Published online Feb 27, 2025. doi: 10.4254/wjh.v17.i2.96506
Revised: September 30, 2024
Accepted: January 2, 2025
Published online: February 27, 2025
Processing time: 287 Days and 20 Hours
Hepatitis B-associated cirrhosis is an important disease burden in China. How
To analyzing the clinical characteristics of patients with hepatitis and cirrhosis, the nomogram model was established and validated.
The clinical data of 1070 patients with hepatitis B who were treated in our hospital from October 2015 to July 2022 were collected. In a 7:3 ratio, 749 cases were divi
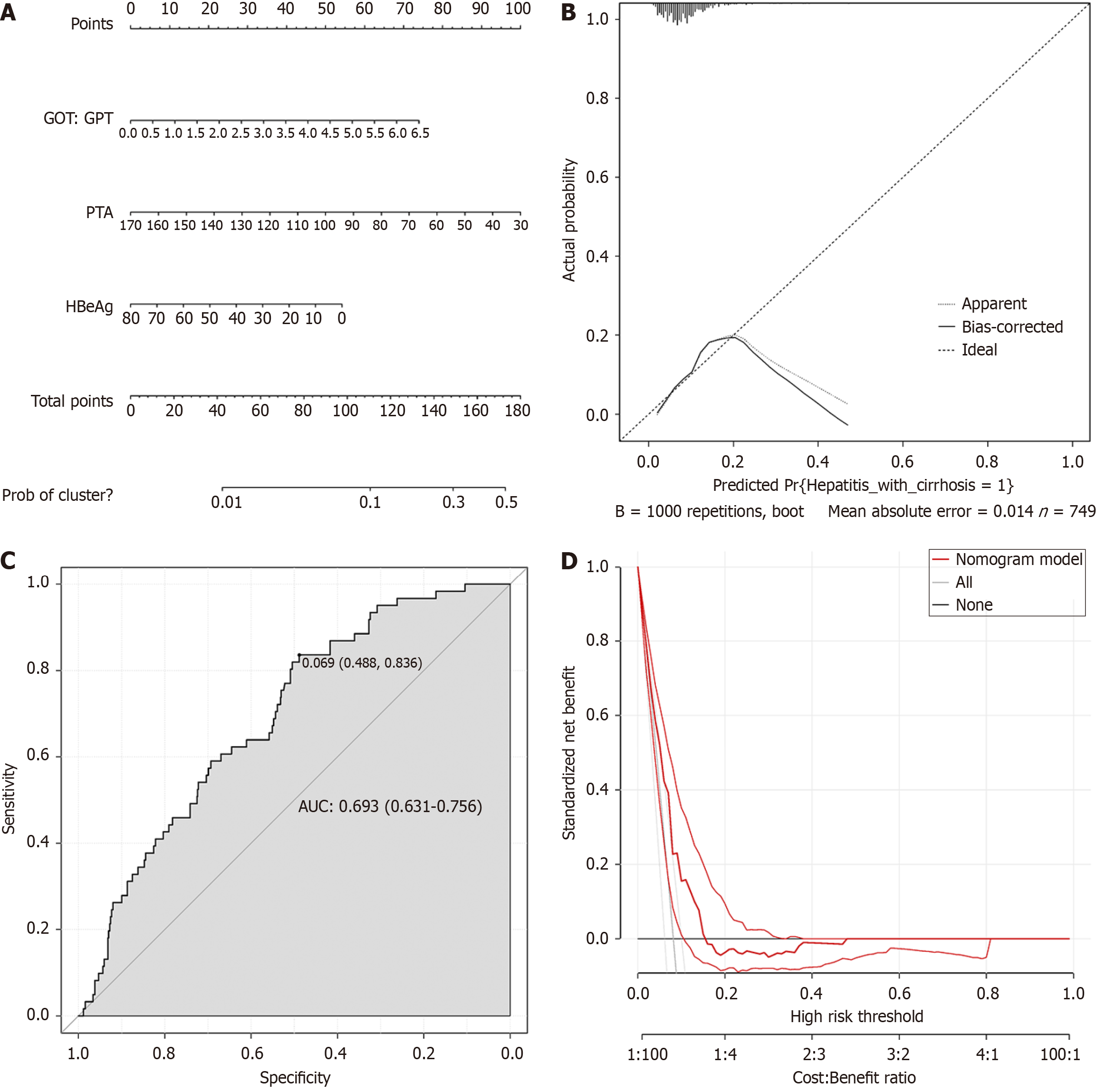
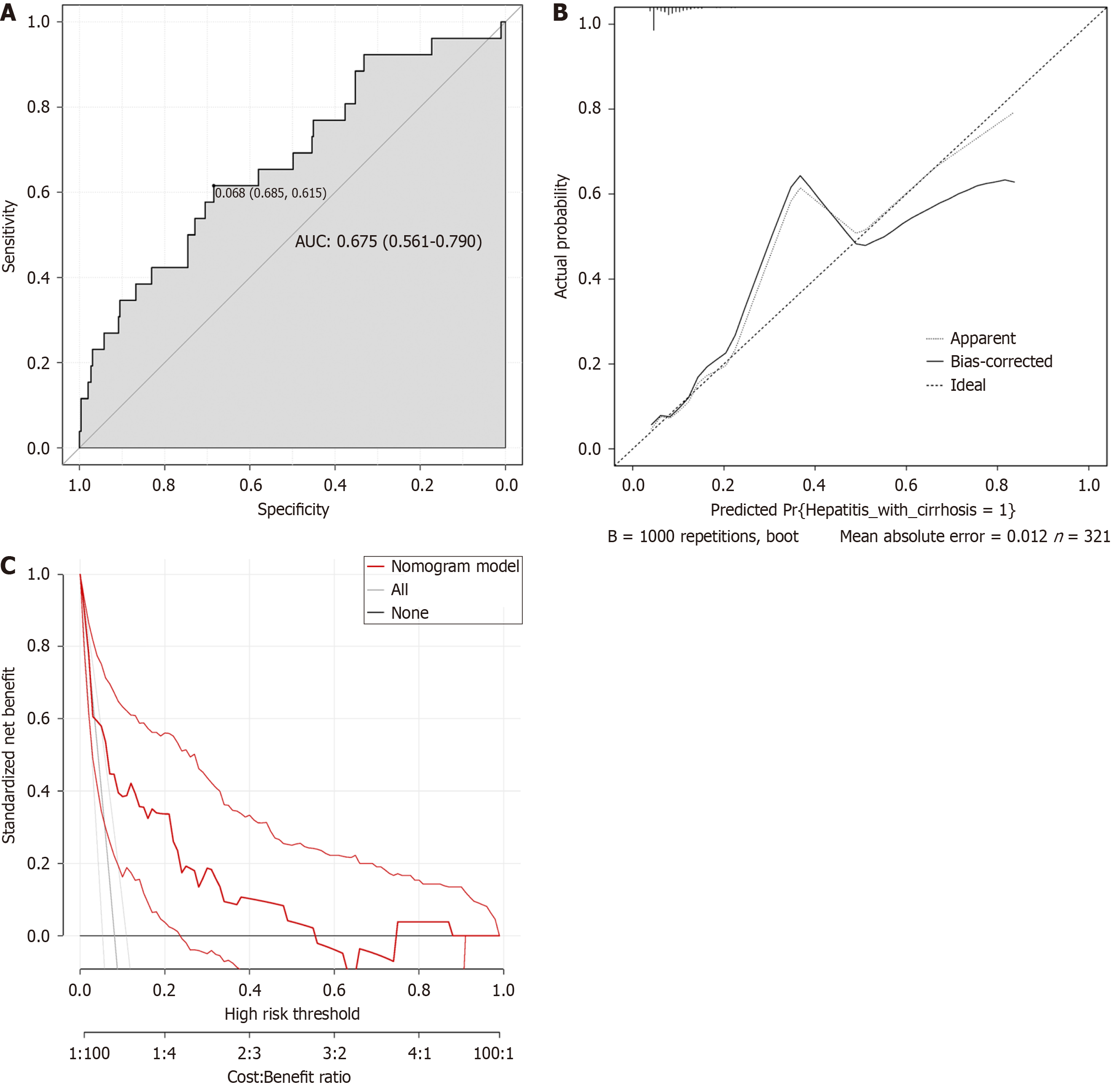
Binary logistic regression analysis was performed using hepatitis B-related cirrhosis = 1 and hepatitis = 0 as dependent variables, and univariate analysis of serological indicators was used as covariates. The results showed that glutamic oxaloacetate aminotransferase/glutamate acetone aminotransferase levels, prothrombin time activity, and hepatitis B e antigen levels were all contributing factors to the progression of hepatitis to cirrhosis. The area under the ROC curve was 0.693 [95% confidence interval (CI): 0.631 to 0.756] for the training cohort and 0.675 (95%CI: 0.561 to 0.790) for the validation cohort. In addition, the decision analysis curves of the prediction models of both the training cohort and the validation cohort confirmed the effectiveness of the nomogram prediction model.
Three independent factors influencing the progression to cirrhosis in patients with hepatitis B were identified. The construction of a nomogram prediction model from hepatitis to cirrhosis has high application value as a tool for predicting the occurrence of liver cirrhosis in hepatitis B patients.
Core Tip: In order to analyze the clinical characteristics of patients with hepatitis and cirrhosis, a nomogram model was established and verified. The clinical records of 1070 patients with hepatitis B who were treated in our hospital were selected for study, and they were divided into 749 cases in the modeling cohort and 321 cases in the model validation cohort in a 7:3 ratio. At the same time, the model validation cohort was divided into hepatitis group (n = 688) and hepatitis cirrhosis group
- Citation: Huang YS, Gao W, Sun AJ, Pu CW, Xu SS. Clinical characteristics of patients with hepatitis and cirrhosis and the construction of a prediction model. World J Hepatol 2025; 17(2): 96506
- URL: https://www.wjgnet.com/1948-5182/full/v17/i2/96506.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i2.96506
The incidence of hepatitis B virus (HBV) infection is relatively high in China, and the mortality rate has obviously increa
Liver biopsy, as the most objective, true and reliable diagnostic criterion for detecting liver function, is not only trau
It is of great clinical significance to find new markers and predictive models that can safely and effectively detect the liver function of patients with hepatitis B cirrhosis. As a graphical tool, the nomogram can not only accelerate more complicated calculations, but also present the influence of various prediction factors on the results through graphical tools, so that we can better understand and use[3]. In addition, this modeling method is not only easy to operate, but also safer. Besides, it can be observed repeatedly and accepted by patients. Based on this, 1070 cases of hepatitis patients were included in this study. By collecting their general clinical data and routine laboratory indicators, a corresponding nomo
A retrospective study was conducted on the clinical medical records of 1070 hepatitis B patients who were treated in our hospital from October 2015 to July 2022. They were divided into a model cohort of 749 cases and a model validation cohort of 321 cases according to the ratio of 7:3. The model validation cohort was divided into hepatitis group (n = 21) and hepatitis cirrhosis group (n = 45). The study was approved by the Hospital Ethics Committee. Inclusion criteria: (1) All patients met the diagnostic criteria of hepatitis B in the Guidelines for the prevention and treatment of chronic hepatitis B[4], and the patients with cirrhosis of liver met the criteria of hepatitis and cirrhosis in the guidelines for the prevention and treatment of chronic hepatitis B[4]; (2) The patient has reached the age of 18; and (3) The clinical data of patients are complete. Exclusion criteria: (1) Patients with combined liver cancer; (2) Patients with severe cardiac system diseases; (3) Liver cirrhosis caused by other reasons; and (4) Pregnant or lactating patients.
Clinical data of patients in the hepatitis group and the liver cirrhosis group were collected through electronic medical records in our hospital. General data of the two groups were compared, including gender, age, hepatitis classification, allergy history, disease history, operation history, smoking history, alcohol consumption history, family disease history, and treatment plan. Meanwhile, laboratory indexes of two groups of patients are counted, albumin (Alb), lactate dehy
To analyze the influencing factors of patients with hepatitis cirrhosis, and establish and verify the road map prediction model.
Statistical product and service solutions 25. 0 software and R software were used to analyze the collected data. The collected measurement data were subjected to Shapiro-Wilk normal distribution test, where P > 0.05 meant the normal distribution data were expressed as mean ± SD, and t test, where P < 0.05 meant the non-normal distribution data were described as the median (quartile), followed by Mann-Whitney U test. Enumeration data collected were expressed as % and disordered data were analyzed using χ2 or Fisher exact test, and ordered data were analyzed using Mann-Whitney U test. Univariate and multivariate logistic regression analysis was used to analyze the influencing factors of hepatitis cirrhosis in patients, and the nomogram prediction model was established. The discriminant ability of the verification set and the calibration map were used to evaluate the accuracy of the nomogram. The area under the receiver operating characteristic (ROC) curve was used to evaluate the resolution of nomograms. The correction curve of the model was calculated, and the consistency of the model was verified by Hosmer-Lemelshaw test. Decision curve analysis was also performed to evaluate the model’s discriminating ability. P < 0.05 indicated that the difference was statistically signifi
A total of 1070 patients with hepatitis B were included, including 749 cases in the modeling cohort and 321 cases in the verification cohort. The average age of the patients was (46.84, 11.73) years old. There were 679 males (63.46%) and 391 females (36.54%). In the modeling cohort, 487 cases (65.02%) were male and 262 cases (34.98%) were female. In the validation cohort, there were 192 males (59.81%) and 129 females (40.19%). There was no significant difference between the two groups in baseline data (P > 0.05) as shown in Table 1 and Supplementary Table 1.
| Index | Total cases (n = 1070) | Modeling queue (n = 749) | Verification queue (n = 321) | t/χ2/Z | P value | |
| Gender | Man | 679 (63.46) | 487 (65.02) | 192 (59.81) | 2.627 | 0.105 |
| Woman | 391 (36.54) | 262 (34.98) | 129 (40.19) | |||
| Age (years) | 46.84 ± 11.73 | 46.58 ± 11.77 | 47.45 ± 11.63 | 1.112 | 0.266 | |
| Hepatitis classification | Mild | 242 (22.62) | 161 (21.50) | 81 (25.23) | 0.849 | 0.396 |
| Moderate | 753 (70.37) | 538 (71.83) | 215 (66.98) | |||
| Serious | 75 (7.01) | 50 (6.68) | 25 (7.79) | |||
| Alb (× 109/L) | 43.11 ± 4.70 | 43.02 ± 4.65 | 43.32 ± 4.79 | 0.958 | 0.338 | |
| LDH (U/L) | 210.73 ± 93.70 | 210.07 ± 73.36 | 212.28 ± 129.41 | 0.353 | 0.724 | |
| CHE (U/L) | 7376.59 ± 2247.89 | 7298.93 ± 2190.67 | 7557.79 ± 2369.69 | 1.728 | 0.084 | |
| WBC (× 109/L) | 5.19 ± 1.53 | 5.19 ± 1.51 | 5.20 ± 1.59 | 0.098 | 0.922 | |
| RBC (× 1012/L) | 4.74 ± 0.50 | 4.73 ± 0.51 | 4.74 ± 0.49 | 0.297 | 0.766 | |
| HGB (g/L) | 145.45 ± 16.13 | 145.56 ± 15.96 | 145.22 ± 16.54 | 0.316 | 0.752 | |
| ANC (× 109/L) | 2.84 ± 16.13 | 2.81 ± 1.07 | 2.89 ± 1.25 | 1.064 | 0.288 | |
| ALC (× 109/L) | 1.86 ± 0.65 | 1.88 ± 0.66 | 1.84 ± 0.64 | 0.917 | 0.360 | |
| GOT/GPT | 0.76 ± 0.43 | 0.75 ± 0.42 | 0.78 ± 0.46 | 1.040 | 0.299 | |
| PTA (%) | 109.50 ± 18.79 | 108.16 ± 40.22 | 112.61 ± 40.40 | 1.545 | 0.123 | |
| HBeAg (S/CO) | 1745.74 ± 32177.8 | 2355.23 ± 38449.88 | 323.60 ± 526.52 | 0.946 | 0.344 | |
| INR | 1.02 ± 0.12 | 1.03 ± 0.31 | 1.00 ± 0.31 | 1.451 | 0.147 | |
| PT (second) | 11.76 ± 1.39 | 11.84 ± 4.38 | 11.57 ± 3.38 | 0.986 | 0.325 | |
| HA (ng/mL) | 141.29 ± 142.55 | 147.90 ± 173.41 | 125.87 ± 160.61 | 1.946 | 0.052 | |
| LN (μg/L) | 54.47 ± 42.36 | 56.44 ± 55.54 | 49.87 ± 43.43 | 1.886 | 0.060 | |
| PLT (× 109/L) | 362.35 ± 4394.84 | 439.84 ± 5251.83 | 181.54 ± 63.11 | 0.881 | 0.379 | |
In the modeling cohort, the levels of Alb, LDH, CHE, PTA, HBeAg, WBC, RBC, HGB, ANC and ALC of patients with hepatitis cirrhosis in the hepatitis cirrhosis group were significantly lower than those in the hepatitis group. The levels of GOT/GPT, INR, PT, HA, LN and PLT were significantly higher than those in the hepatitis group (P < 0.05). See Table 2 and Supplementary Table 2.
| Index | Total cases (n = 749) | Hepatitis group (n = 688) | Hepatitis cirrhosis group (n = 61) | t/χ2/Z | P value | |
| Gender | Man | 487 (65.02) | 447 (64.97) | 40 (65.57) | 0.009 | 0.924 |
| Woman | 262 (34.98) | 241 (35.03) | 21 (34.43) | |||
| Age (years) | 46.12 ± 22.80 | 51.79 ± 10.06 | 1.925 | 0.051 | ||
| Hepatitis classification | Mild | 161 (21.50) | 152 (22.09) | 9 (14.75) | 1.342 | 0.180 |
| Moderate | 538 (71.83) | 491 (71.37) | 47 (77.05) | |||
| Serious | 50 (6.68) | 45 (6.54) | 5 (8.20) | |||
| Allergy history | 44 (5.87) | 40 (5.81) | 4 (6.56) | 0.002 | 0.963 | |
| Disease history | Hypertension | 41 (5.47) | 38 (5.52) | 3 (4.92) | 0.605 | 0.545 |
| Diabetes | 38 (5.07) | 35 (5.09) | 3 (4.92) | |||
| Heart disease | 30 (4.01) | 29 (4.22) | 1 (1.64) | |||
| Operation history | 99 (13.22) | 90 (13.08) | 9 (14.75) | 0.137 | 0.712 | |
| Smoking history | 95 (12.68) | 86 (12.50) | 9 (14.75) | 0.257 | 0.612 | |
| Drinking history | 90 (12.02) | 82 (11.92) | 8 (13.11) | 0.769 | 0.380 | |
| Family history of disease | 572 (76.37) | 520 (75.58) | 52 (85.25) | 2.900 | 0.089 | |
| Treatment regimen | Monotherapy | 623 (83.18) | 568 (82.56) | 55 (90.16) | 2.317 | 0.128 |
| Combination therapy | 126 (16.82) | 120 (17.44) | 6 (9.84) | |||
| Alb (× 109/L) | 43.02 ± 4.65 | 43.10 ± 4.60 | 42.13 ± 1.06 | 2.891 | 0.004 | |
| LDH (U/L) | 210.07 ± 73.36 | 210.33 ± 11.13 | 207.17 ± 10.49 | 2.133 | 0.033 | |
| CHE (U/L) | 7298.93 ± 2190.67 | 7336.36 ± 1184.27 | 6876.75 ± 1236.59 | 2.895 | 0.004 | |
| PTA (%) | 108.16 ± 19.22 | 108.92 ± 19.13 | 99.62 ± 18.22 | 3.653 | 0.000 | |
| HBeAg (S/CO) | 2355.23 ± 38449.88 | 2543.73 ± 314.87 | 229.18 ± 303.99 | 55.175 | 0.000 | |
| WBC (× 109/L) | 5.19 ± 1.51 | 5.25 ± 1.51 | 4.55 ± 1.22 | 3.520 | 0.001 | |
| RBC (× 1012/L) | 4.73 ± 0.51 | 4.74 ± 0.31 | 4.64 ± 0.23 | 2.460 | 0.014 | |
| HGB (g/L) | 145.56 ± 15.96 | 145.70 ± 6.12 | 143.97 ± 3.97 | 2.167 | 0.031 | |
| ANC (× 109/L) | 2.81 ± 1.07 | 2.85 ± 1.09 | 2.45 ± 0.81 | 2.798 | 0.005 | |
| ALC (× 109/L) | 1.88 ± 0.66 | 1.90 ± 0.66 | 1.69 ± 0.60 | 2.399 | 0.017 | |
| GOT/GPT | 0.75 ± 0.42 | 0.74 ± 0.42 | 0.86 ± 0.36 | 2.162 | 0.031 | |
| INR | 1.03 ± 0.11 | 1.02 ± 0.12 | 1.07 ± 0.13 | 3.097 | 0.002 | |
| PT (second) | 11.84 ± 1.38 | 11.79 ± 1.37 | 12.35 ± 1.43 | 3.049 | 0.002 | |
| HA (ng/mL) | 147.90 ± 145.41 | 144.64 ± 142.14 | 184.75 ± 175.34 | 2.069 | 0.039 | |
| LN (μg/L) | 56.44 ± 45.54 | 55.72 ± 24.77 | 64.63 ± 23.16 | 2.706 | 0.007 | |
| PLT (× 109/L) | 439.84 ± 5251.83 | 179.83 ± 54.36 | 3372.49 ± 18283.84 | 4.612 | 0.000 | |
Hepatitis cirrhosis = 1, and hepatitis = 0 were taken as the dependent variables, and the factors significantly different in the above single factor analysis were taken as the covariates for binary logic regression analysis. The results showed that the levels of GOT/GPT, PTA and HBeAg were all the influencing factors of hepatitis cirrhosis.
Log (P) = 6.572 + 0.640 × GOT/GPT - 0.049 × PTA - 0.001 × HBeAg. See Table 3.
| B | SE | Wals | P value | OR | 95%CI | |
| Alb | -0.033 | 0.043 | 0.608 | 0.736 | 0.967 | 0.890 to 1.052 |
| LDH | -0.002 | 0.002 | 1.211 | 0.271 | 0.998 | 0.993 to 1.002 |
| CHE | 0.000 | 0.000 | 1.565 | 0.211 | 1.000 | 1.000 to 1.000 |
| GOT/GPT | 0.640 | 0.305 | 4.401 | 0.036 | 1.896 | 1.043 to 3.448 |
| INR | 14.952 | 9.843 | 2.307 | 0.129 | 2.536 | 0.013 to 7.450 |
| PT | -1.566 | 0.877 | 3.184 | 0.074 | 0.209 | 0.037 to 1.167 |
| PTA | -0.049 | 0.022 | 5.252 | 0.022 | 0.952 | 0.913 to 0.993 |
| HBeAg | -0.001 | 0.000 | 5.310 | 0.021 | 0.999 | 0.999 to 1.000 |
| HA | 0.000 | 0.001 | 0.088 | 0.767 | 1.000 | 0.998 to 1.003 |
| LN | 0.000 | 0.004 | 0.015 | 0.901 | 1.000 | 0.992 to 1.007 |
| PLT | 0.000 | 0.000 | 0.574 | 0.449 | 1.000 | 1.000 to 1.000 |
| WBC | -1.527 | 0.794 | 3.701 | 0.054 | 0.217 | 0.046 to 1.029 |
| RBC | -0.311 | 0.586 | 0.281 | 0.596 | 0.733 | 0.233 to 2.310 |
| HGB | 0.027 | 0.018 | 2.129 | 0.145 | 1.027 | 0.991 to 1.065 |
| ANC | 1.302 | 0.864 | 2.268 | 0.132 | 3.676 | 0.676 to 20.002 |
| ALC | 0.963 | 0.831 | 1.341 | 0.247 | 2.619 | 0.513 to 13.364 |
| Constant | 6.572 | 5.590 | 1.382 | 0.240 | 715.135 |
The obtained three independent risk factors (GOT/GPT, PTA and HBeAg) were used to construct a prediction model using R software, and the nomogram model was established (Figure 1A). After the generated nomogram was calibrated (Figure 1B), the predicted event was in high consistency with the actual event. The area under the ROC curve of the nomogram prediction model was 0.693 [95% confidence interval (CI): 0.631 to 0.756] (Figure 1C). The decision analysis curve is shown in Figure 1D, where the X axis represents the threshold probability, the Y axis represents the net gain, and the solid black line represents the net gain using the nomogram prediction model. The curve shows a high yield, further confirming the effectiveness of the nomogram prediction model.
Based on clinical data from a validation cohort (n = 321) of patients (Table 4), the ROC curve was used for external va
| Index | Total cases (n = 321) | Hepatitis group (n = 295) | Hepatitis cirrhosis group (n = 26) | t/χ2/Z | P value | |
| Gender | Man | 192 (59.81) | 180 (61.02) | 12 (46.15) | 2.196 | 0.138 |
| Woman | 129 (40.19) | 115 (38.98) | 14 (53.85) | |||
| Age (years) | 46.89 ± 21.68 | 53.85 ± 9.03 | 1.623 | 0.106 | ||
| Hepatitis classification | Mild | 81 (25.23) | 78 (26.44) | 3 (11.54) | 1.718 | 0.086 |
| Moderate | 215 (66.98) | 195 (66.10) | 20 (76.92) | |||
| Serious | 25 (7.79) | 22 (7.46) | 3 (11.54) | |||
| Alb (× 109/L) | 43.32 ± 4.79 | 43.44 ± 3.70 | 41.97 ± 2.64 | 1.981 | 0.049 | |
| LDH (U/L) | 212.28 ± 129.41 | 203.94 ± 66.37 | 306.92 ± 390.42 | 3.979 | 0.000 | |
| CHE (U/L) | 7557.79 ± 2369.69 | 7617.54 ± 1352.37 | 6879.85 ± 1506.37 | 2.642 | 0.009 | |
| PTA (%) | 112.61 ± 17.40 | 114.05 ± 16.15 | 96.36 ± 22.54 | 5.166 | 0.000 | |
| HBeAg (S/CO) | 323.60 ± 526.52 | 340.11 ± 333.91 | 136.59 ± 395.57 | 2.933 | 0.004 | |
| WBC (× 109/L) | 5.20 ± 1.59 | 5.28 ± 1.57 | 4.30 ± 1.48 | 3.065 | 0.002 | |
| RBC (× 1012/L) | 4.74 ± 0.49 | 4.76 ± 0.48 | 4.46 ± 0.47 | 3.060 | 0.002 | |
| HGB (g/L) | 145.22 ± 16.54 | 145.96 ± 15.98 | 136.77 ± 20.42 | 2.744 | 0.006 | |
| ANC (× 109/L) | 2.89 ± 1.25 | 2.94 ± 1.26 | 2.24 ± 0.96 | 2.761 | 0.006 | |
| ALC (× 109/L) | 1.84 ± 0.64 | 1.85 ± 0.45 | 1.63 ± 0.41 | 2.406 | 0.017 | |
| GOT/GPT | 0.78 ± 0.46 | 0.77 ± 0.46 | 1.00 ± 0.44 | 2.452 | 0.015 | |
| INR | 1.00 ± 0.11 | 0.99 ± 0.22 | 1.11 ± 0.12 | 2.743 | 0.006 | |
| PT (second) | 11.57 ± 1.38 | 11.45 ± 0.96 | 12.95 ± 3.35 | 5.576 | 0.000 | |
| HA (ng/mL) | 125.87 ± 134.61 | 124.44 ± 34.51 | 142.08 ± 37.40 | 2.482 | 0.014 | |
| LN (μg/L) | 49.87 ± 33.43 | 48.29 ± 31.21 | 67.75 ± 49.89 | 2.878 | 0.004 | |
| PLT (× 109/L) | 181.54 ± 63.11 | 185.44 ± 61.16 | 137.27 ± 69.18 | 3.809 | 0.000 | |
Hepatitis cirrhosis is a severe, chronic liver disease that seriously affects the quality of life and health of patients and places a heavy burden on the health-care system. With the social and economic development and the improvement of living standards, the prevalence of hepatitis and cirrhosis has gradually increased and become an important issue in the field of global public health[5]. Therefore, understanding the clinical characteristics of patients with hepatitis and cirrho
In this study, the patients were divided into a modeling cohort and a verification cohort, and the clinical data of the patients were compared. The results showed that in the modeling cohort, there were 688 patients with hepatitis (91.86%) and 61 patients with hepatitis cirrhosis (8.14%), indicating that the probability of liver cirrhosis for patients with hepatitis was high. This result was also close to that in the previous study by Liao et al[6]. At the same time, through the compa
Related studies have found that patients' liver functions are damaged, which further leads to the reduction in the synthesis of coagulation factors in the body, and thus to a certain extent causes patients to have a certain degree of coa
In order to determine the predictive value of GOT/GPT, PTA and HBeAg levels in patients with hepatitis cirrhosis, in this study, the nomogram model was established using the modeling cohort. The area under the ROC curve of the nomogram prediction model was larger, and the prediction performance was good. The probability of liver failure in the verification group was predicted by the subjects in the verification cohort using the nomogram, which indicated that it had certain predictive value. In addition, the factors of this model are all medical records of patients, so it is easy to obtain and has high clinical adaptability. In addition, verification of the cohort calibration curve revealed a small deviation of the actual result curve from the calibration curve, indicating a high agreement between the predicted and actual events. It could be seen from the decision analysis curve of the verification queue that the decision analysis curve at the top right corner generally indicated that the model had high true positive rate and low false positive rate, which meant that the model had certain accuracy and reliability.
The prediction model in this study is based on retrospective data. The data collection is from a single medical center, and there may be selection bias. Moreover, the sample size is relatively limited, and only 1070 patients were included. This may influence the stability and generalization ability of the model. The accuracy of the predicted results of this model is also influenced by the interpretation and application of the results by clinicians. In actual clinical decision-making, it may be necessary to combine other clinical indicators and doctors’ experience to make comprehensive judg
GOT/GPT, PTA and HBeAg are all independent factors influencing the progression of hepatitis to cirrhosis in patients with hepatitis. The nomogram prediction model for the occurrence of hepatitis cirrhosis constructed in this study showed good prediction ability, and the predicted events were highly consistent with the actual events. As a tool for predicting the occurrence of hepatitis cirrhosis in hepatitis patients, the model has high application value. However, due to the limited clinical data of our patients in this retrospective study, which may also affect the results of this study to some extent, further prospective studies are required to establish more comprehensive prediction models.
Special thanks to Dr. Xu C of Dalian Institute of Public Health and Dalian Public Health Clinical Center for providing scientific guidance and manuscript revision for this manuscript.
| 1. | Pollicino T, Caminiti G. HBV-Integration Studies in the Clinic: Role in the Natural History of Infection. Viruses. 2021;13:368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 2. | Premkumar M, Anand AC. Overview of Complications in Cirrhosis. J Clin Exp Hepatol. 2022;12:1150-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Chen Y, Gong J, Zhou W, Jie Y, Li Z, Chong Y, Hu B. A Novel Prediction Model for Significant Liver Fibrosis in Patients with Chronic Hepatitis B. Biomed Res Int. 2020;2020:6839137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | You H, Wang F, Li T, Xu X, Sun Y, Nan Y, Wang G, Hou J, Duan Z, Wei L, Jia J, Zhuang H; Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the Prevention and Treatment of Chronic Hepatitis B (version 2022). J Clin Transl Hepatol. 2023;11:1425-1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 86] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 5. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 861] [Article Influence: 215.3] [Reference Citation Analysis (1)] |
| 6. | Liao MJ, Li J, Dang W, Chen DB, Qin WY, Chen P, Zhao BG, Ren LY, Xu TF, Chen HS, Liao WJ. Novel index for the prediction of significant liver fibrosis and cirrhosis in chronic hepatitis B patients in China. World J Gastroenterol. 2022;28:3503-3513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Zijlstra MK, Gampa A, Joseph N, Sonnenberg A, Fimmel CJ. Progressive changes in platelet counts and Fib-4 scores precede the diagnosis of advanced fibrosis in NASH patients. World J Hepatol. 2023;15:225-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Samejo SA, Abbas Z, Asim M. Effects of anthropometric and metabolic parameters on transaminase levels and liver stiffness in patients with non-alcohol fatty liver disease. Trop Doct. 2021;51:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Ormeci A, Aydın Y, Sumnu A, Baran B, Soyer OM, Pınarbasi B, Gokturk S, Gulluoglu M, Onel D, Badur S, Akyuz F, Karaca C, Demir K, Besisik F, Kaymakoglu S. Predictors of treatment requirement in HBeAg-negative chronic hepatitis B patients with persistently normal alanine aminotransferase and high serum HBV DNA levels. Int J Infect Dis. 2016;52:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Wen QP, Qian H, Ba S, Lu MJ, Silang LDJ, Shi L. [Exploring the effects of entecavir treatment on the degree of liver fibrosis in patients with non-alcoholic fatty liver combined with chronic hepatitis B in Tibet region]. Zhonghua Gan Zang Bing Za Zhi. 2022;30:304-308. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Peng J, He G, Chen H, Kuang X. Study on correlation between coagulation indexes and disease progression in patients with cirrhosis. Am J Transl Res. 2021;13:4614-4623. [PubMed] |
| 12. | Zhang Q, Shi B, Wu L. Characteristics and risk factors of infections in patients with HBV-related acute-on-chronic liver failure: a retrospective study. PeerJ. 2022;10:e13519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | You X, Jiang F, Zhang Y. Clinical effects of combined treatment of traditional Chinese medicine and western medicine for viral hepatitis B cirrhosis and the effects on serum miR-122, miR-200a. Biotechnol Genet Eng Rev. 2024;40:2803-2817. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Li L, Chen L, Wang H, Li P, Wang D, Zhang W, Mi L, Lin F, Qin Y, Zhou Y. Clinical correlation between coagulation disorders and sepsis in patients with liver failure. Clin Hemorheol Microcirc. 2022;80:219-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Lisman T, Caldwell SH, Intagliata NM. Haemostatic alterations and management of haemostasis in patients with cirrhosis. J Hepatol. 2022;76:1291-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 16. | Roberts LN, Lisman T, Stanworth S, Hernandez-Gea V, Magnusson M, Tripodi A, Thachil J. Periprocedural management of abnormal coagulation parameters and thrombocytopenia in patients with cirrhosis: Guidance from the SSC of the ISTH. J Thromb Haemost. 2022;20:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 17. | Tantai N, Chaikledkaew U, Tanwandee T, Werayingyong P, Teerawattananon Y. A cost-utility analysis of drug treatments in patients with HBeAg-positive chronic hepatitis B in Thailand. BMC Health Serv Res. 2014;14:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Wang J, Wu W, Yan X, Wei J, Yao K, Yang Y, Xiong Y, Xia J, Liu Y, Chen Y, Jia B, Zhang Z, Ding W, Huang R, Wu C. HBeAg Negativity Is Associated With More Advanced Liver Fibrosis in Patients With Chronic Hepatitis B: A Propensity Score-Matching Analysis. J Clin Gastroenterol. 2020;54:826-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Li J, Cheng L, Jia H, Liu C, Wang S, Liu Y, Shen Y, Wu S, Meng F, Zheng B, Yang C, Jiang W. IFN-γ facilitates liver fibrogenesis by CD161(+)CD4(+) T cells through a regenerative IL-23/IL-17 axis in chronic hepatitis B virus infection. Clin Transl Immunology. 2021;10:e1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Peña-Asensio J, Calvo-Sánchez H, Miquel-Plaza J, Sanz-de-Villalobos E, González-Praetorius A, Delgado-Fernandez A, Torralba M, Larrubia JR. HBsAg level defines different clinical phenotypes of HBeAg(-) chronic HBV infection related to HBV polymerase-specific CD8(+) cell response quality. Front Immunol. 2024;15:1352929. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |