Published online Feb 27, 2025. doi: 10.4254/wjh.v17.i2.100451
Revised: December 4, 2024
Accepted: January 23, 2025
Published online: February 27, 2025
Processing time: 187 Days and 18 Hours
Transjugular intrahepatic portosystemic shunt (TIPS) has an important role in the therapy of complications of portal-hypertension-related ascites. Various guide
To compare the clinical outcomes of TIPS for RA and RNRA in patients with complications related to portal hypertension.
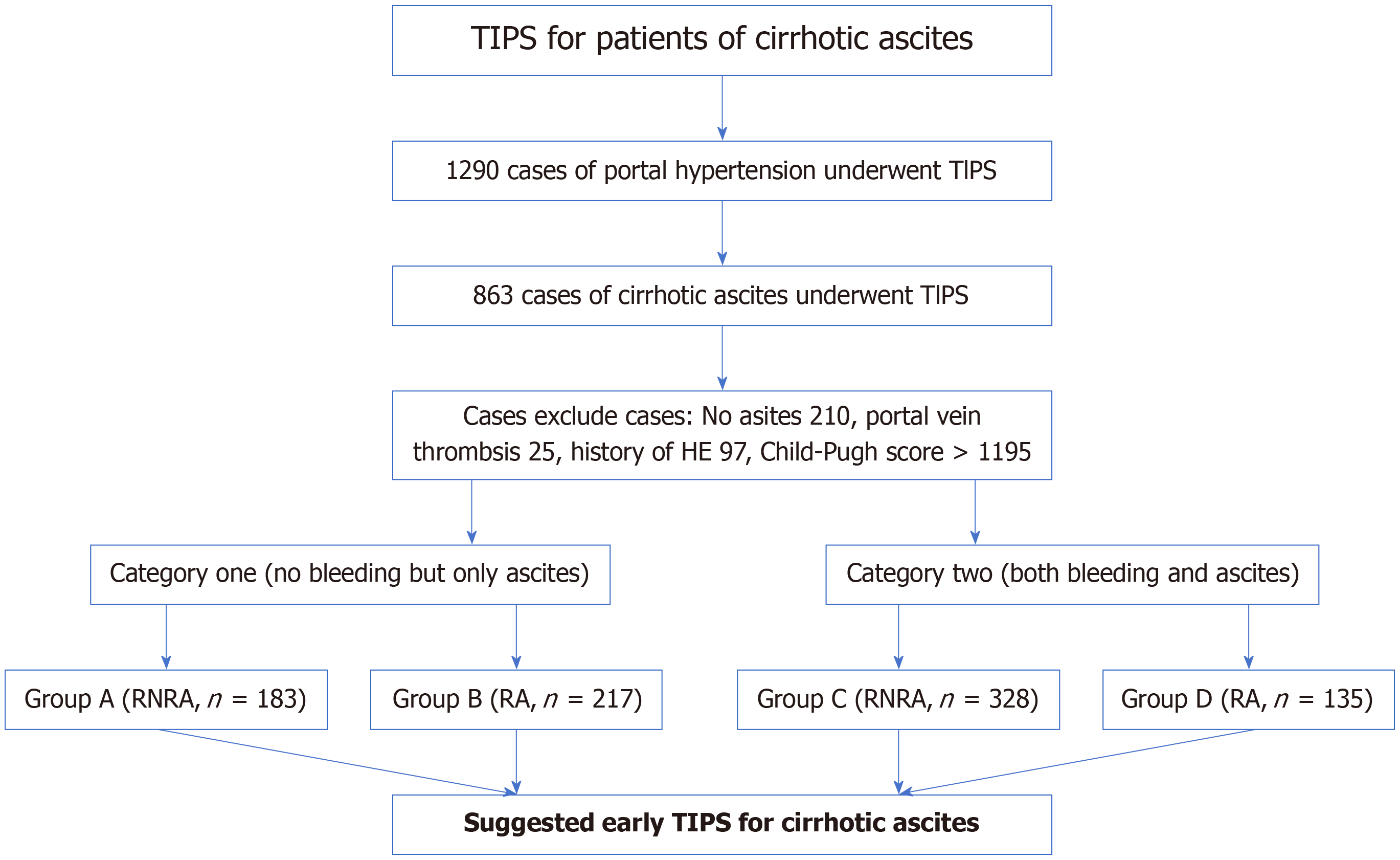
There were 863 patients divided into two main categories who underwent TIPS between September 2016 and September 2021. In category 1, patients had ascites without cirrhotic gastrointestinal bleeding. The patients were divided into group A (RNRA, n = 183) and group B (RA, n = 217). In category 2, patients had ascites and cirrhotic gastrointestinal bleeding. The patients were divided into group C (RNRA, n = 328) and group D (RA, n = 135). The clinical outcomes were proba
The symptoms of ascites disappeared or were relieved within 1 week in group A compared with group B (P = 0.032), and in group C compared with group D (P = 0.027). By the end of follow-up, there were significant di
TIPS has a good therapeutic effect on ascites related to cirrhotic portal hypertension, and early TIPS for RNRA can prolong survival, and prevent progression to RA.
Core Tip: This study aims to retrospectively compare the clinical outcomes of patients with refractory ascites (RA) and recurrent nonrefractory ascites (RNRA) who underwent transjugular intrahepatic portosystemic shunt (TIPS) for complications related to portal hypertension. RA is associated with a significant reduced survival of 50% at 6 months, and is related to development of many complications. Quality of life is poor. TIPS is currently adopted for the treatment of cirrhotic portal hypertension combined with ascites in the absence of progression to RA, that is, at the stage of RNRA. As a result, liver function does not deteriorate, ascites does not recur, survival is prolonged, and quality of life is improved compared with patients with RA.
- Citation: Luo SH, Zhang HF, Liu W, Chu JG, Chen JY. Comparison of clinical outcomes of transjugular intrahepatic portosystemic shunt for refractory ascites and recurrent nonrefractory ascites. World J Hepatol 2025; 17(2): 100451
- URL: https://www.wjgnet.com/1948-5182/full/v17/i2/100451.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i2.100451
Ascites is a common complication of cirrhosis, and some patients may develop refractory ascites (RA), which has a major impact on quality of life[1,2]. The first-line treatment for patients with cirrhotic ascites is sodium restriction and diuretics[3]. In the early stages of ascites, or the presence of small amounts of ascites, sodium restriction and diuretics can achieve good clinical results[4]. When RA develops, both quality of life and survival are severely affected[5]. RA is associated with poor survival of < 1 year, and is related to the development of many complications[6], such as immobility, bloating, early satiety, and hernia, and is associated with a poor outcome.
Transjugular intrahepatic portosystemic shunt (TIPS) is frequently used for the treatment portal hypertension[7]. Various guidelines[8,9] now indicate that TIPS is indicated for RA, but even after TIPS, survival rate in patients with RA is not ideal, and symptoms of ascites can recur[10].
The development of ascites in the natural history of cirrhosis implies the onset of decompensation, which is usually responsive to diuretic therapy at the initial stage. As liver cirrhosis progresses, ascites progresses to moderate or large volume, is effective for drug-treated responses, and is not yet refractory to ascites, symptoms will recur repeatedly, which is defined as recurrent nonrefractory ascites (RNRA)[11]. The symptoms of RNRA tend to be relieved in clinical practice with little or moderate pharmacological therapy[12]. Although drug therapy may be effective, it is sometimes necessary to use large volume paracentesis (LVP) to achieve short-term symptomatic relief. Medium and large volume of ascites often has complications; among which, peritonitis is one of the most harmful[13].
We hypothesized that TIPS could be performed in patients with cirrhotic portal hypertension combined with ascites without progression to RA, that is, at the stage of RNRA. As a result, liver function would not be deteriorated, ascites would not recur, survival would be prolonged, and quality of life would be better compared with that in patients with RA. However, there are few studies and data on this subject.
This study aimed to retrospectively compare the clinical outcomes of patients with RA and RNRA who underwent TIPS for complications related to portal hypertension. We provide some suggestions for TIPS treatment of ascites related to cirrhotic portal hypertension.
Eight hundred and sixty-three patients were admitted to hospital and underwent TIPS between May 2016 and May 2021. All procedures were conducted according to the guidelines approved by the Ethics Committee of Air Force Medical Center of PLA (AB-17.05/06). The indication for a stent graft shunt was ascites related to cirrhotic portal hypertension. The clinical outcomes of RA, probability of total hepatic impairment, incidence of hepatic encephalopathy (HE) and survival rate were compared. A comprehensive analysis of patient medical records and imaging data was conducted to collect information regarding the underlying causes, clinical manifestations, demographic characteristics (including age and gender), and the degree of hepatic fibrosis severity (Table 1).
| Characteristics | Group A, n = 183 | Group B, n = 217 | Group C, n = 328 | Group D, n = 135 |
| Gender, male/female | 106/77 | 137/80 | 173/155 | 74/61 |
| Age (year) | 53.57 ± 11.62 | 51.27 ± 13.29 | 49.24 ± 10.34 | 51.15 ± 9.25 |
| Child-Pugh A/B/C | 13/114/56 | 0/69/148 | 0/221/107 | 0/18/117 |
| MELD score | 9.16 ± 3.46 | 10.37 ± 2.71 | 11.15 ± 1.41 | 11.27 ± 1.05 |
| Viral hepatitis | 135 | 162 | 272 | 112 |
| Chronic ethanol consumption | 31 | 34 | 32 | 14 |
| Cryptogenic hepatitis | 17 | 21 | 24 | 9 |
| Laboratory tests | ||||
| Alanine transaminase (U/L) | 37.14 ± 8.17 | 41.27 ± 7.43 | 46.25 ± 11.38 | 48.36 ± 8.17 |
| Aspartate transaminase (U/L) | 43.74 ± 9.36 | 47.28 ± 8.49 | 54.16 ± 14.25 | 57.21 ± 12.04 |
| Alkaline phosphatase (U/L) | 119.43 ± 17.67 | 126.27 ± 14.17 | 124.16 ± 26.18 | 136.19 ± 21.51 |
| γ-Glutamyl transpeptidase (U/L) | 257.43 ± 29.28 | 262.46 ± 33.45 | 285.43 ± 27.65 | 247.32 ± 16.42 |
| Total bilirubin (mol/L) | 37.23 ± 6.54 | 45.13 ± 7.26 | 49.32 ± 12.17 | 54.72 ± 09.28 |
| Albumin (g/L) | 32.26 ± 2.35 | 29.14 ± 2.86 | 27.02 ± 1.54 | 24.37 ± 2.69 |
| Prothrombin time (second) | 15.16 ± 1.46 | 16.27 ± 1.24 | 16.18 ± 2.64 | 17.25 ± 1.31 |
| Clinical presentation | ||||
| Abdominal distention | 167 | 194 | 283 | 103 |
| Abdominal pain | 26 | 38 | 43 | 17 |
| Weakness | 46 | 62 | 189 | 83 |
| Poor appetite | 135 | 152 | 315 | 132 |
| Jaundice | 24 | 37 | 35 | 16 |
| Splenomegaly | 147 | 169 | 317 | 129 |
| Lower limb edema | 132 | 163 | 289 | 116 |
| Ascites paracentesis | 176 | 217 | 321 | 135 |
This was a multicenter retrospective study. A total of 1290 patients with cirrhosis-related portal hypertension underwent TIPS, and 863 were included in this study. They were divided into two main categories who underwent TIPS between September 2016 and September 2021. In category 1, patients had ascites with no cirrhotic gastrointestinal bleeding. We extracted the patient data and the patients were divided into group A (RNRA, n = 183) and group B (RA, n = 217). In category 2, the patients had ascites and cirrhotic gastrointestinal bleeding. They were divided into group C (RNRA, n = 328) and group D (RA, n = 135; Figure 1).
Patients with RA and RNRA that required TIPS placement due to portal-hypertension-related complications were included. RA is characterized by the persistence of fluid accumulation in the peritoneal cavity that remains unresponsive to therapeutic interventions, including the inability to achieve adequate fluid mobilization or the early reaccumulation of ascites following LVP, despite optimal medical management. Clinically, RA is further classified into two distinct categories: diuretic-resistant ascites, where patients fail to respond to standard diuretic regimens, and diuretic-intolerant ascites, where the use of diuretics is precluded due to adverse effects or contraindications[11]. RNRA refers to those patients who are not RA, had paracentesis more than 3 times a year, reach the standard of massive ascites.
The exclusion criteria were as follows: portal vein thrombosis, prior episodes of HE, advanced right ventricular dysfunction, end-stage hepatic insufficiency (defined as serum bilirubin levels exceeding 4 mg/dL), polycystic liver disease, biliary duct dilation, individuals aged over 75 years, and those with a Child-Pugh score greater than 11, MELD score > 18, hepatic carcinoma, sepsis, spontaneous bacterial peritonitis, and patients who underwent liver transplantation.
TIPS was performed under local anesthesia as described previously[14]. The stent graft (Viatorr, W.L. Gore & Associates, Flagstaff, AZ, United States) was deployed to fully cover the intrahepatic tract. During the procedure, both the hepatic venous pressure gradient and portal vein pressure were monitored, and the shunts were carefully dilated to achieve the desired diameter, aiming for a portosystemic gradient (PSG) of less than 12 mmHg. Gastroesophageal collateral vessels identified during the TIPS procedure were embolized using coils (Cook Inc., Bloomington, IL, United States; or Interlock Coil, Boston Scientific Corporation, Natick, MA, United States). Following this, direct portography was conducted to confirm the complete patency of the portal venous system.
After the TIPS procedure, intravenous Dalteparin Sodium Injection (5000 U/d; Vetter Pharma-Fertigung GmbH & Co. KG, Germany) was administered for 3 d, and no patients had portal vein thrombosis. Oral warfarin was not given.
Following TIPS placement, initial duplex sonography was conducted on the procedure day. Subsequent shunt velocities were systematically compared with these baseline measurements during follow-up assessments. Both patient cohorts underwent identical follow-up protocols. Post-procedure evaluations were scheduled through outpatient consultations at 1 month, followed by intervals of 3 months, 6 months, and annually up to 3 years, with additional visits arranged as clinically indicated. Each follow-up session incorporated a comprehensive clinical evaluation, biochemical profiling, upper abdominal ultrasound imaging, and HE assessment. HE was diagnosed according to the West Haven Criteria[15]. Patients who were unavailable for subsequent follow-up assessments had their data censored at the point of their most recent shunt imaging evaluation, which was conducted using either duplex ultrasonography or shunt venography techniques.
Data are presented as mean values accompanied by standard deviation, with statistical comparisons performed using either independent sample t-tests or one-way analysis of variance (ANOVA). For categorical variables, frequencies are reported, and χ2 tests are employed for comparative analysis. Intergroup differences were assessed through one-way ANOVA, with subsequent post-hoc analysis conducted using least significant difference t-tests. Survival rates were compared between group A and group B at 6 months, and 1, 2 and 3 years, and between group C and group D at 6 months, and 1, 2 and 3 years. Statistical significance was determined at a threshold of P < 0.05. All data analyses were conducted using SPSS software, version 22.0 (IBM Corporation, Armonk, NY, United States).
TIPS decreased PSG from 28.56 ± 4.89 to 9.87 ± 2.92 mmHg in group A (P = 0.003), from 27.46 ± 5.65 to 10.43 ± 3.76 mmHg in group B (P= 0.005), from 28.56 ± 4.89 to 9.87 ± 2.92 mmHg in group C (P = 0.004), and from 27.46 ± 5.65 to 10.43 ± 3.76 mmHg in group D (P = 0.003). There were no significant differences in the decreased PSG among the groups.
None of the patients died within 30 days after TIPS placement, with an early survival rate of 100%. The symptoms of ascites in 172 (93.98%) patients in group A and 156 (71.88%) in group B disappeared or were relieved within the first week without paracentesis and there was no significant difference between the groups (P = 0.032). For group C and group D, 311 (94.81%) and 82 (60.74%) patients had disappearance or relief of symptoms within the first week without paracentesis and there was no significant difference between the groups (P = 0.027; Table 2).
| Symptoms | Group A, n = 183 | Group B, n = 217 | χ2 | P value | Group C, n = 328 | Group D, n = 135 | χ2 | P value |
| First week of ascites | 172 (93.98) | 156 (71.88) | 0.032 | 311 (94.81) | 82 (60.74) | 0.027 | ||
| Hepatic function compromise | 43 (23.49) | 146 (67.28) | 2.072 | 0.024 | 47 (14.32) | 96 (71.11) | 3.043 | 0.019 |
| Recurrence of ascites | 40 (21.85) | 173 (79.72) | 0.016 | 64 (19.51) | 119 (88.14) | 0.012 | ||
| Hepatic dysfunction | 33 (18.03) | 154 (70.96) | 0.007 | 51 (15.54) | 107 (79.25) | 0.004 | ||
| Others | 7 (3.82) | 19 (8.75) | 0.025 | 13 (3.96) | 12 (8.88) | 0.019 | ||
| Ascites recurrence times | 48 | 326 | 0.000 | 103 | 202 | 0.001 |
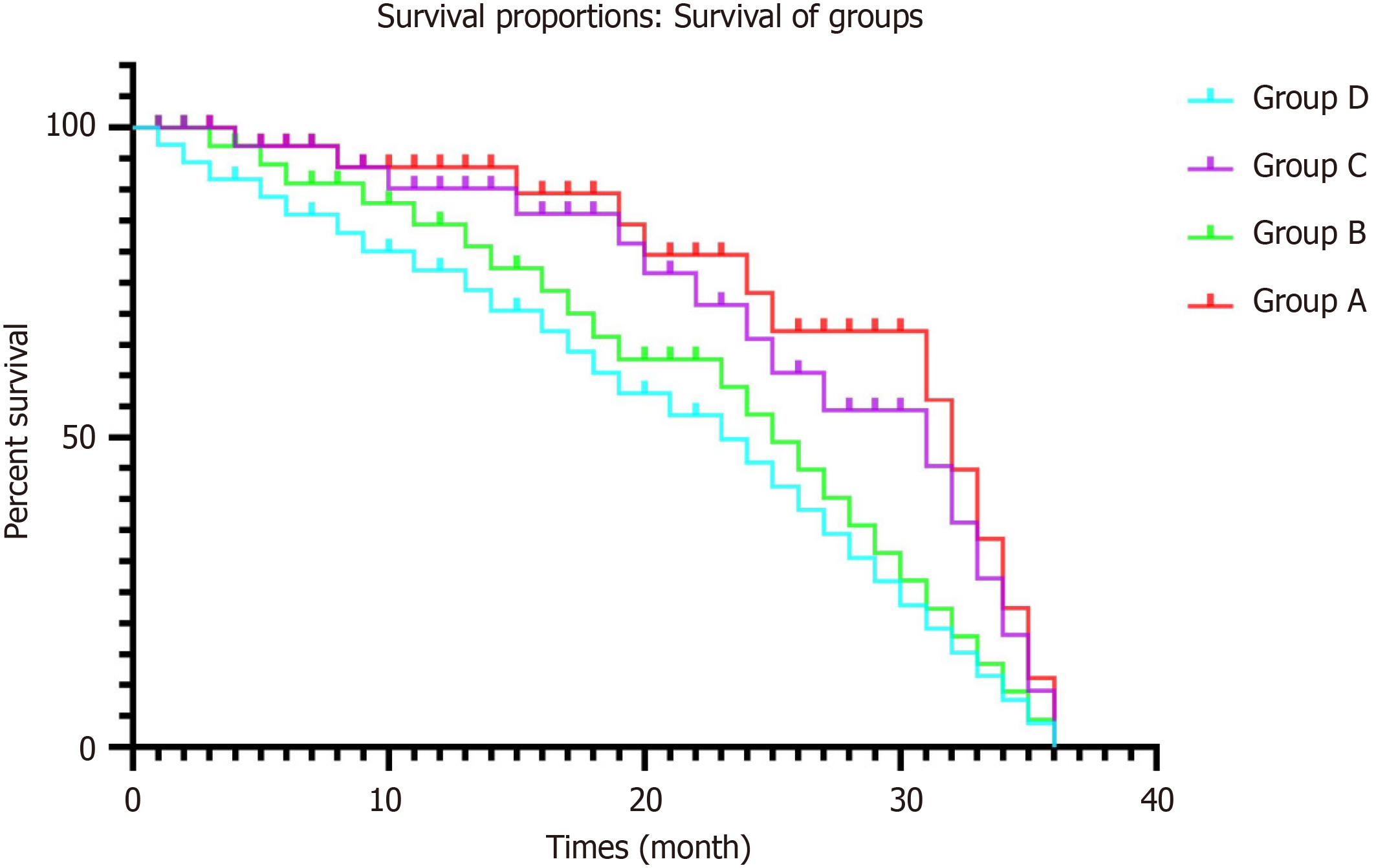
During follow-up, from 1 to 3 months, no patient was lost. The endpoint of this study was 3 years, and 70 patients in group A, 156 in group B, 137 in group C and 132 in group D were lost to follow-up. One hundred and fifty-nine patients died of multiorgan failure, 46 of hepatic tumor, and 59 from other causes. The 6-mo, and 1-, 2- and 3-year survival rates differed significantly between groups A and B (χ2 = 5.938, P = 0.007; χ2 = 5.726, P = 0.003; χ2 = 8.443, P = 0.002 and χ2 = 8.172, P = 0.002, respectively), and between groups C and D (χ2 = 6.357, P = 0.006; χ2 = 6.632, P = 0.002; χ2 = 9.257, P = 0.001 and χ2 = 8.243, P = 0.001, respectively; Table 3 and Figure 2).
| Time | Group | Survival | Survival rate (%) | χ2 | P value | |
| Yes | No | |||||
| 6 months | A | 172 | 11 | 93.98 | 5.938 | 0.007 |
| B | 109 | 108 | 50.23 | |||
| C | 301 | 27 | 91.76 | 6.357 | 0.006 | |
| D | 67 | 68 | 49.62 | |||
| 1 year | A | 159 | 24 | 86.88 | 5.726 | 0.003 |
| B | 28 | 189 | 12.90 | |||
| C | 272 | 56 | 82.92 | 6.632 | 0.002 | |
| D | 13 | 122 | 9.62 | |||
| 2 years | A | 131 | 52 | 71.58 | 8.443 | 0.002 |
| B | 14 | 203 | 6.45 | |||
| C | 229 | 99 | 69.81 | 9.257 | 0.001 | |
| D | 6 | 129 | 4.44 | |||
| 3 years | A | 113 | 70 | 61.74 | 8.172 | 0.002 |
| B | 6 | 156 | 2.76 | |||
| C | 191 | 137 | 58.23 | 8.243 | 0.001 | |
| D | 3 | 132 | 2.22 | |||
Forty-three (24.39%) patients in group A, 146 (67.28%) in group B, 47 (14.32%) in group C and 96 (71.11%) in group D developed hepatic impairment after TIPS placement. The probability of total hepatic impairment within 3 years was significantly different between groups A and B (χ2 = 2.072, P = 0.024, log-rank test), and groups C and D (χ2 = 3.043, P = 0.019, log-rank test; Table 2). Mean aspartate transaminase, alanine transaminase, and total bilirubin concentrations were elevated, albumin levels decreased, and prothrombin time was prolonged compared with before TIPS.
At the end of follow-up, 40 (21.85%) patients in group A, 173 (79.72%) in group B, 64 (19.51%) in group C and 119 (71.11%) in group D had recurrence of ascites, with a significant difference between groups A and B (P = 0.016) and groups C and D (P = 0.012). Of these, 33 (18.03%) cases in group A, and 154 (70.96%) in group B, 51 (15.54%) in group C and 107 (79.25%) in group D were caused by hepatic dysfunction of hypoalbuminemia, with a significant difference between groups A and B and groups C and D (P = 0.007, P = 0.004, respectively). After drug therapy and albumin supplementation, the symptoms recurred many times, and the time to recurrence in group A was less than in group B (P = 0.000), and less in group C than in group D (P = 0.001; Table 2).
The rate of HE in group A was lower than that in group B, and lower in group C than in group D at 1 month (χ2 = 6.462, P = 0.004; χ2 = 5.357, P = 0.007), 3 months (χ2 = 5.153, P = 0.001; χ2 = 6.468, P = 0.003), 6 months (χ2 = 6.269, P = 0.006; χ2 = 7.356, P = 0.005), 1 year (χ2 = 5.045, P = 0.005; χ2 = 6.248, P = 0.007), 2 years (χ2 = 5.327, P = 0.006; χ2 = 4.253, P = 0.003) and 3 years (χ2 = 6.043, P = 0.008; χ2 = 7.375, P = 0.004), and showed a downward trend. There were significant differences in HE occurrence between groups A and B, and groups C and D. Following pharmacological intervention, clinical manifestations resolved in individuals presenting with covert and grade II HE. Among patients diagnosed with grade III or IV HE, symptom resolution was achieved subsequent to shunt reduction procedures. However, five cases persisted with hepatic myelopathy despite undergoing shunt reduction therapy (Table 4).
| Time | Group | HE occurrence | Occurrence rate (%) | χ2 | P value | |
| Yes | No | |||||
| 1 month | A | 25 | 158 | 13.66 | 6.462 | 0.004 |
| B | 81 | 136 | 37.32 | |||
| C | 69 | 259 | 21.03 | 5.357 | 0.07 | |
| D | 56 | 79 | 41.48 | |||
| 3 months | A | 38 | 145 | 20.76 | 5.153 | 0.001 |
| B | 101 | 116 | 46.54 | |||
| C | 76 | 252 | 23.17 | 6.468 | 0.003 | |
| D | 65 | 70 | 48.14 | |||
| 6 months | A | 30 | 142 | 17.44 | 6.269 | 0.006 |
| B | 45 | 64 | 41.28 | |||
| C | 57 | 244 | 18.93 | 7.356 | 0.005 | |
| D | 30 | 37 | 44.77 | |||
| 1 year | A | 19 | 140 | 11.94 | 5.045 | 0.005 |
| B | 9 | 19 | 32.14 | |||
| C | 43 | 229 | 15.80 | 6.248 | 0.007 | |
| D | 4 | 9 | 44.44 | |||
| 2 years | A | 12 | 119 | 9.16 | 5.327 | 0.006 |
| B | 4 | 10 | 28.57 | |||
| C | 25 | 204 | 10.91 | 4.253 | 0.003 | |
| D | 2 | 4 | 33.33 | |||
| 3 years | A | 7 | 106 | 6.19 | 6.403 | 0.008 |
| B | 2 | 4 | 33.33 | |||
| C | 13 | 178 | 6.80 | 7.375 | 0.004 | |
| D | 1 | 2 | 33.33 | |||
Ascites of cirrhosis is defined as an accumulation of fluid in the peritoneal cavity in patients with cirrhotic portal hypertension and is due to cirrhosis in approximately 80% of cases[16]. It is considered to be the most common com
At stages 1 and 2 of ascites, medication therapy tends to achieve better clinical outcomes, although symptoms can recur repeatedly. As cirrhosis continues to progress to grade 3 ascites, paracentesis should also be performed if necessary. In the final stage, this can result in dilutional hyponatremia, which may make the prognosis worse and treatment of ascites more difficult, resulting in progression to RA.
According to the International Club of Ascites, RA is defined as ascites that cannot be mobilized, or early recurrence that cannot be satisfactorily prevented by medical therapy such as sodium restriction and LVP[19].
TIPS is performed to establish a conduit, in order to decrease portal systemic pressure and lower portal pressure[20]. Currently, TIPS guidelines[21,22] for ascites are recommended for RA, but not for RNRA, and consent from the ethics committee and the patient’s family should be required. In addition, diuresis, albumin infusion, and paracentesis also yield good clinical results[23].
RA is a complex condition and is not only induced by hypoproteinemia or excessive direct portal pressure, but also by a myriad of nonhemodynamic factors[24]; however, high portal pressure is the main cause. TIPS plays an important role in the cure or relief of RA. More recent data suggest that TIPS is effective in controlling RA. One study showed that TIPS was more effective than LVP in controlling ascites and could lead to ascites elimination in up to 77.6% of patients[25]. A meta-analysis of individual patient data from four randomized controlled trials comparing LVP vs TIPS in cirrhotic patients with RA found that TIPS was more efficacious in controlling RA (89.4% vs 42%)[26]. Another meta-analysis demonstrated significantly higher transplant-free survival rates in patients with RA treated with TIPS[27]. More recently, it was shown that 1-year transplant-free survival was significantly higher in patients with RA treated with covered TIPS than in those treated with LVP (93% vs 52%, P = 0.003)[28]. It has been suggested that TIPS placement should be considered as first-line treatment in carefully selected patients with RA.
However, TIPS has multiple problems in RA; the first of which is complications. The adverse events associated with TIPS can be categorized into three primary groups: procedural complications, stent-related issues, and consequences of portosystemic shunting[29]. The complications are rare and can include arrhythmias, hemoperitoneum, liver capsule rupture, stent migration, kinking, hemolytic anemia, hepatic ischemia, and infarction that compromises liver function[30]. Among them, HE and liver function deterioration are the main complications in patients undergoing TIPS placement[15].
In the present study, the incidence of HE in group A was lower than in group B, and lower in group C than in group D at 1, 3 and 6 months and 1, 2 and 3 years, and there was a significant difference in HE occurrence between groups A and B and groups C and D. During the 3-year follow-up, the total incidence of HE in group A was lower than in group B, and lower in group C than in group D. This showed that early TIPS prevented the occurrence of HE, deterioration of liver function, and progression to grade 3 ascites, resulting in RA.
In RA patients, liver function is impaired to varying degrees. Our results showed that groups A–D all had hepatic function compromise, and the likelihood of developing complete hepatic dysfunction over a three-year period de
Data suggested that TIPS placement before the development of RA may also be beneficial in improving survival[31]. It has been reported that there is no difference in survival following TIPS compared with LVP[32]. Although TIPS had a transient effect on RA, the symptoms recurred, with multiple complications and short survival. As portal hypertension is only one factor in RA, the prevention of RA and late-stage ascites is essential, and in these patients, early TIPS placement is suggested.
In our study, by the end of follow-up, patients in groups A and B, groups C and D had recurrent ascites, and there were significant differences between the groups. After drug therapy and albumin infusion, the symptoms recurred, and the time to recurrence in group A was less than in groups B, groups C and D. Survival rate in group A was higher than in groups B, groups C and D after 6 months, 1 year and 3 years.
These findings showed that TIPS had a good therapeutic effect on ascites related to cirrhotic portal hypertension, and early TIPS for RNRA can prolong survival, and prevent progression to RA. Diuresis, albumin infusion, and even LVP for ascites can benefit the patients in the short term. However, the recurrence of symptoms affects quality of life, and causes ascites-induced peritonitis, and kidney or other organ dysfunction, which rapidly reduces patient survival; thus, early TIPS for stage 2 ascites is needed.
Our study had several limitations. Firstly, randomized controlled trials are needed to verify the results. Secondly, TIPS is recommended for RA, but not for small amounts of multiple ascites or large amounts of nonrefractory ascites, even though the Baveno VII consensus[33] recommends TIPS when ≥ 3 large-volume paracenteses are required within 1 year. In this study, patients in group A were beyond the scope of the past guidelines, or needed multifaceted data for verification. Finally, our results need to be validated by a comparative study including results of long-term comparative studies of RA survival.
This retrospective study showed that TIPS has a good therapeutic effect on ascites related to cirrhotic portal hypertension, and early TIPS for large and multiple ascites can prolong survival, and prevent progression to RA.
The authors thank all the patients who took part in this study and our colleagues in the Department of Gastroenterology of our hospital for their contributions to data collection. The authors would also like to acknowledge the cooperation of all participating units for the collection and processing of case data.
| 1. | Pose E, Cardenas A. Translating Our Current Understanding of Ascites Management into New Therapies for Patients with Cirrhosis and Fluid Retention. Dig Dis. 2017;35:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Adebayo D, Neong SF, Wong F. Refractory Ascites in Liver Cirrhosis. Am J Gastroenterol. 2019;114:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Neong SF, Adebayo D, Wong F. An update on the pathogenesis and clinical management of cirrhosis with refractory ascites. Expert Rev Gastroenterol Hepatol. 2019;13:293-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Rudler M, Mallet M, Sultanik P, Bouzbib C, Thabut D. Optimal management of ascites. Liver Int. 2020;40 Suppl 1:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Di Pascoli M, Fasolato S, Piano S, Bolognesi M, Angeli P. Long-term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver Int. 2019;39:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (2)] |
| 6. | Stepanova M, De Avila L, Afendy M, Younossi I, Pham H, Cable R, Younossi ZM. Direct and Indirect Economic Burden of Chronic Liver Disease in the United States. Clin Gastroenterol Hepatol. 2017;15:759-766.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 7. | Mauro E, Gadano A. What's new in portal hypertension? Liver Int. 2020;40 Suppl 1:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, Wong F, Kim WR. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 462] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 9. | Italian Association for the Study of the Liver (AISF). Portal Hypertension and Ascites: Patient-and Population-centered Clinical Practice Guidelines by the Italian Association for the Study of the Liver (AISF). Dig Liver Dis. 2021;53:1089-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Rajesh S, George T, Philips CA, Ahamed R, Kumbar S, Mohan N, Mohanan M, Augustine P. Transjugular intrahepatic portosystemic shunt in cirrhosis: An exhaustive critical update. World J Gastroenterol. 2020;26:5561-5596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Tonon M, Piano S. Cirrhosis and Portal Hypertension: How Do We Deal with Ascites and Its Consequences. Med Clin North Am. 2023;107:505-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 12. | Philipp M, Blattmann T, Bienert J, Fischer K, Hausberg L, Kröger JC, Heller T, Weber MA, Lamprecht G. Transjugular intrahepatic portosystemic shunt vs conservative treatment for recurrent ascites: A propensity score matched comparison. World J Gastroenterol. 2022;28:5944-5956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Wong F. Management of refractory ascites. Clin Mol Hepatol. 2023;29:16-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 14. | Luo SH, Chu JG, Huang H, Zhao GR, Yao KC. Targeted puncture of left branch of intrahepatic portal vein in transjugular intrahepatic portosystemic shunt to reduce hepatic encephalopathy. World J Gastroenterol. 2019;25:1088-1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | de Wit K, Schaapman JJ, Nevens F, Verbeek J, Coenen S, Cuperus FJC, Kramer M, Tjwa ETTL, Mostafavi N, Dijkgraaf MGW, van Delden OM, Beuers UHW, Coenraad MJ, Takkenberg RB. Prevention of hepatic encephalopathy by administration of rifaximin and lactulose in patients with liver cirrhosis undergoing placement of a transjugular intrahepatic portosystemic shunt (TIPS): a multicentre randomised, double blind, placebo controlled trial (PEARL trial). BMJ Open Gastroenterol. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Tsochatzis EA, Gerbes AL. Diagnosis and treatment of ascites. J Hepatol. 2017;67:184-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Horn P, von Loeffelholz C, Forkert F, Stengel S, Reuken P, Aschenbach R, Stallmach A, Bruns T. Low circulating chemerin levels correlate with hepatic dysfunction and increased mortality in decompensated liver cirrhosis. Sci Rep. 2018;8:9242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Piano S, Tonon M, Angeli P. Management of ascites and hepatorenal syndrome. Hepatol Int. 2018;12:122-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Velamati PG, Herlong HF. Treatment of refractory ascites. Curr Treat Options Gastroenterol. 2006;9:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Artru F, Moschouri E, Denys A. Direct intrahepatic portocaval shunt (DIPS) or transjugular transcaval intrahepatic portosystemic shunt (TTIPS) to treat complications of portal hypertension: Indications, technique, and outcomes beyond Budd-Chiari syndrome. Clin Res Hepatol Gastroenterol. 2022;46:101858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Aithal GP, Palaniyappan N, China L, Härmälä S, Macken L, Ryan JM, Wilkes EA, Moore K, Leithead JA, Hayes PC, O'Brien AJ, Verma S. Guidelines on the management of ascites in cirrhosis. Gut. 2021;70:9-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 245] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 22. | Faisal MS, Singh T, Amin H, Esfeh JM. A guide to diagnosing and managing ascites in cirrhosis. J Fam Pract. 2021;70:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Pathak R, Yadav AK, Thapaliya S, Kafle B, Jha A, Khadga P. Comparative Study of Slow Infusion versus Bolus Doses of Albumin and Furosemide Infusion to Mobilize Refractory Ascites in Decompensated Chronic Liver Disease. J Nepal Health Res Counc. 2020;18:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | García-Pagán JC, Saffo S, Mandorfer M, Garcia-Tsao G. Where does TIPS fit in the management of patients with cirrhosis? JHEP Rep. 2020;2:100122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 25. | Russo MW, Sood A, Jacobson IM, Brown RS Jr. Transjugular intrahepatic portosystemic shunt for refractory ascites: an analysis of the literature on efficacy, morbidity, and mortality. Am J Gastroenterol. 2003;98:2521-2527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Bai M, Qi XS, Yang ZP, Yang M, Fan DM, Han GH. TIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World J Gastroenterol. 2014;20:2704-2714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 127] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (2)] |
| 27. | Colombato L. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension. J Clin Gastroenterol. 2007;41 Suppl 3:S344-S351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Bureau C, Thabut D, Oberti F, Dharancy S, Carbonell N, Bouvier A, Mathurin P, Otal P, Cabarrou P, Péron JM, Vinel JP. Transjugular Intrahepatic Portosystemic Shunts With Covered Stents Increase Transplant-Free Survival of Patients With Cirrhosis and Recurrent Ascites. Gastroenterology. 2017;152:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 305] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 29. | Khan A, Maheshwari S, Gupta K, Naseem K, Chowdry M, Singh S. Rate, reasons, predictors, and burden of readmissions after transjugular intrahepatic portosystemic shunt placement. J Gastroenterol Hepatol. 2021;36:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Horhat A, Bureau C, Thabut D, Rudler M. Transjugular intrahepatic portosystemic shunt in patients with cirrhosis: Indications and posttransjugular intrahepatic portosystemic shunt complications in 2020. United European Gastroenterol J. 2021;9:203-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Suraweera D, Jimenez M, Viramontes M, Jamal N, Grotts J, Elashoff D, Lee EW, Saab S. Age-related Morbidity and Mortality After Transjugular Intrahepatic Portosystemic Shunts. J Clin Gastroenterol. 2017;51:360-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Bucsics T, Hoffman S, Grünberger J, Schoder M, Matzek W, Stadlmann A, Mandorfer M, Schwabl P, Ferlitsch A, Peck-Radosavljevic M, Trauner M, Karner J, Karnel F, Reiberger T. ePTFE-TIPS vs repetitive LVP plus albumin for the treatment of refractory ascites in patients with cirrhosis. Liver Int. 2018;38:1036-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1506] [Article Influence: 502.0] [Reference Citation Analysis (2)] |