Published online Mar 27, 2025. doi: 10.4254/wjh.v17.i3.105255
Revised: February 23, 2025
Accepted: March 10, 2025
Published online: March 27, 2025
Processing time: 69 Days and 2.1 Hours
In this editorial, we comment on the article by Zhao et al which highlighted how patients having nonalcoholic fatty liver disease (NAFLD) were more susceptible to drug-induced lung injury (DILI). This article looked at the downstream effects of metabolic profiles and biochemical processes after medication and substance use. Although previous studies looked at how NAFLD and DILI were related, there is a lack of information on the consequences of everyday medication and substance use. NAFLD is one of the most common chronic liver diseases wor
Core Tip: This article explores the downstream effects of metabolic profiles and biochemical processes following medication and substance use. While previous studies have examined the relationship between nonalcoholic fatty liver disease (NAFLD) and drug-induced lung injury (DILI), there is a lack of information on the consequences of everyday medication and substance use. NAFLD is one of the most common chronic liver diseases, closely linked to metabolic syndrome and cardiovascular disease. These interconnected mechanisms compromise liver function, requiring tailored therapeutic strategies and cautious medication management to reduce the risk of DILI, helping physicians prevent acute or chronic diseases in vulnerable populations.
- Citation: Salolin Vargas VP, Gasbarra M, Calderon-Martinez E, Shah YR, Dahiya DS, Saenz de Sicilia MG. Non-alcoholic fatty liver disease and drug induced liver injury: A metabolic storm waiting to happen. World J Hepatol 2025; 17(3): 105255
- URL: https://www.wjgnet.com/1948-5182/full/v17/i3/105255.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i3.105255
Nonalcoholic fatty liver disease (NAFLD) is the accumulation of excess lipids in hepatocytes. According to recent research, NAFLD has become an increasingly common condition that is believed to impact more than 25% of adults worldwide[1]. Drug-induced liver injury (DILI) can be caused by medications, dietary and/or herbal supplements. It’s primarily classified into two types: Dose-dependent (intrinsic) and idiosyncratic. DILI Dose-dependent, this type of DILI occurs predictably and is directly related to the dose of the drug. Higher doses lead to increased liver damage. A well-known example is paracetamol (acetaminophen) overdose, which can cause severe liver injury due to the accumulation of toxic metabolites when taken in excessive amounts. In such cases, liver damage occurs shortly after a toxic dose is consumed. The other type of DILI is idiosyncratic which is unpredictable and occurs in a small percentage of individuals, regardless of the drug dose. This form of liver injury is not related to the drug's inherent toxicity but is believed to result from individual susceptibilities, possibly due to genetic factors, immune responses, or environmental influences. For instance, certain antibiotics and antifungals, such as amoxicillin-clavulanate and flucloxacillin, have been associated with idiosyncratic DILI, where liver injury occurs unpredictably in susceptible individuals[2]. DILI has been reported to have an annual incidence of fourteen to nineteen events per 100000 in the general population and is reported to be 1.2% in hospitalized patients[3]. Because patients with NAFLD are most likely being treated for other comorbidities such as hypertension or type 2 diabetes, these patients are more likely to experience downstream effects[4]. Patients with NAFLD are four times more likely to have DILI compared to healthy individuals[5]. Having both NAFLD and DILI raises the concern that consequences such as liver fibrosis, cirrhosis or possible liver failure can eventually occur with continued substance use.
Ferroptosis, a form of regulated cell death characterized by iron-dependent lipid peroxidation, has been implicated in the development and progression of NAFLD. In NAFLD, excessive accumulation of lipids in hepatocytes increases susceptibility to oxidative stress, promoting ferroptosis and contributing to liver injury and inflammation. Therefore, understanding the role of ferroptosis in NAFLD is crucial for developing therapeutic strategies to mitigate both NAFLD progression and the associated risk of DILI. The oxidative environment in NAFLD may also predispose patients to an increased risk of DILI. Drugs that induce oxidative stress or disrupt antioxidant defenses can exacerbate lipid pero
In the context of DILI, AMPK activation and autophagy play protective roles. AMPK activation enhances autophagic processes, facilitating the removal of drug-induced damaged mitochondria and reducing oxidative stress. This protective mechanism can mitigate hepatocyte injury caused by various hepatotoxic drugs. However, specific human studies directly linking AMPK, autophagy, NAFLD, and DILI are currently limited[6].
There are several mechanisms of how medications and supplements can cause liver damage. This article examined how liver damage can be caused by the drugs themselves, their metabolites, reactive oxygen species (ROS), and hypersensitivity reactions. C3 complement protein, IL-4 levels, the development of cholestasis, the activity of detoxifying CYPs, and Glutathione S-transferase were also investigated. This study addressed the limitations of previous studies by looking at clinical data, biochemical indices and liver biopsy results.
It is important to understand what medications and substances can contribute to DILI, what immune factors can possibly be expressed, the chances of cholestasis and what degree of liver injury these drugs can potentially cause. If we know a patient has already been diagnosed with NAFLD, it would be beneficial to the patient if the provider takes into consideration how these medications could interact with their baseline disease and if potentially for DILI can occur[7].
Cytochrome P450 (CYP450) enzymes are heme-containing, membrane-bound oxidases that play a pivotal role in human drug metabolism, cellular biochemical processes, and the regulation of homeostasis[8]. CYP450 enzymes are a critical family of enzymes primarily located in the liver, playing essential roles in drug metabolism, detoxification, and the biosynthesis of endogenous compounds. They are central to Phase I drug metabolism, where they oxidize, reduce, or hydrolyze drugs to more water-soluble forms for excretion, as seen in the conversion of codeine to morphine by CYP2D6. These enzymes also metabolize endogenous substances like steroid hormones, bile acids, and fatty acids, contributing to physiological balance. Clinically, CYP450 enzymes are significant due to their involvement in drug interactions, pharmacogenomics, and adverse drug reactions[9]. Moreover, CYP450 and NAFLD play a crucial role in drug metabolism, and their expression and activity are significantly altered in NAFLD. As NAFLD progresses, it has been shown decreased activity in enzymes such as CYP1A2 and CYP2C19, while others like CYP2A6 and CYP2C9 show increased activity[8]. These alterations can impact the metabolism of medications, potentially affecting their efficacy and safety in individuals with NAFLD. Additionally, CYP450 enzyme alterations in NAFLD lies in its significant impact on drug metabolism, dosing, and patient safety[9]. As NAFLD progresses, changes in CYP450 activity can lead to altered drug clearance, requiring adjustments in dosing to avoid toxicity or therapeutic failure.
NAFLD is linked to oxidative stress, which plays a crucial role in its development and progression[10]. Oxidative stress originates when there's an imbalance between ROS production and when the liver's antioxidant defenses. In NAFLD, excessive fat accumulation in liver cells leads to lipotoxicity, triggering mitochondrial dysfunction, endoplasmic reticulum stress, and inflammation, all of which contribute to increased ROS generation[11]. This oxidative environment damages cellular components, exacerbating liver injury and promoting the transition from simple steatosis to nona
Nevertheless, the clinical importance of oxidative stress in NAFLD arises in its significant role in disease progression and its impact on therapeutic strategies. Oxidative stress not only drives the transition from simple steatosis to more severe forms like NASH and fibrosis, it also contributes to complications such as liver cirrhosis and hepatocellular carcinoma[12]. Clinically, this means that patients with higher oxidative stress markers may have a worse prognosis and may require more aggressive management[12].
Immune dysregulation occurs when the immune system fails to sustain self-tolerance and effectively control immune responses[9]. This imbalance can lead to autoimmunity, where the immune system mistakenly targets the body's own tissues, or immunodeficiency, characterized by a weakened immune response. Contributing factors to immune dysregulation include genetic variations, environmental influences, and disturbances in the regulatory mechanisms that uphold immune equilibrium[9].
Integrating metabolic profiling into regulatory frameworks would improve drug approval processes by providing a deeper understanding of metabolic toxicity and variability. It could also optimize post-market drug surveillance by predicting idiosyncratic reactions and refining guidelines for medications associated with DILI[6]. Research on NAFLD patients has shown that profiling can identify critical markers, such as increased cytokine levels and oxidative stress, which are strong predictors of DILI risk[7]. These advancements could transform regulatory standards, fostering proactive safety measures during drug development.
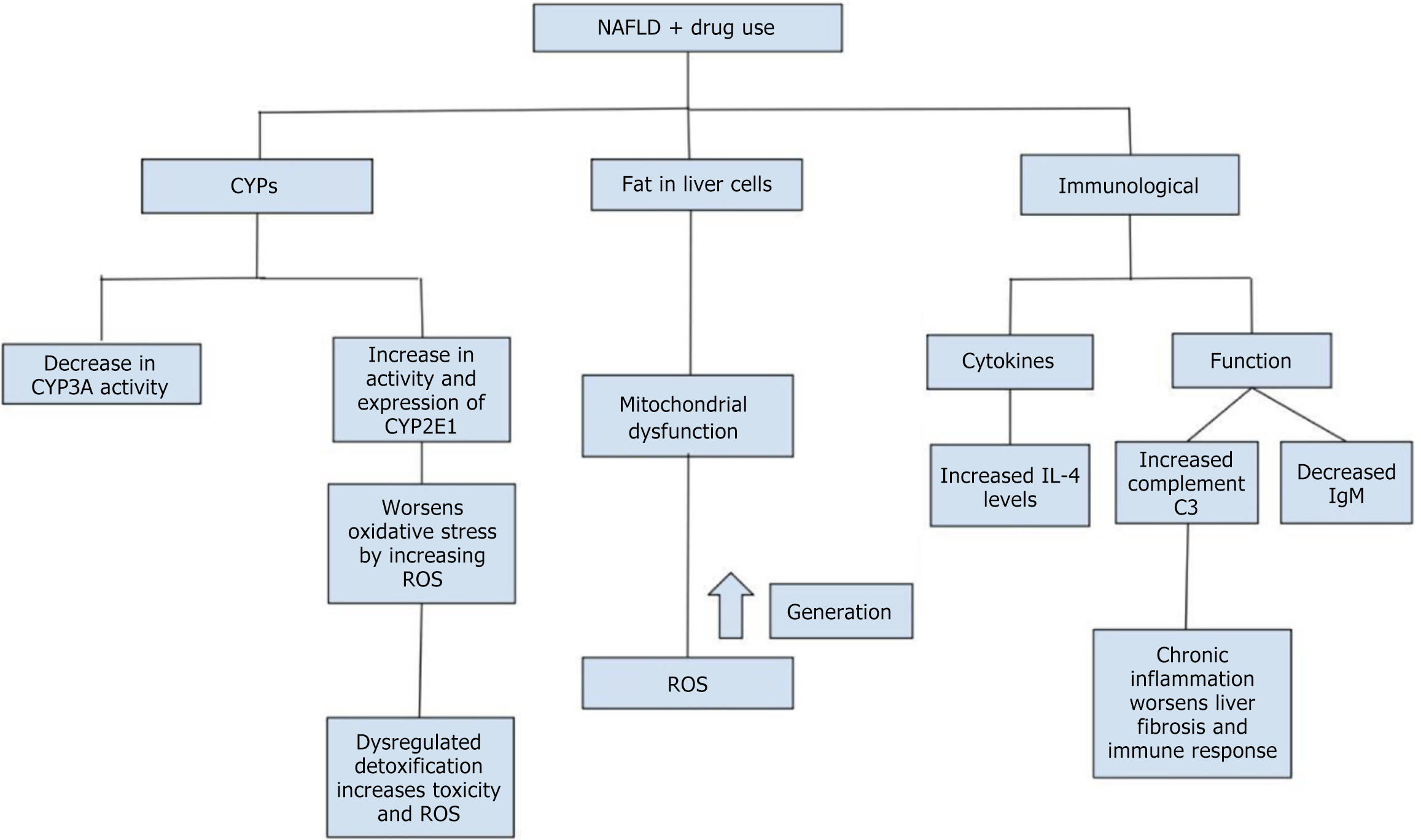
According to Zhao et al[13], investigates the interplay between NAFLD and DILI, with a focus on oxidative stress, CYP450 activity, and immune dysregulation. NAFLD patients were found to be at a significantly higher risk of severe DILI, characterized by prolonged liver injury, immune hyperactivation, and altered bile salt composition. Elevated CYP2E1 activity in NAFLD heightened the production of ROS, exacerbating oxidative stress and promoting hepatotoxicity, especially with drugs like acetaminophen[10]. Concurrently, CYP3A4 activity was downregulated, impairing drug metabolism and prolonging drug toxicity. Immune dysregulation played a pivotal role, with increased levels of IL-4 and complement C3 in NAFLD+DILI patients, indicating heightened immune activation and inflammation. IL-4, in particular, promoted a pro-inflammatory environment by inducing B-cell activity and neoantigen formation, sensitizing hepatocytes to injury. Additionally, the complement system, primarily through C3 overactivation, contributed to immune-mediated hepatocellular damage. These factors made NAFLD patients more susceptible to cholestatic-type DILI, with liver injury severity reaching grade 2 or higher and recovery times extending up to 180 days. The findings underscore the importance of cautious drug use in NAFLD patients, addressing underlying metabolic dysfunctions, and closely monitoring liver function to mitigate risks. This study provides valuable insights into the mechanistic pathways of oxidative stress and immune dysregulation, highlighting the need for personalized therapeutic approaches in managing DILI in NAFLD[13]. In Figure 1, the interplay between NAFLD and drug use is summarized. The figure demonstrates how drug use impacts liver function through three primary mechanisms. Firstly, CYPs are altered, with decreased CYP3A activity and increased CYP2E1 activity, leading to heightened oxidative stress due to increased ROS. Secondly, fat accumulation in liver cells contributes to mitochondrial dysfunction, which exacerbates oxidative stress. Finally, immunological effects are seen with changes in cytokine levels (e.g., increased IL-4) and immune function (e.g., increased complement C3 and decreased IgM), promoting chronic inflammation and liver fibrosis.
In conclusion, patients with NAFLD are at significantly increased risk for DILI due to disrupted CYP450 enzyme activity, excessive oxidative stress from lipid overload, and chronic inflammation with heightened pro-inflammatory cytokines. These interconnected mechanisms compromise liver function and resilience, necessitating tailored therapeutic strategies and cautious medication management to mitigate the risk of DILI in this vulnerable population. Because NAFLD is considered one of the most common chronic liver diseases worldwide, we know that this study would benefit a large number of patients because providers would be aware of the long term consequences of adding certain medications and/or supplements to their patients’ care. However, prevention of DILI in patients with NAFLD involves a multi-faceted approach. Key strategies include regular medication review to avoid hepatotoxic drugs, careful dose adjustments based on liver function, and close monitoring of liver enzymes. Educating patients on the risks of self-medication and encouraging communication about all substances they are consuming is critical. Additionally, promoting lifestyle modifications, such as weight loss and physical activity, can improve liver health and reduce susceptibility to DILI. Early detection through routine liver function tests ensures timely intervention and prevents further liver damage. Providers could take this data and apply it to the healthcare setting by either monitoring patients long term or not start these medications or substances at all due to wanting to prevent acute or chronic outcomes.
| 1. | Duell PB, Welty FK, Miller M, Chait A, Hammond G, Ahmad Z, Cohen DE, Horton JD, Pressman GS, Toth PP; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Hypertension; Council on the Kidney in Cardiovascular Disease; Council on Lifestyle and Cardiometabolic Health; and Council on Peripheral Vascular Disease. Nonalcoholic Fatty Liver Disease and Cardiovascular Risk: A Scientific Statement From the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42:e168-e185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 352] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 2. | Katarey D, Verma S. Drug-induced liver injury. Clin Med (Lond). 2016;16:s104-s109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 3. | Fontana RJ, Liou I, Reuben A, Suzuki A, Fiel MI, Lee W, Navarro V. AASLD practice guidance on drug, herbal, and dietary supplement-induced liver injury. Hepatology. 2023;77:1036-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 93] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 4. | Shen T, Liu Y, Shang J, Xie Q, Li J, Yan M, Xu J, Niu J, Liu J, Watkins PB, Aithal GP, Andrade RJ, Dou X, Yao L, Lv F, Wang Q, Li Y, Zhou X, Zhang Y, Zong P, Wan B, Zou Z, Yang D, Nie Y, Li D, Wang Y, Han X, Zhuang H, Mao Y, Chen C. Incidence and Etiology of Drug-Induced Liver Injury in Mainland China. Gastroenterology. 2019;156:2230-2241.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 384] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 5. | Liu YH, Guo Y, Xu H, Feng H, Chen DY. Impact of Non-Alcoholic Simple Fatty Liver Disease on Antituberculosis Drug-Induced Liver Injury. Infect Drug Resist. 2021;14:3667-3671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | You T, Li Y, Li B, Wu S, Jiang X, Fu D, Xin J, Huang Y, Jin L, Hu C. Caveolin-1 protects against liver damage exacerbated by acetaminophen in non-alcoholic fatty liver disease by inhibiting the ERK/HIF-1α pathway. Mol Immunol. 2023;163:104-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Arslan N. Obesity, fatty liver disease and intestinal microbiota. World J Gastroenterol. 2014;20:16452-16463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 152] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Deodhar M, Al Rihani SB, Arwood MJ, Darakjian L, Dow P, Turgeon J, Michaud V. Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice. Pharmaceutics. 2020;12:846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 9. | Fisher CD, Lickteig AJ, Augustine LM, Ranger-Moore J, Jackson JP, Ferguson SS, Cherrington NJ. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos. 2009;37:2087-2094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 265] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 10. | Nguyen GC, Sam J, Thuluvath PJ. Hepatitis C is a predictor of acute liver injury among hospitalizations for acetaminophen overdose in the United States: a nationwide analysis. Hepatology. 2008;48:1336-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020;152:116-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 809] [Article Influence: 161.8] [Reference Citation Analysis (1)] |
| 12. | Arroyave-Ospina JC, Wu Z, Geng Y, Moshage H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants (Basel). 2021;10:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 292] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 13. | Zhao Y, Li JZ, Liu YG, Zhu YJ, Zhang Y, Zheng WW, Ma L, Li J, Wang CY. Clinical features and prognosis of drug-induced liver injury in patients with non-alcoholic fatty liver. World J Hepatol. 2025;17:101741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |