INTRODUCTION
Acetaminophen (APAP) is a commonly used medication known for its analgesic and antipyretic properties. However, excessive intake-whether due to drug abuse or accidental overdose-can lead to serious adverse effects, such as acute liver injury (ALI) and liver failure[1]. The liver, which serves as the main organ for detoxification processes, is especially susceptible to drug exposure. When hepatocytes are exposed to acute excess APAP, the drug undergoes metabolism through cytochrome P450 (CYP450), resulting in the production of significant amounts of the harmful metabolite N-acetyl-p-benzoquinone imine (NAPQI). This toxic compound rapidly reduces glutathione (GSH) within hepatocytes. Accumulation of NAPQI, when GSH is insufficient for detoxification, leads to its reaction with cysteine residues in proteins, ultimately resulting in hepatocyte necrosis[2]. While numerous studies have elucidated the pathogenesis of liver injury associated with APAP overdose[3,4], the development of effective therapeutic interventions remains limited[5]. Although the antioxidant N-acetylcysteine, a precursor of GSH, is currently recognized as an effective therapeutic agent, its efficacy is greatest when administered within 8 to 10 hours following APAP overdose, with therapeutic effects diminishing significantly if administration is delayed[6,7]. Consequently, there is a pressing need for further research into preventive measures and effective antidotes for APAP toxicity.
In recent years, the evaluation of bioactivity in antioxidants derived from natural sources has been extensively explored, revealing unexpected therapeutic effects[8]. Siraitia grosvenorii, commonly known as Monk fruit, is a traditionally cultivated plant in China[9]. Mogroside V (MV), a triterpenoid glycoside extracted from the Mogrosides present in Siraitia grosvenorii, is widely used as a natural sweetener in commercial applications[10]. Recently, several pharmacological activities of MV have been observed, including anti-oxidative[11], anti-asthmatic[12], and anti-inflammatory effects[13]. In rat models, MV has shown potential hypoglycemic effects by inhibiting intestinal maltase activity[14], and alleviating insulin resistance while increasing glycogen synthesis via the PI3K/AKT pathway[15]. In a cerebral ischemia-reperfusion mice model, MV attenuates inflammation and oxidative stress via regulating the TLR4/TRADD pathway[16]. Additionally, MV shows potential in combating pancreatic cancer by inducing apoptosis and cell cycle arrest through regulating the signal transducer and activator of transcription 3 pathway[17]. However, the impact of MV on ALI has yet to be evaluated. This study aimed to explore the hepatoprotective effects of MV in an APAP overdose model of acute hepatotoxicity.
This study examines the hepatoprotective properties of MV in the context of ALI induced by APAP. The findings suggest that MV could serve as a promising therapeutic candidate for preventing ALI due to APAP exposure.
MATERIALS AND METHODS
Reagents and cell lines
The MV powder, with a purity of at least 98%, was sourced from Chengdu Must Biotechnology Co., Ltd. (Chengdu, China). The APAP powder (purity > 99.98%) was obtained from MCE Co., Ltd (HY-66005, China).
The alpha mouse liver 12 (AML12) cells were cultivated in DMEM/F-12 medium enriched with 5% FBS, dexamethasone (Beyotime, ST1254, Shanghai, China), and ITS liquid media supplement (Sigma, I3146).
Animals and ALI model
Male C57BL/6J mice aged six to eight weeks were acquired from Jiangsu Huachuang Xinnuo Pharmaceutical Technology Co., Ltd. (Jiangsu, China). The mice were housed in a SPF environment and had unlimited access to food, water, and rest. According to the previously reported method[18], an overdose of APAP was used to establish an ALI model by injecting of APAP (300 mg/kg) or vehicle (warm sterile saline) intraperitoneally to the mice that had undergone a 15-hour water-only fasting period.
Prior to the experiment, the mice were randomly assigned to three groups: Saline, APAP and APAP + MV. To examine the protective efficacy of multiple vs single administrations of MV against APAP-induced liver injury, we conducted two separate experiments: (1) In the experiment involving seven consecutive days of MV administrations, mice in the APAP + MV group received MV solution (10 mg/kg) via oral gavage once daily for seven consecutive days[19], after which APAP was administered one hour after the last dose of MV; and (2) In the single dose MV administration experiment, mice in the APAP + MV group received a single dose of MV solution (10 mg/kg) via oral gavage, followed by APAP administration one hour later. Finally, both serum and liver tissues from all mice were collected at specified time points after the injection of APAP.
Alanine aminotransferase and aspartate aminotransferase measurement
The serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using the mouse ALT ELISA kit and the mouse AST ELISA kit, respectively. The kits were obtained from Wuhan Sailuofei Biotechnology Co., Ltd., (Wuhan, China) and the procedure was performed according to the kit instructions.
Hematoxylin and eosin, immunohistochemistry and (terminal deoxynucleotidyl transferase dUTP nick end labeling) TUNEL staining
Fresh liver tissues were fixed in 4% formaldehyde, embedded in paraffin, sliced into 4 μm thick sections, and dewaxed with turpentine for subsequent use. For Hematoxylin and eosin (H&E) staining, the sections were treated sequentially with eosin and hematoxylin. For immunohistochemistry (IHC) staining, the sections were soaked in 3% H2O2 to eliminate endogenous peroxide, followed by microwave heating. The sections were then incubated overnight at 4 ℃ with anti-Nitrotyrosine (Invitrogen, A-21285), anti-CD68 (Abcam, ab283654) or anti-S100A9 (Abcam, ab242945), and subsequently incubated with secondary antibodies for one hour at 37 ℃. Finally, the sections were stained with the DAB substrate kit (CST, 8059S) and hematoxylin in turn. For TUNEL staining, the Fluorescein (FITC) Tunel Cell Apoptosis Detection Kit (Servicebio, China) was utilized as per the manufacturer's protocol. The stained sections were examined and photographed using either light or fluorescence microscopy.
GSH measurement
GSH was measured using the Reduced GSH Assay kit (Solarbio, BC1175). In brief, liver tissue was homogenized in GSH assay lysis buffer. The lysate supernatant was used to detect GSH activity, according to the kit instructions.
Western blotting
The levels of target proteins in the samples were assessed by western blotting. Total protein lysate was collected after lysing liver tissues or cells and were subsequently subjected to denaturation. Equal amounts of protein lysate were placed onto 4%-20% SDS-PAGE gels (Beijing Tsingke Biotech Co., Ltd.) for separation, followed by transfer to 0.22 μm PVDF membranes. The membranes were then blocked using a blocking buffer, followed by incubation overnight at 4 °C with the following primary antibodies: Anti- c-jun-N-terminal kinase (JNK) (CST), anti-p-JNK (CST), or anti-β-actin (Beyotime). Following this, the membranes were treated with the appropriate secondary antibodies at room temperature. Exposure to ECL reagents (ABclonal) allowed for the capture of grayscale images of each protein lane.
Statistical analysis
The intensity of the image signal was measured using ImageJ software. The results are presented as mean ± SEM. To assess statistical significance between the groups, a two-tailed unpaired student’s t test was employed, and comparisons across multiple groups were performed using Ordinary one-way ANOVA. All analyses were carried out with GraphPad Prism software, and a P value of less than 0.05 was regarded as statistically significant.
RESULTS
MV attenuates APAP-induced ALI
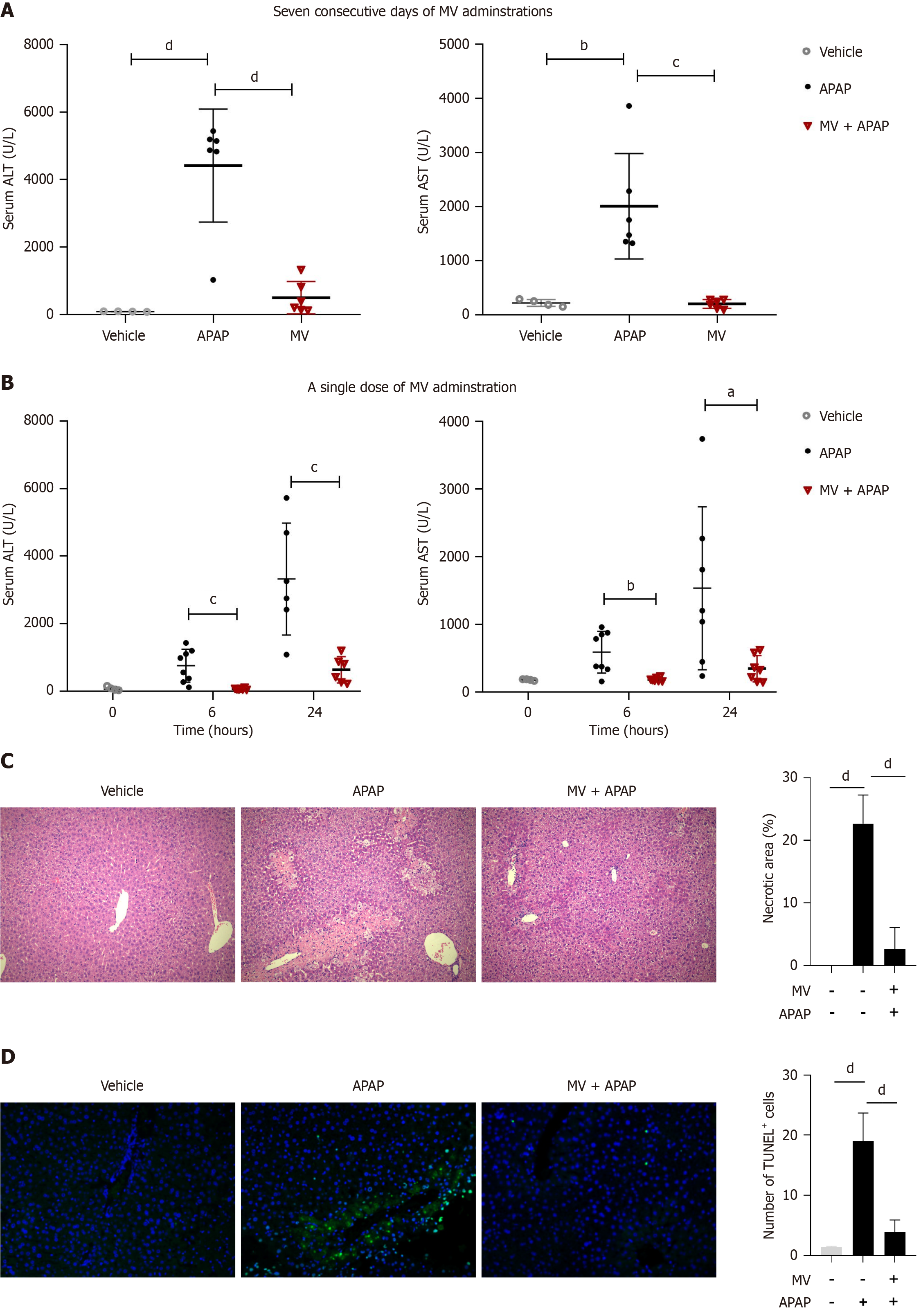
We initially evaluated the impact of oral MV administration over seven consecutive days on APAP-induced liver injury. As anticipated, the serum ALT and AST levels in APAP-treated mice exhibited a marked increase 24 hours post-APAP injection (Figure 1A). However, the elevation in serum ALT and AST levels induced by APAP was significantly mitigated following seven consecutive days of MV administration (Figure 1A). Notably, a single oral administration of MV one hour prior to APAP administration also significantly lowered the serum ALT and AST levels caused by APAP (Figure 1B). Consequently, the single-dose MV administration method was utilized in subsequent experiments. Additionally, we conducted H&E and TUNEL staining to assess the extent of liver damage. As illustrated in Figure 1C and D, the livers of mice in the APAP group exhibited extensive centrilobular necrosis and a substantial number of TUNEL+ cells. In contrast, the livers of mice in the MV + APAP group displayed only mild hepatocyte injury and a reduced number of TUNEL+ cells (Figure 1C and D). These results indicate that pre-treatment with MV can significantly improve APAP-induced ALI.
Figure 1 Mogroside V attenuates acetaminophen-induced acute liver injury.
A: Seven consecutive days of oral Mogroside V (MV) administration decreased serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in mice 24 hours after acetaminophen (APAP) treatment. n = 4-8 per group; B: A single dose of oral MV administration decreased serum ALT and AST levels in mice 6 and 24 hours following APAP treatment. n = 4-8 per group; C and D: A single dose of oral MV administration reduced the necrotic area and TUNEL+ cells in the livers of mice 12 hours after APAP exposure. n = 4 per group. Scale bar, 50 μm. aP < 0.05, bP < 0.01, cP < 0.001, dP < 0.0001. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; MV: Mogroside V; APAP: Acetaminophen.
MV attenuates infiltration of inflammatory cells in the liver of APAP-treated mice
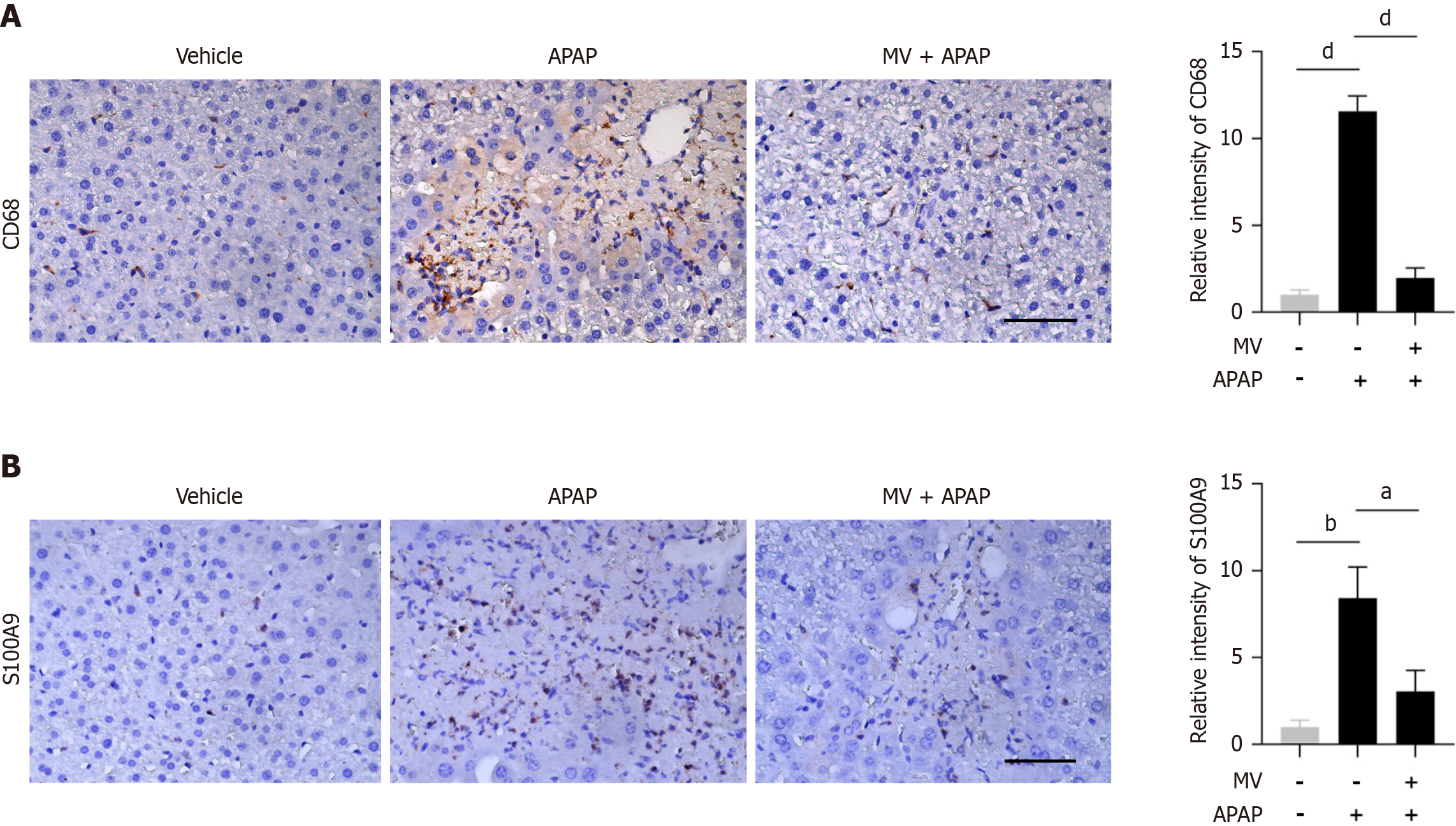
As a central immunological organ, the liver can rapidly initiate the immune system’s response to pathogens, infections, harmful stimuli or inflammation[20]. The calcium-binding protein S100A9 is intensely upregulated during inflammatory processes, and has been recognized as a biomarker for diagnosing inflammation-related diseases[21]. In order to explore the effect of MV on APAP-induced inflammatory responses, we assessed the presence of S100A9+ cells and macrophages (CD68+ cells) in the liver using IHC staining. As illustrated in Figure 2A, CD68+ macrophages were enriched in the injured area of the APAP-treated mice. Conversely, MV pre-treatment decreased the number of CD68+ macrophages in the liver of APAP-treated mice (Figure 2A). Similarly, the MV + APAP group exhibited a notable decrease in the amount of S100A9+ cells when compared to the APAP group (Figure 2B). Collectively, these findings indicate that MV pre-treatment significantly reduces both the recruitment of S100A9+ cells and CD68+ macrophages to the injured area, suggesting that MV alleviates the inflammatory response in the liver of APAP-induced ALI.
Figure 2 Mogroside V reduces the infiltration of inflammatory cells in the liver of acetaminophen-treated mice.
A: Representative images of immunohistochemistry (IHC) staining and the relative intensity of CD68; B: Representative images of IHC staining and the relative intensity of S100A9. Scale bar, 50 μm. n = 4-8 per group. aP < 0.05, bP < 0.01, dP < 0.0001. MV: Mogroside V; APAP: Acetaminophen.
MV has no effect on GSH depletion
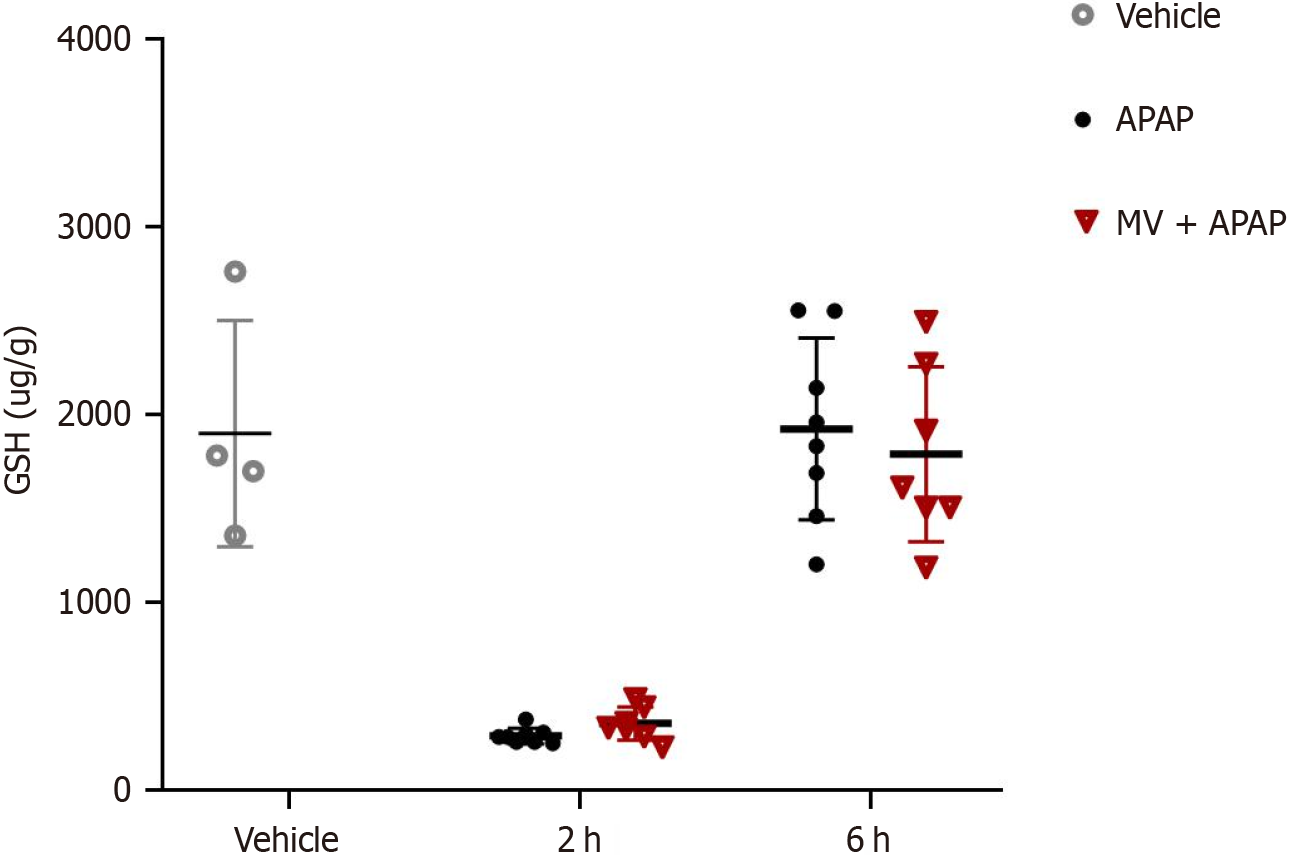
Given that GSH is a vital molecule involved in APAP poisoning, we examined the impact of MV on GSH levels. Two hours post-APAP poisoning, the GSH levels in the liver were significantly lower in mice treated with APAP; however, six hours later, these returned to baseline levels (Figure 3). Notably, the changes in GSH levels over time did not reveal any significant differences between the MV+APAP group and the APAP-only group. These results suggest that MV does not influence GSH depletion during APAP exposure.
Figure 3 Mogroside V has no effect on glutathione depletion in the liver following acetaminophen-induced acute liver injury.
Mice were pretreated with or without Mogroside V for one hour prior to acetaminophen (APAP) administration, and glutathione levels were measured 2 and 6 hours after APAP exposure. n = 4-8 per group. MV: Mogroside V; APAP: Acetaminophen; GSH: Glutathione.
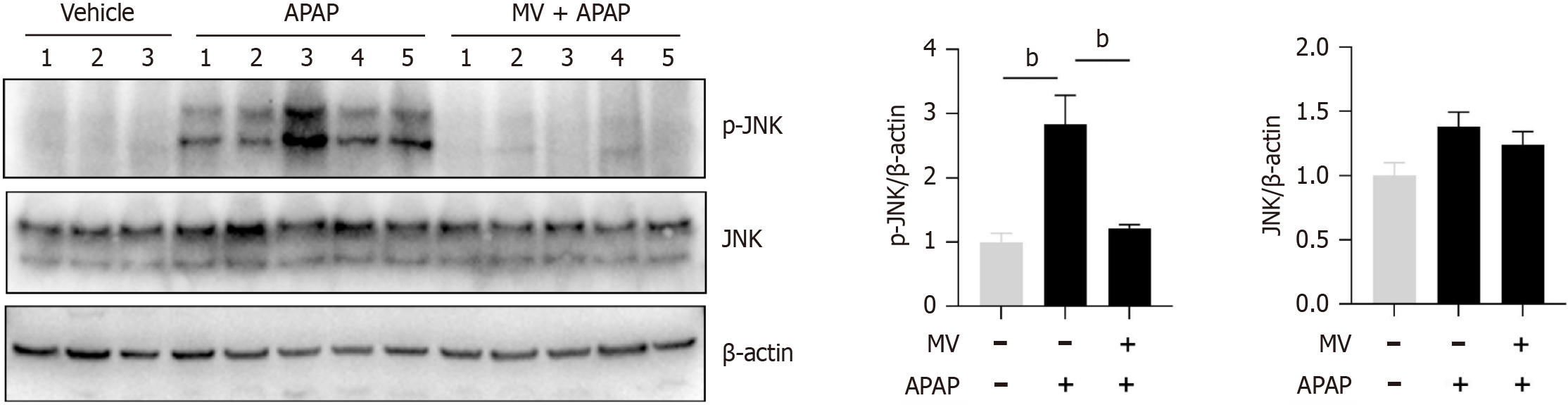
MV inhibits JNK activation and reactive oxygen species production in the liver of APAP-treated mice
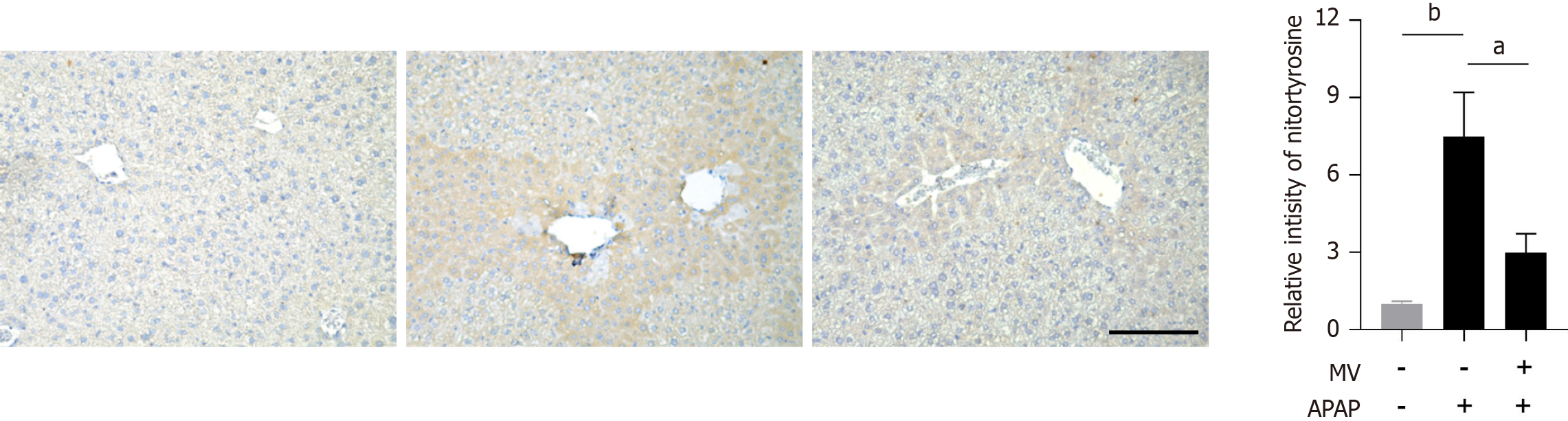
The activation of JNK is crucial for hepatocyte necrosis induced by APAP. We subsequently investigated whether MV affected JNK activation in the liver post-APAP treatment. The levels of p-JNK were found to be considerably increased in the liver of mice treated with APAP (Figure 4). However, MV pre-treatment markedly inhibited JNK activation (Figure 4). Given that MV contains several hydroxyl groups with antioxidant properties, we investigated the effect of MV on reactive oxygen species (ROS) production by APAP by assessing nitrotyrosine (the product of a type of ROS) levels using IHC staining. Nitrotyrosine was predominantly and abundantly expressed in the necrotic areas adjacent to the central vein in the livers of the APAP group (Figure 5). Notably, the nitrotyrosine levels in the MV+APAP group were significantly lower compared to those in the APAP group. These results suggest that MV pre-treatment can inhibit JNK activation by reducing ROS accumulation in the necrotic areas of mice treated with APAP.
Figure 4 Mogroside V inhibits acetaminophen-induced c-jun-N-terminal kinase activation in the liver of acetaminophen-treated mice.
Mice were pretreated with or without Mogroside V for one hour prior to acetaminophen administration, and the protein level of phosphorylated c-jun-N-terminal kinase (JNK) and total JNK were measured six hours later. n = 3-5 per group. aP < 0.05, bP < 0.01. MV: Mogroside V; APAP: Acetaminophen; GSH: Glutathione.
Figure 5 Mogroside V reduces nitrotyrosine levels in the liver of acetaminophen-treated mice.
Representative images of immunohistochemistry staining and the corresponding relative intensity of nitrotyrosine are presented. Scale bar, 50 μm. n = 4 per group. bP < 0.01. MV: Mogroside V; APAP: Acetaminophen; GSH: Glutathione.
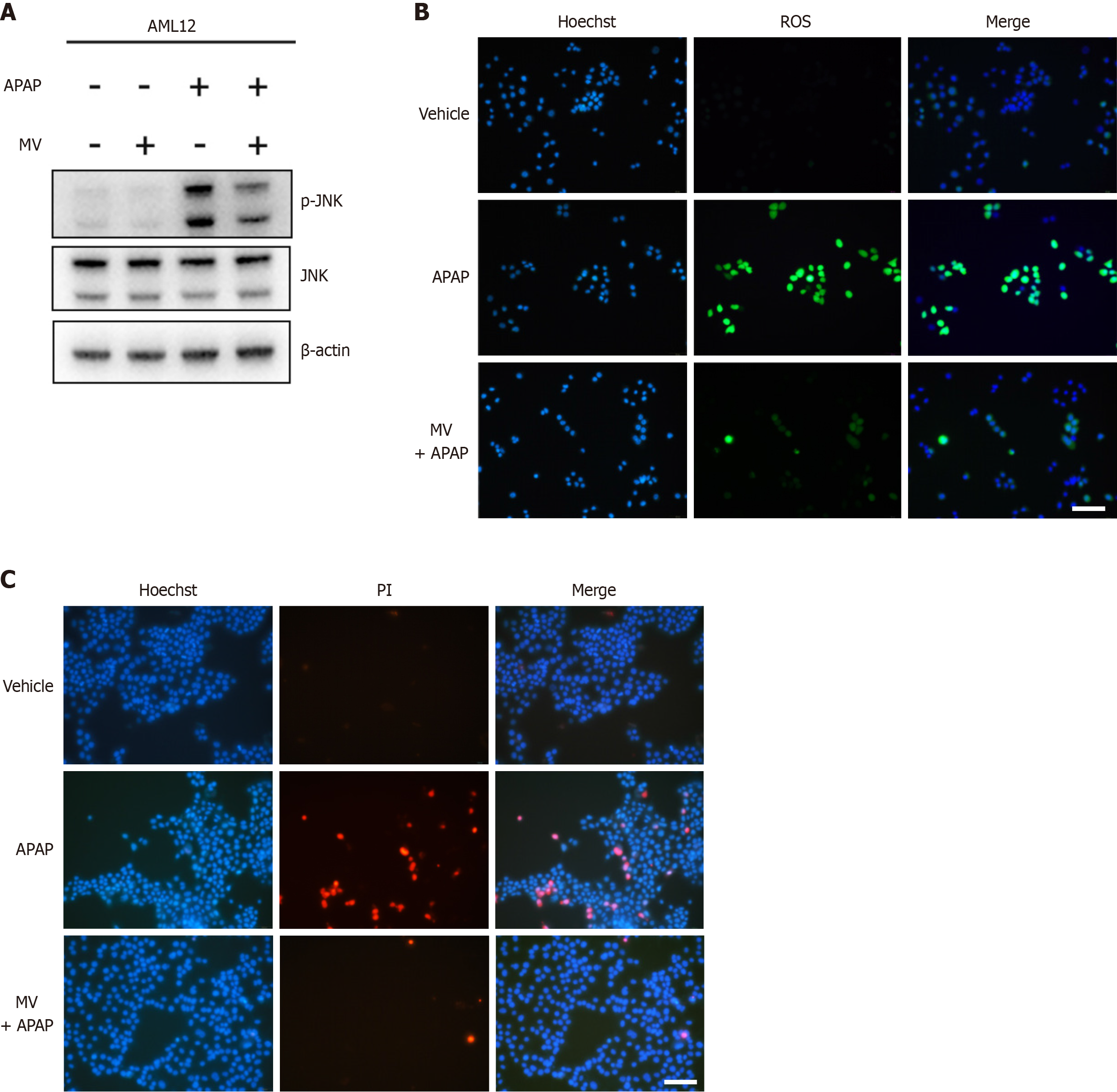
MV mitigates hepatocyte damage induced by APAP in vitro
We further utilized the mouse hepatocytes AML12 Line to evaluate the impact of MV on APAP-induced hepatocyte damage. As expected, MV mitigated APAP-induced phosphorylation of JNK (Figure 6A). ROS are known to activate JNK in APAP-induced ALI; therefore, we used the H2DCFDH fluorescence probe to measure the effect of MV on ROS production in AML12 cells. Intracellular ROS accumulation significantly increased following APAP stimulation (Figure 6B). Notably, MV alleviated this accumulation (Figure 6B). Furthermore, PI staining revealed that MV inhibited APAP-induced cell death in AML12 hepatocytes (Figure 6C). These findings further support the idea that MV may protect against APAP-induced hepatocyte damage through diminishing ROS production and subsequent JNK activation.
Figure 6 Mogroside V attenuates acetaminophen-induced hepatocytes damage in AML12 cells.
AML 12 cells were treated with acetaminophen (APAP) (20 mM) supplemented with or without mogroside V (100 μM). A: Western blotting was used to detect the protein expression of p-c-jun-N-terminal kinase (JNK) and JNK after APAP exposure for six hours; B: Reactive oxygen species levels were detected 3 hours after APAP exposure; C: Cell death was measured by PI/Hoechst 33342 staining 24 hours after APAP exposure. Scale bar, 100 μm. MV: Mogroside V; APAP: Acetaminophen; GSH: Glutathione.
DISCUSSION
In the current research, we performed a preventive intervention by administering MV solution via oral gavage to assess, for the first time, MV's protective role against APAP-induced ALI. The findings revealed that mice pretreated with MV showed significant resistance to APAP-induced ALI, which was linked to a reduction in ROS production and JNK activation.
APAP-induced ALI is characterized by the production of NAPQI, depletion of GSH, formation of mitochondrial protein adducts, generation of ROS, JNK activation, and mitochondrial dysfunction, ultimately culminating in hepatocyte necrosis and sterile inflammation. In our investigation, both the seven-day consecutive treatment and the single dose of MV substantially mitigated APAP-induced ALI, as indicated by lower serum levels of ALT and AST, fewer dead cells, reduced necrotic areas, and less inflammatory cell infiltration in the liver. It is well established that the toxic metabolite NAPQI can react with intracellular GSH during the initial phase of APAP exposure, resulting in a rapid depletion of GSH levels within two hours, subsequently intracellular GSH gradually returns to the initial levels[22,23]. Notably, MV pre-treatment did not alter GSH levels at either two or six hours after APAP exposure, suggesting that MV's protective effect is not linked to the metabolic process involving intracellular GSH consumption triggered by NAPQI.
Activation of JNK is essential in the response to cellular stress; however, persistent activation may result in cellular damage and necrosis. It is widely accepted that JNK activation is critical in the context of APAP-induced ALI, and the inhibition of JNK activation has been demonstrated to prevent APAP-induced ALI[24,25]. Additionally, diacerein offers protection against APAP-related injury by blocking JNK-driven oxidative stress and apoptosis[26]. Importantly, our findings suggest that MV treatment can considerably reduce the sustained JNK activation triggered by APAP exposure.
ROS is an important biomarker of oxidative stress with high biological activity. In hepatocytes after APAP exposure, NAPQI protein adducts form on mitochondria, initiating the opening of mitochondrial membrane permeability transition pores. This process leads to mitochondria oxidative stress and dysfunction, subsequently generating substantial amounts of ROS[2]. ROS has been identified as activators of the intracellular JNK pathway[27]. Thus, JNK activation and ROS production mutually enhance each other and contribute to APAP-induced necrosis in hepatocytes. A previous investigation demonstrated that MV could lower intracellular ROS levels and enhanced the expression of genes associated with oxidative stress in an in vitro porcine oocyte culture model[28]. Given that MV contains several hydroxyl groups with antioxidant properties, it is highly plausible that the bioactivity of MV is intricately linked to ROS in the context of APAP poisoning. To verify this hypothesis, we investigated whether MV could reduce ROS levels following APAP exposure. Our findings confirm that MV significantly decreased intracellular ROS levels in AML12 cells subjected to APAP exposure. In addition to MV, polysaccharides from Dendrobium officinale[29] and a phytoextract of Indian mustard seeds[30], have also been found to alleviate APAP-induced liver toxicity, presumably by decreasing ROS levels.
Nitrotyrosine is produced when protein tyrosine residues undergo nitration by the peroxynitrite anion, and its levels are frequently used as an indicator of ROS levels[31]. Nitration of proteins at the subcellular level can impair their normal conformation and physiological function to some extent[32]. This tyrosine nitration caused by peroxynitrite affects various active molecules in the body, including lipids, proteins and DNA, thereby contributing to disease progression. Previous studies have documented the accumulation of nitrotyrosine in conditions such as inflammation[33], acute lung injury[34], neurodegenerative diseases[35] and other pathological processes. Through IHC staining, we observed a notable accumulation of toxic nitrotyrosine in the damaged areas of the liver in APAP-treated mice, which is consistent with previous reports[36]. Notably, the nitrotyrosine level in the liver of MV-pretreated mice was dramatically reduced, suggesting that MV may also protect hepatocytes from nitration caused by APAP.
As the awareness of APAP hepatotoxicity has increased, various alternative strategies and experimental models, including both animal models and cell lines, have been employed to screen for substances that can prevent or treat APAP-induced hepatotoxicity from multiple perspectives. Herein, we utilized the AML12 cell line to investigate the hepatoprotective activity of MV. Specifically, we analyzed ROS levels, phosphorylated JNK level and cell death in vitro. Our in vitro experimental results indicated that MV alleviated the accumulation of ROS in AML12 cells caused by APAP, further mitigated JNK activation, and reduced cell death, which were consistent with our in vivo experimental findings.