Published online Feb 27, 2025. doi: 10.4254/wjh.v17.i2.103299
Revised: December 26, 2024
Accepted: January 21, 2025
Published online: February 27, 2025
Processing time: 98 Days and 1.9 Hours
Heterozygous familial hypobetalipoproteinemia (FHBL) is a semi-autosomal disorder that is caused mainly by an APOB variant. It is usually asymptomatic and rarely leads to non-alcoholic steatohepatitis (NASH).
A 12-year-old boy was referred to our hospital after prolonged elevation of liver enzymes was observed during health checkups in Kagawa Prefecture. Abdominal ultrasound showed a bright liver, and laboratory investigations revealed low low-density lipoprotein cholesterol and apolipoprotein B protein levels. His family history included fatty liver and hypolipidemia in his father, which led to a clinical diagnosis of FHBL. A liver biopsy was performed on suspicion of liver fibrosis based on biomarkers. The liver tissue showed fatty steatosis, inflammation, hepatocyte ballooning, and fibrosis, indicating NASH. Genetic testing detected the APOB variant, and the patient was treated successfully with vitamin E.
It is important to assess family history and liver dysfunction severity in non-obese patients with hypolipidemia and fatty liver.
Core Tip: This report describes a 12-year-old boy with a prolonged history of liver dysfunction and low levels of low-density lipoprotein cholesterol (LDL-C) who was pathologically diagnosed with nonalcoholic steatohepatitis. His father also had a history of prolonged liver dysfunction and low LDL-C. Genetic testing identified a heterozygous mutation in APOB, which confirmed a diagnosis of familial hypobetalipoproteinemia (FHBL) with nonalcoholic steatohepatitis. Nonalcoholic steatohepatitis is well documented in adults with FHBL. This rare report of FHBL in a child indicates that nonalcoholic steatohepatitis can develop within a relatively short period but responded to treatment with vitamin E.
- Citation: Miyamoto K, Kondo S, Kondo T, Ishikawa R, Tani R, Inoue T, Matsunaga K, Minamino T, Kusaka T. Pathological features of non-alcoholic steatohepatitis in a pediatric patient with heterozygous familial hypobetalipoproteinemia: A case report. World J Hepatol 2025; 17(2): 103299
- URL: https://www.wjgnet.com/1948-5182/full/v17/i2/103299.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i2.103299
Familial hypobetalipoproteinemia (FHBL) is an inherited disorder of lipid metabolism that is characterized by hypolipidemia and is caused by mutations in genes such as APOB, PCSK9, and ANGPTL3[1].
APOB mutations essentially have the same clinical manifestations as abetalipoproteinemia in homozygotes and are typically asymptomatic in heterozygotes, who do not usually require treatment but are at risk of non-alcoholic fatty liver disease (NAFLD)[2]. Approximately 5%–10% of patients with FHBL develop non-alcoholic steatohepatitis (NASH), which is rarely associated with cirrhosis[3,4]. FHBL has been demonstrated to increase the risk of fatty liver and to be associated with NASH in adults, but there are no similar reports in children.
A 12-year-old boy was referred to our hospital due to prolonged liver dysfunction and hypolipidemia.
There was a 2-year history of prolonged liver dysfunction and persistent hypolipidemia.
The patient was first found to have liver dysfunction and hypolipidemia, in particular a low low-density lipoprotein cholesterol (LDL-C) level, during a health checkup for pediatric lifestyle-related diseases in Kagawa Prefecture at the age of 10 years and had been visiting a local hospital since that time. Ultrasound examination revealed fatty liver, for which he received guidance on exercise and nutrition. However, he had persistently elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, fluctuating between 50 and 100 U/L. Viral hepatitis, Wilson's disease, and autoimmune hepatitis were ruled out by blood tests. Type IV collagen 7S was elevated (234 ng/mL), suggesting liver fibrosis, while low levels of LDL-C (28 mg/dL) and apolipoprotein B (ApoB, 19 mg/dL) suggested abnormalities of lipid metabolism. Therefore, he was referred to our hospital for further evaluation and management.
His medical history included asthma for which he had been treated at a local hospital, but he no longer required medication. His father had liver dysfunction, which was thought to be alcohol-related, and was found to have low LDL-C (43 mg/dL) during a health checkup, which was not evaluated further or treated.
At the initial visit, his vital signs were normal and he was not obese (height: 166.5 cm; weight: 64.4 kg; body mass index: 23.2 kg/m2). He generally felt quite well and had a good appetite. He had no abdominal pain, and examination of the skin and conjunctiva did not indicate jaundice.
Laboratory tests showed elevated liver enzyme levels (AST: 63 IU/L; ALT: 123 IU/L) and decreased levels of total cholesterol (83 mg dL), triglycerides (32 mg/dL), LDL-C (31 mg/dL), and ApoB (19 mg/dL). Blood cell counts were normal, with normal renal parameters and thyroid function and no evidence of cholestasis or coagulation abnormalities. Insulin resistance was noted, with an elevated Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) of 4.8.
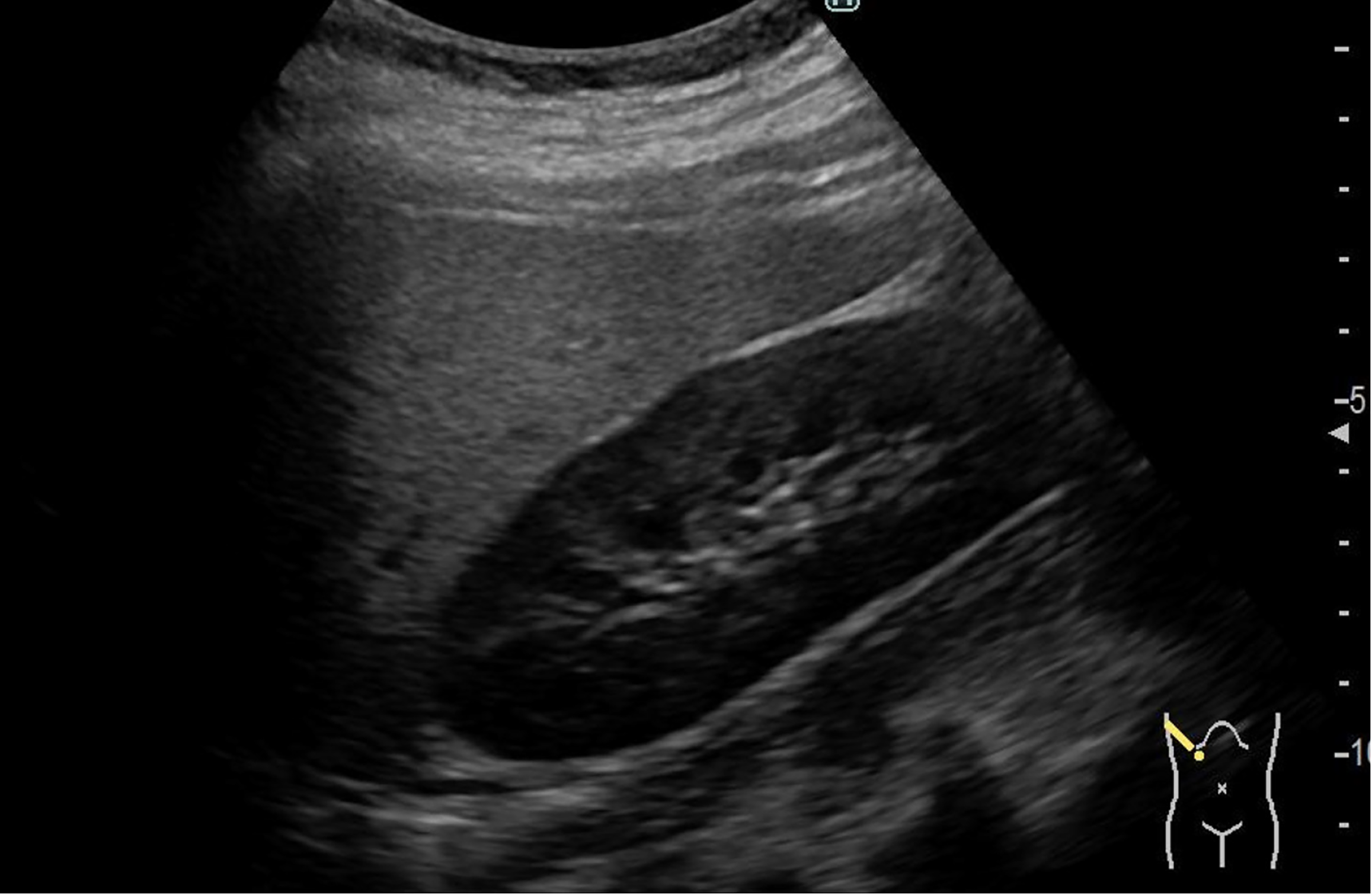
Abdominal ultrasound revealed deep attenuation and liver–kidney contrast, suggesting fatty liver (Figure 1).
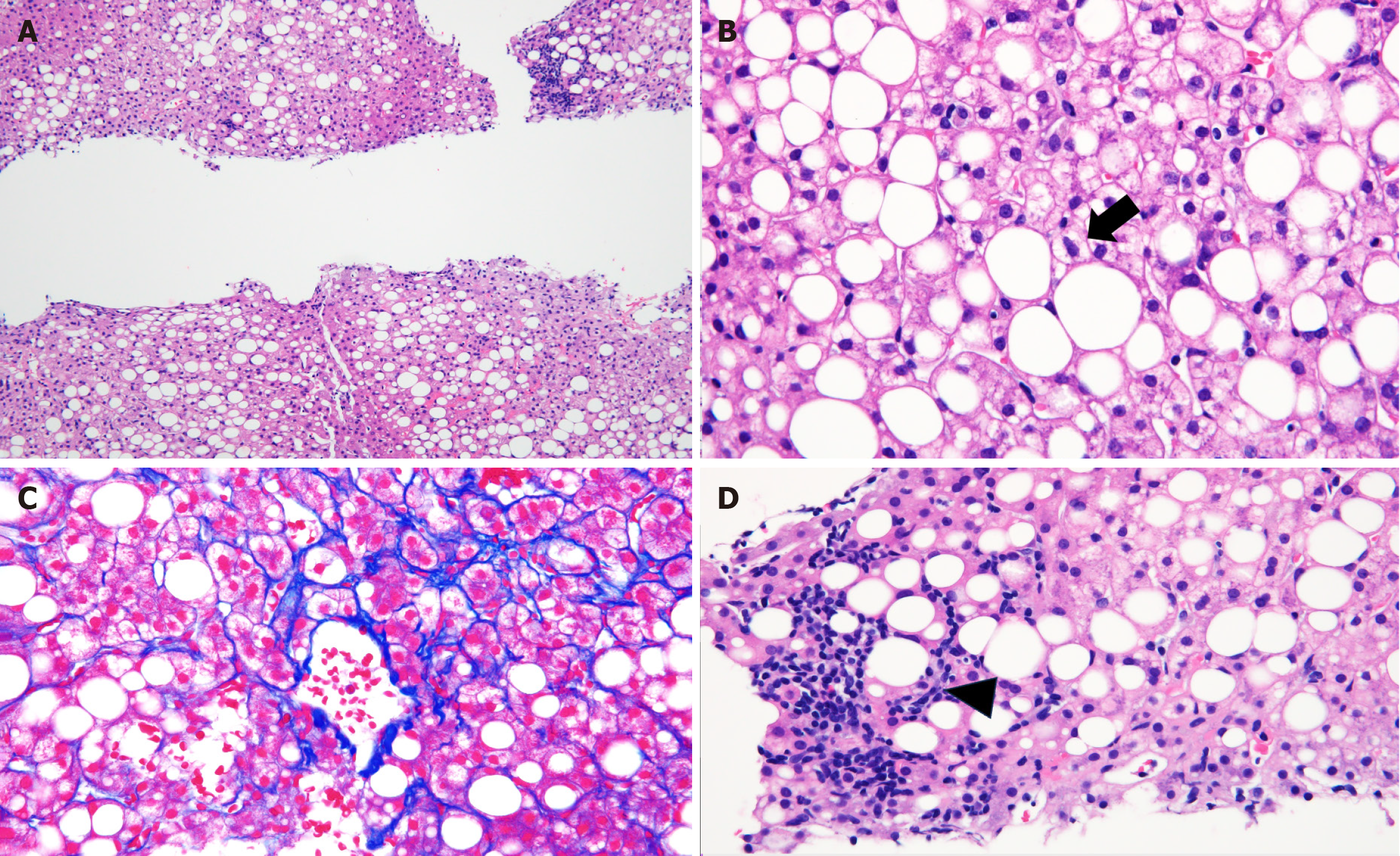
Pathological findings from liver biopsy revealed that more than 50% of hepatocytes showed mixed macro- and microvesicular steatosis, hepatocellular ballooning, fibrosis around the central veins and hepatocytes, and lymphocytic infiltration in the hepatic lobules (Figure 2), suggesting NASH.
Pathologically diagnosed NASH in a pediatric patient with FHBL.
He was discharged as scheduled on the day after the liver biopsy without any complications. FHBL was suspected based on the pathological findings, and the possibility that NASH was associated with this condition was considered. Further nutritional guidance and exercise therapy was provided. However, his AST and ALT levels remained elevated at approximately 100 IU/L. Vitamin E (tocopherol acetate) 1.5 mg/kg/day was initiated for NASH 1 month after discharge.
The patient's AST and ALT levels gradually decreased and returned to the normal range after 8 months. The patient remained asymptomatic. At 1 year after discharge, genetic testing identified a heterozygous mutation (NM_000384.3: c.7537C>T) in the APOB gene. Subsequent examinations did not detect deficiencies in vitamins A, D, or K. However, a deficiency in vitamin E persisted, necessitating continued oral supplementation. Fatty liver was no longer observed on ultrasound examination at 2 years and 6 months after discharge. It has now been 3 years since discharge, and the patient remains well with normalization of liver enzymes and collagen 7S. His father has not undergone genetic testing.
Despite the absence of obesity, this patient developed liver pathology suggestive of NASH, which was associated with FHBL. Oral administration of vitamin E was effective.
NASH is a progressive form of NAFLD characterized by hepatic steatosis, inflammation, and fibrosis. Features of NASH include excessive accumulation of hepatic fat, hepatocyte ballooning, inflammation of the liver, and development of fibrosis, potentially leading to cirrhosis and liver cancer[5,6]. In recent years, the term NAFLD was recognized as not accurately reflecting the underlying disease pathology, leading to the proposal of the concept of metabolic-associated fatty liver disease (MAFLD) in 2020 and its introduction in 2023. The shift from NASH to metabolic-associated steatohepatitis was proposed as an extension of the MAFLD definition, re-defining the disease with a focus on metabolic abnormalities[7]. Cases like the one described here, in which metabolic dysfunction or specific non-alcohol-related causes contribute to fatty liver disease, may be more appropriately classified as “specific etiology steatotic liver disease”. However, the pathological findings were similar to those seen in conventional NASH, even though it may not be suitable to refer to these findings as NASH.
FHBL is an autosomal semi-dominant disorder, known to be caused by mutations in the APOB, PCSK9, ANGPTL3, or NPC1 L1 gene. The most common cause of FHBL is mutations in the APOB gene, with more than 90 types of mutations identified[8]. APOB encodes ApoB, which is the primary component of LDL-C and plays a pivotal role in lipid metabolism and secretion in the liver. ApoB is typically a very long polypeptide chain, and its full-length form, ApoB-100, is essential for the synthesis and secretion of lipoproteins[9]. FHBL is thought to be heterozygous in 1 in 3000 and as homozygous in less than 1 in 1 million[10], and is diagnosed clinically based on hypolipidemia and family history.
Although our patient was not obese, elevated liver enzymes were noted during health checkups and fatty liver was confirmed by ultrasound. Based on the family history and detailed evaluations including genetic testing, the patient was diagnosed as having NASH associated with FHBL and heterozygous APOB mutation.
In our case, the finding was heterozygous FHBL, but it had progressed to NASH. Homozygous cases have a clinical course similar to that observed in abetalipoproteinemia, which is characterized by impaired fat absorption, retinitis pigmentosa, and spinocerebellar degeneration. In contrast, heterozygous cases are typically asymptomatic or manifest with mild liver dysfunction and require minimal or no treatment. It has been suggested that heterozygous FHBL caused by PCSK9 or ANGPLT3 mutation is asymptomatic and may reduce the risk of atherosclerosis. Most cases of heterozygous APOB mutation are asymptomatic, but can lead to fatty liver and gallstones. We suspected some form of lipid metabolism disorder in our patient, who presented with non-obese fatty liver and reduced levels of ApoB detected at another hospital. However, his father also had persistent liver dysfunction, which was thought to be alcohol-related, but the specifics of his lipid profile were unknown, making diagnosis difficult. No significant findings were noted during the physical examination, but pathological analysis showed that more than 50% of the hepatocytes were steatotic with hepatocellular ballooning, fibrosis, and lymphocytic infiltration within the hepatic lobules.
In FHBL, mutations in the APOB gene result in production of truncated ApoB proteins. The abnormally shortened ApoB molecules disrupt the normal structure and function of lipoproteins, particularly the synthesis and secretion of very- LDL-C, which is produced in the liver and transports fats throughout the body. The extent of this disruption is typically correlated with the degree of ApoB truncation and is determined by the location of the genetic mutation. While longer truncations of ApoB retain some lipid-binding capacity, truncations shorter than ApoB-29/30 are predominantly degraded intracellularly and are not secreted as part of lipoprotein particles[11]. The presence of truncations shorter than ApoB-29/30 results in impaired lipid metabolism, which is characterized by neurological dysfunction, fatty liver, acanthocytosis, and deficiencies in fat-soluble vitamins. In contrast, individuals with longer truncations (e.g., those longer than ApoB-75) are considered to be less susceptible, with minimal impact on lipid metabolism and an asymptomatic course. Fifty-seven percent of heterozygous patients are reported to have fatty liver[12] and 5%-10% are reported to have the potential for progression to severe conditions such as NASH[4,13]. The progression of this process is influenced by the degree of the above-mentioned ApoB truncation. Furthermore, excessive fat intake and obesity can be additional risk factors for development of fatty liver even in individuals with longer truncated ApoB.
There have been some reports of NASH in patients diagnosed with FHBL[13,14]. However, it should be noted that those cases are exclusively in adults and are limited in number.
Although Molk et al[12] reported a pediatric case diagnosed with NAFLD with FHBL, to the best of our knowledge, there are no pediatric reports of NASH associated with FHBL diagnosed by pathological findings in a liver biopsy. Previous reports have involved adult cases of NASH, where it is assumed that accumulation of fat in the liver over time leads to worsening of fatty liver. However, our case report indicates that NASH can develop within the relatively short span of childhood, underscoring the need for caution.
Given that NASH was observed in this case, vitamin E was administered with the expectation of antioxidant effects. The effectiveness of antioxidants in treating NAFLD/NASH has been studied with several medications, vitamin E being the only one available for administration in our country. Sanyal et al[15] conducted a 96-week randomized controlled trial and reported the efficacy of vitamin E, while Sato et al[16] reported that vitamin E not only improved blood test results but also improved liver tissue[17]. In this case, vitamin E supplementation normalized AST and ALT levels within 8 months, and they have remained normal during the 3-year follow-up period.
There is no specific treatment recommendations for FHBL. Treatment is not usually required in a heterozygous FHBL case. However appropriate treatment includes dietary restrictions and supplementation with vitamins, which can prevent neurological sequelae. Previous reports have recommended consideration of supplementation when the patient has low lipid-soluble vitamin levels[3,18]. On the other hand, Moutzouri et al[19] reported that vitamin E supplementation may not be necessary in asymptomatic heterozygous FHBL. In this case, although the patient was asymptomatic and there was no observed decrease in other fat-soluble vitamins during treatment, only vitamin E was continued in view of its decrease and because it was already being used for NASH.
Although a repeat liver biopsy was not performed, follow-up abdominal ultrasound showed improvement in the bright appearance of the liver. For assessment of steatosis and fibrosis, transient elastography (FibroScan) is another option, which we plan to utilize during follow-up. Furthermore, we have not yet evaluated HOMA-IR after the initiation of treatment, but we intend to use it to assess insulin resistance during follow-up. We will perform regular monitoring through blood tests and FibroScan measurements as the patient grows up. After discontinuation of vitamin E supplementation, nutritional therapy will be the primary management strategy. If his liver status worsens again, we will consider reintroducing vitamin E or other fat-soluble vitamins based on his current condition. While NASH is considered an irreversible condition and the most severe form of fatty liver disease, the improvement observed in this case following early diagnosis and treatment underscores the need for a more detailed understanding of fatty liver in FHBL.
Our report emphasizes that heterozygous FHBL can lead to NASH, even in young patients, highlighting the importance of early diagnosis and evaluating the severity of fatty liver for preventive intervention. Further genetic studies are needed in both children and adults to determine whether heterozygotes FHBL can cause NASH.
In conclusion, NASH can be caused by heterozygous FHBL in pediatric patients. Our patient responded to treatment with vitamin E and is maintaining remission by sustained dietary restriction. Some cases of heterozygous FHBL might remain undetected, so more cases of pediatric FHBL may occur than currently thought. The possibility of FHBL should be considered in children who has fatty liver without obesity and hypolipidemia.
We thank Dr. Asahiro Morishita and Dr. Tomoko Tadokoro for their help with performing the liver biopsies, which greatly contributed to this study.
| 1. | Bredefeld C, Hussain MM, Averna M, Black DD, Brin MF, Burnett JR, Charrière S, Cuerq C, Davidson NO, Deckelbaum RJ, Goldberg IJ, Granot E, Hegele RA, Ishibashi S, Karmally W, Levy E, Moulin P, Okazaki H, Poinsot P, Rader DJ, Takahashi M, Tarugi P, Traber MG, Di Filippo M, Peretti N. Guidance for the diagnosis and treatment of hypolipidemia disorders. J Clin Lipidol. 2022;16:797-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 2. | Della Corte C, Fintini D, Giordano U, Cappa M, Brufani C, Majo F, Mennini C, Nobili V. Fatty liver and insulin resistance in children with hypobetalipoproteinemia: the importance of aetiology. Clin Endocrinol (Oxf). 2013;79:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Burnett JR, Hooper AJ, Hegele RA. APOB-Related Familial Hypobetalipoproteinemia. 2021 May 13. In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–. [PubMed] |
| 4. | Vilar-Gomez E, Gawrieh S, Liang T, McIntyre AD, Hegele RA, Chalasani N. Interrogation of selected genes influencing serum LDL-Cholesterol levels in patients with well characterized NAFLD. J Clin Lipidol. 2021;15:275-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Sinha RA, Singh BK, Yen PM. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol. 2018;14:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 418] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 6. | Tripathi M, Singh BK, Zhou J, Tikno K, Widjaja A, Sandireddy R, Arul K, Abdul Ghani SAB, Bee GGB, Wong KA, Pei HJ, Shekeran SG, Sinha RA, Singh MK, Cook SA, Suzuki A, Lim TR, Cheah CC, Wang J, Xiao RP, Zhang X, Chow PKH, Yen PM. Vitamin B(12) and folate decrease inflammation and fibrosis in NASH by preventing syntaxin 17 homocysteinylation. J Hepatol. 2022;77:1246-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 7. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1312] [Article Influence: 656.0] [Reference Citation Analysis (1)] |
| 8. | Burnett JR, Bell DA, Hooper AJ, Hegele RA. Clinical utility gene card for: Abetalipoproteinaemia--Update 2014. Eur J Hum Genet. 2015;23:890-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Young SG. Recent progress in understanding apolipoprotein B. Circulation. 1990;82:1574-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 302] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Welty FK, Lahoz C, Tucker KL, Ordovas JM, Wilson PW, Schaefer EJ. Frequency of ApoB and ApoE gene mutations as causes of hypobetalipoproteinemia in the framingham offspring population. Arterioscler Thromb Vasc Biol. 1998;18:1745-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Tarugi P, Averna M, Di Leo E, Cefalù AB, Noto D, Magnolo L, Cattin L, Bertolini S, Calandra S. Molecular diagnosis of hypobetalipoproteinemia: an ENID review. Atherosclerosis. 2007;195:e19-e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Molk N, Bitenc M, Urlep D, Zerjav Tansek M, Bertok S, Trebusak Podkrajsek K, Sustar U, Kovac J, Battelino T, Debeljak M, Groselj U. Non-alcoholic fatty liver disease in a pediatric patient with heterozygous familial hypobetalipoproteinemia due to a novel APOB variant: a case report and systematic literature review. Front Med (Lausanne). 2023;10:1106441. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Lam MC, Singham J, Hegele RA, Riazy M, Hiob MA, Francis G, Steinbrecher UP. Familial hypobetalipoproteinemia-induced nonalcoholic steatohepatitis. Case Rep Gastroenterol. 2012;6:429-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Harada N, Soejima Y, Taketomi A, Yoshizumi T, Uchiyama H, Ikegami T, Saibara T, Nishizaki T, Maehara Y. Recurrent familial hypobetalipoproteinemia-induced nonalcoholic fatty liver disease after living donor liver transplantation. Liver Transpl. 2009;15:806-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2473] [Article Influence: 164.9] [Reference Citation Analysis (2)] |
| 16. | Sato K, Gosho M, Yamamoto T, Kobayashi Y, Ishii N, Ohashi T, Nakade Y, Ito K, Fukuzawa Y, Yoneda M. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Nutrition. 2015;31:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 17. | Kim GH, Chung JW, Lee JH, Ok KS, Jang ES, Kim J, Shin CM, Park YS, Hwang JH, Jeong SH, Kim N, Lee DH, Kim JW. Effect of vitamin E in nonalcoholic fatty liver disease with metabolic syndrome: A propensity score-matched cohort study. Clin Mol Hepatol. 2015;21:379-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Buryska S, Ahn JC, Allen AM, Simha V, Simonetto DA. Familial Hypobetalipoproteinemia: An Underrecognized Cause of Lean NASH. Hepatology. 2021;74:2897-2898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Moutzouri E, Elisaf M, Liberopoulos EN. Hypocholesterolemia. Curr Vasc Pharmacol. 2011;9:200-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |