Published online Feb 27, 2025. doi: 10.4254/wjh.v17.i2.97741
Revised: October 30, 2024
Accepted: January 15, 2025
Published online: February 27, 2025
Processing time: 257 Days and 23 Hours
Cardiovascular health (CVH) plays a crucial role in overall health, but its asso
To investigate the relationship between CVH, measured using Life’s Essential 8 (LE8) and Life’s Simple 7 (LS7), and the prevalence of MAFLD.
This cross-sectional study had a sample of 2234 individuals, representing approximately 120 million individuals in the United States. Baseline parameters were compared between the LE8 and LS7 groups. Logistic regression models were used to evaluate the relationship between LE8, LS7, and MAFLD, while taking into account confounding factors. The investigation employed restricted cubic splines to investigate non-linear associations. Subgroup analyses and sensitivity studies were performed to evaluate the strength and reliability of the results.
Higher LE8 and LS7 scores were significantly associated with a decreased risk of MAFLD, even after controlling for demographic, socioeconomic, and clinical variables. This association demonstrated a non-linear pattern, with the most dramatic risk reduction observed at higher CVH levels. Individual CVH components, notably healthy behaviors and factors, exhibited strong relationships with MAFLD. Subgroup analyses indicated consistent relationships across several demographics. Sensitivity tests utilizing other MAFLD definitions validated the robustness of the findings.
Higher adherence to CVH criteria, as indicated by LE8 and LS7 scores, is associated with a significantly lower risk of MAFLD. These results emphasize the need to advance CVH to control and avoid MAFLD.
Core Tip: Higher adherence to cardiovascular health (CVH) metrics, assessed using Life’s Essential 8 and Life’s Simple 7, is strongly associated with a reduced risk of metabolic-associated fatty liver disease (MAFLD). This large cross-sectional study demonstrates a non-linear relationship, with the most significant risk reduction observed at higher CVH levels, emphasizing the importance of promoting CVH for the prevention and management of MAFLD.
- Citation: Fu W, Cheng GB, Zhao JL, Lv LY, Ding Y. Association of Life’s Essential 8 and Life’s Simple 7 with metabolic-associated fatty liver disease in the United States. World J Hepatol 2025; 17(2): 97741
- URL: https://www.wjgnet.com/1948-5182/full/v17/i2/97741.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i2.97741
Metabolic-associated fatty liver disease (MAFLD), formerly known as non-alcoholic fatty liver disease (NAFLD), is an increasing global health concern[1]. With an estimated 25%–38% of the world’s population affected, the rising incidence of MAFLD is alarming, as it significantly reduces survival rates and poses a considerable threat to public health[2]. The perils of MAFLD are multifaceted, extending beyond liver-related complications. It is a leading cause of advanced liver pathologies, including cirrhosis and hepatocellular carcinoma, which contribute to a substantial number of mortalities annually[3]. The limitations in treatment options, particularly for end-stage liver disease, highlight an urgent need for novel therapeutic approaches. Furthermore, MAFLD is closely associated with type 2 diabetes and obesity, significantly increasing the risk of cardiovascular diseases, metabolic disorders, and various forms of cancer, thereby increasing the burden on healthcare systems and affecting the quality of life for millions of individuals worldwide[4]. Recognizing the complexity of MAFLD, the identification of new indicators that predict the onset and progression of fatty liver disease is of paramount importance. The understanding that not all individuals with obesity develop MAFLD suggests that other factors may play a role in its pathogenesis.
MAFLD has been increasingly recognized for its strong association with cardiovascular diseases[5-7]. Studies have shown that higher Life’s Simple 7 (LS7) scores are associated with a lower prevalence of NAFLD[8,9], suggesting that LS7 not only serves as a cardiovascular health (CVH) metric but may also be instrumental in the early identification and prevention of liver diseases such as MAFLD. In 2022, the American Heart Association (AHA) expanded upon LS7 by introducing Life’s Essential 8 (LE8), which incorporates sleep health as an additional parameter. LE8 has since demonstrated superior predictive capabilities over LS7 for various cardiovascular outcomes[10]. However, despite the evident advantages of LE8, there remains a gap in research concerning its relationship with MAFLD. It is noteworthy that few studies have investigated the applicability of LE8 to the revised MAFLD nomenclature; understanding this re
In this study, we investigate the relationship between LE8, LS7, and MAFLD, hypothesizing that both scoring systems may have a major impact on the onset and course of MAFLD, especially in the United States population. We aim to determine the strength of these correlations using data from 9254 adults from the National Health and Nutrition Examination Survey (NHANES) 2017–2018, therefore providing a more sophisticated clinical screening and diagnosis for MAFLD and associated metabolic diseases. Furthermore, this study will investigate the relationships of LE8 and LS7 with various definitions of non-alcoholic fatty liver and will include a larger number of significant factors compared to previous research[11,12].
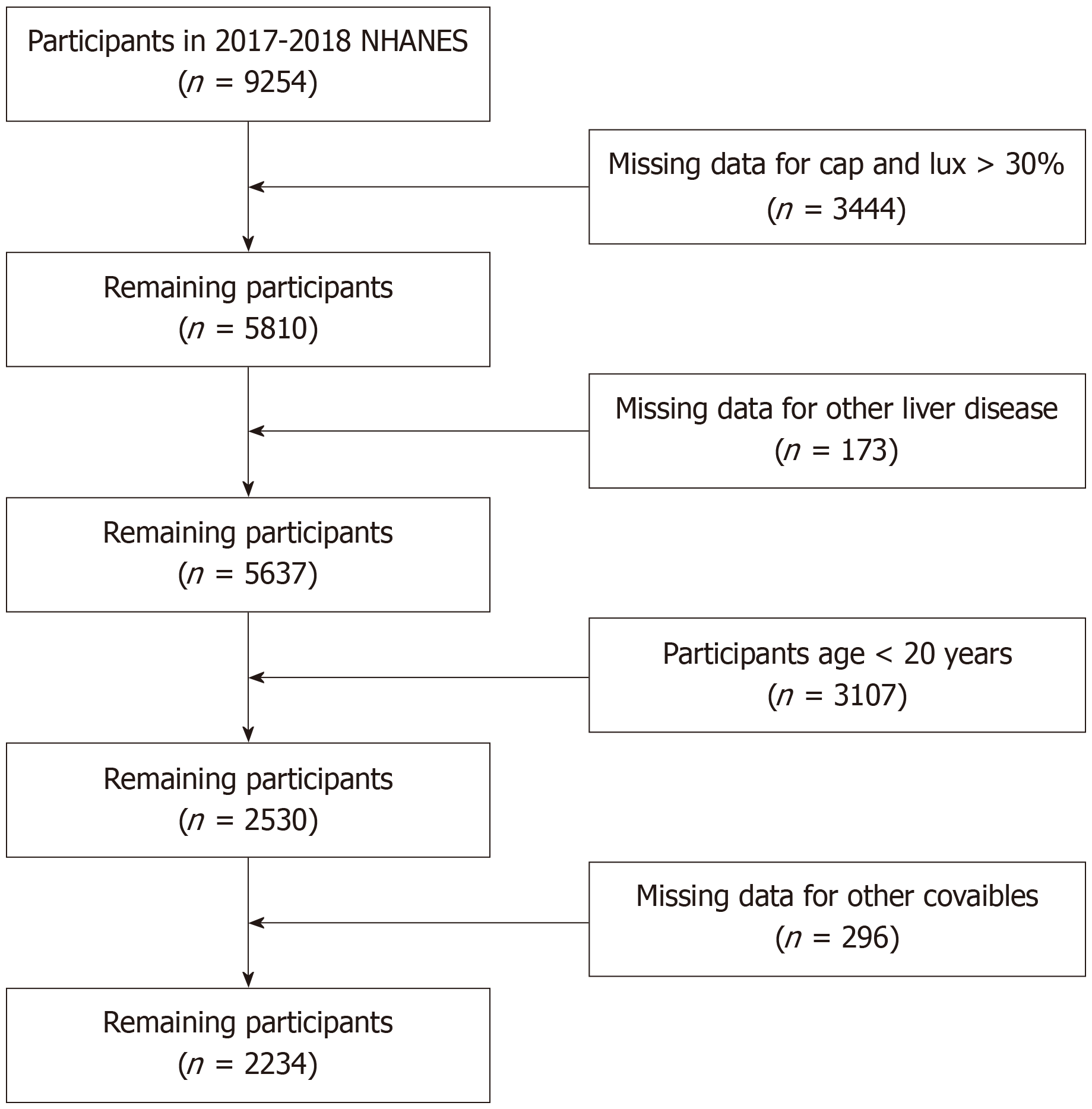
The NHANES study collected data on the dietary habits and general health of Americans, including both adults and children. The National Center for Health Statistics Research Ethics Review Board approved the survey, which used a sophisticated sampling technique. One important addition to the 2017–2018 study was the use of ultrasonography and vibration-controlled transient elastography for liver evaluation. A total of 9000 adults participated in this phase of the study. Individuals aged under 20 years, pregnant women, those ineligible for or with incomplete elastography examinations, and those lacking data on the Dietary Approaches to Stop Hypertension (DASH) were excluded (Figure 1).
This study evaluated liver fibrosis and steatosis using the median controlled attenuation parameter (CAP) and liver stiffness measurement methods. A CAP threshold of 285 dB/m was set along with one of three criteria to detect hepatic steatosis: having type 2 diabetes mellitus (T2DM), being overweight or obese (body mass index [BMI] ≥ 25 kg/m2), or having a BMI < 25 kg/m2 but exhibiting at least two metabolic risk abnormalities. These metabolic risk abnormalities included low-density lipoprotein-cholesterol levels below 40 mg/dL for men and 50 mg/dL for women, prediabetes indicators (fasting glucose of 100–125 mg/dL or hemoglobin A1c levels of 5.7%–6.4%), a homeostasis model assessment of insulin resistance score of 2.5 or higher, and plasma high-sensitivity C-reactive protein (CRP) levels exceeding 2 mg/L[13].
Steatotic liver disease (SLD) was detected using CAP scores equal to or exceeding 263 dB/m (≥ S1), ensuring a sen
A CAP score of ≥ 285 dB/m was established as diagnostic of a high risk of NAFLD, whereas advanced fibrosis (F3-4) was characterized by liver stiffness measurements of ≥ 8.6 kPa, coupled with an interquartile range/median (IQR/median) of liver stiffness below 30%[17,18].
LE8 was introduced by the AHA as an expansion of the LS7 paradigm, comprising four health behaviors (diet, physical activity [PA], nicotine exposure, and sleep health) and four health metrics (BMI, blood lipids, blood glucose, and blood pressure [BP])[19,20]. These characteristics were assessed at baseline to determine LE8 scores. Dietary patterns were evaluated using the DASH technique, conducted via face-to-face 24-hour dietary recall interviews employing the USDA Automated Multiple-Pass technique to quantify food and beverage intake. PA included self-reported time spent on activities such as walking, bicycling, domestic duties, and leisure interests during the week[21].
The LE8 score was assessed on a scale of 0–100, with the final score calculated as the unweighted average of all indicators. The entire LE8 score was then categorized into three levels: high (80–100), moderate (50–79), and low (0–49). Detailed directions for applying the LE8 scoring algorithms to NHANES data for adults were included in the online materials and presidential advisory[22].
LS7, the precursor to LE8, excluded sleep health and employed a simpler scoring algorithm. Each LS7 metric was graded into poor, moderate, or ideal levels, corresponding to scores of 0, 1, and 2, respectively. The aggregate LS7 score was categorized as insufficient (0–7), average (8–10), or optimum (11–14), as indicated in earlier studies. Comprehensive instructions for applying LS7 scoring algorithms to NHANES data for adults were provided in the online materials[23].
In our study, confounders such as age, sex, race/ethnicity, education, poverty-income ratio, marital status, disease history, alcohol consumption, sleep disorders, and depression were considered based on prior research. The NHANES database provided important covariate data, including BMI, gamma-glutamyl transferase (GGT)(IU/L), and CRP (mg/L).
Age at baseline was obtained using participants’ birthdates and baseline assessment dates and then classified into three groups: < 40 years, 40–64 years, and ≥ 65 years. Self-reported race/ethnicity was categorized as Mexican American, non-Hispanic Black, Non-Hispanic White, Other Hispanic, or Other. Educational level was classified as less than high school, high school or equivalent, and above high school. The poverty-to-income ratio (PIR) was divided into three categories: ≤ 1.30, 1.31–3.50, and > 3.50. Participants scoring ≥ 10 on the Patient Health Questionnaire-9 (PHQ-9) were categorized as suffering from depression. Marital status was categorized as married (or living with a partner) or single (or widowed/divorced/separated), while smoking status was categorized as current smoker, past smoker, and never smoker. Par
Owing to the sophisticated four-stage sampling strategy adopted by NHANES, the WTDRD1 weights were produced by altering the Mobile Examination Center sample weights (WTMEC2YR) to account for extra non-response and unequal distribution of food intake data collection by day of the week. Weighted univariate logistic regression analysis was employed to filter covariates and identify relevant factors. The strata (SDMVSTRA) and main sampling units (SDMVPSU) were then used to reconstruct the data to reflect the entire non-institutionalized civilian population of the United States[24].
Continuous variables having a normal distribution were provided as mean ± SD, whereas skewed continuous variables were characterized using the median and IQR. Categorical variables were presented as frequencies and percentages. Statistical analysis included the χ2 test or Fisher’s exact test for categorical data, one-way analysis of variance for normally distributed continuous variables, and the Kruskal-Wallis H test for skewed distributed variables to analyze differences across groups. To address multiple comparisons, modifications to P-values were performed using the Bonferroni correction, Tukey, or LSD techniques, as applicable. Univariate and multivariate binary logistic regression models were applied to evaluate the associations between LE8, LS7, and MAFLD. In these models, LE8 and LS7 were regarded as categorical variables indicative of clinical diagnoses. Confounders were selected based on clinical significance and existing scientific evidence. Three models were constructed: Model 1 accounted for age and sex; Model 2 further corrected for ethnicity, education, marital status, PIR, and alcohol consumption status; and Model 3 also included comorbidities.
This technique attempted to uncover possible nonlinear interactions. To evaluate possible nonlinear dose-response relationships between LE8, LS7, and MAFLD, we applied a limited cubic spline model to construct smooth curves. In this model, LE8 and LS7 were handled as continuous variables, with four knots positioned at the 5th, 35th, 65th, and 95th percentiles, following Harrell’s recommendations. Nonlinearity was tested using a likelihood ratio test, comparing a model having just linear terms to one integrating both linear and cubic spline components. After examining the smoothing curves, we proceeded to develop a two-piecewise linear regression model to discover any threshold effects while adjusting for confounding factors. Subgroup analyses were carried out based on subgroup characteristics, with interactions between subgroups investigated by a likelihood ratio test. No covariate exhibited missing values exceeding 5%. Furthermore, the application of K-Nearest Neighbors imputation to address missing data in all covariates did not result in substantial changes in the study outcomes. Sensitivity assessments included complete-case analysis and alternative definitions of outcome variables, with findings consistent across these approaches, as stated in the Supplementary material.
All statistical analyses were conducted using R Statistical Software (Version 4.2.2) and the Free Statistics Analysis Platform (Version 1.9.2, Beijing, China). Statistical significance was determined at a two-sided P value < 0.05.
The fundamental characteristics of the participants are presented in Table 1, categorized based on their adherence to LE8. After undergoing comprehensive screening following pre-established inclusion and exclusion criteria, a total of 2234 patients were included in the study, which is representative of approximately 120 million individuals in the United States. The weighted prevalence of MAFLD in the study population was 40.51%. The distribution of a number of clinical and demographic factors varied significantly across the LE8 groups. Interestingly, gender distribution differed sig
| Variables | LE8 | ||||
| Total | Low | Moderate | High | P value | |
| N | 2234 | 365 | 1411 | 458 | |
| Weighted N | 126883299.26 | 17279315.83 | 77128139.71 | 32475843.72 | |
| Gender | |||||
| Male | 47.13 | 52.50 | 49.46 | 38.71 | 0.0182 |
| Female | 52.87 | 47.50 | 50.54 | 61.29 | |
| Age | 46.532 ± 16.63 | 53.419 ± 15.20 | 48.173 ± 16.49 | 38.972 ± 14.93 | < 0.0001 |
| Age group (yr) | |||||
| < 40 | 32.00 | 27.78 | 31.99 | 34.28 | 0.6517 |
| 40-64 | 43.96 | 46.96 | 44.37 | 41.37 | |
| ≥ 65 | 24.05 | 25.27 | 23.64 | 24.35 | |
| Poverty-to-income ratio | 3.22 ± 1.62 | 2.71 ± 1.63 | 3.20 ± 1.60 | 3.55 ± 1.58 | 0.0005 |
| Education | |||||
| Less than high school | 1.83 | 1.64 | 2.14 | 1.22 | 0.0010 |
| Completed high school | 5.58 | 11.11 | 5.32 | 3.24 | |
| Beyond high school | 92.59 | 87.25 | 92.54 | 95.54 | |
| Marital | |||||
| Single | 62.86 | 63.24 | 64.58 | 58.55 | 0.0844 |
| Married | 37.14 | 36.76 | 35.42 | 41.45 | |
| Race | |||||
| Mexican Americans | 8.31 | 9.84 | 8.87 | 6.16 | 0.1569 |
| Non-Hispanic Blacks | 6.36 | 4.97 | 6.22 | 7.42 | |
| Non-Hispanic Whites | 64.80 | 63.38 | 64.62 | 66.00 | |
| Other Hispanics | 10.12 | 12.74 | 10.60 | 7.59 | |
| Other races | 10.41 | 9.07 | 9.69 | 12.83 | |
| Smoke | |||||
| Never | 61.58 | 28.14 | 58.75 | 86.07 | < 0.0001 |
| Former | 23.39 | 35.72 | 25.27 | 12.38 | |
| Current | 15.03 | 36.14 | 15.98 | 1.55 | |
| Alcohol | |||||
| No | 79.03 | 77.50 | 80.09 | 77.33 | 0.6295 |
| Yes | 20.97 | 22.50 | 19.91 | 22.67 | |
| Diabetes | |||||
| No | 88.79 | 63.90 | 90.09 | 98.93 | < 0.0001 |
| Yes | 11.21 | 36.10 | 9.91 | 1.07 | |
| Sleep disorder | |||||
| No | 86.99 | 75.95 | 87.18 | 92.42 | 0.0006 |
| Yes | 13.01 | 24.05 | 12.82 | 7.58 | |
| Depression | |||||
| No | 92.58 | 86.01 | 92.70 | 95.78 | 0.0134 |
| Yes | 7.42 | 13.99 | 7.30 | 4.22 | |
| Comorbid | |||||
| No | 74.14 | 50.60 | 73.28 | 88.70 | < 0.0001 |
| Yes | 25.86 | 49.40 | 26.72 | 11.30 | |
| BMI | 29.55 ± 6.91 | 36.19 ± 7.34 | 30.01 ± 6.23 | 24.95 ± 4.51 | < 0.0001 |
| Cap | 261.68 ± 61.39 | 314.67 ± 54.83 | 267.92 ± 55.37 | 218.67 ± 49.18 | < 0.0001 |
| GGT (IU/L) | 20.00 (13.00, 31.00) | 27.00 (19.00, 44.00) | 21.00 (14.00, 34.00) | 15.00 (12.00, 21.00) | < 0.0001 |
| CRP (mg/L) | 1.70 (0.81, 3.86) | 3.70 (1.69, 7.33) | 1.90 (0.94, 3.85) | 0.970 (0.56, 1.93) | < 0.0001 |
Furthermore, lifestyle variables, medical conditions, and biomarkers, such as marital status, race, smoking status, alcohol intake, diabetes (P < 0.0001), sleep disorders (P = 0.0006), depression (P = 0.0134), comorbidities (P < 0.0001), BMI, CAP, GGT (P < 0.0001), and CRP (P < 0.0001), were shown to be significantly correlated with LE8 Levels. These results emphasize the clear relationship between LE8 scores, clinical factors, and general health.
Several logistic regression models were used to analyze the effect of the main predictor variables on the result. In Model 0, the odds ratio (OR) for LE8 (per 10 units increase) was 0.51 (95%CI: 0.46-0.56, P < 0.001). In Model 1, after adjusting for age and sex, the OR remained significant at 0.53 (95%CI: 0.48-0.58, P < 0.001). An identical effect was demonstrated by Model 2, which included adjustments for drinking status, marital status, PIR, and ethnicity (95%CI: 0.43-0.59, P < 0.001). A little reduced but significant OR of 0.51 (95%CI: 0.41-0.64, P = 0.006) was obtained with Model 3, which included extra adjustments for comorbid conditions (Table 2). With regard to LE8, the unadjusted model (Model 0) showed a notable decrease in the risk of MAFLD for the moderate CVH group (OR: 0.28, 95%CI: 0.19–0.42, P < 0.001) and the high CVH group (OR: 0.05, 95%CI: 0.03–0.07, P < 0.001, P for trend < 0.001). With ORs of 0.30 (95%CI: 0.20–0.45, P < 0.001) for the moderate CVH group and 0.06 (95%CI: 0.04–0.09, P < 0.001) for the high CVH group, after additional adjustment for demographic covariates in Model 1, these associations remained strong. In Model 2, which included more socioeconomic factors, the risk reduction persisted, with the high CVH group showing an OR of 0.05 (95%CI: 0.02–0.14, P = 0.006) and the moderate CVH group showing an OR of 0.27 (95%CI: 0.13–0.59, P = 0.018). Finally, in the fully adjusted model (Model 3), which included comorbid conditions, the associations were still significant (Table 2) but moderately attenuated (moderate CVH group: OR: 0.29, 95%CI: 0.13–0.65, P = 0.022; high CVH group: OR: 0.06, 95%CI: 0.02–0.15, P = 0.007, P for trend = 0.001).
| Model 0 | P value | Model 1 | P value | Model 2 | P value | Model 3 | P value | ||
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | ||||||
| LE8 (per 10) | Continuous | 0.51 (0.46-0.56) | < 0.001 | 0.53 (0.48-0.58) | < 0.001 | 0.5 (0.43-0.59) | < 0.001 | 0.51 (0.41-0.64) | 0.006 |
| LE8 group | |||||||||
| Low | Reference | Reference | Reference | Reference | |||||
| Moderate | 0.28 (0.19-0.42) | < 0.001 | 0.30 (0.20-0.45) | < 0.001 | 0.27 (0.13-0.59) | 0.018 | 0.29 (0.13-0.65) | 0.022 | |
| High | 0.05 (0.03-0.07) | < 0.001 | 0.06 (0.04-0.09) | < 0.001 | 0.05 (0.02-0.14) | 0.006 | 0.06 (0.02-0.15) | 0.007 | |
| P for trend | < 0.001 | < 0.001 | < 0.001 | 0.001 | |||||
| LS7 | Continuous | 0.62 (0.57-0.68) | < 0.001 | 0.63 (0.58-0.68) | < 0.001 | 0.61 (0.53-0.70) | 0.001 | 0.62 (0.50-0.76) | 0.01 |
| LS7 group | |||||||||
| Inadequate | Reference | Reference | Reference | Reference | |||||
| Average | 0.31 (0.22-0.44) | < 0.001 | 0.32 (0.22-0.45) | < 0.001 | 0.29 (0.14-0.60) | 0.018 | 0.32 (0.14- 0.74) | 0.028 | |
| Optimal | 0.04 (0.02-0.09) | < 0.001 | 0.05 (0.03-0.10) | < 0.001 | 0.05 (0.01, 0.18) | 0.011 | 0.05 (0.01- 0.22) | 0.013 | |
| P for trend | < 0.001 | < 0.001 | 0.003 | 0.004 | |||||
For LS7 (continuous), the OR in Model 0 was 0.62 (95%CI: 0.57-0.68, P < 0.001). The OR stayed significant at 0.63 (95%CI: 0.58-0.68, P < 0.001) and 0.61 (95%CI: 0.53-0.70, P = 0.001) after adjustments in Models 1 and 2. The OR in Model 3 decreased somewhat to 0.62 (95%CI: 0.50-0.76, P = 0.01). In the unadjusted model (Model 0), for LS7, the optimal CVH group showed a more marked decline in MAFLD (OR: 0.04, 95%CI: 0.02–0.09, P < 0.001, P for trend < 0.001) compared to the average CVH group (OR: 0.31, 95%CI: 0.22–0.44, P < 0.001). The ORs for the average CVH group were 0.32 (95%CI: 0.22–0.45, P < 0.001) after demographic factor adjustments in Model 1, and those for the ideal CVH group were 0.05 (95%CI: 0.03–0.10, P < 0.001). Following additional adjustments for socioeconomic status, the ORs for the average CVH group in Model 2 were 0.29 (95%CI: 0.14–0.60, P = 0.018) and those for the ideal CVH group were 0.05 (95%CI: 0.01–0.18, P = 0.011). In the fully adjusted model (Model 3), these relationships remained significant, with ORs for the average CVH group at 0.32 (95%CI: 0.14–0.74, P = 0.028) and the optimal CVH group at 0.05 (95%CI: 0.01–0.22, P = 0.013, P for trend = 0.004) (Table 2).
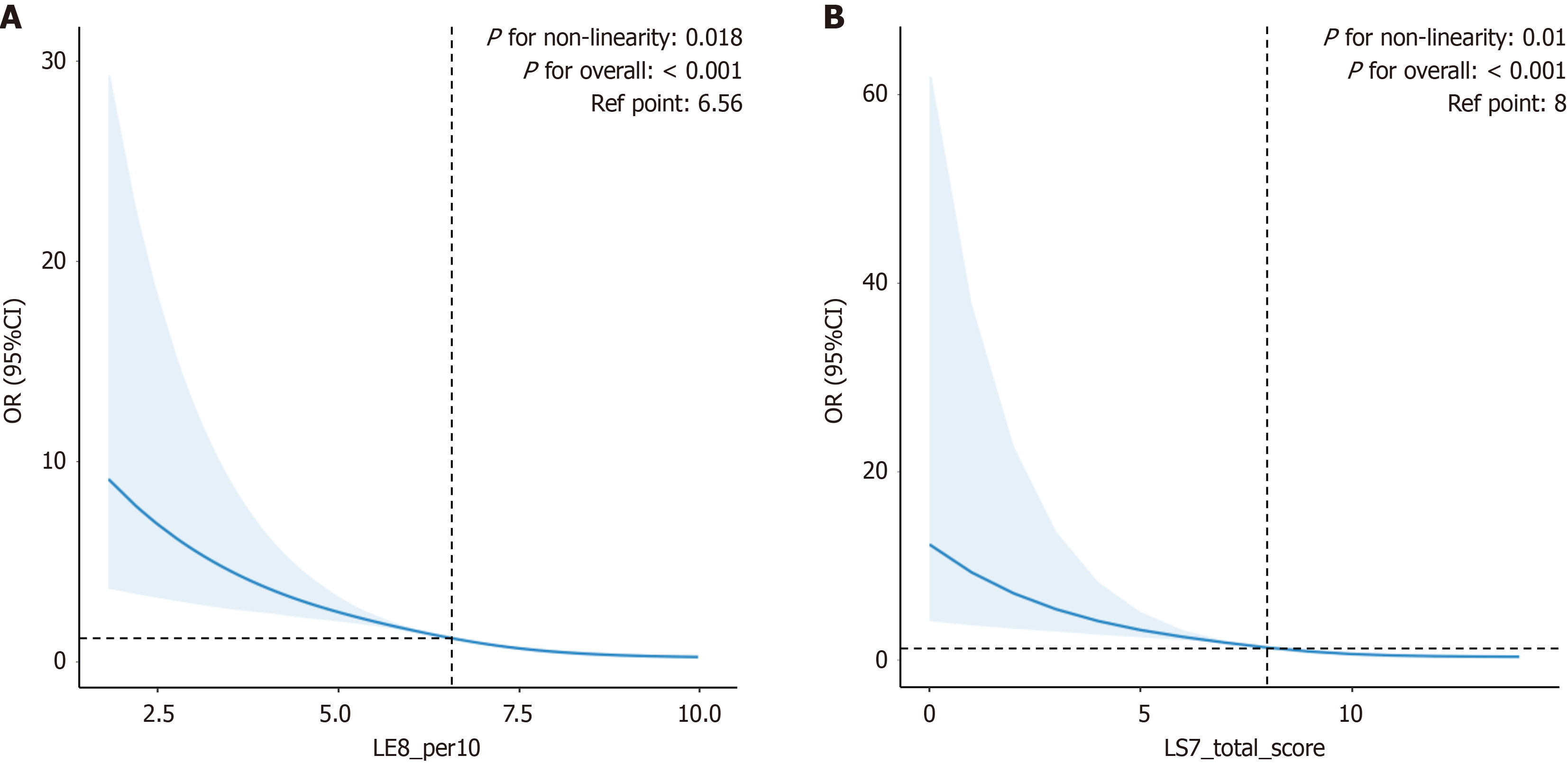
A nonlinear relationship was observed between LE8 and the risk of MAFLD (P for overall < 0.001, nonlinear P = 0.018) (Figure 2A). This shows a strong statistical correlation between the risk of MAFLD and LE8 scores, with considerable variations in risk as LE8 scores increase. The relationship for LS7 remained curvilinear, as seen by the nonlinearity (P for overall < 0.001, nonlinear P = 0.01) (Figure 2B).
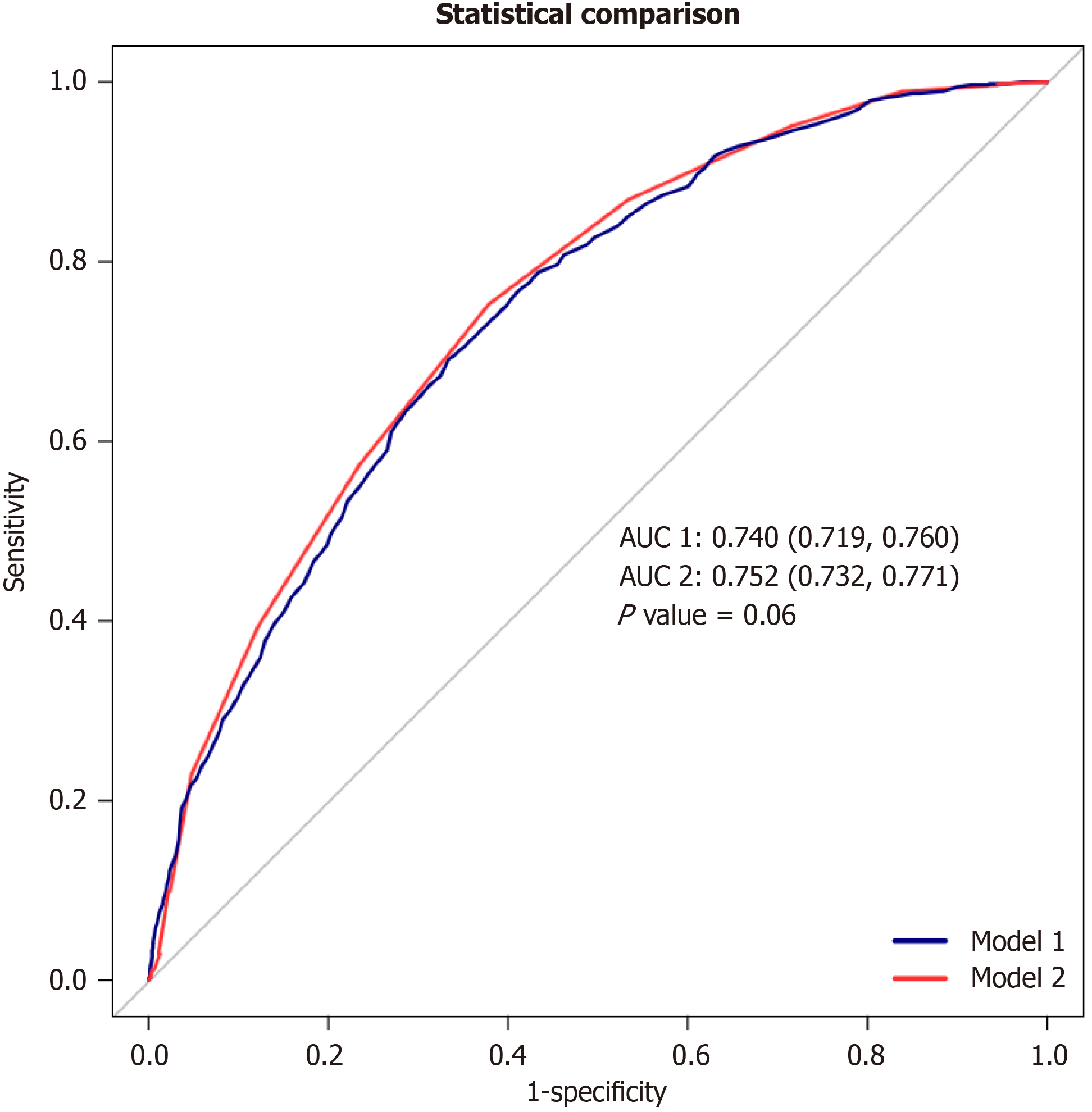
When weights were not taken into account, LE8 (0.740; 95%CI: 0.719-0.760; P = 0.06) and LS7 (0.752; 95%CI: 0.732-0.771; P = 0.06) had noticeably less area under the receiver operating characteristic curves (AUC) for predicting a certain condition. Nevertheless, the observed difference was not considered statistically significant (P DeLong = 0.06; Figure 3).
Table 3 shows how each CVH component relates to the risk of MAFLD. Higher scores in health factors (BMI, high-density lipoprotein, BP, and glucose) and health behaviors (PA) were significantly associated with a reduced risk of MAFLD (P < 0.05 for all). However, no statistically significant relationships were found between MAFLD and scores for diet (DASH), smoking, or sleep.
| CVH component (per 10) | Crude model | Adjusted model | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| LE8 health behaviors | 0.84 | 0.79-0.90 | < 0.001 | 0.85 | 0.74-0.99 | 0.042 |
| LE8 health factors | 0.5 | 0.46-0.55 | < 0.001 | 0.5 | 0.42-0.60 | 0.004 |
| DASH score | 0.95 | 0.91-0.99 | 0.018 | 0.95 | 0.87-1.04 | 0.128 |
| PA score | 0.92 | 0.89-0.94 | < 0.001 | 0.92 | 0.88-0.97 | 0.023 |
| Smoking score | 0.99 | 0.97-1.01 | 0.15 | 0.99 | 0.93-1.06 | 0.636 |
| Sleeping score | 0.98 | 0.93-1.04 | 0.471 | 0.98 | 0.88-1.11 | 0.623 |
| BMI score | 0.7 | 0.66-0.74 | < 0.001 | 0.69 | 0.61-0.77 | 0.006 |
| HDL score | 0.86 | 0.83-0.89 | < 0.001 | 0.88 | 0.81-0.96 | 0.026 |
| Glu score | 0.75 | 0.71-0.80 | < 0.001 | 0.79 | 0.68-0.91 | 0.019 |
| BP score | 0.83 | 0.79-0.88 | < 0.001 | 0.86 | 0.77-0.97 | 0.034 |
These findings highlight the complexity of the relationship between MAFLD and CVH components. More research is needed to completely understand how these components relate to mental health because some components are clearly associated with the risk of MAFLD while others are not.
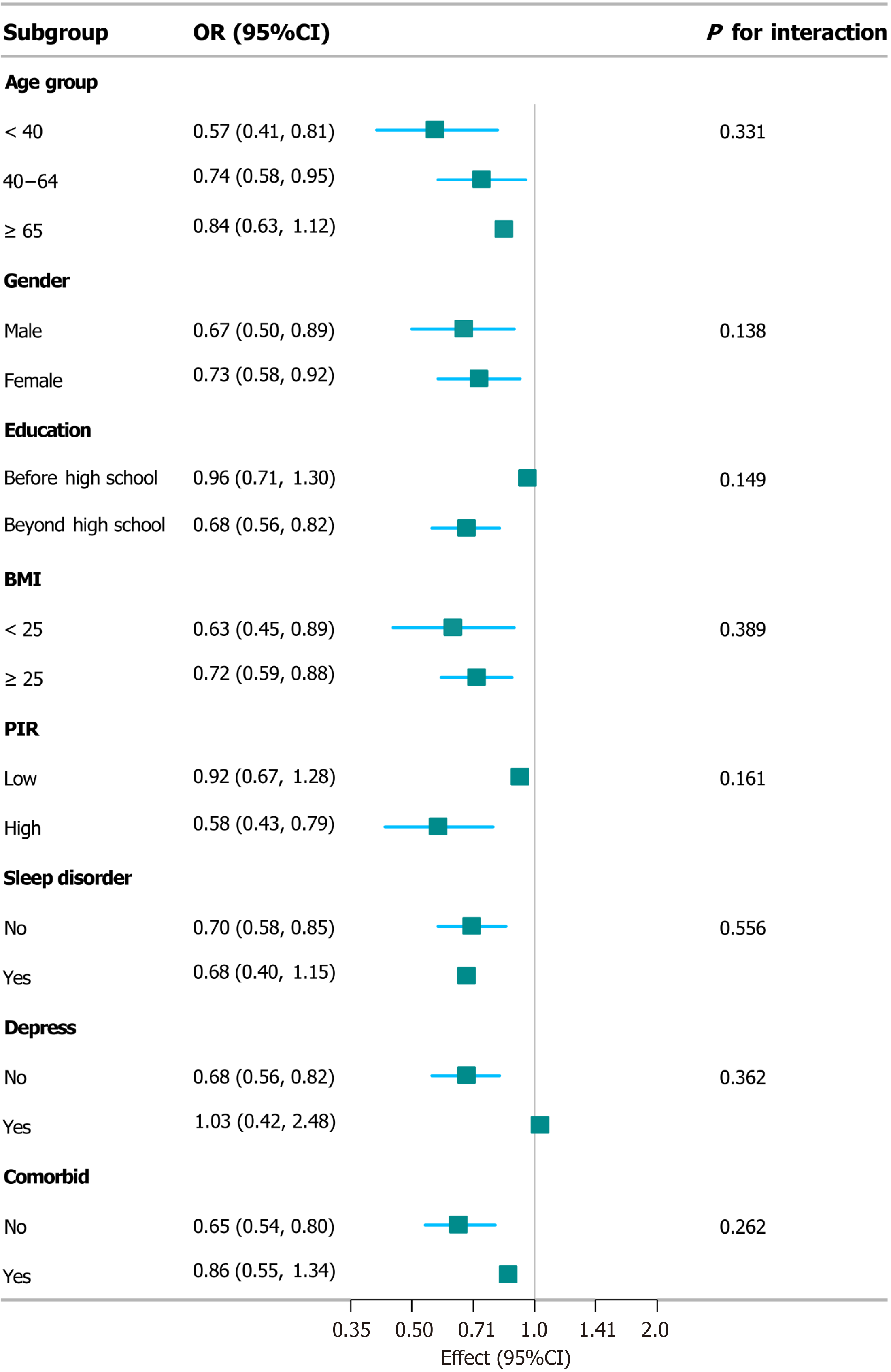
A subgroup analysis of the intervention’s impact, stratified by various demographic and clinical factors, revealed a significant positive effect in individuals aged < 40 years (OR = 0.57, 95%CI: 0.41-0.81) and a potentially significant effect in those aged 40-64 years (OR = 0.74, 95%CI: 0.58-0.95). Significant effects were also observed in both males (OR = 0.67, 95%CI: 0.50-0.89) and females (OR = 0.73, 95%CI: 0.58-0.92), with no significant gender difference (P = 0.138). Moreover, individuals with education beyond high school (OR = 0.68, 95%CI: 0.56-0.82), BMI < 25 kg/m2 (OR = 0.63, 95%CI: 0.45-0.89), and high PIR (OR = 0.58, 95%CI: 0.43-0.79) experienced significant positive effects from the intervention. Although no significant differences were observed in subgroups based on sleep disorders, depression, or comorbid conditions, trends toward significance were noted for depression (P = 0.362) and comorbid conditions (P = 0.262). The consistent findings from our subgroup analyses, stratified across various demographic and clinical factors, provide further evidence for the robustness of our results (Figure 4). Similarly, in the subgroup analysis of LS7, the outcomes were robust for both the association analysis and the outcome analysis (Supplementary Figure 1).
Sensitivity analyses further corroborated the robustness of our findings, as detailed in Supplementary Tables 1 to 3. Initially, in the unweighted data, the results were consistent with those from the weighted data, indicating the robustness of the results. Additionally, within the unweighted data, curve analysis and inflection point analysis revealed the nonlinear relationship between LE8 and LS7 with MAFLD, as well as the possible threshold points (Supplementary Figures 2 and 3). These analyses utilized weighted data and incorporated alternative definitions of outcome variables, including NAFLD, SLD, and MAFLD. Consistently, our results remained stable and reliable across these different definitions (Supplementary Table 4).
Following the AHA’s 2022 upgrade of “Life’s Simple 7” to “Life’s Essential 8,” this study is the first to compare the relationships of both LE8 and LS7 CVH measures with MAFLD. Using data representative of over 120 million individuals in the United States, our study revealed that both LE8 and LS7 scores were significantly and inversely associated with MAFLD risk. In particular, a 51% decrease in MAFLD risk was linked to each 10-unit rise in LE8 score (OR: 0.51, 95%CI: 0.46-0.56, P < 0.001) according to the unadjusted Model 0. The association remained significant after adjusting for age and sex in Model 1 (OR: 0.53, 95%CI: 0.48-0.58, P < 0.001). Similar effects were produced by further adjustments in Model 2 for drinking status, marital status, PIR, and ethnicity (OR: 0.50, 95%CI: 0.43-0.59, P < 0.001). Although somewhat reduced, the correlation was still statistically significant (OR: 0.51, 95%CI: 0.41-0.64, P = 0.006) even after comorbidities were included in Model 3. A comparable pattern was observed in the continuous variable analysis of LS7. In the unadjusted Model 0, the OR was 0.62 (95%CI: 0.57-0.68, P < 0.001). Following corrections in Models 1 and 2 (OR: 0.63, 95%CI: 0.58-0.68, P < 0.001 and OR: 0.61, 95%CI: 0.53-0.70, P = 0.001, respectively), the association remained robust. A little reduced but yet significant OR of 0.62 (95%CI: 0.50-0.76, P = 0.01) was shown by Model 3, which included all modifications. Without taking weights into account, Figure 2A displays the ROC curves for LE8, with an AUC of 0.740 (95%CI: 0.719-0.760, P = 0.06) for LE8 and 0.752 (95%CI: 0.732-0.771, P = 0.06) for LS7. However, this AUC difference was not statistically significant (P DeLong = 0.06).
A statistically significant connection was identified between greater LE8 scores and decreased MAFLD risk. Significant risk reductions were observed in the moderate and high CVH groups compared to the low CVH group, with ORs ranging from 0.28 to 0.05 across the models. These associations remained strong after accounting for comorbidities, socioeconomic status, and demographic factors. Similarly, for LS7, the ideal and moderate CVH groups reported lower MAFLD risks compared to the insufficient CVH group, with ORs ranging from 0.31 to 0.04, showing a clear inverse correlation between LS7 scores and MAFLD risk. These results underline the probable mental and physical health benefits associated with better compliance with LS7 and LE8 recommendations. Higher LE8 scores were associated with a decreased risk in all three types of fatty liver disease: for each 10-unit rise in LE8 score in the fully adjusted models, the likelihood of SLD decreased by 50%, MESLD by 56%, and NAFLD by 49%. For LS7, similar trends were identified; greater scores were always associated with a reduced risk for each condition. The dose-response relationship was further highlighted by categorical analyses of LE8 and LS7. For all three outcomes, the moderate and high adherence groups had far lower risks compared to the low adherence group. The benefits of obtaining optimal CVH scores were underscored by the fact that the high adherence groups constantly demonstrated the greatest risk reduction. The robustness and generalizability of our results are bolstered by the continuous inverse associations seen across numerous outcome variables. These results suggest that the protective effects of LE8 and LS7 extend beyond a single diagnosis of fatty liver disease to encompass a wide range of metabolic health conditions and liver fat-related disorders.
Our analysis demonstrated a significant association between higher LE8 and LS7 scores and a reduced risk of MAFLD, even after adjusting for various demographic and clinical confounders. This finding aligns with previous research highlighting the protective effect of a healthy lifestyle on the development of MAFLD[25,26]. The restricted cubic spline analysis further emphasized this relationship, revealing a nonlinear, inverse association where higher scores on both scales corresponded to a significantly lower MAFLD risk. Interestingly, examining individual LE8 components revealed a nuanced interplay between health behaviors and factors in relation to MAFLD. While higher scores in health behaviors such as PA and BMI were unexpectedly associated with increased MAFLD risk, higher scores in health factors such as high-density lipoprotein cholesterol and glucose levels were associated with a decreased risk. This seemingly paradoxical finding regarding health behaviors might be attributed to complex interactions between lifestyle factors and development of MAFLD, potentially influenced by factors such as dietary habits not captured in the LE8 scoring system. Further investigation is warranted to clarify these relationships[27-30].
Although both LE8 and LS7 demonstrated significant associations with MAFLD risk, no statistically significant difference was observed in their predictive ability for MAFLD, as indicated by the AUC analysis. This suggests that both metrics can be valuable tools for assessing MAFLD risk in clinical settings. However, the choice between LE8 and LS7 may depend on the specific context and the availability of data, considering LE8 incorporates sleep health, which might be relevant for specific populations, while LS7 was designed to provide simpler and more actionable health improvement goals[31-33].
PA emerged as the strongest correlate, consistent with the findings of previous studies[34]. Huang et al found that active leisure-time PA is associated with a reduced risk of MAFLD/MASLD and liver fibrosis. Importantly, this association was not observed for occupational or transportation-related PA, highlighting the specific benefits of leisure-time PA for liver health[35]. This study analyzed the 2017-2018 NHANES dataset and found that both active PA (especially leisure-time PA) and adequate weekday sleep duration were independently associated with a lower MASLD risk. The combination of these lifestyle factors further reduced the risk, except among those with significant alcohol consumption[36]. A case-control study investigated micro-RNAs (miRNAs) as potential biomarkers for SLD, a condition often linked to a high-fat diet and lack of exercise. While individual miRNA levels showed some correlation with disease, combinations of miRNA changes demonstrated stronger associations with disease severity, suggesting potential diagnostic and prognostic applications[37]. Previous studies exploring individual dietary metrics in LS7 showed that the association between diet and MAFLD remains controversial after adjusting for other LS7 metrics[38].
This research examined the LE8 and LS7 scoring systems for predicting the risk of MAFLD. The LE8 score system covers more healthy living characteristics, including sleep health, while the LS7 system focuses on fundamental health behavior markers. This research found that both scoring systems predict MAFLD risk with similar accuracy despite their design variations. In practice, either the LE8 or LS7 scoring system can be used to evaluate and manage MAFLD risk, depending on personal circumstances and preferences. Regardless of the scoring method used, adopting a healthy lifestyle—especially maintaining a healthy BMI and engaging in regular exercise—is essential to prevent MAFLD. Although a balanced diet and smoking cessation are important for overall health, this research indicates that they have a comparatively smaller impact on reducing MAFLD risk. Healthcare practitioners should prioritize promoting and personalizing lifestyle interventions to assess individual needs. However, the intricate relationship between CVH factors and MAFLD warrants further investigation to deepen our understanding and optimize prevention strategies.
We are deeply grateful to Jie Liu from the General Hospital of the People’s Liberation Army (301 Hospital) for her valuable support in clinical research methods and ideas.
| 1. | Lazarus JV, Mark HE, Anstee QM, Arab JP, Batterham RL, Castera L, Cortez-Pinto H, Crespo J, Cusi K, Dirac MA, Francque S, George J, Hagström H, Huang TT, Ismail MH, Kautz A, Sarin SK, Loomba R, Miller V, Newsome PN, Ninburg M, Ocama P, Ratziu V, Rinella M, Romero D, Romero-Gómez M, Schattenberg JM, Tsochatzis EA, Valenti L, Wong VW, Yilmaz Y, Younossi ZM, Zelber-Sagi S; NAFLD Consensus Consortium. Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol. 2022;19:60-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 454] [Article Influence: 151.3] [Reference Citation Analysis (0)] |
| 2. | Chan KE, Koh TJL, Tang ASP, Quek J, Yong JN, Tay P, Tan DJH, Lim WH, Lin SY, Huang D, Chan M, Khoo CM, Chew NWS, Kaewdech A, Chamroonkul N, Dan YY, Noureddin M, Muthiah M, Eslam M, Ng CH. Global Prevalence and Clinical Characteristics of Metabolic-associated Fatty Liver Disease: A Meta-Analysis and Systematic Review of 10 739 607 Individuals. J Clin Endocrinol Metab. 2022;107:2691-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 193] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 3. | Lin H, Wang L, Liu Z, Long K, Kong M, Ye D, Chen X, Wang K, Wu KK, Fan M, Song E, Wang C, Hoo RL, Hui X, Hallenborg P, Piao H, Xu A, Cheng KK. Hepatic MDM2 Causes Metabolic Associated Fatty Liver Disease by Blocking Triglyceride-VLDL Secretion via ApoB Degradation. Adv Sci (Weinh). 2022;9:e2200742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Eslam M, El-Serag HB, Francque S, Sarin SK, Wei L, Bugianesi E, George J. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat Rev Gastroenterol Hepatol. 2022;19:638-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 145] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 5. | Ismaiel A, Dumitrascu DL. Genetic predisposition in metabolic-dysfunction-associated fatty liver disease and cardiovascular outcomes-Systematic review. Eur J Clin Invest. 2020;50:e13331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Yoneda M, Yamamoto T, Honda Y, Imajo K, Ogawa Y, Kessoku T, Kobayashi T, Nogami A, Higurashi T, Kato S, Hosono K, Saito S, Nakajima A. Risk of cardiovascular disease in patients with fatty liver disease as defined from the metabolic dysfunction associated fatty liver disease or nonalcoholic fatty liver disease point of view: a retrospective nationwide claims database study in Japan. J Gastroenterol. 2021;56:1022-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2589] [Cited by in RCA: 3551] [Article Influence: 236.7] [Reference Citation Analysis (0)] |
| 8. | Fan H, Xu C, Li W, Huang Y, Hua R, Xiong Y, Yang Y, Feng X, Wang Z, Yuan Z, Zhou J. Ideal Cardiovascular Health Metrics Are Associated with Reduced Severity of Hepatic Steatosis and Liver Fibrosis Detected by Transient Elastography. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 9. | Oni E, Ogunmoroti O, Allen N, A-Mallah MH, Blankstein R, Martin SS, Zeb I, Cushman M, Joshi PH, Budoff MJ, Blaha MJ, Blumenthal RS, Veledar E, Nasir K. Life's Simple 7 and Nonalcoholic Fatty Liver Disease: The Multiethnic Study of Atherosclerosis. Am J Med. 2021;134:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G, Rosamond W; American Heart Association. Life's Essential 8: Updating and Enhancing the American Heart Association's Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation. 2022;146:e18-e43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 1386] [Article Influence: 462.0] [Reference Citation Analysis (0)] |
| 11. | Tang H. Further insight into the association of Life's Essential 8 and MAFLD. J Hepatol. 2023;79:e87-e88. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Wang X, Wang A, Zhang R, Cheng S, Pang Y. Life's Essential 8 and MAFLD in the United States. J Hepatol. 2023;78:e61-e63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2840] [Article Influence: 568.0] [Reference Citation Analysis (1)] |
| 14. | Siddiqui MS, Vuppalanchi R, Van Natta ML, Hallinan E, Kowdley KV, Abdelmalek M, Neuschwander-Tetri BA, Loomba R, Dasarathy S, Brandman D, Doo E, Tonascia JA, Kleiner DE, Chalasani N, Sanyal AJ; NASH Clinical Research Network. Vibration-Controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2019;17:156-163.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 453] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 15. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1308] [Article Influence: 654.0] [Reference Citation Analysis (1)] |
| 16. | Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Guha IN, Cobbold JF, Deeks JJ, Paradis V, Bedossa P, Newsome PN. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1717-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 971] [Article Influence: 161.8] [Reference Citation Analysis (0)] |
| 17. | Ajmera VH, Cachay ER, Ramers CB, Bassirian S, Singh S, Bettencourt R, Richards L, Hamilton G, Middleton M, Fowler K, Sirlin C, Loomba R. Optimal Threshold of Controlled Attenuation Parameter for Detection of HIV-Associated NAFLD With Magnetic Resonance Imaging as the Reference Standard. Clin Infect Dis. 2021;72:2124-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Zhou J, Long Y, Ding N, Su Y. Association between bedtime at night and nonalcoholic fatty liver disease diagnosed by liver ultrasound transient elastography. Diabetes Res Clin Pract. 2022;184:109195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Herraiz-Adillo Á, Higueras-Fresnillo S, Ahlqvist VH, Berglind D, Syrjälä MB, Daka B, Lenander C, Sundström J, Ortega FB, Östgren CJ, Rådholm K, Henriksson P. Life's Essential 8 and Life's Simple 7 in Relation to Coronary Atherosclerosis: Results From the Population-Based SCAPIS Project. Mayo Clin Proc. 2024;99:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 20. | Howard G, Cushman M, Blair J, Wilson NR, Yuan Y, Safford MM, Levitan EB, Judd SE, Howard VJ. Comparative Discrimination of Life's Simple 7 and Life's Essential 8 to Stratify Cardiovascular Risk: Is the Added Complexity Worth It? Circulation. 2024;149:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 21. | Ning N, Zhang Y, Liu Q, Zhou W, He Y, Liu Y, Jin L, Ma Y. American Heart Association's new 'Life's Essential 8' score in association with cardiovascular disease: a national cross-sectional analysis. Public Health. 2023;225:336-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 22. | Isiozor NM, Kunutsor SK, Voutilainen A, Laukkanen JA. Life's Essential 8 and the risk of cardiovascular disease death and all-cause mortality in Finnish men. Eur J Prev Cardiol. 2023;30:658-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 82] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 23. | Li L, Dai F. Comparison of the associations between Life's Essential 8 and Life's Simple 7 with depression, as well as the mediating role of oxidative stress factors and inflammation: NHANES 2005-2018. J Affect Disord. 2024;351:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Reference Citation Analysis (0)] |
| 24. | Zhan JJ, Hodge RA, Dunlop AL, Lee MM, Bui L, Liang D, Ferranti EP. Dietaryindex: A User-Friendly and Versatile R Package for Standardizing Dietary Pattern Analysis in Epidemiological and Clinical Studies. bioRxiv. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Zhou B, Gong N, He Q, Huang X, Zhu J, Zhang L, Huang Y, Tan X, Xia Y, Zheng Y, Shi Q, Qin C. Clustering of lifestyle behaviours and analysis of their associations with MAFLD: a cross-sectional study of 196,515 individuals in China. BMC Public Health. 2023;23:2303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 26. | Lewis MY, Yonemori K, Ross A, Wilkens LR, Shepherd J, Cassel K, Stenger A, Rettenmeier C, Lim U, Boushey C, Le Marchand L. Effect of Intermittent vs. Continuous Energy Restriction on Visceral Fat: Protocol for The Healthy Diet and Lifestyle Study 2 (HDLS2). Nutrients. 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 27. | van der Heide FCT, Valeri L, Dugravot A, Danilevicz I, Landre B, Kivimaki M, Sabia S, Singh-Manoux A. Role of cardiovascular health factors in mediating social inequalities in the incidence of dementia in the UK: two prospective, population-based cohort studies. EClinicalMedicine. 2024;70:102539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 28. | Zhang H, Chang Q, Yang H, Yu H, Chen L, Zhao Y, Xia Y. Life's Essential 8, genetic predisposition, and risk of incident adult-onset asthma: a prospective cohort study. Am J Clin Nutr. 2024;119:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 29. | Rosendale N, Wood AJ, Leung CW, Kim AS, Caceres BA. Differences in Cardiovascular Health at the Intersection of Race, Ethnicity, and Sexual Identity. JAMA Netw Open. 2024;7:e249060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 30. | López-Bueno R, Núñez-Cortés R, Calatayud J, Salazar-Méndez J, Petermann-Rocha F, López-Gil JF, Del Pozo Cruz B. Global prevalence of cardiovascular risk factors based on the Life's Essential 8 score: an overview of systematic reviews and meta-analysis. Cardiovasc Res. 2024;120:13-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 31. | Suglia SF, Knox N, April-Sanders AK, Aguayo L, López-Cepero A, Cohall A, Wang S, Wall M, Canino G, Bird H, Duarte CS. Prevalence of cardiometabolic risk and health factors among Puerto Rican young adults in the Boricua Youth Study - Health Assessment. Ann Epidemiol. 2024;89:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Hernández-martínez A, Duarte-junior MA, Sotos-prieto M, Ortolá R, Banegas JR, Rodríguez-artalejo F, Soriano-maldonado A, Martínez-gómez D. Salud cardiovascular en España basada en el Life's Essential 8 y su asociación con mortalidad general y cardiovascular: la cohorte ENRICA. Revista Española de Cardiología. 2024;77:372-380. [DOI] [Full Text] |
| 33. | Cai A, Chen C, Wang J, Ou Y, Nie Z, Feng Y. Life's Essential 8 and risk of incident heart failure in community population without cardiovascular disease: Results of the sub-cohort of China PEACE Million Persons Project. Prev Med. 2024;178:107797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 34. | Liu Q, Fan G, Bi J, Qin X, Fang Q, Wu M, Mei S, Wan Z, Lv Y, Song L, Wang Y. Associations of polychlorinated biphenyls and organochlorine pesticides with metabolic dysfunction-associated fatty liver disease among Chinese adults: Effect modification by lifestyle. Environ Res. 2024;240:117507. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 35. | Huang J, Wu Y, Zheng J, Wang M, Goh GB, Lin S. The prognostic role of diet quality in patients with MAFLD and physical activity: data from NHANES. Nutr Diabetes. 2024;14:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 36. | Li Y, Guo Y, Tan S. Independent and joint association of physical activity and adequate weekday sleep duration with metabolic dysfunction-associated steatotic liver disease. Clin Res Hepatol Gastroenterol. 2024;48:102320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 37. | Stoica VC, Apostol D, Diculescu MM, Gârdan IP, Gârdan DA, Mărunțelu I, Constantinescu I. Time for micro-RNAs in steatotic liver disease: a case-control study. Front Endocrinol (Lausanne). 2024;15:1349524. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Van Dongen C, Paik JM, Harring M, Younossi Y, Price JK, Kabbara K, Golabi P, Younossi ZM. Sarcopenia, healthy living, and mortality in patients with chronic liver diseases. Hepatol Commun. 2022;6:3140-3153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |