Published online Mar 27, 2025. doi: 10.4254/wjh.v17.i3.103261
Revised: February 1, 2025
Accepted: March 4, 2025
Published online: March 27, 2025
Processing time: 132 Days and 18 Hours
Portal hypertension (PHT), a complication of liver cirrhosis, is sometimes managed with transjugular intrahepatic portosystemic shunt (TIPS) to reduce portal pressure. Although effective, TIPS poses risks, including hepatic encephalopathy (HE). This study investigates whether a significant reduction in the portal pressure gradient (PPG) after TIPS improves outcomes in PHT patients.
To evaluate the impact of post-TIPS PPG reduction on clinical outcomes and explore the relationship between PPG reduction and portal vein diameter.
This retrospective cohort study included 815 patients with PHT who underwent TIPS at two tertiary hospitals between 2014 and 2022. Patients were categorized based on whether they achieved a 50% reduction in PPG. Propensity score matching was applied to balance baseline characteristics. Kaplan-Meier analysis assessed clinical outcomes, including rebleeding, HE, liver failure, and hepatocellular carcinoma. Cox regression identified risk factors, and Spearman correlation analyzed the relationship between PPG reduction and portal vein diameter.
Patients with a PPG reduction > 50% had significantly lower risks of rebleeding (P = 0.004), shunt dysfunction (P = 0.002), and mortality (P = 0.024) compared to those with a PPG reduction ≤ 50%. However, these patients faced higher risks of HE (P < 0.001) and liver failure (P = 0.003). A significant negative correlation was observed between the percentage of PPG reduction and portal vein diameter (ρ = -0.632, P < 0.001), suggesting that patients with smaller portal vein diameters may achieve greater PPG reductions.
A significant PPG reduction following TIPS is associated with improved clinical outcomes, including reduced risks of rebleeding, shunt dysfunction, hepatocellular carcinoma, and mortality, though it increases HE and liver failure risks. The observed correlation between portal vein diameter and PPG reduction highlights the potential role of portal vein anatomy in predicting TIPS efficacy, warranting further investigation.
Core Tip: This study evaluates the impact of portal pressure gradient (PPG) reduction following transjugular intrahepatic portosystemic shunt (TIPS) on clinical outcomes in portal hypertension patients. Findings reveal that a significant PPG reduction (> 50%) post-TIPS is linked to decreased risks of rebleeding, shunt dysfunction, hepatocellular carcinoma, and mortality. However, it also raises the risk of hepatic encephalopathy and liver failure. Additionally, portal vein diameter influences the degree of PPG reduction. These insights highlight the need for personalized management strategies to optimize TIPS outcomes, balancing benefits and potential risks for improved patient prognosis.
- Citation: Wang ZB, Zhu B, Meng MM, Wu YF, Zhang Y, Li DZ, Tian H, Wang FC, Lv YF, Ye QX, Liu FQ. Effect of portal pressure gradient reduction on outcomes after transjugular intrahepatic portosystemic shunt in portal hypertension patients. World J Hepatol 2025; 17(3): 103261
- URL: https://www.wjgnet.com/1948-5182/full/v17/i3/103261.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i3.103261
Portal hypertension (PHT) is a common complication in patients with cirrhosis and chronic liver disease. The global burden of cirrhosis remains substantial, with approximately 1.32 million deaths attributed to cirrhosis annually, as reported in the 2017 Global Burden of Disease study[1]. Hepatitis B and C have historically been major contributors to cirrhosis, with the highest prevalence of hepatitis C observed in Mediterranean regions, including Egypt[2,3]. Among the complications of PHT, esophagogastric variceal bleeding is associated with the highest mortality, making it one of the most life-threatening outcomes of cirrhosis[4-8].
With advancements in the understanding of PHT complications, interventional treatments like transjugular intrahepatic portosystemic shunt (TIPS) have been developed to effectively reduce portal pressure. Introduced in the late 1980s, TIPS is a well-established therapeutic option for managing complications of PHT, particularly in patients at high risk for recurrent variceal bleeding or refractory ascites[9-13]. It significantly lowers portal pressure, reduces the risk of recurrent bleeding, and improves outcomes in appropriately selected patients. However, TIPS is not without limitations; it is associated with complications such as hepatic encephalopathy (HE), shunt dysfunction, and liver failure[14,15]. Moreover, post-TIPS outcomes, including survival, risk of rebleeding, and liver function deterioration, vary widely among individuals and are influenced by several factors, including the degree of reduction in the portal pressure gradient (PPG)[16].
In clinical practice, the reduction in PPG is widely regarded as a critical indicator for assessing prognosis and long-term survival following TIPS[17]. Previous studies have demonstrated that a significant reduction in PPG is associated with better control of PHT, as well as a reduced risk of liver-related complications and mortality[18,19]. However, the impact of PPG reduction on specific clinical outcomes, such as rebleeding, HE, and mortality, remains insufficiently explored. This study aims to investigate the relationship between PPG reduction and these clinical outcomes post-TIPS, building on prior findings to provide a clearer understanding and offer insights for optimizing patient selection and post-procedure management.
Patients who underwent TIPS for PHT at the Beijing Shijitan Hospital, the Capital Medical University, and the Fifth Medical Center of the Chinese People’s Liberation Army General Hospital, between June 2014 and January 2022, were considered for this study. The primary indication for TIPS was rescue therapy for uncontrolled variceal bleeding seco
The sample size was determined using data from prior prognostic studies[4,9]. With a significance level of α = 0.05 and a power of 0.8, the minimum sample size was calculated based on the expected incidence of positive outcomes. An anticipated follow-up loss rate of between 10% to 15% was factored in. Patients were divided into two groups: Group A (with a PPG reduction ≤ 50%) and group B (with a PPG reduction > 50%). Propensity score matching (PSM) was applied to balance baseline characteristics between the two groups using variables such as age, sex, etiology of liver disease, and Child-Turcotte-Pugh score, with a 2:1 matching ratio and a caliper value of 0.03.
The data for this study were gathered from the patients’ electronic medical records. Demographic and clinical information were documented by the attending physician. Imaging data were collected from computed tomography. Laboratory data were recorded in accordance with standard laboratory protocols[20].
All TIPS procedures in this study were performed by experienced interventional radiologists using a standardized protocol and a transjugular approach under fluoroscopic guidance[10]. Local anesthesia was routinely used to ensure patient comfort during the procedure. A catheter was introduced into the inferior vena cava (IVC) and hepatic vein, followed by angiography to delineate vascular anatomy. The portal vein was punctured through the hepatic segment of the IVC or the hepatic vein, and a catheter was advanced into the splenic vein or superior mesenteric vein to perform portography. Portal vein pressure was measured under stable conditions to establish the baseline PPG. A covered stent was deployed between the portal vein and the hepatic vein/IVC to create the shunt after dilation of the hepatic paren
All patients were monitored for a period of 2 years. Follow-up data were collected through outpatient visits, telephone interviews, and electronic medical records. Repeated PPG measurements were not performed during the follow-up period due to ethical considerations, the invasive nature of the procedure, and concerns regarding patient compliance. The primary outcomes assessed included rebleeding, shunt dysfunction, HE, HCC, liver failure, and death. Rebleeding was defined as any episode of hematemesis or melena requiring medical intervention. Shunt dysfunction was identified via imaging or clinical signs of recurrent PHT. Mortality was defined as death from any cause during follow-up. To address the potential impact of loss to follow-up, survival analyses (Kaplan-Meier method and Cox regression models) were based on the assumption of non-informative censoring, where patients lost to follow-up were censored at the time of their last recorded contact.
The statistical methods of this study were reviewed by Wang ZB from Beijing Shijitan Hospital, Capital Medical University. Statistical analyses were conducted using R software (version 4.4.1, https://www.R-project.org). Continuous variables were tested for normality using the Shapiro-Wilk test and were uniformly expressed as medians (interquartile ranges). Logarithmic (ln) transformation was applied to skewed continuous variables to improve normality before analysis. Group comparisons for continuous variables were performed using the Mann-Whitney U test. Categorical variables were expressed as frequencies and percentages, with comparisons made using the χ2-test or Fisher’s exact test. There were no missing data in this study. Survival analysis was conducted using the Kaplan-Meier method, and differences between groups were assessed with the log-rank test. Least absolute shrinkage and selection operator (LASSO) regression was employed to identify significant variables, followed by univariate and multivariate Cox regression analyses to evaluate risk factors. A two-sided P value of < 0.05 was regarded as statistically significant.
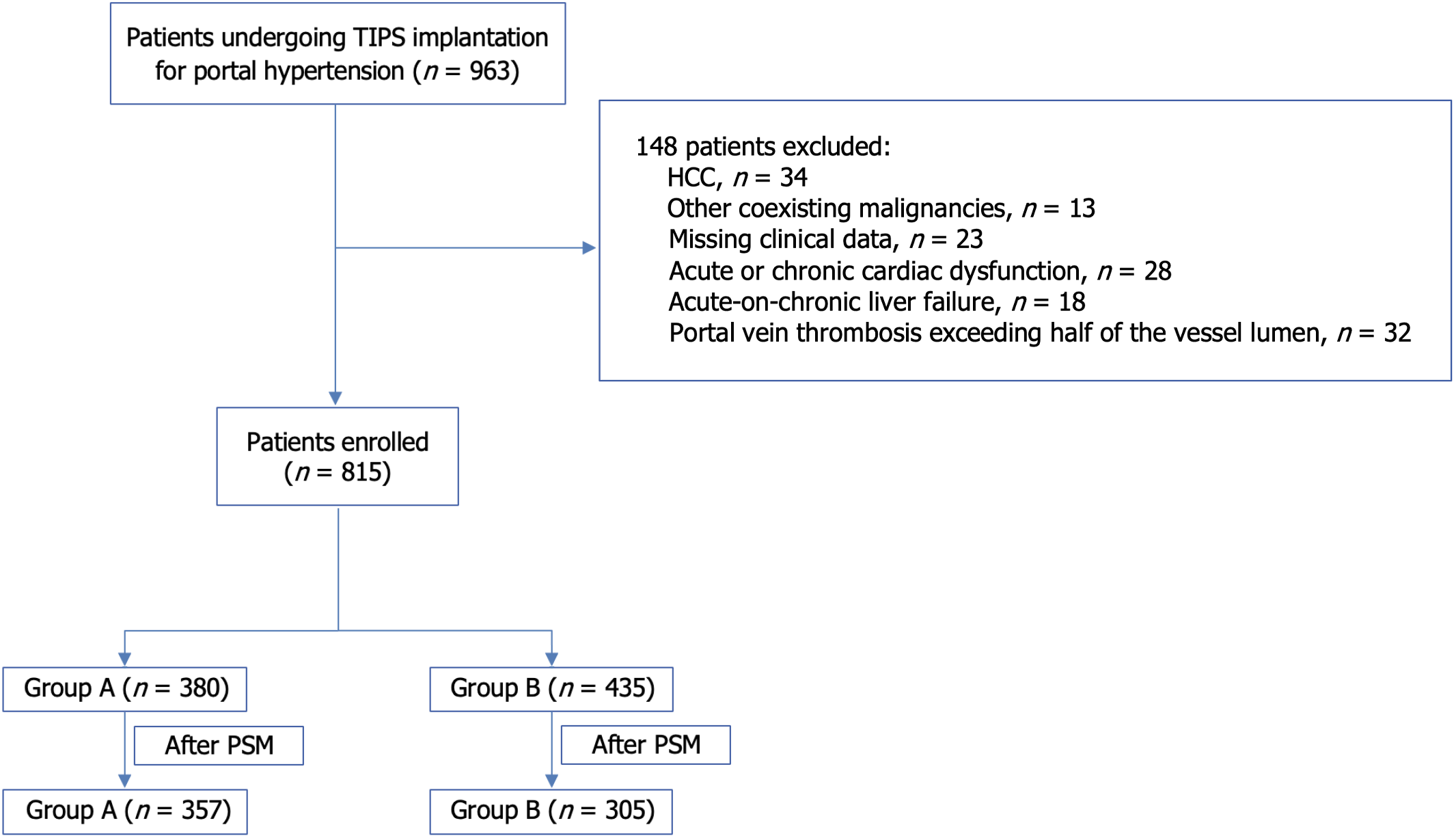
Initially, 963 patients who underwent TIPS for PHT were considered for this study. A total of 148 patients were excluded based on the exclusion criteria. They included those with pre-existing HCC (n = 34), other malignancies (n = 13), missing key clinical data (n = 23), those with acute or chronic cardiac dysfunction (n = 28), those with acute-on-chronic liver failure (n = 18), and patients who had a portal vein thrombosis which occupied more than half of the vessel lumen (n = 32). This resulted in only 815 patients being eligible for inclusion. During the 2-year follow-up period, 93 patients (11.41%) were lost to follow-up.
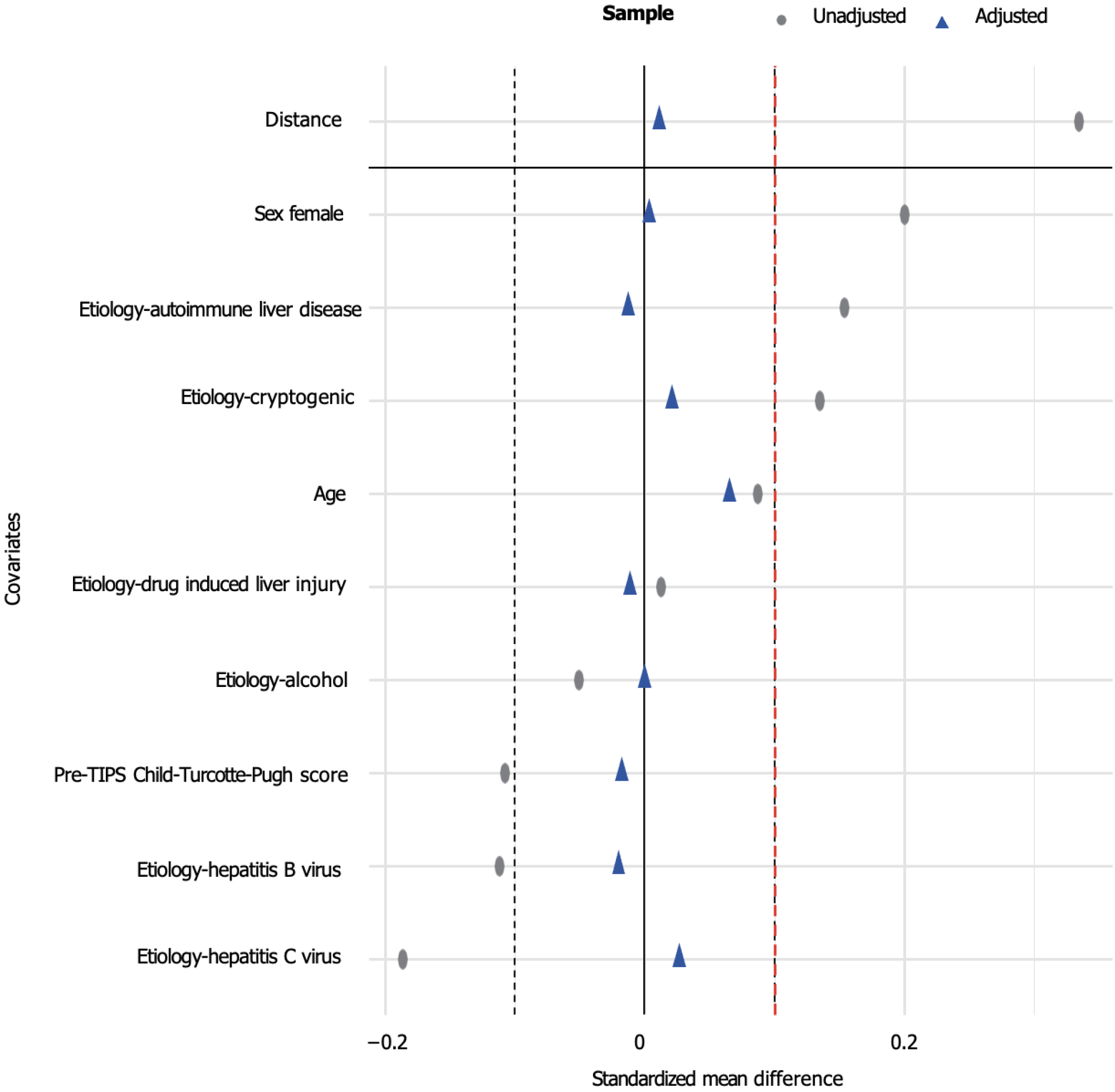
The patients were subsequently divided into two groups based on the percentage reduction in PPG following TIPS: Group A (with a PPG reduction ≤ 50%, n = 380) and group B (with a PPG reduction > 50%, n = 435). Significant differences in baseline characteristics were observed between the two groups. Using the PSM, the differences between the groups were minimized. Prior to matching, the standardized mean difference (SMD) of the propensity scores was 0.3344. After matching, the SMD for all considered variables approached 0, and the SMD for the propensity score decreased to 0.0115. This was an indication that baseline characteristics were well balanced. The final sample included 357 patients in group A and 305 patients in group B. Despite the successful matching, the portal vein diameter remained significantly different between the two groups (P < 0.001), with patients group A having a larger portal vein diameter than those in group B (Supplementary Table 1, Figures 1 and 2).
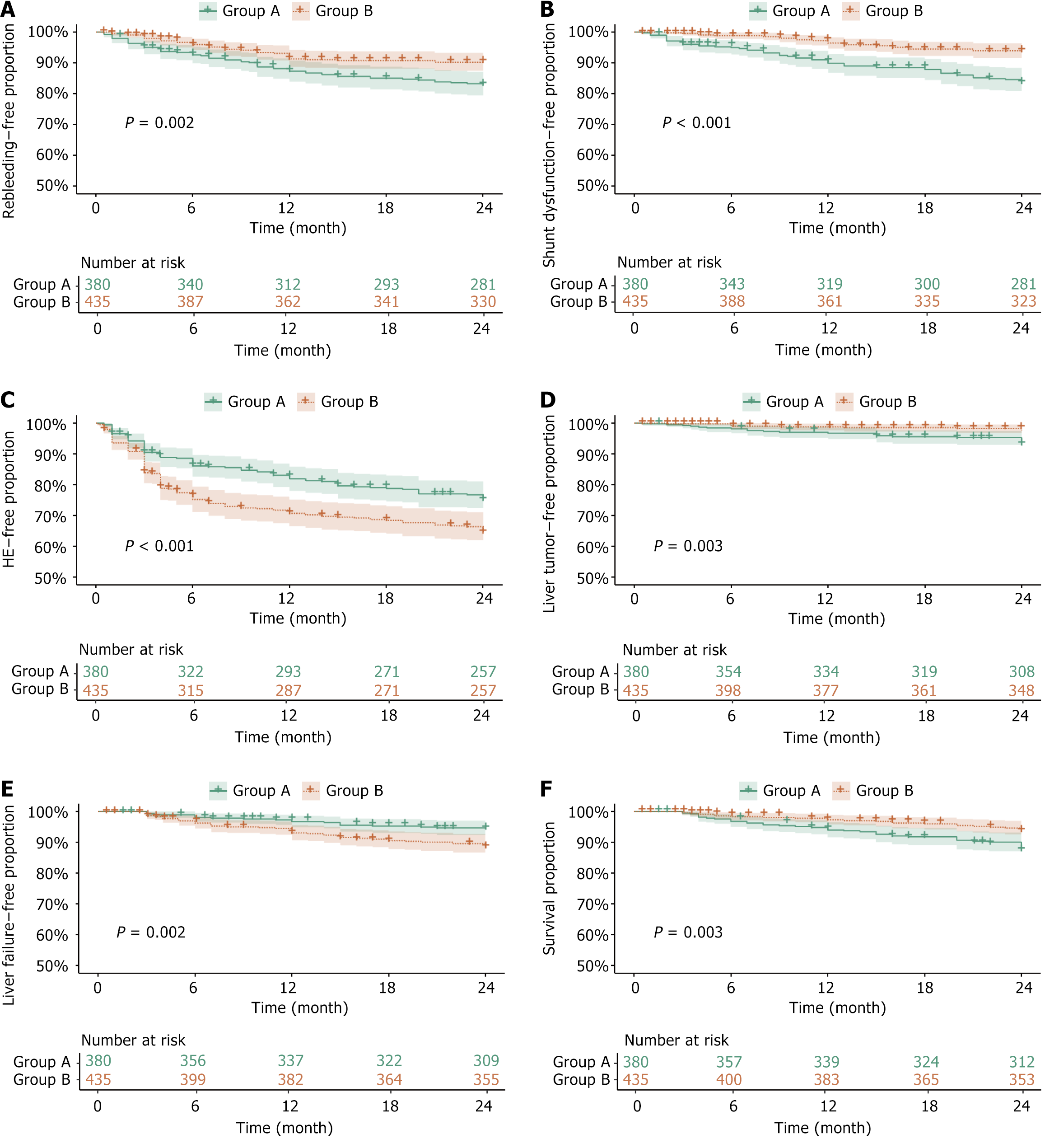
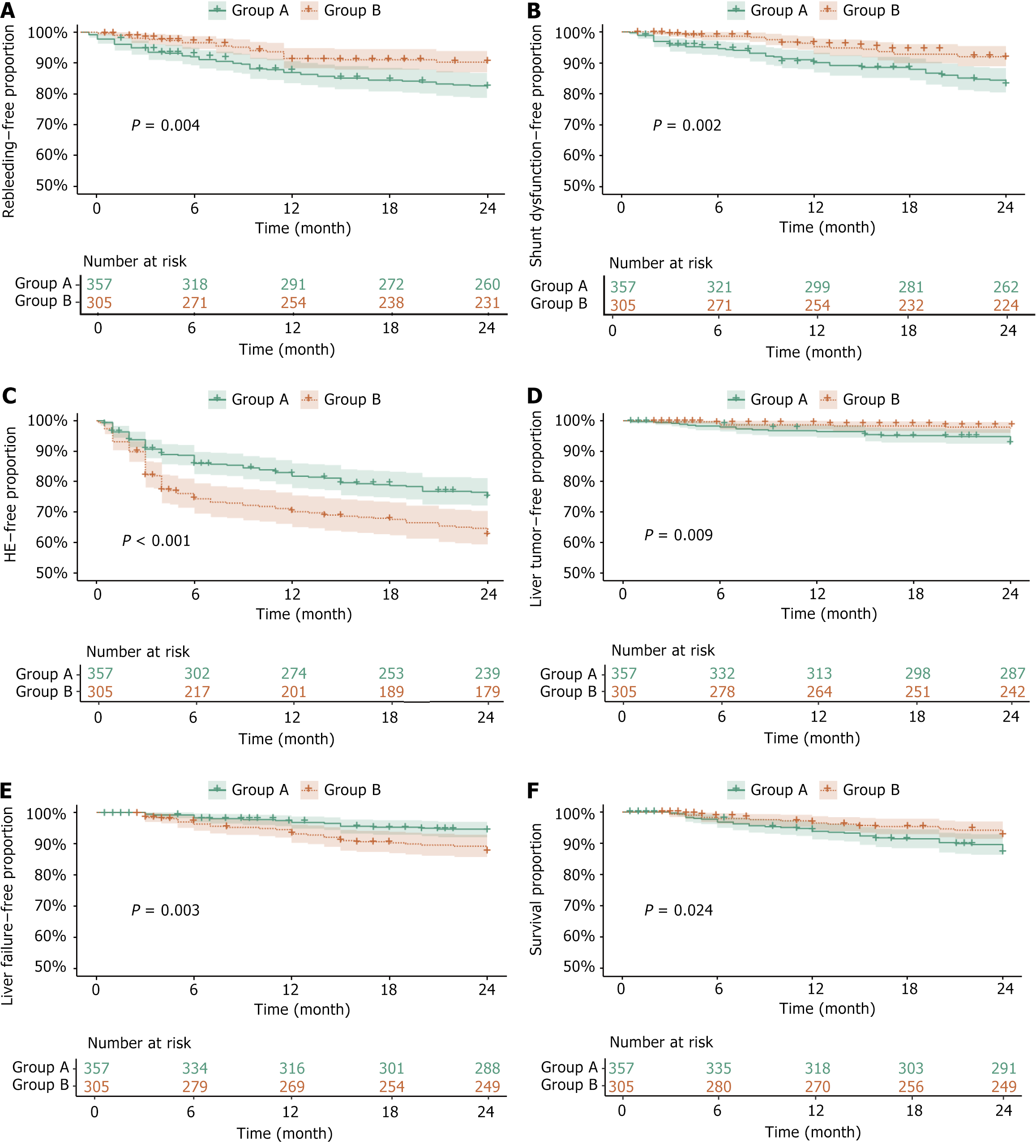
Recurrent variceal bleeding: Before PSM, the rebleeding-free rates for group A and B at 6, 12, 18, and 24 months were 92.63% vs 96.09%, 87.63% vs 91.95%, 85.53% vs 91.49%, and 83.68% vs 91.03% respectively, showing statistically significant differences (P = 0.002). A univariate analysis revealed that the risk of rebleeding was significantly lower in group B compared to group A [hazard ratio (HR) = 0.546, P = 0.003], while a multivariate analysis showed no statistically significant difference (HR = 0.623, P = 0.052). Serum potassium emerged as a protective factor in both univariate and multivariate analyses (HR = 0.305, P = 0.014). Although cancer antigen 153 and creatinine were associated with rebleeding risk in univariate analysis, there was no significant difference in the multivariate analysis. After PSM, the rebleeding-free rates for group A and B at 6, 12, 18, and 24 months were 92.44% vs 96.72%, 87.39% vs 92.13%, 85.15% vs 91.80%, and 83.19% vs 91.15%, respectively, showing statistically significant differences (P = 0.004). The risk of rebleeding remained significantly lower in group B compared to group A after matching (HR = 0.520, P = 0.005, Table 1, Figures 3A and 4A).
| Characteristics | Before PSM, univariate analyses (n = 815) | Before PSM, multivariate analyses (n = 815) | After PSM, univariate analyses (n = 662) | After PSM, univariate analyses (n = 662) | ||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| Group B | 0.546 | 0.366-0.815 | 0.003b | 0.623 | 0.387-1.004 | 0.052 | 0.520 | 0.330-0.818 | 0.005b | 0.520 | 0.330-0.818 | 0.005b |
| Kalium | 0.305 | 0.120-0.774 | 0.012a | 0.305 | 0.119-0.783 | 0.014a | ||||||
| Cancer antigen 153 | 0.691 | 0.493-0.968 | 0.032a | 0.713 | 0.500-1.016 | 0.061 | ||||||
| Creatinine | 1.952 | 1.123-3.394 | 0.018a | 1.679 | 0.943-2.990 | 0.078 | ||||||
| Pre-TIPS inferior vena cava pressure | 1.629 | 1.010-2.628 | 0.046a | 1.556 | 0.950-2.547 | 0.079 | ||||||
| Pre-TIPS Child-Turcotte-Pugh score | 2.513 | 1.095-5.766 | 0.030a | 1.983 | 0.841-4.679 | 0.118 | ||||||
| Portal vein diameter | 2.487 | 1.216-5.084 | 0.013a | 1.279 | 0.548-2.984 | 0.569 | ||||||
| Alkaline phosphatase | 0.680 | 0.460-1.006 | 0.054 | |||||||||
| Alanine aminotransferase | 0.774 | 0.563-1.065 | 0.116 | |||||||||
Shunt dysfunction: Before PSM, the shunt dysfunction-free rates for group A and B at 6, 12, 18, and 24 months were 95.00% vs 98.85%, 90.26% vs 96.78%, 88.42% vs 95.17%, and 84.47% vs 94.25%, respectively, showing significant differences (P < 0.001). Univariate Cox regression analysis revealed that the risk of shunt dysfunction was significantly lower in group B compared to group A (HR = 0.372, P < 0.001). Multivariate analysis confirmed these findings, showing similar results (HR = 0.452, P = 0.005). Alkaline phosphatase (HR = 0.545, P = 0.007) and serum potassium (HR = 0.302, P = 0.035) were also identified as protective factors. In contrast, the portal vein diameter did not retain statistical significance in the multivariate analysis (P = 0.284). After PSM, the shunt dysfunction-free rates for group A and B at 6, 12, 18, and 24 months were 94.68% vs 98.69%, 90.48% vs 95.74%, 88.52% vs 93.77%, and 84.31% vs 92.46%, respectively, showing significant differences (P = 0.002). The risk of shunt dysfunction remained significantly lower in group B compared to group A (HR = 0.496, P = 0.005), while alkaline phosphatase continued to be a protective factor (HR = 0.640, P = 0.049, Table 2, Figures 3B and 4B).
| Characteristics | Before PSM, univariate analyses (n = 815) | Before PSM, multivariate analyses (n = 815) | After PSM, univariate analyses (n = 662) | After PSM, univariate analyses (n = 662) | ||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| Group B | 0.372 | 0.233-0.594 | < 0.001b | 0.452 | 0.261-0.783 | 0.005b | 0.482 | 0.297-0.783 | 0.003b | 0.496 | 0.305-0.807 | 0.005b |
| Alkaline phosphatase | 0.538 | 0.349-0.830 | 0.005b | 0.545 | 0.350-0.849 | 0.007b | 0.615 | 0.394-0.958 | 0.031a | 0.64 | 0.411-0.998 | 0.049a |
| Kalium | 0.338 | 0.117-0.974 | 0.045a | 0.302 | 0.099-0.922 | 0.035a | ||||||
| Portal vein diameter | 4.075 | 1.885-8.808 | < 0.001b | 1.663 | 0.656-4.214 | 0.284 | ||||||
| C-Reactive protein | 0.926 | 0.854-1.004 | 0.062 | |||||||||
| Serum urea nitrogen | 0.69 | 0.422-1.128 | 0.139 | |||||||||
| Right hepatic vein free pressure | 1.535 | 0.846-2.786 | 0.159 | |||||||||
| Natrium | 0.699 | 0.412-1.187 | 0.185 | |||||||||
| Age | 1.549 | 0.671-3.577 | 0.305 | |||||||||
HE: Before PSM, the HE-free rates for group A and B at 6, 12, 18, and 24 months were 86.32% vs 75.63%, 82.37% vs 71.49%, 79.47% vs 69.43%, and 76.05% vs 65.75%, respectively, showing statistically significant differences (P < 0.001). Univariate and multivariate Cox regression analyses revealed that a larger portal vein diameter was significantly associated with a reduced risk of HE (HR = 0.309, P < 0.001). After PSM, the HE-free rates for group A and B at 6, 12, 18, and 24 months were 86.27% vs 74.75%, 82.35% vs 70.82%, 79.55% vs 68.52%, and 76.19% vs 63.93%, respectively, showing the differences remaining statistically significant (P < 0.001). The portal vein diameter remained a significant protective factor against HE (HR = 0.372, P = 0.001). The risk of HE was significantly higher in group B compared to group A (HR = 1.672, P < 0.001), however, this association showed no statistical significance in the multivariate analysis (P = 0.163, Table 3, Figures 3C and 4C).
| Characteristics | Before PSM, univariate analyses (n = 815) | Before PSM, multivariate analyses (n = 815) | After PSM, univariate analyses (n = 662) | After PSM, univariate analyses (n = 662) | ||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| Portal vein diameter | 0.309 | 0.193-0.494 | < 0.001b | 0.309 | 0.193-0.494 | < 0.001b | 0.299 | 0.179-0.500 | < 0.001b | 0.372 | 0.204-0.676 | 0.001b |
| Group B | 1.672 | 1.260-2.219 | < 0.001b | 1.263 | 0.909-1.755 | 0.163 | ||||||
HCC: Before PSM, the HCC-free rates for group A and B at 6, 12, 18, and 24 months were 98.16% vs 99.08%, 96.84% vs 98.85%, 95.79% vs 98.62%, and 93.68% vs 97.63%, respectively, showing statistically significant differences (P = 0.003). Univariate Cox regression revealed that the risk of HCC was significantly lower in group B compared to group A (HR = 0.332, P = 0.005), with similar results reported in the multivariate analysis (HR = 0.370, P = 0.012). Elevated serum creatinine was associated with a higher risk of HCC (HR = 3.098, P = 0.005), although this association did not retain statistical significance in the multivariate analysis (P = 0.098). The model for end-stage liver disease score showed statistical significance in univariate analysis (HR = 58.775, P = 0.038) but did not retain the significance in the multivariate analysis (P = 0.251). After PSM, the HCC-free rates for group A and B at 6, 12, 18, and 24 months were 98.04% vs 98.69%, 96.64% vs 98.69%, 95.52% vs 98.36%, and 93.28% vs 97.70%, respectively, showing significant differences (P = 0.009). Both univariate (HR = 0.346, P = 0.013) and multivariate (HR = 0.374, P = 0.023) analyses demonstrated a significantly lower risk of HCC in group B compared to group A. Serum creatinine retained its association with an increased risk of HCC after PSM (HR = 3.253, P = 0.004), while prothrombin time activity was identified as a protective factor (HR = 0.128, P = 0.011, Table 4, Figures 3D and 4D).
| Characteristics | Before PSM, univariate analyses (n = 815) | Before PSM, multivariate analyses (n = 815) | After PSM, univariate analyses (n = 662) | After PSM, univariate analyses (n = 662) | ||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| Group B | 0.332 | 0.154-0.713 | 0.005b | 0.37 | 0.171-0.800 | 0.012a | 0.346 | 0.149-0.802 | 0.013a | 0.374 | 0.160-0.874 | 0.023a |
| Creatinine | 3.098 | 1.406-6.828 | 0.005b | 2.144 | 0.868-5.297 | 0.098 | 3.091 | 1.434-6.662 | 0.004b | 3.253 | 1.442-7.340 | 0.004b |
| Prothrombin time activity | 0.593 | 0.293-1.201 | 0.147 | 0.178 | 0.039-0.813 | 0.026a | 0.128 | 0.026-0.623 | 0.011a | |||
| Pre-TIPS MELD score | 58.775 | 1.249-2766.772 | 0.038a | 13.367 | 0.160-1115.608 | 0.251 | ||||||
| Female | 0.422 | 0.163-1.093 | 0.075 | |||||||||
| Alanine aminotransferase | 0.63 | 0.377-1.053 | 0.078 | |||||||||
| Pre-TIPS inferior vena cava pressure | 0.579 | 0.281-1.193 | 0.139 | |||||||||
| White blood cell count | 0.635 | 0.337-1.194 | 0.159 | |||||||||
| Chlorine | 26.688 | 0.261-2726.917 | 0.164 | |||||||||
| Blood ammonia | 0.737 | 0.390-1.393 | 0.348 | |||||||||
| C-reactive protein | 1.058 | 0.931-1.203 | 0.387 | |||||||||
| Hemoglobin | 1.59 | 0.402-6.288 | 0.508 | |||||||||
Liver failure: Before PSM, the liver failure-free rates for group A and B at 6, 12, 18, and 24 months were 98.16% vs 96.32%, 96.84% vs 93.33%, 95.53% vs 91.03%, and 94.74% vs 88.74%, respectively, showing statistically significant differences (P = 0.002). Both univariate and multivariate Cox regression analyses revealed that the risk of liver failure was significantly higher in group B compared to group A (HR = 2.523, P = 0.001). Furthermore, the elevated serum creatinine levels (HR = 3.096, P < 0.001) and right hepatic vein wedge pressure (HR = 2.643, P = 0.013) were associated with an increased risk of liver failure. After PSM, the liver failure-free rates for group A and B at 6, 12, 18, and 24 months were 98.32% vs 96.39%, 96.92% vs 93.44%, 95.52% vs 90.82%, and 94.68% vs 88.20%, respectively, showing significant differences still present (P = 0.003). The risk of liver failure continued to be higher in group B than in group A (HR = 2.241, P = 0.005). The serum creatinine (HR = 3.120, P = 0.002), alanine aminotransferase (HR = 1.541, P = 0.048), and C-reactive protein (CRP, HR = 1.138, P = 0.019) emerged as significant predictors (Table 5, Figures 3E and 4E).
| Characteristics | Before PSM, univariate analyses (n = 815) | Before PSM, multivariate analyses (n = 815) | After PSM, univariate analyses (n = 662) | After PSM, univariate analyses (n = 662) | ||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| Group B | 2.166 | 1.288-3.643 | 0.004b | 2.523 | 1.486-4.283 | 0.001b | 2.238 | 1.284-3.901 | 0.005b | 2.241 | 1.276-3.937 | 0.005b |
| Creatinine | 2.802 | 1.536-5.110 | 0.001b | 3.096 | 1.702-5.630 | < 0.001b | 2.529 | 1.287-4.970 | 0.007b | 3.12 | 1.500-6.486 | 0.002b |
| Right hepatic venous wedge pressure | 2.614 | 1.223-5.586 | 0.013a | 2.643 | 1.229-5.680 | 0.013a | 1.941 | 0.858-4.392 | 0.112 | |||
| Alanine aminotransferase | 1.45 | 0.979-2.146 | 0.064 | 1.683 | 1.091-2.598 | 0.019a | 1.541 | 1.003-2.366 | 0.048a | |||
| Cancer antigen 153 | 0.727 | 0.484-1.092 | 0.124 | 0.814 | 0.511-1.299 | 0.389 | ||||||
| Platelet count | 1.265 | 0.893-1.794 | 0.186 | 1.261 | 0.855-1.859 | 0.242 | ||||||
| Carcinoembryonic antigen | 0.877 | 0.639-1.202 | 0.414 | 0.852 | 0.597-1.218 | 0.38 | ||||||
| C-Reactive protein | 1.166 | 1.049-1.296 | 0.004b | 1.138 | 1.022-1.267 | 0.019a | ||||||
| Alkaline phosphatase | 1.461 | 0.909-2.348 | 0.117 | |||||||||
| Pre-TIPS right atrial pressure | 1.595 | 0.882-2.884 | 0.123 | |||||||||
| International normalized ratio | 0.283 | 0.044-1.845 | 0.187 | |||||||||
| Blood ammonia | 0.704 | 0.440-1.129 | 0.145 | |||||||||
| Pre-TIPS MELD score | 0.6 | 0.024-14.884 | 0.755 | |||||||||
| Activated partial thromboplastin time | 2.952 | 0.880-9.905 | 0.08 | |||||||||
| Hemoglobin | 2.551 | 0.903-7.205 | 0.077 | |||||||||
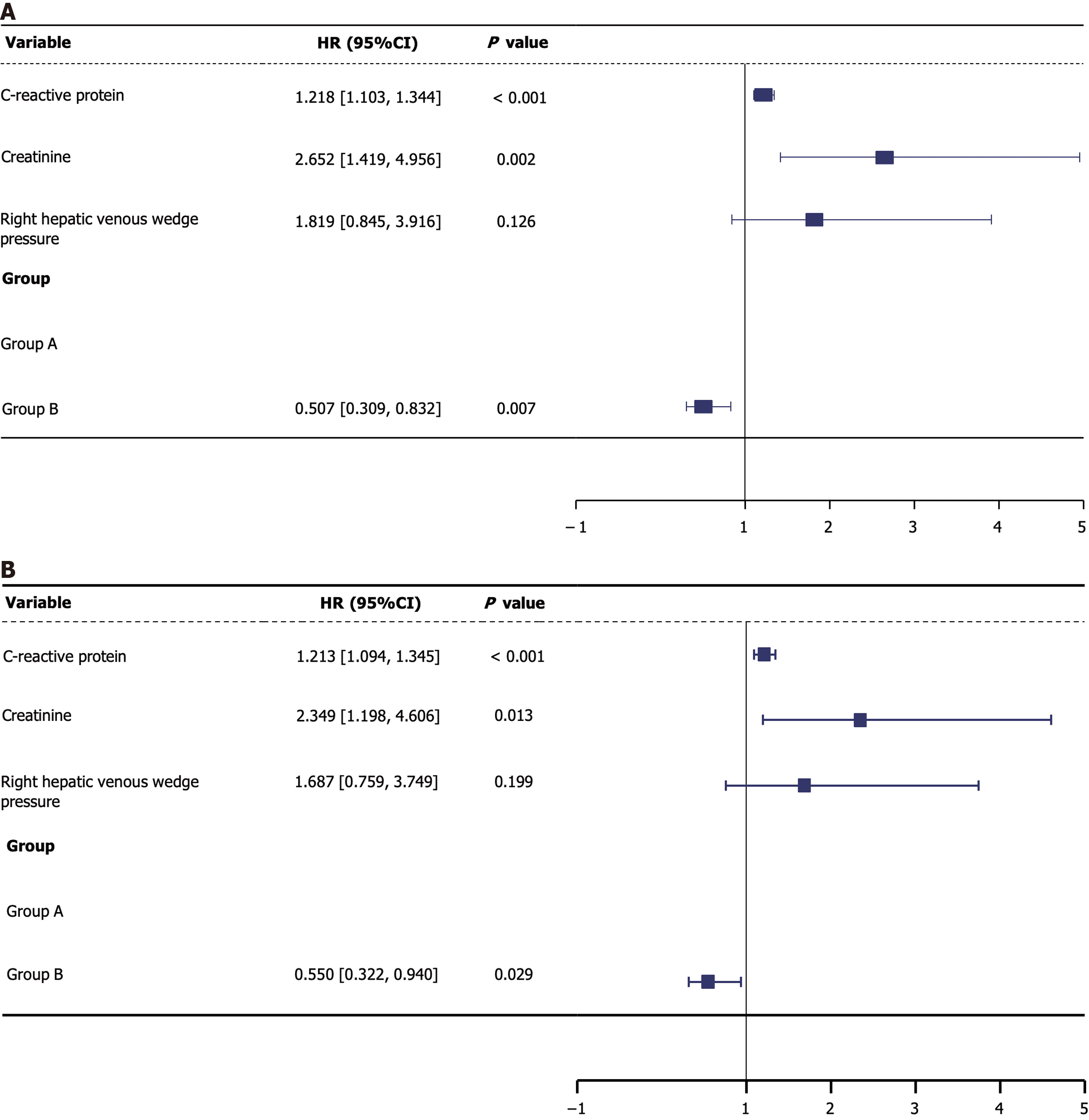
Overall survival: Before PSM, the survival rates for group A and B at 6, 12, 18, and 24 months were 96.84% vs 98.39%, 94.21% vs 97.47%, 92.11% vs 96.32%, and 88.16% vs 94.25%, respectively, showing statistically significant differences (P = 0.003). Univariate Cox regression analysis showed that the risk of death was significantly lower in group B compared to group A (HR = 0.491, P = 0.004) with similar results being reported in the multivariate analysis (HR = 0.507, P = 0.007). Elevated CRP levels were associated with a higher risk of death (HR = 1.218, P < 0.001). The creatinine levels were also statistically significant (HR = 2.652, P = 0.002). Although the right hepatic vein wedge pressure showed statistical significance in the univariate analysis (P = 0.016), it had no significance in the multivariate analysis (P = 0.126). After PSM, the survival rates for group A and B at 6, 12, 18, and 24 months were 96.92% vs 98.03%, 94.12% vs 96.72%, 91.88% vs 95.74%, and 87.96% vs 93.44%, respectively, showing statistically significant differences still observed (P = 0.024). The risk of death remained significantly lower in group B compared to group A (HR = 0.550, P = 0.029). Both CRP (HR = 1.213, P < 0.001) and creatinine (HR = 2.349, P = 0.013) retained significant predictors of mortality after PSM. The right hepatic vein wedge pressure was significant in univariate analysis after PSM (P = 0.048), but not in the multivariate analysis (P = 0.199, Table 6, Figures 3F, 4F and 5).
| Characteristics | Before PSM, univariate analyses (n = 815) | Before PSM, multivariate analyses (n = 815) | After PSM, univariate analyses (n = 662) | After PSM, univariate analyses (n = 662) | ||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| Group B | 0.491 | 0.301-0.801 | 0.004b | 0.507 | 0.309-0.832 | 0.007b | 0.549 | 0.323-0.933 | 0.027a | 0.550 | 0.322-0.940 | 0.029a |
| C-reactive protein | 1.217 | 1.104-1.342 | < 0.001b | 1.218 | 1.103-1.344 | < 0.001b | 1.213 | 1.095-1.344 | < 0.001b | 1.213 | 1.094-1.345 | < 0.001b |
| Creatinine | 2.974 | 1.687-5.243 | < 0.001b | 2.652 | 1.419-4.956 | 0.002b | 2.548 | 1.375-4.721 | 0.003b | 2.349 | 1.198-4.606 | 0.013a |
| Right hepatic venous wedge pressure | 2.514 | 1.187-5.322 | 0.016a | 1.819 | 0.845-3.916 | 0.126 | 2.194 | 1.008-4.772 | 0.048a | 1.687 | 0.759-3.749 | 0.199 |
| Glutamyl transferase | 1.231 | 0.961-1.577 | 0.100 | 1.297 | 0.999-1.683 | 0.051 | ||||||
| Portal vein diameter | 0.642 | 0.271-1.521 | 0.314 | 0.488 | 0.196-1.214 | 0.123 | ||||||
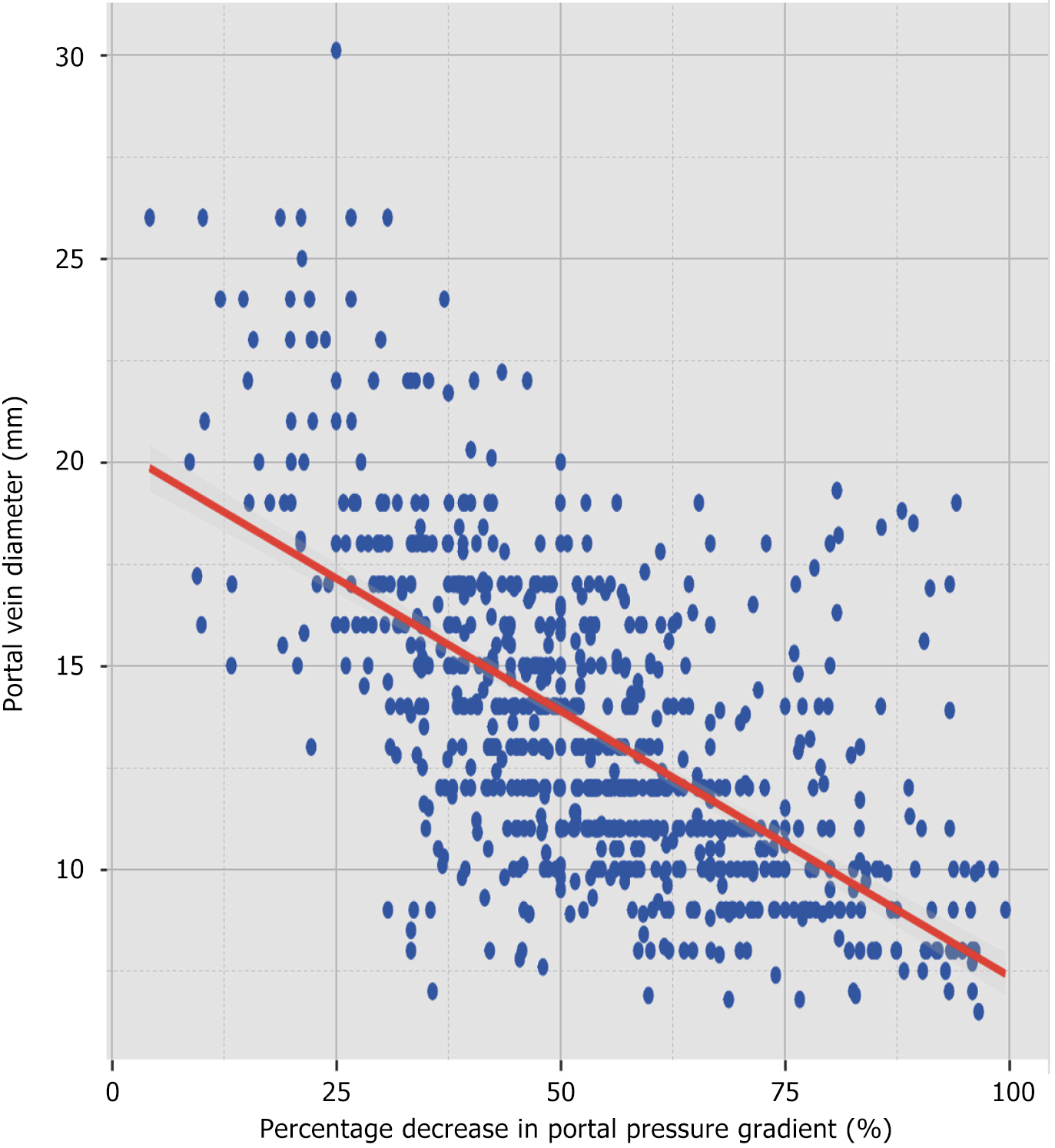
Given that a significant difference in portal vein diameter persisted between group A and B after PSM (P < 0.001), further analysis of the correlation between portal vein diameter and the percentage decrease in PPG was done to investigate the probable cause of this difference. The Shapiro-Wilk normality test showed that neither the percentage decrease in PPG (W = 0.991, P < 0.001) nor the portal vein diameter (W = 0.954, P < 0.001) adhered to a normal distribution, so the Spearman’s rank correlation coefficient was used. The analysis showed a significantly negative correlation between portal vein diameter and the percentage decrease in PPG, with a correlation coefficient of ρ = -0.632 (P < 0.001, Figure 6).
This study evaluated patient prognosis based on the degree of PPG reduction following TIPS. The findings showed that patients with a > 50% reduction in PPG experienced lower rates of rebleeding, shunt dysfunction, HCC, and mortality. This suggested that a substantial decrease in PPG can effectively reduce these adverse effects[24]. However, patients with a > 50% PPG reduction also faced a significantly higher risk of HE and liver failure compared to those with a ≤ 50% reduction. The LASSO regression was employed to identify variables which were significantly associated with positive outcomes, while univariate and multivariate Cox regression analyses were used to confirm main influencing factors, clarifying the roles of various pre- and post-operative variables in patient prognosis.
This research showed that PPG reduction of more than 50% significantly decreased the risk of rebleeding. Some studies have reported that reductions of over 60% were more successful in reducing rebleeding rates, while reductions under 30% were associated with higher risks of rebleeding. Patients with a > 50% PPG reduction had significantly lower rates of HCC compared to those with reductions ≤ 50%. Rössle et al[13] highlighted that the risks of shunt dysfunction, rebleeding, and HCC, were significantly reduced with effective PPG control after TIPS. This view was supported by Praktiknjo et al[14], who suggested that PPG reduction may indirectly lower the occurrence of liver cancer. This study further confirmed that a reduction in PPG effectively lowers mortality, aligning with the findings of Moon et al[2]. While a reduction in PPG can decrease certain complications, exceeding the 50% mark increases the risks of HE and liver failure. This study was able to establish that patients with > 50% PPG reduction, had a significantly higher incidence of liver failure compared to those with reductions ≤ 50%. This suggests that excessive PPG reduction may worsen liver function. Consequently, PPG control after TIPS should be carefully managed, with treatment strategies tailored to factors such as age, model for end-stage liver disease score, and international normalized ratio[2,10,20,25,26]. Correlation analysis revealed a significant negative correlation between the percentage reduction in PPG and portal vein diameter (ρ = -0.632, P < 0.001). This suggested that a greater reduction in PPG corresponds to a smaller portal vein diameter, elucidating the optimization of TIPS treatment strategies. Vizzutti et al[27] also highlighted that portal vein diameter could influence clinical outcomes after TIPS. Further research is required to investigate this mechanism and assess the predictive value of portal vein diameter in TIPS evaluation and PPG reduction.
This study employed several specific methodologies to ensure the reliability and scientific validity of the results. Firstly, PSM was used to balance the baseline characteristics between group A and B, thus minimizing the influence of potential confounders. Additionally, the LASSO regression model was used to identify significant risk factors, which were further validated through univariate and multivariate Cox regression analyses. Secondly, log transformation (ln) was applied to the variables to improve data normality, which is essential when handling skewed data, as it enhances the accuracy of a model. Finally, the significant negative correlation between portal vein diameter and PPG reduction is a key finding of this study. This study confirms this important correlation between portal vein diameter and PPG reduction, suggesting that preoperative assessment of the portal vein diameter may help predict post-TIPS prognosis.
While this study presents compelling evidence for the association between PPG reduction after TIPS and various positive outcomes, it still has some limitations. Firstly, as a two-center retrospective study, there may be issues of selection and information bias. While PSM was used to reduce confounding factors, the generalizability and external validity of these findings need to be confirmed through larger, multi-center prospective studies to ensure broader applicability[15]. Furthermore, this study did not account for potential genetic or hereditary factors, which could significantly have an impact on how patients respond to TIPS and their postoperative outcomes. Future research should also explore the impact of varying degrees of PPG reduction on specific high-risk populations, such as those with severe complications. Multi-center, long-term follow-up studies that integrate individual characteristics and genetic backgrounds, will better assess the generalizability and external validity of post-TIPS strategies, and provide stronger evidences for personalized therapy. Future research should also aim to identify biomarkers related to PPG changes, as well as explore potential genetic or hereditary factors to enhance postoperative prognosis assessment and intervention strategies.
This study comprehensively examined the impact of the percentage reduction in PPG after TIPS on multiple clinical outcomes, further contributing to the theoretical foundation of PHT treatment. The findings indicated that a significant reduction in PPG post-TIPS correlates with reduced risk of rebleeding, shunt dysfunction, HCC, and mortality. However, it also increases the risk of HE and liver failure. Additionally, the portal vein diameter had a significant influence on the degree of PPG reduction.
| 1. | GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1080] [Cited by in RCA: 1008] [Article Influence: 201.6] [Reference Citation Analysis (4)] |
| 2. | Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650-2666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 719] [Article Influence: 143.8] [Reference Citation Analysis (0)] |
| 3. | Fang K, Yang Q, Lin Y, Zheng L, Wang HL, Wu J. Global cirrhosis prevalence trends and attributable risk factors-an ecological study using data from 1990-2019. Liver Int. 2022;42:2791-2799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 4. | Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 638] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 5. | Biecker E. Portal hypertension and gastrointestinal bleeding: diagnosis, prevention and management. World J Gastroenterol. 2013;19:5035-5050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Jakab SS, Garcia-Tsao G. Evaluation and Management of Esophageal and Gastric Varices in Patients with Cirrhosis. Clin Liver Dis. 2020;24:335-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 7. | Alqahtani SA, Jang S. Pathophysiology and Management of Variceal Bleeding. Drugs. 2021;81:647-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Lu HL, Xuan FF, Luo YC, Qin X. Efficacy and safety of transjugular intrahepatic portosystemic shunt combined with transcatheter embolization/chemoembolization in hepatocellular carcinoma with portal hypertension and arterioportal shunt. Abdom Radiol (NY). 2021;46:5417-5427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Bettinger D, Thimme R, Schultheiß M. Implantation of transjugular intrahepatic portosystemic shunt (TIPS): indication and patient selection. Curr Opin Gastroenterol. 2022;38:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Manekeller S, Kalff JC. [Esophageal variceal bleeding: management and tips on transjugular intrahepatic portosystemic shunt]. Chirurg. 2019;90:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Büttner L, Aigner A, Pick L, Brittinger J, Steib CJ, Böning G, Streitparth F. 25 years of experience with transjugular intrahepatic portosystemic shunt (TIPS): changes in patient selection and procedural aspects. Insights Imaging. 2022;13:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 12. | Wang G, Zhang F, Ojeda A, Shalaby S, Hernandez-Gea V, Garcia-Pagan JC. The evolution of the TIPS placement technique and its applications over four decades. Dig Liver Dis. 2024;56:1980-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Rössle M, Siegerstetter V, Olschewski M, Ochs A, Berger E, Haag K. How much reduction in portal pressure is necessary to prevent variceal rebleeding? A longitudinal study in 225 patients with transjugular intrahepatic portosystemic shunts. Am J Gastroenterol. 2001;96:3379-3383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Praktiknjo M, Abu-Omar J, Chang J, Thomas D, Jansen C, Kupczyk P, Schepis F, Garcia-Pagan JC, Merli M, Meyer C, Strassburg CP, Pieper CC, Trebicka J. Controlled underdilation using novel VIATORR® controlled expansion stents improves survival after transjugular intrahepatic portosystemic shunt implantation. JHEP Rep. 2021;3:100264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Trebicka J, Gu W, Ibáñez-Samaniego L, Hernández-Gea V, Pitarch C, Garcia E, Procopet B, Giráldez Á, Amitrano L, Villanueva C, Thabut D, Silva-Junior G, Martinez J, Genescà J, Bureau C, Llop E, Laleman W, Palazon JM, Castellote J, Rodrigues S, Gluud L, Ferreira CN, Barcelo R, Cañete N, Rodríguez M, Ferlitsch A, Mundi JL, Gronbaek H, Hernández-Guerra M, Sassatelli R, Dell'Era A, Senzolo M, Abraldes JG, Romero-Gómez M, Zipprich A, Casas M, Masnou H, Primignani M, Weiss E, Catalina MV, Erasmus HP, Uschner FE, Schulz M, Brol MJ, Praktiknjo M, Chang J, Krag A, Nevens F, Calleja JL, Robic MA, Conejo I, Albillos A, Rudler M, Alvarado E, Guardascione MA, Tantau M, Bosch J, Torres F, Pavesi M, Garcia-Pagán JC, Jansen C, Bañares R; International Variceal Bleeding Observational Study Group and Baveno Cooperation. Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J Hepatol. 2020;73:1082-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 16. | Xia Y, Tie J, Wang G, Zhuge Y, Wu H, Xue H, Xu J, Zhang F, Zhao L, Huang G, Zhang M, Wei B, Li P, Wu W, Chen C, Tang C, Zhang C. Individualized portal pressure gradient threshold based on liver function categories in preventing rebleeding after TIPS. Hepatol Int. 2023;17:967-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Larrue H, D'Amico G, Olivas P, Lv Y, Bucsics T, Rudler M, Sauerbruch T, Hernandez-Gea V, Han G, Reiberger T, Thabut D, Vinel JP, Péron JM, García-Pagán JC, Bureau C. TIPS prevents further decompensation and improves survival in patients with cirrhosis and portal hypertension in an individual patient data meta-analysis. J Hepatol. 2023;79:692-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 52] [Article Influence: 26.0] [Reference Citation Analysis (1)] |
| 18. | Gunarathne LS, Rajapaksha H, Shackel N, Angus PW, Herath CB. Cirrhotic portal hypertension: From pathophysiology to novel therapeutics. World J Gastroenterol. 2020;26:6111-6140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (6)] |
| 19. | Sánchez J, González S, Poyatos P, Escudero MD, Montón C, Carbonell JA, Casula E, Guijarro J, Lluch P, Ballester MP. Recompensation after TIPS reduces the incidence of hepatocellular carcinoma and increases survival in patients with cirrhosis. Liver Int. 2024;44:3072-3082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 20. | Dariushnia SR, Haskal ZJ, Midia M, Martin LG, Walker TG, Kalva SP, Clark TW, Ganguli S, Krishnamurthy V, Saiter CK, Nikolic B; Society of Interventional Radiology Standards of Practice Committee. Quality Improvement Guidelines for Transjugular Intrahepatic Portosystemic Shunts. J Vasc Interv Radiol. 2016;27:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 21. | Kaplan DE, Ripoll C, Thiele M, Fortune BE, Simonetto DA, Garcia-Tsao G, Bosch J. AASLD Practice Guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2024;79:1180-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 136] [Article Influence: 136.0] [Reference Citation Analysis (1)] |
| 22. | Büttner L, Pick L, Jonczyk M, Fehrenbach U, Collettini F, Auer TA, Schnapauff D, De Bucourt M, Wieners G, Gebauer B, Aigner A, Böning G. Shunt dysfunction and mortality after transjugular intrahepatic portosystemic shunt (TIPS) in patients with portal hypertension. Insights Imaging. 2024;15:193. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Caines A, Selim R, Salgia R. The Changing Global Epidemiology of Hepatocellular Carcinoma. Clin Liver Dis. 2020;24:535-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Xiao T, Chen L, Chen W, Xu B, Long Q, Li R, Li L, Peng Z, Fang D, Wang R. Comparison of transjugular intrahepatic portosystemic shunt (TIPS) alone versus TIPS combined with embolotherapy in advanced cirrhosis: a retrospective study. J Clin Gastroenterol. 2011;45:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Coronado WM, Ju C, Bullen J, Kapoor B. Predictors of Occurrence and Risk of Hepatic Encephalopathy After TIPS Creation: A 15-Year Experience. Cardiovasc Intervent Radiol. 2020;43:1156-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Chung HH, Razavi MK, Sze DY, Frisoli JK, Kee ST, Dake MD, Hellinger JC, Kang BC. Portosystemic pressure gradient during transjugular intrahepatic portosystemic shunt with Viatorr stent graft: what is the critical low threshold to avoid medically uncontrolled low pressure gradient related complications? J Gastroenterol Hepatol. 2008;23:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Vizzutti F, Schepis F, Arena U, Fanelli F, Gitto S, Aspite S, Turco L, Dragoni G, Laffi G, Marra F. Transjugular intrahepatic portosystemic shunt (TIPS): current indications and strategies to improve the outcomes. Intern Emerg Med. 2020;15:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |