Published online Mar 27, 2025. doi: 10.4254/wjh.v17.i3.97767
Revised: November 24, 2024
Accepted: February 24, 2025
Published online: March 27, 2025
Processing time: 291 Days and 12.5 Hours
The portal vein thrombosis (PVT) can exacerbate portal hypertension and lead to complications, increasing the risk of mortality.
To evaluate the predictive capacity of artificial neural networks (ANNs) in quan
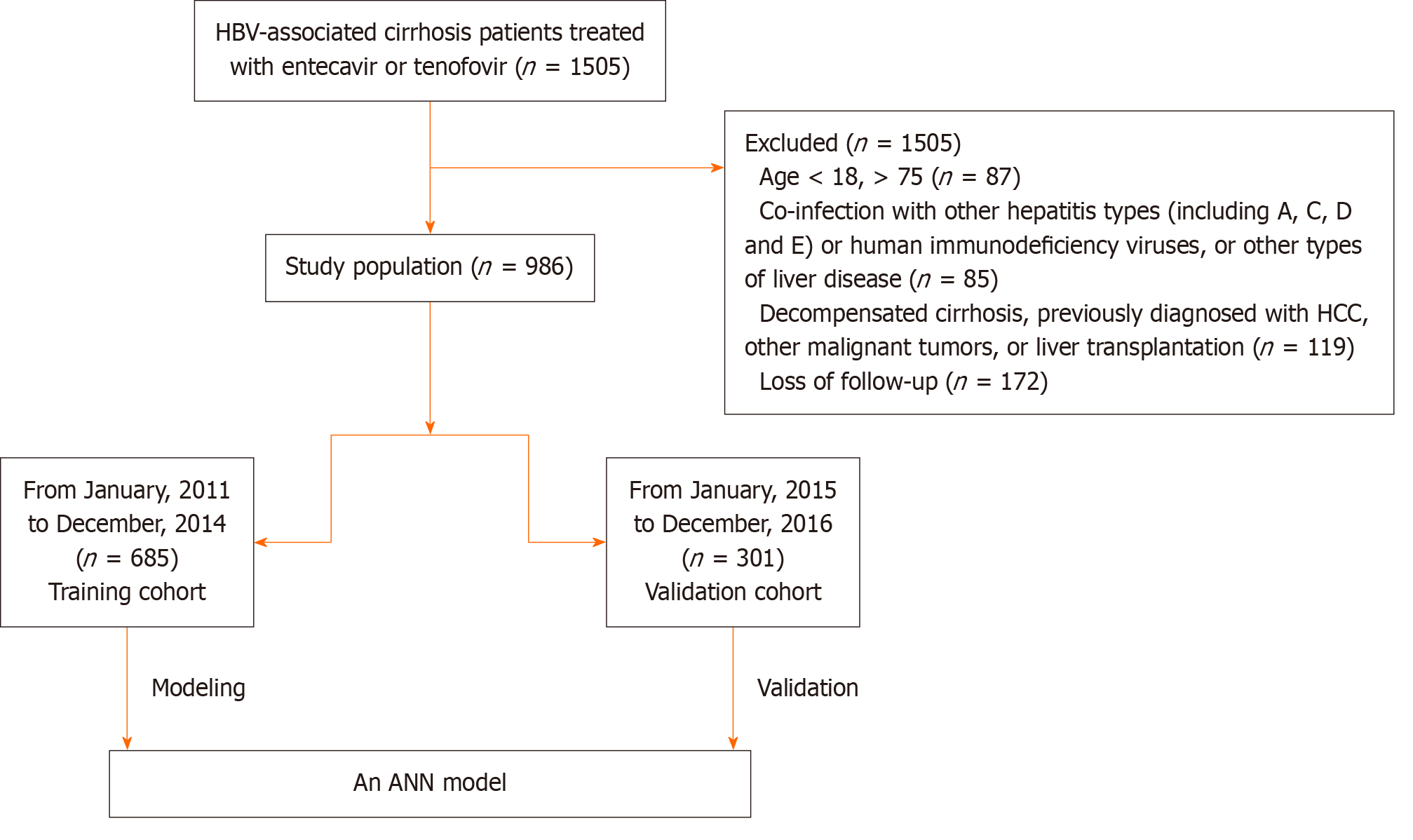
A retrospective study was conducted at Beijing Ditan Hospital, affiliated with Capital Medical University, including 986 hospitalized patients. Patients admitted between January 2011 and December 2014 were assigned to the training set (685 cases), while those hospitalized from January 2015 to December 2016 were divided into the validation cohort (301 cases). Independent risk factors for PVT were identified using COX univariate analysis and used to construct an ANN model. Model performance was evaluated through metrics such as the area under the receiver operating characteristic curve (AUC) and concordance index.
In the training set, PVT occurred in 19.0% of patients within three years and 23.7% within five years. In the validation cohort, PVT developed in 16.7% of patients within three years and 24.0% within five years. The ANN model incorporated nine independent risk factors: Age, ascites, hepatic encephalopathy, gastrointestinal varices with bleeding, Child-Pugh classification, alanine aminotransferase levels, albumin levels, neutrophil-to-lymphocyte ratio, and platelet. The model achieved an AUC of 0.967 (95%CI: 0.960–0.974) at three years and 0.975 (95%CI: 0.955–0.992) at five years, significantly outperforming existing models such as model for end-stage liver disease and Child-Pugh-Turcotte (all P < 0.001).
The ANN model demonstrated effective stratification of patients into high- and low-risk groups for PVT deve
Core Tip: An artificial neural network was developed to predict portal vein thrombosis (PVT) risk in hepatitis B-induced cirrhosis. The model outperformed existing scoring systems in predicting PVT incidence at three and five years intervals. Decision curve analysis and calibration curves highlighted superior clinical utility and benefits.
- Citation: Meng PP, Xiong FX, Chen JL, Zhou Y, Liu XL, Ji XM, Jiang YY, Hou YX. Establish and validate an artificial neural networks model used for predicting portal vein thrombosis risk in hepatitis B-related cirrhosis patients. World J Hepatol 2025; 17(3): 97767
- URL: https://www.wjgnet.com/1948-5182/full/v17/i3/97767.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i3.97767
Chronic hepatitis B virus infection remains a significant global public health issue and is the leading cause of liver cirrhosis in the Chinese population[1]. Among patients with cirrhosis, the risk of developing portal vein thrombosis (PVT) reaches approximately 40%, contributing to increased healthcare costs and reduced life expectancy[2]. PVT involves thrombosis in the main portal vein and its branches, causing partial or complete vascular obstruction. Traditionally, cirrhosis has been associated with clotting factor synthesis disorders and thrombocytopenia, predisposing patients to bleeding. However, recent studies indicate that the anticoagulant and procoagulant systems in cirrhotic livers are highly dynamic and unstable. This imbalance, combined with blood flow stagnation in the portal vein and endothelial dysfunction, increases the risk of bleeding and thrombosis. Non-tumoral PVT is particularly prevalent among patients with cirrhosis, with an annual incidence estimated between 4.6% and 26%[2,3], and occurs more frequently in advanced stages of liver disease. In hepatitis B-related cirrhosis, PVT is a common complication, further aggravating conditions such as refractory ascites and upper gastrointestinal bleeding by reducing hepatic blood flow and increasing portal pressure. Early detection and management of PVT remain challenging due to the absence of specific clinical symptoms in its initial stages.
Most studies on PVT rely on retrospective analyses of influencing factors, and no reliable model currently exists for predicting its occurrence at an early stage. As a result, identifying high-risk individuals for early prevention is chal
Machine learning (ML) is increasingly utilized in liver disease research. Some studies have applied ML methods to predict PVT occurrence in patients with cirrhosis and those undergoing splenectomy and cardia devascularization surgeries[17,18]. However, these studies had limitations, such as a relatively small number of baseline indicators, insufficient cohort size, and limited population. Artificial neural networks (ANNs), a form of ML, simulate the infor
A total of 1505 patients diagnosed with hepatitis B cirrhosis (HBC) were retrospectively enrolled at the Beijing Ditan Hospital of Capital Medical University in Beijing, China, from January 2011 to December 2016. Eligibility criteria required patients to have a first-time diagnosis of HBC. Inclusion criteria included: (1) Age between 18 and 75 years; (2) Diagnosis consistent with chronic hepatitis B, as defined by Asian Pacific Association for the Study of the Liver guidelines[24], with treatment involving entecavir or tenofovir; (3) Absence of prior hepatocellular carcinoma (HCC) or organ transplantation; and (4) No history of decompensated cirrhosis. Exclusion criteria were: (1) Age below 18 or above 75 years; (2) Co-infection with other hepatitis types (A, C, D, or E), human immunodeficiency virus, or other liver diseases such as autoimmune hepatitis, alcoholic hepatitis, drug-induced liver disease, fatty liver disease, idiopathic noncirrhotic portal hypertension, or genetic metabolic liver diseases; (3) Prior decompensated cirrhosis, HCC, other malignant tumors, or liver transplantation; (4) Development of HCC within the first six months of follow-up; and (5) Loss to follow-up within five years. To ensure a representative sample, 685 patients from January 2011 to December 2014 were assigned to the training cohort, and 301 patients from January 2015 to December 2016 were assigned to the validation cohort (Figure 1). This study received ethical approval from the Ethics Committee of Beijing Ditan Hospital.
Chronic hepatitis B was defined as the presence of hepatitis B surface antigen for more than six months. Compensatory cirrhosis was diagnosed using the following criteria: (1) Pathological findings of F4 stage cirrhosis on biopsy; (2) Detection of esophageal varices during endoscopy, with noncirrhotic portal hypertension excluded; and (3) In the absence of histological and endoscopic evidence, at least two of the following three criteria: (1) Imaging findings [ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI)] showing liver morphology changes such as nodules and an irregular surface; (2) PLT below 100 × 109 cells/L without other identifiable causes; and (3) Serum albumin (ALB) < 35.0 g/L, international normalized ratio (INR) > 1.3, or prothrombin time prolonged by more than three seconds. Decompensated cirrhosis was diagnosed based on the presence of cirrhosis accompanied by complications related to portal hypertension and/or liver dysfunction. The diagnostic criteria included: (1) Evidence of cirrhosis; and (2) Complications such as ascites, bleeding from esophageal or gastric varices, or hepatic encephalopathy (HE)[25]. PVT is diagnosed through a meticulous process that involves the detection of non-malignant thrombosis within the portal vein or its tributaries. The diagnostic procedure relies on the utilization of advanced imaging techniques, specifically CT Angiography or MRI, which provide high-resolution cross-sectional views of the venous system. PVT was classified as occlusive if blood flow was completely absent in the vein and partial if the vein's lumen was partially occluded with residual blood flow.
The starting point of this research was the initial cirrhosis diagnosis at the hospital. The conclusion of this study focused on the recent identification of PVT within the first year and subsequent follow-up over five years. Clinical data collection included several categories: Demographics (age and gender), complications (gastrointestinal variceal bleeding, ascites, and HE, and biochemical indicators such as alanine aminotransferase (ALT), aspartate aminotransferase, total bilirubin, ALB, gamma-glutamyl transpeptidase, white blood cell count, neutrophil count, lymphocyte count, PLT, creatinine, prothrombin time, INR, and alpha-fetoprotein. Additional parameters included hepatitis B e-antigen status, HBV-DNA levels, and routine laboratory assessments. Radiological evaluations, including CT and MRI, were conducted every 3–6 months.
An ANN is a sophisticated computational system composed of a modular structure, featuring interconnected neuronal units. This architecture organizes units into three fundamental compartments: An input layer, an output layer, and one or more hidden layers[26,27]. These neurons are linked through weighted connections, which are adjusted during the learning process. ANNs offer advantages such as self-learning, self-adaptation, and inference capabilities. The learning process involves analyzing examples and adjusting connection weights to establish relationships between inputs and outputs. Input data flows through the network layers, producing an output. An error signal is generated if the output differs from the desired result. The backpropagation (BP) method uses this error to adjust connection weights to minimize overall network error. Through iterative adjustments, the error between predicted and desired outputs gradually decreases until reaching a minimum, indicating convergence of the network. After convergence, the ANN applies its trained knowledge to new input data, generating accurate predictions or outputs across various datasets[28-30].
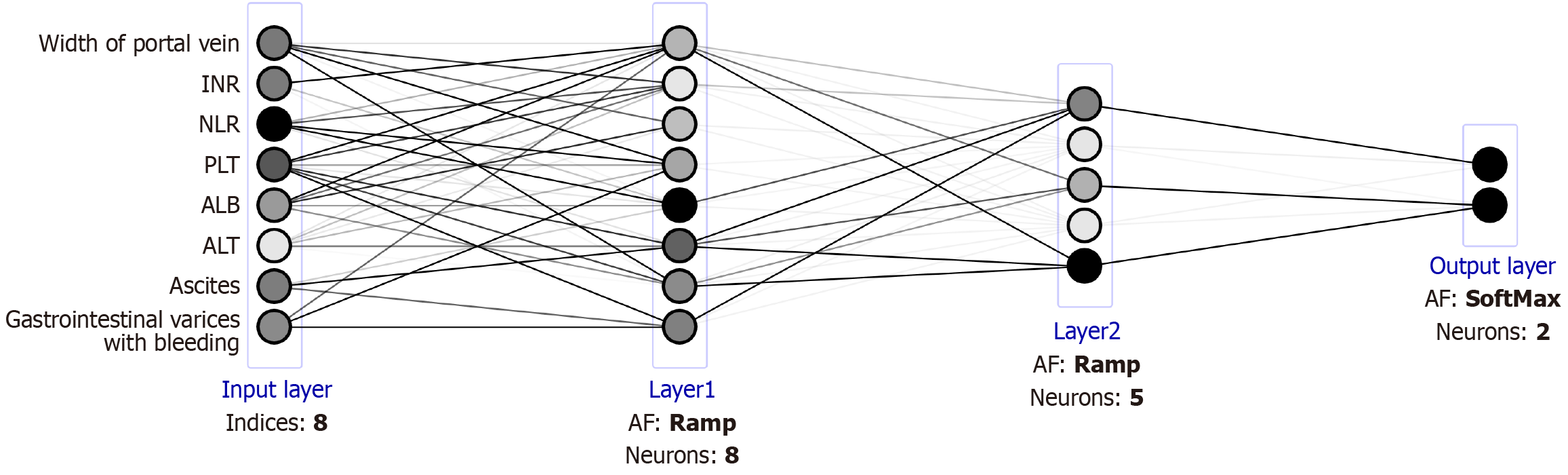
In this study, the 5-year progression of PVT in HBC was analyzed using an ANN model. The input layer comprised 637 neurons that imported clinical, demographic, and laboratory data. The output layer comprised neurons that generated the corresponding predictive results. Hidden layers facilitated complex interactions between the input and output neurons. A novel ANN was meticulously designed to unravel the pivotal predictors of PVT in cirrhosis. Mathematica 11.1.1 for Microsoft Windows (64-bit), a powerful software platform designed specifically for the efficient and interactive construction of neural networks. The BP algorithm guided the learning process by calculating errors between predicted and desired outputs. Neuron connections were adjusted by modifying weights to minimize overall network errors. Learning was terminated when the sum of squared errors reached a minimum against the cross-validation dataset. The final model provided individualized PVT risk predictions over the years for each patient.
Quantitative variables that follow a normal distribution were expressed as the mean and SD, while those not following a normal distribution were presented as quartiles. As appropriate, continuous data were compared using either the Student's t-test (for normally distributed data) or the non-parametric Mann-Whitney U test (for non-normal distributions). Categorical variables were analyzed using either the χ2-distribution goodness-of-fit test (χ2-d) for ample sample sizes or Fisher's exact test. Variables showing statistically significant differences or clinical relevance were selected as input layers for constructing ANN models to predict PVT development over 3 and 5 years. Hazard ratios (HR) with 95%CI and P-values were reported. Discriminative prowess of models was systematically assessed through the computation of receiver operating characteristic (ROC) curves, which visually portrayed the trade-off between true positive and false positive rates. The diagnostic accuracy was quantified by calculating the area under the ROC curve (AUC), commonly referred to as Harrell's c-index, serving as a robust measure of predictive discriminatory power. The performance of the ANN model was compared to the MELD score using ROC curves[31,32]. MELD scores were cal
Between 2008 and 2016, a study involving 986 patients was conducted. A total of 685 patients admitted from January 2011 to December 2014 were assigned to the training cohort, while 301 patients admitted from January 2015 to December 2016 were assigned to the validation cohort. In the training cohort, the majority of participants were male (439, 69.0%), with a median age of 52.8 years (range: 40.0–74.0). In the validation cohort, 62.5% were male, with a median age of 52.9 years (43.0–75.0). No significant differences in baseline characteristics were observed between the two cohorts (Table 1). Within three years, 121 patients (19.0%) in the training group developed PVT, increasing to 151 patients (23.7%) within five years. In the validation group, 46 patients (16.7%) developed PVT within three years, and 66 patients (24.0%) within five years. The characteristics of the two cohorts were comparable, as detailed in Table 1.
| Variables | All patients (n = 912) | Training cohort (n = 637) | Validation cohort (n = 275) | P value |
| Age, years | 53.0 (41.0-73.0) | 52.8.0 (40.0-74.0) | 52.9 (43.0-75.0) | 0.436 |
| Male sex | 611 (67.0) | 439 (69.0) | 172 (62.5) | 0.320 |
| Smoking | 210 (23.0) | 149 (23.4) | 61 (22.2) | 0.846 |
| Alcohol consumption | 195(21.4) | 123 (19.3) | 62 (22.5) | 0.579 |
| Diabetes | 152 (16.7) | 103 (16.2) | 49 (17.8) | 0.174 |
| Hypertension | 129 (14.1) | 95 (14.9) | 34 (12.4) | 0.835 |
| Ascites | 197 (21.6) | 134 (21.0) | 63 (22.9) | 0.485 |
| Encephalopathy | 41 (4.5) | 30 (4.7) | 11 (4.0) | 0.458 |
| Gastrointestinal varices with bleeding | 134 (14.7) | 88 (13.8) | 46 (16.7) | 0.348 |
| HBeAg positivity | 365 (40.0) | 253 (39.9) | 112 (40.7) | 0.115 |
| CTP score | 7.0 (5.0-10.0) | 7.0 (6.0-9.0) | 7.0 (5.0-10.0) | 0.626 |
| MELD score | 10.1 (7.8-12.9) | 10.1 (8.0-12.6) | 10.1 (7.7-13.6) | 0.851 |
| Alanine aminotransferase (U/L) | 41.3 (26.5-105.8) | 43.6 (27.1-112.4) | 37.5 (25.1-95.5) | 0.164 |
| Aspartate aminotransferase (U/L) | 47.9 (30.8-106.1) | 48.6 (32.1-107.9) | 46.7 (29.7-98.1) | 0.282 |
| Total bilirubin (μmol/L) | 22.4 (14.2-39.2) | 22.7 (14.1-38.5) | 22.3 (14.5-40.7) | 0.805 |
| Albumin (g/L) | 33.6 (30.3-39.2) | 33.8 (30.4-40.1) | 33.2 (30.1-38.4) | 0.258 |
| Gamma-glutamyl transpeptidase (U/L) | 56.3 (37.2-97.8) | 55.3 (36.1-99.7) | 57.3 (40.4-95.6) | 0.497 |
| White blood cell count (× 109/L) | 3.9 (2.8-5.3) | 3.9 (2.8-5.3) | 3.8 (2.7-5.3) | 0.451 |
| Neutrophil count (× 109/L) | 2.2 (1.5-3.1) | 2.2 (1.5-3.2) | 2.2 (1.5-3.0) | 0.434 |
| Lymphocyte count (× 109/L) | 1.1 (0.8-1.6) | 1.1 (0.8-1.6) | 1.1 (0.7-1.6) | 0.921 |
| Neutrophil-lymphocyte ratio | 2.0 (1.4-2.7) | 2.0 (1.4-2.8) | 1.9 (1.4-2.6) | 0.598 |
| Platelets (× 109/L) | 87.0 (65.8-118.6) | 87.0 (65.0-116.0) | 89.0 (67.0-121.0) | 0.199 |
| Creatinine (μmol/L) | 66.0 (56.0-76.0) | 66.1 (56.1-76.2) | 65.0 (56.0-73.9) | 0.602 |
| Blood urea nitrogen (mmol/L) | 5.1 (4.0-6.7) | 5.1 (4.0-6.7) | 5.2 (4.1-6.7) | 0.717 |
| Prothrombin time (s) | 14.3 (12.7-16.3) | 14.3 (12.8-16.1) | 14.1 (12.5-16.7) | 0.801 |
| Prothrombin activity (%) | 67.0 (54.0-80.0) | 67.0 (54.0-80.0) | 67.0 (53.0-81.0) | 0.944 |
| International normalized ratio | 1.2 (1.1-1.3) | 1.2 (1.1-1.3) | 1.2 (1.1-1.4) | 0.865 |
| Width of portal vein, mm | 7.1 (3.4-30.1) | 7.2 (3.4-30.5) | 6.7 (3.3-26.0) | 0.375 |
| HBV DNA (log 10IU/mL) | 2.7(1.2-5.9) | 2.7(1.2-5.8) | 2.7(1.2-6.0) | 0.340 |
| NA(s) ETV/TDF | 614/298 | 421/216 | 193/82 | 0.783 |
| 3-year PVT | 167(18.3) | 121(19.0) | 46(16.7) | 0.202 |
| 5-year PVT | 217(23.8) | 151(23.7) | 66(24.0) | 0.172 |
Table 2 shows the results of the Cox regression analysis, highlighting significant associations between various factors and PVT occurrence. Age (HR = 1.045, 95%CI: 1.029–1.061, P < 0.001), gastrointestinal varices with bleeding (HR = 0.767, 95%CI: 1.420–3.265, P < 0.001), ALT (HR = 0.998 95%CI: 0.996–0.999, P = 0.004), ALB (HR = 0.972, 95%CI: 0.947–0.997, P = 0.028), NLR (HR = 1.331, 95%CI: 1.262–1.403, P < 0.001), PLT (HR = 0.979, 95%CI: 0.972–0.985, P < 0.001), INR (HR = 1.749, 95%CI: 0.936–3.628, P = 0.008), and portal vein width (HR = 0.998, 95%CI: 0.996–1.000, P = 0.003) were all identified as significant predictors of PVT in the training group.
| Variables | Univariate analysis | P value | |
| β | HR (95%CI) | ||
| Age (year) | 0.044 | 1.045 (1.029-1.061) | < 0.001 |
| Sex (male) | 0.267 | 1.306 (0.873-1.954) | 0.194 |
| Smoking | 0.081 | 0.922 (0.605-1.405) | 0.706 |
| Alcohol consumption | 0.161 | 1,175 (0.767-1.800) | 0.458 |
| Diabetes | 0.255 | 1.291 (0.807-2.064) | 0.287 |
| Ascites | 0.660 | 1.935 (1.354-2.766) | < 0.001 |
| Hepatic encephalopathy | 0.255 | 1.291 (0.807-2.064) | 0.287 |
| Gastrointestinal varices with bleeding | 0.767 | 2.153 (1.420-3.265) | < 0.001 |
| Alanine aminotransferase (U/L) | -0.002 | 0.998 (0.996-0.999) | 0.004 |
| Aspartate aminotransferase (U/L) | -0.002 | 0.998 (0.996-0.999) | 0.323 |
| Total bilirubin (mg/dL) | -0.003 | 0.997 (0.992-1.001) | 0.114 |
| Albumin (g/L) | -0.029 | 0.972 (0.947-0.997) | 0.028 |
| gamma-glutamyl transpeptidase (U/L) | 0.001 | 1.001 (0.998-1.003) | 0.637 |
| White blood cell count (× 109/L) | -0.007 | 0.993 (0.913-1.079) | 0.861 |
| NLR | 0.286 | 1.331 (1.262-1.403) | < 0.001 |
| Platelets (× 109/L) | -0.022 | 0.979 (0.972-0.985) | < 0.001 |
| Creatinine (μmol/L) | 0.001 | 1.001 (0.995-1.007) | 0.758 |
| International normalized ratio | 0.559 | 1.749 (0.936-3.268) | 0.080 |
| Width of portal vein | -0.002 | 0.998 (0.996-1.000) | 0.063 |
| HBeAg positivity | 0.726 | 1.242 (0.681–2.267) | 0.487 |
| HBV DNA (log 10IU/mL) | 0.828 | 1.237 (0.677–2.244) | 0.567 |
| NA(s) ETV/TDF | 0.925 | 1.245 (0.755–1.956) | 0.789 |
The identified factors were incorporated into the construction of an ANN model, available at https://Lixuan.me/annmodel/myg-v4/. The ANN deployed a sophisticated architecture known as a Multi-Layer Perceptron (MLP). This network was composed of three fundamental layers: An input layer, an intermediate hidden layer, and an output layer. The input layer served as a bridge, taking clinical and biochemical parameters as input variables, reflecting patient characteristics. The hidden layer, characterized by its non-linear processing capabilities, extracted intricate features from these inputs. Finally, the output layer delivered precise predictions for the prognosis, effectively translating the processed information into actionable insights[25]. The predictive model for 3- and 5-year PVT risk in cirrhotics is available at https://houyixin.math.ink/PVTR/index.html. Employing a deep-learning architecture, the model featured weighted synapses connecting 8 input and 2 output neurons. Four intricately designed hidden layers were systematically integrated post-thorough debugging and optimization, significantly boosting the multilayer perceptron's (MLP) pre
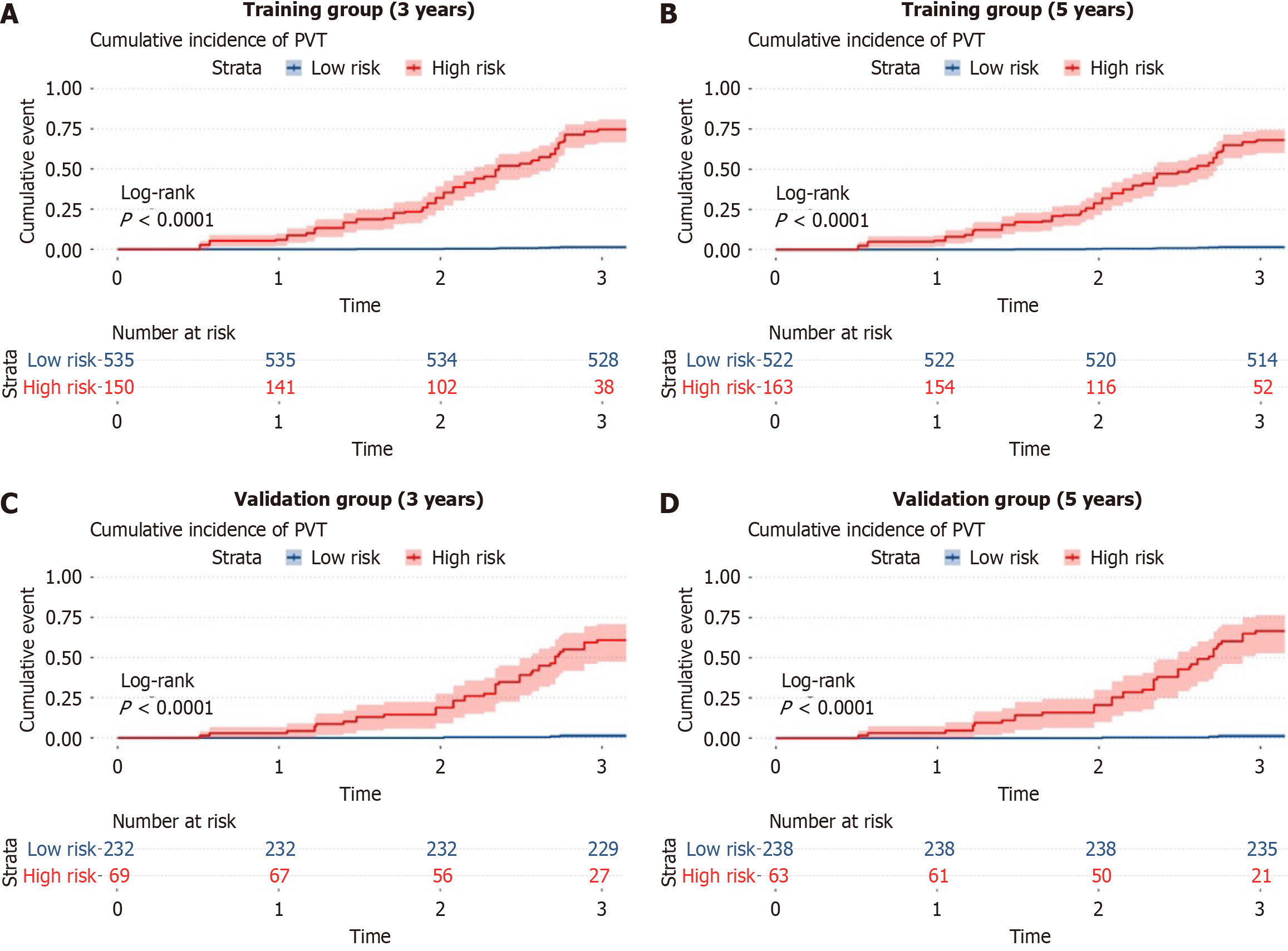
Patient information was input into the ANN model to estimate the 3- to 5-year risk of developing PVT, allowing for risk classification into high- and low-risk groups. In the training cohort, the incidence of PVT was significantly higher in the high-risk group compared to the low-risk group (P < 0.0001) (Figure 3A-B). Similarly, in the validation cohort, a marked difference in PVT incidence was observed between the two groups (P < 0.0001) (Figure 3C-D). The ANN model effe
| Cohort | Models | 3-year risk of PVT | 5-year risk of PVT | ||
| Positive (%) | Negative (%) | Positive (%) | Negative (%) | ||
| Training cohort | ANN (low risk) | 26.2 (25.0-27.4) | 98.7 (95.2-99.7) | 23.2 (21.0-28.4) | 97.7 (95.2-99.7) |
| ANN (high risk) | 54.7 (48.6-60.7) | 98.6 (89.4-99.4) | 52.7 (49.6-60.7) | 98.6 (88.4-99.5) | |
| Validation cohort | ANN (low risk) | 20.9 (19.6-22.2) | 100 (-) | 19.9 (19.6-24.2) | 97.9 (89.6-98.3) |
| ANN (high risk) | 41.5 (32.8-50.8) | 91.9 (88.6-94.3) | 31.5 (28.8-51.8) | 98.9 (88.6-99.3) | |
In the training cohort, the ANN model achieved outstanding performance in predicting PVT occurrence over 3 and 5 years, with area under the receiver operating characteristic curve (AUROC) values of 0.967 (95%CI: 0.960–0.974) and 0.975 (95%CI: 0.955–0.992), respectively, and corresponding C-index values of 0.954 and 0.958 (Table 4). These results demonstrated significantly superior predictive accuracy compared to the MELD and CTP models (P < 0.001). Similarly, in the validation cohort, the ANN model maintained excellent predictive capability for PVT over 3 and 5 years, with AUROC values of 0.958 (95%CI: 0.944–0.973) and 0.973 (95%CI: 0.958–0.987), respectively, and C-index values of 0.941 and 0.948 (Table 4). The predictive performance of the ANN model was significantly better than that of the MELD and CTP models (P < 0.001) (Table 4).
| Cohort | Models | 3-year risk of PVT | 5-year risk of PVT | ||||
| AUROC | C-index | P value | AUROC | C-index | P value | ||
| Training cohort | ANN | 0.967 (0.960-0.974) | 0.954 | 0.975 (0.955–0.992) | 0.958 | ||
| CTP | 0.593 (0.568-0.618) | 0.591 | < 0.001 | 0.602 (0.579-0.625) | 0.592 | < 0.001 | |
| MELD | 0.530 (0.550-0.560) | 0.535 | < 0.001 | 0.555 (0.528-0.581) | 0.544 | < 0.001 | |
| Validation cohort | ANN | 0.958 (0.944–0.973) | 0.941 | 0.973 (0.958–0.987) | |||
| CTP | 0.541 (0.497-0.585) | 0.552 | < 0.001 | 0.547 (0.508-0.585) | 0.541 | < 0.001 | |
| MELD | 0.539 (0.492-0.586) | 0.544 | < 0.001 | 0.553 (0.512-0.594) | 0.546 | < 0.001 | |
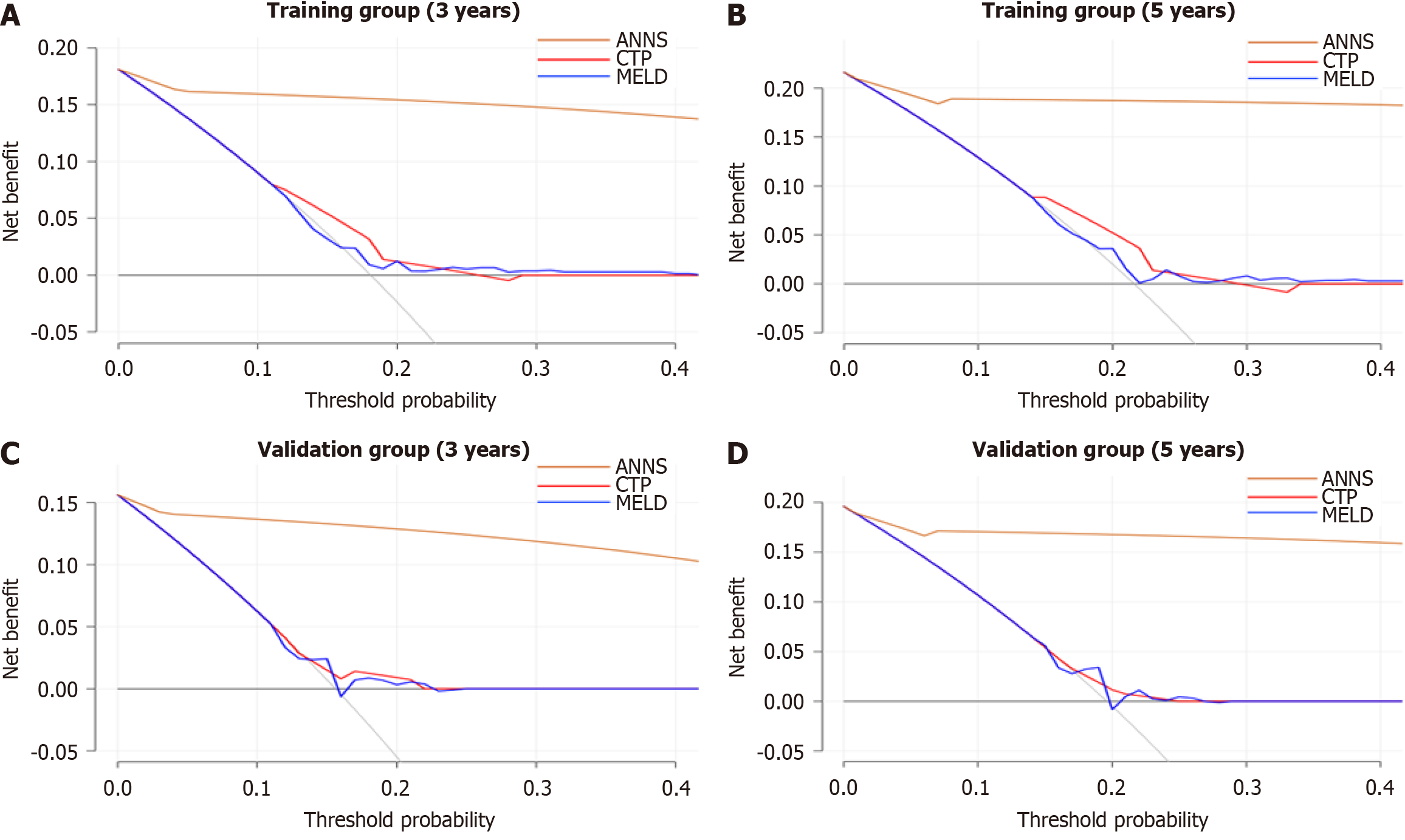
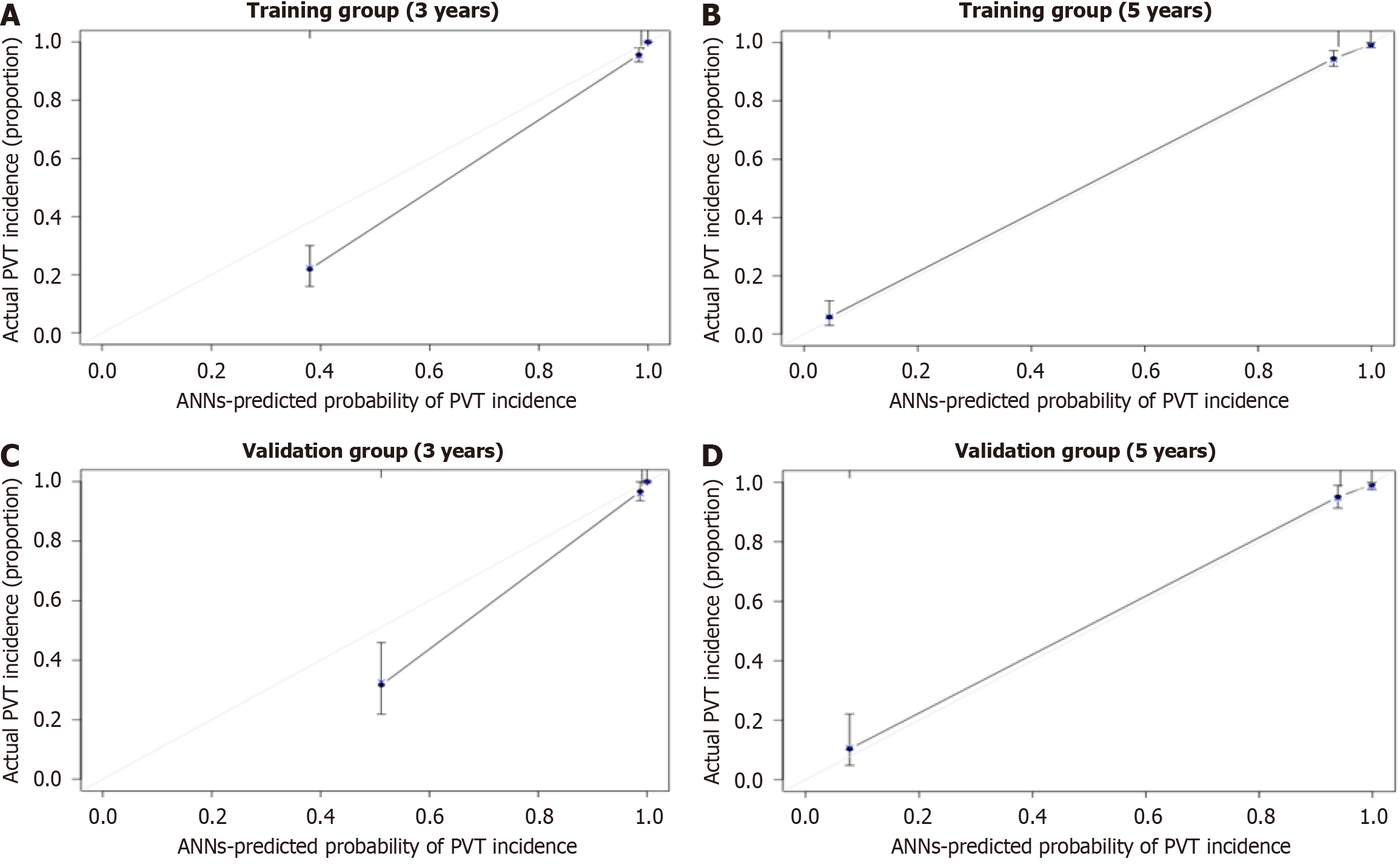
DCA further demonstrated the superior performance of the ANN model compared to the MELD and CTP models in both the training (Figure 4A and B) and validation cohorts (Figure 4C and D). Calibration curves further confirmed the strong agreement between the predicted probability of being PVT-free and the observed probability over 3 and 5 years in both the training (Figure 5A and B) and validation cohorts (Figure 5C and D). These results highlight the clinical practicability and reliability of the ANN model, making it a more effective tool for predicting PVT risk than the MELD and CTP models.
Our research identifies several factors closely associated with PVT development, including gastrointestinal varices with bleeding, ALB levels, PLT levels, INR, and portal vein width. Previous studies have similarly suggested that decompen
The ANN model demonstrated exceptional performance in predicting the occurrence of PVT at 3 and 5 years, as reflected by a significantly high AUC of 0.956 in both training and calibration curves. In comparison, conventional models such as MELD and CTP exhibited lower AUC, indicating the better predictive ability of the ANN model, particularly in cirrhosis patients. ANN models are able to learn from data and optimize prediction accuracy by iteratively adjusting the connections among variables. Unlike traditional logistic or Cox regression models, ANN models are non-linear, allowing them to train factors relevant to the outcome through repeated iterations. This non-linear framework enables ANN models to achieve higher prediction accuracy[35,36].
The study had some limitations which should be acknowledged. First, the retrospective design introduces potential selection bias. Key indicators, such as portal vein blood flow velocity, splenic vein diameter, spleen thickness, and thromboelastographic parameters, were incomplete in the dataset. Incorporating these variables in future studies could further enhance the accuracy of the prediction model. Prospective studies that systematically collect these indicators at admission are necessary to address this limitation. Second, baseline data for an external validation group were unav
This model provides a user-friendly and easily implementable tool for clinical use. Regular evaluation of relevant indicators during the management of cirrhosis patients is essential. This approach enables the early identification of high-risk PVT patients and supports timely clinical decision-making to improve patient prognosis.
We utilized an ANN model to develop a predictive tool for estimating the 3- and 5-year risk of PVT in patients with HBC. The ANN model exhibited excellent performance in individualized risk prediction, providing a valuable tool for assessing PVT risk in clinical practice and facilitating improved management of patients with HBC.
We gratefully recognize the patients who participated in this study. We thank for Li-Hua Yu helping with the data collection.
| 1. | Ambrosino P, Tarantino L, Di Minno G, Paternoster M, Graziano V, Petitto M, Nasto A, Di Minno MN. The risk of venous thromboembolism in patients with cirrhosis. A systematic review and meta-analysis. Thromb Haemost. 2017;117:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 2. | Francoz C, Valla D, Durand F. Portal vein thrombosis, cirrhosis, and liver transplantation. J Hepatol. 2012;57:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 3. | Nery F, Chevret S, Condat B, de Raucourt E, Boudaoud L, Rautou PE, Plessier A, Roulot D, Chaffaut C, Bourcier V, Trinchet JC, Valla DC; Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology. 2015;61:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 348] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 4. | Zocco MA, Di Stasio E, De Cristofaro R, Novi M, Ainora ME, Ponziani F, Riccardi L, Lancellotti S, Santoliquido A, Flore R, Pompili M, Rapaccini GL, Tondi P, Gasbarrini GB, Landolfi R, Gasbarrini A. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol. 2009;51:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 356] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 5. | Maruyama H, Okugawa H, Takahashi M, Yokosuka O. De novo portal vein thrombosis in virus-related cirrhosis: predictive factors and long-term outcomes. Am J Gastroenterol. 2013;108:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 6. | Noronha Ferreira C, Marinho RT, Cortez-Pinto H, Ferreira P, Dias MS, Vasconcelos M, Alexandrino P, Serejo F, Pedro AJ, Gonçalves A, Palma S, Leite I, Reis D, Damião F, Valente A, Xavier Brito L, Baldaia C, Fatela N, Ramalho F, Velosa J. Incidence, predictive factors and clinical significance of development of portal vein thrombosis in cirrhosis: A prospective study. Liver Int. 2019;39:1459-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Nery F, Correia S, Macedo C, Gandara J, Lopes V, Valadares D, Ferreira S, Oliveira J, Gomes MT, Lucas R, Rautou PE, Miranda HP, Valla D. Nonselective beta-blockers and the risk of portal vein thrombosis in patients with cirrhosis: results of a prospective longitudinal study. Aliment Pharmacol Ther. 2019;49:582-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Gaballa D, Bezinover D, Kadry Z, Eyster E, Wang M, Northup PG, Stine JG. Development of a Model to Predict Portal Vein Thrombosis in Liver Transplant Candidates: The Portal Vein Thrombosis Risk Index. Liver Transpl. 2019;25:1747-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Stine JG, Wang J, Shah PM, Argo CK, Intagliata N, Uflacker A, Caldwell SH, Northup PG. Decreased portal vein velocity is predictive of the development of portal vein thrombosis: A matched case-control study. Liver Int. 2018;38:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 10. | Francoz C, Belghiti J, Vilgrain V, Sommacale D, Paradis V, Condat B, Denninger MH, Sauvanet A, Valla D, Durand F. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut. 2005;54:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 392] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 11. | Basili S, Carnevale R, Nocella C, Bartimoccia S, Raparelli V, Talerico G, Stefanini L, Romiti GF, Perticone F, Corazza GR, Piscaglia F, Pietrangelo A, Violi F; PRO‐LIVER Collaborators. Serum Albumin Is Inversely Associated With Portal Vein Thrombosis in Cirrhosis. Hepatol Commun. 2019;3:504-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Giannitrapani L, Granà W, Licata A, Schiavone C, Montalto G, Soresi M. Nontumorous Portal Vein Thrombosis in Liver Cirrhosis: Possible Role of β-Blockers. Med Princ Pract. 2018;27:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Yerdel MA, Gunson B, Mirza D, Karayalçin K, Olliff S, Buckels J, Mayer D, McMaster P, Pirenne J. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation. 2000;69:1873-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 525] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 14. | Xu X, Guo X, De Stefano V, Silva-Junior G, Goyal H, Bai Z, Zhao Q, Qi X. Nonselective beta-blockers and development of portal vein thrombosis in liver cirrhosis: a systematic review and meta-analysis. Hepatol Int. 2019;13:468-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 447] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 16. | Kalambokis GN, Oikonomou A, Christou L, Baltayiannis G. High von Willebrand factor antigen levels and procoagulant imbalance may be involved in both increasing severity of cirrhosis and portal vein thrombosis. Hepatology. 2016;64:1383-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Li Y, Gao J, Zheng X, Nie G, Qin J, Wang H, He T, Wheelock Å, Li CX, Cheng L, Li X. Diagnostic Prediction of portal vein thrombosis in chronic cirrhosis patients using data-driven precision medicine model. Brief Bioinform. 2023;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Wang M, Ding L, Xu M, Xie J, Wu S, Xu S, Yao Y, Liu Q. A novel method detecting the key clinic factors of portal vein system thrombosis of splenectomy & cardia devascularization patients for cirrhosis & portal hypertension. BMC Bioinformatics. 2019;20:720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | La Mura V, Tripodi A, Tosetti G, Cavallaro F, Chantarangkul V, Colombo M, Primignani M. Resistance to thrombomodulin is associated with de novo portal vein thrombosis and low survival in patients with cirrhosis. Liver Int. 2016;36:1322-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Martinelli I, Primignani M, Aghemo A, Reati R, Bucciarelli P, Fabris F, Battaglioli T, Dell'Era A, Mannucci PM. High levels of factor VIII and risk of extra-hepatic portal vein obstruction. J Hepatol. 2009;50:916-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Lancellotti S, Basso M, Veca V, Sacco M, Riccardi L, Pompili M, De Cristofaro R. Presence of portal vein thrombosis in liver cirrhosis is strongly associated with low levels of ADAMTS-13: a pilot study. Intern Emerg Med. 2016;11:959-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Ma SD, Wang J, Bezinover D, Kadry Z, Northup PG, Stine JG. Inherited thrombophilia and portal vein thrombosis in cirrhosis: A systematic review and meta-analysis. Res Pract Thromb Haemost. 2019;3:658-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Mangia A, Villani MR, Cappucci G, Santoro R, Ricciardi R, Facciorusso D, Leandro G, Caruso N, Andriulli A. Causes of portal venous thrombosis in cirrhotic patients: the role of genetic and acquired factors. Eur J Gastroenterol Hepatol. 2005;17:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1958] [Article Influence: 217.6] [Reference Citation Analysis (0)] |
| 25. | Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 964] [Cited by in RCA: 1347] [Article Influence: 103.6] [Reference Citation Analysis (0)] |
| 26. | Jiménez-Alcázar M, Kim N, Fuchs TA. Circulating Extracellular DNA: Cause or Consequence of Thrombosis? Semin Thromb Hemost. 2017;43:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Berzigotti A, Piscaglia F. Ultrasound in portal hypertension--part 1. Ultraschall Med. 2011;32:548-68; quiz 569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | La Mura V, Reverter JC, Flores-Arroyo A, Raffa S, Reverter E, Seijo S, Abraldes JG, Bosch J, García-Pagán JC. Von Willebrand factor levels predict clinical outcome in patients with cirrhosis and portal hypertension. Gut. 2011;60:1133-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Raffa S, Reverter JC, Seijo S, Tassies D, Abraldes JG, Bosch J, García-Pagán JC. Hypercoagulability in patients with chronic noncirrhotic portal vein thrombosis. Clin Gastroenterol Hepatol. 2012;10:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Ariëns RA, Kohler HP, Mansfield MW, Grant PJ. Subunit antigen and activity levels of blood coagulation factor XIII in healthy individuals. Relation to sex, age, smoking, and hypertension. Arterioscler Thromb Vasc Biol. 1999;19:2012-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Martínez-Zamora MA, Tàssies D, Creus M, Reverter JC, Puerto B, Monteagudo J, Carmona F, Balasch J. Higher levels of procoagulant microparticles in women with recurrent miscarriage are not associated with antiphospholipid antibodies. Hum Reprod. 2016;31:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | von Meijenfeldt FA, Burlage LC, Bos S, Adelmeijer J, Porte RJ, Lisman T. Elevated Plasma Levels of Cell-Free DNA During Liver Transplantation Are Associated With Activation of Coagulation. Liver Transpl. 2018;24:1716-1725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Lisman T, de Groot PG, Meijers JC, Rosendaal FR. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood. 2005;105:1102-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 34. | Aleman MM, Byrnes JR, Wang JG, Tran R, Lam WA, Di Paola J, Mackman N, Degen JL, Flick MJ, Wolberg AS. Factor XIII activity mediates red blood cell retention in venous thrombi. J Clin Invest. 2014;124:3590-3600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 35. | Lisman T, Ariëns RA. Alterations in Fibrin Structure in Patients with Liver Diseases. Semin Thromb Hemost. 2016;42:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Hugenholtz GC, Macrae F, Adelmeijer J, Dulfer S, Porte RJ, Lisman T, Ariëns RA. Procoagulant changes in fibrin clot structure in patients with cirrhosis are associated with oxidative modifications of fibrinogen. J Thromb Haemost. 2016;14:1054-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |