Published online Feb 27, 2025. doi: 10.4254/wjh.v17.i2.99092
Revised: January 6, 2025
Accepted: January 24, 2025
Published online: February 27, 2025
Processing time: 221 Days and 19.4 Hours
Hepatocellular carcinoma (HCC) surveillance is crucial for patients with com
To assess the predictive value of TH, Ig, and complements for HCC development.
Data from 142 patients, comprising 72 patients with CC and 70 patients with DC, were analysed as a training set. Among them, 100 patients who underwent complement and Ig tests were considered for internal validation. Logistic regres
The median follow-up duration was 32 (24-37 months) months. The incidence of HCC was significantly higher in the DC group (16/70, 22.9%) compared to the CC group (3/72, 4.2%) (χ² = 10.698, P < 0.01). Patients with DC exhibited lower total tetraiodothyronine (TT4), total triiodothyronine (TT3), free triiodothyronine, complement C3, and C4 (all P < 0.01), and higher IgA and IgG (both P < 0.01). In both CC and DC patients, TT3 and TT4 positively correlated with alanine transaminase (ALT), aspartate transaminase (AST), and gamma-glutamyl transpeptidase (GGT). IgG positively correlated with IgM, IgA, ALT, and AST, while it negatively correlated with C3 and C4. Multivariable analysis indicated that age, DC status, and GGT were independent risk factors for HCC development.
The predictive value of TH, Ig, and complements for HCC development is suboptimal. Age, DC, and GGT emerge as more significant factors during HCC surveillance in hepatitis B virus-related LC.
Core Tip: Hepatocellular carcinoma (HCC) surveillance is crucial for patients with compensated cirrhosis (CC) and decompensated cirrhosis (DC). During a median follow-up duration of 32 months, the incidence of HCC was significantly higher in the DC group compared to the CC group. Total triiodothyronine and total tetraiodothyronine positively correlated with alanine transaminase (ALT), aspartate transaminase (AST), and gamma-glutamyl transpeptidase (GGT). Immunoglobulin (Ig) G positively correlated with IgM, IgA, ALT, and AST, while it negatively correlated with C3 and C4. The predictive value of thyroid hormone, Ig, and complements for HCC development is suboptimal. Multivariable analysis indicated that age, DC, and GGT were independent risk factors for HCC development.
- Citation: Tong XC, Liu K, Huang ZY, Zhang XJ, Xue Y. Thyroid hormone, immunoglobin and complements for predicting hepatocellular carcinoma development in patients with hepatitis B virus-related liver cirrhosis. World J Hepatol 2025; 17(2): 99092
- URL: https://www.wjgnet.com/1948-5182/full/v17/i2/99092.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i2.99092
Hepatocellular carcinoma (HCC) stands as a leading cause of global tumor-related mortality[1,2]. Approximately one-third of patients with decompensated cirrhosis (DC) develop HCC during a 3-year follow-up, with the incidence significantly higher than in patients with compensated cirrhosis (CC)[3]. HCC surveillance is imperative for those with DC, yet only around 25% of such patients undergo such surveillance[2]. Despite advancements in systemic HCC therapy over the last decade, the prognosis remains poor for advanced-stage HCC[4]. With the prolonged survival in liver cirrhosis (LC), identifying biomarkers predictive of HCC development becomes crucial.
Increasing evidence has revealed a connection between thyroid hormone (TH) and HCC, although this relationship remains contentious[5-9]. TH production is regulated by the hypothalamus-pituitary-thyroid axis, and its metabolism is intricately tied to the liver. TH binding proteins are synthesized in the liver, and the conversion of tetraiodothyronine to triiodothyronine by deiodinase enzymes predominantly occurs in the liver[9]. Furthermore, TH strongly promotes liver regeneration and enhances the restoration of liver tissue[10]. A case-control study demonstrated that a history of hypothyroidism exceeding 10 years was associated with a higher risk of HCC in women[5]. In contrast, several scholars have reported that hypothyroidism exhibits a protective association with HCC[6,9]. Additionally, hypothyroidism has been identified as an independent predictor for poor prognosis in patients with advanced HCC[11]. Conversely, data from another study suggested that elevated free tetraiodothyronine (FT4) was associated with a poor prognosis in HCC[7].
Moreover, dysregulated innate and adaptive immunity predict disease progression in LC[12]. Immunoglobulin (Ig), produced by plasma cells under antigen stimulation, can be classified into IgG, IgA, IgM, IgD, and IgE. IgG, the primary Ig in serum, activates complement and regulates antibody-dependent cell-mediated cytotoxicity. IgG positively correlates with fibrosis and inflammation stage in patients with chronic hepatitis B (CHB)[13]. Patients with hypersplenism and Child-Pugh class C had higher levels of IgA and IgG than those with Child-Pugh class A and B[14]. IgM showed no significant difference among the three Child-Pugh classes[14]. For patients with HCC who received immune checkpoint inhibitors (ICIs), it has been reported that patients with low IgM had shorter survival times[15]. Another study showed that a higher increase in IgG was a negative prognostic marker in patients with HCC and ICIs treatment[16]. Recently, increased circulating levels of anti-flagellin IgA were reported to be associated with a 76% to 93% increased risk of HCC[17]. Besides Ig, lower levels of C3 and C4 in patients with cirrhotic portal hypertension paralleled impairment of liver function[14]. C3 and C4 negatively correlated with fibrosis and inflammation stage[13]. C3 has also shown promise in predicting outcomes of acute-on-chronic liver failure[18].
In patients with advanced chronic liver disease, complements negatively correlated with systemic inflammation (SI), while Ig positively correlated with SI[12]. These results suggest that complements and Ig, which serve as surrogates of cirrhosis-associated immune dysfunction, are associated with the severity and outcomes of LC[12]. To date, whether complements and Ig can predict HCC development in patients with LC, especially in patients with DC, remains largely unknown.
Herein, the present study aims to investigate the potential of TH, complements, and Ig to predict HCC development in patients with LC.
From March 2017 to June 2020, 274 patients with LC admitted to Changzhou Third People’s Hospital were enrolled and followed up until the last visit in January 2022. Hepatitis B virus (HBV)-related CC and DC were diagnosed according to the Chinese guidelines for the prevention and treatment of CHB (2019 version)[19]. All the patients had a history of positive hepatitis B surface antigen for more than 6 months, and histological or ultrasonographic examinations showed nodules in the hepatic parenchyma. Patients with LC and complications, including ascites, encephalopathy, variceal bleeding, or hepatorenal syndrome, were diagnosed with DC. HCC was diagnosed based on evidence from abdominal ultrasonography, enhanced computer tomography, or magnetic resonance imaging examinations, alpha-fetoprotein, and/or histological findings[20]. Patients with alcoholic liver disease, autoimmune liver disease, co-infection with other hepatitis viruses, or malignant tumors at the time of enrollment were excluded. The primary endpoint of the study was HCC development or death during follow-up.
All patients received first-line antiviral agents during follow-up. HCC surveillance, including physical examination, abdominal ultrasonography, and alpha-fetoprotein assessment, was conducted every 3 months to 6 months. Laboratory data, comprising alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin (TBil), gamma-glutamyl transpeptidase (GGT), albumin (ALB), total tetraiodothyronine (TT4), total triiodothyronine (TT3), free triiodothyronine (FT3), FT4, thyroid-stimulating hormone (TSH), complement C3 and C4, IgM, IgA, IgG, international standard ratio (INR), HBV DNA, and blood cell count, were collected.
The study was approved by the Ethics Committee of The Third People’s Hospital of Changzhou. All methods were carried out according to the Declaration of Helsinki, 2013, and written informed consent was obtained from all participants.
TH was determined using chemiluminescence immunoassay according to the manufacturers’ instructions. Ig and complements were measured through turbidimetry.
Statistical Package for the Social Sciences version 25.0 (Armonk, NY, United States) was employed for statistical analysis. Data were presented as median (interquartile range) and analyzed using Mann-Whitney U tests for continuous variables. Categorical values were presented as frequencies and compared using the χ2 test. The Spearman correlation test was applied to analyze the correlation between TH, complements, and Ig. Univariate and multivariate logistic regression analyses were conducted to identify independent risk factors for HCC development. A significance level of P < 0.05 was considered statistically significant.
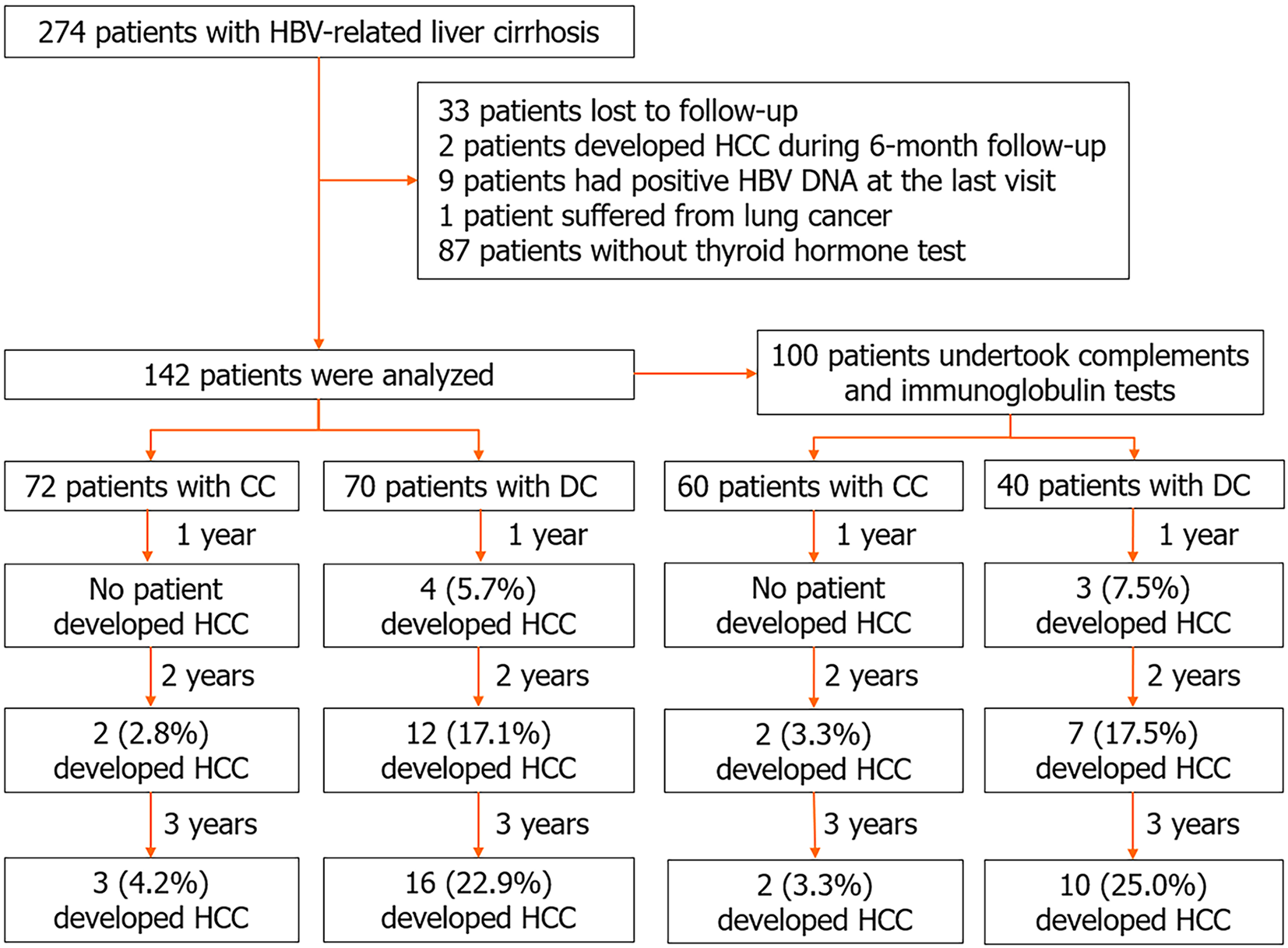
As depicted in Figure 1, nine patients with positive HBV DNA at the end of follow-up were excluded. Data from 142 patients, comprising 72 patients with CC and 70 patients with DC, were analyzed as the training set. The median follow-up duration was 32 (24-37 months) months. A total of 3 (4.2%) patients in the CC group and 16 (22.9%) patients in the DC group developed HCC. The incidence of HCC was significantly higher in the DC group than in the CC group (χ2 = 10.698, P < 0.01).
The characteristics of patients in the training set are presented in Table 1. Patients who developed HCC were older and had a higher proportion of diabetes than those who did not develop HCC (P < 0.01 and P = 0.03, respectively). There was no significant difference in TT3, TT4, FT3, FT4, and TSH between patients who did and did not develop HCC (all P > 0.05).
| Variables | Patients did not develope HCC (n = 123) | Patients developed HCC (n = 19) | Z or χ² | P value |
| Age (years) | 50 (45, 61) | 62 (55, 67) | 3.622 | < 0.01 |
| Male | 80 (65) | 14 (73.7) | 0.549 | 0.46 |
| Decompensated cirrhosis | 54 (43.9) | 16 (84.2) | 10.698 | < 0.01 |
| Diabetes | 39 (31.7) | 11 (57.9) | 4.947 | 0.03 |
| Alanine transaminase (U/L) | 33 (21, 67) | 28 (21, 53) | 0.947 | 0.34 |
| Aspartate transaminase (U/L) | 33 (24, 54) | 37 (27, 47) | 0.258 | 0.80 |
| Gamma-glutamyl transpeptidase (U/L) | 56 (26, 82) | 60 (26, 152) | 0.665 | 0.51 |
| Total bilirubin (µmol/L) | 22.5 (15.7, 31.6) | 24 (16.5, 28.4) | 0.012 | 0.99 |
| Albumin (g/L) | 39.9 (32, 43.3) | 40 (32.2, 44.9) | 0.515 | 0.61 |
| Creatinine (µmol/L) | 77.3 (68.4, 89.9) | 82.4 (69.1, 93.5) | 0.809 | 0.42 |
| International normalized ratio | 1.1 (1.0, 1.3) | 1.1 (1.0, 1.3) | 0.254 | 0.80 |
| Platelet (E+09/L) | 99 (63, 145) | 81 (56, 105) | 1.840 | 0.07 |
| Total triiodothyronine (nmol/L) | 1.5 (1.2, 1.8) | 1.4 (1.2, 1.6) | 1.319 | 0.19 |
| Total tetraiodothyronine (nmol/L) | 112 (93.1, 127.8) | 109.9 (95.5, 131.1) | 0.088 | 0.93 |
| Free triiodothyronine (pmol/L) | 4.7 (4.1, 5.3) | 4.6 (4.0, 5.2) | 0.896 | 0.37 |
| Free tetraiodothyronine (pmol/L) | 11.3 (9.8, 12.5) | 11.5 (9.1, 12.8) | 0.084 | 0.93 |
| Thyroid-stimulating hormone (μIU/mL) | 1.5 (1.0, 2.0) | 1.3 (0.9, 2.5) | 0.285 | 0.78 |
| Duration of follow-up (months) | 33 (28, 38) | 21 (14, 27) | 4.825 | < 0.01 |
Among the aforementioned 142 patients, 100 patients who underwent complements and Ig tests were re-analyzed as the internal validation set. The median follow-up duration was 31 (24.3-36 months) months. At the end of follow-up, 2 (3.3%) patients in the CC group and 10 (25%) patients in the DC group developed HCC. The incidence of HCC was significantly higher in the DC group than in the CC group (χ2 = 10.669, P < 0.01).
The characteristics of patients in the validation set are presented in Table 2. Consistent with the training set, patients who developed HCC were older than those who did not develop HCC (P < 0.01). Patients who developed HCC had lower levels of platelet (PLT) (Z = 2.127, P = 0.03). Moreover, there was no significant difference in TT3, TT4, FT3, FT4, TSH, C3, C4, IgM, IgA, and IgG between the two groups (all P > 0.05).
| Variables | Patients did not develope HCC (n = 88) | Patients developed HCC (n = 12) | Z or χ² | P value |
| Age (years) | 50.5 (46, 61.8) | 63 (55, 67.8) | 3.010 | < 0.01 |
| Male | 58 (65.9) | 9 (75.0) | 0.395 | 0.75 |
| Decompensated cirrhosis | 30 (34.1) | 10 (83.3) | 10.669 | < 0.01 |
| Diabetes | 22 (25) | 6 (50) | 3.274 | 0.09 |
| Alanine transaminase (U/L) | 35 (21, 68.8) | 28.5 (18.8, 54.5) | 0.960 | 0.34 |
| Aspartate transaminase (U/L) | 36 (25, 57.8) | 43.5 (33, 54.5) | 0.642 | 0.52 |
| Gamma-glutamyl transpeptidase (U/L) | 60 (28.3, 90) | 130.5 (28.5, 169.8) | 1.681 | 0.09 |
| Total bilirubin (µmol/L) | 21.8 (15.6, 30.7) | 24.5 (16.9, 32.2) | 0.536 | 0.59 |
| Albumin (g/L) | 40.3 (33.8, 43.9) | 40.2 (33.2, 44.3) | 0.042 | 0.97 |
| Creatinine (µmol/L) | 77.4 (70.1, 88.9) | 81.3 (63.2, 90.8) | 0.127 | 0.90 |
| International normalized ratio | 1.1 (1.0, 1.2) | 1.1 (0.9, 1.2) | 0.244 | 0.81 |
| Platelet (E+09/L) | 103.5 (65.5, 142.8) | 74 (51.5, 90) | 2.127 | 0.03 |
| Total triiodothyronine (nmol/L) | 1.6 (1.3, 1.9) | 1.5 (1.3, 1.7) | 1.008 | 0.31 |
| Total tetraiodothyronine (nmol/L) | 118.2 (102.5, 134.3) | 113.2 (94.4, 135.5) | 0.525 | 0.60 |
| Free triiodothyronine (pmol/L) | 4.8 (4.3, 5.3) | 4.9 (4.3, 5.5) | 0.106 | 0.92 |
| Free tetraiodothyronine (pmol/L) | 11.2 (9.8, 12.4) | 12.2 (9.6, 13.1) | 0.981 | 0.33 |
| Thyroid-stimulating hormone (μIU/mL) | 1.4 (1.1, 2.0) | 1.3 (1.0, 2.6) | 0.377 | 0.71 |
| IgM (g/L) | 1.4 (0.9, 1.8) | 1.2 (1.0, 1.9) | 0.048 | 0.96 |
| IgA (g/L) | 2.8 (2.2, 3.8) | 2.8 (1.9, 4.1) | 0.191 | 0.85 |
| IgG (g/L) | 14.9 (12.9, 18.8) | 15.5 (11.1, 19.3) | 0.472 | 0.64 |
| Complement C3 (g/L) | 0.8 (0.7, 0.9) | 0.8 (0.6, 1.0) | 0.074 | 0.94 |
| Complement C4 (g/L) | 0.2 (0.1, 0.2) | 0.2 (0.1, 0.2) | 0.871 | 0.38 |
| Duration of follow-up (months) | 32 (26.3, 36.8) | 21.5 (12.5, 26.3) | 3.759 | < 0.01 |
Patients with HBV-related DC were older (Z = 4.247, P < 0.01) and had a higher proportion of diabetes (χ2 = 4.762, P = 0.03) than those with CC. TBil and INR were higher, while ALB and PLT count were lower in patients with DC (all P < 0.01). Additionally, patients with DC had lower TT3, TT4, FT3, C3, and C4 (all P < 0.01) and higher IgA and IgG (both P < 0.01). There was no significant difference in FT4 and IgM between patients with CC and DC (Table 3).
| Variables | Compensated cirrhosis (n = 60) | Decompensated cirrhosis (n = 40) | Z or χ² | P value |
| Age (years) | 50.1 (45.3, 55) | 60.2 (55, 66.8) | 4.247 | < 0.01 |
| Male | 38 (63.3) | 29 (72.5) | 0.912 | 0.34 |
| Hepatocellular carcinoma | 2 (3.3) | 10 (25) | 10.669 | < 0.01 |
| Diabetes | 12 (20) | 16 (40) | 4.762 | 0.03 |
| Alanine transaminase (U/L) | 105.5 (21, 95.3) | 52.1 (21.3, 59.5) | 1.116 | 0.27 |
| Aspartate transaminase (U/L) | 71.3 (23.3, 56.5) | 57.5 (29.5, 57.3) | 1.165 | 0.24 |
| Gamma-glutamyl transpeptidase (U/L) | 74.2 (26.3, 82) | 92.4 (35.5, 123) | 1.530 | 0.13 |
| Total bilirubin (µmol/L) | 21 (14.9, 23.7) | 35.3 (18.7, 47.7) | 3.912 | < 0.01 |
| Albumin (g/L) | 41.9 (39.8, 45.1) | 34.3 (29.5, 40.1) | 5.717 | < 0.01 |
| Creatinine (µmol/L) | 79.4 (71.4, 89.5) | 87.4 (66.8, 88.7) | 0.310 | 0.76 |
| International normalized ratio | 1.1 (0.99, 1.1) | 1.3 (1.1, 1.5) | 5.365 | < 0.01 |
| Platelet (E+09/L) | 122.1 (84.5, 153.5) | 87.2 (50.3, 107.8) | 3.652 | < 0.01 |
| Total triiodothyronine (nmol/L) | 1.8 (1.6, 2.0) | 1.3 (1.1, 1.5) | 6.414 | < 0.01 |
| Total tetraiodothyronine (nmol/L) | 125.3 (107.6, 140.8) | 108.3 (90.9, 126.5) | 2.698 | < 0.01 |
| Free triiodothyronine (pmol/L) | 5.1 (4.6, 5.5) | 4.3 (3.8, 4.9) | 4.655 | < 0.01 |
| Free tetraiodothyronine (pmol/L) | 11.3 (10.3, 12.3) | 11.3 (9.6, 12.8) | 0.127 | 0.90 |
| Thyroid-stimulating hormone (μIU/mL) | 1.6 (1.1, 1.9) | 1.8 (1.1, 2.2) | 0.454 | 0.65 |
| IgM (g/L) | 1.4 (0.9, 1.7) | 1.6 (1.0, 2.0) | 1.168 | 0.24 |
| IgA (g/L) | 2.6 (2.0, 3.1) | 3.9 (2.6, 5.1) | 4.208 | < 0.01 |
| IgG (g/L) | 15.0 (12.3, 16.5) | 17.4 (13.9, 19.8) | 2.712 | < 0.01 |
| Complement C3 (g/L) | 0.9 (0.7, 0.9) | 0.7 (0.6, 1.0) | 3.087 | < 0.01 |
| Complement C4 (g/L) | 0.2 (0.1, 0.3) | 0.1 (0.1, 0.2) | 2.915 | < 0.01 |
| Duration of follow-up (months) | 32 (25.3, 36.8) | 27.9 (23, 35) | 1.972 | 0.05 |
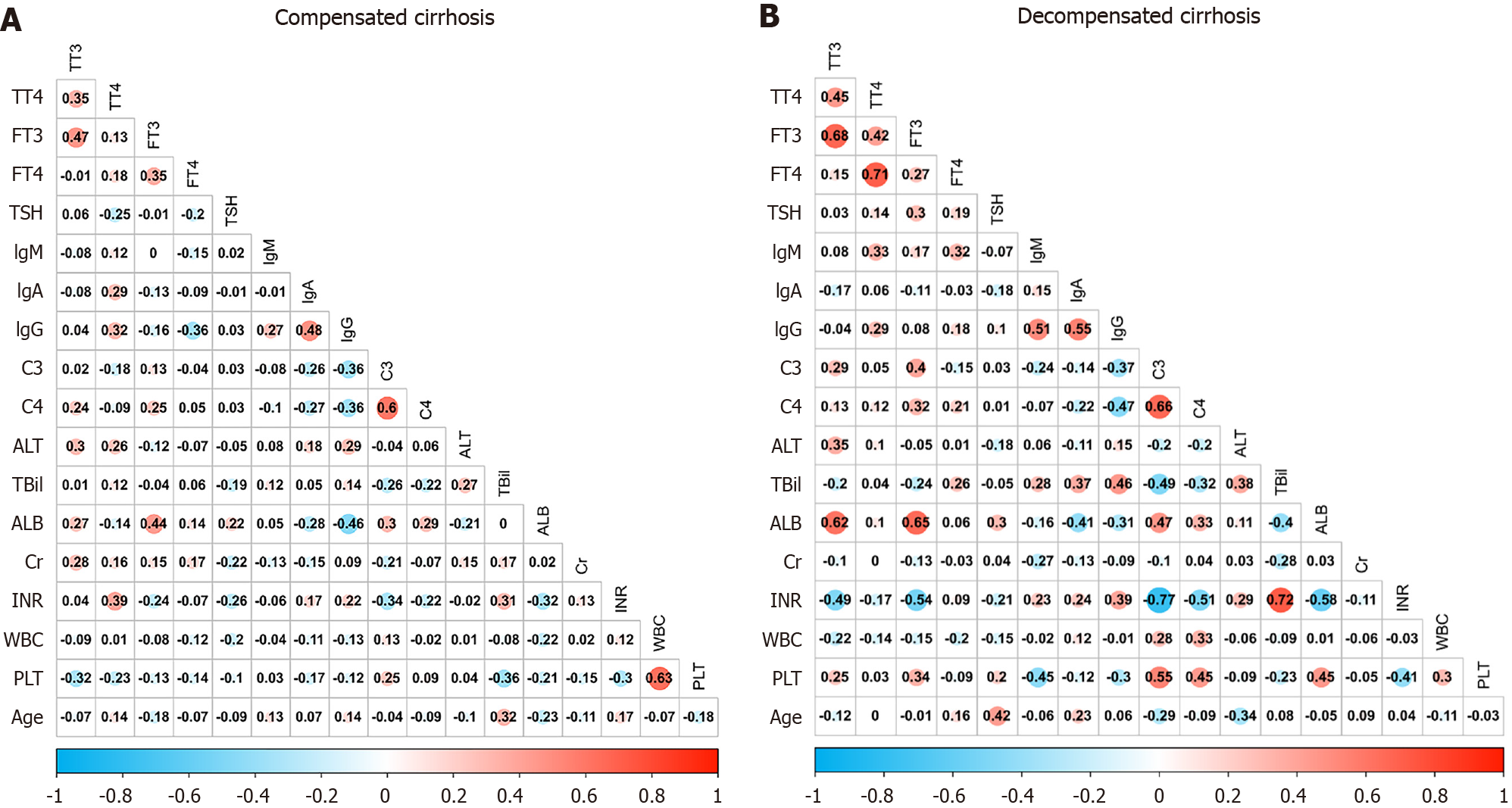
As shown in Figure 2A, TT3 positively correlated with TT4 and FT3 (both P < 0.01). It also positively correlated with ALT (P = 0.01), AST (P < 0.01), GGT (P < 0.01), and creatinine (P = 0.03), while it negatively correlated with the PLT count (P = 0.02). TT4 positively correlated with ALT (P = 0.03), AST (P = 0.04), GGT (P < 0.01), INR (P < 0.01), IgA (P < 0.01), and IgG (P = 0.04). FT3 positively correlated with FT4 and ALB (both P < 0.01). In addition, FT4 negatively correlated with TSH (P = 0.03) and IgG (P < 0.01).
IgG positively correlated with IgM (P = 0.02), IgA (P < 0.01), ALT (P < 0.01), AST (P < 0.01), and GGT (P = 0.03), while it negatively correlated with C3 (P = 0.03) and C4 (P < 0.01). IgA but not IgM positively correlated with ALT (P = 0.03), AST (P = 0.01), and GGT (P = 0.02).
C3 positively correlated with C4 (P < 0.01), ALB (P = 0.03), and PLT (P = 0.03), and negatively correlated with INR (P = 0.01).
As depicted in Figure 2B, TT3 positively correlated with TT4 (P = 0.01) and FT3 (P < 0.01), ALT (P < 0.01), AST (P = 0.02), GGT (P < 0.01), and ALB (P < 0.01), while it negatively correlated with INR (P < 0.01). TT4 positively correlated with FT3
IgG positively correlated with TT4 (P = 0.03), IgM (P < 0.01), IgA (P < 0.01), ALT (P < 0.01), AST (P < 0.01), and TBil (P < 0.01), while it negatively correlated with C3 (P = 0.049) and C4 (P < 0.01) and PLT (P = 0.01). IgM positively correlated with TT4 (P = 0.02), FT4 (P = 0.02), TBil (P < 0.01), and INR (P = 0.047), while it negatively correlated with ALB (P = 0.03) and PLT (P < 0.01). IgA positively correlated with TBil (P = 0.03) and negatively correlated with ALB (P = 0.01).
C3 positively correlated with C4 (P < 0.01), GGT (P = 0.03), ALB (P < 0.01), and PLT (P < 0.01), and negatively correlated with TBil and INR (both P < 0.01).
Both C3 and C4 positively correlated with FT3 (P < 0.01 and P = 0.03), ALB (P < 0.01 and P = 0.03), and PLT (both P < 0.01), and negatively correlated with IgG (P = 0.045 and P < 0.01), TBil (P < 0.01 and P = 0.02), and INR (both P < 0.01).
For patients in the training set, the univariate analysis indicated associations between HCC development and age, DC, diabetes, GGT, and PLT. Multivariable analysis revealed that age (P = 0.01), DC status (P = 0.03), and GGT (P = 0.03) were independent risk factors for HCC development (all P < 0.05) (Table 4).
| Baseline variables | Univariate | Multivariate | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age (years) | 1.081 | 1.029-1.135 | < 0.01 | 1.068 | 1.014-1.125 | 0.01 |
| Male | 0.664 | 0.224-1.969 | 0.46 | |||
| Decompensated cirrhosis | 6.815 | 1.888-24.597 | < 0.01 | 4.703 | 1.180-18.745 | 0.03 |
| Diabetes | 2.962 | 1.104-7.944 | 0.03 | |||
| Alanine transaminase (U/L) | 0.991 | 0.978-1.004 | 0.18 | |||
| Gamma-glutamyl transpeptidase (U/L) | 1.005 | 0.999-1.011 | 0.08 | 1.007 | 1.001-1.014 | 0.03 |
| Total bilirubin (µmol/L) | 1.007 | 0.982-1.033 | 0.57 | |||
| International normalized ratio | 0.746 | 0.081-6.888 | 0.80 | |||
| Platelet (E+09/L) | 0.990 | 0.979-1.000 | 0.05 | |||
| Total triiodothyronine (nmol/L) | 0.395 | 0.120-1.298 | 0.13 | |||
| Total tetraiodothyronine (nmol/L) | 0.997 | 0.980-1.014 | 0.73 | |||
| Free triiodothyronine (pmol/L) | 0.738 | 0.423-1.290 | 0.29 | |||
| Free tetraiodothyronine (pmol/L) | 0.949 | 0.749-1.203 | 0.67 | |||
| Thyroid-stimulating hormone (μIU/mL) | 1.101 | 0.683-1.776 | 0.69 | |||
Similar findings emerged in the validation set, where univariate analysis identified associations between HCC development and age, DC, diabetes, GGT, and PLT. Subsequent multivariable analysis demonstrated that age (P = 0.04), DC status (P = 0.06), and GGT (P = 0.01) remained independent risk factors for HCC development (all P < 0.05) (Table 5).
| Baseline variables | Univariate | Multivariate | ||||
| 95%CI | P value | 95%CI | P value | 95%CI | P value | |
| Age (years) | 1.088 | 1.022-1.158 | < 0.01 | 1.08 | 1.002-1.163 | 0.04 |
| Male | 1.552 | 0.391-6.162 | 0.53 | |||
| Decompensated cirrhosis | 9.667 | 1.989-46.972 | < 0.01 | 5.476 | 0.906-33.101 | 0.06 |
| Diabetes | 3 | 0.877-10.265 | 0.08 | |||
| Alanine transaminase (U/L) | 0.992 | 0.978-1.006 | 0.26 | |||
| Gamma-glutamyl transpeptidase (U/L) | 1.007 | 1.001-1.014 | 0.03 | 1.01 | 1.002-1.019 | 0.01 |
| Total bilirubin (µmol/L) | 1.003 | 0.969-1.038 | 0.87 | |||
| International normalized ratio | 0.248 | 0.008-7.998 | 0.43 | |||
| Platelet (E+09/L) | 0.985 | 0.971-1.000 | 0.04 | |||
| Total triiodothyronine (nmol/L) | 0.519 | 0.113-2.384 | 0.4 | |||
| Total tetraiodothyronine (nmol/L) | 0.994 | 0.973-1.015 | 0.55 | |||
| Free triiodothyronine (pmol/L) | 1.008 | 0.472-2.152 | 0.98 | |||
| Free tetraiodothyronine (pmol/L) | 1.102 | 0.807-1.505 | 0.54 | |||
| Thyroid-stimulating hormone (μIU/mL) | 1.173 | 0.670-2.055 | 0.58 | |||
| IgM (g/L) | 1.574 | 0.792-3.126 | 0.2 | |||
| IgA (g/L) | 0.986 | 0.638-1.522 | 0.95 | |||
| IgG (g/L) | 0.96 | 0.834-1.105 | 0.57 | |||
| Complement 3 (g/L) | 1.588 | 0.081-31.080 | 0.76 | |||
| Complement 4 (g/L) | 0.096 | 0-462.908 | 0.59 | |||
For patients in both the training and validation sets, univariate analysis revealed no significant associations between TT3, TT4, FT3, FT4, TSH, and HCC development (all P > 0.05). Additionally, C3, C4, IgM, IgA, and IgG showed no significant associations with HCC development in the validation group (all P > 0.05).
In the present study, patients with DC exhibited a higher incidence of HCC development than those with CC (22.9% vs 4.2%, P < 0.01) during a median follow-up of 32 months, and this observation was validated in an internal validation set. Patients with DC manifested lower levels of TT3, TT4, FT3, C3, and C4, coupled with higher levels of IgA and IgG. In both CC and DC patients, TT3 and TT4 exhibited positive correlations with ALT, AST, and GGT. IgG demonstrated positive correlations with IgM, IgA, ALT, and AST, while displaying negative correlations with C3 and C4. In both the training and validation sets, univariate analysis revealed associations between age, DC, diabetes, GGT, PLT count, and HCC development. Subsequent multivariable analysis identified age, DC status, and GGT as independent risk factors for HCC development. Baseline levels of TH, complements, and Ig did not emerge as risk factors for HCC development in patients with CC or DC.
The role of TH in predicting HCC development remains a matter of controversy. Several studies have identified hypothyroidism as a potential risk factor for HCC development[5,7,8,11], while contradictory findings have been reported in other studies[6,8]. The prevalence of hypothyroidism was notably high among patients with HCC (11.7%)[5]. Speculation suggests that underlying conditions, such as non-alcoholic fatty liver disease and hepatitis C virus infection, might contribute to this elevated prevalence of hypothyroidism[5]. Conversely, data from a different study indicated that HCC development in patients with HCV-related cirrhosis correlated with a significant increase in TT3, but not in FT3 or FT4[21]. Discrepancies in results could be attributed to variations in etiologies of chronic liver diseases, sample sizes, and the racial composition of the study populations. Additionally, differing diagnostic criteria for hypothyroidism and exclusion criteria for participant enrolment may contribute to these conflicting outcomes[5,8,9]. In the current study, baseline TH was not found to be associated with HCC development in patients with HBV-related CC or DC. However, given that TT3, TT4, and FT3 were lower in patients with DC, who are at a higher risk of HCC, it is essential to monitor dynamic changes in TH during HCC surveillance.
In patients with CC or DC, IgG exhibited a positive correlation with ALT and AST, while demonstrating a negative correlation with C3 and C4. These findings imply a notable association between IgG and liver inflammation. Considering that antiviral treatment can potentially reverse liver inflammation and fibrosis stage in a considerable number of patients with CC or DC, assessing the dynamic changes in IgG and complements during follow-up holds significance. It is important to acknowledge that the effects of antiviral agents on HCC development may vary, and the dynamic alterations in IgG and complements could differ between patients with CC and DC. In line with a previous study, age, DC, and GGT were identified as independent risk factors for HCC development in patients with LC[3]. However, the present study did not reveal optimal predictive values for IgG and complements. This suggests a lack of substantial evidence to advocate for IgG or complements tests during HCC surveillance, emphasizing the need to alleviate the economic burden on patients.
The present study has several limitations. Firstly, the sample size is relatively small, warranting the necessity for a multicenter study with a larger sample size. Monitoring of TH, Ig, and complements may be of potential values during follow-up, and further studies focusing on the mechanisms of these biomarker in cirrhosis progression are needed in future. Secondly, the internal validation set is derived from the training set; however, the inclusion of an external validation set would enhance the persuasiveness of the findings. Thirdly, the present study did not collect information on HBV genotypes and the family history of HCC.
In conclusion, the predictive value of TH, Ig, and complements for HCC development is suboptimal. Age, DC status, and GGT emerged as more significant factors during HCC surveillance in HBV-related LC.
We thank Professor Yan B from Malmö Lund University, Professor Wan L and You L from Nanjing Medical University for their kind guidance.
| 1. | Su GL, Altayar O, O'Shea R, Shah R, Estfan B, Wenzell C, Sultan S, Falck-Ytter Y. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology. 2022;162:920-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 128] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 2. | Huang DQ, Singal AG, Kanwal F, Lampertico P, Buti M, Sirlin CB, Nguyen MH, Loomba R. Hepatocellular carcinoma surveillance - utilization, barriers and the impact of changing aetiology. Nat Rev Gastroenterol Hepatol. 2023;20:797-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 86] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 3. | Mao HD, Zheng SQ, Yang SH, Huang ZY, Xue Y, Zhou M. A new model predicts hepatocellular carcinoma in patients with HBV-related decompensated liver cirrhosis and long-term antiviral therapy: a prospective study. PeerJ. 2023;11:e15014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Jacobson IM, Brown RS Jr, McMahon BJ, Perrillo RP, Gish R. An Evidence-based Practical Guide to Vaccination for Hepatitis B Virus. J Clin Gastroenterol. 2022;56:478-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 5. | Hassan MM, Kaseb A, Li D, Patt YZ, Vauthey JN, Thomas MB, Curley SA, Spitz MR, Sherman SI, Abdalla EK, Davila M, Lozano RD, Hassan DM, Chan W, Brown TD, Abbruzzese JL. Association between hypothyroidism and hepatocellular carcinoma: a case-control study in the United States. Hepatology. 2009;49:1563-1570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Lu L, Wan B, Li L, Sun M. Hypothyroidism has a protective causal association with hepatocellular carcinoma: A two-sample Mendelian randomization study. Front Endocrinol (Lausanne). 2022;13:987401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Pinter M, Haupt L, Hucke F, Bota S, Bucsics T, Trauner M, Peck-Radosavljevic M, Sieghart W. The impact of thyroid hormones on patients with hepatocellular carcinoma. PLoS One. 2017;12:e0181878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Reddy A, Dash C, Leerapun A, Mettler TA, Stadheim LM, Lazaridis KN, Roberts RO, Roberts LR. Hypothyroidism: a possible risk factor for liver cancer in patients with no known underlying cause of liver disease. Clin Gastroenterol Hepatol. 2007;5:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Sahin T, Oral A, Turker F, Kocak E. Can hypothyroidism be a protective factor for hepatocellular carcinoma in cirrhosis? Medicine (Baltimore). 2020;99:e19492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Alisi A, Demori I, Spagnuolo S, Pierantozzi E, Fugassa E, Leoni S. Thyroid status affects rat liver regeneration after partial hepatectomy by regulating cell cycle and apoptosis. Cell Physiol Biochem. 2005;15:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Shao YY, Cheng AL, Hsu CH. An Underdiagnosed Hypothyroidism and Its Clinical Significance in Patients with Advanced Hepatocellular Carcinoma. Oncologist. 2021;26:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Simbrunner B, Hartl L, Jachs M, Bauer DJM, Scheiner B, Hofer BS, Stättermayer AF, Marculescu R, Trauner M, Mandorfer M, Reiberger T. Dysregulated biomarkers of innate and adaptive immunity predict infections and disease progression in cirrhosis. JHEP Rep. 2023;5:100712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Lian M, Wang Q, Chen S, Yang Y, Hong G. The association of serum immunoglobulin and complement levels and liver fibrosis and inflammation stage in patients with chronic hepatitis B. J Viral Hepat. 2023;30:437-447. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Zhang K, Zeng M, Li YJ, Wu HF, Wu JC, Zhang ZS, Zheng JF, Lv YF. Antibody and complement levels in patients with hypersplenism associated with cirrhotic portal hypertension and therapeutic principles. World J Clin Cases. 2022;10:13208-13215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Liu C, Zhao H, Wang P, Guo Z, Qu Z. The combination of circulating IgM and geriatric nutritional risk index predicts the prognostic of hepatocellular carcinoma patients who underwent immune checkpoint inhibitors. Int Immunopharmacol. 2023;123:110704. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Balcar L, Bauer D, Pomej K, Meischl T, Mandorfer M, Reiberger T, Trauner M, Scheiner B, Pinter M. Early changes in immunoglobulin G levels during immune checkpoint inhibitor treatment are associated with survival in hepatocellular carcinoma patients. PLoS One. 2023;18:e0282680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Petrick JL, Florio AA, Zen J, Wang Y, Gewirtz AT, Pfeiffer RM, Loftus S, Inglefield J, Koshiol J, Yang B, Yu K, Hildesheim A, Chen CJ, Yang HI, Lee MH, McGlynn KA. Biomarkers of gut barrier dysfunction and risk of hepatocellular carcinoma in the REVEAL-HBV and REVEAL-HCV cohort studies. Int J Cancer. 2023;153:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Chen C, Yuan Z, Li W, Fei L, Ji L, Huang Q, Zhang S, Chen L. Complement C3 Facilitates Stratification of Stages of Chronic Hepatitis B and Signifies Development of Acute-on-Chronic Liver Failure in Acute Decompensated Cirrhosis. Adv Ther. 2023;40:1171-1186. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Jia JD, Hou JL, Wei L, Zhuang H. [Highlights of the guidelines of prevention and treatment for chronic hepatitis B (2019 version)]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:21-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer. 2018;7:235-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 443] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 21. | Sorvillo F, Mazziotti G, Carbone A, Morisco F, Cioffi M, Rotondi M, Stornaiuolo G, Amato G, Gaeta GB, Caporaso N, Carella C. Increased serum reverse triiodothyronine levels at diagnosis of hepatocellular carcinoma in patients with compensated HCV-related liver cirrhosis. Clin Endocrinol (Oxf). 2003;58:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |