Published online Feb 27, 2025. doi: 10.4254/wjh.v17.i2.99292
Revised: December 5, 2024
Accepted: January 18, 2025
Published online: February 27, 2025
Processing time: 216 Days and 7.3 Hours
Hepatitis B virus (HBV) evades the innate immunity and leads to persistent chronic infection, but the molecular mechanism is still not well known.
To investigate whether HBV-miR-3 is involved in HBV immune evasion.
HBV-miR-3 agomir and antagomir were employed to verify the effectiveness of HBV-miR-3 on cGAS-Sting-IFN pathway through the experiments on relative luciferase activity, cGAS protein expression, Sting phosphorylation and interferon (IFN) production.
HBV-miR-3 down-regulates cGAS protein expression post-transcriptionally by inhibition of cGAS 3’-untranslated region (3’-UTR) activity, which results in lower Sting phosphorylation and IFN production. HBV-miR-3 antagomir rescued cGAS protein expression, Sting phosphorylation and IFN-β production.
HBV-miR-3 plays an important role in HBV immunity evasion by targeting cGAS 3’-UTR and interfering with cGAS-Sting-IFN pathway.
Core Tip: This study uncovers a novel mechanism of hepatitis B virus (HBV) immunity evasion through HBV-miR-3, a recently identified HBV-encoded microRNA. HBV-miR-3 down-regulates the cGAS-STING-IFN pathway by inhibiting cGAS 3’ untranslated region activity, leading to decreased cGAS protein expression, STING phosphorylation, and interferon production. The findings highlight the crucial role of HBV-miR-3 in modulating host immune responses, providing insights into potential therapeutic targets for HBV infection.
- Citation: Xu ZY, Gao JS, He Y, Xiao XQ, Gong GZ, Zhang M. Hepatitis B virus confers innate immunity evasion through hepatitis B virus-miR-3 down-regulation of cGAS-Sting-IFN signaling. World J Hepatol 2025; 17(2): 99292
- URL: https://www.wjgnet.com/1948-5182/full/v17/i2/99292.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i2.99292
Hepatitis B virus (HBV) infection, leading to liver cirrhosis and hepatocellular carcinoma (HCC), is a major global health problem[1]. The interaction between HBV and the host immune system plays a critical role in establishing persistent HBV infection[2]. HBV has developed many strategies to evade and weaken the host immunological defenses[3], including disrupting antigen processing and presentation, preventing MHC molecules from binding peptide segments, producing various viral proteins that inhibit antibody, cytokine production, and T cell activation[4-6]. The cGAS-Sting pathway is crucial in innate immunity against viruses[7]. When viral DNA is sensed, cGAS generates cGAMP, which binds Sting, triggering cascades leading to type I interferon (IFN) production and antiviral molecules. Compounds like Schisandrin C or other Sting agonists can activate the cGAS-Sting pathway, resulting in the suppression of HBV replication[8,9]. Conversely, disrupting the cGAS-Sting pathway can facilitate the establishment of persistent viral infections[10,11]. HBV is a double-stranded DNA virus that theoretically serve as a substrate for activating the cGAS-Sting pathway, and then inhibiting HBV replication. In actuality, HBV can evade the innate immunity by inhibiting cGAS pathway and estab
pHBV 1.3 was preserved by the Infectious Disease Laboratory of the Second Xiangya Hospital. To perform target gene assays, a wild-type (WT) fragment of the 3’-untranslated region (3’-UTR) of cGAS containing the predicted HBV-miR-3 binding sequence was inserted into the pmirGLO Dual-luciferase miRNA target expression vector (Promega, Madison, Wisconsin, United States); pmirGLO-cGAS-WT, and a mutant (MUT) fragment of the 3’-UTR of cGAS was also cloned into the vector to generate a pmirGLO-cGAS-MUT construct with a mutated binding site; The dual-fluorescence plasmids were generated, then confirmed by sequencing.
The human hepatoma cell lines HepG2 and HepG2.2.15 were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). HepG2.2.15 cells are derived from HepG2 cells and stably transfected with the HBV genome, continuously producing infectious HBV particles, making them suitable for studying HBV replication. The HepG2-NTCP cell line, stably expressing the sodium taurocholate cotransporting polypeptide (NTCP), was purchased from BLUEFBIO™ (Cat. No. BFN 60805958, Shanghai, China). HepG2-NTCP cells are highly permissive to HBV infection, serving as an in vitro HBV infection model.
All cells were cultured in Dulbecco's modified Eagle's medium (DMEM, HyClone, Shanghai, China) supplemented with 10% fetal bovine serum (FBS, Gibco, New York, United States) and 100 U/mL penicillin and streptomycin (Cat. No. ST488, Beyotime, Beijing, China) at 37 °C in a humidified atmosphere with 5% CO2. The cells were seeded in 6-well plates at a density of 1 × 106 cells/well. After 24 hours, a concentration gradient of HBV-miR-3 agomir or negative control (NC) RNA (synthesized by GenePharma, Shanghai, China) was transfected into HepG2 cells.
To establish an HBV infection model, collect the culture supernatant from HepG2.2.15 cells and measure the HBV DNA concentration using quantitative PCR. Count the HepG2-NTCP cells to ensure accurate seeding. Infect the HepG2-NTCP cells with the HBV-containing supernatant at an multiplicity of infection of 300.
HepG2 cells, grown to 60% confluence, were transfected with pmirGLO-cGAS-WT or pmirGLO-cGAS-MUT plasmids and either HBV-miR-3 agomir or NC using Lipofectamine™ 2000 Transfection Reagent (Cat. No. 11668-019, Invitrogen, Carlsbad, CA, United States). After 48 hours, luciferase activity was analyzed with the luciferase assay kit (Cat. No. N1610, Promega, Madison, WI, United States).
Protein extraction was performed using RIPA buffer (Cat. No. P0013C, Beyotime). After 12% SDS-PAGE and transfer to nitrocellulose membranes (Cat. No. FFN02, Beyotime), membranes were blocked, incubated with cGAS antibody (1:1000, Cat. No. 26416-1-AP, Proteintech), and then with HRP-conjugated secondary antibody (Cat. No. sc-2005, Santa Cruz). Detection used enhanced chemiluminescence (Beyo ECL Star, Cat. No. P1008AS, Beyotime). The membrane was washed three times for five minutes each in wash buffer. After that, it was incubated with p-Sting antibody solution (1:1000, Cat. No. 50907, Cell Signaling, MA, United States), followed by secondary antibody incubation and exposure steps. The membrane was washed again, then incubated with Sting and IFN-β antibodies (1:1000, Cat. No. 19851-1-AP/27506-1-AP, Proteintech, Chicago, United States), and finally, incubated with the HRP-conjugated internal control GAPDH antibody solution (1:3000, Cat. No. HRP-60004, Proteintech, Chicago, United States). Each experiment was conducted in triplicate, with each sample also tested in triplicate.
RT-PCR analysis of cGAS mRNA was performed using the following steps. Firstly, total RNA was extracted from cells or tissues using TRIzol reagent (Takara, Japan; Cat. No. 9109). Subsequently, the total RNA was reverse transcribed into cDNA using a reverse transcription kit (Thermo Fisher, United States; Cat. No. K1622). During the reverse transcription process, random primers and reverse transcriptase were used to synthesize cDNA. Next, PCR amplification was carried out using the TB Green PCR Master Mix (Takara, Japan; Cat. No. RR820A) on a 7500 real-time PCR system. The PCR reaction conditions were as follows: Stage 1: Pre-denaturation, with 1 cycle of 95 °C for 30 seconds; Stage 2: PCR reaction, with 40 cycles of 95 °C for 5 seconds and 60 °C for 30-34 seconds. In this study, the mRNA expression level of cGAS was quantitatively detected and analyzed under different conditions using the described RT-PCR method. The primers used for cGAS mRNA were as follows: cGAS: Forward primer (F): 5'-GGGAGCCCTGCTGTAACACTTCTTAT-3'; Reverse primer (R): 5'-CCTTTGCATGCTTGGGTACAAGGT-3'. GAPDH: Forward primer (F): 5'-CCACTCCTCCACCTTTGAC-3'; Reverse primer (R): 5'-ACCCTGTTGCTGTAGCCA-3'.
HBV DNA levels were assessed using fluorescent quantitative PCR with kits from (Sansure Biotech Inc. China). HBV pgRNA was extracted from 200 μL of sample using a magnetic bead-based kit (Sansure Biotech Inc. China) and reverse-transcribed into cDNA at 50 °C for 30 minutes. cDNA amplification included an initial step at 95 °C for 2 minutes, 50 cycles of 95 °C for 15 seconds and 60 °C for 30 seconds, and a final cooling at 25 °C for 10 seconds. Fluorescence signals were quantified using the 7500 real-time PCR System (Applied Biosystems®). PCR data were normalized to the expression of GAPDH as an internal control. The relative expression levels were calculated using the ΔΔCt method, where Ct represents the threshold cycle. All results are expressed as fold changes relative to the control group. Each experiment was evaluated using three PCR reactions and was repeated three times. Data are presented as mean ± SD.
Data were analyzed with IBM SPSS 20.0. Group comparisons used Student's t-test, paired t-tests for cGAS and HBV-miR-3, and one-way ANOVA for other data. Correlation analysis assessed dose-response relationships, with significance at P < 0.05.
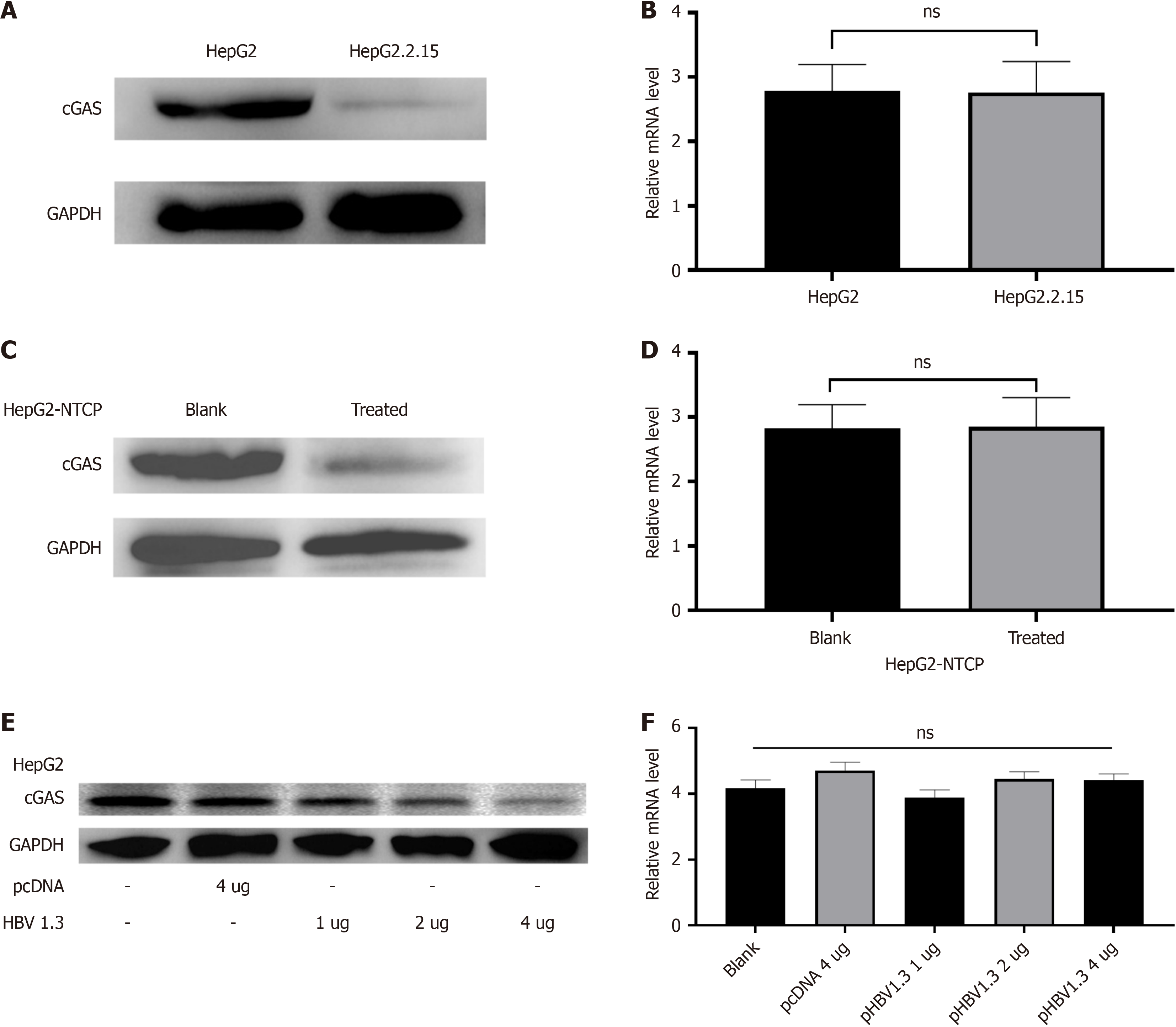
Our previous studies have confirmed that HBV-miR-3 is specifically expressed during HBV infection[14,15]. cGAS protein and mRNA levels between HepG2 and HepG2.2.15 cells were studied, and we found cGAS protein were evidently lower in HepG2.2.15 cells, but the cGAS mRNA level was observed similar in these two cell line cells (Figure 1A and B). Similar results were obtained from the HepG2-NTCP cells infected or not infected with HBV from the HepG2.2.15 supernatant (Figure 1C and D). To further evidence this finding, we did the experiment with different doses of pHBV1.3 to transiently transfect the HepG2 cells. The results showed with increasing doses of pHBV1.3, HBV-miR-3, HBV DNA, and HBV pgRNA increased gradually, and the cGAS protein expression decreased accordingly, but the cGAS mRNA between these different doses of pHBV1.3 transfected HepG2 cell groups was kept almost similar (Figure 1E and F). These findings indicated that HBV down-regulated cGAS protein expression post-transcriptionally.
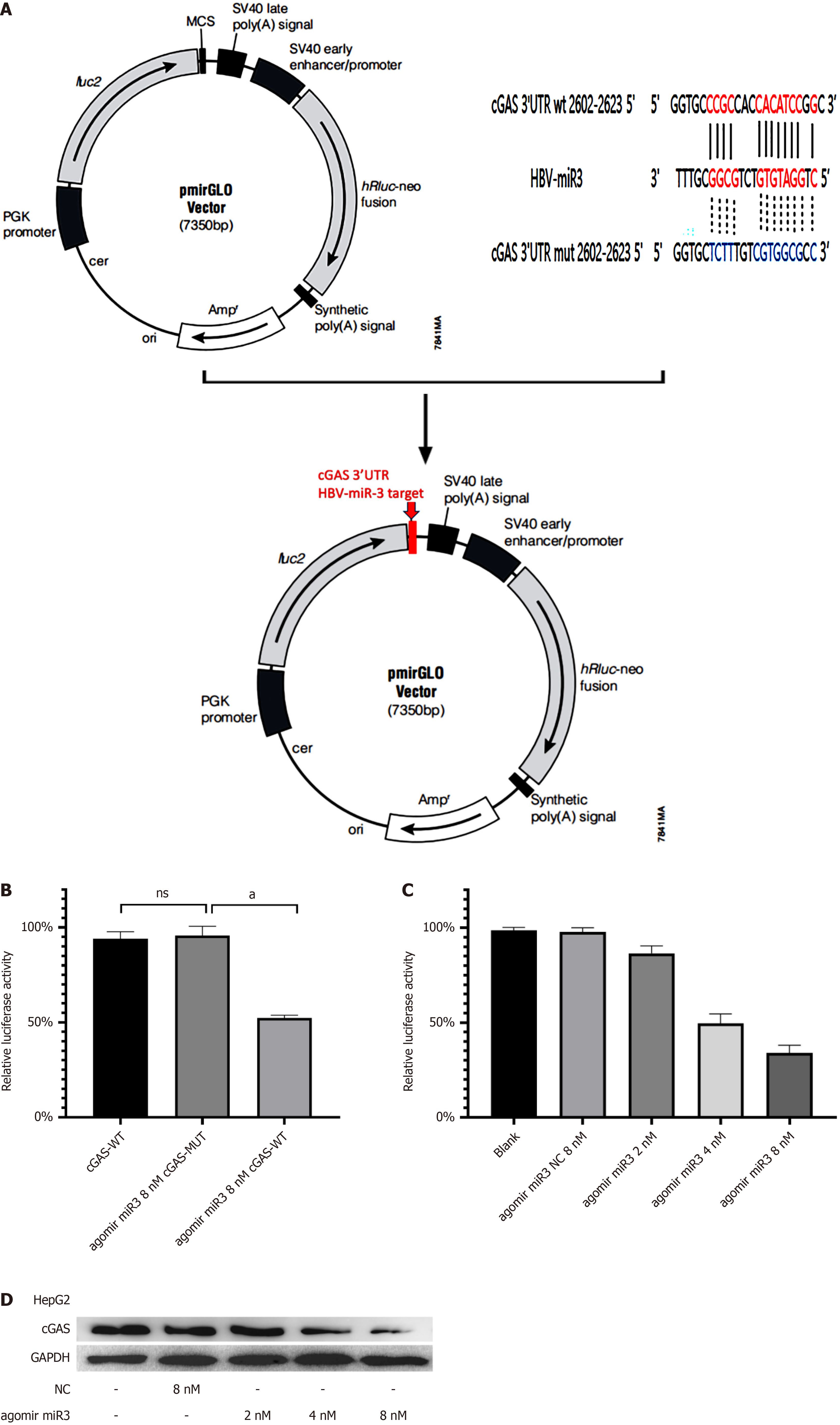
Using bioinformatics analysis, we predicted a binding site between HBV-miR-3 and cGAS 3’-UTR (Figure. 2A). A fluorescent reporter construct was designed based on this finding. Artificially synthesized HBV-miR-3 agomir was employed in this experiment. HBV-miR-3 agomir suppressed relative luciferase activity from wild-type cGAS 3’-UTR, but no effect was observed on relative luciferase activity from the mutant-type cGAS 3’-UTR (Figure 2B). Subsequently, we performed a dose dependent experiment to further confirm the inhibitory effect of HBV-miR-3 agomir on cGAS 3’-UTR activity. The result showed with increasing doses of HBV-miR-3 agomir, the relative luciferase activity from wild-type cGAS 3’-UTR decreased gradually, accompanying with cGAS protein changed similar (Figure 2C and D).
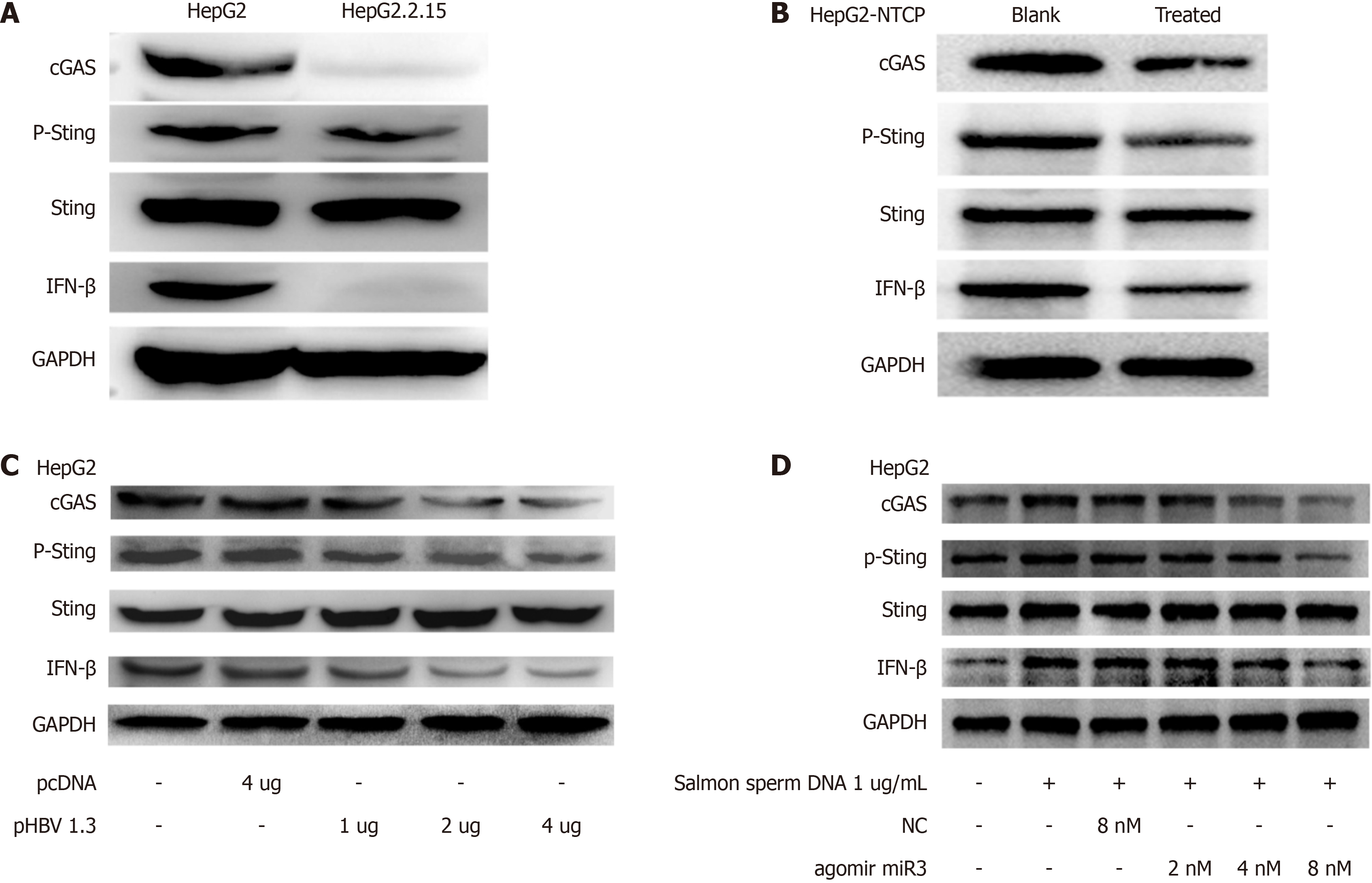
HBV-miR-3 downregulation reduced the expression of the cGAS protein, resulting in decreased Sting phosphorylation and IFN-β production. Three models were used to confirm these findings: HepG2 cells vs HepG2.2.15 cells (Figure 3A), a cell culture model involving HBV infection in HepG2-NTCP cells (Figure 3B), and transfection of varying concentrations of HBV plasmids into HepG2 cells (Figure 3C). Notably, a consistent trend suggests that HBV-miR-3 influences the phosphorylation of the Sting protein rather than its expression. Salmon sperm DNA stimulation of HepG2 cells led to a significant increase in the cGAS-Sting pathway compared to untreated HepG2 cells (Figure 3D). This resulted in elevated levels of cGAS, enhanced Sting phosphorylation, IFN-β production, while Sting expression remained unchanged. This suggests that salmon sperm DNA promotes IFN-β expression through the cGAS-Sting pathway. We investigated the inhibitory effect of HBV-miR-3 on the cGAS-Sting pathway by adding different concentrations (2 nM, 4 nM, and 8 nM) to HepG2 cells stimulated with salmon sperm DNA. The study indicated that HBV-miR-3 agomir decreases cGAS levels, which leads to decreased production of P-Sting and IFN-β. Furthermore, the inhibitory action of HBV-miR-3 agomir has a dose-dependent response.
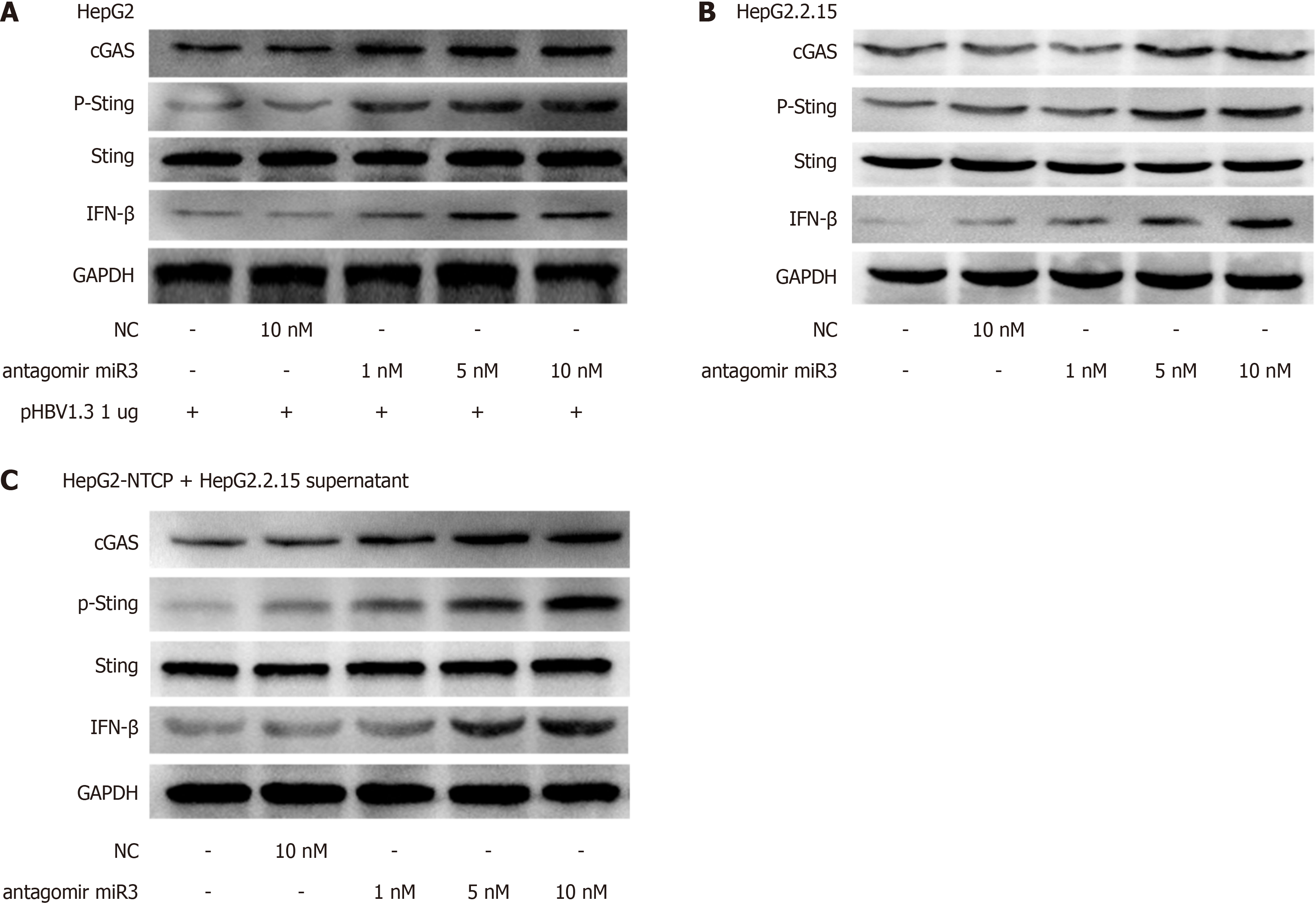
First, we confirmed that the HBV-miR-3 antagomir worked as intended. Figure 4A shows that the inhibitor can effectively restore the reduced fluorescence activity caused by HBV-miR-3 agomir suppression. Next, we noticed that HBV-miR-3 antagomir could increase the production of the cGAS-Sting pathway in HepG2 cells transfected with pHBV1.3 (Figure 4B). Following treatment with varying doses of HBV-miR-3 antagomir, the cGAS-Sting pathway was increased in HepG2.2.15 cells (Figure 4C).
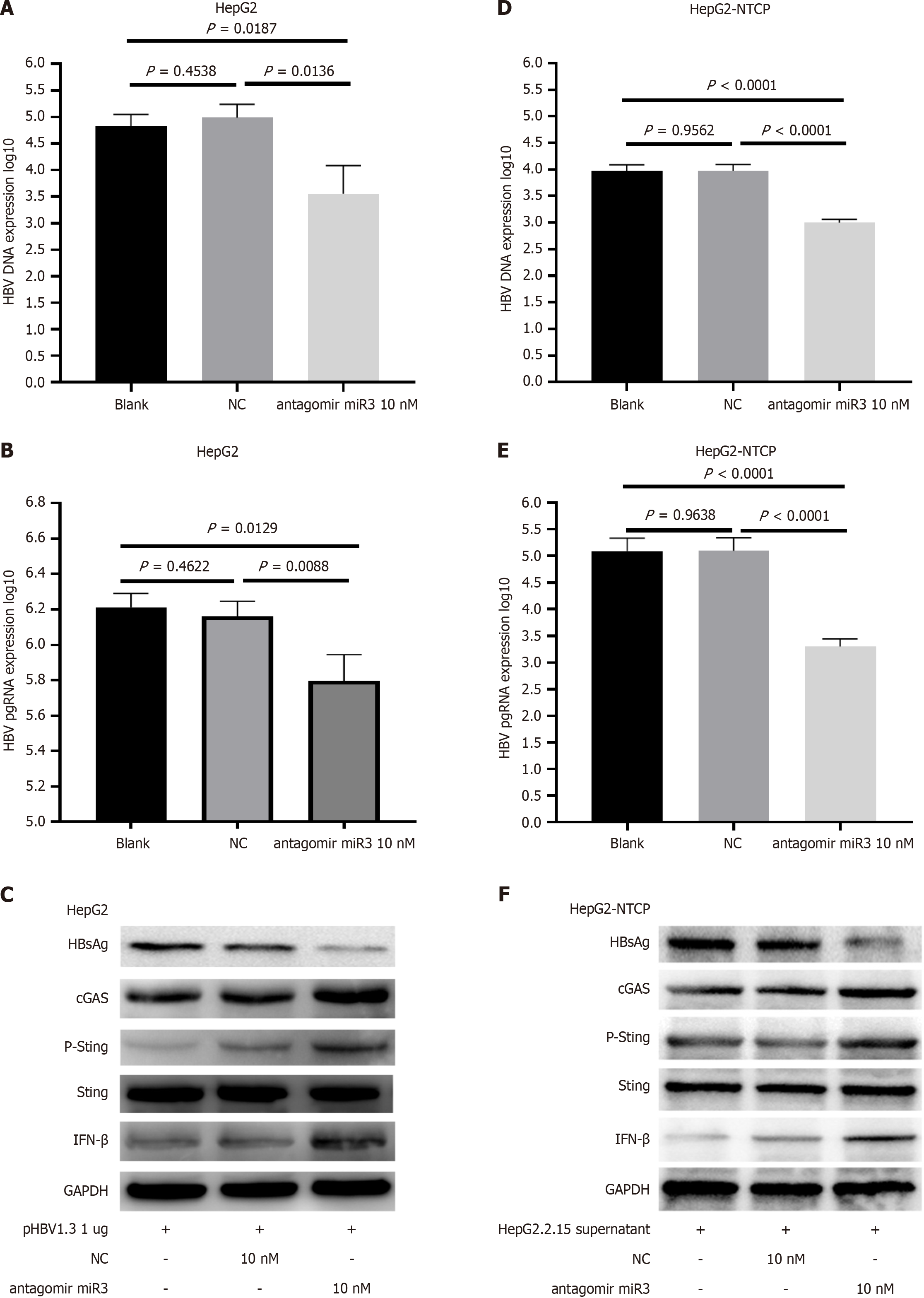
HepG2 cells were transfected with pHBV1.3 and HBV-miR-3 antagomir, then HBV DNA, HBV pgRNA, HBsAg protein, and cGAS-Sting-IFN pathway were evaluated (Figure 5A-C). Similar results were obtained when HepG2-NTCP cells infected with HepG2.2.15 supernatant were treated with HBV-miR-3 antagomir (Figure 5D-F).
This study identifies HBV-miR-3 as a novel regulator of cGAS in HBV-infected hepatocytes. We also introduced a po
Our data suggest that HBV-miR-3 plays a crucial role in mediating immune evasion during chronic HBV infection. Previous studies have mainly focused on the role of HBV-miR-3 in HBV-related HCC and viral replication regulation[14-16]. In 2015, Li et al[17] demonstrated that PPM1A regulates the STING pathway, a crucial component of the innate immune response, suggesting its role in immune modulation during HBV infection. Building on this, in 2020, Chavalit et al[18] showed that HBV miR-3 interacts with PPM1A, promoting HCC development. This underscores a complex network between HBV miR-3, PPM1A, and STING in liver disease and cancer. Earlier research has demonstrated that HBV is capable of suppressing IFN production, thereby reducing viral clearance ability[19]. More recent investigations have further confirmed that this immune evasion effect is primarily associated with the upregulation of HBV-miR-3 in hepatocytes[20]. Therefore, in this study, we employed chronic HBV-infected cell models and utilized HBV-miR-3 mimics (agomir) and inhibitors (antagomir) to investigate the role of HBV-miR-3 in modulating host immune responses. Consistent with previous research, the presence of HBV significantly downregulates the expression of cGAS in hepato
This study reveals the role of HBV-miR-3 in HBV immune escape for the first time; different from previous studies focusing on HBV-related liver cancer or replication. The innate immune system determines the outcome of HBV infection through complex interactions with the virus. Innate immune receptors (PRRs), such as cGAS, and RIG[21-23], can re
Following that, we utilized miRDB (http://mirdb.org/cgi-bin/custom_predict/customDetail.cgi) to perform a com
In order to clarify the association between HBV-miR-3 and IFN, we pursued further investigations downstream of the cGAS-Sting pathway. This exploration unveiled that the reduction of cGAS triggered by HBV exerted downstream consequences, culminating in diminished Sting phosphorylation and reduced IFN-β production. Intriguingly, the addition of salmon sperm DNA, or HBV-miR-3 inhibitors prompted a restoration of the cGAS-Sting pathway's activity in the presence of HBV or HBV-miR-3, countering the previous downregulation.
The results indicate that stimulation with salmon sperm DNA (Figure 3D) or treatment with the HBV-miR3 inhibitor (Figure 5) can partially restore the cGAS-Sting pathway previously inhibited by HBV-miR3. Salmon sperm DNA, being double-stranded, is recognized by cGAS or other factors that activate the cGAS-Sting pathway, leading to increased IFN expression and ultimately reducing HBV replication, as reported in the literature[27,28]. Similarly, we discovered that the HBV-miR3 inhibitor relieved HBV's immunosuppressive effects, resulting in lower replication. This was specifically evidenced by a statistically significant reduction in HBV DNA and HBV pgRNA in the supernatant as shown by PCR results, the restoration of the cGAS-Sting pathway in cells as indicated by Western blot analysis, and a reduction in HBsAg levels, increasing the likelihood of HBV clearance. Also, the activation of the cGAS-Sting pathway could potentially help control chronic HBV infection and reduce the incidence of HBV-related liver cancer. Additionally, the immunological control of HBV-miR-3 is likewise quite complex. In a previous clinical retrospective analysis involving 650 individuals, we found that HBV-miR-3 exhibited a strong predictive effect for HBeAg seroconversion in HBeAg-positive patients undergoing IFN-α 2b treatment. Specifically, lower levels of HBV-miR-3 correlated with greater reductions, indicating a higher likelihood of seroconversion[15]. Additionally, studies have shown that post-IFN-α treatment, HBV-miR-3 targets the SOCS5 protein enhancing IFN's antiviral activity[20]. These findings collectively indicate that the role of HBV-miR-3 in immune regulation is not fixed but varies depending on the stage of HBV infection and the presence of exogenous IFN treatment, targeting multiple genes across various cells to exert a comprehensive effect.
These results could give a possible explanation for the controversial point: Whether HBV can be monitored by cGAS. That is, HBV inhibits the activation of cGAS-Sting pathway through HBV-miR-3, including Sting phosphorylation and IFN production. This phenomenon is similar to hepatitis E virus (HEV) evasion of immune clearance via HEV-miR-A6 expression. HEV-miR-A6 inhibits IRF3 phosphorylation, thereby inhibiting IFN expression and facilitating viral rep
The immune evasion mechanisms of HBV are highly complex, employing multiple strategies to evade the immune system. HBV does not directly destroy hepatocytes but synthesizes large amounts of viral components within them, including HBV DNA, HBsAg, HBeAg, and HBx. It is generally believed that these molecules continuously stimulate the immune system, leading to the suppression and exhaustion of immune cells in the liver. HBsAg negatively affects the normal immune responses of various innate immune cells, including monocytes/macrophages, Kupffer cells, NK cells, dendritic cells, and monocytic myeloid-derived suppressor cells[32]. HBeAg can inhibit the NF-kB pathway and ROS production, weakening LPS-induced NLRP3 inflammasome activation and IL-1β production[33]. HBV-miR-3 inhibition of cGAS represents a more sophisticated approach. It directly interferes with the cellular machinery responsible for reco
The chemical nature of the HBV-miR3 inhibitor is an antisense miRNA of HBV-miR3. This aligns with current deve
Immune evasion by HBV is facilitated through the action of HBV-miR-3, which inhibits cGAS and suppresses cGAS-Sting-IFN signaling. The observed differential expression of HBV-miR-3 and cGAS provides evidence for their negative correlation. Inhibition of HBV-miR-3 results in enhanced IFN expression, ultimately leading to a reduction in HBV proliferation. These findings collectively establish HBV-miR-3 as a promising therapeutic target for CHB treatment.
| 1. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1216] [Article Influence: 173.7] [Reference Citation Analysis (2)] |
| 2. | Riedl T, Faure-Dupuy S, Rolland M, Schuehle S, Hizir Z, Calderazzo S, Zhuang X, Wettengel J, Lopez MA, Barnault R, Mirakaj V, Prokosch S, Heide D, Leuchtenberger C, Schneider M, Heßling B, Stottmeier B, Wessbecher IM, Schirmacher P, McKeating JA, Protzer U, Durantel D, Lucifora J, Dejardin E, Heikenwalder M. Hypoxia-Inducible Factor 1 Alpha-Mediated RelB/APOBEC3B Down-regulation Allows Hepatitis B Virus Persistence. Hepatology. 2021;74:1766-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Zhou L, He R, Fang P, Li M, Yu H, Wang Q, Yu Y, Wang F, Zhang Y, Chen A, Peng N, Lin Y, Zhang R, Trilling M, Broering R, Lu M, Zhu Y, Liu S. Hepatitis B virus rigs the cellular metabolome to avoid innate immune recognition. Nat Commun. 2021;12:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (1)] |
| 4. | Deng F, Xu G, Cheng Z, Huang Y, Ma C, Luo C, Yu C, Wang J, Xu X, Liu S, Zhu Y. Hepatitis B Surface Antigen Suppresses the Activation of Nuclear Factor Kappa B Pathway via Interaction With the TAK1-TAB2 Complex. Front Immunol. 2021;12:618196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Lin J, Yin L, Xu XZ, Sun HC, Huang ZH, Ni XY, Chen Y, Lin X. Bay41-4109-induced aberrant polymers of hepatitis b capsid proteins are removed via STUB1-promoted p62-mediated macroautophagy. PLoS Pathog. 2022;18:e1010204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Lang T, Lo C, Skinner N, Locarnini S, Visvanathan K, Mansell A. The hepatitis B e antigen (HBeAg) targets and suppresses activation of the toll-like receptor signaling pathway. J Hepatol. 2011;55:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (3)] |
| 7. | Zheng W, Zhou Z, Rui Y, Ye R, Xia F, Guo F, Liu X, Su J, Lou M, Yu XF. TRAF3 activates STING-mediated suppression of EV-A71 and target of viral evasion. Signal Transduct Target Ther. 2023;8:79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Zhao J, Xu G, Hou X, Mu W, Yang H, Shi W, Wen J, Liu T, Wu Z, Bai J, Zhang P, Wang Z, Xiao X, Zou W, Bai Z, Zhan X. Schisandrin C enhances cGAS-STING pathway activation and inhibits HBV replication. J Ethnopharmacol. 2023;311:116427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 9. | Amin OE, Colbeck EJ, Daffis S, Khan S, Ramakrishnan D, Pattabiraman D, Chu R, Micolochick Steuer H, Lehar S, Peiser L, Palazzo A, Frey C, Davies J, Javanbakht H, Rosenberg WMC, Fletcher SP, Maini MK, Pallett LJ. Therapeutic Potential of TLR8 Agonist GS-9688 (Selgantolimod) in Chronic Hepatitis B: Remodeling of Antiviral and Regulatory Mediators. Hepatology. 2021;74:55-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 10. | Albright ER, Mickelson CK, Kalejta RF. Human Cytomegalovirus UL138 Protein Inhibits the STING Pathway and Reduces Interferon Beta mRNA Accumulation during Lytic and Latent Infections. mBio. 2021;12:e0226721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Acerbi G, Montali I, Ferrigno GD, Barili V, Schivazappa S, Alfieri A, Laccabue D, Loglio A, Borghi M, Massari M, Rossi M, Vecchi A, Penna A, Boni C, Missale G, Lampertico P, Del Rio D, Ferrari C, Fisicaro P. Functional reconstitution of HBV-specific CD8 T cells by in vitro polyphenol treatment in chronic hepatitis B. J Hepatol. 2021;74:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Verrier ER, Yim SA, Heydmann L, El Saghire H, Bach C, Turon-Lagot V, Mailly L, Durand SC, Lucifora J, Durantel D, Pessaux P, Manel N, Hirsch I, Zeisel MB, Pochet N, Schuster C, Baumert TF. Hepatitis B Virus Evasion From Cyclic Guanosine Monophosphate-Adenosine Monophosphate Synthase Sensing in Human Hepatocytes. Hepatology. 2018;68:1695-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 13. | Chen H, Jiang L, Chen S, Hu Q, Huang Y, Wu Y, Chen W. HBx inhibits DNA sensing signaling pathway via ubiquitination and autophagy of cGAS. Virol J. 2022;19:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Tang J, Xiao X, Jiang Y, Tian Y, Peng Z, Yang M, Xu Z, Gong G. miR-3 Encoded by Hepatitis B Virus Downregulates PTEN Protein Expression and Promotes Cell Proliferation. J Hepatocell Carcinoma. 2020;7:257-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Xu Z, Xu Y, Wu Z, Wang S, Zhang M, Jiang Y, Gong G. HBV-miR-3 is closely related to HBV replication and strongly predictive of HBeAg seroconversion in PegIFN-α treated patients. Sci Rep. 2024;14:1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Yang X, Li H, Sun H, Fan H, Hu Y, Liu M, Li X, Tang H. Hepatitis B Virus-Encoded MicroRNA Controls Viral Replication. J Virol. 2017;91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Li Z, Liu G, Sun L, Teng Y, Guo X, Jia J, Sha J, Yang X, Chen D, Sun Q. PPM1A regulates antiviral signaling by antagonizing TBK1-mediated STING phosphorylation and aggregation. PLoS Pathog. 2015;11:e1004783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Chavalit T, Nimsamer P, Sirivassanametha K, Anuntakarun S, Saengchoowong S, Tangkijvanich P, Payungporn S. Hepatitis B Virus-Encoded MicroRNA (HBV-miR-3) Regulates Host Gene PPM1A Related to Hepatocellular Carcinoma. Microrna. 2020;9:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Liu CJ, Sheen IS, Chen CY, Chuang WL, Wang HY, Tseng KC, Chang TT, Yang J, Massetto B, Suri V, Camus G, Jiang D, Zhang F, Gaggar A, Hu TH, Hsu YC, Lo GH, Chu CJ, Chen JJ, Peng CY, Chien RN, Chen PJ. Ledipasvir/Sofosbuvir for Patients Coinfected With Chronic Hepatitis C and Hepatitis B in Taiwan: Follow-up at 108 Weeks Posttreatment. Clin Infect Dis. 2022;75:453-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Zhao X, Sun L, Mu T, Yi J, Ma C, Xie H, Liu M, Tang H. An HBV-encoded miRNA activates innate immunity to restrict HBV replication. J Mol Cell Biol. 2020;12:263-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 21. | Morrison AH, Diamond MS, Hay CA, Byrne KT, Vonderheide RH. Sufficiency of CD40 activation and immune checkpoint blockade for T cell priming and tumor immunity. Proc Natl Acad Sci U S A. 2020;117:8022-8031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 22. | Liu G, Lee JH, Parker ZM, Acharya D, Chiang JJ, van Gent M, Riedl W, Davis-Gardner ME, Wies E, Chiang C, Gack MU. ISG15-dependent activation of the sensor MDA5 is antagonized by the SARS-CoV-2 papain-like protease to evade host innate immunity. Nat Microbiol. 2021;6:467-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 223] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 23. | Riazalhosseini B, Mohamed R, Apalasamy YD, Langmia IM, Mohamed Z. Circulating microRNA as a marker for predicting liver disease progression in patients with chronic hepatitis B. Rev Soc Bras Med Trop. 2017;50:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Irieda H, Takano Y. Epidermal chloroplasts are defense-related motile organelles equipped with plant immune components. Nat Commun. 2021;12:2739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Gan W, Chen X, Wu Z, Zhu X, Liu J, Wang T, Gao Z. The relationship between serum exosome HBV-miR-3 and current virological markers and its dynamics in chronic hepatitis B patients on antiviral treatment. Ann Transl Med. 2022;10:536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Zhao L, Yuan H, Wang Y, Geng Y, Yun H, Zheng W, Yuan Y, Lv P, Hou C, Zhang H, Sun J, Sun L, Suo Y, Wang S, Zhang N, Lu W, Yang G, Zhang X. HBV confers innate immune evasion through triggering HAT1/acetylation of H4K5/H4K12/miR-181a-5p or KPNA2/cGAS-STING/IFN-I signaling. J Med Virol. 2023;95:e28966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 27. | He J, Hao R, Liu D, Liu X, Wu S, Guo S, Wang Y, Tien P, Guo D. Inhibition of hepatitis B virus replication by activation of the cGAS-STING pathway. J Gen Virol. 2016;97:3368-3378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Li Y, He M, Wang Z, Duan Z, Guo Z, Wang Z, Gong R, Chu T, Cai J, Gao B. STING signaling activation inhibits HBV replication and attenuates the severity of liver injury and HBV-induced fibrosis. Cell Mol Immunol. 2022;19:92-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 29. | Qian Z, Yang C, Xu L, Mickael HK, Chen S, Zhang Y, Xia Y, Li T, Yu W, Huang F. Hepatitis E virus-encoded microRNA promotes viral replication by inhibiting type I interferon. FASEB J. 2022;36:e22104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 30. | Iizasa H, Kim H, Kartika AV, Kanehiro Y, Yoshiyama H. Role of Viral and Host microRNAs in Immune Regulation of Epstein-Barr Virus-Associated Diseases. Front Immunol. 2020;11:367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 31. | Hu M, Wang C, Li W, Lu W, Bai Z, Qin D, Yan Q, Zhu J, Krueger BJ, Renne R, Gao SJ, Lu C. A KSHV microRNA Directly Targets G Protein-Coupled Receptor Kinase 2 to Promote the Migration and Invasion of Endothelial Cells by Inducing CXCR2 and Activating AKT Signaling. PLoS Pathog. 2015;11:e1005171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Fang Z, Zhang Y, Zhu Z, Wang C, Hu Y, Peng X, Zhang D, Zhao J, Shi B, Shen Z, Wu M, Xu C, Chen J, Zhou X, Xie Y, Yu H, Zhang X, Li J, Hu Y, Kozlowski M, Bertoletti A, Yuan Z. Monocytic MDSCs homing to thymus contribute to age-related CD8+ T cell tolerance of HBV. J Exp Med. 2022;219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 33. | Yu X, Lan P, Hou X, Han Q, Lu N, Li T, Jiao C, Zhang J, Zhang C, Tian Z. HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1β production via suppressing the NF-κB pathway and ROS production. J Hepatol. 2017;66:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 262] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 34. | Wildum S, Korolowicz KE, Suresh M, Steiner G, Dai L, Li B, Yon C, De Vera Mudry MC, Regenass-Lechner F, Huang X, Hong X, Murreddu MG, Kallakury BV, Young JAT, Menne S. Toll-Like Receptor 7 Agonist RG7854 Mediates Therapeutic Efficacy and Seroconversion in Woodchucks With Chronic Hepatitis B. Front Immunol. 2022;13:884113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |