities each year. Although approved antiviral nucleos(t)ide analogues can effectively su
Core Tip: As we know, chronic hepatitis B virus (HBV) infection can lead to liver failure and cirrhosis, resulting in significantly increased morbidity and mortality rates. Current antiviral nucleos(t)ide analogues suppress HBV replication but are limited in reducing hepatitis B surface antigen (HBsAg) levels. Interferon alpha, an immunomodulator, is restricted by safety concerns and adverse reactions. New drug development aims for a functional cure, defined as HBsAg clearance and sustained HBV DNA suppression. This review highlights recent advancements in novel therapies targeting HBV, including HBsAg entry inhibitors, monoclonal antibodies, transcription inhibitors, and immunomodulatory agents like TLR-7/8 agonists, immune checkpoint inhibitors, and therapeutic vaccines.
- Citation: Liu T, Wang H, Zhao Y, Wang YX, Xing X, Gao P. Drug development for chronic hepatitis B functional cure: Recent progress. World J Hepatol 2025; 17(4): 105797
- URL: https://www.wjgnet.com/1948-5182/full/v17/i4/105797.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i4.105797
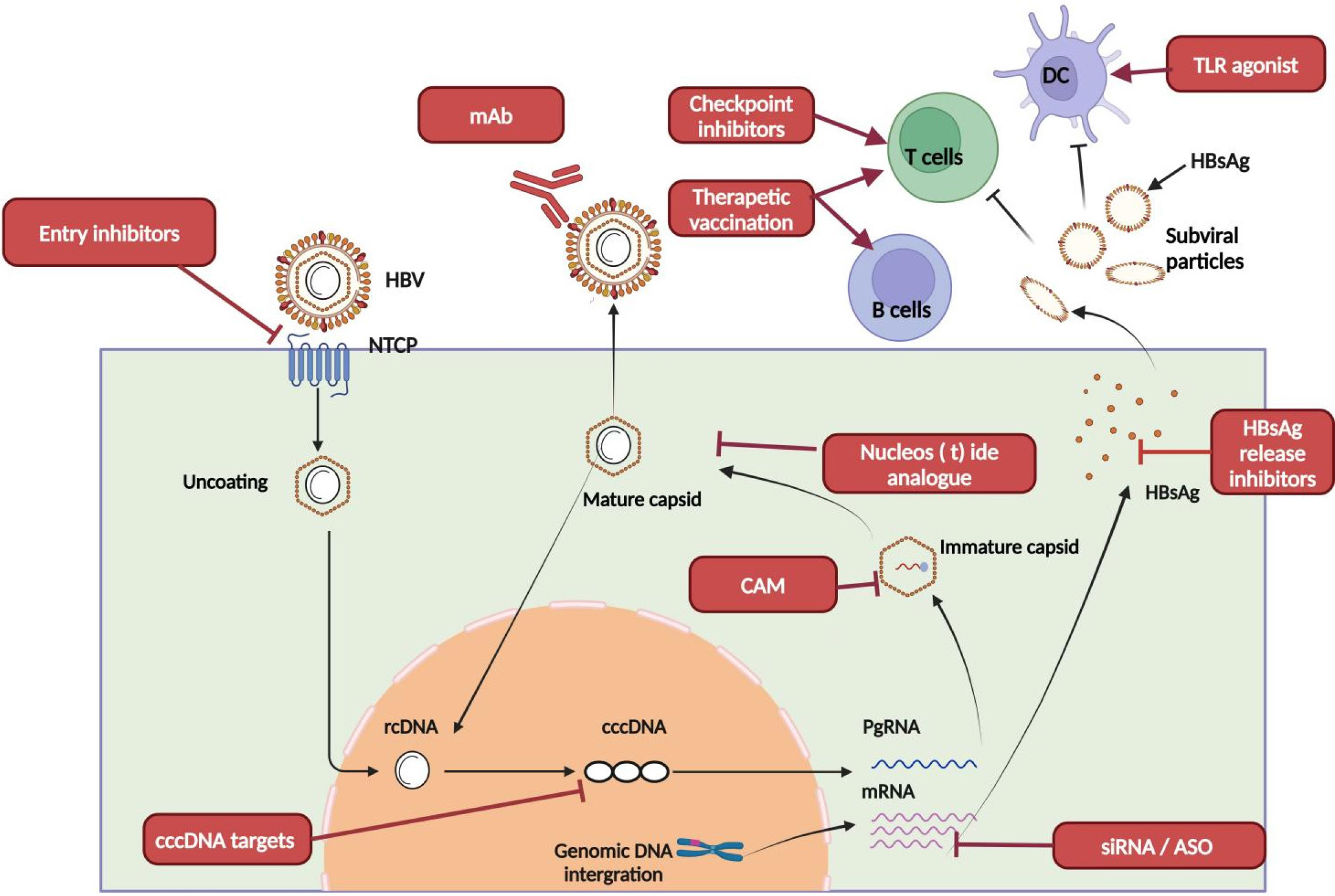
Chronic hepatitis B virus (HBV) infection represents a significant global public health challenge. According to the World Health Organization, an estimated 254 million individuals are living with chronic hepatitis B in 2022, with approximately 1.1 million deaths attributed to HBV-related complications such as cirrhosis and hepatocellular carcinoma (primary liver cancer)[1]. The challenges in achieving a cure for HBV infection are attributable to the virological and immunological features of chronic HBV infection, including the persistence of covalently closed circular DNA (cccDNA) in the nuclei of infected hepatocytes as a viral transcription template; the accumulation of viral integration events throughout the long-term infection that contributes to hepatitis B surface antigen (HBsAg) production; and the high viral antigen load that drives the exhaustion of HBV-specific immunity[2]. As current available antiviral therapies are rarely effective in treating HBV, a group of novel antiviral drugs with distinct mechanisms are being developed, which can be categorized into the following two classes: Direct-acting antivirals that further block viral replication and lower the viral antigen load, and immunomodulators that boost immunity and restore anti-HBV immune surveillance.
HBV is a partially double-stranded DNA virus belonging to the Hepadnaviridae family, has a structure composed of an envelope surrounding a nucleocapsid that houses the relaxed circular DNA (rcDNA) genome. The virus specifically infects hepatocytes, initiating a complex replication cycle. The process begins with the interaction between the Pre-S1 glycoprotein on the viral envelope and the sodium taurocholate co-transporting polypeptide (NTCP) receptor on the hepatocyte surface, which facilitates the entry of the viral nucleocapsid into the cell. After entry, the rcDNA is transported to the nucleus, where it is converted into cccDNA, serving as a stable template for the transcription of pre-genomic RNA (pgRNA) and messenger RNA (mRNA), which are essential for viral replication and protein synthesis. The pgRNA undergoes reverse transcription, generating new rcDNA molecules while simultaneously encoding core proteins and polymerase, which are required for the assembly of new nucleocapsids. Concurrently, the mRNA directs the production of other viral proteins. The mature nucleocapsids acquire viral envelope proteins to form mature virions that are released from the cell. A subset of nucleocapsids returns to the nucleus, where rcDNA is reconverted into cccDNA, perpetuating the intracellular reservoir of viral DNA. This recycling mechanism enables the persistence of HBV within hepatocytes, even in the absence of new infections. Moreover, HBV DNA can integrate into the host genome, contributing to the development and progression of chronic infection[3].
The treatment of chronic hepatitis B relies on two main classes of drugs approved by the United States FDA, the European Medicines Agency, and regulatory bodies in many Asian countries: Immunomodulators and nucleos(t)ide analog antiviral drugs as listed in Table 1. The approved immunomodulators include conventional interferon alpha-2α (IFN-2α) and pegylated IFN-2α (PEG-IFN-2α)[4]. The nucleos(t)ide analog family has three drugs used as first-line therapy, namely, entecavir, tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide. Adefovir dipivoxil, lamivudine, and telbivudine are less ideal in terms of potency, resistance, and long-term safety profiles.
| Types of drugs | Name | Antiviral mechanism | Limitations/comments |
| Immunomodulators | IFN-2α and PEG-IFN-2α | Inhibit HBV replication Activate antiviral immune responses | Poor tolerability; Anti-PEG antibody mediated immune clearance[4] |
| Nucleoside analogue | ETV | HBV DNA polymerase inhibitor | High potency and low resistance |
| Nucleotide analogue | TDF | HBV DNA polymerase inhibitor | Potent, but with kidney and bone toxicity concern in long-term use |
| Nucleotide analogue | TAF | HBV DNA polymerase inhibitor | TDF analog with improved renal and bone safety profiles |
| Nucleotide analogue | ADV | HBV DNA polymerase inhibitor | Less potent, nephrotoxicity |
| Nucleoside analogue | 3TC | HBV DNA polymerase inhibitor | Less potent, high rate of resistance |
| Nucleoside analogue | LdT | HBV DNA polymerase inhibitor | High rate of resistance |
A hallmark of chronic HBV infection is the presence of an enormous number of subviral particles composed of hepatitis B surface antigen (HBsAg) in the plasma that outnumber complete virions by a ratio of 10000-100000: 1[5]. This excess of subviral particles interfere with the function of innate and adaptive immune cells, impeding the clearance of circulating viruses and infected hepatocytes. Thus, hepatitis B treatment goals are categorized into functional and complete cure, whereby functional cure is defined as HBsAg clearance or levels below 0.05 IU/mL, undetectable HBV DNA or levels below 10 IU/mL, with or without HBsAg seroconversion[6], the complete cure is considered as the total eradication of HBV DNA, including cccDNA and integrated HBV DNA, from the liver and serum[7]. However, the development of therapies targeting cccDNA remains a significant unmet need, making complete cure currently una
Nucleos(t)ide analog therapy is effective in suppressing HBV replication but demonstrates limited efficacy in reducing serum HBsAg, with an annual HBsAg clearance rate of approximately 1%[8]. Combination therapy using PEG-IFN-2α and TDF achieves higher rates of HBsAg seroclearance, with approximately 10% of patients achieving this outcome. However, the use of interferon (IFN) is frequently associated with severe adverse effects[9]. It is worth noting that discontinuation of nucleos(t)ide analogs has recently been suggested as a novel treatment strategy to enhance the rate of HBsAg loss by allowing immune reconstitution. Although the STOP-NUC and FINITE trials that enrolled HBsAg-negative patients found that stopping nucleos(t)ide analogs substantially increased the rate of HBsAg loss (10.1% vs 0%; 19% vs 0%, respectively), data from other trials do not suggest such favorable outcomes. A significant proportion of patients suffer clinical relapse and hepatic flares after stopping treatment[10-14]. This discrepancy suggests that multiple factors such as patient ethnicity and baseline HBsAg levels may impact clinical outcomes and strongly argues that treatment discontinuation should be carefully considered based on patient qualifications and that the patients should be sub
Taken together, the limited cure rate with currently available treatment options underscores the urgent needs for the development of new, safe, and effective drugs for the treatment of chronic hepatitis B.
New therapeutic strategies aim to achieve long-lasting viral suppression with shorter treatment durations while also restoring the body’s antiviral immune response[16,17], and are divided into two main categories: Direct-acting antiviral agents and immunomodulators (Figure 1)[18].
HBV replication involves several key steps, such as viral entry, transcription, translation, DNA synthesis, assembly, and virion secretion. Direct-acting antiviral drugs target these processes to block viral entry, disrupt RNA transcription, prevent nucleocapsid assembly, inhibit DNA synthesis, or stop the secretion of HBsAg.
HBV entry inhibitors: HBV enters hepatocytes by interacting with the Pre-S1 glycoprotein on the viral envelope and the sodium taurocholate cotransporting polypeptide (NTCP) receptor[19]. Bulevirtide, formerly called Myrcludex B, is a linear lipopeptide derived from HBV PreS1 region that potently blocks this interaction by competing with the virus for NTCP binding, thereby preventing viral entry[20,21]. This drug was approved by the European Union in 2020 for treating chronic HBV/hepatitis delta virus (HDV) co-infection, and clinical studies have shown that bulevirtide monotherapy or combination with PEG-IFN-α for 48 weeks significantly reduces HDV RNA levels and HDV infected hepatocytes, and even leads to HBsAg dropping by more than 1 Log IU/mL[22-24]. Bulevirtide is also being investigated in clinical trials for chronic hepatitis B. The success of bulevirtide in treating HDV infections largely relies on HDV being an RNA virus with a much higher rate of spread and re-infection than HBV, making it more vulnerable to blocking entry. However, it is still unclear whether preventing new HBV infections by bulevirtide in patients with previously established chronic infections can significantly improve treatment outcomes. Nevertheless, considering the recent discovery in HBV-infected mice with humanized liver that a new HBV infection is necessary to reactivate previously silenced cccDNA, blocking new infections by bulevirtide shows potential especially in combination therapies[25].
HBV monoclonal antibodies: HBV-neutralizing monoclonal antibodies target the virus’s envelope proteins to block viral entry into hepatocytes, reduce circulating HBsAg levels and activate immune cells through Fc-mediated mechanisms[26]. Several monoclonal antibodies are currently under investigation in Phase II clinical trials, including lenvervimab (GC1102), HH-003, and tobevimab (VIR3434) (Table 2).
| Types of drugs | Mechanism of action | Representative drugs | Global clinical R&D phase | Efficacy |
| HBV entry inhibitors | Acts on NTCP to inhibit virus entry into cells | Bulevirtide | Clinical phase II | Profound HDV RNA reduction but minimal HBsAg reduction |
| HBV monoclonal antibodies | Binding to HBV virus indicates that HBsAg binds and neutralizes the virus | Lenvervimab | Clinical phase II | HBsAg decreased after each administration but rebounded rapidly; 6 out of 27 patients had HBsAg loss when combined with siRNA and IFN-α |
| Tobevibart (VIR-3434) | Clinical phase II | |||
| HH-003 | Clinical phase II | N/A on HBsAg loss rate; Efficacious against HDV | ||
| BJT-778 | Clinical phase II | N/A for HBsAg loss rate; Efficacious against HDV | ||
| RNA interference (siRNA) | Small interfering RNA targeted degradation of HBV mRNA | Daplusiran + tomligisiran (JNJ-3989) | Clinical phase II | Mean HBsAg reduction of 1.89 Log10 IU/mL after a 48-week therapy |
| Elebsiran (VIR-2218) | Clinical phase II | 11 out of 69 patients had HBsAg loss after different regimens of VIR-2218 plus IFN-α | ||
| Imdusiran (AB-729) | Clinical phase II | 4 out of 12 patients had HBsAg loss after 48 weeks Imdusiran plus 24 weeks IFN-α and ongoing nucleos(t)ide analogs | ||
| RBD1016 | Clinical phase I | HBsAg decline of 1.26 Log10 IU/mL by week 12 after two 3 mg/kg dosing | ||
| Xalnesiran (RG6346) | Clinical phase II | 30% HBsAg loss by Xalnesiran 200 mg and IFN-α combination at the end of 48-week treatment | ||
| RNA interference (ASO) | Targeted degradation of HBV mRNA by antisense oligonucleotides | Bepirovirsen | Clinical phase III | 9%-10% patients had HBsAg loss after 300 mg weekly for 24 weeks |
| AHB-137 | Clinical phase II | 62% patients had HBsAg loss after 12-week 300 mg dosing (interim results) | ||
| Inhibitor of capsid assembly | By affecting nucleocapsid formation, it reduces virus formation and cccDNA internal recruitment | JNJ-6379 | Clinical phase I | HBV DNA reduction after a 28-day therapy, no HBsAg loss |
| Morphothiadine (GLS4) | Clinical phase III | HBV DNA and RNA suppression. HBsAg decline but no HBsAg loss | ||
| Canocapavir | Clinical phase II | HBV DNA and pregenomic RNA reduction after a 28-day therapy | ||
| EDP-514 | Clinical phase I | HBV DNA and RNA reduction in treatment-naïve and viral suppressed patients after a 28-day therapy | ||
| ALG-000184 | Clinical phase I | HBV DNA and RNA reduction, and modest HBsAg reduction after a 28-day monotherapy or ETV combination | ||
| HBsAg secretion inhibitors | Nucleic acid polymers interfere with the release of subviral particles by interacting with chaperone proteins, such as DNAJB12, and increase the intracellular degradation of subviral particles | REP 2139 | Clinical phase II | 14 of 40 patients had HBsAg loss after 48 weeks of combination therapy with TDF and peg-IFN |
| REP 2165 | Clinical phase II | 14 of 40 patients had HBsAg loss after 48 weeks of combination therapy with TDF and peg-IFN | ||
| Direct-acting cccDNA drugs | Gene editing technology was used to knock out cccDNA | PBGENE-HBV | Clinical phase I | Reduction in HBsAg in 2 out of 3 patients after the first administration at 0.2 mg/kg dose |
| TLR-7 agonist | TLR-7 is activated to activate innate immune cells | Vesatolimod (GS-9620) | Clinical phase II | No patient has HBsAg loss after 12-week therapy with TDF combination |
| TQ-A3334 | Clinical phase II | N/A | ||
| TLR-8 agonist | TLR-8 is activated to activate innate immune cells | Selgantolimod | Clinical phase II | 2 out of 39 achieved HBsAg loss after 24-week therapy |
| HRS9950 | Clinical phase II | N/A | ||
| TQA3810 | Clinical phase II | N/A | ||
| Immune checkpoint inhibitors | Anti-PD-1 antibody, by inhibiting PD-1, activates immune cells | Nivolumab | Clinical phase II | 1 out of 12 patients had HBsAg loss after 12-week therapy |
| Serplulimab | Clinical phase II | N/A | ||
| Sintilimab | Clinical phase I | N/A | ||
| Anti-PD-L1 antibody, by inhibiting PD-L1, activates immune cells | Envafolimab | Clinical phase II | 3 out of 33 patients had HBsAg loss after 24-wekk therapy | |
| Therapeutic vaccines | Enhanced T- and B-cell immunity | VBI-2601 (BRII-179) | Clinical phase II | A 9 doses regimen with HBV siRNA induced anti-HBs antibody (16/40) but no HBsAg loss |
| VVX001 | Clinical phase II | N/A | ||
| CVI-HBV-002 | Clinical phase II | 2 out 18 patients had HBsAg loss after ChAdOx1-HBV on Day 0 and MVA-HBV on Day 28 (VTP-300) |
Lenvervimab has demonstrated modest efficacy in clearing HBsAg. In clinical trials, a 12.5% HBsAg clearance rate was observed in the 80000 IU dose group, while the 240000 IU dose group achieved a 22.2% clearance rate after one month of treatment. However, HBsAg levels rebounded rapidly following the cessation of therapy[27]. HH-003 is a fully human monoclonal antibody that specifically targets the pre-S1 region of the HBV envelope protein. By inhibiting the binding of the pre-S1 region to the NTCP receptor, HH-003 effectively prevents HBV entry into hepatocytes. A single-center, open-label Phase IIb clinical trial showed that treatment with HH-003 for 24 weeks in patients with chronic hepatitis B and co-existing chronic hepatitis D resulted in a 2.18 Log10 IU/mL reduction in HDV RNA levels[28]. VIR3434, another fully human monoclonal antibody, targets the antigenic loop shared by large, medium, and small HBsAg proteins. This antibody has been engineered to enhance its half-life through LS mutation in Fc region to increase its affinity for Fc-γ receptors (Fc-γRIIa and Fc-γRIIIA). These modifications improve its ability to activate immune cells. VIR3434 mediates its therapeutic effects via three mechanisms: (1) Neutralizing HBV to prevent its entry into hepatocytes; (2) Enhancing antigen presentation and stimulating T-cell responses, producing a vaccine-like effect; and (3) Neutralizing and faci
Overall, HBV monoclonal antibodies are predicted to block viral entry, clear HBsAg, and enhance immune control. However, similar to bulevirtide, monotherapy with HBV monoclonal antibodies is unlikely to cure HBV infections and is further limited by potential viral rebound after treatment discontinuation. Therefore, proper drug combinations with additional directacting antivirals or immunomodulators are necessary to improve treatment outcomes.
RNA interference: RNA interference agents, including siRNA and antisense oligonucleotides (ASO), are designed to reduce viral antigen burden by targeting viral transcripts as well as block HBV replication by limiting replication templates and factors. siRNA is a double-stranded RNA molecule that recognizes HBV transcripts to trigger RNA-induced silencing complex and Argonaute 2 mediated viral RNA degradation and translation repression. Distinct from siRNA, ASO is single-stranded DNA oligo that binds complementary HBV RNA to promote viral RNA degradation by recruiting RNA-degrading enzyme RNaseH. Overall, facilitated by liver targeted delivery and chemical modification to extend the half-life, RNA interference agents have demonstrated exceptional efficiency and durability in lowering HBsAg.
Currently, several siRNA drugs, including JNJ-3989, AB-729, RG-6346, and VIR-2218, are being investigated in Phase II clinical trials. JNJ-3989 is a combination of two siRNA molecules (JNJ-3976 and JNJ-3924 in a 2: 1 ratio) designed to target the viral RNAs encoding HBV surface antigens as well as all viral transcripts including the HBx mRNAs, with N-acetylgalactosamine (GalNAc) conjugation for liver-targeting delivery. The Phase IIb REEF 1 trial (CNT-03982186) evaluated its efficacy in patients randomized into three groups: (1) Nucleoside analog (NA) monotherapy; (2) NA with JNJ-3989 at doses of 40, 100, or 200 mg every four weeks; and (3) NA with JNJ-3989 (100 mg every four weeks) combined with JNJ-6379, a core-capsid assembly regulator. The primary clinical endpoint was defined as alanine aminotransferase levels < 3 times the upper limit of normal, HBV DNA below the lower limit of quantification, and HBsAg < 10 IU/mL after 48 weeks of treatment. The results demonstrated a dose-dependent reduction in HBsAg levels with JNJ-3989, with mean reductions of 1.5 Log10 IU/mL (40 mg), 2.1 Log10 IU/mL (100 mg), and 2.6 Log10 IU/mL (200 mg). At 48 weeks, 19% of patients receiving the 200 mg dose achieved the primary clinical endpoint. The reduction in HBsAg levels in the group treated with the combination of JNJ-3989 and JNJ-6379 was 1.8 Log10 IU/mL, which was lower than the reduction achieved with JNJ-3989 monotherapy, indicating that JNJ-6379 may not contribute significantly to the anti-HBsAg therapeutic effect[32].
In the REEF 2 trial (NCT04129554), 130 HBeAg-negative patients with chronic hepatitis B were randomized to receive either NA plus JNJ-3989 (200 mg every 4 weeks) with JNJ-6379 (250 mg daily) or NA with placebo for 48 weeks, followed by treatment discontinuation. The primary endpoint was serum clearance of HBsAg at week 72 (24 weeks after treatment cessation). At week 48, the combination therapy group achieved a mean HBsAg reduction of 1.89 Log10 IU/mL. Although no patients met the primary endpoint after drug withdrawal, 67.1% of the combination therapy group maintained HBsAg levels below 100 IU/mL, and 57.9% showed sustained reductions at the 24-week follow-up[33].
VIR-2218 is also a GalNAc-conjugated siRNA therapeutic targeting conserved regions within the HBx coding sequence. In patients with chronic hepatitis B, the treatment regimen involved administering 200 mg every four weeks for six doses, followed by a 40-week observation period after treatment cessation. At the end of the six-dose treatment, the mean reduction in HBsAg levels was 1.96 Log10 IU/mL. After the 40-week post-treatment observation period, the mean HBsAg reduction remained at 1.61 Log10 IU/mL[34]. When VIR-2218 was combined with IFN-α, the HBsAg seroconversion rate reached 15.9% (11 out of 69 patients), which was significantly higher than that with IFN-α or VIR-2218 monotherapy[35]. This promising result indicates that HBsAg reduction by siRNA could potentially create a conducive environment in the liver to improve treatment outcomes by concurrent or subsequent immune modulators including IFN-α.
In addition to siRNA therapeutics, several ASO drugs, including bepirovirsen and AHB-137, are under clinical investigation. Bepirovirsen is currently in the Phase III development stage. Results from its Phase IIb trial showed that patients receiving 300 mg weekly for 24 weeks, followed by a 24-week drug-free observation period, achieved HBsAg seroclearance rates of 9%-10%[36]. It should be noted that the immune-modulation function of bepirovirsen may be partially responsible for its efficacy. Interestingly, a recent study comparing 24-week bepirovirsen vs 12-week bepirovirsen treatment followed by 24-week PEG-IFN-α administration in virus-suppressed patients found that longer duration of antigen reduction before the sequential addition of PEG-IFN-α could reduce HBsAg relapse rates[37]. This finding further implies that lower antigen load by RNA interference is associated with better treatment outcomes upon the subsequent addition of immunomodulators.
Capsid assembly modulators: Capsid assembly is an essential step in the HBV life cycle, involving core proteins as building blocks to form a capsid which houses the reverse transcription of pgRNA by HBV polymerase, with direct connections with viral particle assembly and amplification of cccDNA. Core protein allosteric modulators (CpAMs) disrupt this process by inducing the formation of unstable capsids (class I) or empty capsids, which then reduce viral particle production and impair the nuclear trafficking of rcDNA-containing capsids, limiting the accumulation of cccDNA within hepatocytes[38,39]. Monotherapy with CpAMs has shown reductions in serum HBV DNA and RNA levels; however, HBsAg levels remained largely unaffected after 12 weeks of treatment. In a Phase Ib clinical trial, JNJ-6379 significantly reduced HBV DNA levels after 28 days of continuous treatment, although no changes in HBsAg levels were observed[40]. Similarly, investigational class II CpAMs, such as EDP-514 and ALG-000184, are currently in Phase I trials but have not demonstrated significant effects on HBsAg levels. It is not completely surprising that CpAMs are limited in their ability in reducing HBsAg, given their primary mechanism of action is blocking HBV DNA replication. However, cell culture studies have revealed that certain CpAMs can disrupt nucleocapsid disassembly in normal HBV during entry, thereby reducing cccDNA formation. It remains to be elucidated whether more potent CpAMs can provide such benefits in a clinical setting. Of note, being another HBV DNA replication inhibitor, orally available potent CpAMs with high resistant barrier may be potential alternatives as nucleos(t)ide analogs if early treatment is to be initiated for chronic HBV infections[41,42].
HBsAg secretion inhibitors: Nucleic acid polymers (NAPs) are single-stranded thiophosphate oligonucleotides that inhibit the release of subviral particles to enhance the intracellular degradation of subviral particles without affecting HBV RNA levels, Dane particles, or the production of HBc/HBeAg[43,44]. While its precise mechanism of action is still debatable, it seems to involve host chaperone proteins such as DNAJB12[45]. REP 2139 and REP 2165 are notable NAPs under investigation. A Phase II clinical trial demonstrated that combining REP 2139/REP 2165 with TDF and pegylated PEG-IFN effectively reduced serum HBsAg levels over 24 weeks, with 60% of patients achieving HBsAg levels below 0.05 IU/mL. At 48 weeks post-treatment cessation, 35% of patients (14 out of 40) achieved complete serum HBsAg clearance[46]. While the clinical data appear promising, larger-sized clinical trials are warranted to gain deeper insights into its efficacy, safety, and mechanism of action.
Direct-acting cccDNA drugs: Drugs that can eradicate cccDNA need to be developed to completely cure chronic hepatitis B. However, cccDNA removal from the already infected hepatocytes poses a significant challenge due to its chromatinized nature, making targeting structurally difficult when using the currently available small-molecule drugs. To date, no drug targeting cccDNA has entered clinical trials. Experimentally, a high-throughput screening study of 846000 compounds in HBV-infected primary human hepatocytes has led to the identification of ccc_R08, which reduces cccDNA in a dose-dependent manner[47,48]. Additional studies on this compound and a deeper understanding of HBV cccDNA biology are necessary to develop more potent compounds and design novel approaches to specifically reduce cccDNA in HBV-infected livers. In addition to the use of small-molecule inhibitors, in vitro and in vivo studies have shown that the CRISPR/Cas system can significantly reduce cccDNA levels in the liver based on the unique sequence of HBV, which is distinct from the chromosomal DNA of the host[49-51]. Despite the potential of the CRISPR/Cas system as a cccDNA-targeting tool, several challenges persist, including issues related to delivery efficiency and the risk of off-target effects[52]. An alternative strategy for eradicating cccDNA is by the transcriptional silencing of cccDNA. Accordingly, several in vitro studies, including the CRISPR baseediting approach and HBV X protein-disruption approach, have shown promising results[53-55]. While these results are preliminary, specifically silencing cccDNA by novel virological and immunological strategies represents an exciting research direction with immense translational potential.
Chronic HBV infection suppresses both innate and adaptive immunity, indicating that immune-related targets could play a pivotal role in combination therapies. Persistent HBV infection has been associated with the exhaustion and depletion of HBV-specific T cells, potentially driven by continuous exposure to high level of HBsAg[56]. Additionally, inadequate activation of the innate immune system and the presence of an immunotolerant liver microenvironment hinder the clearance of HBV[57]. Therefore, strategies to enhance the body’s immune response to HBV are essential for virus elimination and achieving a cure for chronic hepatitis B. We herein discuss several classes of HBV immune modulation approaches that are under clinical investigation.
TLR-7/TLR-8 agonists: TLRs are essential components of the innate immune system as they can recognize pathogen-associated molecular patterns. TLR-7 is primarily expressed in plasmacytoid dendritic cells, B cells, and macrophages. Upon ligand binding, TLR-7 induces the secretion of type I IFNs, tissue necrosis factor-α (TNF-α), and chemokines such as CXCL10, and enhances antigen presentation, T-cell activation, and plasma cell differentiation[58,59]. Conversely, TLR-8 is predominantly expressed in macrophages and myeloid dendritic cells, contributing to the secretion of pro-inflammatory cytokines[60]. Several TLR-7 and TLR-8 agonists are being investigated in clinical trials, however, current reports suggest that their efficacy in reducing HBsAg levels is limited. For instance, Vesatolimod, a TLR-7 agonist, showed that it can induce ISG15 expression but did not significantly increase plasma IFN-α levels or reduce HBsAg levels in Phase II trials, failing to meet its primary endpoint[61,62]. Another TLR-7 agonist, TQ-A3334, is undergoing Phase II clinical trials, with Phase I data confirming its potential safety and tolerability in healthy adults[63].
Selgantolimod (GS-9688), a TLR-8 agonist, stimulates the secretion of cytokines such as p40, IL-6, IL-12, TNF-α and IFN-γ to enhance NK cell function and suppress myeloid-derived suppressor cells[64]. In a Phase II trial, patients with chronic hepatitis B on nucleos(t)ide analogs received weekly doses of 3 mg, 1.5 mg, or placebo for 24 weeks and were followed for 24 weeks after Selgantolimod treatment. Results revealed that 26% (10/39) of patients experienced a reduction in HBsAg levels (> 0.1 Log10 IU/mL) after 24 weeks of Selgantolimod administration, and 5% (2/39) achieved HBsAg loss[65]. These data suggest that TLR8 agonist exhibit modest but durable clinical benefits for chronic HBV infection and may constitute a component of future combination therapies.
Immune checkpoint inhibitors: The increasing clinical use of immune checkpoint inhibitors in treating malignant tumors has prompted research into their potential role in HBV infection. The PD-1/PD-L1 signaling pathway is known to regulate T-cell proliferation, activation, and cytokine release[66]. Chronic hepatitis B is associated with elevated ex
Therapeutic vaccines: Despite having a highly effective preventive vaccine for HBV, there is currently no approved therapeutic vaccine for HBV. The primary goal of therapeutic vaccine development is to activate HBV-specific B and T cells to restore adaptive immunity. In this regard, several therapeutic vaccines, including TG1050, VTP-300, GS-4774 and BRII-179, are undergoing Phase II clinical trials.
TG1050 is an adenovirus (Ad5)-based vaccine that encodes multiple HBV antigens, including polymerase, HBcAg, and HBsAg domains. Preclinical studies in murine models have shown that TG1050 induces both immunogenic and antiviral effects, promoting the activation and proliferation of HBV-specific T cells[72]. However, its clinical impact on HBsAg levels in patients with chronic hepatitis B has been minimal, with most patients experiencing reductions of less than 0.2 Log10 IU/mL[73]. VTP-300 is a therapeutic vaccine derived from a chimpanzee adenovirus vector (ChAdOx1 HBV) combined with a Modified Vaccinia Ankara Enhanced (MVA-HBV) vaccine. These encode polymerase, core, and S antigens from a genotype C HBV sequence. In a Phase Ib/IIa clinical trial, patients received an intramuscular injection of ChAdOx1 HBV on day 0 and MVA-HBV on day 28. The results showed HBsAg reductions of 0.7, 0.7, and 1.4 Log10 IU/mL in 3 of the 18 participants at two months post-treatment, with 2 patients achieving durable undetectable HBsAg level. However, these patients had baseline HBsAg levels below 50 IU/mL before the therapy[74]. It remains to be further elucidated whether the combination with HBsAg-reducing agents, such as siRNA, to lower baseline HBsAg levels could further increase the functional cure rate.
BRII-179 (VBI-2601) is a protein-based recombinant HBV immunotherapy expressing Pre-S1, Pre-S2, and S HBV surface antigens. In a recent phase 2a study, 40% (16/40) of patients with chronic hepatitis B treated with nine 4-weekly doses of HBV-targeted siRNA elebsiran (BRII835) in combination with the therapeutic vaccine BRII-179 developed robust anti-HBs antibodies, whereas none of the patients in the siRNA-alone arm showed the presence of antiHBs antibodies despite a 1.2-2.6-fold log reduction in serum HBsAg levels[75]. Overall, this vaccine enhanced HBV-specific B cells and PreS1/S2-specific CD4+ T cells but not Sspecific CD8+ T cells. Another striking finding in this trial was that although neutralizing anti-HBs antibodies induced by BRII-179 contributed to HBsAg reduction, it did not lead to HBsAg seroconversion (no HBsAg loss). Concurrent treatment with IFN-α also failed to provide additional clinical benefits. This study once again highlights the complex interplay between chronic HBV infections and host immunity and implies that efficient reactivation of both humoral and cellular adaptive immunities against HBV may be needed for a durable functional cure.
The use of approved nucleos(t)ide analogues and IFN-α in the treatment of chronic hepatitis B has been associated with low functional cure rates and a limited range of treatment options. These limitations are primarily attributed to the persistent nature of HBV cccDNA and ineffective immune control over HBV-infected cells. To overcome these challenges, novel therapeutic approaches with diverse mechanisms of action are currently under development and urgently needed, targeting a functional cure for patients with chronic hepatitis B. Results from multiple clinical trials indicate that monotherapy may be insufficient to achieve the serological clearance of HBsAg. Thus, future treatment directions should focus on multidrug combinations including siRNA or ASO drugs with immunomodulators on the backbone of nu
| 1. | Boora S, Sharma V, Kaushik S, Bhupatiraju AV, Singh S, Kaushik S. Hepatitis B virus-induced hepatocellular carcinoma: a persistent global problem. Braz J Microbiol. 2023;54:679-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 2. | Revill PA, Penicaud C, Brechot C, Zoulim F. Meeting the Challenge of Eliminating Chronic Hepatitis B Infection. Genes (Basel). 2019;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1157] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 4. | Nishio A, Bolte FJ, Takeda K, Park N, Yu ZX, Park H, Valdez K, Ghany MG, Rehermann B. Clearance of pegylated interferon by Kupffer cells limits NK cell activation and therapy response of patients with HBV infection. Sci Transl Med. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Vaillant A. HBsAg, Subviral Particles, and Their Clearance in Establishing a Functional Cure of Chronic Hepatitis B Virus Infection. ACS Infect Dis. 2021;7:1351-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Cornberg M, Lok AS, Terrault NA, Zoulim F; 2019 EASL-AASLD HBV Treatment Endpoints Conference Faculty. Guidance for design and endpoints of clinical trials in chronic hepatitis B - Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference(‡). J Hepatol. 2020;72:539-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 237] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 7. | Revill PA, Chisari FV, Block JM, Dandri M, Gehring AJ, Guo H, Hu J, Kramvis A, Lampertico P, Janssen HLA, Levrero M, Li W, Liang TJ, Lim SG, Lu F, Penicaud MC, Tavis JE, Thimme R; Members of the ICE-HBV Working Groups; ICE-HBV Stakeholders Group Chairs; ICE-HBV Senior Advisors, Zoulim F. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol Hepatol. 2019;4:545-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 379] [Article Influence: 63.2] [Reference Citation Analysis (1)] |
| 8. | CME Exam 3: Factors Associated With Rates of HBsAg Seroclearance in Adults With Chronic HBV Infection: A Systematic Review and Meta-analysis. Gastroenterology. 2019;156:e18. [DOI] [Full Text] |
| 9. | Hu C, Song Y, Tang C, Li M, Liu J, Liu J, Liao M, Zhou F, Zhang YY, Zhou Y. Effect of Pegylated Interferon Plus Tenofovir Combination on Higher Hepatitis B Surface Antigen Loss in Treatment-naive Patients With Hepatitis B e Antigen -positive Chronic Hepatitis B: A Real-world Experience. Clin Ther. 2021;43:572-581.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | van Bömmel F, Stein K, Heyne R, Petersen J, Buggisch P, Berg C, Zeuzem S, Stallmach A, Sprinzl M, Schott E, Pathil-Warth A, von Arnim U, Keitel V, Lohmeyer J, Simon KG, Trautwein C, Trein A, Hüppe D, Cornberg M, Lammert F, Ingiliz P, Zachoval R, Hinrichsen H, Zipprich A, Klinker H, Schulze Zur Wiesch J, Schmiedeknecht A, Brosteanu O, Berg T. A multicenter randomized-controlled trial of nucleos(t)ide analogue cessation in HBeAg-negative chronic hepatitis B. J Hepatol. 2023;78:926-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (1)] |
| 11. | Berg T, Simon KG, Mauss S, Schott E, Heyne R, Klass DM, Eisenbach C, Welzel TM, Zachoval R, Felten G, Schulze-Zur-Wiesch J, Cornberg M, Op den Brouw ML, Jump B, Reiser H, Gallo L, Warger T, Petersen J; FINITE CHB study investigators [First investigation in stopping TDF treatment after long-term virological suppression in HBeAg-negative chronic hepatitis B]. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients - FINITE study. J Hepatol. 2017;67:918-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 236] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 12. | Liem KS, Fung S, Wong DK, Yim C, Noureldin S, Chen J, Feld JJ, Hansen BE, Janssen HLA. Limited sustained response after stopping nucleos(t)ide analogues in patients with chronic hepatitis B: results from a randomised controlled trial (Toronto STOP study). Gut. 2019;68:2206-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 13. | Jeng WJ, Chen YC, Chien RN, Sheen IS, Liaw YF. Incidence and predictors of hepatitis B surface antigen seroclearance after cessation of nucleos(t)ide analogue therapy in hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2018;68:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 249] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 14. | Liem KS, Gehring AJ, Feld JJ, Janssen HLA. Challenges With Stopping Long-term Nucleos(t)ide Analogue Therapy in Patients With Chronic Hepatitis B. Gastroenterology. 2020;158:1185-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 15. | Choi HSJ, Tonthat A, Janssen HLA, Terrault NA. Aiming for Functional Cure With Established and Novel Therapies for Chronic Hepatitis B. Hepatol Commun. 2022;6:935-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Tout I, Loureiro D, Mansouri A, Soumelis V, Boyer N, Asselah T. Hepatitis B surface antigen seroclearance: Immune mechanisms, clinical impact, importance for drug development. J Hepatol. 2020;73:409-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 17. | Lim SG, Baumert TF, Boni C, Gane E, Levrero M, Lok AS, Maini MK, Terrault NA, Zoulim F. The scientific basis of combination therapy for chronic hepatitis B functional cure. Nat Rev Gastroenterol Hepatol. 2023;20:238-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 87] [Reference Citation Analysis (0)] |
| 18. | Li Y. Overview of chronic hepatitis B drug therapy targets. 2025. Available from: https://BioRender.com/x17c085. |
| 19. | Yuen MF, Lai CL. Hepatitis B in 2014: HBV research moves forward--receptors and reactivation. Nat Rev Gastroenterol Hepatol. 2015;12:70-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Uhl P, Helm F, Hofhaus G, Brings S, Kaufman C, Leotta K, Urban S, Haberkorn U, Mier W, Fricker G. A liposomal formulation for the oral application of the investigational hepatitis B drug Myrcludex B. Eur J Pharm Biopharm. 2016;103:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Volz T, Allweiss L, Ben MBarek M, Warlich M, Lohse AW, Pollok JM, Alexandrov A, Urban S, Petersen J, Lütgehetmann M, Dandri M. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J Hepatol. 2013;58:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 22. | Asselah T, Chulanov V, Lampertico P, Wedemeyer H, Streinu-Cercel A, Pântea V, Lazar S, Placinta G, Gherlan GS, Bogomolov P, Stepanova T, Morozov V, Syutkin V, Sagalova O, Manuilov D, Mercier RC, Ye L, Da BL, Chee G, Lau AH, Osinusi A, Bourliere M, Ratziu V, Pol S, Hilleret MN, Zoulim F. Bulevirtide Combined with Pegylated Interferon for Chronic Hepatitis D. N Engl J Med. 2024;391:133-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 23. | Allweiss L, Volmari A, Suri V, Wallin JJ, Flaherty JF, Manuilov D, Downie B, Lütgehetmann M, Bockmann JH, Urban S, Wedemeyer H, Dandri M. Blocking viral entry with bulevirtide reduces the number of HDV-infected hepatocytes in human liver biopsies. J Hepatol. 2024;80:882-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 24. | Wedemeyer H, Schöneweis K, Bogomolov PO, Chulanov V, Stepanova T, Viacheslav M, Allweiss L, Dandri M, Ciesek S, Dittmer U, Haefeli W, Alexandrov A, Urban S. 48 weeks of high dose (10 mg) bulevirtide as monotherapy or with peginterferon alfa-2a in patients with chronic HBV/HDV co-infection. J Hepatol. 20;73:S52-S53. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 25. | Allweiss L, Giersch K, Pirosu A, Volz T, Muench RC, Beran RK, Urban S, Javanbakht H, Fletcher SP, Lütgehetmann M, Dandri M. Therapeutic shutdown of HBV transcripts promotes reappearance of the SMC5/6 complex and silencing of the viral genome in vivo. Gut. 2022;71:372-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 26. | Beretta M, Mouquet H. Advances in human monoclonal antibody therapy for HBV infection. Curr Opin Virol. 2022;53:101205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Lee HW, Park JY, Hong T, Park MS, Ahn SH. Efficacy of Lenvervimab, a Recombinant Human Immunoglobulin, in Treatment of Chronic Hepatitis B Virus Infection. Clin Gastroenterol Hepatol. 2020;18:3043-3045.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Wang X, Chi X, Zhang Y, Gu Y, Xiao L, Qi Y, Zou L, Wen J, Zhang Y, Chen P, Lei C, Ye B, Sui J, Li W, Niu J. Safety and efficacy of anti-pre-S1 domain monoclonal antibody (HH-003) treatment in patients with co-infection of chronic hepatitis B virus (HBV) and hepatitis D virus (HDV): a single center, open-label, phase 2 trial. J Hepatol. 2023;78:S117. [DOI] [Full Text] |
| 29. | Lempp FA, Volz T, Cameroni E, Benigni F, Zhou J, Rosen LE, Noack J, Zatta F, Kaiser H, Bianchi S, Lombardo G, Jaconi S, Vincenzetti L, Imam H, Soriaga LB, Passini N, Belnap DM, Schulze A, Lütgehetmann M, Telenti A, Cathcart AL, Snell G, Purcell LA, Hebner CM, Urban S, Dandri M, Corti D, Schmid MA. Potent broadly neutralizing antibody VIR-3434 controls hepatitis B and D virus infection and reduces HBsAg in humanized mice. J Hepatol. 2023;79:1129-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 30. | Agarwal K, Yuen M, Wedemeyer H, Cloutier D, Shen L, Arizpe A, Gupta SV, Fanget MC, Seu L, Cathcart A, Lau AH, Hwang C, Gane EJ. Dose-dependent durability of hepatitis B surface antigen reductions following administration of a single dose of VIR-3434, a novel neutralizing vaccinal monoclonal antibody. J Hepatol. 2022;77:S831-S832. [DOI] [Full Text] |
| 31. | Gane EJ, Jucov A, Dobryanksa M, yoon KT, Lim TH, Arizpe A, Cloutier D, Chattergoon M, Mao S, Gupta SV, Camus G, Hwang C, Lim Y. Safety and antiviral activity of short-duration combinations of the investigational small interfering ribonucleic acid VIR-2218 with the neutralizing, vaccinal monoclonal antibody VIR-3434: post-treatment follow-up from the Phase 2 MARCH trial. J Hepatol. 2023;78:S34-S35. [DOI] [Full Text] |
| 32. | Yuen M, Asselah T, Jacobson IM, Brunetto M, Janssen H, Takehara T, Hou J, Kakuda T, Lambrecht T, Kalmeijer R, Guinard-azadian C, Mayer C, Jerzorwski J, Verbinnen T, Lenz O, Shukla U, Biermer M. Effects of the siRNA JNJ-3989 and/or the capsid assembly modulator (CAM-N) JNJ-6379 on viral markers of chronic hepatitis B (CHB): results from the REEF-1 study. J Hepatol. 2022;77:S864-S865. [DOI] [Full Text] |
| 33. | Agarwal K, Buti M, van Bömmel F, Lampertico P, Janczewska E, Bourliere M, Vanwolleghem T, Lenz O, Verbinnen T, Kakuda T, Mayer C, Jerzorwski J, Beumont-mauviel M, Kalmeijer R, Biermer M, Lonjon-domanec I. Efficacy and safety of finite 48-week treatment with the siRNA JNJ-3989 and the capsid assembly modulator (CAM-N) JNJ-6379 in HBeAg negative virologically suppressed (VS) chronic hepatitis B (CHB) patients: results from REEF-2 study. J Hepatol. 22;77:S8. [DOI] [Full Text] |
| 34. | Lim Y, Yuen M, Cloutier D, Thanawala V, Shen L, Gupta SV, Arizpe A, Cathcart A, Hwang C, Gane EJ. Longer treatment duration of monthly VIR-2218 results in deeper and more sustained reductions in hepatitis B surface antigen in participants with chronic hepatitis B infection. J Hepatol. 2022;77:S69-S70. [DOI] [Full Text] |
| 35. | Yuen MF, Lim YS, Yoon KT, Lim TH, Heo J, Tangkijvanich P, Tak WY, Thanawala V, Cloutier D, Mao S, Arizpe A, Cathcart AL, Gupta SV, Hwang C, Gane E. VIR-2218 (elebsiran) plus pegylated interferon-alfa-2a in participants with chronic hepatitis B virus infection: a phase 2 study. Lancet Gastroenterol Hepatol. 2024;9:1121-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 36. | Yuen MF, Lim SG, Plesniak R, Tsuji K, Janssen HLA, Pojoga C, Gadano A, Popescu CP, Stepanova T, Asselah T, Diaconescu G, Yim HJ, Heo J, Janczewska E, Wong A, Idriz N, Imamura M, Rizzardini G, Takaguchi K, Andreone P, Arbune M, Hou J, Park SJ, Vata A, Cremer J, Elston R, Lukić T, Quinn G, Maynard L, Kendrick S, Plein H, Campbell F, Paff M, Theodore D; B-Clear Study Group. Efficacy and Safety of Bepirovirsen in Chronic Hepatitis B Infection. N Engl J Med. 2022;387:1957-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 136] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 37. | Buti M, Heo J, Tanaka Y, Andreone P, Atsukawa M, Cabezas J, Chak E, Coffin CS, Fujiwara K, Gankina N, Gordon SC, Janczewska E, Komori A, Lampertico P, McPherson S, Morozov V, Plesniak R, Poulin S, Ryan P, Sagalova O, Sheng G, Voloshina N, Xie Q, Yim HJ, Dixon S, Paff M, Felton L, Lee M, Greene T, Lim J, Lakshminarayanan D, McGonagle G, Plein H, Youssef AS, Elston R, Kendrick S, Theodore D. Sequential Peg-IFN after bepirovirsen may reduce post-treatment relapse in chronic hepatitis B. J Hepatol. 2025;82:222-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Reference Citation Analysis (0)] |
| 38. | Mak LY, Wong DK, Seto WK, Lai CL, Yuen MF. Hepatitis B core protein as a therapeutic target. Expert Opin Ther Targets. 2017;21:1153-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Mak LY, Seto WK, Yuen MF. Novel Antivirals in Clinical Development for Chronic Hepatitis B Infection. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Zoulim F, Lenz O, Vandenbossche JJ, Talloen W, Verbinnen T, Moscalu I, Streinu-Cercel A, Bourgeois S, Buti M, Crespo J, Manuel Pascasio J, Sarrazin C, Vanwolleghem T, Shukla U, Fry J, Yogaratnam JZ. JNJ-56136379, an HBV Capsid Assembly Modulator, Is Well-Tolerated and Has Antiviral Activity in a Phase 1 Study of Patients With Chronic Infection. Gastroenterology. 2020;159:521-533.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 41. | Lim YS, Kim WR, Dieterich D, Kao JH, Flaherty JF, Yee LJ, Roberts LR, Razavi H, Kennedy PTF. Evidence for Benefits of Early Treatment Initiation for Chronic Hepatitis B. Viruses. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 42. | Block TM, Guo JT, Zoulim F, Rice CM, Thio CL, Schneider WM, Alter HJ, Jacobson IM, Gish RG, Block PD, Sulkowski M, Feld JJ, Cohen CA. New potent HBV replication inhibitors for the management of chronic hepatitis B are needed. Nat Rev Gastroenterol Hepatol. 2025;22:150-151. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 43. | Boulon R, Blanchet M, Lemasson M, Vaillant A, Labonté P. Characterization of the antiviral effects of REP 2139 on the HBV lifecycle in vitro. Antiviral Res. 2020;183:104853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 44. | Vaillant A. REP 2139: Antiviral Mechanisms and Applications in Achieving Functional Control of HBV and HDV Infection. ACS Infect Dis. 2019;5:675-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 45. | Vaillant A. Editorial: In vitro mechanistic evaluation of nucleic acid polymers: A cautionary tale. Mol Ther Nucleic Acids. 2022;28:168-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Bazinet M, Pântea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, Jimbei P, Iarovoi L, Smesnoi V, Musteata T, Jucov A, Dittmer U, Krawczyk A, Vaillant A. Safety and Efficacy of 48 Weeks REP 2139 or REP 2165, Tenofovir Disoproxil, and Pegylated Interferon Alfa-2a in Patients With Chronic HBV Infection Naïve to Nucleos(t)ide Therapy. Gastroenterology. 2020;158:2180-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 47. | Wang L, Zhu Q, Zhang JD, Zhang Y, Ni X, Xiang K, Jiang J, Li B, Yu Y, Hu H, Zhang M, Wu W, Zeng J, Yan Z, Dai J, Sun K, Zhang X, Chen D, Feng S, Sach-Peltason L, Young JAT, Gao L. Discovery of a first-in-class orally available HBV cccDNA inhibitor. J Hepatol. 2023;78:742-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 48. | Chen D, Tan X, Chen W, Liu Y, Li C, Wu J, Zheng J, Shen HC, Zhang M, Wu W, Wang L, Xiong J, Dai J, Sun K, Zhang JD, Xiang K, Li B, Ni X, Zhu Q, Gao L, Wang L, Feng S. Discovery of Novel cccDNA Reducers toward the Cure of Hepatitis B Virus Infection. J Med Chem. 2022;65:10938-10955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 49. | Martinez MG, Combe E, Inchauspe A, Mangeot PE, Delberghe E, Chapus F, Neveu G, Alam A, Carter K, Testoni B, Zoulim F. CRISPR-Cas9 Targeting of Hepatitis B Virus Covalently Closed Circular DNA Generates Transcriptionally Active Episomal Variants. mBio. 2022;13:e0288821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 50. | Ramanan V, Shlomai A, Cox DB, Schwartz RE, Michailidis E, Bhatta A, Scott DA, Zhang F, Rice CM, Bhatia SN. CRISPR/Cas9 cleavage of viral DNA efficiently suppresses hepatitis B virus. Sci Rep. 2015;5:10833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 51. | Stone D, Long KR, Loprieno MA, De Silva Feelixge HS, Kenkel EJ, Liley RM, Rapp S, Roychoudhury P, Nguyen T, Stensland L, Colón-Thillet R, Klouser LM, Weber ND, Le C, Wagoner J, Goecker EA, Li AZ, Eichholz K, Corey L, Tyrrell DL, Greninger AL, Huang ML, Polyak SJ, Aubert M, Sagartz JE, Jerome KR. CRISPR-Cas9 gene editing of hepatitis B virus in chronically infected humanized mice. Mol Ther Methods Clin Dev. 2021;20:258-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 52. | Yang HC, Chen PJ. The potential and challenges of CRISPR-Cas in eradication of hepatitis B virus covalently closed circular DNA. Virus Res. 2018;244:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Yang YC, Chen YH, Kao JH, Ching C, Liu IJ, Wang CC, Tsai CH, Wu FY, Liu CJ, Chen PJ, Chen DS, Yang HC. Permanent Inactivation of HBV Genomes by CRISPR/Cas9-Mediated Non-cleavage Base Editing. Mol Ther Nucleic Acids. 2020;20:480-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 54. | Qu B, Nebioglu F, Leuthold MM, Ni Y, Mutz P, Beneke J, Erfle H, Vondran FWR, Bartenschlager R, Urban S. Dual role of neddylation in transcription of hepatitis B virus RNAs from cccDNA and production of viral surface antigen. JHEP Rep. 2022;4:100551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 55. | Prescott NA, Biaco T, Mansisidor A, Bram Y, Rendleman J, Faulkner SC, Lemmon AA, Lim C, Tiersky R, Salataj E, Garcia-Martinez L, Borges RL, Morey L, Hamard PJ, Koche RP, Risca VI, Schwartz RE, David Y. A nucleosome switch primes hepatitis B virus infection. Cell. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Iannacone M, Guidotti LG. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol. 2022;22:19-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 291] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 57. | Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, Peters MG, Lai CL. Hepatitis B virus infection. Nat Rev Dis Primers. 2018;4:18035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 538] [Article Influence: 76.9] [Reference Citation Analysis (1)] |
| 58. | Fitzgerald KA, Kagan JC. Toll-like Receptors and the Control of Immunity. Cell. 2020;180:1044-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 1406] [Article Influence: 281.2] [Reference Citation Analysis (0)] |
| 59. | Petes C, Odoardi N, Gee K. The Toll for Trafficking: Toll-Like Receptor 7 Delivery to the Endosome. Front Immunol. 2017;8:1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 60. | Moen SH, Ehrnström B, Kojen JF, Yurchenko M, Beckwith KS, Afset JE, Damås JK, Hu Z, Yin H, Espevik T, Stenvik J. Human Toll-like Receptor 8 (TLR8) Is an Important Sensor of Pyogenic Bacteria, and Is Attenuated by Cell Surface TLR Signaling. Front Immunol. 2019;10:1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 61. | Janssen HLA, Brunetto MR, Kim YJ, Ferrari C, Massetto B, Nguyen AH, Joshi A, Woo J, Lau AH, Gaggar A, Subramanian GM, Yoshida EM, Ahn SH, Tsai NCS, Fung S, Gane EJ. Safety, efficacy and pharmacodynamics of vesatolimod (GS-9620) in virally suppressed patients with chronic hepatitis B. J Hepatol. 2018;68:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 62. | Agarwal K, Ahn SH, Elkhashab M, Lau AH, Gaggar A, Bulusu A, Tian X, Cathcart AL, Woo J, Subramanian GM, Andreone P, Kim HJ, Chuang WL, Nguyen MH. Safety and efficacy of vesatolimod (GS-9620) in patients with chronic hepatitis B who are not currently on antiviral treatment. J Viral Hepat. 2018;25:1331-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 63. | Hu Y, Zhang H, Wu M, Liu J, Li X, Zhu X, Li C, Chen H, Liu C, Niu J, Ding Y. Safety, pharmacokinetics and pharmacodynamics of TQ-A3334, an oral toll-like receptor 7 agonist in healthy individuals. Expert Opin Investig Drugs. 2021;30:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Amin OE, Colbeck EJ, Daffis S, Khan S, Ramakrishnan D, Pattabiraman D, Chu R, Micolochick Steuer H, Lehar S, Peiser L, Palazzo A, Frey C, Davies J, Javanbakht H, Rosenberg WMC, Fletcher SP, Maini MK, Pallett LJ. Therapeutic Potential of TLR8 Agonist GS-9688 (Selgantolimod) in Chronic Hepatitis B: Remodeling of Antiviral and Regulatory Mediators. Hepatology. 2021;74:55-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 65. | Gane EJ, Dunbar PR, Brooks AE, Zhang F, Chen D, Wallin JJ, van Buuren N, Arora P, Fletcher SP, Tan SK, Yang JC, Gaggar A, Kottilil S, Tang L. Safety and efficacy of the oral TLR8 agonist selgantolimod in individuals with chronic hepatitis B under viral suppression. J Hepatol. 2023;78:513-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 66. | Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 558] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 67. | Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 68. | Gane E, Verdon DJ, Brooks AE, Gaggar A, Nguyen AH, Subramanian GM, Schwabe C, Dunbar PR. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J Hepatol. 2019;71:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 69. | Ma H, Lim TH, Leerapun A, Weltman M, Jia J, Lim YS, Tangkijvanich P, Sukeepaisarnjaroen W, Ji Y, Le Bert N, Li D, Zhang Y, Hamatake R, Tan N, Li C, Strasser SI, Ding H, Yoon JH, Stace NH, Ahmed T, Anderson DE, Yan L, Bertoletti A, Zhu Q, Yuen MF. Therapeutic vaccine BRII-179 restores HBV-specific immune responses in patients with chronic HBV in a phase Ib/IIa study. JHEP Rep. 2021;3:100361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 70. | Wang G, Cui Y, Xie Y, Mao Q, Xie Q, Gu Y, Chen X, Hu G, Yang Y, Lu J, Zou G, Zhang Q, Fu L, Chen Y, Guo X, Hou J, Yan Y, He H, Wu J. ALT flares were linked to HBsAg reduction, seroclearance and seroconversion: interim results from a phase IIb study in chronic hepatitis B patients with 24-week treatment of subcutaneous PD-L1 Ab ASC22 (Envafolimab) plus nucleos (t)ide analogs. J Hepatol. 2022;77:S70. [DOI] [Full Text] |
| 71. | Andreata F, Laura C, Ravà M, Krueger CC, Ficht X, Kawashima K, Beccaria CG, Moalli F, Partini B, Fumagalli V, Nosetto G, Di Lucia P, Montali I, Garcia-Manteiga JM, Bono EB, Giustini L, Perucchini C, Venzin V, Ranucci S, Inverso D, De Giovanni M, Genua M, Ostuni R, Lugli E, Isogawa M, Ferrari C, Boni C, Fisicaro P, Guidotti LG, Iannacone M. Therapeutic potential of co-signaling receptor modulation in hepatitis B. Cell. 2024;187:4078-4094.e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 72. | Martin P, Dubois C, Jacquier E, Dion S, Mancini-Bourgine M, Godon O, Kratzer R, Lelu-Santolaria K, Evlachev A, Meritet JF, Schlesinger Y, Villeval D, Strub JM, Van Dorsselaer A, Marchand JB, Geist M, Brandely R, Findeli A, Boukhebza H, Menguy T, Silvestre N, Michel ML, Inchauspé G. TG1050, an immunotherapeutic to treat chronic hepatitis B, induces robust T cells and exerts an antiviral effect in HBV-persistent mice. Gut. 2015;64:1961-1971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 73. | Zoulim F, Fournier C, Habersetzer F, Sprinzl M, Pol S, Coffin CS, Leroy V, Ma M, Wedemeyer H, Lohse AW, Thimme R, Lugardon K, Martin P, Bastien B, Sansas B, Adda N, Halluard C, Bendjama K, Brandely M, Inchauspé G. Safety and immunogenicity of the therapeutic vaccine TG1050 in chronic hepatitis B patients: a phase 1b placebo-controlled trial. Hum Vaccin Immunother. 2020;16:388-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 74. | Tak WY, Chuang WL, Chen CY, Tseng KC, Lim YS, Lo GH, Heo J, Agarwal K, Bussey L, Teoh SL, Tria A, Brown A, Anderson K, Vardeu A, O'Brien S, Kopycinski J, Kolenovska R, Barnes E, Evans T. Phase Ib/IIa randomized study of heterologous ChAdOx1-HBV/MVA-HBV therapeutic vaccination (VTP-300) as monotherapy and combined with low-dose nivolumab in virally-suppressed patients with CHB. J Hepatol. 2024;81:949-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Reference Citation Analysis (0)] |
| 75. | Ji Y, Bert NL, Wong GL, Douglas MW, Lee A, Zhu C, Wang B, Lv J, Li D, Tan Y, Ma H, Chen J, Chen X, Zhu Q, Yuen MF, Bertoletti A. The impact of HBsAg reduction via siRNA treatment on natural and vaccine (BRII-179)-induced HBV-specific humoral and cellular immune responses. Gastroenterology. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 76. | Tang L, Zhao Q, Wu S, Cheng J, Chang J, Guo JT. The current status and future directions of hepatitis B antiviral drug discovery. Expert Opin Drug Discov. 2017;12:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |