Published online Feb 27, 2025. doi: 10.4254/wjh.v17.i2.100033
Revised: December 4, 2024
Accepted: January 24, 2025
Published online: February 27, 2025
Processing time: 198 Days and 16 Hours
This review evaluated the diagnostic effectiveness of various ultrasound (US) methods compared to liver biopsy.
To determine the diagnostic accuracy of US techniques in assessing liver fibrosis and steatosis in adults, using the area under the receiver operating characteristic curve (AUROC) as the standard measure.
The review included original retrospective or prospective studies published in the last three years in peer-reviewed medical journals, that reported AUROC values. Studies were identified through PubMed searches on January 3 and April 30, 2024. Quality was assessed using the QUADAS-2 tool. Results were tabulated according to the diagnostic method and the type of liver pathology.
The review included 52 studies. For liver fibrosis detection, 2D-shear wave elastography (SWE) AUROCs ranged from 0.54 to 0.994, showing better accuracy for advanced stages. Modifications, including 2D-SWE with propagation map guidance and supersonic imagine achieved AUROCs of 0.84 to nearly 1.0. point SWE and classical SWE had AUROCs of 0.741-0.99, and 0.507-0.995, respectively. Transient elastography (TE), visual TE, vibration-controlled TE (VCTE), and FibroTouch reported AUROCs close to 1.0. For steatosis, VCTE with controlled attenuation parameter showed AUROCs up to 0.89 (for ≥ S1), acoustic radiation force impulse ranged from 0.762 to 0.784, US attenuation parameter from 0.88 to 0.93, and normalized local variance measurement from 0.583 to 0.875. Most studies had a low risk of bias across all or most domains, but evidence was limited by variability in study quality and small sample sizes. Innovative SWE variants were evaluated in a single study.
Modern US techniques can serve as effective noninvasive diagnostic tools for liver fibrosis and steatosis, with the potential to reduce the reliance on biopsies.
Core Tip: This study demonstrates that advancements in shear wave elastography and other ultrasound (US) methods allow for increasingly higher diagnostic accuracy. Several methods with area under the receiver operating characteristic curves slightly worse than 1.0 are described. For fibrosis, lower diagnostic accuracy was observed in earlier stages. However, the diagnostic value of US methods will probably resemble that of biopsy, questioning the rationale of sampling. For steatosis, the options remain less effective, and studies are fewer, but promising US modalities are emerging.
- Citation: Pozowski P, Bilski M, Bedrylo M, Sitny P, Zaleska-Dorobisz U. Modern ultrasound techniques for diagnosing liver steatosis and fibrosis: A systematic review with a focus on biopsy comparison. World J Hepatol 2025; 17(2): 100033
- URL: https://www.wjgnet.com/1948-5182/full/v17/i2/100033.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i2.100033
The prevalence of liver diseases, including nonalcoholic fatty liver disease (NAFLD) and alcoholic liver disease, is generally increasing[1-3], necessitating reliable diagnostic techniques that can effectively assess both the presence and progression of functional liver degeneration, especially using noninvasive methods that do not introduce additional risk.
Liver biopsy remains the ‘gold standard’ (or at least the best standard) for diagnostics[4-6] but it presents several challenges, including invasiveness, potential complications, and variability in results due to sampling error[6-9].
The growing prevalence of liver diseases increases the number of patients at risk of the harmful effects of biopsy and adds to the burden on pathologists. This highlights the need to minimize the proportion of patients referred for liver biopsy.
Evaluation of diagnostic methods that may eventually replace liver biopsy in a significant proportion of patients is essential. However, this may only be the last opportunity for such an evaluation. This is because, for ethical reasons, biopsies are currently rarely performed in diagnostic efficacy studies[10-12]. Advances in diagnostic imaging that reduce the number of patients referred for biopsy, particularly by selecting techniques and modalities with excellent diagnostic efficacy, will lead to a decrease in the number of biopsies.
Therefore, it is necessary to summarize the results achieved to date to identify the methods that have the greatest potential to replace liver biopsy in most clinical cases. This will enable appropriate planning in the development of medical diagnostics toward minimizing the need for liver biopsies.
This review aimed to determine the diagnostic effectiveness of ultrasound (US) using various techniques and modalities for assessing liver fibrosis and steatosis in adults. This review focuses on US rather than magnetic resonance imaging (MRI) or other imaginary methods because of its cost-effectiveness, wide availability, and minimally invasive nature, making it a promising and potentially superior alternative for routine clinical use.
This review follows the PRISMA declaration guidelines[13,14].
No review protocol was formally established or registered for this systematic review. Therefore, no protocol or registration information is available.
Articles presenting the latest advances in liver US diagnostics were included in this review. Given the dynamic nature of the field, it was deemed reasonable to include articles published within the last three years. Eligible articles featured original retrospective, or prospective studies with randomized or consecutive groups, providing area under the receiver operating characteristic curve (AUROC) calculations, which is a recognized international standard for comparing diagnostic imaging methods.
Articles that did not specify the key parameters, or lacked sufficient details for reliability analysis (e.g., unclear study flow) were excluded. Other exclusion criteria included publication outside peer-reviewed medical journals, studies involving children, and review articles. Data were obtained in table format and synthesized according to the examined liver pathology, diagnostic modality, and technique.
The PubMed database was searched on January 3, 2024, and repeated on April 30, 2024, for further review. The filters applied included publications from the last three years, abstract availability, and the exclusion of prescriptions.
The search used keyword combinations, including biopsy, ultrasonography, liver, steatosis, and/or fibrosis.
Articles that met the inclusion criteria were identified by double-screening the titles and abstracts. Because the procedure was performed twice, duplicates were excluded. After retrieving the articles, full texts were reviewed to apply the inclusion and exclusion criteria. All steps were conducted by two researchers working independently without automation. Two researchers reviewed studies on liver fibrosis and two others reviewed studies on steatosis. A fifth team member, a full-time professor, conducted an additional assessment of the results from both parts.
Data for each part of the review were extracted directly from the text by two independent researchers. However, the process was not automated. The reported values were subjected to an assessment of the probability of an obvious clerical error (e.g., if the reported mean was not within its confidence interval). The verification method was used to determine whether the goals set by the authors were consistent with the data presented. This was done directly by reading the text and using classical logical reasoning (deductive reasoning).
Outcomes measured in the randomized group of participants or at the time point closest to the biopsy were selected. The reported data included AUROC values, for the US modality or method, patient group, and liver histology stage. If the Scheuer fibrosis score was used, it was converted into the METAVIR score. The results were organized into tables based on the degree of fibrosis. For each study, a qualitative interpretation of the obtained AUROCs was performed to compare the effectiveness of the methods. Additionally, the arithmetic mean was calculated.
The risk of bias was independently investigated by two scientists. As part of the initial evaluation, a flowchart was created for each article and studies were categorized as retrospective and prospective. QUADAS-2 tool[15] was used for this assessment.
This assessment was carried out in four domains: Patient selection, index test, reference standard, and flow/timing. Yes/no questions derived from the tool were used, with applicability assessed for the first three domains.
Notably, the QUADAS-2 tool is a two-stage assessment system, categorizing criteria as either unmet or uncertain, without an option for ‘some concerns’.
The primary outcome measure in the synthesis was the AUROC, with the effect measured as the difference between the AUROC values. Data tabulation was used as the synthesis method. The results were divided according to the analyzed techniques, and the descriptive statistics for the tables were determined.
The methods used to assess certainty in the body of evidence included the QUADAS-2 tool for evaluating the risk of bias, stringent inclusion and exclusion criteria, meticulous data extraction and verification for accuracy, and the synthesis of data focusing on reported AUROC values across different fibrosis stages.
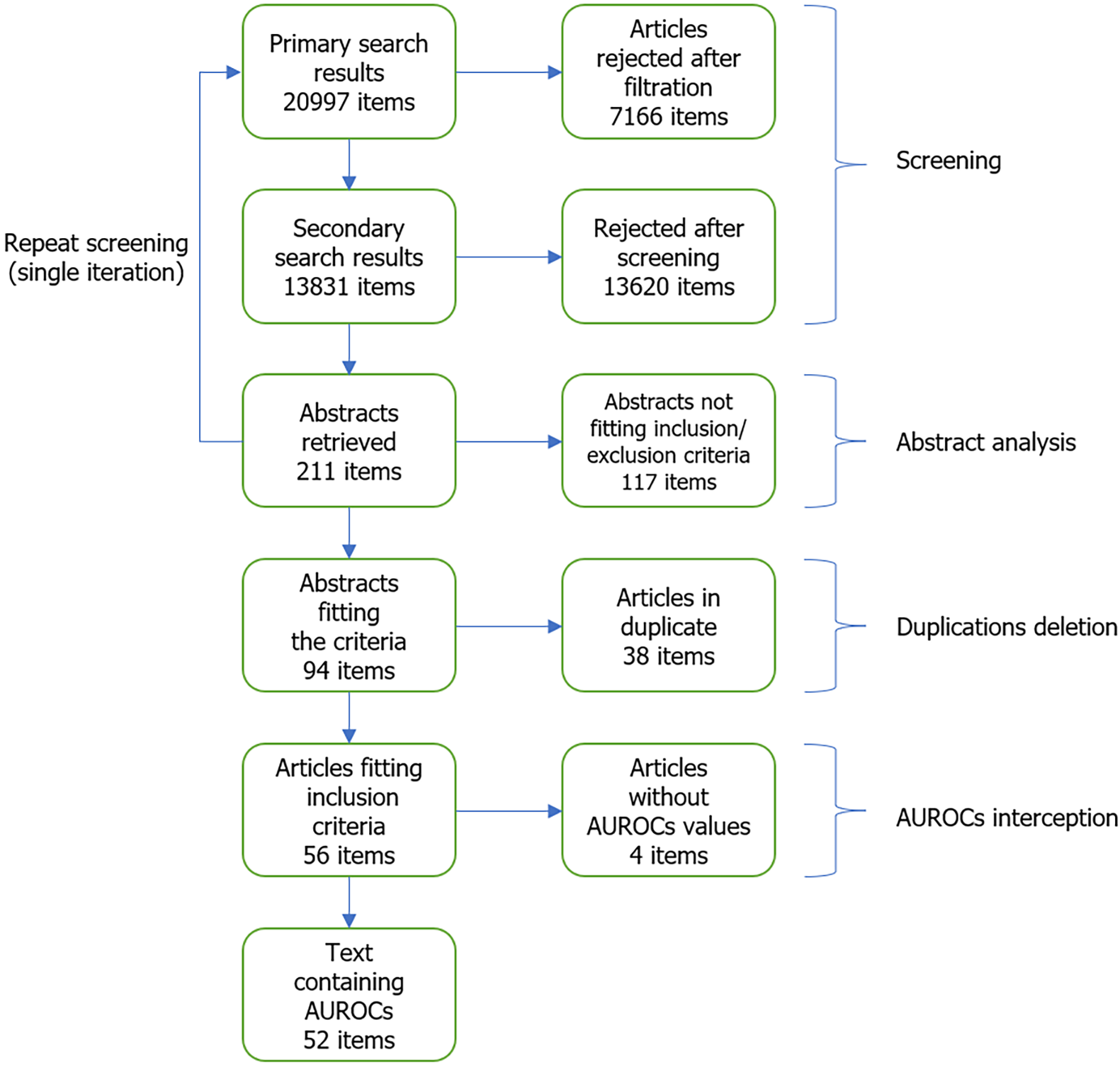
A total of 13831 results were screened, and 52 texts were included (Figure 1).
Unfortunately, four studies that initially met the inclusion/exclusion criteria were excluded due to the unavailability of AUROC values. The excluded studies were: Przybyłkowski et al[16] in 2021; Kalaiyarasi et al[17] in 2024; Chimoriya et al[18] in 2023; and Tosun and Uslu[19] in 2022.
A 2021 study by Aksakal et al[20] used a prospective approach to investigate 2D-shear wave elastography (SWE) efficacy in fibrosis staging and determined disease-specific cut-off values in 103 adult patients with chronic hepatitis B (CHB) and chronic hepatitis C (CHC).
Alcantara-Diaz et al[21] conducted a cross-sectional study to evaluate 2D-SWE in 227 obese adults who underwent bariatric surgery between 2020 and 2021 in 2023.
Atzori et al[22] evaluated the accuracy of the following three SWE methods: Philips Elast point quantification, Siemens Virtual TouchTM Quantification (VTQ) acoustic radiation force impulse, and transient elastography (TE) measured using the Echosens FibroScan in a cohort of 160 patients in 2024. The study correlated liver stiffness measurements (LSM) from these methods with same-day liver biopsy results.
The Cassinotto et al[23] study evaluated the concept of a multistep strategy, including laboratory tests, vibration-controlled TE (VCTE), and 2D-SWE with supersonic imagine (SSI), for diagnosing advanced fibrosis in 577 patients with NAFLD in 2021.
Damjanovska et al[24] compared the accuracy of TE using FibroScan and the fibrosis-4 index (FIB-4) against biopsy in ruling out cirrhosis among 93 obese patients with NAFLD at a tertiary transplant referral center in 2022.
Fang et al[25] conducted a prospective study to assess the accuracy of point SWE (p-SWE) and 2D-SWE in 121 patients with chronic liver disease (CLD).
Garcovich et al[26] assessed the correlation and accuracy of a new p-SWE method, marked X + p-SWE for staging liver fibrosis in patients with CLD alongside 2D-SWE-SSI. Notably, while the study included 253 patients, only 122 underwent liver biopsy.
Gatos et al[27] evaluated and compared the accuracy of visual TE (ViTE) with those of VCTE, SWE, and sound touch elastography (STE) in assessing liver fibrosis in 152 consecutive patients with CLD.
The 2023 study by Jocius et al[28] compared 2D-SWE and 99mTc mebrofenin dynamic scintigraphy using 99mTc in 72 patients with CHB/CHC.
Kavak et al[29] evaluated fibrosis in 253 patients with CHB using 2D-SWE with propagation map guidance, compared the results with histopathology and assessed changes in liver stiffness following antiviral therapy.
Laroia et al[30] evaluated SWE in patients with normal body mass index (BMI) and CLD of mixed etiology. The study included 124 patients who had undergone liver biopsy, analyzing both liver and spleen stiffness.
Lee et al[31] compared the diagnostic performance of 2D-SWE and p-SWE in 87 patients.
The 2021 study by Manesis et al[32] evaluated the correlation between 2D-SWE and the liver biopsy-confirmed fibrosis stage in primary biliary cholangitis (PBC).
Martonik et al[33] analyzed the consistency between 2D-SWE measurements and biopsy-confirmed fibrosis stages in hepatitis B virus (HBV) and hepatitis C virus (HCV) and compared the consistency between 2D-SWE and TE.
The 2022 study by Mendoza et al[34] evaluated the impact of steatosis and inflammatory activity on the accuracy of TE and 2D-SWE in patients with NAFLD.
The 2021 study by Mikolasevic et al[35] assessed the diagnostic accuracy of controlled attenuation parameter (CAP), and TE by FibroScan against biopsy in 179 patients with NAFLD.
Nogami et al[36] evaluated VCTE and two MRI techniques, magnetic resonance elastography (MRE) and MRI-proton density fat fraction (PDFF), for assessing liver fibrosis and steatosis in overweight and obese patients with NAFLD. The study included 163 patients with biopsy-proven NAFLD classified according to BMI.
Ogino et al[37] assessed the diagnostic abilities of S-map strain elastography and SWE in patients with NAFLD who underwent liver biopsies between 2015 and 2019.
Paisant et al[38] analyzed the reliability of 2D-SWE for the assessment of liver fibrosis by comparing a relatively high number (4277) of measurements from 788 patients with liver biopsy results to establish reliability criteria based on measurement variability and stiffness values.
Patidar et al[39] published a prospective single-center study that determined the optimal site for liver biopsy in patients with diffuse liver disease by comparing the diagnostic accuracies of real-time SWE (RT-SWE) and TE.
Roccarina et al[40] compared p-SWE with ElastPQ and TE with FibroScan for staging liver fibrosis in 671 patients with NAFLD in a multidisciplinary clinic. This was a retrospective cross-sectional study.
Saadi et al[41] investigated the utility of p-SWE in CLD of various etiologies at a single center with 202 patients and 14 healthy controls.
Seyrek et al[42] conducted a prospective study evaluating 2D-SWE, TE, and shear wave dispersion (SWD) for detecting stages of hepatic fibrosis and necroinflammation using liver biopsy as the reference standard in patients with CLD.
Sharpton et al[43] compared the effectiveness of 2D-SWE and VCTE in 114 patients with CLD.
Soh et al[44] evaluated the utility of 2D-SWE in the assessment of both liver stiffness and response to treatment in patients with autoimmune hepatitis (AIH). This retrospective study included 69 patients.
Song et al[45] conducted a prospective multicenter study to evaluate the diagnostic performance of 2D-SWE for staging liver fibrosis in 602 patients with CHB.
Taibbi et al[46] prospectively compared p-SWE and TE in 56 patients with NAFLD.
Wang et al[47] assessed 2D-SWE, the aspartate aminotransferase to platelet ratio index (APRI), and the gamma-glutamyl transferase to platelet ratio to evaluate liver fibrosis and high-risk esophageal varices in 141 patients with AIH-PBC overlap syndrome.
Wang et al[48] explored STE and sound touch quantification (STQ) in patients with CHB for staging fibrosis. The study included 524 patients with CHB and 97 healthy volunteers.
Wang et al[49] compared the diagnostic performances of SWE and SWD in 210 patients with hepatocellular carcinoma (HCC) scheduled for hepatectomy.
Yang et al[50] investigated the effectiveness and feasibility of SWE, STE, and ViTE for the noninvasive quantitative diagnosis of liver fibrosis in 106 patients with CLD. Yang et al[51] evaluated the diagnostic performance of shear wave-based STE for staging liver fibrosis in patients with autoimmune liver diseases (AILD), involving a total of 102 patients. Both these studies[50,51] failed to identify an association between their findings.
The 2022 study by Yoo et al[52] evaluated 2D-SWE using LOGIQ S8 and E9 for staging hepatic fibrosis compared with TE in 203 patients with CLD. Reference biopsies were obtained on the same day.
In 2021, Yoo et al[53] determined the accuracy of 2D-SWE relative to TE for assessing liver fibrosis in 115 patients with CLD, With all biopsies performed on the same day.
Although both studies by Yoo et al[52,53] share several similar elements and some of the same authors, the lead scientists (both surnamed Yoo) are distinct individuals.
The 2022 prospective study by Yamaoka et al[54] compared the performance of 2D-SWE with that of TE in 116 patients with CLD after liver biopsy. Liver staging in this study was based on surgical specimens rather than biopsy results.
The 2022 study by Zougmoré et al[55] compared the diagnostic accuracies of SWE and TE using FibroScan in 121 patients with CLDs, including NAFLD.
The 2022 study by Nogami et al[36] evaluated the diagnostic performance of VCTE and MRI techniques (MRE and MRI-PDFF) in assessing both liver fibrosis and steatosis in 163 overweight and obese patients with NAFLD classified according to BMI.
Hsu et al[56] aimed to evaluate the effectiveness of US-based attenuation imaging (ATI) as a novel method for diagnosing steatosis in 28 patients with CLD.
Kjaergaard et al[57] evaluated the accuracy of hepatic steatosis or fibrosis-related index (HRI) by the B-mode ratio in diagnosing hepatic steatosis compared to the US steatosis score, CAP, and fatty liver index, involving a prospective cohort of 137 participants with alcohol-related CLD or NAFLD. All biopsies were performed on the same day.
Kuroda et al[58] investigated the usefulness of combining 2D-SWE and the US attenuation parameter (UAP) for assessing the risk of progressive nonalcoholic steatohepatitis (NASH). This prospective study included 202 patients with NAFLD.
The observational study by Liu et al[59] validated the diagnostic efficacy of acoustic ATI and SWE for grading steatosis in 100 patients with NAFLD.
The 2021 study by Qu et al[60] evaluated the diagnostic performance of the UAP using FibroTouch for diagnosing hepatic steatosis and fibrosis in 237 patients with NAFLD, using liver biopsy as the reference standard.
The 2023 pilot study by Yazdani et al[61] assessed the diagnostic performance of shear wave attenuation imaging in 13 volunteers and 49 patients with NAFLD, along with SWD and MRI-PDFF.
The prospective study by Yu et al[62] evaluated the efficacy of FibroTouch in diagnosing liver steatosis and fibrosis in 85 patients with NAFLD marked as ‘metabolic-associated fatty liver disease’ combined with type 2 diabetes mellitus (T2DM).
Welman et al[63] aimed to assess the diagnostic accuracy of ATI compared to histological hepatosteatosis grading in 76 patients who underwent liver biopsy and multiparametric liver US within four weeks.
The 2022 study by Zhou et al[64] evaluated the accuracy of 2D-SWE as well as four noninvasive fibrotic biomarker scores (NFS, FIB-4, BARD, and APRI) for both steatohepatitis and fibrosis in biopsy-proven NAFLD. The study involved 116 patients with NAFLD and 23 controls.
Zhao et al[65] evaluated the diagnostic performance of a novel US technology, normalized local variance (NLV), and NLV standard deviation (NLV-SD) for hepatic steatosis in 34 patients with metabolic-associated fatty liver disease (MAFLD) in 2022.
Imajo et al[66] compared VCTE, 2D-SWE, and MRE in 231 patients with NAFLD.
Kim et al[67] conducted a diagnostic occuracy study on 60 patients who underwent liver biopsy, gray-scale US (HRI and SWE), and Fibroscan (CAP and TE) for the evaluation of liver steatosis and liver fibrosis in NASH.
Prieto Ortiz et al[68] conducted a retrospective study evaluating 2D-SWE. The study used a training dataset, consisting of subjects with a biopsy-to-2D-SWE interval of ≤ 6 months, to establish cut-off values, and a test dataset, consisting of subjects with an interval of > 6 months, for validation.
The 2022 study by Yan et al[69] evaluated the clinical utility of 2D-SWE for staging liver fibrosis and monitoring treatment responses in 148 patients with AIH-PBC overlap syndrome.
The 2022 study by Zhang et al[70] compared SWE and MRE for classifying fibrosis stages in 100 patients with biopsy-proven NAFLD.
Most of the studies had a low risk of bias across all domains.
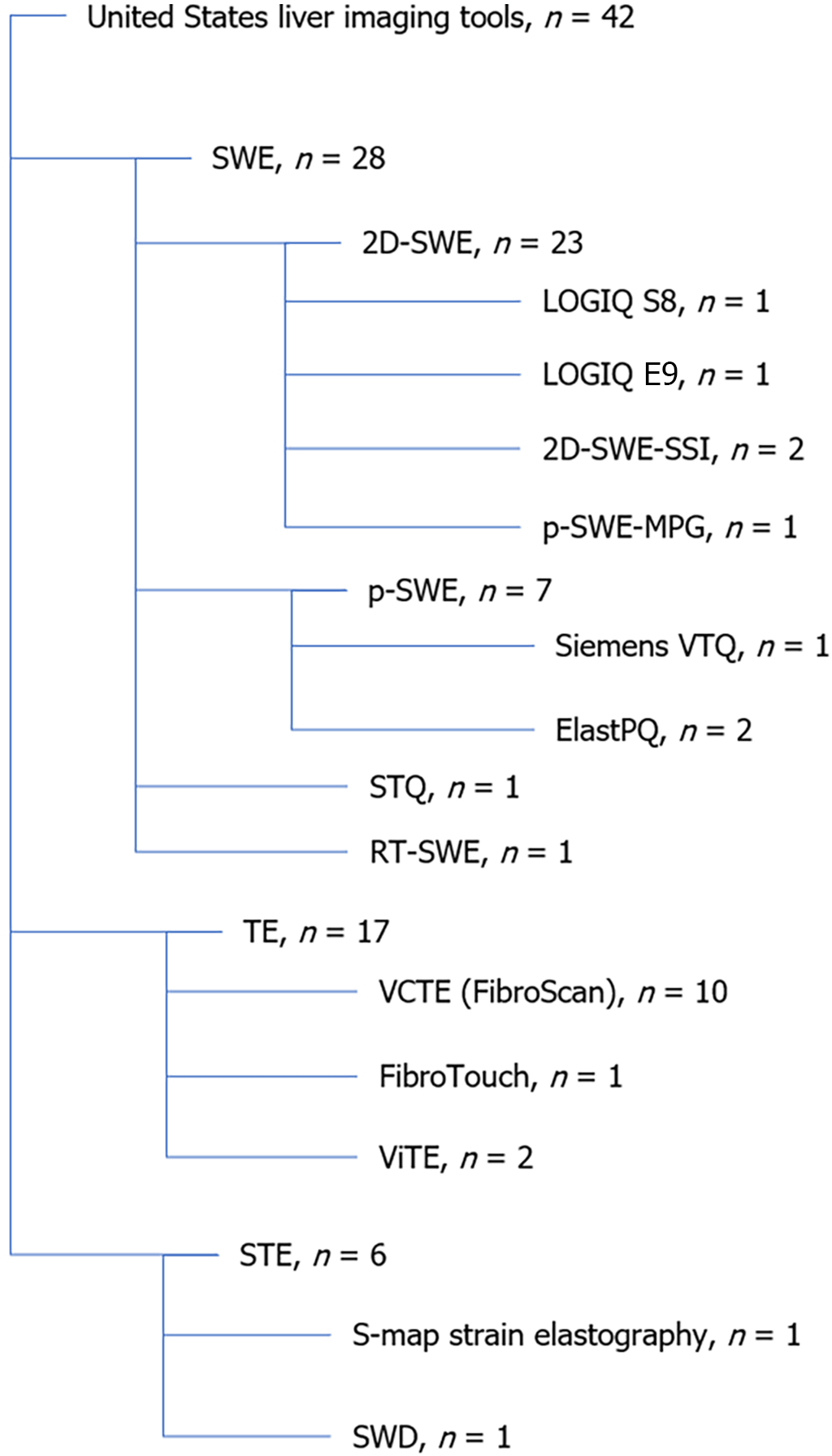
A total of 42 studies assessed liver fibrosis using US methods, primarily through LSM. While the most commonly researched modality was SWE, the most frequently used technique was VCTE using FibroScan (Figure 2).
Aksakal et al[20] obtained AUROCs using 2D-SWE for fibrosis stages with the following values 0.85 (CI: 0.75-0.94) for F ≥ 1, 0.98 (CI: 0.94-1.00) for F ≥ 2, 0.97 (CI: 0.94-1.00) for F ≥ 3, and 0.94 (CI: 0.89-1.00) for F ≥ 4. No P values provided in 2021.
Alcantara-Diaz et al[21] reported AUROCs using 2D-SWE for obesity with the following results 0.54 (CI: 0.47-0.62) for F ≥ 2, 0.73 (CI: 0.60-0.87) for F ≥ 3 (whole), 0.82 (CI: 0.59-1.00) for F ≥ 3 (women), and 0.78 (CI: 0.61-0.99) for F ≥ 3 (morbid obesity), without specifying P values in 2023.
Atzori et al[22] calculated AUROCs using various methods. Philips ElastPQ showed AUROCs of 0.828 (CI: 0.762-0.895) for F0/1, 0.812 (CI: 0.734-0.889) for F3/4, and 0.856 (CI: 0.753-0.938) for F4. Siemens VTQ reported 0.741 (CI: 0.659-0.823) for F0/1, 0.782 (CI: 0.702-0.861) for F3/4, and 0.826 (CI: 0.734-0.918) for F4. TE via FibroScan demonstrated AUROCs of 0.810 (CI: 0.746-0.884) for F0/1, 0.841 (CI: 0.771-0.910) for F3/4, and 0.939 (CI: 0.896-0.982) for F4 in 2024. No P values were provided.
Cassinotto et al[23] reported AUROC values of 0.80 (CI: 0.76-0.84) for F ≥ 2, 0.82 (CI: 0.78-0.86) for F ≥ 3, and 0.85 (CI: 0.80-0.90) for F4 with VCTE all with P values of < 0.001. Using 2D-SWE-SSI, the values were 0.84 (CI: 0.81-0.88) for F ≥ 2, 0.88 (CI: 0.84-0.91) for F ≥ 3, and 0.86 (CI: 0.82-0.90) for F4, also with P values of < 0.001 in 2021.
Damjanovska, et al[24] recorded an AUROC of 0.77 (CI: 0.66-0.88) for F4 using TE (FibroScan), without a specified P value in 2022.
Fang et al[25] reported AUROC values of 0.855 (CI: 0.778-0.932) for F ≥ 3 and 0.890 (CI: 0.826-0.954) for F ≥ 5 using p-SWE. For 2D-SWE, the corresponding AUROC values were 0.884 (CI: 0.817-0.951) for F ≥ 3 and 0.926 (CI 0.88-0.973) for F ≥ 5, with no P values provided in 2022.
Garcovich et al[26] achieved AUROCs of 0.96 (CI 0.93-0.99) for F ≥ 2, 0.98 (CI 0.97-1.00) for F ≥ 3, and 0.99 (CI 0.98-1.00) for F4 in 2023.
Gatos et al[27] reported AUROCs with ViTE of 0.9481 for F ≥ F1, 0.9698 for F ≥ F2, 0.9846 for F ≥ F3, and 0.9524 for F = F4. With VCTE, their AUROCs were 0.9900 for F ≥ F1, 0.9767 for F ≥ F2, 0.9651 for F ≥ F3, and 0.9645 for F = F4. With SWE they obtained 0.9621 for F ≥ F1, 0.9931 for F ≥ F2, 0.9835 for F ≥ F3, and 0.9656 for F = F4. Using STE, they noted 0.9683 for F ≥ F1, 0.9834 for F ≥ F2, 0.9763 for F ≥ F3, and 0.9509 for F = F4.
Imajo et al[66] obtained AUROCs of 0.87 (CI: 0.80-0.91) for F4 using VCTE and 0.88 (CI: 0.83-0.92) for F4 using 2D-SWE, both with P < 0.001.
Jocius et al[28] found the AUROCs of 0.75 for differentiating F1 vs F2-F4, 0.93-for F1-F2 vs F3-F4, and 0.91 for F1-F3 vs F4.
Kavak et al[29], using 2D-SWE-propagation map guidance (MPG), reported AUROCs of 0.956 (CI: 0.920-0.991) for significant fibrosis, 0.978 (CI: 0.945-1.000) for severe fibrosis, both with P values < 0.05.
For SWE, Laroia et al[30] documented AUROCs of 0.995 (CI: 0.988-1.00) for F0, 0.676 (CI: 0.589-0.763) for F1, 0.507 (CI: 0.409-0.605) for F2, 0.708 (CI: 0.621-0.795) for F3, 0.932 (CI: 0.889-0.975) for F4, 0.612 (CI: 0.516-0.708) for F1+F2, and 0.961 (CI: 0.933-0.990) for F3 + F4.
Lee et al[31] achieved AUROC values of 0.965 (CI: 0.895-0.993) for ≥ F2 and 0.994 (CI: 0.943-1.00) for F4 with 2D-SWE; and 0.872 (CI: 0.777-0.937) for ≥ F2 and 0.886 (CI: 0.794-0.947) for F4 with p-SWE, with P values of 0.022 and 0.042 respectively.
Manesis et al[32] reported AUROCs of 0.874 for F1, 0.853 for F2, 095.3 for F3, and 0.953 for F4 (P < 0.001).
Martonik et al[33] obtained AUROCs of 0.83 for F0-F1 vs ≥ F2, 0.84 for F2 vs ≥ F3, and 0.94 for F3 vs F4, using 2D-SWE
Mikolasevic et al[35] showed AUROCs of 0.98 for both ≥ F3 and F4 using TE FibroScan.
Nogami et al[36] reported AUROCs of 0.83-0.94 for ≥ F2, 0.90-0.95 for ≥ F3, and 0.87-0.89 for F4 using VCTE.
Ogino et al[37] reported AUROCs of 0.75 for F2, 0.80 for F3, and 0.85 for F4; using S-map strain elastography and AUROCs of 0.88 for F2, 0.87 for F3, and 0.92 for F4, using SWE, with multiple comparisons P > 0.05 or P > 0.01 for differentiation.
Paisant et al[38] achieved AUROCs of 0.825 (SD ± 0.006) for > F2 and 0.880 (SD ± 0.006) for F4, by 2D-SWE, with P < 0.001 for both.
Patidar et al[39] found AUROCs by TE 0.824 (CI: 0.72-1) for F0-F1, 0.935 (CI: 0.906-1) for F1-F2, 0.964 (CI: 0.883-1) for F2-F3, and 0.979 (CI: 0.633-1) for F3-F4; and using RT-SWE 0.867 (CI: 0.72-1) for F0-F1, 0.955 (CI: 0.906-1) for F1-F2, 0.946 (CI: 0.883-1) for F2-F3, and 0.93 (CI: 0.633-1) for F3-F4.
Prieto Ortiz et al[68] reported AUROCs of 0.75 for mild fibrosis, 0.83 for significant fibrosis, 0.89 for advanced fibrosis, and 0.94 for cirrhosis, using 2D-SWE.
Qu et al[60] reported AUROCs of 0.71 for ≥ F2, 0.71 for ≥ F3, and 0.77 for F4 by FibroTouch TE.
Roccarina et al[40] obtained AUROCs of 0.835 for F > 1, 0.831 for F > 2, 0.864 for F > 3, and 0.952 for F = 4 using p-SWE with ElastPQ, and AUROCs of 0.792 for F > 1, 0.849 for F > 2, 0.851 for F > 3, and 0.911 for F = 4 using TE with FibroScan.
Saadi et al[41] used p-SWE and reported AUROCs of 0.744 for F0-F1, 0.82 for F3, and 0.95 for F4.
Seyrek et al[42] found AUROCs of 0.86 for > F2, 0.87 for > F3, and 0.93 for F4 using 2D-SWE, with a P value of 0.1 for F4; and AUROCs of 0.79 for > F2 using TE, with a P value of < 0.001.
Sharpton et al[43] reported AUROCs of 0.88 for F3-F4, using 2D-SWE and 0.86 for F2-F4, 0.91 for F3-F4, and 0.96 for F4, using VCTE in 2021.
Soh et al[44] achieved AUROCs using 2D-SWE of 0.903 for ≥ S2, 0.815 for ≥ S3, and 0.854 for S4, with P < 0.002 across all stages (Scheuer scale).
Song et al[45] reported AUROCs using 2D-SWE of 0.817 for ≥ S1, 0.887 for ≥ S2, 0.912 for ≥ S3, and 0.832 for F4 (Scheuer scale).
Wang et al[47] found AUROCs using 2D-SWE of 0.748 for S2-S4, 0.818 for S3-S4, and 0.879 for S4, with the latter two stages having P values of < 0.001 (Scheuer scale).
Wang et al[48] noted AUROCs using STE and STQ of 0.87 for F > 4 and 0.76 for F > 2 with STE, and 0.86 for F > 4 and 0.73 for F > 2 with STQ, with F > 4 stages showing P values < 0.05.
Yan et al[69] calculated the AUROCs using 2D-SWE of 0.91 for ≥ S2, 0.97 for ≥ S3, and 0.96 for S4.
Taibbi et al[46] obtained AUROCs using various p-SWE techniques of 0.787 for F2-F4, 0.797 for F3-F4, and slightly lower for other stages, with all advanced fibrosis stages showing P values ranging from 0.002 to < 0.001. For ten measurements they obtained values of 0.787 (0.646-0.927, P = 0.002) for F2-F4, 0.797 (0.659-0.935, < 0.001) for F3-F4. For three measurements it was 0.714 (0.560-0.869, P = 0.021) for F2-F4, and 0.736 (0.587-0.885, P = 0.003) for F3-F4. In five measurements by p-SWE, they reported 0.809 (0.676-0.942, < 0.001) for F2-F4, and 0.809 (0.684-0.933, < 0.001) for F3-F4. Using TE, they reported 0.719 for F2-F4 and 0.799 for F3-F4, both with significant P values (0.016 and < 0.001, respectively).
Yang et al[50] reported AUROC values of 0.88 for F1 ≤ , 0.84 for F2 ≤, 0.80 for F3 ≤, and 0.80 for F4 using ViTE in 2021. With SWE, they found 0.92 for F1 ≤, 0.84 for F2 ≤, 0.79 for F3 ≤, and 0.76 for F4. By STE they reported, 0.91 for F1 ≤, 0.84 for F2 ≤, 0.77 for F3 ≤, and 0.71 for F4.
Yang et al[51], using STE, reported AUROCs of 0.82 for F2, 0.87 for F3, and 0.91 for F4 (P < 0.001 across all stages).
Yoo et al[52] utilized 2D-SWE and achieved AUROCs of 0.908 for F2, 0.905 for F3, and 0.931 for F4 using LOGIQ S8, and 0.910 for F2, 0.897 for F3, and 0.931 for F4 using LOGIQ E9 (all P < 0.001).
Yoo et al[53] reported AUROCs of 0.851 for F2, 0.917 for F3, and 0.889 for F4 using 2D-SWE, and 0.859 for F2, 0.881 for F3, and 0.938 for F4 using TE.
Zhang et al[70] documented AUROCs of 0.65 for ≥ 1, 0.81 for ≥ 2, 0.85 for ≥ 3, and 0.91 for F > 4, (P 0.005-0.720 across all stages, and 0.005 and 0.009 for F1-2 respectively).
Yamaoka et al[54] using 2D-SWE achieved AUROCs of 0.85 for ≥ F2, 0.91 for ≥ F3, and 0.88 for F4.
Wang et al[49] reported AUROCs of 0.895 for S ≥ 2, 0.877 for S ≥ 3, and 0.854 for S = 4 with SWE, and 0.857 for S ≥ 2, 0.815 for S ≥ 3, and 0.791 for S = 4 with SWD (Scheuer scale).
Zougmoré et al[55] calculated AUROCs of 0.91 for F > 2, 0.93 for F > 3, and 0.96 for F = 4 with SWE, and 0.86 for F > 2, 0.89 for F > 3, and 0.90 for F = 4 with TE, all with P < 0.001.
Kim et al[67] calculated AUROCs of 0.777 for ≥ F1, 0.747 for ≥ F2, 0.861 for ≥ F3, and 0.846 for ≥ F4 with SWE, and 0.733 for ≥ F1, 0.828 for ≥ F2, 0.869 for ≥ F3, and 0.891 for ≥ F4 with TE in patients with NASH.
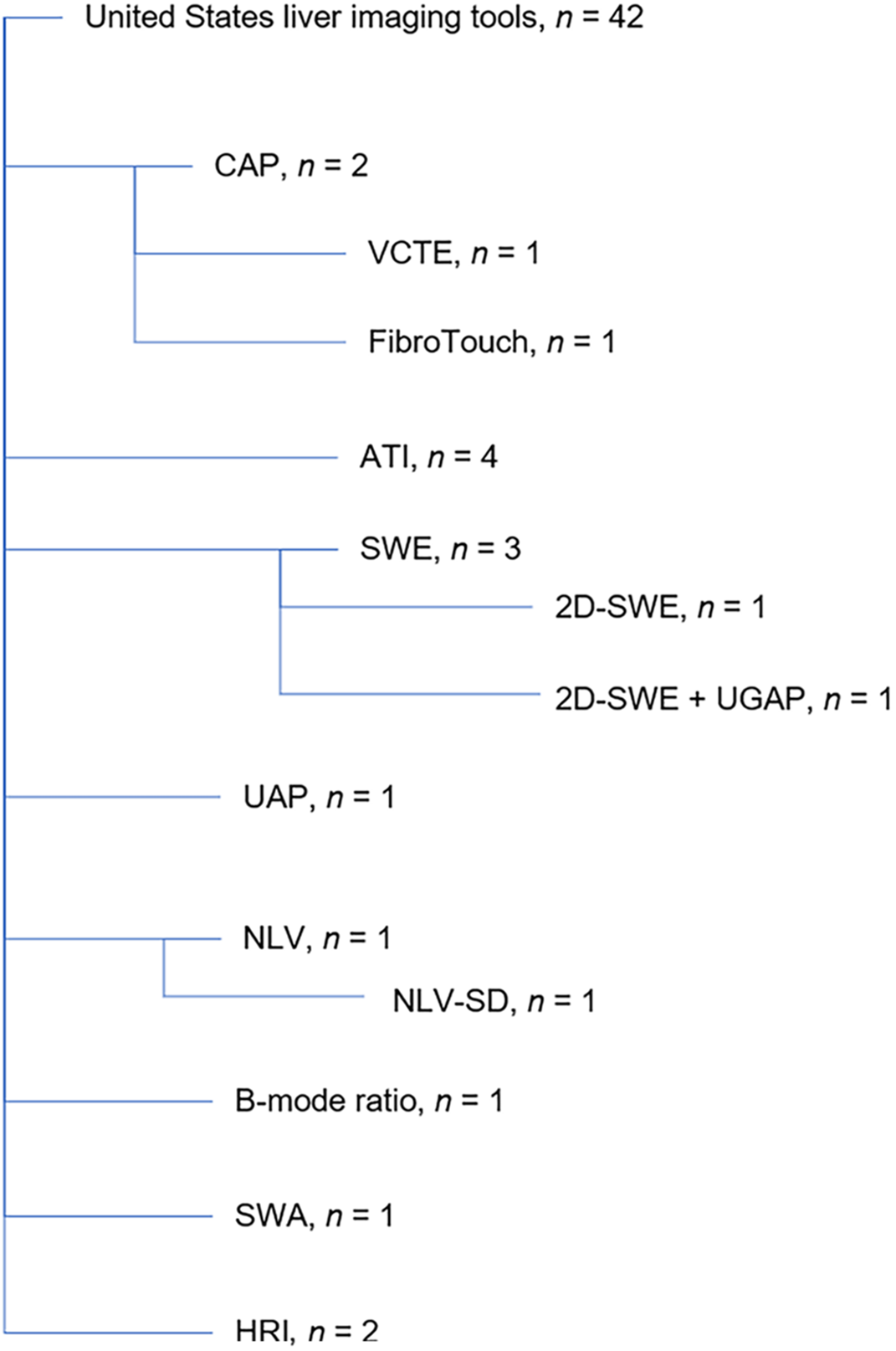
A total of 11 studies focused on steatosis. ATI, SWE, and CAP were assessed most frequently (Figure 3).
Nogami et al[36] reported AUROCs of 0.89 for ≥ S1, 0.77 for S2, and 0.69 for S3, each with P < 0.001.
A study by Liu et al[59] reported AUROCs of 0.762 for S ≥ 1, 0.774 for S ≥ 2, and 0.784 for S ≥ 3 using ATI, and 0.764 for S ≥ 1, 0.783 for S ≥ 2, and 0.802 for S ≥ 3 using SWE (P < 0.05).
Qu et al[60] calculated AUROCs of 0.88 for S1, 0.93 for S2, and 0.88 for S3.
Zhao et al[65] reported AUROCs of 0.875 for S ≥ 1, 0.735 for S ≥ 2, and 0.583 for S ≥ 3 using NLV, and 0.900 for S ≥ 1, 0.745 for S ≥ 2, and 0.603 for S ≥ 3 using NLV-SD.
Kjaergaard et al[57] reported AUROCs of 0.79 for S ≥ 1, 0.76 for S ≥ 2, and 0.74 for S ≥ 3.
Zhou et al[64] observed an AUROC of 0.88 for 2D-SWE in steatohepatis detection.
Yazdani et al[61] reported AUROCs of 0.99 for S0 vs ≥ S1, 0.98 for ≤ S1 vs ≥ S2, and 0.93 for ≤ S2 vs S3.
Yu et al[62] achieved AUROCs of 0.84 for S ≥ S1, 0.88 for S ≥ S2, and 0.89 for S = S3.
Hsu et al[56] calculated AUROCs of 0.97 for S ≥ 1, 0.99 for S ≥ 2, and 0.97 for S = 3, all with P < 0.001.
Kim et al[67] reported AUROCs of 0.871 for ≥ S2 and 0.851 for ≥ S3 for HRI.
Kuroda et al[58] documented AUROCs of 0.89 for ≥ S1, 0.91 for ≥ S2, and 0.92 for S3, with P < 0.05.
Welman et al[63] reported AUROCs of 0.85 for S1-S3, 0.91 for S2-S3, and 0.89 for S0-S1 vs S2-S3.
In the present study, a typical meta-analysis was not performed; however, a synthesis and summary of the results were performed.
The Scheuer scale is used to assess the severity of fibrosis in chronic hepatitis by dividing its progression into four stages. It begins with the initial development of enlarged fibrotic portal tracts and progresses through more severe structural changes, including periportal or portoportal septa, significant fibrosis with some architectural distortion, and ultimately cirrhosis. The METAVIR scoring system is a simplified framework of four distinct stages, focused specifically on fibrosis. It starts with F0, which indicates no fibrosis, and gradually progresses to cirrhosis[71].
This does not prevent conversions between scales, as shown in Table 1.
| Scheuer | METAVIR |
| S0 (no fibrosis) | F0 |
| S1 (portal fibrosis) | F1 |
| S2 (periportal fibrosis, few septa) | F2 |
| S3 (numerous septa without cirrhosis) | F3 |
| S4 (cirrhosis) | F4 |
AUROCs for US fibrosis stages are generally calculated as follows[72-75]: (1) Mild fibrosis - F0 vs F1 + F2 + F3 + F4; (2) Significant fibrosis - F0 + F1 vs F2 + F3 + F4; and (3) Advanced fibrosis - F0 + F1 + F2 vs F3 + F4; (4) Cirrhosis - F0 + F1 + F2 + F3 vs F4. Therefore whenever ≥ F1 is reported, it corresponds to (1), ≥ F2 to (2), ≥ F3 to (3), and F4 to (4).
In most scientific evaluations, efficacy results are considered acceptable at > 0.55, good at > 0.65, very good at > 0.75, and excellent at > 0.85[76] as a coin toss achieves 0.5 in both specificity and sensitivity. Although AUROC is a geometric measure of both specificity and sensitivity, a value of 0.5 indicates no diagnostic ability. An AUROC of 0.5 forms a diagonal line in the ROC space, showing the test performs no better than random guessing.
In medical diagnostics, being nonrandom and more accurate is not always sufficient for making rational diagnostic decisions. It is crucial to accurately distinguish between varying stages of liver fibrosis, providing clinicians with reliable and precise diagnostic tools to guide treatment decisions effectively. This necessitates a stricter assessment of AUROC quality. AUROCs are typically classified as “excellent” if > 90%, “good” if 80%-90%, and “acceptable” if 70%-80%[77].
The presented 2D-SWE data included results from a relatively large but diverse set of studies with sample sizes ranging from 53 to 788 patients. The diseases studied mainly encompass different types of CLD, including CHB, CHC, NAFLD, PBC, HBV/HCV, and AIH-PBC.
AUROC values ranged from 0.54 to 0.994, indicating a wide range of diagnostic performances of a single method. Higher AUROC values were generally observed in association with the detection of advanced fibrosis (F3 and F4). Conversely, lower AUROC values (e.g., 0.54 in patients with obesity reported by Alcantara-Diaz et al[21] in 2023) suggest poorer discrimination in specific contexts or patient groups. This study[21] highlighted that obesity might affect the efficacy of 2D-SWE, resulting in lower AUROC values.
The mean 2D-SWE AUROC value based on all the reports was 0.871 (SD = 0.076), with a median value of 0.877. The minimal value was 0.54 obtained by Alcantara-Diaz et al[21] in 2023 in a group of 227 obese patients with F > 2, while the highest value was calculated by Lee et al[31] based on their assessment of 87 patients with CLD for F > 2 (Table 2).
| Ref. | Number of patients | Disease/diagnose | Diagnostic method | Fibrosis stage | AUROC (CI) | Assessment |
| Fang et al[25] | 121 | CLD | 2D-SWE | F ≥ 2 | 0.884 (0.817-0.951) | Good |
| F ≥ 3 | 0.926 (0.88-0.973) | Excellent | ||||
| Aksakal et al[20] | 103 | CHB/CHC | 2D-SWE | F ≥ 1 | 0.85 (0.75-0.94) | Good |
| F ≥ 2 | 0.98 (0.94-1.00) | Excellent | ||||
| F ≥ 3 | 0.97 (0.94-1.00) | Excellent | ||||
| F ≥ 4 | 0.94 (0.89-1.00) | Excellent | ||||
| Alcantara-Diaz et al[21] | 227 | Obesity | 2D-SWE | F ≥ 2 | 0.54 (0.47-0.62) | Unacceptable |
| F ≥ 3 (whole) | 0.73 (0.60-0.87) | Acceptable | ||||
| F ≥ 3 (women) | 0.82 (0.59-1.00) | Good | ||||
| F ≥ 3 (morbid obesity) | 0.78 (0.61-0.99) | Acceptable | ||||
| Imajo et al[66] | 231 | NAFLD | 2D-SWE | F4 | 0.88 (0.83-0.92) | Good |
| Jocius et al[28] | 72 | CLD | 2D-SWE | F1 vs F2-F4 | 0.75 | Acceptable |
| F1-F2 vs F3-F4 | 0.93 | Excellent | ||||
| F1-F3 vs F4 | 0.91 | Excellent | ||||
| Lee et al[31] | 87 | CLD | 2D-SWE | ≥ F2 | 0.965 (0.895-0.993) | Excellent |
| F4 | 0.994 (0.943-1.00) | Excellent | ||||
| Manesis et al[32] | 53 | PBC | 2D-SWE | F1 | 0.874 | Good |
| F2 | 0.853 | Good | ||||
| F3 | 0.953 | Excellent | ||||
| F4 | 0.953 | Excellent | ||||
| Martonik et al[33] | 231 | HBV/HCV | 2D-SWE | F0-F1 vs ≥ F2 | 0.83 | Good |
| F2 vs ≥ F3 | 0.84 | Good | ||||
| F3 vs F4 | 0.94 | Excellent | ||||
| Mendoza et al[34] | 200 | NAFLD | 2D-SWE | ≥ F2 | 0.83 (0.72-0.93) | Good |
| ≥ F3 | 0.84 (0.76-0.92) | Good | ||||
| F4 | 0.94 (0.89-0.99) | Excellent | ||||
| Paisant et al[38] | 788 | Liver fibrosis | 2D-SWE | >F2 | 0.825 (SD ± 0.006) | Good |
| F4 | 0.880 (SD ± 0.006) | Good | ||||
| Prieto Ortiz et al[68] | 453 | Liver fibrosis | 2D-SWE | F > 1 | 0.75 | Acceptable |
| F > 2 | 0.83 | Good | ||||
| F > 3 | 0.89 | Good | ||||
| F = 4 | 0.94 | Excellent | ||||
| Seyrek et al[42] | 146 | CLD | 2D-SWE | > F2 | 0.86 (0.75-0.96) | Good |
| > F3 | 0.87 (0.78-0.97) | Good | ||||
| F2-F4 | 0.84 (0.76-0.92) | Good | ||||
| F4 | 0.93 (0.86-0.99) | Excellent | ||||
| Sharpton et al[43] | 114 | CLD | 2D-SWE | F2-F4 | 0.84 (0.76-0.92) | Good |
| F3-F4 | 0.88 (0.81-0.96) | Good | ||||
| F4 | 0.93 (0.86-0.99) | Excellent | ||||
| Soh et al[44] | 69 | AIH | 2D-SWE | ≥ F2 | 0.903 (0.807-0.961) | Excellent |
| ≥ F3 | 0.815 (0.703-0.898) | Good | ||||
| F4 | 0.854 (0.748-0.927) | Good | ||||
| Song et al[45] | 602 | CHB | 2D-SWE | ≥ F1 | 0.807 (0.742-0.861) | Good |
| ≥ F2 | 0.868 (0.810-0.914) | Good | ||||
| ≥ F3 | 0.855 (0.796-0.903) | Good | ||||
| F4 | 0.851 (0.791-0.900) | Good | ||||
| Wang et al[47] | 141 | AIH-PBC | 2D-SWE | F2-F4 | 0.748 (0.668-0.817) | Acceptable |
| F3-F4 | 0.818 (0.745-0.878) | Good | ||||
| F4 | 0.879 (0.813-0.928) | Good | ||||
| Yan et al[69] | 148 | AIH-PBC | 2D-SWE | ≥ F2 | 0.91 (0.85-0.96) | Excellent |
| ≥ F3 | 0.97 (0.94-0.99) | Excellent | ||||
| F4 | 0.96 (0.92-0.99) | Excellent | ||||
| Yoo et al[52] | 115 | CLD | 2D-SWE | F2 | 0.851 (0.773-0.911) | Good |
| F3 | 0.917 (0.851-0.960) | Excellent | ||||
| F4 | 0.889 (0.817-0.940) | Good | ||||
| Yamaoka et al[54] | 116 | CLD | 2D-SWE | ≥ F2 | 0.85 (0.773-0.911) | Good |
| ≥ F3 | 0.91 (0.81-0.97) | Excellent | ||||
| F4 | 0.88 (0.79-1.00) | Good | ||||
| Zhou et al[64] | 116 | NAFLD | 2D-SWE | ≥ F2 | 0.86 (0.77-0.94) | Good |
| ≥ F3 | 0.89 (0.81-0.97) | Good | ||||
| F4 | 0.90 (0.79-1.00) | Good |
Innovative 2D-SWE modifications (Table 3) are generally more effective than 2D-SWE.
| Ref. | Number of patients | Disease/diagnose | Diagnostic method | Fibrosis stage | AUROC (CI) | Assessment |
| Yoo et al[52] | 203 | CLD | 2D-SWE LOGIQ E9 | F2 | 0.910 (0.871-0.944) | Excellent |
| F3 | 0.897 (0.844-0.939) | Good | ||||
| F4 | 0.931 (0.874-0.969) | Excellent | ||||
| 2D-SWE LOGIQ S8 | F2 | 0.908 (0.874-0.940) | Excellent | |||
| F3 | 0.905 (0.864-0.954) | Excellent | ||||
| F4 | 0.931 (0.889-0.964) | Excellent | ||||
| Kavak et al[29] | 253 | CHB | 2D-SWE-MPG | F > 2 | 0.956 (0.920-0.991) | Excellent |
| F > 3 | 0.978 (0.945-1.000) | Excellent | ||||
| Garcovich et al[26] | 253 | CLD | 2D-SWE-SSI | F ≥ 2 | 0.96 (0.93-0.99) | Excellent |
| F ≥ 3 | 0.98 (0.97-1.00) | Excellent | ||||
| F4 | 0.99 (0.98-1.00) | Excellent | ||||
| Cassinotto et al[23] | 577 | NAFLD | 2D-SWE-SSI | F ≥ 2 | 0.84 (0.81-0.88) | Exclusion |
| F ≥ 3 | 0.88 (0.84-0.91) | Excellent | ||||
| F4 | 0.86 (0.82-0.90) | Excellent |
Excluding the results of Garcovich et al[26] which reported AUROCs nearing 1, the mean AUROC across all studies was 0.91 (SD 0.04), with a median of 0.908 in 2023. The highest AUROC value (0.978) was reported by Kavak et al[29] using 2D-SWE-MPG, for F > 3, in patients with CHB, while the lowest was recorded by Cassinotto et al[23] using 2D-SWE-SSI in patients with NAFLD for F ≥ 2, which still represents a high value (0.84) in 2021. The study by Garcovich et al[26] involving 253 patients with CLD using 2D-SWE-SSI, demonstrated exceptionally high AUROC values, of 0.96, 0.98, 0.99 for F ≥ 2, F ≥ 3, and F4, respectively in 2023. These results approach 1 in the upper confidence intervals, significantly exceeding the outcomes reported in most other studies.
The AUROC values for 2D-SWE modifications ranged from 0.84 to 0.978, with some studies, like Garcovich et al[26], exceeding this range. These values suggest that 2D-SWE holds significant promise for development and, in certain forms, is highly effective in distinguishing between the different stages of liver fibrosis. AUROC values consistently above 0.9, often observed in datasets, indicate excellent diagnostic accuracy. However, similar higher values are also noted in other data subsets (Tables 2, 3, 4, 5, 6, 7, 8 and 9). Drawing more categorical conclusions will require an increased number of comparable studies.
| Ref. | Number of patients | Disease/diagnose | Diagnostic method | Fibrosis stage | AUROC (CI) | Assessment |
| Lee et al[31] | 87 | CLD | p-SWE | ≥ F2 | 0.872 (0.777-0.937) | Good |
| F4 | 0.886 (0.794-0.947) | Good | ||||
| Fang et al[25] | 121 | CLD | p-SWE | ≥ F2 | 0.855 (0.778-0.932) | Good |
| ≥ F3 | 0.890 (0.826-0.954) | Good | ||||
| Saadi et al[41] | 216 | CLD | p-SWE | F0-F1 | 0.744 (0.67-0.82) | Acceptable |
| F3 | 0.82 (0.74-0.89) | Good | ||||
| F4 | 0.95 (0.91-0.98) | Acceptable | ||||
| Atzori et al[22] | 160 | CLD | p-SWE - Philips ElastPQ | F0/1 | 0.828 (0.762-0.895) | Good |
| F3/4 | 0.812 (0.734-0.889) | Good | ||||
| F4 | 0.856 (0.753-0.938) | Good | ||||
| Atzori et al[22] | 160 | CLD | p-SWE - Siemens VTQ | F0/1 | 0.741 (0.659-0.823) | Acceptable |
| F3/4 | 0.782 (0.702-0.861) | Acceptable | ||||
| F4 | 0.826 (0.734-0.918) | Good | ||||
| Roccarina et al[40] | 671 | NAFLD | p-SWE by ElastPQ | F > 1 | 0.835 (0.72-0.93) | Good |
| F > 2 | 0.831 (0.78-0.90) | Good | ||||
| F > 3 | 0.864 (0.82-0.93) | Good | ||||
| F = 4 | 0.952 (0.92-0.99) | Excellent | ||||
| Garcovich et al[26] | 253 | CLD | X + p-SWE | F ≥ 2 | 0.96 (0.93-0.99) | Excellent |
| F ≥ 3 | 0.98 (0.97-1.00) | Excellent | ||||
| F4 | 0.99 (0.98-1.00) | Excellent |
| Ref. | Number of patients | Disease/diagnose | Diagnostic method | Fibrosis stage | AUROC (CI) | Assessment |
| Gatos et al[27] | 152 | CLD | SWE | F ≥ F1 | 0.9621 | Excellent |
| F ≥ F2 | 0.9931 | Excellent | ||||
| F ≥ F3 | 0.9835 | Excellent | ||||
| F = F4 | 0.9656 | Excellent | ||||
| F0 | 0.995 (0.988-1.00) | Excellent | ||||
| F1 | 0.676 (0.589-0.763) | Unacceptable | ||||
| F2 | 0.507 (0.409-0.605) | Unacceptable | ||||
| F3 | 0.708 (0.621-0.795) | Good | ||||
| F4 | 0.932 (0.889-0.975) | Excellent | ||||
| Laroia et al[30] | 124 | CLD | SWE | Combined F1 + F2 | 0.612 (0.516-0.708) | Unacceptable |
| Combined F3 + F4 | 0.961 (0.933-0.990) | Excellent | ||||
| Ogino e t al[37] | 107 | NAFLD | SWE | F2 | 0.88 | Good |
| F3 | 0.87 | Good | ||||
| F4 | 0.92 | Excellent | ||||
| Zhang et al[70] | 100 | NAFLD | SWE | ≥ 1 | 0.65 (0.54-0.76) | Unacceptable |
| ≥ 2 | 0.81 (0.71-0.91) | Good | ||||
| ≥ 3 | 0.85 (0.74-0.96) | Good | ||||
| 4 | 0.91 (0.79-1.00) | Excellent | ||||
| Wang et al[49] | 210 | HCC | SWE | ≥ F2 | 0.895 (0.842-0.947) | Good |
| ≥ F3 | 0.877 (0.826-0.927) | Good | ||||
| = F4 | 0.854 (0.803-0.905) | Good | ||||
| Zougmoré et al[55] | 476 | CLD | SWE | ≥ F2 | 0.91 (0.88-0.96) | Excellent |
| ≥ F3 | 0.93 (0.89-0.97) | Excellent | ||||
| ≥ F4 | 0.96 (0.94-0.98) | Excellent | ||||
| Kim et al[67] | 60 | NASH | SWE | ≥ F1 | 0.777 (0.653-0.777) | Acceptable |
| ≥ F2 | 0.747 (0.611-0.854) | Acceptable | ||||
| ≥ F3 | 0.861 (0.742-0.940) | Good | ||||
| ≥ F4 | 0.846 (0.730-0.926) | Good | ||||
| Yang et al[50] | 106 | CLD | SWE | F0 vs F1-3 | 0.91 | Excellent |
| F0-1 vs F2-4 | 0.84 | Good | ||||
| F0-2 vs F3-4 | 0.79 | Acceptable | ||||
| F0-3 vs F4 | 0.76 | Acceptable |
| Ref. | Number of patients | Disease/diagnose | Diagnostic method | Fibrosis stage | AUROC (CI) | Assessment |
| Patidar et al[39] | 127 | Diffuse liver diseases | RT-SWE | F0-F1 | 0.867 (0.72-1) | Good |
| F1-F2 | 0.955 (0.906-1 | Excellent | ||||
| F2-F3 | 0.946 (0.883-1) | Excellent | ||||
| F3-F4 | 0.93 (0.633-1) | Excellent | ||||
| Wang et al[48] | 524 | CHB | STQ | F > 4 | 0.86 | Good |
| F > 2 | 0.73 | Acceptable | ||||
| Taibbi et al[46] | 56 | NAFLD | SWE (10 measurements) | F2-F4 | 0.787 (0.646-0.927) | Acceptable |
| F3-F4 | 0.797 (0.659-0.935) | Acceptable | ||||
| SWE (5 measurements) | F2-F4 | 0.809 (0.676-0.942) | Good | |||
| F3-F4 | 0.809 (0.684-0.933) | Good | ||||
| SWE (3 measurements) | F2-F4 | 0.714 (0.560-0.869) | Acceptable | |||
| F3-F4 | 0.736 (0.587-0.885) | Acceptable |
| Ref. | Number of patients | Disease/diagnose | Diagnostic method | Fibrosis stage | AUROC (CI) | Assessment |
| Mendoza et al[34] | 200 | NAFLD | TE | ≥ F2 | 0.76 (0.64-0.88) | Acceptable |
| ≥ F3 | 0.72 (0.63-0.82) | Acceptable | ||||
| F4 | 0.89 (0.78-1.00) | Good | ||||
| Patidar et al[39] | 127 | Diffuse liver diseases | TE | F0-F1 | 0.824 (0.72-1) | Good |
| F1-F2 | 0.935 (0.906-1) | Good | ||||
| F2-F3 | 0.964 (0.883-1) | Good | ||||
| F3-F4 | 0.979 (0.633-1) | Good | ||||
| Seyrek et al[42] | 146 | CLD | TE | > F2 | 0.79 (0.65-0.94) | Acceptable |
| Taibbi et al[46] | 56 | NAFLD | TE | F2-F4 | 0.719 (0.572-0.867) | Acceptable |
| F3-F4 | 0.799 (0.646-0.952) | Acceptable | ||||
| Yoo et al[52] | 115 | CLD | TE | F2 | 0.859 (0.781-0.916) | Good |
| F3 | 0.881 (0.807-0.934) | Good | ||||
| F4 | 0.938 (0.877-0.974) | Excellent | ||||
| Kim et al[67] | 60 | NASH | TE | ≥ F1 | 0.733 (0.603-0.839) | Acceptable |
| ≥ F2 | 0.828 (0.709-0.913) | Good | ||||
| ≥ F3 | 0.869 (0.756-0.942) | Good | ||||
| ≥ F4 | 0.891 (0.783-0.957) | Good |
| Ref. | Number of patients | Disease/diagnose | Diagnostic method | Fibrosis stage | AUROC (CI) | Assessment |
| Cassinotto et al[23] | 577 | NAFLD | VCTE | F ≥ 2 | 0.80 (0.76-0.84) | Acceptable |
| F ≥ 3 | 0.82 (0.78-0.86) | Good | ||||
| F4 | 0.85 (0.80-0.90) | Good | ||||
| Gatos et al[27] | 152 | CLD | VCTE | F ≥ F1 | 0.9900 | Excellent |
| F ≥ F2 | 0.9767 | Excellent | ||||
| F ≥ F3 | 0.9651 | Excellent | ||||
| F = F4 | 0.9645 | Excellent | ||||
| Imajo et al[66] | 231 | NAFLD | VCTE | F4 | 0.87 (0.80-0.91) | Good |
| Nogami et al[36] | 163 | NAFLD | VCTE | ≥ F2 | 0.855 (0.83-0.94) | Good |
| ≥ F3 | 0.925 (0.90-0.95) | Excellent | ||||
| F4 | 0.88 (0.87-0.89) | Good | ||||
| Sharpton et al[43] | 114 | CLD | VCTE | F2-F4 | 0.86 (0.80-0.93) | Good |
| F3-F4 | 0.91 (0.82-0.99) | Excellent | ||||
| F4 | 0.96 (0.91-1.00) | Excellent | ||||
| Atzori et al[22] | 160 | CLD | VCTE | F0/1 | 0.810 (0.746-0.884) | Good |
| F3/4 | 0.841 (0.771-0.910) | Good | ||||
| F4 | 0.939 (0.896-0.982) | Excellent | ||||
| Damjanovska et al[24] | 93 | NAFLD | VCTE | F4 | 0.77 (0.66-0.88) | Acceptable |
| Mikolasevic et al[35] | 179 | NAFLD | VCTE | ≥ F3 | 0.98 | Excellent |
| F4 | 0.98 | Excellent | ||||
| Roccarina et al[40] | 671 | NAFLD | VCTE | F > 1 | 0.792 (0.60-0.91) | Acceptable |
| F > 2 | 0.849 (0.78-0.91) | Good | ||||
| F > 3 | 0.851 (0.79-0.91) | Good | ||||
| F = 4 | 0.911 (0.83-0.96) | Excellent | ||||
| Zougmoréet al[55] | 476 | CLD | VCTE | ≥ F2 | 0.86 (0.81-0.91) | Good |
| ≥ F3 | 0.89 (0.85-0.93) | Good | ||||
| ≥ F4 | 0.90 (0.86-0.94) | Good |
| Ref. | Number of patients | Disease/diagnose | Diagnostic method | Fibrosis stage | AUROC (CI) | Assessment |
| Ogino et al[37] | 107 | NAFLD | S-map | F2 | 0.75 | Acceptable |
| F3 | 0.80 | Acceptable | ||||
| F4 | 0.85 | Good | ||||
| Gatos et al[27] | 152 | CLD | STE | F ≥ F1 | 0.9683 | Excellent |
| F ≥ F2 | 0.9834 | Excellent | ||||
| F ≥ F3 | 0.9763 | Excellent | ||||
| F = F4 | 0.9509 | Excellent | ||||
| Wang et al[48] | 524 | CHB | STE | F > 4 | 0.87 | Good |
| F > 2 | 0.76 | Acceptable | ||||
| Yang et al[51] | 102 | AILD | STE | F2 | 0.82 (0.73-0.89) | Good |
| F3 | 0.87 (0.78-0.93) | Good | ||||
| F4 | 0.91 (0.83-0.96) | Excellent | ||||
| Yang et al[50] | 106 | CLD | STE | F0 vs F1-3 | 0.92 | Excellent |
| F0-1 vs F2-4 | 0.84 | Good | ||||
| F0-2 vs F3-4 | 0.77 | Acceptable | ||||
| F0-3 vs F4 | 0.71 | Acceptable | ||||
| Wang et al[49] | 210 | HCC | SWD | ≥ F2 | 0.857 (0.784-0.920) | Good |
| ≥ F3 | 0.815 (0.757-0.874) | Good | ||||
| F4 | 0.791 (0.730-0.852) | Acceptable |
These data cover a less extensive range of liver diseases, including CLD, CHB, and NAFLD. Each disease showed a strong response to imaging with 2D-SWE modifications, which indicates that this method is versatile and applicable to various liver diseases, even when new modalities are being tested.
The use of p-SWE to assess fibrosis indicated the overall diagnostic performance of p-SWE, as illustrated by the AUROC values ranging from 0.741 to 0.99. This range suggests varying levels of diagnostic accuracy but generally indicates a good-to-excellent ability to detect different stages of liver fibrosis.
The diseases studied-CLD and NAFLD-did not provide a complete picture of the performance of this method for different diseases.
Moreover, early fibrosis yielded lower AUROC values, suggesting that although p-SWE is useful, it may be less sensitive in detecting mild fibrotic changes. Values in this category often range from 0.741-0.828.
For moderate-to-severe fibrosis, starting at F2, the AUROC values generally increase with the severity of fibrosis, showing improved diagnostic performance for these conditions (ranging from 0.831 to 0.99). Advanced fibrosis (F3-F4) showed very high AUROC values, especially for detecting F4, often reaching almost perfect accuracy (close to 1.00), suggesting excellent sensitivity and specificity.
Some studies refer to the equipment used to modify p-SWE (Philips ElastPQ, Siemens VTQ). There was some variability in the AUROC values depending on the technology used, suggesting that the specific implementation of p-SWE may have an impact on diagnostic accuracy. For example, the Philips ElastPQ tended to have slightly higher AUROC values than the Siemens VTQ in the same study by Atzori et al[22] in 2024.
Among the studies analyzing p-SWE (Table 4), excluding X + p-SWE, the mean AUROC value was 0.8438 (SD: 0.0587), with a median of 0.835. The lowest value (0.741) was obtained by Atzori et al[22] using Siemens VTQ for differentiating F0/F1 in patients with CLD in 2024. The highest value (0.952) was reported by Roccarina et al[40] in patients with NAFLD using p-SWE-ElastPQ with F = 4. X + p-SWE, as assessed by Garcovich et al[26], demonstrated AUROC values close to 1 in patients with CLD.
For classical SWE, the AUROC values varied considerably, ranging from 0.507 to 0.995. Therefore, the diagnostic accuracy levels vary depending on the stage of fibrosis and other factors such as disease type and patient population.
The early stages (F0, F1, and F2) generally had lower AUROC values, indicating a poorer discriminatory ability. For example, stages F0 and F1 showed moderate-to-high AUROC values in some studies, but F2 dropped significantly in some studies (0.507). Furthermore, advanced stages (F3 and F4) had much better AUROC values, often exceeding 0.9, suggesting that SWE is more reliable in detecting more advanced stages of fibrosis.
The diseases studied encompassed CLD, NAFLD, HCC, and NASH. The performance of SWE varied, and generally better results were achieved in more severe stages of fibrosis in these diseases.
For pure and unmodified SWE (Table 5), the AUROC values ranged from 0.507 to 0.995, both reported by Gatos et al[27]. The lowest value (0.507) was for detecting F2 and the highest (0.995) was for F0, although the F0 AUROC value was not statistically significant. The mean AUROC across all studies was 0.848 (SD = 0.119).
For the modified SWE observational studies (Table 6), techniques such as RT-SWE and multiple-measurement SWE demonstrated higher AUROCs compared to SWE; however, the differences were not significant. Taibbi et al[46] suggested that increasing the number of measurements enhances SWE effectiveness. Meanwhile, STQ assessed in a single study did not achieve better results than SWE.
The TE studies included 56 to 200 patients, and the reported AUROC values ranged from 0.72 to 0.979. The study encompassed a variety of liver diseases, including NAFLD, CLD, and diffuse liver disease, an unexplored condition in previous research using these techniques. Lower fibrosis stages (including F2) had lower AUROC values, whereas more advanced stages (especially F4) showed higher AUROC values, which is consistent with the results presented thus far.
The mean AUROC values across all fibrosis stages studied was 0.846 (SD = 0.084), indicating a good overall efficacy of TE (Table 7).
For studies on VCTE, the sample size ranged from 93 to 671 patients. The minimum AUROC value was 0.77 reported by Damjanovska et al[24] for stage F4 in 2022, whereas the maximum was 0.9900 noted by Gatos et al[27] for stage F ≥ F1, an unusually high result.
The dataset included studies involving patients with CLD and NAFLD. Generally, higher stages of liver fibrosis (except in the study by Gatos et al[27]) showed higher AUROC values, indicating a better diagnostic performance with increasing fibrosis severity.
The calculated mean AUROC across all studies and stages of liver fibrosis was approximately 0.889 (SD = 0.063).
Data on other TE techniques, including studies using ViTE and FibroTouch, encompassed studies with sample sizes ranging from 85 to 237 patients. These studies primarily focused on CLD, T2DM, and NAFLD.
AUROC values ranged from 0.71 (Qu et al[60] for stages ≥ F2 and ≥ F3) to 0.9846 (Gatos et al[27] for stages F ≥ F3 in 2022). These results showed a tendency for AUROC values to increase with more advanced stages of fibrosis. The mean AUROC was 0.847 (SD = 0.092).
ViTE appeared to be more effective than FibroTouch with means AUROC values of 0.897 (SD = 0.072) and 0.780 (SD = 0.072).
The STE dataset (Table 10) includes results from studies using both ’pure’ STE and its modifications, namely S-map and SWD. These studies included patients with NAFLD, CLD, AILD, CHB, and HCC.
| Ref. | Number of patients | Disease/diagnose | Diagnostic method | Fibrosis stage | AUROC (CI) | Assessment |
| Gatos et al[27] | 152 | CLD | ViTE | F ≥ F1 | 0.9481 | Excellent |
| F ≥ F2 | 0.9698 | Excellent | ||||
| F ≥ F3 | 0.9846 | Excellent | ||||
| F = F4 | 0.9524 | Excellent | ||||
| Yang et al[50] | 106 | CLD | ViTE | F0 vs F1-3 | 0.88 | Good |
| F0-1 vs F2-4 | 0.84 | Good | ||||
| F0-2 vs F3-4 | 0.80 | Acceptable | ||||
| F0-3 vs F4 | 0.80 | Acceptable | ||||
| Yu et al[62] | 85 | T2DM | FibroTouch | ≥ F2 | 0.76 (0.66-0.86) | Acceptable |
| ≥ F3 | 0.81 (0.71-0.91) | Good | ||||
| ≥ F4 | 0.92 (0.85-1.00 | Excellent | ||||
| Qu et al[60] | 237 | NAFLD | FibroTouch | ≥ F2 | 0.71 | Acceptable |
| ≥ F3 | 0.71 | Acceptable | ||||
| F4 | 0.77 | Acceptable |
The number of patients ranged from 102 to 524. The lowest AUROC value of 0.71 was reported by Yang et al[50] for differentiating liver cirrhosis (F0-3 vs F4), while the highest (0.9834) was obtained by Gatos et al[27] for F ≥ F2. Higher grades of fibrosis generally showed higher AUROC values.
Regarding steatosis, the provided dataset (Table 11) includes studies that evaluated the diagnostic performance of different elastography methods in patients primarily diagnosed with NAFLD, alcoholic liver disease, CLD, and NASH.
| Ref. | Number of patients | Disease | Diagnostic method | Steatosis stage | AUROC (CI) | Assessment |
| Nogami et al[36] | 163 | NAFLD | VCTE (CAP) | ≥ S1 | 0.89 (0.73-0.95) | Good |
| S2 | 0.77 (0.82-0.82) | Acceptable | ||||
| S3 | 0.69 (0.75-0.75) | Unacceptable | ||||
| Liu et al[59] | 100 | NAFLD | ATI | S ≥ 1 | 0.762 | Acceptable |
| S ≥ 2 | 0.774 | Acceptable | ||||
| S ≥ 3 | 0.784 | Acceptable | ||||
| SWE | S ≥ 1 | 0.764 | Acceptable | |||
| S ≥ 2 | 0.783 | Acceptable | ||||
| S ≥ 3 | 0.802 | Good | ||||
| Qu et al[60] | 237 | NAFLD | UAP | S1 | 0.88 | Good |
| S2 | 0.93 | Excellent | ||||
| S3 | 0.88 | Good | ||||
| Zhao et al[65] | 34 | MAFLD | NLV | S ≥ 1 | 0.875 (0.716-0.963) | Good |
| S ≥ 2 | 0.735 (0.556-0.871) | Acceptable | ||||
| S ≥ 3 | 0.583 (0.402-0.749) | Unacceptable | ||||
| NLV-SD | S ≥ 1 | 0.900 (0.748-0.976) | Good | |||
| S ≥ 2 | 0.745 (0.567-0.878) | Acceptable | ||||
| S ≥ 3 | 0.603 (0.422-0.766) | Unacceptable | ||||
| Kjaergaard et al[57] | 137 | ALD/NAFLD | B-mode ratio | S ≥ 1 | 0.79 (0.70-0.88) | Acceptable |
| S ≥ 2 | 0.76 (0.66-0.85) | Acceptable | ||||
| S ≥ 3 | 0.74 (0.62-0.86) | Acceptable | ||||
| Zhou et al[64] | 139 | NAFLD | 2D-SWE | Steatohepatitis | 0.88 | Good |
| Yazdani et al[61] | 49 | NAFLD | SWA | S0 vs ≥ S1 | 0.99 | Excellent |
| ≤ S1 vs ≥ S2 | 0.98 | Excellent | ||||
| ≤ S2 vs S3 | 0.93 | Excellent | ||||
| Yu et al[62] | 85 | MAFLD | CAP (FibroTouch) | S ≥ S1 | 0.84 (0.67-1.01) | Good |
| S ≥ S2 | 0.88 (0.81-0.95) | Good | ||||
| S = S3 | 0.89 (0.82-0.95) | Good | ||||
| Hsu et al[56] | 28 | CLD | ATI | S ≥ 1 | 0.97 (0.83-1.00) | Acceptable |
| S ≥ 2 | 0.99 (0.86-1.00) | Acceptable | ||||
| S = 3 | 0.97 (0.82-1.00) | Acceptable | ||||
| Kim et al[67] | 60 | NASH | HRI | ≥ S2 | 0.871 (0.783-0.956) | Good |
| ≥ S3 | 0.851 (0.735-0.930) | Good | ||||
| Kuroda et al[58] | 202 | NAFLD | 2D-SWE + UGAP | ≥ S1 | 0.89 (P < 0.05) | Good |
| ≥ S2 | 0.91 (P < 0.05) | Excellent | ||||
| S3 | 0.92 (P < 0.05) | Excellent | ||||
| Welman et al[63] | 76 | Not specified or mixed | ATI | S1-S3 | 0.85 (0.75-0.91) | Good |
| S2-S3 | 0.91 (0.83-0.99) | Excellent | ||||
| S0-S1 vs S2-S3 | 0.89 (0.65-0.98) | Good |
The number of patients in these studies ranged from 28 to 237. The lowest AUROC of 0.583 was reported by Zhao et al[65] for S ≥ 3, while the maximum AUROC of 0.99 was reported by Yazdani et al[61] for differentiating S0 from ≥ S1.
Overall, the diagnostic performance (measured by the AUROC index) tends to vary across different stages of steatosis. However, unlike fibrosis, no consistent relationship was observed. Some studies have shown a decrease in the AUROC values with increasing steatosis grades, whereas others maintained high AUROC values across all stages.
For 2D-SWE diagnostic efficacy (AUROCs for fibrosis detection), not all studies assessed or reported on every stage of fibrosis, with some focusing primarily on higher stages (usually F2 and above). This may be due to the lack of statistical significance for lower stages of the disease owing to the small sample sizes. Despite this limitation, a clear trend is observed with advanced stages of fibrosis tending to yield more accurate results. Therefore, the synthesis carries a moderate risk of bias due to selective reporting of higher fibrosis stages, potentially limiting a comprehensive assessment of diagnostic effectiveness across all stages.
Similarly, for 2D-SWE modifications, there could be incomplete reporting across all fibrosis stages and a moderate risk of bias if the early stages of fibrosis (F0 and F1) were not consistently evaluated, which could imply a selective emphasis on more advanced fibrosis.
The p-SWE studies likely focused on selected stages of fibrosis without comprehensive coverage from F0 to F4. This introduces a moderate-to-high risk of bias if the full spectrum of fibrosis is not assessed, potentially overlooking diagnostic performance in the early stages.
For SWE, some studies may not report on early stages, such as F0 and F1. This exclusion introduces a high risk of bias and may misrepresent the diagnostic utility of SWE across the progression of liver fibrosis.
Modified or specific SWE modalities also demonstrated similar gaps in stage coverage, with a focus possibly skewed toward the mid to late stages. Consequently, a moderate risk of bias was observed.
TE diagnostic efficacy is likely incomplete in the coverage of all fibrosis stages for the same reasons, with a moderate-to-high risk of bias due to the potential exclusion of early fibrosis stages.
Missing VCTE data for early fibrosis stages in some studies led to a moderate risk of bias, as excluding early stages could skew the understanding of the effectiveness of VCTE in early detection.
For STE techniques, incomplete assessment across all stages of fibrosis remains a concern. The exclusion of early stages, if frequent, presents a high risk of bias.
For STE and related techniques, early stages are often not reported, similar to that observed in other tables, introducing moderate-to-high risk of bias, potentially limiting the reliability of conclusions drawn about their diagnostic performance in early fibrosis detection.
As for steatosis, the data were across a range of steatosis stages, from S0 (only one study) to S3, and most studies reported on stages S1 to S3, with varying degrees of granularity. Some studies specifically addressed all three stages (S1, S2, and S3), whereas others focused on combined categories or provided less detail for certain stages. Only one study[61] reported on S0. A moderate-to-high risk of bias may be assumed owing to inconsistent reporting across the full spectrum of steatosis stages, while the underrepresentation or absence of data for S0 in most studies could skew the overall understanding of the diagnostic efficacy of US techniques, potentially leading to an overestimation of their effectiveness in early steatosis detection.
2D-SWE has moderate to high certainty of evidence. Most studies reported significant AUROCs with confidence intervals; however, not all reported statistical significance (P values). The absence of P values in some results may slightly reduce certainty, owing to potential biases or unaccounted variability.
For 2D-SWE modifications, the certainty of evidence was high. The AUROCs were generally very high (often > 0.9), indicating excellent diagnostic performance. The high AUROCs, along with the reported confidence intervals and P values, highlighted the improvements over standard 2D-SWE. However, some studies lacked P value assessments.
For the diagnostic efficacy of p-SWE, the certainty of evidence was moderate. The range of the AUROCs was broader, varying from moderate to high, reflecting the variability in diagnostic accuracy across different studies and research setups. However, the lower end of AUROCs and the missing P values for some entries, suggest the need for cautious interpretation.
For the diagnostic efficacy of SWE, the certainty of evidence was questionable (low to moderate). AUROCs varied widely, with some results indicating low diagnostic accuracy (as low as 0.507). This variability combined with the inconsistencies across studies and the absence of P values in some cases, affects the overall certainty of the evidence.
For modified or specific SWE modalities the certainty of evidence was moderate. Most modifications demonstrated good diagnostic efficacy, but the evidence base is small and the variability in AUROC scores suggests that further validation is needed. P values were not provided in some studies.
TE diagnostic efficacy has a moderate to high certainty of evidence. TE generally shows very good to excellent AUROC scores, particularly for higher stages of fibrosis; however, a relatively narrower scope of disease stages (often only advanced stages are thoroughly reported) may limit its generalizability. P values were not provided in some studies.
For VCTE, the certainty of evidence was high, with consistently high AUROCs across multiple studies. P values were not provided in some studies, although the data on confidence intervals were nearly complete.
The diagnostic efficacy of other STE techniques demonstrated moderate certainty of evidence. Variability in AUROC scores and the innovative nature of the techniques suggests that, while promising, these results should be interpreted with caution, particularly as they may represent early-stage evaluations of the technology.
The diagnostic efficacy of STE and other related techniques for steatosis also shows moderate certainty of evidence. High AUROCs in some studies are promising; however, the limited number of studies for each modality reduces overall confidence in the findings.
The risk of bias across the four domains (D1, D2, D3, and D4) varied among studies. Most showed low risk in all domains, with generally acceptable methodological quality. However, certain studies exhibited high or uncertain risks in specific domains owing to limitations in these aspects of the study design or execution. Not all the researchers ensured that the study sample size was adequate. In some cases (Table 12), we also had radiological concerns regarding the lack of comprehensive descriptions that would allow for the assessment of the quality of the measurements performed using the US.
| Ref. | Risk of bias by domain | Applicability by domain | |||||
| D1 | D2 | D3 | D4 | D1 | D2 | D3 | |
| Aksakal et al[20] | Low | Low | Low | Low | Low | Low | Low |
| Alcantara-Diaz et al[21] | High | Uncertain | Uncertain | Low | High | Low | Low |
| Atzori et al[22] | Low | Low | Low | Low | Low | Low | Low |
| Cassinotto et al[23] | Low | Low | Low | Low | Low | Low | Low |
| Damjanovska et al[24] | High | Uncertain | Uncertain | High | Low | Low | Low |
| Fang et al[25] | Low | Uncertain | Uncertain | Low | Low | Low | Low |
| Garcovich et al[26] | Low | Low | Low | Low | Low | Low | Low |
| Gatos et al[27] | Low | Low | Low | Low | Low | Low | Low |
| Hsu et al[56] | Low | Low | Low | Low | Low | Low | Low |
| Imajo et al[66] | Low | Low | Low | Low | Low | Low | Low |
| Jocius et al[28] | Low | Uncertain | Uncertain | Low | Low | Low | Low |
| Kavak et al[29] | Uncertain | Uncertain | Uncertain | Uncertain | Low | Low | Low |
| Kim et al[67] | Low | Low | Low | Low | Low | Low | Low |
| Kjaergaard et al[57] | Low | Low | Low | Low | Low | Low | Low |
| Kuroda et al[58] | Low | Low | Low | Low | Low | Low | Low |
| Laroia et al[30] | Low | Low | Low | Low | Low | Low | Low |
| Lee et al[31] | Low | Low | Low | Low | Low | Low | Low |
| Liu et al[59] | Low | Uncertain | Uncertain | Low | Low | Low | Low |
| Manesis et al[32] | Uncertain | Uncertain | Uncertain | Low | Low | Low | Low |
| Martonik et al[33] | Low | Low | Low | Low | Low | Low | Low |
| Mendoza et al[34] | Low | Uncertain | Uncertain | Low | Low | Low | Low |
| Mikolasevic et al[35] | Low | Uncertain | Uncertain | Low | Low | Low | Low |
| Nogami et al[36] | Uncertain | Low | Low | Low | Low | Low | Low |
| Ogino et al[37] | Low | Low | Low | Uncertain | Low | Low | Low |
| Paisant et al[38] | Low | Low | Low | Low | Low | Low | Low |
| Patidar et al[39] | Low | Low | Low | Uncertain | Low | Low | Low |
| Prieto Ortiz et al[68] | High | Uncertain | Uncertain | High | High | High | High |
| Qu et al[60] | Low | Low | Low | Low | Low | Low | Low |
| Roccarina et al[40] | Low | Low | Low | Low | Low | Low | Low |
| Saadi et al[41] | Low | Low | Low | Low | Low | Low | Low |
| Seyrek et al[42] | Low | Low | Low | Low | Low | Low | Low |
| Sharpton et al[43] | Low | Low | Low | Low | Low | Low | Low |
| Soh et al[44] | Low | Uncertain | Uncertain | Low | Low | Low | Low |
| Song et al[45] | Low | Low | Low | Low | Low | Low | Low |
| Taibbi et al[46] | High | Low | Low | High | Low | Low | Low |
| Wang et al[47] | High | Uncertain | Uncertain | Low | Low | Low | Low |
| Wang et al[48] | Uncertain | Low | Low | Uncertain | Low | Low | Low |
| Wang et al[49] | Low | Low | Low | Low | Low | Low | Low |
| Welman et al[63] | High | Low | Low | High | Low | Low | Low |
| Yamaoka et al[54] | Low | Low | Low | Low | Low | Low | Low |
| Yan et al[69] | Low | Low | Low | Low | Low | Low | Low |
| Yang et al[50] | High | Low | Low | Uncertain | Low | Low | Low |
| Yang et al[51] | High | Low | Low | Uncertain | Low | Low | Low |
| Yazdani et al[61] | Low | Low | Low | Low | Low | Low | Low |
| Yoo et al[53] | High | Low | Low | Uncertain | Low | Low | Low |
| Yoo et al[52] | High | Low | Low | Uncertain | Low | Low | Low |
| Yu et al[62] | Low | Low | Low | Low | Low | Low | Low |
| Zhang et al[70] | Low | Low | Low | Low | Low | Low | Low |
| Zhao et al[65] | Low | Low | Low | Low | Low | Low | Low |
| Zhou et al[64] | Low | Low | Low | Low | Low | Low | Low |
| Zougmoré et al[55] | High | Low | Low | Low | Low | Low | Low |
The applicability of the findings across the three domains (D1, D2, and D3) generally indicated low concern as they were relevant to the review aim. Notably, one study[68] displayed high concerns about both bias and applicability across all domains, which may affect the interpretation of its findings.
No additional analyses were performed.
This review provides a comprehensive evaluation of modern US techniques for diagnosing liver steatosis and fibrosis compared to biopsy. The evidence confirms that Modern US techniques, especially SWE modifications, demonstrate high diagnostic accuracy (with AUROCs reaching nearly 1.0) in assessing liver fibrosis. For steatosis, newer US methods have also shown promising results, albeit with smaller datasets. The studies incorporated mostly exhibited a low risk of bias, enhancing the credibility of US techniques as reliable and noninvasive diagnostic tools.
Unfortunately, the scientific evidence obtained has numerous limitations that negatively affect its interpretation. The studies had varied designs, including sample size, patient demographics, and clinical settings. This affected the generalizability of the results. The focus on specific liver diseases, predominantly CLD and NAFLD, may not fully represent the efficacy of the diagnostic techniques for other liver conditions. There is a lack of research addressing autoimmune and neoplastic diseases (with a few exceptions), and data on viral and alcohol-related diseases do not cover all research methods.
Some studies failed to report critical metrics such as P values or confidence intervals, which are essential for assessing the statistical significance (and reliability of the findings). Not all studies provide a comprehensive assessment across all stages of fibrosis, and some focus primarily on advanced stages, which could bias the results and underestimate the diagnostic accuracy in early-stage fibrosis, which is crucial for timely intervention. Moreover, some studies had relatively small sample sizes, which limited the statistical power to detect a true effect, and increased the likelihood of random errors influencing the results. This would not pose a significant concern if there were enough studies to relate or synthesize with studies involving larger populations. Unfortunately, for most techniques, especially the more modern and promising ones, the number of available studies does not adequately compensate for the small size of the groups.
The review process has several limitations that may affect the power and generalizability of the conclusions drawn. All activities, including data extraction, risk of bias assessment, and initial article screening, were conducted by two separate researchers. Nevertheless, the lack of process automation caused by human factors may have been a limitation.
Although the search was not restricted to articles available in English, which is common in many systematic reviews, no studies in other languages were found to be relevant by title or abstract. However, this might be due to such studies not containing appropriately defined English keywords. At the discussion stage, it is challenging to determine why this was the case. Several studies published in Chinese and Spanish appeared in the search results but did not meet the inclusion/exclusion criteria. This could indicate that the scientific discourse in radiology has become so internationalized that studies on the most modern diagnostic techniques are rarely published in non-English languages. Alternatively, such studies might appear in databases with a delay due to technical processes.
The search was confined to PubMed, which is seen as a comprehensive source of medical literature, excluding other databases such as Scopus, Web of Science, and Embase, which might have omitted relevant studies. This justifies further research using multiple databases to identify additional articles. Moreover, the exclusion of automated tools for searching and screening might have increased the workload of a single researcher and could lead to human errors.
High AUROC values for some US techniques suggest that they are reliable for diagnosing different stages of liver fibrosis or fatty liver disease. They can already be used with high confidence, potentially reducing the reliance on invasive procedures such as biopsies.
Variability in diagnostic performance across stages of liver disease and US techniques means that specialist training is needed for healthcare professionals, especially because some conditions reduce diagnostic performance. Special attention should be paid to patients with coexisting fibrosis, steatosis, and obesity. Professionalism in examinations and a high load on the learning curve is essential for eliminating the uncertainties associated with the obtained results.
Similarly, the evaluation of the effect of the number of measurements showed that increasing the number of measurements improved the accuracy of the results obtained. Diagnostic recommendations and software design should aim to support a higher number of measurements over time, ideally exceeding five, and preferably reaching several dozens. This is especially important for less-studied and diagnostically less-reliable early-stages of liver fibrosis. Improved diagnostic accuracy not only provides a clearer picture of treatment progress to the hepatologist but also enhances the patient’s understanding, leading to more informed consent for continuing therapy.
These results may prompt an update of the clinical guidelines for the diagnosis and treatment of liver diseases. This applies particularly to the implementation of SWE and 2D-SWE modifications, which approach the accuracy of the results obtained with fine-needle biopsies.
Future studies should directly compare different US modalities to determine the most effective modality while maintaining the reference standard in the form of a biopsy, as long as it is ethically justified.
Regarding SWE, given the wide range of AUROC values, further research is needed to standardize SWE applications and investigate the reasons for the observed variability. Modified SWE technologies, especially RT-SWE and multiple-measurement SWE, suggest improvements over classical SWE. Similarly, p-SWE has shown a promising diagnostic accuracy. This was particularly evident in the moderate-to-severe liver fibrosis stages. The impact of new technologies
TE and VCTE consistently show high diagnostic accuracy, particularly in advanced stages of fibrosis. Further research should focus on early-stage fibrosis detection, in which the sensitivity appears lower, especially where current data seem lacking or inconclusive.
2D-SWE, given its promising AUROC values, especially with certain modifications, such as 2D-SWE-MPG, appears promising for liver fibrosis detection across all stages, including the early stages. However, some studies have suggested variability in the diagnostic performance among different patient groups (e.g., patients with obesity). This indicates that there is a need for more targeted research rather than focusing on the early stages of fibrosis.
For steatosis, the available data indicate less consistency in diagnostic performance compared to that of fibrosis, with some studies showing decreasing AUROC values as the steatosis grade increases. Therefore, there is an evident need for improvement. This might involve the development of new US techniques or the refinement of existing techniques. Research should aim to precisely determine the usefulness of elastography in patients with increasing degrees of steatosis. Furthermore, we need to determine whether the knowledge already accumulated from fibrosis research can be applied to improve the efficiency of steatosis detection.
Considering the high efficiency of modifications of US diagnostic methods, and the significant cost of advanced devices, it may be reasonable to explore modernizing and upgrading existing medical equipment by local forces in regions where providing every patient with access to state-of-the-art diagnostic methods is not feasible. This approach could help expand access to reliable diagnostics globally, ensuring equitable healthcare outcomes while leveraging available resources efficiently.
This review emphasizes the need for longitudinal studies to assess the effectiveness of US techniques over time as the effectiveness of diagnostics in the early stages of the disease is poorly understood. In terms of clinical implications, it appears that newer variants of 2D-SWE and advanced TE methods, even today, offer the potential to replace liver biopsy for more advanced fibrosis. They should already be included in therapeutic guidelines as primary diagnostic tools. It would be beneficial to use both 2D-SWE and ViTE modalities simultaneously; however, there are no studies on their combined effectiveness or algorithms that would enable reliable staging assessment in cases with conflicting results from different methods. Device manufacturers should be expected to facilitate conditions for obtaining simultaneous and multiple measurements, which will increase the certainty of the results. Unfortunately, the results of this review do not yet provide sufficient evidence for eliminating the use of liver biopsies in cases of fibrosis. The limited data available are insufficient to confirm that the reported very high effectiveness of noninvasive methods is error-free and consistently replicable. This highlights the need for further research, especially on new 2D-SWE modalities. Additionally, it is evident that physicians cannot yet rely on diagnostic results for steatosis to the same extent as those for liver fibrosis. This presents a significant obstacle to the goal of minimizing the use of liver biopsies. To address this challenge, there is a pressing need for intensified and focused research on methods that enable a more reliable assessment of steatosis.
| 1. | Mitra S, De A, Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol. 2020;5:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 336] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 2. | Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524-530.e1; quiz e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 790] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 3. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2296] [Article Influence: 382.7] [Reference Citation Analysis (0)] |
| 4. | Berger D, Desai V, Janardhan S. Con: Liver Biopsy Remains the Gold Standard to Evaluate Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Liver Dis (Hoboken). 2019;13:114-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | Saleh HA, Abu-Rashed AH. Liver biopsy remains the gold standard for evaluation of chronic hepatitis and fibrosis. J Gastrointestin Liver Dis. 2007;16:425-426. [PubMed] |
| 6. | Chowdhury AB, Mehta KJ. Liver biopsy for assessment of chronic liver diseases: a synopsis. Clin Exp Med. 2023;23:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 7. | Kouvari M, Valenzuela-Vallejo L, Guatibonza-Garcia V, Polyzos SA, Deng Y, Kokkorakis M, Agraz M, Mylonakis SC, Katsarou A, Verrastro O, Markakis G, Eslam M, Papatheodoridis G, George J, Mingrone G, Mantzoros CS. Liver biopsy-based validation, confirmation and comparison of the diagnostic performance of established and novel non-invasive steatotic liver disease indexes: Results from a large multi-center study. Metabolism. 2023;147:155666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 8. | Chi H, Hansen BE, Tang WY, Schouten JN, Sprengers D, Taimr P, Janssen HL, de Knegt RJ. Multiple biopsy passes and the risk of complications of percutaneous liver biopsy. Eur J Gastroenterol Hepatol. 2017;29:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Vuppalanchi R, Unalp A, Van Natta ML, Cummings OW, Sandrasegaran KE, Hameed T, Tonascia J, Chalasani N. Effects of liver biopsy sample length and number of readings on sampling variability in nonalcoholic Fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:481-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Nagasawa T, Kuroda H, Abe T, Saiki H, Takikawa Y. Shear wave dispersion to assess liver disease progression in Fontan-associated liver disease. PLoS One. 2022;17:e0271223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Doyle A, Sherman M. Liver Biopsy for Hepatocellular Carcinoma (HCC): Should this be a Routine? Curr Hepatology Rep. 2017;16:46-50. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Papatheodoridis GV. Ethics related to liver biopsies and antiviral therapies in chronic viral hepatitis. Dig Dis. 2008;26:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40601] [Article Influence: 10150.3] [Reference Citation Analysis (2)] |
| 14. | Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4127] [Cited by in RCA: 4685] [Article Influence: 1171.3] [Reference Citation Analysis (0)] |
| 15. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 9589] [Article Influence: 684.9] [Reference Citation Analysis (0)] |
| 16. | Przybyłkowski A, Szeligowska J, Januszewicz M, Raszeja-Wyszomirska J, Szczepankiewicz B, Nehring P, Górnicka B, Litwin T, Członkowska A. Evaluation of liver fibrosis in patients with Wilson's disease. Eur J Gastroenterol Hepatol. 2021;33:535-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Kalaiyarasi K, Sanchalika A, Hsien Min L, Wei Ming Y, Vishalkumar S, Kuo Chao Y, Jee Keem L, Sameer J, Terence HCW, Yen Ping T. Transient Elastography Is the Best-Performing Non-Invasive Test of Liver Fibrosis in Obese Asian Patients with Non-Alcoholic Fatty Liver Disease: A Pilot, Cross-Sectional Study. Medicina (Kaunas). 2024;60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Chimoriya R, Ho V, Wang ZV, Chang R, Boumelhem BB, Simmons D, Kormas N, Gorrell MD, Piya MK. Application and Diagnostic Performance of Two-Dimensional Shear Wave Elastography and Liver Fibrosis Scores in Adults with Class 3 Obesity. Nutrients. 2023;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Tosun M, Uslu H. Comparison of superb microvascular imaging and shear wave elastography for assessing liver fibrosis in chronic hepatitis B. Ultrasonography. 2022;41:394-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 20. | Aksakal M, Oktar SO, Sendur HN, Esendaglı G, Ozenirler S, Cindoruk M, Hızel K. Diagnostic performance of 2D shear wave elastography in predicting liver fibrosis in patients with chronic hepatitis B and C: a histopathological correlation study. Abdom Radiol (NY). 2021;46:3238-3244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Alcantara-Diaz AL, Ruiz-Fernandez JF, Salazar-Alarcon JL, Salinas-Sedo G, Toro-Huamanchumo CJ. Diagnostic Performance of 2D Shear Wave (2D-SWE) for Liver Fibrosis in Adults Undergoing Bariatric Surgery. Obes Surg. 2023;33:3120-3126. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Atzori SM, Pasha Y, Maurice JB, Taylor-Robinson SD, Campbell L, Lim AKP. Prospective evaluation of liver shearwave elastography measurements with 3 different technologies and same day liver biopsy in patients with chronic liver disease. Dig Liver Dis. 2024;56:484-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Cassinotto C, Boursier J, Paisant A, Guiu B, Irles-Depe M, Canivet C, Aube C, de Ledinghen V. Transient Versus Two-Dimensional Shear-Wave Elastography in a Multistep Strategy to Detect Advanced Fibrosis in NAFLD. Hepatology. 2021;73:2196-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Damjanovska S, Karb DB, Tripathi A, Asirwatham J, Delozier S, Perez JA, Falck-Ytter Y, Cohen S. Accuracy of Ultrasound Elastography and Fibrosis-4 Index (FIB-4) in Ruling Out Cirrhosis in Obese Non-Alcoholic Fatty Liver Disease (NAFLD) Patients. Cureus. 2022;14:e29445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 25. | Fang C, Rafailidis V, Konstantatou E, Yusuf GT, Barrow I, Pagkalidou E, Romanos O, Agarwal K, Quaglia A, Sidhu PS. Comparison Between Different Manufacturers' 2-D and Point Shear Wave Elastography Techniques in Staging Liver Fibrosis in Chronic Liver Disease Using Liver Biopsy as the Reference Standard: A Prospective Study. Ultrasound Med Biol. 2022;48:2229-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Garcovich M, Paratore M, Riccardi L, Zocco MA, Ainora ME, Mingrone G, Gasbarrini A, Pompili M. Correlation between a New Point-Shear Wave Elastography Device (X+pSWE) with Liver Histology and 2D-SWE (SSI) for Liver Stiffness Quantification in Chronic Liver Disease. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 27. | Gatos I, Yarmenitis S, Theotokas I, Koskinas J, Manesis E, Zoumpoulis SP, Zoumpoulis PS. Comparison of Visual Transient Elastography, Vibration Controlled Transient Elastography, Shear Wave Elastography and Sound Touch Elastography in Chronic liver Disease assessment using liver biopsy as 'Gold Standard'. Eur J Radiol. 2022;157:110557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Jocius D, Vajauskas D, Samuilis A, Mikelis K, Jokubauskiene S, Strupas K, Tamosiunas AE. Assessing Liver Fibrosis Using 2D-SWE Liver Ultrasound Elastography and Dynamic Liver Scintigraphy with 99mTc-mebrofenin: A Comparative Prospective Single-Center Study. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Kavak S, Kaya S, Senol A, Sogutcu N. Evaluation of liver fibrosis in chronic hepatitis B patients with 2D shear wave elastography with propagation map guidance: a single-centre study. BMC Med Imaging. 2022;22:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 30. | Laroia ST, Vellore Srinivasan S, Yadav K, Rastogi A, Kumar S, Kumar G, Kumar M. Performance of shear wave elastography: A single centre pilot study of mixed etiology liver disease patients with normal BMI. Australas J Ultrasound Med. 2021;24:120-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Lee SM, Ha HI, Lee IJ, Lee K, Lee JW, Park JW, Kim SE, Kwon MJ, Choe JY, Yoon SY, Yeo SG, Kim MJ. Comparison between Two-Dimensional and Point Shear Wave Elastography Techniques in Evaluating Liver Fibrosis Using Histological Staging as the Reference Standard: A Prospective Pilot Study. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Manesis EK, Schina M, Vafiadis I, Gatos I, Theotokas J, Zoumpoulis P, Drazinos P, Ketikoglou J, Delladetsima IK, Tiniakos DG. Liver stiffness measurements by 2-dimensional shear wave elastography compared to histological and ultrasound parameters in primary biliary cholangitis. Scand J Gastroenterol. 2021;56:1187-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Martonik D, Wandałowicz A, Supronowicz Ł, Panasiuk A, Parfieniuk-Kowerda A, Flisiak R. Shear-wave elastography for evaluation of hepatic stiffness in chronic viral hepatitis B and C. Clin Exp Hepatol. 2023;9:179-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 34. | Mendoza YP, Rodrigues SG, Delgado MG, Murgia G, Lange NF, Schropp J, Montani M, Dufour JF, Berzigotti A. Inflammatory activity affects the accuracy of liver stiffness measurement by transient elastography but not by two-dimensional shear wave elastography in non-alcoholic fatty liver disease. Liver Int. 2022;42:102-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 35. | Mikolasevic I, Domislovic V, Klapan M, Juric T, Lukic A, Krznaric-Zrnic I, Fuckar-Cupic D, Stimac D, Filipec Kanizaj T, Krznaric Z, Radic-Kristo D, Milic S, Martinovic M, Grubesic A, Grgurevic I. Accuracy of Controlled Attenuation Parameter and Liver Stiffness Measurement in Patients with Non-alcoholic Fatty Liver Disease. Ultrasound Med Biol. 2021;47:428-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Nogami A, Yoneda M, Iwaki M, Kobayashi T, Kessoku T, Honda Y, Ogawa Y, Imajo K, Higurashi T, Hosono K, Kirikoshi H, Saito S, Nakajima A. Diagnostic comparison of vibration-controlled transient elastography and MRI techniques in overweight and obese patients with NAFLD. Sci Rep. 2022;12:21925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Ogino Y, Wakui N, Nagai H, Matsuda T. Comparison of strain elastography and shear wave elastography in diagnosis of fibrosis in nonalcoholic fatty liver disease. J Med Ultrason (2001). 2023;50:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 38. | Paisant A, Lemoine S, Cassinotto C, de Lédinghen V, Ronot M, Irlès-Depé M, Vilgrain V, Le Bail B, Paradis V, Canivet CM, Michalak S, Rousselet MC, Rautou PE, Lebigot J, Hunault G, Crouan A, Aubé C, Boursier J. Reliability Criteria of Two-Dimensional Shear Wave Elastography: Analysis of 4277 Measurements in 788 Patients. Clin Gastroenterol Hepatol. 2022;20:400-408.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 39. | Patidar Y, Singh J, Chatterjee N, Mukund A, Rastogi A, Kumar G, Sharma MK. Real-Time Shear Wave Elastography for Determining the Ideal Site of Liver Biopsy in Diffuse Liver Disease. Indian J Radiol Imaging. 2024;34:44-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 40. | Roccarina D, Iogna Prat L, Pallini G, Guerrero Misas M, Buzzetti E, Saffioti F, Aricò FM, Mantovani A, Koutli E, Goyale A, Rosselli M, Luong TV, Pinzani M, Tsochatzis EA. Comparison of point-shear wave elastography (ElastPQ) and transient elastography (FibroScan) for liver fibrosis staging in patients with non-alcoholic fatty liver disease. Liver Int. 2022;42:2195-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 41. | Saadi T, Khoury J, Toukan W, Krimasky R, Veitsman E, Baruch Y, Gaitini D, Beck-Razi N. Point Shear Wave Elastography for Assessing Liver Stiffness in Chronic Liver Diseases of Different Etiologies Compared to Biopsy. Isr Med Assoc J. 2021;23:223-228. [PubMed] |
| 42. | Seyrek S, Ayyildiz H, Bulakci M, Salmaslioglu A, Seyrek F, Gultekin B, Cavus B, Berker N, Buyuk M, Yuce S. Comparison of Fibroscan, Shear Wave Elastography, and Shear Wave Dispersion Measurements in Evaluating Fibrosis and Necroinflammation in Patients Who Underwent Liver Biopsy. Ultrasound Q. 2024;40:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 43. | Sharpton SR, Tamaki N, Bettencourt R, Madamba E, Jung J, Liu A, Behling C, Valasek MA, Loomba R. Diagnostic accuracy of two-dimensional shear wave elastography and transient elastography in nonalcoholic fatty liver disease. Therap Adv Gastroenterol. 2021;14:17562848211050436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Soh EG, Lee YH, Kim YR, Yoon KH, Choi KH. Usefulness of 2D shear wave elastography for the evaluation of hepatic fibrosis and treatment response in patients with autoimmune hepatitis. Ultrasonography. 2022;41:740-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 45. | Song L, Zhao L, Deng J, Meng F, Wu X, Lu Q, Xiang H, Jing X, Luo Y. Staging liver fibrosis in patients with chronic hepatitis B using two-dimensional shear wave elastography based on histopathological findings: a prospective multicenter study. Quant Imaging Med Surg. 2023;13:2376-2387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 46. | Taibbi A, Petta S, Matranga D, Caruana G, Cannella R, Busè G, Marco VD, Midiri M, Bartolotta TV. Liver stiffness quantification in biopsy-proven nonalcoholic fatty liver disease patients using shear wave elastography in comparison with transient elastography. Ultrasonography. 2021;40:407-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Wang X, Li Y, Jiang L, Zhou M, Zhang X, Wen H. Performance of 2D-shear wave elastography in autoimmune hepatitis-primary biliary cholangitis overlap syndrome. Abdom Radiol (NY). 2023;48:1290-1297. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Wang J, Wu M, Linghu R, Chang J, Wu M, Feng C, Ren X, Liu C, Lin J, Song T, Gu J, Zhang Y, Fang Y, Ma S, Hu P, Wu L, Han X, Chen K, Shi Q, Zhang R, Zhou Q, Du R, Gao Y, Jing X, Yang S, Zhou C, Zheng J, Liang P, Zheng RQ. Usefulness of New Shear Wave Elastography Technique for Noninvasive Assessment of Liver Fibrosis in Patients with Chronic Hepatitis B: A Prospective Multicenter Study. Ultraschall Med. 2022;43:e1-e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Wang K, Yu D, Li G, Wen L, Zhang S. Comparison of the diagnostic performance of shear wave elastography with shear wave dispersion for pre-operative staging of hepatic fibrosis in patients with hepatocellular carcinoma. Eur J Radiol. 2022;154:110459. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 50. | Yang J, Li J, Ye G, Luo Y. Comparison of Visual Transient Elastography and Shear Wave Elastography in Evaluating Liver Fibrosis in Patients with Chronic Liver Disease. Int J Gen Med. 2021;14:3553-3561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Yang L, Ling W, He D, Lu C, Ma L, Tang L, Luo Y, Chen S. Shear wave-based sound touch elastography in liver fibrosis assessment for patients with autoimmune liver diseases. Quant Imaging Med Surg. 2021;11:1532-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Yoo HW, Kim SG, Jang JY, Yoo JJ, Jeong SW, Kim YS, Kim BS. Two-dimensional shear wave elastography for assessing liver fibrosis in patients with chronic liver disease: a prospective cohort study. Korean J Intern Med. 2022;37:285-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Yoo JJ, Kim SG, Kim YS. The Diagnostic Accuracy of LOGIQ S8 and E9 Shear Wave Elastography for Staging Hepatic Fibrosis, in Comparison with Transient Elastography. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Yamaoka K, Saitoh S, Kinowaki K, Fujiyama S, Kawamura Y, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Suzuki F, Suzuki Y, Arase Y, Ikeda K, Fukusato T, Kumada H. Clinicopathological assessment of steatohepatitic hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2022;46:101799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Zougmoré HT, Cadranel JFD, Fantognon G, Azzi B, Smadhi R, Ngele Efole JR, Mrabti S, Heng R, Ntsama MA, Medmoun M, Kazerouni F, Le Magoarou T. Fibroscan® and Shear Wave correlated well in hepatic fibrosis evaluation of patients with chronic liver diseases "in real life situation". Medicine (Baltimore). 2022;101:e30025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 56. | Hsu PK, Wu LS, Yen HH, Huang HP, Chen YY, Su PY, Su WW. Attenuation Imaging with Ultrasound as a Novel Evaluation Method for Liver Steatosis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 57. | Kjaergaard M, Lindvig KP, Hansen CD, Detlefsen S, Krag A, Thiele M. Hepatorenal Index by B-Mode Ratio Versus Imaging and Fatty Liver Index to Diagnose Steatosis in Alcohol-Related and Nonalcoholic Fatty Liver Disease. J Ultrasound Med. 2023;42:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 58. | Kuroda H, Abe T, Fujiwara Y, Nagasawa T, Takikawa Y. Diagnostic accuracy of ultrasound-guided attenuation parameter as a noninvasive test for steatosis in non-alcoholic fatty liver disease. J Med Ultrason (2001). 2021;48:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Liu GT, Ni QF, Zhang YH, Dong XM, Zhou C, Shen B, Zhu JY, Chen YJ, Zhu Z. Application of noninvasive test (acoustic attenuation imaging and ultrasonic shear wave elastography) to grade nonalcoholic fatty liver disease: An observational study. Medicine (Baltimore). 2023;102:e34550. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 60. | Qu Y, Song YY, Chen CW, Fu QC, Shi JP, Xu Y, Xie Q, Yang YF, Zhou YJ, Li LP, Xu MY, Cai XB, Zhang QD, Yu H, Fan JG, Lu LG. Diagnostic Performance of FibroTouch Ultrasound Attenuation Parameter and Liver Stiffness Measurement in Assessing Hepatic Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Transl Gastroenterol. 2021;12:e00323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 61. | Yazdani L, Rafati I, Gesnik M, Nicolet F, Chayer B, Gilbert G, Volniansky A, Olivié D, Giard JM, Sebastiani G, Nguyen BN, Tang A, Cloutier G. Ultrasound Shear Wave Attenuation Imaging for Grading Liver Steatosis in Volunteers and Patients With Non-alcoholic Fatty Liver Disease: A Pilot Study. Ultrasound Med Biol. 2023;49:2264-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 62. | Yu H, Liu H, Zhang J, Jia G, Yang L, Zhang Q, Li G, Liu F, Di F, Wang F. Accuracy of FibroTouch in assessing liver steatosis and fibrosis in patients with metabolic-associated fatty liver disease combined with type 2 diabetes mellitus. Ann Palliat Med. 2021;10:9702-9714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Welman CJ, Saunders J, Zelesco M, Abbott S, Boardman G, Ayonrinde OT. Hepatic steatosis: Ultrasound assessment using attenuation imaging (ATI) with liver biopsy correlation. J Med Imaging Radiat Oncol. 2023;67:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Zhou J, Yan F, Xu J, Lu Q, Zhu X, Gao B, Zhang H, Yang R, Luo Y. Diagnosis of steatohepatitis and fibrosis in biopsy-proven nonalcoholic fatty liver diseases: including two-dimension real-time shear wave elastography and noninvasive fibrotic biomarker scores. Quant Imaging Med Surg. 2022;12:1800-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Zhao Y, Zhang C, Xu S, Zhang H, Wei S, Huang P, Zhang L, Wong YN, Xu W, Huang P. Quantitative evaluation of hepatic steatosis using novel ultrasound technology normalized local variance (NLV) and its standard deviation with different ROIs in patients with metabolic-associated fatty liver disease: a pilot study. Abdom Radiol (NY). 2022;47:693-703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 66. | Imajo K, Honda Y, Kobayashi T, Nagai K, Ozaki A, Iwaki M, Kessoku T, Ogawa Y, Takahashi H, Saigusa Y, Yoneda M, Kirikoshi H, Utsunomiya D, Aishima S, Saito S, Nakajima A. Direct Comparison of US and MR Elastography for Staging Liver Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2022;20:908-917.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 67. | Kim JW, Lee CH, Kim BH, Lee YS, Hwang SY, Park BN, Park YS. Ultrasonographic index for the diagnosis of non-alcoholic steatohepatitis in patients with non-alcoholic fatty liver disease. Quant Imaging Med Surg. 2022;12:1815-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 68. | Prieto Ortiz JE, Sánchez Pardo S, Prieto Ortiz RG, Garzón Orjuela N, S Ford J, Eslava Schmalbach J. Comparison of shear wave elastography (supersonic) with liver biopsy in a cohort of patients from a medium-income country: an observational study. Rev Esp Enferm Dig. 2021;113:318-323. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 69. | Yan YL, Xing X, Wang Y, Wang XZ, Wang Z, Yang L. Clinical utility of two-dimensional shear-wave elastography in monitoring disease course in autoimmune hepatitis-primary biliary cholangitis overlap syndrome. World J Gastroenterol. 2022;28:2021-2033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Zhang YN, Fowler KJ, Boehringer AS, Montes V, Schlein AN, Covarrubias Y, Wolfson T, Hong CW, Valasek MA, Andre MP, Loomba R, Sirlin CB. Comparative diagnostic performance of ultrasound shear wave elastography and magnetic resonance elastography for classifying fibrosis stage in adults with biopsy-proven nonalcoholic fatty liver disease. Eur Radiol. 2022;32:2457-2469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 71. | Ni M, Wang L, Yu H, Wen X, Yang Y, Liu G, Hu Y, Li Z. Radiomics Approaches for Predicting Liver Fibrosis With Nonenhanced T(1) -Weighted Imaging: Comparison of Different Radiomics Models. J Magn Reson Imaging. 2021;53:1080-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | Lewin M, Poujol-Robert A, Boëlle PY, Wendum D, Lasnier E, Viallon M, Guéchot J, Hoeffel C, Arrivé L, Tubiana JM, Poupon R. Diffusion-weighted magnetic resonance imaging for the assessment of fibrosis in chronic hepatitis C. Hepatology. 2007;46:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 73. | Coccia F, Testa M, Guarisco G, Bonci E, Di Cristofano C, Silecchia G, Leonetti F, Gastaldelli A, Capoccia D. Noninvasive assessment of hepatic steatosis and fibrosis in patients with severe obesity. Endocrine. 2020;67:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1214-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 364] [Article Influence: 20.2] [Reference Citation Analysis (1)] |
| 75. | Poynard T, Halfon P, Castera L, Munteanu M, Imbert-Bismut F, Ratziu V, Benhamou Y, Bourlière M, de Ledinghen V; FibroPaca Group. Standardization of ROC curve areas for diagnostic evaluation of liver fibrosis markers based on prevalences of fibrosis stages. Clin Chem. 2007;53:1615-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 76. | Elliott R, Axelin A, Richards KC, Vahlberg T, Ritmala-Castren M. Sensitivity and specificity of proposed Richards-Campbell Sleep Questionnaire cut-off scores for good quality sleep during an ICU stay. J Clin Nurs. 2023;32:2700-2708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 77. | Cassinotto C, Boursier J, de Lédinghen V, Lebigot J, Lapuyade B, Cales P, Hiriart JB, Michalak S, Bail BL, Cartier V, Mouries A, Oberti F, Fouchard-Hubert I, Vergniol J, Aubé C. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 390] [Article Influence: 43.3] [Reference Citation Analysis (0)] |