Published online Mar 27, 2025. doi: 10.4254/wjh.v17.i3.101340
Revised: January 19, 2025
Accepted: February 21, 2025
Published online: March 27, 2025
Processing time: 195 Days and 1 Hours
The pathogenesis of hepatic encephalopathy (HE) remains unclear, and the classical theory of ammonia toxicity lacks sufficient justification.
To investigate the potential of bile acids as intervention targets for HE.
This study employed 42 wild-type male SD rats weighing 200 ± 20 g. Using a random number table method, two rats were randomly selected to undergo common bile duct ligation (BDL). The remaining 40 rats were randomly assigned to four groups serving as controls: The vehicle + control diet (VC) group, the thioacetamide (TAA) group, the TAA + total bile acids (TAAT) group, and the TAA + cholestyramine (TAAC) group. Except for the VC group, all rats were intraperitoneally injected with 100 mg/kg TAA solution once daily for ten consecutive days to establish a HE model. Simultaneously, the TAAT and TAAC groups were administered a diet containing 0.3% bile acids (derived from BDL rats) and 2% cholestyramine, respectively, by gavage for ten days. For the BDL rat model group, the common BDL procedure was performed following the aforementioned protocol. After four weeks, laparotomy revealed swollen bile ducts at the ligation site, and bile was collected. Following successful modeling, behavioral tests, including the elevated plus maze and open field test, were conducted to assess the HE status of the rats. Peripheral blood, liver, and cerebral cortex tissue samples were collected, and the total bile acid content in the serum and cerebral cortex was measured using an enzyme cycling method. The levels of inflammatory factors in the serum and cerebral cortex were analyzed using enzyme-linked immunosorbent assay. Liver histological examination was performed using the hematoxylin-eosin double-labeling method. Reverse transcription polymerase chain reaction, western blot, immunohistochemistry, and other techniques were employed to observe the expression of microglial activation marker ionized calcium-binding adaptor molecule-1 and Takeda G protein-coupled receptor 5 (TGR5) protein.
Compared to the VC group, the TAA group exhibited an exacerbation of HE in rats. The total bile acid content, pro-inflammatory factors [interleukin-1β (IL-1β), IL-6], and the anti-inflammatory factor IL-10 in both the serum and cerebral cortex were significantly elevated. Similarly, the expression of the TGR5 receptor in the cerebral cortex was upregulated. To investigate the impact of total bile acids on HE in rats, comparisons were made with the TAA group. In the TAAT group, the severity of HE was further aggravated, accompanied by increased total bile acid content in the serum and cerebral cortex, elevated pro-inflammatory factors (IL-1β, IL-6), reduced levels of the anti-inflammatory factor IL-10, and decreased expression of the TGR5 receptor in the cerebral cortex. In the TAAC group, the severity of HE was alleviated. This group showed reductions in total bile acid content in the serum and cerebral cortex, decreased pro-inflammatory factors (IL-1β, IL-6), increased levels of the anti-inflammatory factor IL-10, and enhanced expression of the TGR5 receptor in the cerebral cortex.
This study demonstrated that the total bile acid content in the serum and cerebral cortex of TAA-induced liver cirrhosis rats was elevated. Furthermore, total bile acids exacerbate the progression of HE in rats. This effect may be attributed to bile acids’ involvement in the development of neurological dysfunction by mediating TGR5 expression and regulating neuroinflammation.
Core Tip: There are many pathogenic factors for hepatic encephalopathy (HE). This experiment confirmed that total bile acids contribute to the development of HE in liver cirrhosis. The main mechanism is that bile acids mediate the expression of bile acid Takeda G protein-coupled receptor 5 in the brain and then affect microglia activation. However, this experiment only verified the role of total bile acids in HE, but there are many types of bile acids, and we still need to continue to explore in depth which bile acids play and what role they play. In short, bile acids and Takeda G protein-coupled receptor 5 in the brain may become effective targets for treating HE in the future.
- Citation: Ren C, Cha L, Huang SY, Bai GH, Li JH, Xiong X, Feng YX, Feng DP, Gao L, Li JY. Dysregulation of bile acid signal transduction causes neurological dysfunction in cirrhosis rats. World J Hepatol 2025; 17(3): 101340
- URL: https://www.wjgnet.com/1948-5182/full/v17/i3/101340.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i3.101340
Hepatic encephalopathy (HE) is a neuropsychiatric syndrome of varying severity resulting from acute or chronic liver dysfunction or portal-systemic shunt abnormalities. It is fundamentally driven by metabolic disorders[1]. The incidence of liver cirrhosis accompanied by HE ranges from 30% to 45%, and HE is independently associated with increased mortality in patients with liver cirrhosis[2]. While the exact pathogenesis of HE remains unclear, elevated blood ammonia levels are widely recognized as the primary cause of HE[3]. However, in clinical practice, some HE patients present with normal or low blood ammonia levels. Studies suggest that abnormal elevations in bile acids may also contribute to the development of HE[4].
Bile acids are crucial metabolic products synthesized from cholesterol in the body. They play a vital role in emulsifying fats and act as signaling molecules by activating bile acid receptors such as Takeda G protein-coupled receptor 5 (TGR5), sphingosine-1-phosphate receptor 2 (S1PR2), and farnesoid X receptor (FXR). Studies have reported that bile acid levels in the brains of HE patients with acute liver failure are elevated[5], and acute liver failure induces increased bile acid content in the serum and cerebral cortex of HE mice[4]. Interestingly, other research has shown that activation of bile acid receptors may alleviate HE symptoms. For instance, the expression of TGR5 in the brains of HE mice with acute liver failure caused by azoxymethane (AOM) was significantly upregulated, which mitigated neurological dysfunction[6]. However, the relationship between bile acids and HE remains controversial, underscoring the need for further investigation.
Microglial activation and the release of neuroinflammatory cytokines are critical factors in the pathogenesis of HE. Bile acids regulate neuroinflammation and play a significant role in the onset and progression of HE. Previous studies have shown that in mouse models of HE induced by acute liver failure, taurocholic acid (TCA) activates the bile acid receptor S1PR2 in brain neurons, leading to microglial activation and the release of neuroproinflammatory factors[7]. Conversely, in an acute liver failure model induced by AOM, the expression of TGR5 in the cerebral cortex was upregulated, suppressing the release of inflammatory factors such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α), thereby mitigating neurological decline[6]. In AOM-induced HE mouse models, microglial activation was accompanied by the release of neuroproinflammatory factors, including TNF-α and IL-1β.
TGR5 was the first G-protein-coupled receptor identified as specific to bile acids[8]. It is widely expressed in neurons and glial cells within the brain, as well as in other tissues such as the liver and lungs[9]. In a rat model of AOM-induced acute liver failure, TGR5 expression was significantly elevated during the later stages of neurological decline. Central infusion of TGR5 agonists delayed the onset of neurological decline and ataxia[10]. In rodent models of HE, TGR5 activation has been shown to inhibit microglial activation and reduce neuroinflammation by downregulating C-C motif ligand 2 (CCL2) expression in neurons[10]. TGR5 mRNA is detectable in neurons and astrocytes within the brain. However, studies have reported a decrease in TGR5 mRNA levels in astrocyte culture media treated with ammonia. Additionally, prior research has demonstrated that in rat models of acute liver injury, TGR5 expression in liver tissue is increased, suppressing macrophage activation via the cyclic adenosine monophosphate (cAMP) pathway and sub
The relationship between bile acids and HE remains unclear, and no studies have yet explored how bile acids influence the expression of the TGR5 receptor in the brain. Therefore, this study utilized thioacetamide (TAA) to establish a rat model of HE, aiming to elucidate the relationship between bile acids and HE and to investigate how bile acids affect brain TGR5 expression and the downstream mechanisms contributing to HE pathogenesis.
Total bile acid measurement kit (Nanjing Jiancheng Institute of Bioengineering, China); alanine aminotransferase and aspartate aminotransferase measurement kits (Nanjing Jiancheng Institute of Bioengineering, China); rat IL-1β ELISA/rat IL-6 ELISA/rat IL-10 ELISA measurement Kit (Jingmei Biotechnology, China); ionized calcium-binding adaptor molecule-1 (IBA-1) primary antibody (Abcam Company, United Kingdom); TGR5 primary antibody (Abacm Company, United Kingdom); enzyme-labeled goat anti-rat IgG polymer (PV-6000) (Beijing Zhongshan Jinqiao Biotechnology, China); TGR5, GAPDH primers (Thermo Fisher Technology Company, China).
Forty-two specific pathogen-free male SD rats, weighing 200 ± 20 g, aged 6-8 weeks, were retrieved from the Animal Center of Shanxi Medical University and housed at the Public Health Center of Shanxi Medical University. The rearing environment is maintained on a 12-hour light/dark cycle at a temperature of 25 ± 2 °C and a humidity of 25% ± 2%. The study protocol was approved by the Ethics Committee of the First Hospital of Shanxi Medical University. Subsequently, bile duct ligation (BDL) and TAA rat models were established using random number statistical methods, following the 2021 ISNEN Guidelines for Animal Models of HE[12]. Two rats were randomly selected for BDL surgery, and the remaining 40 rats were randomly divided into 4 groups: Vehicle + control diet (VC) group, TAA group, TAA + total bile acids (TAAT) group, and TAA + cholestyramine (TAAC). Except for the VC group, the rat HE model was established by intraperitoneal injection of 100 mg/kg TAA solution once a day for 10 days. During TAA administration, the TAAT and TAAC groups were gavaged with a diet containing 0.3% bile (provided by BDL rats) and 2% cholestyramine once daily for 10 consecutive days. It has been previously reported that a 0.3% bile diet (provided by BDL rats)[13] and a 2% cholestyramine diet[14]. Bile acid content in rodents can be altered. For the BDL rat model group, perform the BDL procedure according to the outlined guidelines. After 4 weeks, the laparotomy revealed swelling of the bile ducts at the ligation site and bile was collected.
All experimental data were presented as mean ± SD and were statistically analyzed using SPSS 27.0 software. Pairwise comparisons between groups were performed using the LSD-t test. Pearson’s correlation analysis is used to test the correlation between measurements that satisfy the assumption of normality, while Spearman’s correlation analysis is used for dose data that does not conform to normality. P < 0.05 was considered to indicate a statistically significant difference.
All rats in each group survived. In the BDL and TAA experimental groups, food intake significantly decreased, mental condition worsened, fur appeared dull, skin color turned yellow, and both stool and urine became yellow. In addition, the rats experienced gradual weight loss.
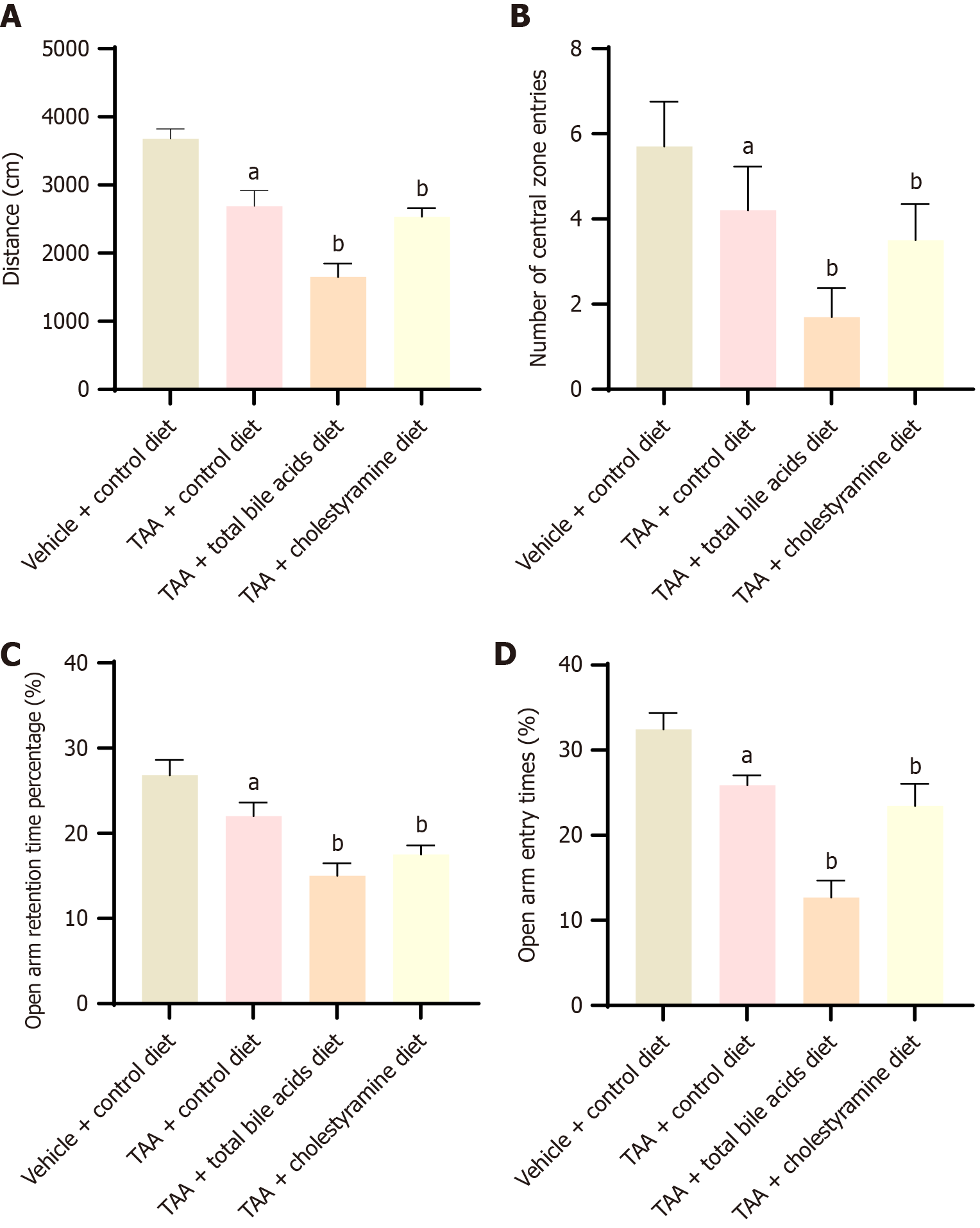
To investigate whether the occurrence of HE following liver cirrhosis is associated with bile acids, open-field and elevated plus-maze tests were conducted to evaluate the neurological characteristics of the rats. In the open-field test, compared with the VC group, the total travel distance and the number of entries into the central area were significantly reduced in the TAA group, indicating successful model establishment. In comparison with the TAA group, the total distance traveled and the number of entries into the central area were further decreased in both TAAT and TAAC groups, with the differences being statistically significant (P < 0.05), and the greatest decrease observed in the TAAT group (Figure 1A and B). The elevated plus-maze test revealed significant differences between all groups according to one-way analysis of variance. LSD-t comparative analysis between groups also identified statistically significant differences. Compared with the VC group, the number of open-arm entries and the percentage of time spent in the open arms were significantly reduced in the TAA group, confirming effective model establishment. Compared with the TAA group, both the number of open-arm entries and the percentage of time spent in the open arms were significantly reduced in the TAAT and TAAC groups (P < 0.05), with the most significant decrease in the TAAT group (Figure 1C and D). These experimental results demonstrate that the TAA-induced liver cirrhosis HE model exhibited significant neurobehavioral changes, including decreased exploratory behavior and increased anxiety. Exogenous intervention with bile acid intake can affect mental and behavioral status, suggesting that bile acids may play a role in the development of HE following liver cirrhosis.
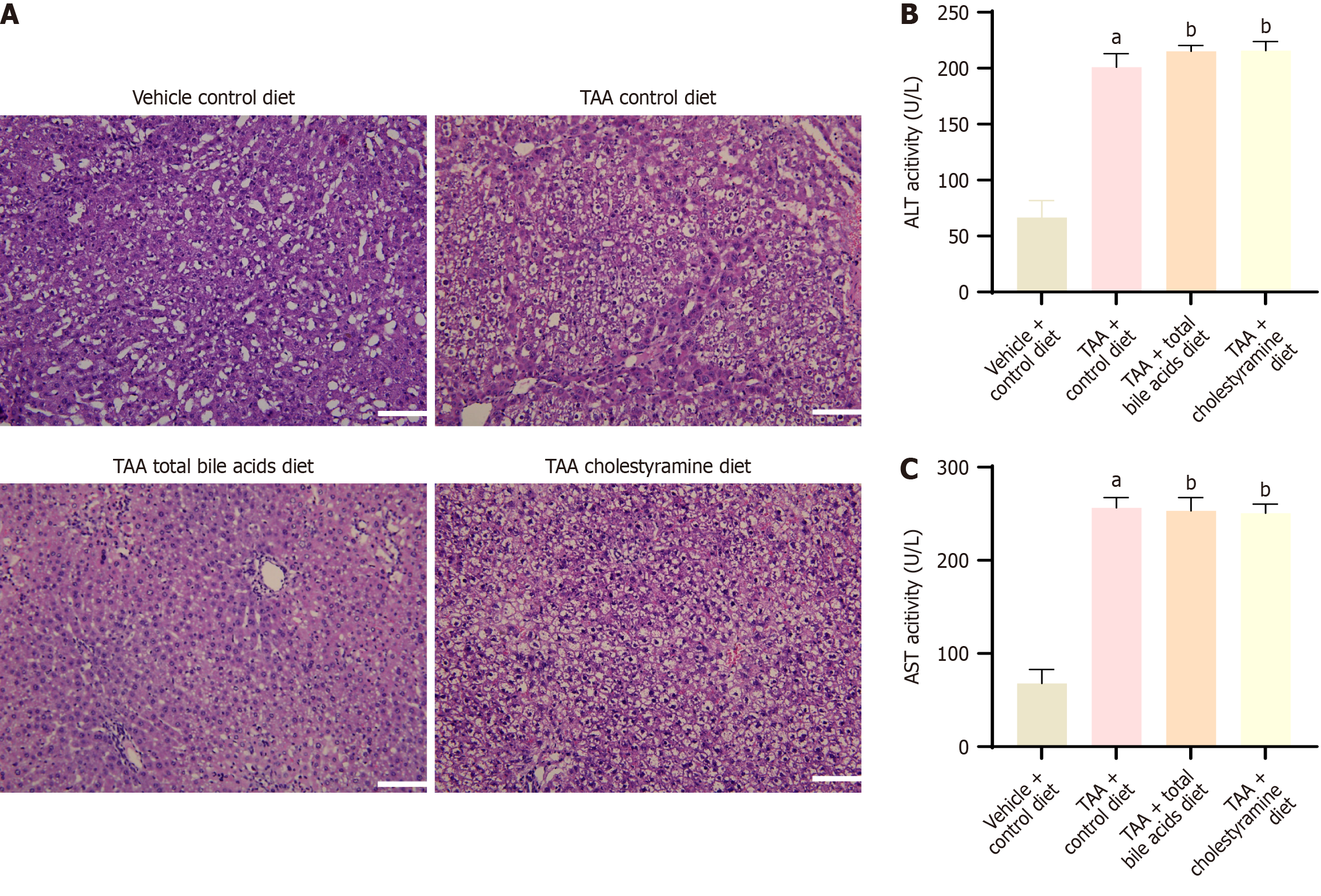
In the VC group, liver tissue appeared normal, with well-formed lobules and no signs of inflammatory cell infiltration. However, in the TAA, TAAT, and TAAC groups, the liver cells were severely damaged, and false lobules had formed (Figure 2A). Compared with the VC group, both aspartate aminotransferase and alanine aminotransferase levels were significantly elevated in the TAA group, with statistical significance (P < 0.05), indicating that TAA induced liver damage in rats. However, no significant differences were observed between the TAA, TAAT, and TAAC groups (Figure 2B and C). Based on these findings, exogenous intervention with bile acids did not appear to affect liver function.
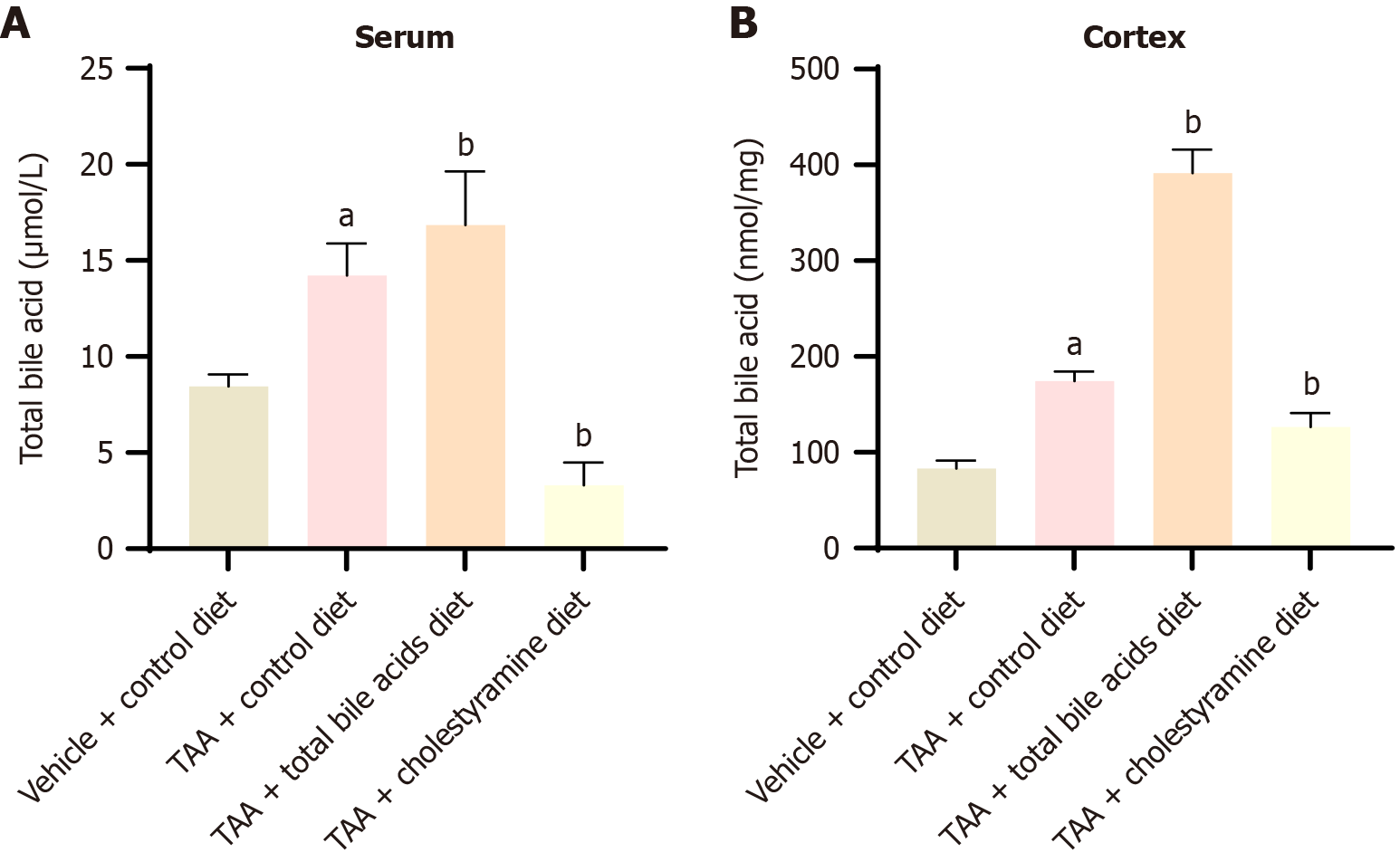
The total bile acid content in the peripheral blood and cerebral cortex of rats from all four groups was measured (Figure 3). LSD-t comparative analysis between the groups revealed significant differences in total bile acid content. Compared with the VC group, total bile acid levels in both peripheral blood and the cerebral cortex were significantly elevated in the TAA group (P < 0.05). In comparison with the TAA group, the total bile acid levels in peripheral blood and the cerebral cortex were increased in the TAAT group (P < 0.01) but decreased in the TAAC group (P < 0.01). These results suggest that interfering with the intake of exogenous bile acids can elevate the total bile acid levels in both peripheral blood and the brain of rats.
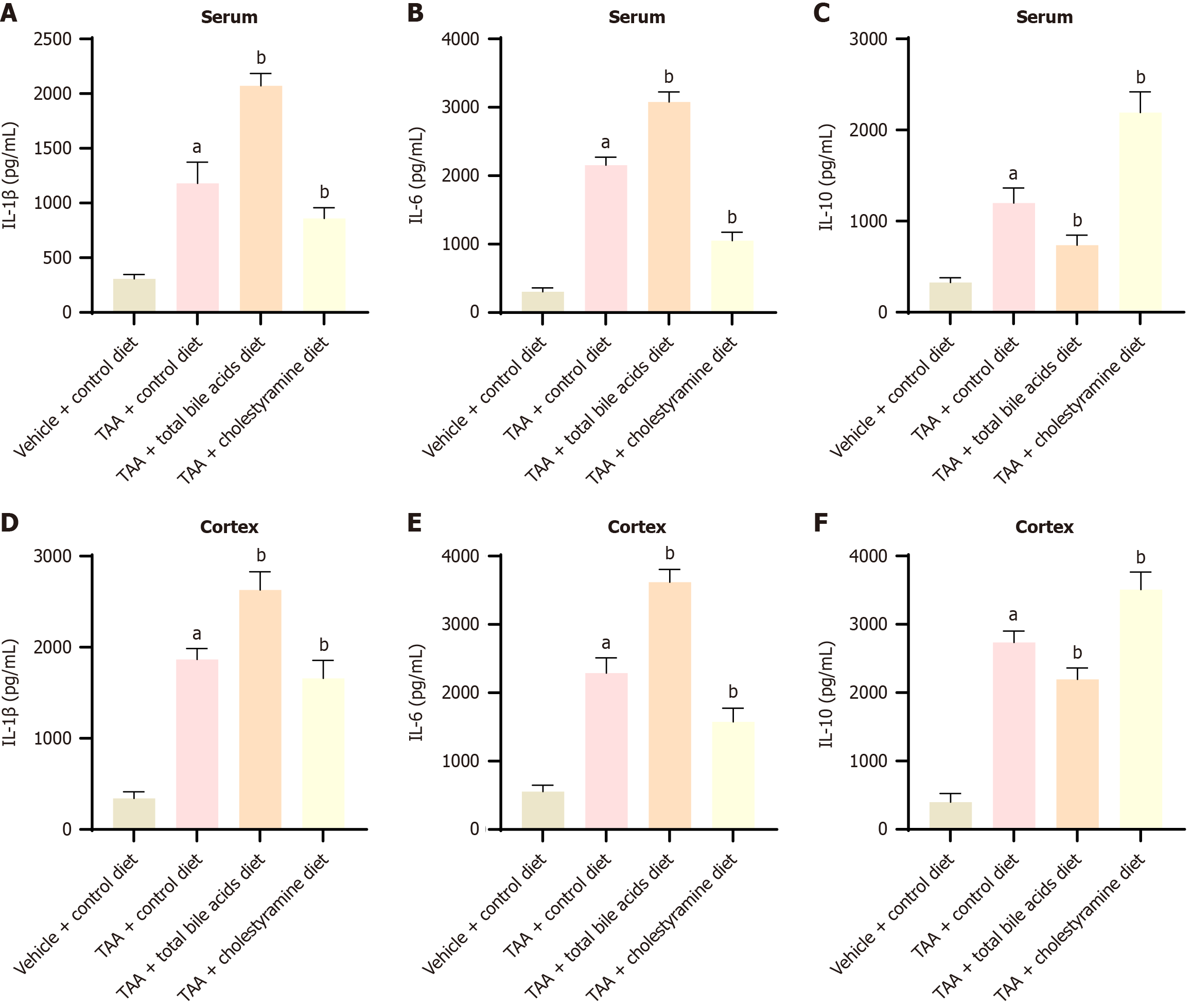
In the serum and cerebral cortex, compared with the TAA group, the levels of pro-inflammatory factors IL-1β and IL-6 were elevated in the TAAT group, while the level of the anti-inflammatory factor IL-10 was decreased. In contrast, the TAAC group exhibited reduced levels of IL-1β and IL-6, accompanied by an increase in IL-10 levels (P < 0.05). These findings indicate that administering bile acids or cholestyramine can regulate inflammation in peripheral blood and the cerebral cortex (Figure 4).
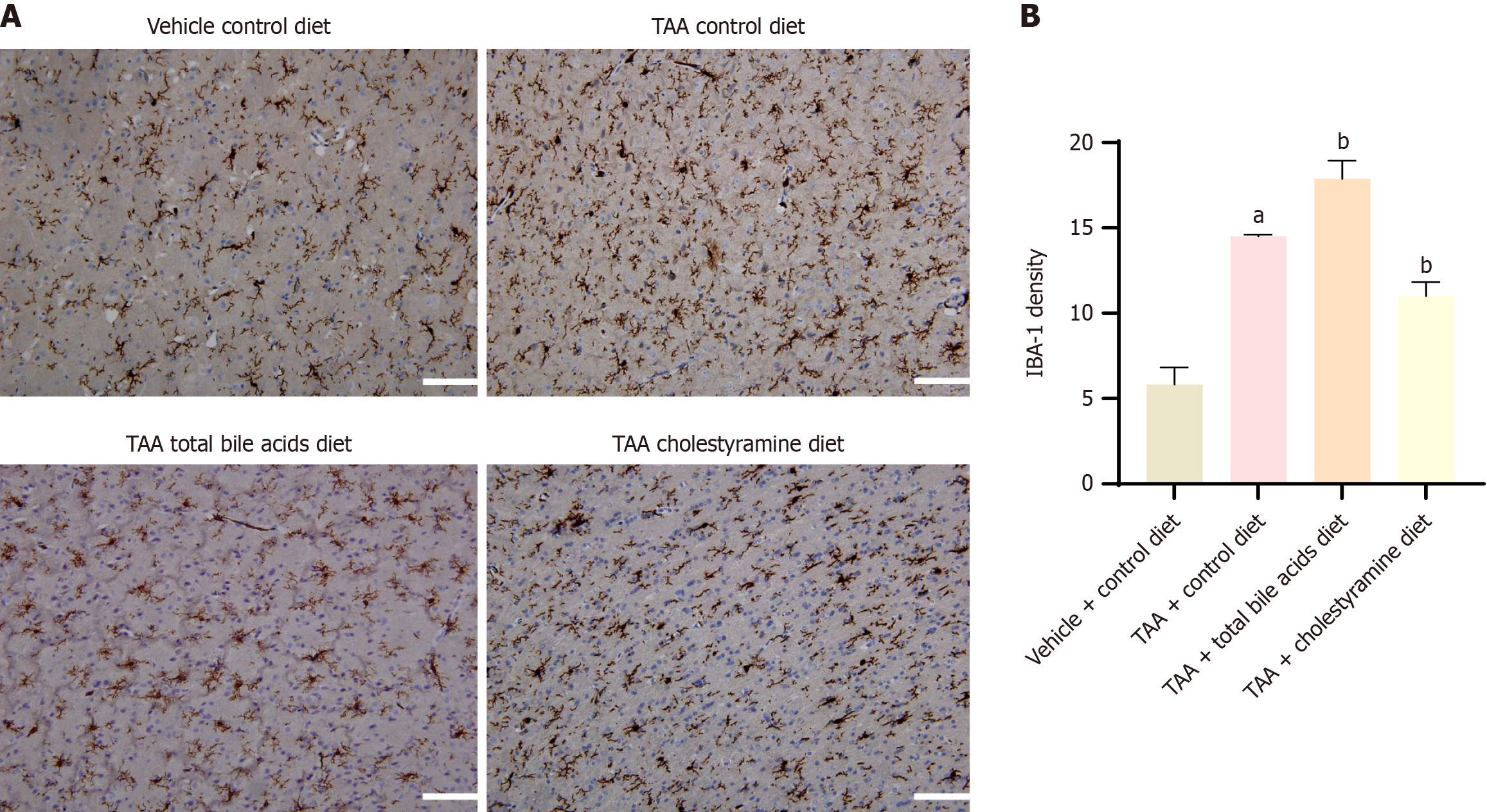
The results demonstrated that the expression of IBA-1 in the cerebral cortex of rats in the TAA group was significantly elevated, indicating notable microglial activation (P < 0.05). Compared with the TAA group, the TAAT group showed a further significant increase in IBA-1 expression (P < 0.01), whereas the TAAC group exhibited a decrease in IBA-1 expression (P < 0.05) (Figure 5). These findings suggest that liver cirrhosis in rats induces microglial activation in the cerebral cortex. Moreover, altering the intake of peripheral blood bile acids can modulate the activation state of microglial cells in the cerebral cortex.
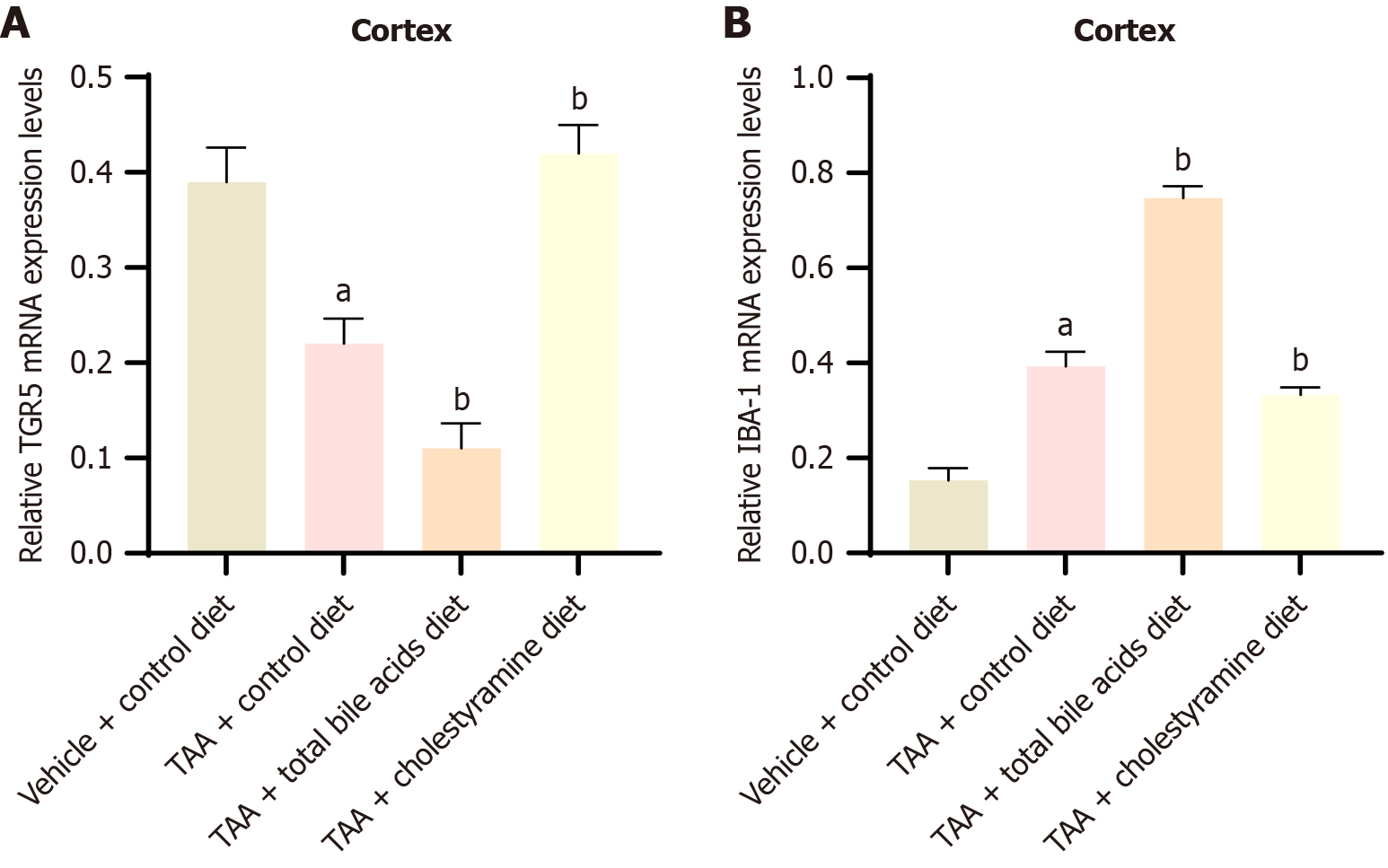
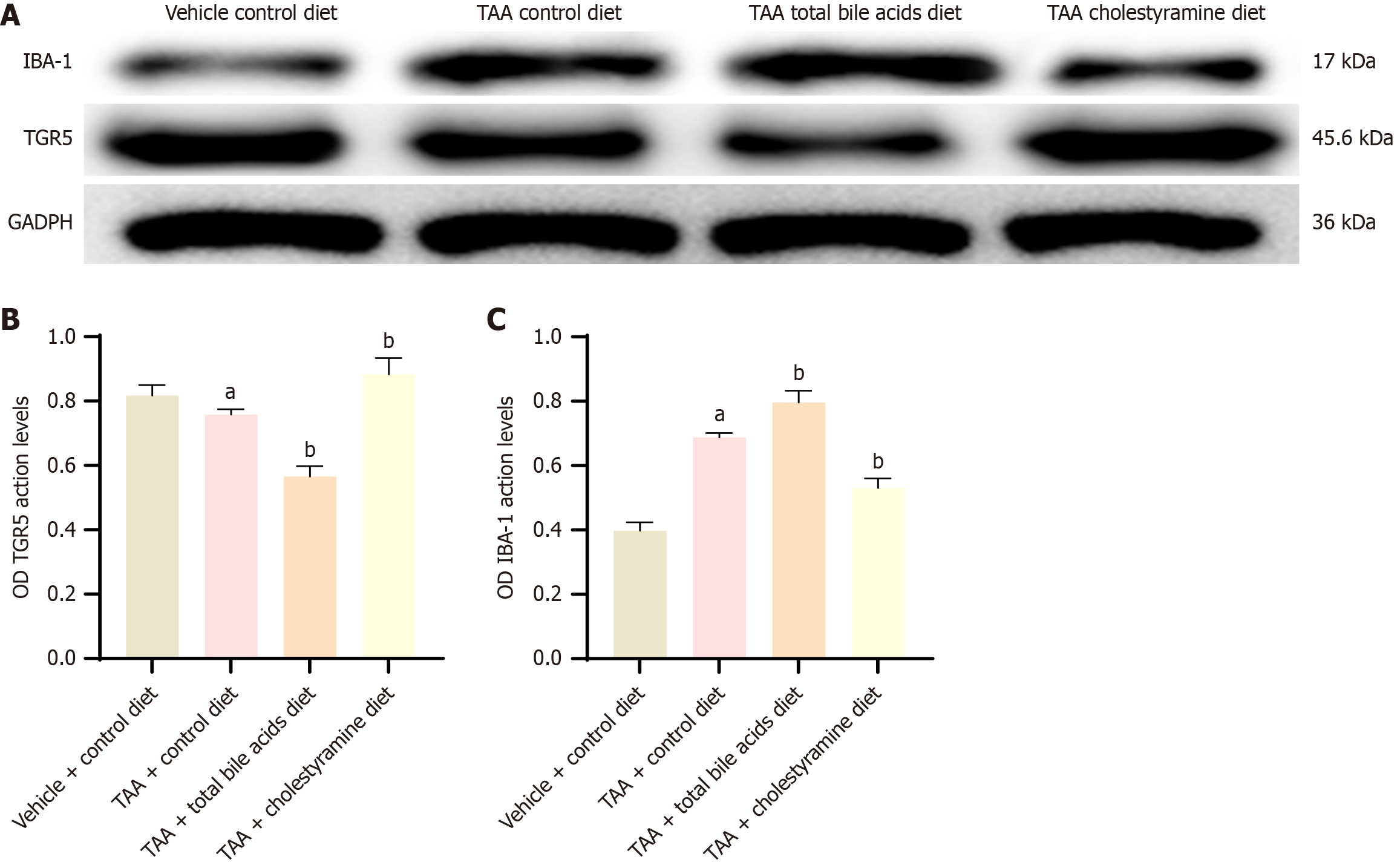
Previous studies have indicated that TGR5 signaling reduces neuroinflammation during HE; however, the impact of total bile acids on TGR5 expression remains unexplored. This study investigated the expression of TGR5 receptors in the cerebral cortex under conditions of exogenous regulation of bile acid intake (Figures 6 and 7). Western blot analysis revealed a significant increase in TGR5 expression in the TAA mouse model (P < 0.05). Compared with the TAA group, the expression of TGR5 was significantly reduced in the TAAT group (P < 0.01) but was slightly elevated in the TAAC group (P < 0.05). Polymerase chain reaction analysis corroborated the results obtained from western blotting. These findings suggest that altering bile acid intake can modulate TGR5 receptor expression in the cerebral cortex of rats.
HE is a critical neurological complication of end-stage liver disease, characterized by cognitive impairment and unconsciousness, often progressing to disturbance of consciousness and ultimately death. The development of HE is influenced by multiple factors, including blood ammonia, bile acids[15], branched-chain amino acids[16], and intestinal flora[17]. In this study, a TAA-induced liver cirrhosis HE rat model was established, revealing that serum bile acids can cross the blood-brain barrier and regulate the expression of TGR5 protein in the cerebral cortex.
Studies have demonstrated increased cerebrospinal fluid (CSF) bile acid levels in patients with HE[18], and elevated total bile acid content in the brains of HE rodents[19]. In an acute liver failure model induced by AOM, bile acid levels in the cerebral cortex were shown to rise and bind to S1PR2 receptors on cortical neurons, leading to neurological dysfunction[7]. However, other studies suggest that bile acid supplementation or activation of bile acid receptors may ameliorate HE symptoms. Activation of TGR5 signaling[6] has been reported to delay neurological decline, elevate c-AMP levels in the cerebral cortex, inhibit microglia activation, and suppress the secretion of inflammatory factors. The current findings highlight the complex and potentially contradictory roles of bile acids in HE. This underscores the need for further research to elucidate the intricate relationship between bile acids and the pathogenesis of HE.
An animal model of TAA-induced HE was established following the ISHEN Animal Guidelines for Hepatic Encephalopathy[12]. The validity of the model was confirmed through open-field tests and elevated cross-tests. Rats in the TAA-induced liver cirrhosis and HE group exhibited significant psychobehavioral changes, as evidenced by reduced total travel distance and fewer entries into the central area in the open-field test. Similarly, in the elevated cross-test, there was a marked decrease in the percentage of open arm entries and the percentage of open arm dwell time. Psychobehavioral disorders were more pronounced in the TAAT group compared to the TAA-induced liver cirrhosis and HE group, while the TAAC group demonstrated an improvement in psychobehavioral performance relative to the TAA-induced liver cirrhosis and HE group. These experimental findings suggest that bile acids contribute to the development of neu
This experiment demonstrated that the serum total bile acid content can be modulated through dietary supplementation with total bile acids or cholestyramine, aligning with findings from previous studies. Specifically, feeding bile acids was observed to increase total bile acid levels in both the serum and cerebral cortex, whereas cholestyramine supplementation reduced these levels. These results suggest that serum bile acid content may influence bile acid levels in the brain by crossing the blood-brain barrier. Two mechanisms have been reported for bile acid entry into the brain: First, bile acids can traverse the blood-brain barrier via bile acid transporters located in the choroid plexus[20]; second, in animal models of acute and chronic liver failure, blood-brain barrier damage permits bile acids to cross more readily. Studies have indicated that bile acids can increase Rac1 activity at the blood-brain barrier, promoting the phosphorylation of occlusive proteins, which compromises tight junction integrity and facilitates bile acid entry into the brain[21]. While bile acids are known to impair the blood-brain barrier, the specific types of bile acids capable of crossing the barrier and their underlying mechanisms remain unclear and warrant further investigation.
Increased total bile acid content in the cerebral cortex plays a critical role in the progression of HE. Previous studies have indicated that the severity of HE is closely associated with the level of systemic inflammation[22]. For instance, pro-inflammatory factors such as IL-1β and TNF-α were shown to increase in the cerebral cortex of rats four weeks post-BDL[23]. It has been reported that increased bile acid levels in the brain can induce neuroinflammation and affect the progression of HE. In AOM-induced acute liver failure, increased cortical bile acid content activates the brain bile acid receptor S1PR2, leading to microglial activation and subsequent neurological dysfunction. The above findings indicate that bile acids can affect neuroinflammation and contribute to HE. We further measured the cerebral cortical microglial marker IBA-1, which showed increased microglial activation after bile acid supplementation. This suggests that bile acid can lead to increased activation of microglia, subsequently causing neuroinflammatory changes.
Numerous studies have investigated the roles of bile acid receptors FXR[24] and S1PR2 in the brain. Increased bile acid content in the brains of rat models with acute liver failure subsequently elevates the expression of FXR and its down
In summary, increased TGR5 expression leads to a reduction in the release of pro-inflammatory factors. Previous studies using animal models of acute liver failure or liver cirrhosis have shown that the onset and progression of HE are closely linked to the activation of microglia and the release of inflammatory factors[25-27]. Administration of the TGR5 agonist betulinic acid into the cerebral ventricle can elevate the concentration of cAMP in the cerebral cortex[28] and reduce the release of the pro-inflammatory chemokine CCL2[25] from neuron-activated microglia, thus limiting the production of pro-inflammatory factors and improving neurological function in HE. These findings suggest that activation of TGR5 may mitigate the progression of neuroinflammation and enhance the development of HE in AOM-induced liver failure through neuron and microglia paracrine signaling.
Our research has important translational significance. Matkowsky et al[29] found that in a mouse model of AOM-induced acute liver failure, brain ammonia levels only increased during hepatic coma, indicating that factors other than ammonia influence the progression of HE. Recent studies have identified a close relationship between several neu
There are some limitations to our study that are worth considering. Firstly, the types and concentrations of bile acids in the brain or CSF differ between rodent and human HE groups. Compared with patients with cirrhosis but without HE, the total bile acid, especially glucourosodeoxycholic acid and glycocholic acid, in the CSF of HE patients with cirrhosis were significantly increased[18]. The concentration of lithocholic acid in the brain of HE rats induced by BDL was higher than that of rats without HE, accounting for 87.4% of the total bile acid content in the brain[31]. The content of TCA in the cerebral cortex of AOM-induced HE mice was increased[7]. When HE occurred in both rodents and humans, the types and concentrations of bile acids in the brain were different; however, the total bile acid levels were increased, which aligns with the findings of our experiment. At present, we have only administered total bile acids to rodents, and it has been demonstrated that bile acids can reduce the expression of the TGR5 receptor in the brain. In the next phase, specific bile acids and TGR5 receptor inhibitors can be further explored as potential treatments for HE.
Currently, no studies have investigated the use of bile acids to interfere with TGR5 expression during HE, nor have they examined the progression of HE and its downstream mechanisms. This experiment found that oral administration of bile acids led to an increase in bile acid levels in the brain, which reduced the expression of the bile acid receptor TGR5 and heightened microglial activation. This activation subsequently led to an increase in the release of pro-inflammatory factors (IL-1β, IL-6) and a decrease in the release of anti-inflammatory factors (IL-10), mediating neurological dysfunction. In addition, this experiment has not yet explored how changes in bile acids within the body affect typical brain bile acid receptors, FXR and S1PR2. It is possible that the interaction between bile acids and their related receptors contributes to the onset of HE, rather than the effect of a single receptor. This experiment only observed a decrease in TGR5 expression in the cerebral cortex following bile acid intake. Future investigations should focus on elucidating the mechanisms by which bile acids alter TGR5 expression in the brain and the subsequent downstream effects.
In conclusion, there are many pathogenic factors for HE. This experiment confirmed that total bile acids contribute to the development of HE in liver cirrhosis. The main mechanism is that bile acids mediate the expression of bile acid TGR5 in the brain and then affect microglia activation. However, this experiment only verified the role of total bile acids in HE, but there are many types of bile acids, and we still need to continue to explore in depth which bile acids play and what role they play. In short, bile acids and bile acid receptor (FXR, TGR5, S1PR2) in the brain may become effective targets for treating HE in the future.
| 1. | Williams E, Chu C, DeMorrow S. A critical review of bile acids and their receptors in hepatic encephalopathy. Anal Biochem. 2022;643:114436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Bajaj JS, O'Leary JG, Tandon P, Wong F, Garcia-Tsao G, Kamath PS, Maliakkal B, Biggins SW, Thuluvath PJ, Fallon MB, Subramanian RM, Vargas HE, Lai J, Thacker LR, Reddy KR. Hepatic Encephalopathy Is Associated With Mortality in Patients With Cirrhosis Independent of Other Extrahepatic Organ Failures. Clin Gastroenterol Hepatol. 2017;15:565-574.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 3. | Aldridge DR, Tranah EJ, Shawcross DL. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol. 2015;5:S7-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 4. | McMillin M, Frampton G, Quinn M, Ashfaq S, de los Santos M 3rd, Grant S, DeMorrow S. Bile Acid Signaling Is Involved in the Neurological Decline in a Murine Model of Acute Liver Failure. Am J Pathol. 2016;186:312-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Bron B, Waldram R, Silk DB, Williams R. Serum, cerebrospinal fluid, and brain levels of bile acids in patients with fulminant hepatic failure. Gut. 1977;18:692-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | McMillin M, Frampton G, Tobin R, Dusio G, Smith J, Shin H, Newell-Rogers K, Grant S, DeMorrow S. TGR5 signaling reduces neuroinflammation during hepatic encephalopathy. J Neurochem. 2015;135:565-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | McMillin M, Frampton G, Grant S, Khan S, Diocares J, Petrescu A, Wyatt A, Kain J, Jefferson B, DeMorrow S. Bile Acid-Mediated Sphingosine-1-Phosphate Receptor 2 Signaling Promotes Neuroinflammation during Hepatic Encephalopathy in Mice. Front Cell Neurosci. 2017;11:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Duboc H, Taché Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46:302-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 347] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 9. | Keitel V, Görg B, Bidmon HJ, Zemtsova I, Spomer L, Zilles K, Häussinger D. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia. 2010;58:1794-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 10. | Huang R, Gao Y, Chen J, Duan Q, He P, Zhang J, Huang H, Zhang Q, Ma G, Zhang Y, Nie K, Wang L. TGR5 Agonist INT-777 Alleviates Inflammatory Neurodegeneration in Parkinson's Disease Mouse Model by Modulating Mitochondrial Dynamics in Microglia. Neuroscience. 2022;490:100-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 11. | Yoneno K, Hisamatsu T, Shimamura K, Kamada N, Ichikawa R, Kitazume MT, Mori M, Uo M, Namikawa Y, Matsuoka K, Sato T, Koganei K, Sugita A, Kanai T, Hibi T. TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn's disease. Immunology. 2013;139:19-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | DeMorrow S, Cudalbu C, Davies N, Jayakumar AR, Rose CF. 2021 ISHEN guidelines on animal models of hepatic encephalopathy. Liver Int. 2021;41:1474-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Zhang Y, Klaassen CD. Effects of feeding bile acids and a bile acid sequestrant on hepatic bile acid composition in mice. J Lipid Res. 2010;51:3230-3242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Frampton G, Invernizzi P, Bernuzzi F, Pae HY, Quinn M, Horvat D, Galindo C, Huang L, McMillin M, Cooper B, Rimassa L, DeMorrow S. Interleukin-6-driven progranulin expression increases cholangiocarcinoma growth by an Akt-dependent mechanism. Gut. 2012;61:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Hadjihambi A, Arias N, Sheikh M, Jalan R. Hepatic encephalopathy: a critical current review. Hepatol Int. 2018;12:135-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 16. | Marrone G, Serra A, Miele L, Biolato M, Liguori A, Grieco A, Gasbarrini A. Branched chain amino acids in hepatic encephalopathy and sarcopenia in liver cirrhosis: Evidence and uncertainties. World J Gastroenterol. 2023;29:2905-2915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (3)] |
| 17. | Won SM, Oh KK, Gupta H, Ganesan R, Sharma SP, Jeong JJ, Yoon SJ, Jeong MK, Min BH, Hyun JY, Park HJ, Eom JA, Lee SB, Cha MG, Kwon GH, Choi MR, Kim DJ, Suk KT. The Link between Gut Microbiota and Hepatic Encephalopathy. Int J Mol Sci. 2022;23:8999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Weiss N, Barbier Saint Hilaire P, Colsch B, Isnard F, Attala S, Schaefer A, Amador MD, Rudler M, Lamari F, Sedel F, Thabut D, Junot C. Cerebrospinal fluid metabolomics highlights dysregulation of energy metabolism in overt hepatic encephalopathy. J Hepatol. 2016;65:1120-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Claeys W, Van Hoecke L, Geerts A, Van Vlierberghe H, Lefere S, Van Imschoot G, Van Wonterghem E, Ghesquière B, Vandenbroucke RE, Van Steenkiste C. A mouse model of hepatic encephalopathy: bile duct ligation induces brain ammonia overload, glial cell activation and neuroinflammation. Sci Rep. 2022;12:17558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Quinn M, McMillin M, Galindo C, Frampton G, Pae HY, DeMorrow S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig Liver Dis. 2014;46:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 21. | Kida T, Omori K, Hori M, Ozaki H, Murata T. Stimulation of G protein-coupled bile acid receptor enhances vascular endothelial barrier function via activation of protein kinase A and Rac1. J Pharmacol Exp Ther. 2014;348:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Häussinger D, Dhiman RK, Felipo V, Görg B, Jalan R, Kircheis G, Merli M, Montagnese S, Romero-Gomez M, Schnitzler A, Taylor-Robinson SD, Vilstrup H. Hepatic encephalopathy. Nat Rev Dis Primers. 2022;8:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 113] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 23. | Claeys W, Van Hoecke L, Lefere S, Geerts A, Verhelst X, Van Vlierberghe H, Degroote H, Devisscher L, Vandenbroucke RE, Van Steenkiste C. The neurogliovascular unit in hepatic encephalopathy. JHEP Rep. 2021;3:100352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | McMillin M, Grant S, Frampton G, Petrescu AD, Kain J, Williams E, Haines R, Canady L, DeMorrow S. FXR-Mediated Cortical Cholesterol Accumulation Contributes to the Pathogenesis of Type A Hepatic Encephalopathy. Cell Mol Gastroenterol Hepatol. 2018;6:47-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | McMillin M, Frampton G, Thompson M, Galindo C, Standeford H, Whittington E, Alpini G, DeMorrow S. Neuronal CCL2 is upregulated during hepatic encephalopathy and contributes to microglia activation and neurological decline. J Neuroinflammation. 2014;11:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Czarnecka AM, Milewski K, Albrecht J, Zielińska M. The Status of Bile Acids and Farnesoid X Receptor in Brain and Liver of Rats with Thioacetamide-Induced Acute Liver Failure. Int J Mol Sci. 2020;21:7750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Jiang W, Desjardins P, Butterworth RF. Minocycline attenuates oxidative/nitrosative stress and cerebral complications of acute liver failure in rats. Neurochem Int. 2009;55:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Karababa A, Groos-Sahr K, Albrecht U, Keitel V, Shafigullina A, Görg B, Häussinger D. Ammonia Attenuates LPS-Induced Upregulation of Pro-Inflammatory Cytokine mRNA in Co-Cultured Astrocytes and Microglia. Neurochem Res. 2017;42:737-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Matkowskyj KA, Marrero JA, Carroll RE, Danilkovich AV, Green RM, Benya RV. Azoxymethane-induced fulminant hepatic failure in C57BL/6J mice: characterization of a new animal model. Am J Physiol. 1999;277:G455-G462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Xie G, Wang X, Jiang R, Zhao A, Yan J, Zheng X, Huang F, Liu X, Panee J, Rajani C, Yao C, Yu H, Jia W, Sun B, Liu P, Jia W. Dysregulated bile acid signaling contributes to the neurological impairment in murine models of acute and chronic liver failure. EBioMedicine. 2018;37:294-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 31. | Tripodi V, Contin M, Fernández MA, Lemberg A. Bile acids content in brain of common duct ligated rats. Ann Hepatol. 2012;11:930-934. [PubMed] |