Published online Apr 27, 2025. doi: 10.4254/wjh.v17.i4.105127
Revised: March 22, 2025
Accepted: April 9, 2025
Published online: April 27, 2025
Processing time: 103 Days and 3.1 Hours
The traditional view of the decompensated stage as a point of no return in the natural history of liver cirrhosis (LC) is currently being questioned. This is due to the appearance of data indicating the possibility of restoring the structure and function of the liver, reducing the portal pressure with a positive effect on com
Core Tip: Numerous publications in recent years have shown the efficacy of etiological therapy in achieving recompensation of decompensated liver cirrhosis (LC). The criteria for the recompensation of alcohol-related, as well as hepatitis B virus-related and hepatitis C virus-related decompensated LC were developed at the Baveno VII consensus workshop. This review provides up-to-date information on the role of etiological therapy in achieving recompensation of decompensated LC according to these criteria.
- Citation: Garbuzenko DV. Role of etiological therapy in achieving recompensation of decompensated liver cirrhosis. World J Hepatol 2025; 17(4): 105127
- URL: https://www.wjgnet.com/1948-5182/full/v17/i4/105127.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i4.105127
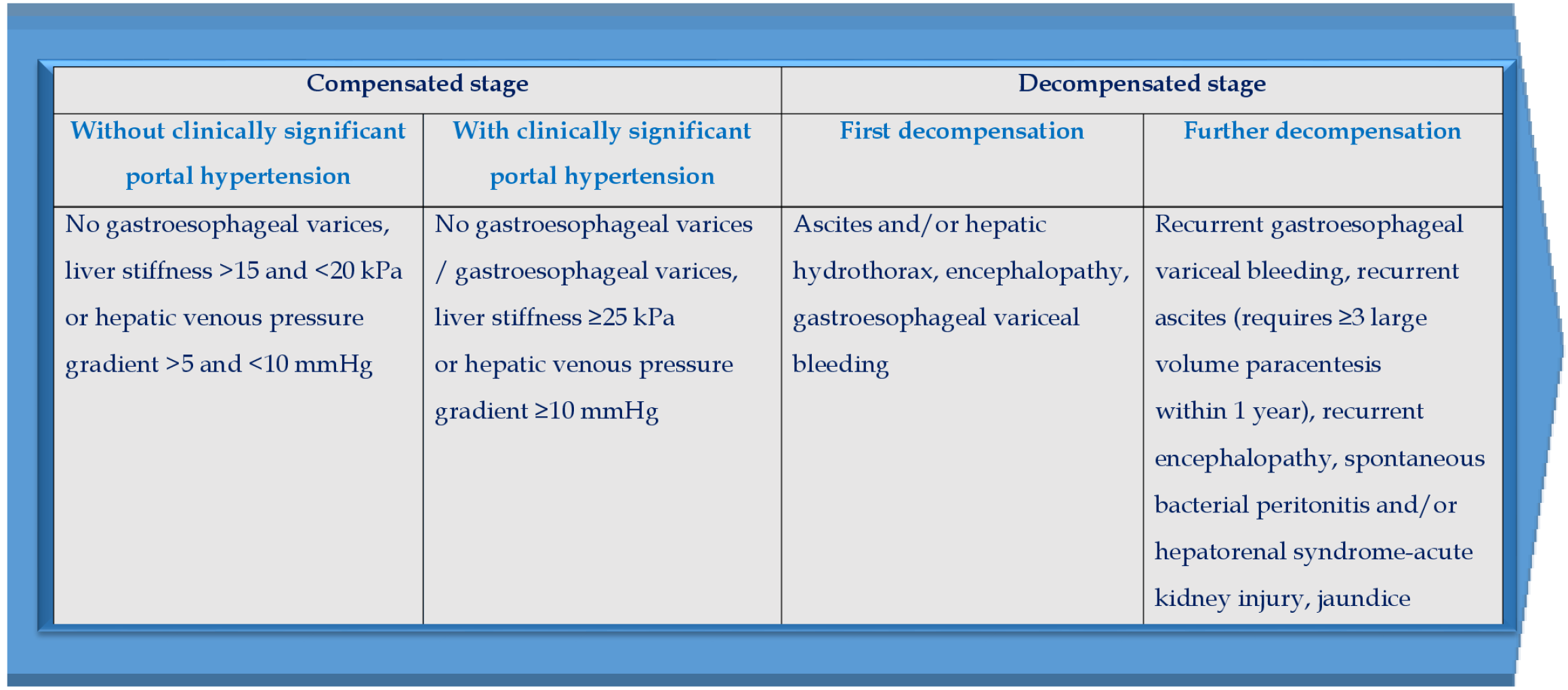
Liver cirrhosis (LC) is an unfavorable event in the evolution of most chronic liver diseases. In the natural history of LC there are a compensated stage (without or with clinically significant portal hypertension (PH) and a decompensated stage (Figure 1). The development of clinically significant PH in compensated LC patients serves as a key prognostic factor, since it leads to an increased risk of the first decompensation, the most important manifestation of which is gastroesophageal variceal bleeding (GEVB)[1]. The diagnostic criteria for clinically significant PH are hepatic venous pressure gradient (HVPG) ≥ 10 mmHg, or liver stiffness values by transient elastography ≥25 kPa[2]. Further LC decompensation is a stratification variable of poor prognosis and is accompanied by a decrease in median survival to 2-4 years[3]. This is due to the development of such life-threatening complications as recurrent GEVB, recurrent ascites (requires ≥ 3 large volume paracentesis within 1 year), recurrent encephalopathy, spontaneous bacterial peritonitis and/or hepatorenal syndrome-acute kidney injury, jaundice[4].
The traditional view of the decompensated stage as a point of no return in the natural history of LC is currently being questioned. Moreover, in 2021, at the Baveno VII consensus workshop has defined the concept of recompensation of decompensated LC. It implies that after elimination of the etiological factor, there is at least a partial regression of structural and functional disorders in the liver, reduction of portal pressure with a positive effect on PH-related complications[5]. At the moment, the efficacy of etiological therapy in achieving developed criteria for recompensation has been evaluated only in patients with alcohol-related, as well as hepatitis B virus (HBV)-related and hepatitis C virus (HCV)-related decompensated LC, which is due to the lack of clear ideas about the etiology of other chronic liver diseases[6,7]. Nevertheless, current data indicate the prospects of this approach, for example, in patients with non-alcoholic steatohepatitis-related LC, when, under certain circumstances, regression of its characteristic histological signs may occur[8]. In a study by Hofer et al[9] recompensation of decompensated LC in patients with primary biliary cholangitis was achieved by ursodeoxycholic acid therapy, especially in those who showed an adequate biochemical response after a 1-year of treatment according to Paris-II criteria.
The purpose of the review is to provide up–to-date information on the role of etiological therapy in achieving recompensation of decompensated LC according to Baveno VII criteria. The PubMed and EMBASE databases, the Web of Science platform, the Google Scholar retrieval system, the Cochrane Database of Systematic Reviews, Reference Citation Analysis (https://www.referencecitationanalysis.com/), and the reference lists from related articles were used to search for relevant publications. Articles corresponding to the aim of the review were selected for 1996-2024. Articles were selected that evaluated the efficacy of etiological therapy in achieving recompensation of decompensated LC.
According to the decisions of the Baveno VII consensus workshop, clinical confirmation of recompensation of de
It should be noted that the resolution of ascites while on diuretics or after transjugular intrahepatic portosystemic shunt (TIPS) and/or the absence of recurrent GEVB while on traditional non-selective β-blockers (NSBBs) + endoscopic band ligation (EBL) or carvedilol + EBL or after TIPS without removal/suppression/cure of the primary etiological factor of LC and without improvement in liver synthetic function, is not evidence of recompensation[5].
In addition, normalization of the clinical status after removal/suppression/cure of the etiological factor is often not enough to achieve stable recompensation of decompensated LC. In order to prevent further decompensation, it is also necessary to influence the pathophysiological mechanisms of its development, which include: (1) Liver fibrosis and associated increase in intrahepatic vascular resistance; (2) Hyperdynamic circulatory state characteristic of clinically significant PH; and (3) Disturbance of the gut-liver axis contributing to systemic inflammation and immune dysfunction[10].
Currently, it has been established that etiological therapy of diseases, the natural history of which is accompanied by liver fibrosis, is an effective method not only for their prevention, but also for the reversal of histological disorders with the restoration of liver structure and function to a normal state, contributing to a reduction in intrahepatic vascular resistance underlying the development of PH[11]. However, in some cases, clinically significant PH may persist despite achieving sustained virological response (SVR) and a decrease in liver stiffness values by transient elastography[12]. That is because the PH in LC is a consequence not only of morphofunctional rearrangement of the hepatic vasculature[13], but also of the subsequent formation of portosystemic shunts and the development of hyperdynamic circulatory state as a result of complex processes of angiogenesis, vascular remodeling and endothelial dysfunction[14]. Considering that maintaining elevated portal pressure contributes to further LC decompensation, NSBBs intake should not be discontinued until signs of clinically significant PH are eliminated, even in LC patients who have achieved recompensation[15].
Systemic inflammation and immune dysfunction are also important triggers for further LC decompensation. In this regard, it is obvious that in order to prevent further LC decompensation, it is necessary to affect key links in the pathogenesis, for example, a disturbed gut-liver axis, where specific alterations in the composition and function of gut microbiota play a crucial role[16].
Worldwide, approximately 2.4 billion people consume alcohol, while alcohol-related liver disease are one of the 30 main causes of death. In 2010, the rate of alcohol-related LC death worldwide was 7.2 per 100000 people[17]. According to the aggregate data of the Global Burden of Diseases, Injuries, and Risk Factors Study 2017 Cirrhosis Collaborators, the proportion of alcohol-related decompensated LC among other causes was 23%[18]. It is predicted that by 2040, the incidence of alcohol-related decompensated LC will increase from 9.9 to 17.5 per 100000 patient-years[19].
Alcohol abstinence is a top priority and one of the main therapeutic approaches for all forms of alcohol related liver diseases, including LC[20]. Long-term alcohol abstinence has a positive effect on the natural history of alcohol-related LC and significantly reduces the risk of decompensation[21]. Back in the mid-90s of the last century, Vorobioff et al[22] revealed a relationship between alcohol abstinence in patients with alcohol-related decompensated LC and improved liver function assessed by the Child-Turcotte-Pugh (CTP) score, reduction of HVPG and regression of gastroesophageal varices. Recent studies have shown that a significant proportion of liver transplant candidates with alcohol-related decompensated LC can be removal from the waiting list due to abstinence-associated recompensation[23,24]. In
In a retrospective, single-center, observational study involving 204 patients with alcohol-related decompensated LC, long-term alcohol abstinence contributed to recompensation in 18.1% of cases at 2 years, which resulted in a > 90% risk reduction in liver-related mortality[26]. In a retrospective, single-center, observational study involving 320 patients with alcohol-related decompensated LC and a median HVPG of 20 mmHg (interquartile range: 17-23 mmHg), alcohol abstinence was linked to a significantly reduced risk of further decompensation [adjusted hazard ratio (aHR): 0.391, P < 0.001] as in groups with HVPG 10-19 mmHg (P < 0.001) and HVPG ≥ 20 mmHg (P = 0.002), as well as liver-related (aHR: 0.428, P < 0.001) and all-cause (aHR: 0.453, P < 0.001) mortality, after adjusting for baseline HVPG, MELD, and previous decompensation. The 3-year decompensation probability was 32.4% vs 60.0% in HVPG 10-19 mmHg and 57.5% vs 82.6% in HVPG ≥ 20 mmHg for abstinent patients vs active drinkers, respectively[27].
Chronic HBV and HCV infections damage liver cells, leading to liver fibrosis and the progression to LC. Statistically, the risk of developing LC is as high as 40% for HBV infection and 10%–20% for HCV infection if not treated with antiviral therapy[28]. At the same time, recent studies have shown that achieving SVR in patients with HBV-related and HCV-related decompensated LC can stop the progression of the disease, contributes to a significant improvement of his
Chronic HBV infection is an important cause of morbidity and mortality worldwide. A systematic analysis for the Global Burden of Disease Study 2019 showed the presence of chronic HBV infection in 316 million people. HBV-related diseases were the cause of 555000 (487000-630000) deaths, and HBV-related LC was responsible for 331000 (279000-392000) deaths[31].
Currently, pegylated interferon and 6 nucleos(t)ide analogues (NAs), including lamivudine, adefovir dipivoxil, entecavir, telbivudine, tenofovir disoproxil fumarate and tenofovir alafenamide fumarate, have been approved for the treatment of chronic HBV infection. In patients with HBV-related LC, indefinite treatment with NAs is recommended, regardless of the serum level of HBV DNA. Patients with HBV-related decompensated LC should be treated with NAs with high barrier to HBV resistance (entecavir, tenofovir disoproxil fumarate, tenofovir alafenamide), irrespective of the level of HBV replication[32]. Many studies have shown the efficacy of NAs in achieving recompensation of HBV-related decompensated LC (Table 1)[33-42]. For example, in a systematic review and meta-analysis of 39 RCTs and observational studies involving a total of 14212 patients with HBV-related decompensated LC and clinically significant PH, therapy with NAs prevented further decompensation [relative risk (RR): 0.51, 95%CI: 0.37-0.71], decreased the risk of GEVB (RR: 0.44, 95%CI: 0.26-0.74), ascites (RR: 0.10, 95%CI: 0.01-1.59) and hepatocellular carcinoma (RR: 0.48, 95%CI: 0.30-0.75), and also reduced the likelihood of liver transplantation or death (RR: 0.36, 95%CI: 0.25-0.53). The first-line NAs (entecavir or tenofovir) were superior to non-first-line NAs in improving these outcomes (RR: 0.85, 95%CI: 0.75-0.97 and RR: 0.85, 95%CI: 0.73-0.99, respectively)[43].
| Ref. | Research design, number of patients | CTP score, points | MELD score points | Antiviral therapy | Treatment period, SVR | Main results1 |
| Nikolaidis et al[33] | Prospective, single-center, N = 20 | B/C, 9.3 ± 2.0 | 16.2 ± 3.1 | LAM | 12-24-36 months, 55.0% | In 55% of patients, the CTP score decreased by more than 2 points, and in 45% of patients they reached CTP class A |
| Manolakopoulos et al[34] | Prospective, single-center, N = 19 | A/B/C, 6 (5–12) | 12 (7–26) | LAM | 12 months, 78.9% | In 10 out of 13 patients with clinically significant portal hypertension and those who achieved SVR, there was a reduction in hepatic venous pressure gradient to less than 12 mmHg or 20% of the baseline |
| Shim et al[35] | Prospective, single-center, N = 55 | B/C, 8.1 ± 1.7 | 11.5 ± 3.9 | ETV | 12 months, 89.1% | The CTP and MELD scores have improved. In 49% of patients, the CTP score values decreased by more than 2 points, and in 65.5% of patients they reached CTP class A |
| Liaw et al[36] | Randomized, open-label, comparative, N = 100, N = 91 | B/C, ≥ 7, B/C, ≥ 7 | 17.1 (SE = 0.50), 15.3 (SE = 0.48) | ETV, ADV | 12 months, 57.0%, 12 months, 20.0% | In 2/3 of the patients in both groups, the CTP score improved. The MELD score decreased by 2.6 points when treated with entecavir, and by 1.7 points when treated with adefovir |
| Jang et al[37] | Prospective, multicenter, N = 423 | A/B/C, 8.7 ± 2.0 | 13.9 ± 6.4 | LAM/ETV/ADV/clevudine/LdT | 12 months, 57.9% | In 2/3 of the patients in both groups, the CTP score improved. The MELD score decreased by 2.6 points when treated with entecavir, and by 1.7 points when treated with adefovir |
| Lee et al[38] | Retrospective, multicenter, N = 57 | B/C, 8.0 ± 1.5 | 13.4 ± 4.7 | TDF | 12 months, 70.2% | In 14.7% of patients, the initial values of the CTP score ≥ 7 decreased by more than 2 points, and in 12% of patients they reached CTP class A. Within 12 months of starting treatment, 33.9% of liver transplant candidates were excluded from the waiting list |
| Wang et al[39] | Prospective, multicenter, N = 283 | B/C, 8.3 ± 1.9 | 13.4 ± 4.4 | ETV | 120 weeks, 92.2% | The CTP and MELD scores have improved. In 49.1% of patients, the initial values of the CTP score ≥ 7 decreased by more than 2 points, and in 68.4% of patients they reached CTP class A |
| Hui et al[40] | Retrospective, cohort, N = 1109 | B, 6, 7 | 7.3 ± 4.5 | ETV/TAF | 12 months, N/A | For at least 12 months, 60.4% of patients had no ascites (off diuretics), encephalopathy (off lactulose/rifaximin) and recurrent gastroesophageal variceal bleeding. The serum albumin levels increased from 31.7 ± 6.4 to 42.4 ± 6.2, international normalized ratio values, serum levels of total bilirubin, ALT and aspartate aminotransferase decreased. The CTP and MELD scores improved: 5.77 ± 1.37 vs 8.33 ± 1.90 and 10.45 ± 4.58 vs 13.37 ± 4.44, respectively |
| Zhang et al[41] | Retrospective, two-center, cohort, N = 71 | B/C, 8.5 ± 1.6 | 13.3 ± 4.3 | ETV/TDF/TAF | 12 months, N/A | Patients with decompensated LC who achieved recompensation showed a similar 5-year survival rate with those with compensated LC (76% and 89.3%, respectively) |
| Li et al[42] | Retrospective, cohort, N = 196 | A/B/C | 11.0 (8.0-15.0) | ETV/LAM + ADV/LdT + ADV/TDF/TAF | 12 months, 78.1% | The cumulative incidence of hepatocellular carcinoma after 2 years, 4 years, and 6 years in patients with decompensated LC who achieved recompensation was the same as in those with compensated LC, which was 1.2%, 5.2%, 24.5%, and 1.3%, 5.4%, 20.0%, respectively. The rate of ascites regression was higher in SVR cohort when compared with that in non-SVR cohort. The serum ALT levels and load of serum hepatitis B virus DNA at baseline were predictors of ascites regression |
A number of studies have focused on determining predictors of recompensation in NAs-treated patients with HBV-related decompensated LC. In a study by Deng et al[44], a serum albumin levels ≥ 34 g/L at treatment with entecavir week 24 predicted recompensation of HBV-related decompensated LC by week 120. The accuracy of the BC2AID score, developed to predicted recompensation of HBV-related decompensated LC and based on six independent clinical parameters, such as serum total bilirubin levels ≤ 5 mg/dL, absence of severe complications, α-fetoprotein ≥ 50 ng/mL, alanine aminotransferase (ALT) ≥ 200 IU/L, INR ≤ 1.5, and ≤ 6 months from initial decompensation until initiation of NAs therapy, was significantly higher than that of the CTP, MELD, MELDNa and BE3A scores (0.813 vs 0.691, 0.638, 0.645 and 0.624, respectively; all P < 0.05)[45]. The nomogram created by Wen et al[46] of the six independent factors, including age, ALT, serum albumin levels, serum sodium, α-fetoprotein, and SVR, predicted recompensation in NAs-treated patients with HBV-related decompensated LC significantly better than CTP, MELD, MELDNa, MELD 3.0, and albumin–bilirubin scores.
The criteria for recompensation in NAs-treated patients with HBV-related decompensated LC can be values of the MELD score < 10 points and/or liver function tests within CTP class A (serum albumin levels > 35 g/L, INR < 1.5 and serum total bilirubin levels < 34 μmol/L)[39].
HCV infection is one of the main causes of chronic liver disease worldwide[47], which remains the leading cause of global deaths related to LC[48]. Currently, direct acting antivirals (DAAs) have been approved for the treatment of chronic HCV infection within interferon-free combination regimens. Their action is aimed at simultaneous inhibition of several targets in the HCV life cycle, namely non-structural proteins NS3, NS4A, NS5A. The choice of DAAs treatment regimen depends on the HCV genotype, the presence or absence of LC, clinical experience and financial capabilities. In patients with HСV-related decompensated LC, only NS5A inhibitors can be used[49]. Many studies have shown the efficacy of DAAs in achieving recompensation of HCV-related decompensated LC (Table 2)[50-66]. For example, in a systematic review and meta-analysis of 49 studies involving a total of 7,886 patients with HCV-related decompensated LC of genotypes 1, 3, and 4, after DAAs treatment for 12-96 weeks, the SVR rate was 86% (95%CI: 0.83-0.88). Improvement in liver function was observed in 51% (95%CI: 0.44-0.58) of patients, and 16% (95%CI: 0.05-0.40) were delisting from liver transplantation[67].
| Ref. | Research design, number of patients | CTP score, points | MELD score points | Hepatitis C virus genotype | Antiviral therapy | Treatment period, SVR | Main results1 |
| Belli et al[50] | Retrospective, multicenter, N = 103 | B/C | 16 (6-31) | 1a, 1b, 2, 3, 4 | SOF, SOF/LED, SOF/DAC, SOF/SIM ± RBV | 12 weeks, 98% | The median CTP score decreased from 10.0 to 8.0, and the median MELD score decreased from 15.5 to 14.0. The median serum albumin levels increased by 0.5 g/dL, the median serum total bilirubin levels decreased by 0.9 mg/dL, and the median international normalized ratio values reduced by 0.13 points. Of the entire cohort of 103 patients, 33% of liver transplant candidates were excluded from the waiting list due to clinical improvement |
| Foster et al[51] | Prospective, multicenter, N = 409 | B/C, ≥ 7 | 12 (7-32) | 1, 3 | SOF/LED, SOF/DAC ± RBV | 12 weeks, 81.6% | The MELD score decreased by an average of 0.85 points. Patients with baseline serum albumin levels < 35 g/L, sodium < 135 mmol/L, and over 65 years of age were least likely to benefit from therapy |
| Mandorfer et al[52] | Retrospective, single-center, N = 41 | A/B | 8 (7-9) | 1, 2, 3, 4 | SOF/DAC, SOF/LED, SOF/SIM ± RBV | 12/24 weeks, 63% | The HVPG reduced by more than 10% of the baseline. The probability of HVPG reduction in CTP class B patients was lower compared with CTP class A patients |
| Perricone et al[53] | Prospective, cohort, N = 142 | A/B/C | 16 (13-18) | 1a, 1b, 2, 3, 4 | SOF, SOF/LED, SOF/DAC, SOF/SIM ± RBV | 12 weeks, N/A | The CTP and MELD scores have improved. In 79.5% of patients, ascites was completely gone, and in 20.5% of patients it required low doses of diuretics. The hepatic encephalopathy disappeared. Within 12 weeks of starting treatment, 30.9% of liver transplant candidates were excluded from the waiting list |
| Macken et al[54] | Prospective, cohort, N = 39 | N/A | 6 (6-7) | 1, 3 | OMB/PAR/DAS, SOF/LED, SOF/DAC ± RBV, SOF/pegylated interferon alpha-2a/RBV | 12 weeks, 77% | The recompensation was recorded in 51% of patients. The associated criterion was a lower baseline serum creatinine levels |
| Hanafy et al[55] | Interventional, N = 160 | B/C, 11.2 ± 1.2 | 20.6 ± 2.04 | 4 | SOF/DAC/RBV | 12/24 weeks, 90% | There were improvements in platelet count, serum albumin levels, CTP and MELD scores, a significant reduction in the frequency of hepatic encephalopathy. Hepatocellular carcinoma developed in 10% of patients within 6.8 months ± 2.5 months after DAAs, survival was higher in the treated vs the control group |
| Moon et al[56] | Prospective, cohort, N = 9399 | N/A | > 9 (70%) | 1 (approximately 60%) | PAR/RIT/OMB/DAS, SOF ± DAC, SOF + SIM | 12 weeks, 84.3% | On average, 5.1% of patients (1.66 cases per 100 patient-years) developed GEVB over a follow-up of 3.1 years. This complication was less common in patients who achieved SVR (1.55 cases per 100 patient-years) than without it (2.96 cases per 100 patient-years) |
| Puigvehí et al[57] | Prospective, multicenter, N = 247 | A | 6 (6–14) | N/A | SOF/SIM, SOF/DAC, SOF/LED ± RBV, PAR + RIT/OMB/OMB | 12 weeks, 93.1% | Over a follow-up of 3 years, GEV developed in 12.5% of patients who had not had it before and increased in 33.1% of patients with low-risk GEV (< 5 mm) |
| Liu et al[58] | Prospective, multicenter, N = 107 | B/C | 10 (7–13) | 1, 1a, 1b, 2, 3, 6 | SOF/VEL + RBV | 12 weeks, 89.7% | The CTP and MELD scores have improved in 84.4% and 64.6% of patients, respectively. The initial values of the MELD score ≥ 15 points decreased by more than 3 points |
| Tada et al[59] | Retrospective, multicenter, N = 65 | B/C, ≥ 7 | N/A | 1, 2 | SOF/VEL | 12 weeks, 92.3% | The albumin–bilirubin score have improved during and after treatment |
| Tahata et al[60] | Prospective, multicenter, N = 82 | A/B/C | N/A | 1, 2, 3, 4 | SOF/VEL | 12 weeks, 90.2% | In 50% of CTP class B patients, the CTP score decreased to class A, in 27% of CTP class C patients, the CTP score decreased to class B, and in 9% of CTP class C patients, the CTP score decreased to class A. The serum albumin level increased when its initial value exceeded 28 g/L |
| Takaoka et al[61] | Prospective, multicenter, N = 72 | B/C | 9 (7-11) | 1, 2 | SOF/VEL | 12 weeks, 95.8% | In 75% of patients who achieved SVR, there was a decrease in CTP score, and in 5.9% of patients they increased. The serum albumin levels and prothrombin time values increased, ascites decreased, while serum total bilirubin levels and the severity of hepatic encephalopathy did not change significantly |
| Meunier et al[62] | Retrospective, multicenter, N = 75 | A/B/C | 14 (11-18) | 1 | SOF/DAC | 24 weeks, 92% | Five years after treatment, 25.3% of liver transplant candidates were excluded from the waiting list due to clinical improvement. The predictors of this were the absence of ascites, the MELD score ≤ 15 points and the CTP score ≤ 7 points |
| Su et al[63] | Retrospective, single-center, N = 50 | B/C | 12 (6–21) | 1, 2, 6 | SOF/DAC, SOF/LED, SOF/VEL ± RBV | 12 weeks, 96% | The values of the following scores decreased: Fibrosis-4 (8.1 ± 4.0 vs 11.2 ± 6.9), CTP (6.8 ± 1.4 vs 8.0 ± 1.2), and MELD (11.6 ± 3.0 vs 12.7 ± 3.6) |
| Kotani et al[64] | Observational, N = 50 | B/C, 8 (7–9) | 10 (9–13) | 1b, 2a, 2b | SOF/VEL | 24 weeks, 89% | In 42% of patients who achieved SVR, the HVPG reduced by more than 20% of the baseline, and the percentage of patients with HVPG > 12 mmHg decreased from 92% to 58%. At the same time, clinically significant PH persisted in 75% of patients |
| Premkumar et al[65] | Prospective, cohort, N = 1152 | A/B/C, 12.7 ± 1.6 | 16.6 (16.5 ± 4.6) | 1, 2, 3 (87.1%), 4, 5, 6 | SOF/DAC, SOF/VEL | 12 weeks, 81.8% | The SVR resulted in recompensation in 24.7% of patients over a follow-up of 4 years. The ascites resolved in 86% of patients (diuretic withdrawal achieved in 24% of patients). Despite SVR, new hepatic decompensation evolved in 19% of patients. PH progressed in 13.7% of patients, with the development of recurrence GEVB in 4%. The hepatocellular carcinoma developed in 2.9% of patients |
| Yuri et al[66] | Retrospective, single-center, N = 109 | A/B/C | N/A | N/A | N/A (DAAs) | 24 weeks, 34,9% | At 7 years, the cumulative GEV progression rate in the DAA-SVR group was significantly lower than that in the non-SVR group. GEVB occurred in 11.3% of patients in the non-SVR group, while no GEVB events were observed in the DAA-SVR group during the observational period |
El-Sherif et al[68] performed a retrospective analysis of data from four RCTs (SOLAR-1, SOLAR-2, ASTRAL-4, and GS-US-334-0125) examining the efficacy of sofosbuvir-based therapy in patients with HCV-related decompensated LC (502-CTP class B and 120-CTP class C) to determine factors associated with recompensation, namely, a reduction of CTP score to class A. In these trials, patients were given 12 or 24 weeks of treatment with ledipasvir, sofosbuvir, and ribavirin or velpatasvir, sofosbuvir, and/or ribavirin, or 48 weeks of treatment with sofosbuvir and ribavirin. It turned out that the presence of ascites or encephalopathy, serum albumin levels < 3.5 g/dL or ALT < 60 U/L, and body mass index (BMI) > 25 kg/m2 were associated with an increased risk of not achieving a reduction in CTP to class A, independent of SVR to therapy. The serum albumin levels < 2.8 g/dL and abnormal serum total bilirubin levels were associated with an increased likelihood of liver transplantation or death. The authors developed a BE3A score based on five baseline factors (BMI, encephalopathy, ascites, and serum levels of ALT and albumin) associated significantly with patient outcomes. For patients with scores of 4-5, the HR for reduction of CTP score to class A was 52.3 (95%CI: 15.2-179.7).
Numerous publications in recent years have shown the efficacy of etiological therapy in achieving recompensation of alcohol-related, as well as HBV-related and HCV-related decompensated LC according to Baveno VII criteria. So far, only the first steps have been taken in studying this problem. To further understand it, research is needed to identify pathophysiological mechanisms, modifying factors, predictors, and potential noninvasive biomarkers of recompensation of decompensated LC.
| 1. | D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, Valsecchi MG. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 367] [Article Influence: 52.4] [Reference Citation Analysis (1)] |
| 2. | Roccarina D, Rosselli M, Genesca J, Tsochatzis EA. Elastography methods for the non-invasive assessment of portal hypertension. Expert Rev Gastroenterol Hepatol. 2018;12:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Jalan R, D'Amico G, Trebicka J, Moreau R, Angeli P, Arroyo V. New clinical and pathophysiological perspectives defining the trajectory of cirrhosis. J Hepatol. 2021;75 Suppl 1:S14-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 4. | Tonon M, D'Ambrosio R, Calvino V, Tosetti G, Barone A, Incicco S, Gambino C, Gagliardi R, Borghi M, Zeni N, Piano S, Lampertico P, Angeli P. A new clinical and prognostic characterization of the patterns of decompensation of cirrhosis. J Hepatol. 2024;80:603-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 5. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1477] [Article Influence: 492.3] [Reference Citation Analysis (2)] |
| 6. | Reiberger T, Hofer BS. The Baveno VII concept of cirrhosis recompensation. Dig Liver Dis. 2023;55:431-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 7. | Feng G, Valenti L, Wong VW, Fouad YM, Yilmaz Y, Kim W, Sebastiani G, Younossi ZM, Hernandez-Gea V, Zheng MH. Recompensation in cirrhosis: unravelling the evolving natural history of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2024;21:46-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 96] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 8. | Sanyal AJ, Anstee QM, Trauner M, Lawitz EJ, Abdelmalek MF, Ding D, Han L, Jia C, Huss RS, Chung C, Wong VW, Okanoue T, Romero-Gomez M, Muir AJ, Afdhal NH, Bosch J, Goodman Z, Harrison SA, Younossi ZM, Myers RP. Cirrhosis regression is associated with improved clinical outcomes in patients with nonalcoholic steatohepatitis. Hepatology. 2022;75:1235-1246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 9. | Hofer BS, Burghart L, Halilbasic E, Simbrunner B, Petrenko O, Mandorfer M, Stättermayer AF, Trauner M, Reiberger T. Evaluation of potential hepatic recompensation criteria in patients with PBC and decompensated cirrhosis. Aliment Pharmacol Ther. 2024;59:962-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Sharma S, Roy A. Recompensation in Cirrhosis: Current Evidence and Future Directions. J Clin Exp Hepatol. 2023;13:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Garbuzenko DV. Current strategies for targeted therapy of liver fibrosis. Bûll sib med. 2022;21:154-165. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 12. | Lens S, Baiges A, Alvarado-Tapias E, LLop E, Martinez J, Fortea JI, Ibáñez-Samaniego L, Mariño Z, Rodríguez-Tajes S, Gallego A, Bañares R, Puente Á, Albillos A, Calleja JL, Torras X, Hernández-Gea V, Bosch J, Villanueva C, García-Pagán JC, Forns X. Clinical outcome and hemodynamic changes following HCV eradication with oral antiviral therapy in patients with clinically significant portal hypertension. J Hepatol. 2020;73:1415-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 13. | Garbuzenko DV. [Morphofunctional rearrangement of the hepatic vasculature in the pathogenesis of portal hypertension in liver cirrhosis]. Ter Arkh. 2014;86:90-95. [PubMed] |
| 14. | Garbuzenko DV, Arefyev NO, Belov DV. Restructuring of the vascular bed in response to hemodynamic disturbances in portal hypertension. World J Hepatol. 2016;8:1602-1609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Garbuzenko DV. Contemporary concepts of prevention and management of gastroesophageal variceal bleeding in liver cirrhosis patients. World J Hepatol. 2024;16:126-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (1)] |
| 16. | Garbuzenko DV. Therapeutic possibilities of gut microbiota modulation in acute decompensation of liver cirrhosis. World J Hepatol. 2023;15:525-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 17. | Thursz M, Kamath PS, Mathurin P, Szabo G, Shah VH. Alcohol-related liver disease: Areas of consensus, unmet needs and opportunities for further study. J Hepatol. 2019;70:521-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 18. | GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1080] [Cited by in RCA: 1006] [Article Influence: 201.2] [Reference Citation Analysis (4)] |
| 19. | Julien J, Ayer T, Bethea ED, Tapper EB, Chhatwal J. Projected prevalence and mortality associated with alcohol-related liver disease in the USA, 2019-40: a modelling study. Lancet Public Health. 2020;5:e316-e323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 20. | Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology. 2020;71:306-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 566] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 21. | Lim WH, Tay P, Ng CH, Tan DJH, Ong C, Koh JH, Teng M, Chee D, Wong ZY, Kawaguchi T, Takahashi H, Muthiah M, Tan EXX, Wijarnpreecha K, Lee GH, Noureddin M, Lee BP, Mathurin P, Loomba R, Huang DQ. Meta-analysis: Prevalence and impact of alcohol abstinence in alcohol-associated cirrhosis. Aliment Pharmacol Ther. 2024;59:730-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 22. | Vorobioff J, Groszmann RJ, Picabea E, Gamen M, Villavicencio R, Bordato J, Morel I, Audano M, Tanno H, Lerner E, Passamonti M. Prognostic value of hepatic venous pressure gradient measurements in alcoholic cirrhosis: a 10-year prospective study. Gastroenterology. 1996;111:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 213] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Giard JM, Dodge JL, Terrault NA. Superior Wait-List Outcomes in Patients with Alcohol-Associated Liver Disease Compared With Other Indications for Liver Transplantation. Liver Transpl. 2019;25:1310-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Pose E, Torrents A, Reverter E, Perez-Campuzano V, Campos-Varela I, Avitabile E, Gratacós-Ginès J, Castellote J, Castells L, Colmenero J, Tort J, Ginès P, Crespo G. A notable proportion of liver transplant candidates with alcohol-related cirrhosis can be delisted because of clinical improvement. J Hepatol. 2021;75:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Aravinthan AD, Barbas AS, Doyle AC, Tazari M, Sapisochin G, Cattral MS, Ghanekar A, McGilvray ID, Selzner M, Greig PD, Bhat M, Selzner N, Grant DR, Lilly LB, Renner EL. Characteristics of liver transplant candidates delisted following recompensation and predictors of such delisting in alcohol-related liver disease: a case-control study. Transpl Int. 2017;30:1140-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Hofer BS, Simbrunner B, Hartl L, Jachs M, Balcar L, Paternostro R, Schwabl P, Semmler G, Scheiner B, Trauner M, Mandorfer M, Reiberger T. Hepatic recompensation according to Baveno VII criteria is linked to a significant survival benefit in decompensated alcohol-related cirrhosis. Liver Int. 2023;43:2220-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 27. | Hofer BS, Simbrunner B, Hartl L, Jachs M, Bauer DJM, Balcar L, Paternostro R, Schwabl P, Semmler G, Scheiner B, Staettermayer AF, Trauner M, Mandorfer M, Reiberger T. Alcohol Abstinence Improves Prognosis Across All Stages of Portal Hypertension in Alcohol-Related Cirrhosis. Clin Gastroenterol Hepatol. 2023;21:2308-2317.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 58] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 28. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 850] [Article Influence: 212.5] [Reference Citation Analysis (1)] |
| 29. | Garbuzenko DV. [The role of antiviral therapy in the management of patients with liver cirrhosis associated with chronic HBV and HCV infection]. Vopr Virusol. 2021;66:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Sinclair S 3rd, Shearen S, Ghobrial Y, Trad G, Abdul Basit S, Shih D, Ryan JK. Review of the Effects of Antiviral Therapy on Hepatitis B/C-Related Mortality and the Regression of Fibrosis. Viruses. 2024;16:1531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 31. | GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:796-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 412] [Article Influence: 137.3] [Reference Citation Analysis (0)] |
| 32. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3790] [Article Influence: 473.8] [Reference Citation Analysis (1)] |
| 33. | Nikolaidis N, Vassiliadis T, Giouleme O, Tziomalos K, Grammatikos N, Patsiaoura K, Orfanou-Koumerkeridou E, Balaska A, Eugenidis N. Effect of lamivudine treatment in patients with decompensated cirrhosis due to anti-HBe positive/HBeAg-negative chronic hepatitis B. Clin Transplant. 2005;19:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Manolakopoulos S, Triantos C, Theodoropoulos J, Vlachogiannakos J, Kougioumtzan A, Papatheodoridis G, Tzourmakliotis D, Karamanolis D, Burroughs AK, Archimandritis A, Raptis S, Avgerinos A. Antiviral therapy reduces portal pressure in patients with cirrhosis due to HBeAg-negative chronic hepatitis B and significant portal hypertension. J Hepatol. 2009;51:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Shim JH, Lee HC, Kim KM, Lim YS, Chung YH, Lee YS, Suh DJ. Efficacy of entecavir in treatment-naïve patients with hepatitis B virus-related decompensated cirrhosis. J Hepatol. 2010;52:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 36. | Liaw YF, Raptopoulou-Gigi M, Cheinquer H, Sarin SK, Tanwandee T, Leung N, Peng CY, Myers RP, Brown RS Jr, Jeffers L, Tsai N, Bialkowska J, Tang S, Beebe S, Cooney E. Efficacy and safety of entecavir versus adefovir in chronic hepatitis B patients with hepatic decompensation: a randomized, open-label study. Hepatology. 2011;54:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 37. | Jang JW, Choi JY, Kim YS, Woo HY, Choi SK, Lee CH, Kim TY, Sohn JH, Tak WY, Han KH. Long-term effect of antiviral therapy on disease course after decompensation in patients with hepatitis B virus-related cirrhosis. Hepatology. 2015;61:1809-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 38. | Lee SK, Song MJ, Kim SH, Lee BS, Lee TH, Kang YW, Kim SB, Song IH, Chae HB, Ko SY, Lee JD. Safety and efficacy of tenofovir in chronic hepatitis B-related decompensated cirrhosis. World J Gastroenterol. 2017;23:2396-2403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Wang Q, Zhao H, Deng Y, Zheng H, Xiang H, Nan Y, Hu J, Meng Q, Xu X, Fang J, Xu J, Wang X, You H, Pan CQ, Xie W, Jia J. Validation of Baveno VII criteria for recompensation in entecavir-treated patients with hepatitis B-related decompensated cirrhosis. J Hepatol. 2022;77:1564-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 40. | Hui VW, Wong GL, Wong VW, Chan HL, Lai JC, Tse YK, Lai MS, Yam TF, Li D, Fan X, Yip TC. Baveno VII criteria for recompensation predict transplant-free survival in patients with hepatitis B-related decompensated cirrhosis. JHEP Rep. 2023;5:100814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Zhang Y, Liu X, Li S, Lin C, Ye Q, Wang Y, Wu J, Zhang Y, Gao H, Li T, Qu Y, Wang Y. Risk of HCC decreases in HBV-related patients with cirrhosis acquired recompensation: A retrospective study based on Baveno VII criteria. Hepatol Commun. 2024;8:e0355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 42. | Li M, Zong Z, Xiong X, Fan J, Zhong H, Liu N, Ye W, Jing J. Ascites re-compensation in HBV-related first decompensated cirrhosis after anti-viral therapy. Front Cell Infect Microbiol. 2022;12:1053608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 43. | Kong Y, Lv T, Li M, Zhao L, Meng T, Wu S, Wei W, Zhang Q, Chen S, You H, Lens S, Yoshiji H, Francque S, Tsochatzis E, Sarin SK, Mandorfer M, Jia J; BAVENO Cooperation: an EASL consortium. Systematic review and meta-analysis: impact of anti-viral therapy on portal hypertensive complications in HBV patients with advanced chronic liver disease. Hepatol Int. 2022;16:1052-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 44. | Deng Y, Kang H, Xiang H, Nan Y, Hu J, Meng Q, Zhao H, Wang Q, Fang J, Xu J, Wang X, Pan CQ, You H, Xu X, Xie W, Jia J. Durability and on-treatment predictors of recompensation in entecavir-treated patients with hepatitis B and decompensated cirrhosis. JHEP Rep. 2024;6:101091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Reference Citation Analysis (0)] |
| 45. | Kim TH, Um SH, Lee YS, Yim SY, Jung YK, Seo YS, Kim JH, An H, Yim HJ, Yeon JE, Byun KS. Determinants of re-compensation in patients with hepatitis B virus-related decompensated cirrhosis starting antiviral therapy. Aliment Pharmacol Ther. 2022;55:83-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 46. | Wen S, Ruan J, Shen J, Wang X, Yang G, Fu J, Li L, Pan X. Development and validation of a nomogram to predict recompensation in HBV-related cirrhosis with ascites as the single first decompensating event. Scand J Gastroenterol. 2023;58:915-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Topi S, Gaxhja E, Charitos IA, Colella M, Santacroce L. Hepatitis C Virus: History and Current Knowledge. Gastroenterol Insigh. 2024;15:676-707. [DOI] [Full Text] |
| 48. | Huang DQ, Terrault NA, Tacke F, Gluud LL, Arrese M, Bugianesi E, Loomba R. Global epidemiology of cirrhosis - aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023;20:388-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 363] [Article Influence: 181.5] [Reference Citation Analysis (0)] |
| 49. | Bhattacharya D, Aronsohn A, Price J, Lo Re V; AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2023 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 99] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 50. | Belli LS, Berenguer M, Cortesi PA, Strazzabosco M, Rockenschaub SR, Martini S, Morelli C, Donato F, Volpes R, Pageaux GP, Coilly A, Fagiuoli S, Amaddeo G, Perricone G, Vinaixa C, Berlakovich G, Facchetti R, Polak W, Muiesan P, Duvoux C; European Liver and Intestine Association (ELITA). Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: A European study. J Hepatol. 2016;65:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 253] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 51. | Foster GR, Irving WL, Cheung MC, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, Brown A, Gelson WT, MacDonald DC, Agarwal K; HCV Research, UK. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 360] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 52. | Mandorfer M, Kozbial K, Schwabl P, Freissmuth C, Schwarzer R, Stern R, Chromy D, Stättermayer AF, Reiberger T, Beinhardt S, Sieghart W, Trauner M, Hofer H, Ferlitsch A, Ferenci P, Peck-Radosavljevic M. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J Hepatol. 2016;65:692-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 241] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 53. | Perricone G, Duvoux C, Berenguer M, Cortesi PA, Vinaixa C, Facchetti R, Mazzarelli C, Rockenschaub SR, Martini S, Morelli C, Monico S, Volpes R, Pageaux GP, Fagiuoli S, Belli LS; European Liver and Intestine Transplant Association (ELITA). Delisting HCV-infected liver transplant candidates who improved after viral eradication: Outcome 2 years after delisting. Liver Int. 2018;38:2170-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 54. | Macken L, Gelson W, Priest M, Abouda G, Barclay S, Fraser A, Healy B, Irving W, Verma S. Efficacy of direct-acting antivirals: UK real-world data from a well-characterised predominantly cirrhotic HCV cohort. J Med Virol. 2019;91:1979-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Hanafy AS, Bassiony MA, Basha MAA. Management of HCV-related decompensated cirrhosis with direct-acting antiviral agents: who should be treated? Hepatol Int. 2019;13:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Moon AM, Green PK, Rockey DC, Berry K, Ioannou GN. Hepatitis C eradication with direct-acting anti-virals reduces the risk of variceal bleeding. Aliment Pharmacol Ther. 2020;51:364-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 57. | Puigvehí M, Londoño MC, Torras X, Lorente S, Vergara M, Morillas RM, Masnou H, Serrano T, Miquel M, Gallego A, Lens S, Carrión JA. Impact of sustained virological response with DAAs on gastroesophageal varices and Baveno criteria in HCV-cirrhotic patients. J Gastroenterol. 2020;55:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 58. | Liu CH, Chen CY, Su WW, Liu CJ, Lo CC, Huang KJ, Chen JJ, Tseng KC, Chang CY, Peng CY, Shih YL, Huang CS, Kao WY, Yang SS, Tsai MC, Wu JH, Chen PY, Su PY, Hwang JJ, Fang YJ, Lee PL, Tseng CW, Lee FJ, Lai HC, Hsieh TY, Chang CC, Chang CH, Huang YJ, Kao JH. Sofosbuvir/velpatasvir plus ribavirin for Child-Pugh B and Child-Pugh C hepatitis C virus-related cirrhosis. Clin Mol Hepatol. 2021;27:575-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Tada T, Kurosaki M, Nakamura S, Hasebe C, Kojima Y, Furuta K, Kobashi H, Kimura H, Ogawa C, Yagisawa H, Uchida Y, Joko K, Akahane T, Arai H, Marusawa H, Narita R, Ide Y, Sato T, Kusakabe A, Tsuji K, Mori N, Kondo M, Mitsuda A, Izumi N. Real-world clinical outcomes of sofosbuvir and velpatasvir treatment in HCV genotype 1- and 2-infected patients with decompensated cirrhosis: A nationwide multicenter study by the Japanese Red Cross Liver Study Group. J Med Virol. 2021;93:6247-6256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 60. | Tahata Y, Hikita H, Mochida S, Kawada N, Enomoto N, Ido A, Yoshiji H, Miki D, Hiasa Y, Takikawa Y, Sakamori R, Kurosaki M, Yatsuhashi H, Tateishi R, Ueno Y, Itoh Y, Yamashita T, Kanto T, Suda G, Nakamoto Y, Kato N, Asahina Y, Matsuura K, Terai S, Nakao K, Shimizu M, Takami T, Akuta N, Yamada R, Kodama T, Tatsumi T, Yamada T, Takehara T. Sofosbuvir plus velpatasvir treatment for hepatitis C virus in patients with decompensated cirrhosis: a Japanese real-world multicenter study. J Gastroenterol. 2021;56:67-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 61. | Takaoka Y, Miura K, Morimoto N, Ikegami T, Kakizaki S, Sato K, Ueno T, Naganuma A, Kosone T, Arai H, Hatanaka T, Tahara T, Tano S, Ohtake T, Murohisa T, Namikawa M, Asano T, Kamoshida T, Horiuchi K, Nihei T, Soeda A, Kurata H, Fujieda T, Ohtake T, Fukaya Y, Iijima M, Watanabe S, Isoda N, Yamamoto H; Liver Investigators in the Northern Kanto Study (LINKS) group. Real-world efficacy and safety of 12-week sofosbuvir/velpatasvir treatment for patients with decompensated liver cirrhosis caused by hepatitis C virus infection. Hepatol Res. 2021;51:51-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 62. | Meunier L, Belkacemi M, Pageaux GP, Radenne S, Vallet-Pichard A, Houssel-Debry P, Duvoux C, Botta-Fridlund D, de Ledinghen V, Conti F, Anty R, Di Martino V, Debette-Gratien M, Leroy V, Gerster T, Lebray P, Alric L, Abergel A, Dumortier J, Besch C, Montialoux H, Samuel D, Duclos-Vallée JC, Coilly A. Patients Treated for HCV Infection and Listed for Liver Transplantation in a French Multicenter Study: What Happens at Five Years? Viruses. 2022;15:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Su PS, Wu SH, Chu CJ, Su CW, Lin CC, Lee SD, Wang YJ, Lee FY, Huang YH, Hou MC. Sofosbuvir-based antiviral therapy provided highly treatment efficacy, safety, and good tolerability for Taiwanese chronic hepatitis C patients with decompensated cirrhosis. J Chin Med Assoc. 2022;85:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Kotani K, Enomoto M, Uchida-Kobayashi S, Tamori A, Yukawa-Muto Y, Odagiri N, Motoyama H, Kozuka R, Kawamura E, Hagihara A, Fujii H, Kageyama K, Yamamoto A, Yoshida A, Higashiyama S, Kawabe J, Kawada N. Short-term hepatocyte function and portal hypertension outcomes of sofosbuvir/velpatasvir for decompensated hepatitis C-related cirrhosis. J Gastroenterol. 2023;58:394-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Premkumar M, Dhiman RK, Duseja A, Mehtani R, Taneja S, Gupta E, Gupta P, Sandhu A, Sharma P, Rathi S, Verma N, Kulkarni AV, Bhujade H, Chaluvashetty SB, Kalra N, Grover GS, Nain J, Reddy KR. Recompensation of Chronic Hepatitis C-Related Decompensated Cirrhosis Following Direct-Acting Antiviral Therapy: Prospective Cohort Study From a Hepatitis C Virus Elimination Program. Gastroenterology. 2024;167:1429-1445. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 66. | Yuri Y, Nishimura T, Ikeda N, Takashima T, Aizawa N, Kimura T, Yoshihara K, Yoshioka R, Kawata S, Kawase Y, Nakano R, Shiomi H, Fukunishi S, Shinzaki S, Enomoto H. Long-term Effect of the HCV Elimination With Direct-acting Antivirals on the Progression of Gastroesophageal Varices. In Vivo. 2024;38:2968-2972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 67. | An J, Park DA, Ko MJ, Ahn SB, Yoo JJ, Jun DW, Yim SY. Direct-Acting Antivirals for HCV Treatment in Decompensated Liver Cirrhosis Patients: A Systematic Review and Meta-Analysis. J Pers Med. 2022;12:1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 68. | El-Sherif O, Jiang ZG, Tapper EB, Huang KC, Zhong A, Osinusi A, Charlton M, Manns M, Afdhal NH, Mukamal K, McHutchison J, Brainard DM, Terrault N, Curry MP. Baseline Factors Associated With Improvements in Decompensated Cirrhosis After Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection. Gastroenterology. 2018;154:2111-2121.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |