Published online Apr 27, 2025. doi: 10.4254/wjh.v17.i4.105120
Revised: February 27, 2025
Accepted: March 25, 2025
Published online: April 27, 2025
Processing time: 103 Days and 17.8 Hours
Bile cast nephropathy (BCN) is suspected in the setting of liver disease and hyperbilirubinemia and is characterized by the formation of tubular bile casts and acute tubular injury. While postmortem studies reveal a high prevalence of BCN, little is known about this orphan acute kidney injury syndrome.
To address this knowledge gap, we performed a systematic review of case reports and case series of BCN, focusing on risk factors, diagnostic criteria, clinical presentation, kidney biopsy findings, severity, treatment approaches, and out
Electronic databases were searched to identify eligible studies of patients with possible, probable, or definite BCN, using pre-established criteria. Relevant va
Sixty-seven case reports and six case series (involving 2 patients each) met the inclusion criteria, totaling 79 cases of BCN. The mean age was 48.3 years, and 83.5% were men. The most common cause of liver disease was drug-induced injury (30.4%), followed by infection (18.9%) and alcoholism (12.7%). BCN diagnosis was deemed definite, probable, and possible in 65.8%, 32.9%, and 1.3% of cases, respectively. Levels of serum creatinine, dialysis requirement, and renal recovery did not differ among the total bilirubin tertile groups. However, both initial and peak serum creatinine were significantly higher in the alcoholic liver disease group compared to the non-alcoholic group (P = 0.011 and P = 0.012, respectively). There was also a non-significant trend toward a higher incidence of dialysis requirement or death in the alcoholic liver disease group (80% vs 52%, P = 0.098). Finally, higher initial serum creatinine (per 1 mg/dL increase) was independently associated with dialysis requirement or death (adjusted odds ratio 1.291, 95% confidence interval: 1.032-1.615, P = 0.025).
BCN is a common and potentially serious cause of acute kidney injury in patients with liver disease. The degree of hyperbilirubinemia does not appear to correlate with BCN severity or outcomes. However, in alcoholic liver disease, BCN is associated with a greater rise in serum creatinine and a trend toward worse outcomes compared to non-alcoholic liver disease. Serum creatinine may be a valuable predictor of BCN prognosis. Further studies are needed to develop non-invasive diagnostic tools and establish effective treatments for BCN.
Core Tip: This systematic review highlights bile cast nephropathy (BCN) as a serious yet underrecognized cause of acute kidney injury in patients with liver disease. Despite its clinical significance, universally accepted diagnostic criteria and therapeutic approaches are currently lacking. Among the various liver disease etiologies implicated in BCN, alcohol-related liver disease appears to be associated with more severe acute kidney injury. Additionally, higher initial serum creatinine was identified as a predictor of dialysis requirement or death. These findings underscore the need for further research into non-invasive diagnostic tools and viable therapeutic strategies for BCN.
- Citation: Alabdul Razzak I, El Naamani H, Dimitrov D, Morin R, Jaber BL. Bile cast nephropathy: A systematic review of case reports and case series. World J Hepatol 2025; 17(4): 105120
- URL: https://www.wjgnet.com/1948-5182/full/v17/i4/105120.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i4.105120
Bile cast nephropathy (BCN), also known as cholemic nephropathy, is a rare condition that causes acute kidney injury (AKI) in patients with liver disease and severe hyperbilirubinemia[1]. First described in 1899, with documented morphological and histological renal alterations in autopsies of patients with jaundice[2], in 1922, a causal link between hyperbilirubinemia and a decline in kidney function was suggested[3], with some evidence of kidney function recovery following resolution of jaundice[4]. BCN is now recognized as a structural kidney disease with characteristic histological features, including tubular epithelial injury usually accompanied by bile casts[5], and this may be the result of the nephrotoxic effects of bile on the renal tubules through several postulated mechanisms[1,5]. While the pathogenesis of BCN continues to be elucidated[6-11], less is known about the clinical course of this orphan AKI syndrome, and its clinical manifestations, natural history, and prognosis have not been systematically examined. To address this knowledge gap, inform clinical practice, and provide future research directions, we performed a systematic review of patients presenting with BCN with a focus on elucidating risk factors, diagnostic criteria, clinical presentation, kidney biopsy findings, disease severity measures, and both kidney- and patient-related outcomes.
This systematic review was conducted in accordance with the Case Report guidelines[12] and the Preferred Reporting Items for Systematic Reviews of Individual Participant Data Statement[13]. The following electronic databases were searched for relevant citations: PubMed, Scopus, EMBASE, and Cochrane Central Register of Controlled Trials for the period of January 01, 1980, to October 31, 2023. Eligible reports were identified using the following Medical Subject Headings search terms: (“bile cast nephropathy” OR “bile nephropathy” OR “bile cast” OR “cholemic nephropathy” OR “cholemic nephrosis” OR “cholemic nephritis“ OR “bilirubin nephropathy” OR “biliuria” OR “hyperbilirubinemia” OR “bile acid” OR “bilirubin”) AND (“renal insufficiency” OR “renal replacement therapy” OR “kidney disease” OR “kidney failure” OR “CKD or CKF or CRD or CRF or ESKD or ESRD or ESRF” OR “hemodialysis” OR “dialysis" OR “kidney transplant” OR “renal transplant” OR “acute renal failure” OR “acute kidney failure” OR “acute renal insufficiency” OR “acute kidney insufficiency” OR “acute tubular necrosis” OR “acute kidney injury” OR “acute renal injury” OR “nephropathy”). Bibliographies of retrieved articles were also inspected to identify additional studies of interest. The search strategy was limited to human studies with no restrictions on language, sample size, or duration of study.
Due to the paucity of retrospective and prospective cohort studies with adequate case descriptions, we focused on case reports and case series (defined as reports involving ≥ 2 patients) of patients with presumed/confirmed BCN. Criteria for excluding articles from further review were duplicates, irrelevant articles, inadequate information, narrative reviews, book chapters, editorial comments or letters to the editor, animal studies, pediatric studies, and cases where kidney involvement was deemed unrelated to BCN. The criteria listed in Table 1 were used to determine the level of evidence for diagnosing BCN. AKI was defined in accordance with the Kidney Disease: Improving Global Outcomes clinical practice guideline[14]. In brief, two of the authors (El Naamani H and Alabdul Razzak I) independently reviewed each report to establish the level of evidence for an unlikely, possible, probable and definite diagnosis of BCN. Disagreement between the two reviewers was resolved through adjudication by a third author (Dimitrov D).
| Criterion | Level of evidence |
| 1 AKI, defined by the KDIGO clinical practice guideline[14] | Definite: 1, 2, 3, and 4 or 5 met |
| 2 Elevated serum total bilirubin level | Probable: 1, 2, and 3, 4, or 5 met |
| 3 Presence of bile casts in the urine sediment or in the tubular lumen (on kidney biopsy/autopsy) | Possible: 1 and 2 met |
| 4 Other causes of AKI excluded (including acute tubular injury and hepato-renal syndrome) | Unlikely: 1 or 2 not met |
| 5 Direct relationship between the degree of hyperbilirubinemia and the AKI |
We extracted data in duplicate using a data extraction spreadsheet. Study-level variables included country, year of publication, study design (case report or case series), publication format (abstract or full manuscript), population setting (alive or post-mortem), and duration of follow-up. Demographic variables were age, sex, and race. Clinical variables encompassed cause of liver disease (e.g., drug-induced, infection-related, obstructive, alcohol-related, malignancy, or other), initial, peak, and end-of-follow-up serum total bilirubin level, initial serum direct and indirect bilirubin, initial serum level of alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase, initial prothrombin time/international normalized ratio (INR), baseline, initial, peak, and end-of-follow-up serum creatinine, preexisting chronic kidney disease, urinalysis findings, type of casts on urine sediment (bilirubin, granular, other, or no casts), kidney biopsy (or autopsy) findings, including the use of the Fouchet histochemical stain for bile pigments, and renal ultrasound findings (presence/absence of urinary obstruction).
Treatment-related variables of interest included use of corticosteroids, intravenous albumin, plasmapheresis, the use and mode of dialysis (intermittent hemodialysis and continuous venovenous hemodiafiltration), liver dialysis, and liver transplantation. Clinical outcomes were categorized as kidney-related, including full recovery, partial recovery, dialysis dependence, and kidney transplantation, and patient-related, namely death. We also ascertained a composite outcome of dialysis requirement or death. In our systematic review, approximations and estimations were employed to maintain consistency and enable a standardized analysis across various reports. For example, duration of follow-up was recorded in months, with approximation to the nearest week. Regarding laboratory values, the serum INR for nine patients reported as “normal” was estimated at 1.0. In cases where laboratory test results exceeded a value, that value was used. In terms of demographic data, when ages were ambiguously reported as “30s” and “late 40s”, they were estimated as 35 and 48 years old, respectively.
The data were synthesized and analyzed using a meta-analytical framework. Data from included cases were tabulated and quantitatively synthesized. Due to paucity of data on BCN, we opted to analyze the results stratified according to tertiles of initial serum total bilirubin level as well as by the underlying cause of liver disease (alcohol-related or other), to inform clinical practice. Continuous variables are reported as mean (with standard deviation or range), and binary variables as counts (with percentage). The Mann-Whitney U and the Kruskal-Wallis tests were used for comparison of continuous variables, and the χ2-test for comparison of categorical variables.
Univariate and multivariable logistic regression analyses were also conducted to examine factors associated with the composite outcome of dialysis requirement or death. The results are displayed as odds ratio (OR) with 95% confidence interval (CI). To account for missing data that is assumed to have occurred randomly for two of our candidate variables, mainly the initial serum creatinine (5.1% missing) and the initial total bilirubin (2.5% missing), we imputed the data using the series mean method prior to repeating the univariate logistic regression analysis. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 22 (IBM Corporation, Armonk, NY, United States). Differences were considered statistically significant at a P value of less than 0.05.
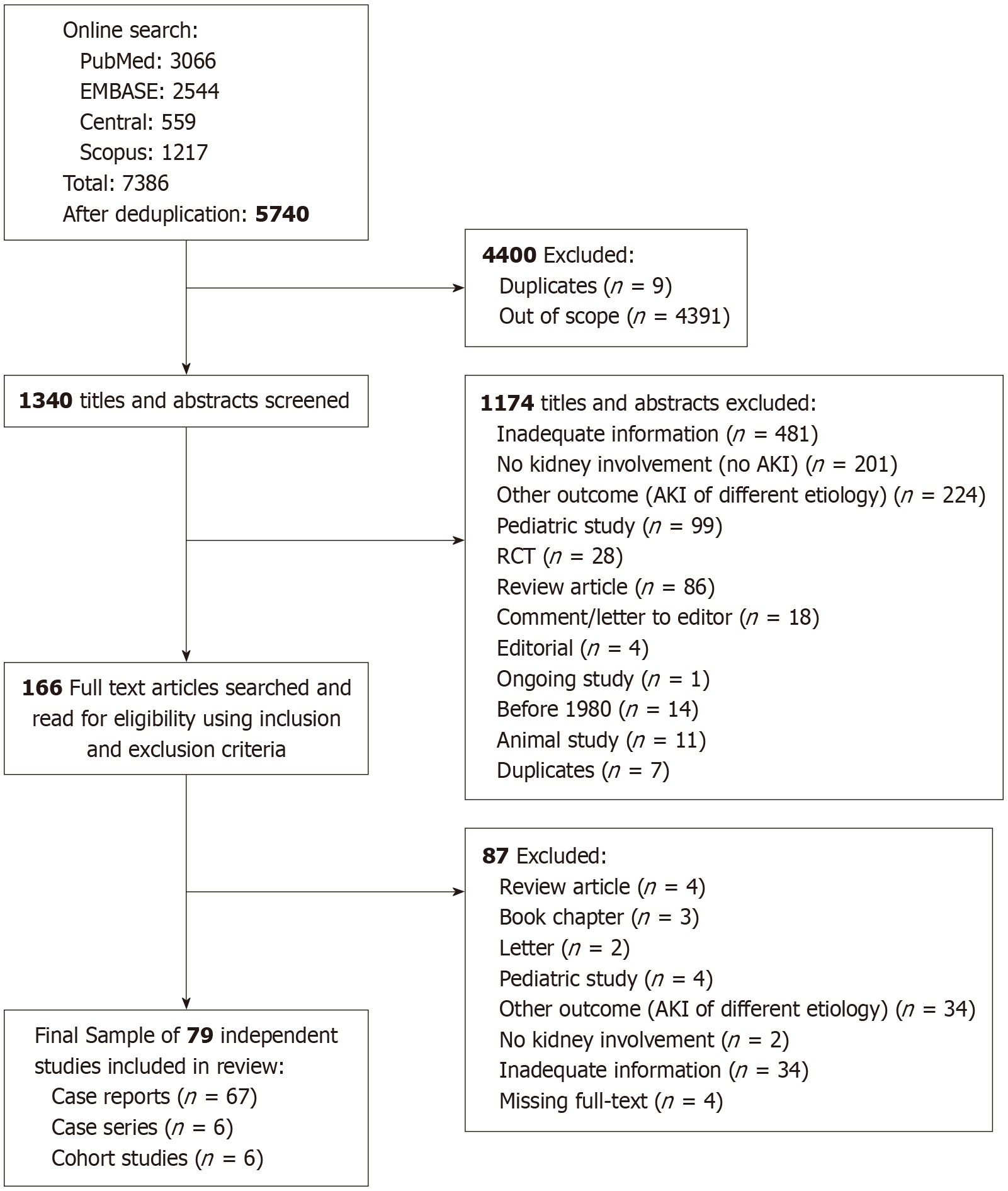
A total of 7386 potentially relevant citations were identified, which dropped to 5740 after removing duplicates. Of these, 4400 citations were excluded after title screening: 4391 were out of scope, and 9 were additional duplicates. Of the remaining 1340 citations, 1174 were excluded based on abstract screening. Hence, 166 full-text articles were retrieved for a detailed evaluation to assess their eligibility against our inclusion and exclusion criteria. Ultimately, 79 independent studies met the eligibility criteria. These included 67 case reports[15-81], 6 case series[82-87], and 6 cohort studies[88-93]. However, the 6 cohort studies were not included in the quantitative analysis as they were not analyzable (Figure 1 and Supplementary Table 1). In total, 79 cases were included: 67 (92%) were individual case reports[15-81], and 6 (8%) were case series[82-87], each involving 2 patients. These reports originated from North America (49%), Europe (23%), Asia (21%), Australia and New Zealand (4%), and Africa (3%). A detailed description of each case is summarized in Supplementary Table 2. These 79 cases were analyzed quantitatively. Using our pre-established criteria, the level of evidence for diagnosing BCN was definite in 52 cases (66%), probable in 26 cases (33%), and possible in 1 case (1%).
Table 2 displays the demographic and clinical characteristics, risk factors, treatment modalities, and outcomes of patients with BCN. In brief, mean age was 48 (range 25-87) years and 66 (84%) patients were men. In terms of liver-related parameters, the most common cause of liver disease was drug-induced liver injury (30.4%), followed by infectious hepatitis (19.0%), malignant biliary obstruction (13.9%), alcohol-related (12.7%), other (12.7%), and benign biliary obstruction (11.4%). At initial presentation, mean serum total bilirubin was 30.3 (range 4.7-53) mg/dL, with a direct bilirubin of 17.8 (range 3.3-45) mg/dL. Mean serum albumin (available in 33 cases) was 2.9 (range 1.6-4.7) gm/dL, and the INR was 1.9 (range 1.0-6.0). Initial alanine aminotransferase and aspartate aminotransferase levels were 705 (range 20-5592) and 619 (21-6205) U/L, respectively, and alkaline phosphatase level was 404 (range 40-1419) U/L. Mean serum bile acid level measured in 2 patients was 84.5 (range 34-135 mmol/L).
| Number of evaluable patients | mean ± SD or n (%) | Range | |
| Demographic variables | |||
| Age, years | 79 | 48 ± 15 | 25-87 |
| Male gender | 79 | 66 (83.5) | |
| White race | 9 | 6 (66.7) | |
| Liver-related parameters | |||
| Cause of liver disease or cholestasis | 79 | ||
| Drug-induced | 79 | 24 (30.4) | |
| Infection-related | 79 | 15 (18.9) | |
| Benign obstructive cholestasis | 79 | 9 (11.4) | |
| Alcohol-related | 79 | 10 (12.7) | |
| Malignant obstructive cholestasis | 79 | 11 (13.9) | |
| Other | 79 | 10 (12.7) | |
| Total bilirubin, mg/dL | |||
| Initial | 77 | 27.1 ± 13.2 | 4.7-53.0 |
| Peak | 49 | 36.0 ± 15.4 | 7.3-102.0 |
| Indirect bilirubin, mg/dL | |||
| Initial | 44 | 8.5 ± 6.2 | 1.1-22.7 |
| Albumin, gm/dL | 33 | 2.9 ± 0.7 | 1.6-4.7 |
| ALT, U/L | 61 | 705 ± 1337 | 20-5592 |
| AST, U/L | 61 | 619 ± 1184 | 21-6205 |
| ALP, U/L | 52 | 404 ± 358 | 40-1419 |
| PT, seconds | 18 | 24 ± 23 | 10-75 |
| INR | 33 | 1.9 ± 1.1 | 1.0-6.0 |
| Kidney-related parameters | |||
| AKI | 79 | 70 (88.6) | |
| Preexisting CKD | 76 | 9 (11.8) | |
| Acute on CKD | 76 | 9 (11.8) | |
| Serum creatinine, mg/dL | |||
| Baseline | 26 | 1.1 ± 0.4 | 0.6-2.1 |
| Initial | 75 | 3.9 ± 3.2 | 0.6-14.3 |
| Peak | 49 | 5.9 ± 2.9 | 1.6-14.5 |
| End-of-follow-up | 50 | 2.1 ± 1.7 | 0.8-8.0 |
| Dipstick urinalysis findings | |||
| Blood | 36 | 15 (41.7) | |
| Protein | 39 | 22 (56.4) | |
| Bilirubin | 26 | 17 (65.4) | |
| Urobilinogen | 21 | 11 (52.4) | |
| Urine sediment findings | |||
| Bilirubin/pigmented casts | 49 | 15 (30.6) | |
| Granular casts | 49 | 7 (14.3) | |
| Bilirubin and granular casts | 49 | 4 (8.2) | |
| Other casts | 49 | 7 (14.3) | |
| No casts | 49 | 16 (32.7) | |
| Random urine sodium, mEq/L | 12 | 41 ± 17 | 15-69 |
| Fractional excretion of sodium, % | 4 | 1.4 ± 1.1 | 0.1-2.7 |
| Histopathological ascertainment | |||
| Kidney biopsy | 79 | 52 (65.8) | |
| Kidney autopsy | 79 | 4 (5.1) | |
| None | 79 | 23 (29.1) | |
| Histopathological findings | 56 | ||
| Bile/bilirubin/green casts or green on autopsy | 56 | 46 (82.1) | |
| Other casts | 56 | 7 (12.5) | |
| Absence of casts | 56 | 3 (5.4) | |
| Sonographic urinary obstruction | 31 | 1 (3.2) | |
| Treatment modalities | |||
| Corticosteroids | 79 | 10 (12.7) | |
| Intravenous albumin | 27 | 5 (18.5) | |
| Plasmapheresis | 27 | 11 (40.7) | |
| Dialysis | 77 | 39 (50.6) | |
| Liver dialysis | 27 | 3 (11.1) | |
| Clinical outcomes | |||
| Duration of follow-up, months | 55 | 4.0 ± 5.7 | 0.0-36.0 |
| Liver transplantation | 27 | 5 (18.5) | |
| Liver and kidney transplantation | 79 | 2 (2.5) | |
| Kidney function | |||
| Full recovery | 73 | 30 (41.1) | |
| Partial recovery | 73 | 29 (39.7) | |
| Dialysis dependence | 73 | 6 (8.2) | |
| Death | 76 | 11 (14.5) |
In terms of kidney-related parameters, 70 (89%) patients experienced AKI, and 9 (11%) had acute on chronic kidney disease. At presentation, the mean serum creatinine was 3.85 (range 0.6-14.3) mg/dL, and at last follow-up was 2.1 (range 0.8-8.0) mg/dL. Results of the dipstick urinalysis were as follows: 15 (41.7%) of 36 tested patients had blood, and 22 (56.4%) of 39 tested patients had protein. The presence of bilirubinuria was reported in 26 cases, with 17 patients (65.4%) testing positive. Histological examination of kidney tissue was reported in 56 (70.8%) cases, including 52 biopsies and 4 autopsies. ‘Bile’, ‘bilirubin’, or ‘green’ casts (including green appearance of the kidney on autopsy) were present on 46 (82.1%) histological or gross anatomical samples.
Treatment modalities employed in the setting of BCN included corticosteroids (12.7%), intravenous albumin (18.5%), plasmapheresis (40.7%), dialysis (50.6%) and liver dialysis (11.1%). Among the 39 patients requiring dialysis, 35 (89.7%) received intermittent hemodialysis and 4 (10.3%) received continuous venovenous hemodiafiltration. The mean duration of follow-up was 2.8 months (range 0-36 months). Longitudinal follow-up data on kidney function were available for 73 patients. Thirty (41.1%) patients had complete renal recovery, 29 (39.7%) patients had partial renal recovery, and 6 (8.2%) patients remained dialysis dependent. Five patients underwent liver transplantation, and 2 patients underwent combined liver and kidney transplantation. Among the 76 evaluable patients, 11 (14.5%) died.
To explore the potential role of serum bilirubin levels as a proxy for nephrotoxicity of bilirubin and bile acids and the severity of AKI, we stratified the cohort into tertiles based on initial serum total bilirubin levels and compared clinical characteristics and outcomes. In brief, as shown in Table 3, there were no significant differences in the demographic and clinical characteristics, liver- and kidney-related parameters, treatment modalities, and outcomes of patients with BCN according to tertiles of initial serum total bilirubin levels. Specifically, there were no significant differences in initial and peak serum creatinine between the three tertile groups, arguing against a link between hyperbilirubinemia and kidney disease severity in patients with BCN. Similarly, dialysis requirement, the degree of renal recovery at the end of follow-up period, and overall mortality did not differ amongst the total bilirubin tertile groups.
| Number of evaluable patients | Total bilirubin, tertile 1 | Total bilirubin, tertile 2 | Total bilirubin, tertile 3 | P value | |
| Demographic variables | |||||
| Age, years | 77 | 53 ± 15 | 47 ± 14 | 46 ± 17 | 0.222 |
| Male gender | 77 | 21 (80.8) | 18 (69.2) | 25 (100) | 0.013 |
| White race | 9 | 3 (100.0) | 2 (66.7) | 1 (33.3) | 0.316 |
| Liver-related variables | |||||
| Cause of liver disease or cholestasis | 77 | 0.062 | |||
| Drug-induced | 8 (30.8) | 5 (19.2) | 11 (44) | ||
| Infection-related | 6 (23.1) | 1 (3.8) | 7 (28) | ||
| Obstructive | 4 (15.4) | 5(19.2) | 0 (0) | ||
| Alcohol-related | 2 (7.7) | 6 (23.1) | 1 (4.0) | ||
| Malignancy | 4 (15.4) | 4 (15.4) | 3 (12.0) | ||
| Other | 2 (7.7) | 5 (19.2) | 3(12.0) | ||
| Total bilirubin, mg/dL | |||||
| Initial | 77 | 12.5 ± 4.8 | 27.0 ± 4.4 | 42.5 ± 5.7 | < 0.001 |
| Peak | 47 | 28.4 ± 11.8 | 40.0 ± 19.8 | 46.1 ± 6.7 | < 0.001 |
| Initial indirect bilirubin, mg/dL | 44 | 3.6 ± 1.9 | 8.8 ± 5.3 | 13.3 ± 6.1 | < 0.001 |
| Albumin, gm/dL | 33 | 2.8 ± 0.5 | 2.8 ± 0.9 | 3.3 ± 0.7 | 0.280 |
| ALT, U/L | 60 | 967 ± 1690 | 169 ± 159 | 966 ± 1484 | 0.228 |
| AST, U/L | 60 | 893 ± 1756 | 251 ± 260 | 611 ± 913 | 0.410 |
| ALP, U/L | 52 | 412 ± 357 | 457 ± 451 | 323 ± 186 | 0.892 |
| PT, seconds | 18 | 36 ± 33 | 16 ± 7 | 21 ± 24 | 0.429 |
| INR | 33 | 2.0 ± 1.1 | 2.1 ± 1.4 | 1.4 ± 0.5 | 0.286 |
| Kidney-related variables | |||||
| AKI | 77 | 23 (88.5) | 22 (84.6) | 23 (92) | 0.714 |
| Preexisting CKD | 74 | 3 (11.5) | 4 (15.4) | 2 (8) | 0.672 |
| Acute on CKD | 74 | 3 (11.5) | 4 (15.4) | 2 (8) | 0.672 |
| Creatinine, mg/dL | |||||
| Baseline | 24 | 1.2 ± 0.5 | 1.1 ± 0.4 | 1.0 ± 0.3 | 0.732 |
| Initial | 73 | 3.2 ± 3.2 | 4.4 ± 2.9 | 3.7 ± 2.7 | 0.136 |
| Peak | 47 | 6.5 ± 3.0 | 5.7 ± 2.1 | 4.9 ± 2.9 | 0.190 |
| End-of-follow-up | 48 | 2.7 ± 2.1 | 2.1 ± 1.6 | 1.6 ± 0.6 | 0.563 |
| Urinalysis findings | |||||
| Blood | 36 | 6 (40) | 4 (33.3) | 5 (55.6) | 0.584 |
| Protein | 39 | 5 (38.5) | 8 (57.1) | 9 (75.0) | 0.183 |
| Bilirubin | 26 | 8 (66.7) | 7 (70.0) | 2 (50.0) | 0.771 |
| Urobilinogen | 21 | 6 (60.0) | 2 (28.6) | 3 (75.0) | 0.267 |
| Urine sediment findings | 49 | 0.191 | |||
| Bilirubin/pigmented casts | 4 (26.7) | 8 (47.1) | 3 (17.6) | ||
| Granular casts | 1 (6.7) | 1 (5.9) | 5 (29.4) | ||
| Bilirubin and granular casts | 1 (6.7) | 0 (0.0) | 3 (17.6) | ||
| Other casts | 3 (20.0) | 2 (11.8) | 2 (11.8) | ||
| No casts | 6 (40.0) | 6 (35.3) | 4 (23.5) | ||
| Random urine sodium, mEq/L | 12 | 35 ± 26 | 48 ± 1 | 42 ± 15 | 0.603 |
| Fractional excretion of sodium, % | 4 | 0.9 ± 0.0 | 0.9 ± 1.2 | 2.7 ± 0.0 | 0.407 |
| Histopathological ascertainment | 77 | 0.250 | |||
| Kidney biopsy | 13 (50) | 18 (69.2) | 20 (80) | ||
| Kidney autopsy | 2 (7.7) | 1 (3.8) | 1 (4.0) | ||
| None | 11 (42.3) | 7 (26.9) | 4 (16) | ||
| Histopathological findings | 55 | 0.232 | |||
| Bile/bilirubin/green casts1 | 14 (93.3) | 13 (68.4) | 18 (85.7) | ||
| Other casts | 0 (0.0) | 5 (26.3) | 2 (9.5) | ||
| Absence of casts | 1 (6.7) | 1 (5.3) | 1 (4.8) | ||
| Sonographic urinary obstruction | 31 | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0.442 |
| Treatment modalities | |||||
| Corticosteroids | 77 | 2 (7.7) | 4 (15.4) | 4 (16) | 0.613 |
| Intravenous albumin | 25 | 1 (20) | 3 (23.1) | 1 (14.3) | 0.465 |
| Plasmapheresis | 25 | 2 (40.0) | 3 (23.1) | 4 (57.1) | 0.465 |
| Dialysis | 75 | 14 (56) | 14 (56) | 11 (44) | 0.618 |
| Liver dialysis | 25 | 1 (20) | 1 (7.7) | 1 (14.3) | 0.465 |
| Clinical outcomes | |||||
| Duration of follow-up, months | 54 | 3.1 ± 3.4 | 5.9 ± 8.3 | 3.2 ± 4.5 | 0.114 |
| Liver transplantation | 25 | 1 (20) | 4 (30.8) | 0 (0.0) | 0.465 |
| Liver and kidney transplantation | 77 | 1 (3.8) | 1 (3.8) | 0 (0.0) | 0.610 |
| Kidney function | 71 | 0.268 | |||
| Full recovery | 9 (36) | 8 (34.8) | 12 (52.2) | ||
| Partial recovery | 8 (32) | 10 (43.5) | 10 (43.5) | ||
| Dialysis dependence | 2 (8) | 3(13) | 1(4.3) | ||
| Death | 74 | 5 (20) | 2 (8) | 4 (16.7) | 0.469 |
We next evaluated the potential impact of alcoholic liver disease on outcomes of patients with BCN relative to other causes of liver disease (Supplementary Table 3). In brief, patients with BCN in the context of alcoholic liver disease had significantly higher initial serum creatinine compared to the setting of non-alcoholic liver disease (6.3 ± 4.2 mg/dL vs 3.5 ± 2.8 mg/dL, P = 0.011), as well as higher peak serum creatinine (9.5 ± 3.2 mg/dL vs 5.5 ± 2.7 mg/dL, P = 0.012). Moreover, mean INR was also significantly higher in the alcohol-related compared to the non- alcohol-related liver disease group (2.4 ± 0.4 vs 1.8 ± 1.2, P = 0.011). Finaly, there was a non-significant trend toward a higher incidence of the composite of dialysis requirement or death in the alcoholic compared to the non-alcoholic liver disease group (80% vs 52%, P = 0.098), suggesting a trend towards worse outcomes in patients with alcoholic liver disease. To address other potential sources of heterogeneity, we performed two additional analyses stratified by gender and by age tertiles. The relevant results are summarized in Supplementary Tables 4 and 5.
We next examined factors that are associated with the composite outcome of dialysis requirement or death to account for the competing risk. Candidate factors included the underlying liver disease, namely alcoholic liver disease, as well as the initial total bilirubin level and initial serum creatinine. Table 4 displays the results of univariate and multivariable logistic regression analyses. In brief, on univariate analysis, alcoholic liver disease was associated with higher odds for dialysis requirement or death, but this did not reach statistical significance (OR: 3.667, 95%CI: 0.726-18.526, P = 0.116). While higher initial serum total bilirubin was not associated with dialysis requirement or death (OR: 0.986, 95%CI: 0.939-1.037,
| Variable | Odds ratio | 95% confidence interval | P value |
| Univariate analyses | |||
| Alcoholic-liver disease, vs other | 3.667 | 0.726-18.526 | 0.116 |
| Initial serum creatinine, per 1 mg/dL, increase | 1.183 | 0.998-1.401 | 0.052 |
| Initial serum total bilirubin, per 1 mg/dL, increase | 0.986 | 0.939-1.037 | 0.589 |
| Multivariable analyses | |||
| Alcoholic liver disease, vs other | 5.062 | 0.553-46.310 | 0.151 |
| Initial serum creatinine, per 1 mg/dL, increase | 1.291 | 1.032-1.615 | 0.025 |
| Initial serum total bilirubin, per 1 mg/dL, increase | 0.968 | 0.930-1.008 | 0.112 |
In the present systematic review, we aimed to describe the clinical presentation, diagnostic and therapeutic approaches, and clinical outcomes of patients with BCN. Additionally, we sought to identify factors predictive of disease severity. We identified a total of 79 cases of presumed BCN derived from case reports and case series. A notable heterogeneity in patients’ demographics, clinical presentations, underlying liver disease, and outcomes was observed. Hyperbilirubinemia did not correlate with the severity of kidney injury, as indicated by serum creatinine elevation. Alcoholic liver disease was associated with more severe kidney injury and a trend towards worse BCN-related clinical outcomes compared to patients with non-alcoholic liver diseases. Finally, AKI severity, defined by higher initial serum creatinine, was associated with a higher likelihood of dialysis requirement or death.
To date, the pathogenesis of BCN remains poorly understood and under studied. A school of thought suggests bilirubin to be the culprit. This stems from the known oxidative stress exerted on tubular cells by excess bilirubin[85,94]. Additionally, bilirubin inhibits mitochondrial oxidative phosphorylation with subsequent decrease in adenosine triphosphate activity and impairment of cellular membrane permeability[5,94,95]. Hyperbilirubinemia is also thought to compromise renal perfusion by depressing cardiac output[96]. However, a direct link between bilirubin and the structural changes of BCN is lacking. In fact, numerous studies suggest a renoprotective effect of elevated bilirubin in various contexts, including pre-liver transplantation[5,97]. Further, in our systematic review, hyperbilirubinemia did not correlate with more severe kidney injury.
More recently, bile acids rose as the offending agent in BCN. In cholestasis, renal handling of bile acids switches from tubular reuptake of filtered bile acids to excretion of excess serum bile acids[8]. Increased renal handling of bile acids is thought to result in BCN[11]. Firstly, when urinary excretion of bile acids exceeds physiological capacity, it is hypo
Concerning the etiology of BCN, a wide spectrum of liver diseases has been implicated with the hallmark being marked hyperbilirubinemia[11]. This is true for acute, subacute, chronic, obstructive, and non-obstructive etiologies. Among non-obstructive etiologies, viral hepatitis, drug-induced liver injury, and acute alcoholic hepatitis stand as common precipitants[1,5,11]. Special attention is warranted for patients with decompensated cirrhosis or acute on chronic liver failure as post-mortem studies indicate a prevalence of BCN of up to 55%[90,99], and given the tendency to label these cases as hepatorenal syndrome. Alcoholic liver disease seems to be a more common player in these clinical settings[90,99], and, in our analysis, BCN in the context of alcoholic liver disease presented with a higher serum creatinine and yielded a higher absolute incidence of dialysis requirement or death.
Although there are no agreed-upon diagnostic criteria for BCN, kidney biopsy remains the gold standard test[11]. However, relying solely on kidney biopsy has notable shortcomings and potential negative sequelae. Firstly, in patients with suspected BCN, the associated liver injury and coagulopathy pose a significant bleeding risk during biopsy[92]. Additionally, the limited sensitivity of Hall’s stain in detecting intratubular bilirubin-containing casts - the diagnostic cornerstone of BCN - and the lack of established treatments for BCN create a scenario where the risks of kidney biopsy outweigh the benefits[5,88]. This has led to underdiagnosis and subsequent limited study of BCN in terms of diagnostic and therapeutic approaches. Identifying non-invasive diagnostic tests for BCN is therefore highly valuable. Although lacking, available evidence points towards limited utility of the urinary sediment in detecting bilirubin casts[11]. In our analysis, only 15 of 49 patients who had urinary sediment examination were found to have bilirubin casts. Urinary neutrophil gelatinase-associated lipocalin, an iron-transporting protein excreted in nephrotoxic or ischemic kidney injury, has been found to correlate with tubular epithelial damage and therapeutic response in mouse models of BCN[1]. Another biomarker of tubular injury, kidney injury molecule-1, is a transmembrane glycoprotein upregulated in proximal tubular cells following injury from various causes, including ischemia-reperfusion and sepsis. Although urinary and serum neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 have demonstrated utility in predicting AKI development and severity[100,101], their specific role in BCN is yet to be investigated in humans. Lastly, bile acids may serve as a valuable biomarker for AKI related to BCN, given their proposed role in mediating tubular injury[1,11]. In our review, serum bile acid levels were reported in only two cases, both of which were elevated. Given the limitations of kidney biopsy, further research into non-invasive diagnostic tools, particularly bile acids, is of paramount importance in BCN.
With more than half of patients with BCN failing to achieve full renal recovery, becoming dialysis dependent, or requiring simultaneous liver and kidney transplantation, our review highlights the seriousness of BCN. To date, there is no agreed upon marker to predict BCN outcomes. Our attempt to address this gap yielded serum creatinine as the only significant predictor of dialysis requirement or death. Thus, the degree of initial serum creatinine elevation may play a role in identifying patients who could benefit from BCN-specific treatments. Such treatments can be extracorporeal or intracorporeal with the common goal of resolving hyperbilirubinemia[11]. Of note, however, treatment of BCN has not been adequately studied in humans or widely accepted. A study on CBDL mice showed improved renal histology, serum creatinine, and blood urea nitrogen in mice fed with N-acetyl cysteine for 28 days compared to control animals[102]. Two other agents, norursodeoxycholic acid and high dose vitamin E, also showed a protective effect against BCN in CBDL mice[9,103]. As for humans, we found that seven (63.6%) of eleven presumed BCN cases treated with plasma exchange or plasmapheresis achieved full renal recovery. Finally, we encountered two cases that utilized extracorporeal albumin dialysis or “liver dialysis” with one achieving partial renal recovery and one requiring simultaneous liver-kidney tran
To our knowledge, this is the first and largest systematic review of reported BCN cases and case series. The main strength lies in describing BCN’s natural history, diagnosis, and response to experimental treatments, as well as iden
In conclusion, BCN is a serious yet underappreciated cause of AKI in patients with liver disease and resultant hyperbilirubinemia. The diagnosis of BCN still relies on kidney biopsy, despite its limitations, and serum creatinine at pre
| 1. | Somagutta MR, Jain MS, Pormento MKL, Pendyala SK, Bathula NR, Jarapala N, Mahadevaiah A, Sasidharan N, Gad MA, Mahmutaj G, Hange N. Bile Cast Nephropathy: A Comprehensive Review. Cureus. 2022;14:e23606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 2. | Krones E, Pollheimer MJ, Rosenkranz AR, Fickert P. Cholemic nephropathy - Historical notes and novel perspectives. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1356-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Haessler H, Rous P, Broun GO. The renal elimination of bilirubin. J Exp Med. 1922;35:533-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Elsom KA. Renal function in obstructive jaundice. Arch Intern Med. 1937;60:1028. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Fickert P, Rosenkranz AR. Cholemic Nephropathy Reloaded. Semin Liver Dis. 2020;40:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Kaler B, Morgan W, Bomzon A, Bach PH. The effects of Bile Acids on Freshly Isolated Rat Glomeruli and Proximal Tubular Fragments. Toxicol In Vitro. 1997;12:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Webster CR, Boria P, Usechak P, Anwer MS. S-adenosylmethionine and cAMP confer differential cytoprotection against bile acid-induced apoptosis in canine renal tubular cells and primary rat hepatocytes. Vet Ther. 2002;3:474-484. [PubMed] |
| 8. | Krones E, Wagner M, Eller K, Rosenkranz AR, Trauner M, Fickert P. Bile acid-induced cholemic nephropathy. Dig Dis. 2015;33:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Fickert P, Krones E, Pollheimer MJ, Thueringer A, Moustafa T, Silbert D, Halilbasic E, Yang M, Jaeschke H, Stokman G, Wells RG, Eller K, Rosenkranz AR, Eggertsen G, Wagner CA, Langner C, Denk H, Trauner M. Bile acids trigger cholemic nephropathy in common bile-duct-ligated mice. Hepatology. 2013;58:2056-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Tsai YL, Liu CW, Hsu CF, Huang CC, Lin MW, Huang SF, Li TH, Lee KC, Hsieh YC, Yang YY, Lee TY, Liu HM, Huang YH, Hou MC, Lin HC. Obeticholic acid ameliorates hepatorenal syndrome in ascitic cirrhotic rats by down-regulating the renal 8-iso-PGF2α-activated COX-TXA2 pathway. Clin Sci (Lond). 2020;134:2055-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Pinter K, Rosenkranz A. Cholemic Nephropathy: Role in Acute Kidney Injury in Cholestasis and Cirrhosis. Adv Kidney Dis Health. 2024;31:111-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D; CARE Group*. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Health Med. 2013;2:38-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 653] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 13. | Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF; PRISMA-IPD Development Group. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA. 2015;313:1657-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1377] [Cited by in RCA: 1562] [Article Influence: 156.2] [Reference Citation Analysis (0)] |
| 14. | Work Group Membership. Kidney Int Suppl (2011). 2012;2:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Yoshida EM, Karim MA, Shaikh JF, Soos JG, Erb SR. At what price, glory? Severe cholestasis and acute renal failure in an athlete abusing stanozolol. CMAJ. 1994;151:791-793. [PubMed] |
| 16. | Griffin MD, Grande JP, Wiesner RH, Velosa JA. Prolonged anuria complicating primary sclerosing cholangitis: successful outcome following orthotopic liver transplantation. Am J Kidney Dis. 1998;31:360-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Wolf M, Oneta CM, Jornod P, Seld D, Wauters JP, Blum AL, Delarive J. Cholestatic hepatitis A complicated by acute renal insufficiency. Z Gastroenterol. 2001;39:519-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Kiewe P, Korfel A, Loddenkemper C, Fischer L, Jahnke K, Notter M, Mühr-Wilkenshoff F, Stein H, Thiel E. Unusual sites of Hodgkin's lymphoma: CASE 3. Cholemic nephrosis in Hodgkin's lymphoma with liver involvement. J Clin Oncol. 2004;22:4230-4231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Hörl MP, Loddenkemper C, Korfel A, Tepel M. Biliary casts in the kidney tubule. Nephrol Dial Transplant. 2005;20:651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Bredewold OW, de Fijter JW, Rabelink T. A case of mononucleosis infectiosa presenting with cholemic nephrosis. NDT Plus. 2011;4:170-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Rafat C, Burbach M, Brochériou I, Zafrani L, Callard P, Rondeau E, Hertig A. Bilirubin-associated acute tubular necrosis in a kidney transplant recipient. Am J Kidney Dis. 2013;61:782-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Sciancalepore AG, Sallustio F, Girardo S, Passione LG, Camposeo A, Mele E, Di Lorenzo M, Costantino V, Schena FP, Pisignano D, Casino FG, Mostacci SD, Di Carlo M, Sabato A, Procida C, Creput C, Vanholder R, Stolear JC, Lefrancois G, Hanoy M, Nortier J, Potier J, Sereni L; For The Midemm Study Group. , Ferraresi M, Pereno A, Nazha M, Barbero S, Piccoli GB, Ficheux A, Gayrard N, Duranton F, Guzman C, Szwarc I, Bismuth -Mondolfo J, Brunet P, Servel MF, Argiles A, Bernardo A, Demers J, Hutchcraft A, Marbury TC, Minkus M, Muller M, Stallard R, Culleton B, Krieter DH, Korner T, Devine E, Ruth M, Jankowski J, Wanner C, Lemke H, Surace A, Rovatti P, Steckiph D, Mancini E, Santoro A, Leypoldt JK, Agar BU, Bernardo A, Culleton BF, Vankova S, Havlin J, Klomp DJ, Van Beijnum F, Day JPR, Wieringa FP, Kooman JP, Gremmels H, Hazenbrink DH, Simonis F, Otten ML, Wester M, Boer WH, Joles JA, Gerritsen KG, Umimoto K, Shimamoto Y, Mastushima K, Miyata M, Muller M, Naik A, Pokropinski S, Bairstow S, Svatek J, Young S, Johnson R, Bernardo A, Rikker C, Juhasz E, Gaspar R, Rosivall L, Rusu E, Zilisteanu D, Balanica S, Achim C, Atasie T, Carstea F, Voiculescu M, Monzon Vazquez T, Saiz Garcia S, Mathani V, Escamilla Cabrera B, Cornelis T, Van Der Sande FM, Eloot S, Cardinaels E, Bekers O, Damoiseaux J, Leunissen KM, Kooman J, Baamonde Laborda E, Bosch Benitez-parodi E, Perez Suarez G, Anton Perez G, Batista Garcia F, Lago Alonso M, Garcia Canton C, Hashimoto S, Seki M, Tomochika M, Yamamoto R, Okamoto N, Nishikawa A, Koike T, Ravagli E, Maldini L, Badiali F, Perazzini C, Lanciotti G, Steckiph D, Surace A, Rovatti P, Severi S, Rigotti A, Mcfarlane P, Marticorena R, Dacouris N, Pauly R, Nikitin S, Amdahl M, Bernardo A, Culleton B, Calabrese G, Mancuso D, Mazzotta A, Vagelli G, Balenzano C, Steckiph D, Bertucci A, Della Volpe M, Gonella M, Uchida T, Ando K, Kofuji M, Higuchi T, Momose N, Ito K, Ueda Y, Miyazawa H, Kaku Y, Nabata A, Hoshino T, Mori H, Yoshida I, Ookawara S, Tabei K, Umimoto K, Suyama M, Shimamoto Y, Miyata M, Kamada A, Sakai R, Minakawa A, Fukudome K, Hisanaga S, Ishihara T, Yamada K, Fukunaga S, Inagaki H, Tanaka C, Sato Y, Fujimoto S, Potier J, Bouet J, Queffeulou G, Bell R, Nolin L, Pichette V, Provencher H, Lamarche C, Nadeau-fredette A, Ouellet G, Leblanc M, Bezzaoucha S, Kouidmir Y, Kassis J, Alonso M, Lafrance J, Vallee M, Fils J, Mailley P, Cantaluppi V, Medica D, Quercia AD, Dellepiane S, Ferrario S, Gai M, Leonardi G, Guarena C, Caiazzo M, Biancone L, Enos M, Culleton B, Wiebenson D, Potier J, Hanoy M, Duquennoy S, Tingli W, Ling Z, Yunying S, Ping F, Dolley-hitze T, Hamel D, Lombart M, Leypoldt JK, Bernardo A, Hutchcraft AM, Vanholder R, Culleton BF, Movilli E, Camerini C, Gaggia P, Zubani R, Feller P, Pola A, Carli O, Salviani C, Manenti C, Cancarini G, Bozzoli L, Colombini E, Ricchiuti G, Pisanu G, Gargani L, Donadio C, Sidoti A, Lusini ML, Biagioli M, Ghezzi PM, Sereni L, Caiazzo M, Palladino G, Tomo T, Ishida K, Nakata T, Hamel D, Dolley-hitze T. Haemodialysis techniques and adequacy 1. Nephrology Dial Transplant. 2014;29:iii209-iii222. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Bile cast nephropathy: An often forgotten diagnosis. Am J Kidney Dis. 2014;63:B65. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | 2014 Annual Meeting of the North American Congress of Clinical Toxicology (NACCT). Clinical Toxicol. 2014;52:682-818. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Luciano RL, Castano E, Moeckel G, Perazella MA. Bile acid nephropathy in a bodybuilder abusing an anabolic androgenic steroid. Am J Kidney Dis. 2014;64:473-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Im DD, Essien U, DePasse JW, Chiappa V. Acute on chronic liver failure in a patient with sickle cell anaemia (HbSS). BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Jain K, Gupta A, Singh HK, Nickeleit V, Kshirsagar AV. Bile cast nephropathy. Kidney Int. 2015;87:484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Rodriguez AC, Clayton S, Brady P, Mamel J. Images of the Month: A Rare Cause of Overt Gastrointestinal Bleeding. Am J Gastroenterol. 2016;111:171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Clinical Vignettes/Case Reports - Biliary/Pancreas. Am J Gastroenterol. 2015;110 Suppl 1:S40-S550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Ryan M, Lazar I, Nadasdy GM, Nadasdy T, Satoskar AA. Acute kidney injury and hyperbilirubinemia in a young male after ingestion of Tribulus terrestris. Clin Nephrol. 2015;83:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Sequeira A, Gu X. Bile cast nephropathy: an often forgotten diagnosis. Hemodial Int. 2015;19:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Oral Free Paper Sessions. Virchows Arch. 2016;469 Suppl 1:1-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Alkhunaizi AM, ElTigani MA, Rabah RS, Nasr SH. Acute bile nephropathy secondary to anabolic steroids. Clin Nephrol. 2016;85:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Alnasrallah B, Collins JF, Zwi LJ. Bile Nephropathy in Flucloxacillin-Induced Cholestatic Liver Dysfunction. Case Rep Nephrol. 2016;2016:4162674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Flores A, Nustas R, Nguyen HL, Rahimi RS. Severe Cholestasis and Bile Acid Nephropathy From Anabolic Steroids Successfully Treated With Plasmapheresis. ACG Case Rep J. 2016;3:133-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Hoshino T, Takagi H, Suzuki Y, Naganuma A, Sato K, Kakizaki S, Nishizawa T, Okamoto H, Yamada M. Fatal fulminant hepatitis caused by infection with subgenotype A1 hepatitis B virus with C1766T/T1768A core promoter mutations. Clin J Gastroenterol. 2016;9:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Castrale C, Azar R, Piquet MA, Lobbedez T. [The specific nutritionnal care in peritoneal dialysis]. Nephrol Ther. 2016;12:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Leclerc M, Lanot A, Béchade C, Le Naoures C, Comoz F, Lobbedez T. [Bile salt nephropathy/cholemic nephrosis]. Nephrol Ther. 2016;12:460-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Clinical Vignettes/Case Reports - Biliary/Pancreas. Am J Gastroenterol. 2016;111:S501-S592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Patel J, Walayat S, Kalva N, Palmer-Hill S, Dhillon S. Bile cast nephropathy: A case report and review of the literature. World J Gastroenterol. 2016;22:6328-6334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Sens F, Bacchetta J, Rabeyrin M, Juillard L. Efficacy of extracorporeal albumin dialysis for acute kidney injury due to cholestatic jaundice nephrotoxicity. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Aniort J, Poyet A, Kemeny JL, Philipponnet C, Heng AE. Bile Cast Nephropathy Caused by Obstructive Cholestasis. Am J Kidney Dis. 2017;69:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | El Khoury C, Sabbouh T, Farhat H, Ferzli A. Severe Cholestasis and Bile Cast Nephropathy Induced by Anabolic Steroids Successfully Treated with Plasma Exchange. Case Rep Med. 2017;2017:4296474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Jung JH. Bile Cast Nephropathy Associated with Acute Hepatitis A. Chonnam Med J. 2017;53:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Novel Catin E, Durupt S, de Laforcade L, Lecoq M, Durieu I, Reynaud Q. Acute kidney injury, a rare complication of acute hepatitis E infection. Med Mal Infect. 2017;47:502-503. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 46. | Woldemichael JA, Rogers TE, Johnson SA. School of Medicine, Atlanta, GA.A Case of Granulomatous Interstitial Nephritis Likely Associated with Lisinopril. [cited 15 December 2024]. Available from: https://regroup-production.s3.amazonaws.com/documents/ReviewReference/864284361/Pages%20from%20KW18Abstracts.pdf?response-content-type=application%2Fpdf&X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Credential=AKIAYSFKCAWYQ4D5IUHG%2F20250323%2Fus-east-1%2Fs3%2Faws4_request&X-Amz-Date=20250323T203507Z&X-Amz-Expires=604800&X-Amz-SignedHeaders=host&X-Amz-Signature=cc0e6d8d5433a868eb6abfb9f20c68a72f4fc7b6dde29fa20f73555e1ecb700f. |
| 47. | Fisler A, Breidthardt T, Schmidlin N, Hopfer H, Dickenmann M, König K, Hirt-Minkowski P. Bile Cast Nephropathy: The Unknown Dangers of Online Shopping. Case Rep Nephrol Dial. 2018;8:98-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Kritmetapak K, Sathidatekoonchorn T, Papanrueng W. Bile cast nephropathy in a patient with cholangiocarcinoma - a case report. Clin Case Rep. 2018;6:779-783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Milla Castellanos M, Gutiérrez Martínez E, Sevillano Prieto Á, Rodríguez Ramos P, Praga Terente M. Bile cast nephropathy associated with severe liver dysfunction caused by anabolic steroids. Nefrología (English Edition). 2018;38:221-223. [DOI] [Full Text] |
| 50. | Nayak S, Sharma M, Kataria A, Tiwari SC, Rastogi A, Mukund A. Cholemic Nephrosis from Acute Hepatitis E Virus Infection: A Forgotten Entity? Indian J Nephrol. 2018;28:250-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Ravi R, Suthar K, Murlidharan P, Lakshmi K, Balan S, Safeer M. Bile cast nephropathy causing acute kidney injury in a patient with nonfulminant acute hepatitis A. Saudi J Kidney Dis Transpl. 2018;29:1498-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Torrealba J, Sweed NT, Burguete D, Hendricks AR. Bile Cast Nephropathy: A Pathologic Finding with Manifold Causes Displayed in an Adult with Alcoholic Steatohepatitis and in a Child with Wilson's Disease. Case Rep Nephrol Dial. 2018;8:207-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | van de Ven SE, Pavlov KV, Beutler JJ, Scheffer RC. Bile Cast Nephropathy Caused by Obstructive Pancreatic Carcinoma and Failed ERCP. ACG Case Rep J. 2018;5:e88. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 54. | Chan S, Spraggon ES, Francis L, Wolley MJ. Bile Cast Nephropathy in a Patient With Obstructive Jaundice. Kidney Int Rep. 2019;4:338-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Mukherjee T, Khan ID, Guha R, Ganguly T. Cholemic nephrosis (bile cast nephropathy) with severe liver dysfunction. Med J Armed Forces India. 2019;75:216-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Ocon AJ, Rosenblum M, Desemone J, Blinkhorn R. Severe cholestatic hyperbilirubinaemia secondary to thyrotoxicosis complicated with bile cast nephropathy treated with plasma exchange and haemodialysis. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Akl A, Oweil A, Al-shawbaki R, Bazarah S, Hammad N, Al-khatib N. Sun-055 successful management of acute kidney injury secondary to ascending cholangitis and bile cast nephropathy: case report and review of literature. Kidney Int Rep. 2020;5:S226-S227. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 58. | Chango Azanza JJ, Lopetegui Lia N, Calle Sarmiento PM. Bile Cast Nephropathy Secondary to Hemophagocytic Lymphohistiocytosis With Liver Failure. Cureus. 2020;12:e10226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 59. | Giuliani KTK, Kassianos AJ, Kildey K, Grivei A, Wang X, Ungerer J, Francis L, Healy H, Gois PFH. Role of inflammation and inflammasome activation in human bile cast nephropathy. Nephrology (Carlton). 2020;25:502-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Jamshaid MB, Iqbal P, Shahzad A, Yousaf Z, Mohamedali M. Acute Renal Failure Due to Bile Cast Nephropathy: An Overlooked Cause of Kidney Injury. Cureus. 2020;12:e9724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | 247 Severe Acute Kidney Injury with Hepatitis A Infection – A Double Hit of Cholemic Nephrosis and Heme Pigment Nephropathy. Am J Kidney Dis. 2020;75:607. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 62. | Mrzljak A, Jurekovic Z, Novak R, Maksimovic B, Mikulic D, Ljubanovic DG. Liver Graft Failure and Bile Cast Nephropathy. Korean J Gastroenterol. 2020;75:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Nguyen C, Baliss M, Sonstein L. S1419 Bile Cast Nephropathy From Acute Liver Failure Due to Unintentional Acetaminophen Overdose. Am J Gastroenterol. 2020;115:S694-S694. [DOI] [Full Text] |
| 64. | Al Awadhi H, Al Qassimi S, Akhras A, Herlitz L, Ghosn M. Bile acid nephropathy induced by anabolic steroids: A case report and review of the literature. Clin Nephrol Case Stud. 2021;9:123-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | 142 Therapeutic Plasma Exchange as Treatment for Bilirubin Cast Nephropathy. Am J Kidney Dis. 2021;77:611. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 66. | Abstracts of the 20th Biennial European Society for Organ Transplantation (ESOT) Congress, Milan, Italy, 29 August - 1 September 2021. Transpl Int. 2021;34 Suppl 1:5-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 67. | Yusuf F, Weissman S, Qureshi N, Ibrahim M, Kurtz D, Manandhar L, Sciarra M, Elias S. Bile Cast Nephropathy an Important Biliary Culprit of Kidney Injury. J Community Hosp Intern Med Perspect. 2021;11:253-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Golloshi K, Skaf DA, Neurgaonkar S. S1818 Rare Sequelae of Obstructive Jaundice: Hyperferritinemia and Bile Cast Nephropathy. Am J Gastroenterol. 2022;117:e1273-e1274. [DOI] [Full Text] |
| 69. | Huang G, Lee W, El-hennawy AS, Frolova E. Clinically Diagnosed Bile Cast Nephropathy in a Patient With Severe Alcoholic Hepatitis and COVID-19 Pneumonia. J Am Soc Nephrol. 2022;33:902-902. [DOI] [Full Text] |
| 70. | Yaseen W, Zipursky JS, Auguste BL. Severe AKI in a Patient With G6PD Deficiency and Acute Hepatitis A Infection. J Am Soc Nephrol. 2022;33:902-903. [DOI] [Full Text] |
| 71. | 304 A Case of Green Kidney: An Underreported Story. Am J Kidney Dis. 2022;79:S93. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 72. | Ahmed K, Jaber F, Pappoppula L, Mohammed E, Aloysius MM. Bile Cast Nephropathy Due to Hepatitis A-induced Hyperbilirubinemia: A Case Report and Literature Review. Cureus. 2023;15:e35779. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 73. | Annavarajula SK, Tandra VR, Ranga SK, Vennavalli S. Bile Cast Nephropathy, An Often-Missed Diagnosis. Indian J Nephrol. 2023;33:315-316. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 74. | Arai M, Moriyama T, Iemura F, Suzuki R, Miyaoka Y, Kanno Y, Tokyo Medical University Hospital. . A Case of Bile Cast Nephropathy Treated with Plasma Exchange Therapy for AKI Associated with Acute Hepatitis A. J Am Soc Nephrol. 2023;34:105-105. [DOI] [Full Text] |
| 75. | Arayangkool C, Gozun M, Tanariyakul M, Techasatian W, Leesutipornchai T, Nishimura Y. Bile Cast Nephropathy Because of Acute Liver Injury Associated With Selective Androgen Receptor Modulators. ACG Case Rep J. 2023;10:e01105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 76. | 69 Prolonged Bile Cast Nephropathy Following Orthotopic Liver Transplant, a Diagnostic and Management Challenge. Am J Kidney Dis. 2023;81:S20. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 77. | Issac AG, Yu MA, Rogers DM, Subramanian RM. Case Report: Efficacy of albumin dialysis for the reversal of bile cast nephropathy-induced acute kidney injury. Front Nephrol. 2023;3:1256672. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 78. | Kliea M, Alsultan M, Basha K. Antinuclear antibodies positive acute nonfulminant hepatitis A associated with acute renal failure and hives: a case report. Ann Med Surg (Lond). 2023;85:1073-1077. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 79. | Me HM, Budhiraja P, Nair S, Kodali L, Ryan M, Khamash H, Heilman R, Wagler J, Ruch B, Jadlowiec CC, Moss A, Reddy KS. Utilizing kidneys from a donor with bile-cast nephropathy. Am J Transplant. 2024;24:141-144. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 80. | Mizuno F, Imai N, Yasuda K, Yokoyama S, Yamamoto K, Ito T, Ishizu Y, Honda T, Ishigami M, Kawashima H. Successful Treatment with Steroids in a Patient with Vanishing Bile Duct Syndrome and Acute Tubular Necrosis. Intern Med. 2024;63:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 81. | Samant SM, Cortesi C. Bile Cast Nephropathy: A Diagnostic Odyssey Beyond Hepatorenal Syndrome. J Am Soc Nephrol. 2023;34:105-105. [DOI] [Full Text] |
| 82. | Betjes MG, Bajema I. The pathology of jaundice-related renal insufficiency: cholemic nephrosis revisited. J Nephrol. 2006;19:229-233. [PubMed] |
| 83. | 2013 Annual Meeting of the North American Congress of Clinical Toxicology (NACCT). Clinical Toxicol. 2013;51:575-724. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Tabatabaee SM, Elahi R, Savaj S. Bile cast nephropathy due to cholestatic jaundice after using stanozolol in 2 amateur bodybuilders. Iran J Kidney Dis. 2015;9:331-334. [PubMed] |
| 85. | Pitlick M, Rastogi P. All That Glitters Yellow Is Not Gold: Presentation and Pathophysiology of Bile Cast Nephropathy. Int J Surg Pathol. 2017;25:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Zhao X, Huang R, Wong P, Fiset PO, Deschênes M. Renal tubular injury in hyperbilirubinemia: Bile cast nephropathy. Can Liver J. 2021;4:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 87. | Mohamed A, Peniston M, Mahmood R. Acute Kidney Injury in Patients Admitted to the Intensive Care Unit: A Case Report. Cureus. 2023;15:e40380. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 88. | van Slambrouck CM, Salem F, Meehan SM, Chang A. Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney Int. 2013;84:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 89. | Mohapatra MK, Behera AK, Karua PC, Bariha PK, Rath A, Aggrawal KC, Nahak SR, Gudaganatti SS. Urinary bile casts in bile cast nephropathy secondary to severe falciparum malaria. Clin Kidney J. 2016;9:644-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 90. | Nayak SL, Kumar M, Bihari C, Rastogi A. Bile Cast Nephropathy in Patients with Acute Kidney Injury Due to Hepatorenal Syndrome: A Postmortem Kidney Biopsy Study. J Clin Transl Hepatol. 2017;5:92-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 91. | Agrawal P, Kumar V, Kumar A, Sachdeva MUS, Malhotra P, Nada R. Monoclonal Gammopathy of Renal Significance Triggered by Viral E Hepatitis. Indian J Nephrol. 2019;29:50-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 92. | Bräsen JH, Mederacke YS, Schmitz J, Diahovets K, Khalifa A, Hartleben B, Person F, Wiech T, Steenbergen E, Großhennig A, Manns MP, Schmitt R, Mederacke I. Cholemic Nephropathy Causes Acute Kidney Injury and Is Accompanied by Loss of Aquaporin 2 in Collecting Ducts. Hepatology. 2019;69:2107-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 93. | Priyaa V, Srinivas BH, Gochhait D, Ganesh RN, Badhe BA, Priyamvada PS, Amalnath D, DAS S, Shaha KK. Cholemic Nephrosis: An Autopsy Study of a Forgotten Entity. Turk Patoloji Derg. 2021;37:212-218. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 94. | Elías MM, Comin EJ, Grosman ME, Galeazzi SA, Rodríguez Garay EA. Possible mechanism of unconjugated bilirubin toxicity on renal tissue. Comp Biochem Physiol A Comp Physiol. 1987;87:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 95. | El Chediak A, Janom K, Koubar SH. Bile cast nephropathy: when the kidneys turn yellow. Ren Replace Ther. 2020;6:15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 96. | Padillo J, Puente J, Gómez M, Dios F, Naranjo A, Vallejo JA, Miño G, Pera C, Sitges-Serra A. Improved cardiac function in patients with obstructive jaundice after internal biliary drainage: hemodynamic and hormonal assessment. Ann Surg. 2001;234:652-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 97. | Deetman PE, Zelle DM, Homan van der Heide JJ, Navis GJ, Gans RO, Bakker SJ. Plasma bilirubin and late graft failure in renal transplant recipients. Transpl Int. 2012;25:876-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 98. | Kaler B, Karram T, Morgan WA, Bach PH, Yousef IM, Bomzon A. Are bile acids involved in the renal dysfunction of obstructive jaundice? An experimental study in bile duct ligated rats. Ren Fail. 2004;26:507-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 99. | Foshat M, Ruff HM, Fischer WG, Beach RE, Fowler MR, Ju H, Aronson JF, Afrouzian M. Bile Cast Nephropathy in Cirrhotic Patients: Effects of Chronic Hyperbilirubinemia. Am J Clin Pathol. 2017;147:525-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Zhang CF, Wang HJ, Tong ZH, Zhang C, Wang YS, Yang HQ, Gao RY, Shi HZ. The diagnostic and prognostic values of serum and urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin in sepsis induced acute renal injury patients. Eur Rev Med Pharmacol Sci. 2020;24:5604-5617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 101. | Schrezenmeier EV, Barasch J, Budde K, Westhoff T, Schmidt-Ott KM. Biomarkers in acute kidney injury - pathophysiological basis and clinical performance. Acta Physiol (Oxf). 2017;219:554-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 251] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 102. | Abdoli N, Sadeghian I, Mousavi K, Azarpira N, Ommati MM, Heidari R. Suppression of cirrhosis-related renal injury by N-acetyl cysteine. Curr Res Pharmacol Drug Discov. 2020;1:30-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 103. | Ortiz MC, Manriquez MC, Nath KA, Lager DJ, Romero JC, Juncos LA. Vitamin E prevents renal dysfunction induced by experimental chronic bile duct ligation. Kidney Int. 2003;64:950-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |