Published online Jan 27, 2025. doi: 10.4254/wjh.v17.i1.97797
Revised: August 29, 2024
Accepted: September 19, 2024
Published online: January 27, 2025
Processing time: 211 Days and 11.2 Hours
Chronic hepatitis B (CHB) affects > 300 million people worldwide. The combi
To investigate the prevalence of cardiometabolic comorbidities in patients with CHB and matched non-CHB comparison group.
We examined patients with CHB and age-, sex-, body mass index (BMI)-, and country-of-birth matched comparison group. Defining cardiometabolic co-morbidity: Obesity (BMI > 25 kg/m2/abnormal waist-to-hip ratio), metabolic dysfunction-associated steatotic liver disease (MASLD), hypercholesterolemia (total-cholesterol > 5 mmol/L/statin use), hypertension (systolic ≥ 135 mmHg/ diastolic ≥ 85 mmHg/antihypertensive medication) and type 2 diabetes (T2D) (2-hour oral glucose tolerance test glucose > 11.1 mmol/L/HbA1c > 48 mmol/mol/ antidiabetic medication). Physical activity was evaluated using maximal oxygen consumption (VO2max), activity monitors, and a questionnaire.
We included 98 patients with CHB and 49 persons in the comparison group. The two groups were well-matched, showing no significant differences in age, sex, BMI, country-of-birth, education, or employment. Among patients with CHB, the following prevalence of cardiometabolic co-morbidity was found: 77% were obese, 45% had MASLD, 38% had hypercholesterolemia, 26% had hypertension, and 7% had T2D, which did not differ significantly from the comparison group, apart from lower prevalence of hemoglobin A1c (HbA1c) ≥ 48 mmol/L or known T2D. Both groups had low VO2max of 27 mL/kg/minute in the patients with CHB and 30 mL/kg/minute in the comparison group, and the patients with CHB had a shorter self-assessed sitting time.
The patients with CHB and the comparison group were well-matched and had a similar prevalence of car
Core Tip: This study assessed the prevalence of cardiometabolic comorbidities in patients with chronic hepatitis B (CHB) compared with a matched non-CHB group in Denmark. Both groups demonstrated similar prevalence of obesity, metabolic dysfunction-associated steatotic liver disease, hypercholesterolemia, hypertension, and type 2 diabetes (T2D). Notably, patients with CHB had a lower prevalence of hemoglobin A1c ≥ 48 mmol/mol or known T2D. Additionally, both groups exhibited low levels of physical fitness. This highlights the need for tailored management strategies to address cardiometabolic health issues in patients with CHB despite the comparable prevalence of comorbidities in the general population.
- Citation: Jespersen S, Fritt-Rasmussen A, Madsbad S, Pedersen BK, Krogh-Madsen R, Weis N. Prevalence of cardiometabolic co-morbidities in patients with vs persons without chronic hepatitis B: The FitLiver cohort study. World J Hepatol 2025; 17(1): 97797
- URL: https://www.wjgnet.com/1948-5182/full/v17/i1/97797.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i1.97797
Chronic hepatitis B (CHB) is caused by persistent infection of the liver > 6 months with hepatitis B virus (HBV). The global prevalence of CHB is estimated to be > 300 million individuals[1], and it is the most common type of hepatitis worldwide. Untreated CHB can cause liver cirrhosis and hepatocellular carcinoma (HCC)[2]. Currently, there is no curative therapy; however, medical treatment can reduce the amount of virus in the blood, reducing CHB-induced morbidity and mortality by decreasing liver inflammation and fibrosis[3,4].
In Asian populations, it has been shown that patients with CHB have higher body mass index (BMI), consume more alcohol, and have poorer physical fitness compared to people without CHB[5]. In addition, approximately 33% are overweight and 50% physically inactive[6]. In general, obesity is associated with metabolic syndrome (i.e., abdominal obesity, dyslipidaemia, hypertension, and glucose abnormality) and metabolic diseases such as type 2 diabetes (T2D), hypertension, hyperlipidaemia[7] and metabolic dysfunction-associated steatotic liver disease (MASLD)[8]. In studies investigating cardiometabolic co-morbidity in patients with CHB, inconsistent findings have been observed, and both lower and higher prevalence of cardiometabolic co-morbidities have been reported compared to the general population[9,10]. T2D and obesity are risk factors for HCC in patients with CHB[11,12]. However, none of the acknowledged HCC risk scoring systems incorporate cardiometabolic comorbidities as risk factors[3], and there is a lack of specific clinical guidance for managing patients with CHB with cardiometabolic comorbidities. The Danish and European guidelines for CHB management recommend screening patients for metabolic liver disease, with no recommendations for follow-up examinations or treatment of patients with CHB and cardiometabolic comorbidities, apart from those with impaired renal function[13]. A study investigating mortality among patients with CHB in Denmark found a prevalence of 4% for T2D compared to 2% in sex- and age-matched persons from the general population[14]. However, little information is available on patients with CHB living in Denmark regarding cardiometabolic co-morbidities.
This study aimed to assess the prevalence of cardiometabolic co-morbidities (obesity, MASLD, hypercholesterolemia, hypertension, and T2D) among patients with CHB compared with a comparison group without CHB. Furthermore, we investigated whether there were any differences in physical fitness or activity.
To investigate the effects of lifestyle-related behaviours and cardiometabolic co-morbidities in a population of patients with CHB living in the capital area of Copenhagen, Denmark, we initiated a prospective observational cohort study that included patients with CHB and an age-, sex-, BMI-, and country-of-birth matched comparison group without CHB. The cohort, called the “FitLiver Cohort”, will include future follow-up visits planned two, five and ten years after inclusion.
This study assessed the baseline prevalence of cardiometabolic co-morbidities in a cohort of patients with CHB and a comparison group of persons without CHB. Furthermore, we compared baseline physical fitness and physical activity between the groups.
The inclusion criteria of the patients with CHB were CHB, defined as hepatitis B surface antigen (HBsAg) positivity > 6 months and age > 18 years. The exclusion criteria were hepatitis C virus (HCV) or human immunodeficiency virus (HIV) co-infection. The inclusion criteria for the matched comparison group was age > 18 years. Exclusion criteria were positive HBsAg, HCV, or HIV infection and uneven matching regarding age, sex, BMI, and country-of-birth to the patients with CHB. For both groups, pregnancy and the inability to understand and read Danish or English information to provide written informed consent were exclusion criteria.
Patients with CHB were recruited during regular outpatient visits to the Department of Infectious Diseases at the Copenhagen University Hospital, Hvidovre, Denmark. Eighteen patients with CHB were also included in a randomised clinical trial[15], and their results will be published elsewhere (paper submitted).
The participants in the comparison group were recruited through social media advertisements, posters in the hospital departments, and a medical trial recruitment company: “forskning.nu.” We primarily included and examined patients with CHB and following sought participants for the comparison group. The matching criteria included age, sex, BMI, and country-of-birth.
The examinations were performed during two different visits to the Centre for Physical Activity Research, Copenhagen University Hospital, Rigshospitalet, Denmark, and the Department of Infectious Diseases, Copenhagen University Hospital, Hvidovre, Denmark.
General examination: All participants were examined by a medical doctor who collected information about their medical history (including the level of education, relationship status, and moderate alcohol use), current medical treatment and diagnoses, electrocardiogram, and physical examination. Moderate alcohol use was defined as less than 10 units/week as defined by the Danish health authorities.
Body anthropometrics: The body composition, weight, height, waist circumference, and hip circumference were measured. Dual-energy X-ray absorptiometry was performed (Lunar Prodigy GE Healthcare, Madison, Wisconsin, encore software version 14, 10, 022) to determine total body fat mass and lean body mass. The participants were instructed to urinate immediately before the scans were performed.
Liver parameters: The general liver health was assessed through blood sampling of alanine aminotransferase (ALT), HBsAg status, hepatitis B viral load (HBV DNA), hepatitis D (HDV) status, hepatitis B core antibody (anti-HBc), hepatitis B surface antibody (anti-HBs), hepatitis B e antigen (HBeAg), and Fibrosis Index-4 (FIB-4) through routine blood samples analysed by the Department of Biochemistry and the Department of Clinical Microbiology at Copenhagen University Hospital, Rigshospitalet, Denmark. We used anti-HBs levels ≥ 10 IU/L to indicate vaccine protection or immunity of HBV infection[15]. Transient elastography (TE) was performed using vibration-controlled TE (Fibroscan 502 Touch, DELRUS, Europe) to assess the fibrosis (TE-score) and controlled attenuation parameter (CAP) to assess hepatic steatosis. CAP cut-off values for hepatic steatosis were set at ≥ 248[16]. Participants were instructed to fast for three hours prior to the scan.
Cardiovascular parameters: Blood pressure measurements (Microlife BP B2 basic, Switzerland) were obtained after at least 15 minutes of rest. Fasting blood lipid profiles (total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides) were analysed using standard procedures at the Department of Clinical Biochemistry, Copenhagen University Hospital, Rigshospitalet, Denmark.
Glucose metabolism: The oral glucose tolerance test (OGTT) was performed to assess glucose metabolism. Participants arrived after an overnight fast. An intravenous catheter was placed in the anterior cubital region, and baseline blood samples, including hemoglobin A1c (HbA1c), were obtained. The participants then drank a glucose solution consisting of 75 g of glucose (water-free) dissolved in 300 mL of water in less than 2 minutes and rested for two hours in a semi-supine position. Following the intake of glucose solution, blood samples were drawn after 15, 30, 60, 90, and 120 minutes. Blood samples were analysed for glucose and insulin levels using standard procedures at the Department of Clinical Biochemistry, Copenhagen University Hospital, Rigshospitalet. Denmark. The area under the curve (AUC) for the glucose and insulin levels was calculated by computing a trapezoidal approximation of the integral under the curve. The Matsuda index, an index of whole-body insulin sensitivity, was calculated as 10000/square root of (fasting glucose × fasting insulin) × (mean glucose × mean insulin during OGTT). The conversion factor of insulin from pmol/L to µIU/L was set to 6.0[17]. The homeostasis model assessment for insulin resistance (HOMA-IR), was calculated from fasting plasma insulin and fasting plasma glucose levels using the HOMA2 calculator[18], which primarily is an index of hepatic insulin resistance.
Collectively assessed cardiometabolic co-morbidities: To classify the cardiometabolic co-morbidities, we used pooled definitions based on examinations, medical treatment, and known diagnoses to define collectively assessed cardio
Physical activity and fitness: We used graded cardiopulmonary exercise testing as described elsewhere[15,25] to determine the level of fitness indicated by maximal oxygen consumption (VO2max), using a prediction model for choosing the watts and watt increase for each individual[26]. A Borg Rating of perceived exertion scale of 6–20 was used to obtain the self-assessed level of exhaustion[27]. Physical activity was measured for seven consecutive days at baseline and after the intervention using an axial accelerometer-based activity monitor (AX3; Axivity, Newcastle upon Tyne, United Kingdom). Moderate-to-vigorous physical activity (MVPA) was defined after the Freedson cut point, ≥ 1952 counts per minute[28], by use of the vertical axis of the accelerometer placed on the back, and days were removed if non-wear time was > 2700 minutes. The R packages devtools, and jbrond/physaccel were used to determine the intensity and physical activity types[29]. We had to remove day one because of the late start of activity registration (after 5:00 am), resulting in a mean of 5.6 days of AX3 wear for all participants, ranging from 1 to 7 days. We used the International Physical Activity Questionnaire-Short Form to assess self-assessed physical activity in Danish or English (Supplementary material).
This study was approved by the Regional Committee of the Danish National Committee on Health Research Ethics (J.no. H-22055480), is regulated by the Danish Data Protection Agency (registration P-2023-24), and was carried out in accordance with the Helsinki Declaration for Ethics Standards in Human Trials.
Baseline characteristics were described using either the mean ± SD or medians [interquartile range (IQR)], depending on whether the data were symmetrically distributed.
Quantitative outcome variables were compared using the independent t-test or Wilcoxon rank-sum test according to the normality distribution. Fisher’s exact test was used to analyse categorical data. R version 4.3.0 was used for the statistical analysis. For baseline characteristics, a P value of < 0.05 was considered significant. For the outcomes, the initial significance level for the study was P < 0.05 (reported as a tendency and P < 0.05; Table 1 and Table 2). However, we used Bonferroni corrections due to multiple outcomes, resulting in a significance level of P < 0.002 (reported as significant and P < 0.05; Table 1 and Table 2).
| | All participants | Chronic hepatitis B | Comparison group | P value |
| Participants, n | 147 | 98 | 49 | |
| Age, years (range) | 45 (19-78) | 44 (25-74) | 46 (19-78) | 0.15 |
| Sex, female | 74 (50) | 50 (51) | 24 (49) | 0.86 |
| Country of birth | 0.30 | |||
| Denmark | 20 (14) | 12 (12) | 8 (16) | |
| Eastern Europe | 9 (6) | 5 (5) | 4 (8) | |
| Turkey | 28 (19) | 21 (21) | 7 (14) | |
| MENA region | 19 (13) | 9 (9) | 10 (20) | |
| Sub-Saharan Africa | 11 (7) | 7 (7) | 4 (8) | |
| Asia | 58 (39) | 43 (44) | 15 (31) | |
| South America | 2 (1) | 1 (1) | 1 (2) | |
| Highest level of education | 0.50 | |||
| Primary school | 17 (12) | 14 (14) | 3 (6) | |
| High school | 10 (7) | 6 (6) | 4 (8) | |
| Vocational education | 31 (21) | 20 (20) | 11 (22) | |
| Academic higher education | 85 (58) | 54 (55) | 31 (63) | |
| Employment | 0.29 | |||
| Student | 11 (7) | 7 (7) | 4 (8) | |
| Employed/self-employed | 109 (74) | 68 (69) | 41 (84) | |
| Unemployed | 10 (7) | 8 (8) | 2 (4) | |
| Retired | 14 (10) | 12 (12) | 2 (4) | |
| Relationship status | ||||
| Married or with a partner | 111 (76) | 79 (80) | 32 (65) | < 0.05 |
| Substance intake | ||||
| Alcohol use currently | 76 (52) | 42 (43) | 34 (69) | < 0.05 |
| Smoking ever | 61 (41) | 41 (42) | 20 (41) | 1.00 |
| Drug-use ever | 12 (8) | 7 (7) | 5 (10) | 0.53 |
| | All participants | Chronic hepatitis B | Comparison group | P value |
| Participants, n | 147 | 98 | 49 | |
| Body anthropometrics | ||||
| Body weight, mean (± SD), kg | 74.6 (± 17.4) | 74.5 (± 17.7) | 74.8 (± 16.8) | 0.94 |
| BMI, mean (± SD), kg/m2 | 26.2 (± 4.9) | 26.3 (± 5.16) | 26.1 (± 4.43) | 0.83 |
| Waist-to-hip ratio (WHR), mean (± SD) | 0.88 (± 0.09) | 0.88 (± 0.09) | 0.88 (± 0.08) | 1.00 |
| Total body fat, mean (± SD), % | 32.6 (± 9.5) | 32.9 (± 9.7) | 32.0 (± 9.3) | 0.59 |
| Total lean body mass, median (IQR), kg | 45.6 (17.5) | 46.5 (18.3) | 45.1 (13.3) | 0.48 |
| Abdominal obesity by WHR | 85 (58) | 56 (57) | 29 (59) | 0.86 |
| Liver parameters | ||||
| ALT, median (IQR), U/L | 27 (17) | 28 (18) | 24 (13) | < 0.05 |
| Viral load, median (IQR), IU/mL | - | 310 (2020) | - | |
| Anti-HDV positive | - | 1 (1) | - | |
| HBeAg positive | - | 5 (5) | - | |
| Anti-HBc positive | 106 (72) | 98 (100) | 8 (16) | < 0.05a |
| Anti-HBs positive, level ≥ 10 IU/L | 28 (19) | 1 (1) | 27 (55) | < 0.05a |
| Fib-4 score, median (IQR), | 0.89 (0.53) | 0.89 (0.52) | 0.87 (0.58) | 0.84 |
| TE-score, median (IQR), kPa | 4.5 (1.6) | 4.7 (1.5) | 4.4 (1.3) | < 0.05 |
| CAP, mean (± SD), dB/m | 250 (± 66) | 248 (± 64) | 255 (± 69) | 0.52 |
| Hepatic steatosis, CAP ≥ 248 | 73 (50) | 47 (48) | 26 (53) | 0.60 |
| Cardiovascular parameters | ||||
| Cholesterol, mmol/L | 4.8 (1.1) | 4.7 (1.1) | 4.9 (1.1) | 0.29 |
| Hypercholesterolemia by cholesterol > 5 mmol/L | 52 (35) | 30 (31) | 22 (45) | 0.10 |
| Triglycerides, median (IQR), mmol/L | 1.0 (0.7) | 1.0 (0.6) | 1.1 (0.8) | 0.43 |
| LDL-cholesterol, median (IQR), mmol/L | 3.0 (1.0) | 2.8 (0.9) | 3.2 (1.3) | 0.13 |
| HDL-cholesterol, median (IQR), median (IQR), mmol/L | 1.3 (0.6) | 1.3 (0.5) | 1.4 (0.5) | 0.12 |
| Systolic blood pressure, mean (± SD), mmHg | 119 (± 15) | 118 (± 15) | 122 (± 15) | 0.13 |
| Diastolic blood pressure, mean (± SD), mmHg | 75 (± 10) | 75 (± 11) | 75 (± 9) | 0.95 |
| Glucose metabolism | ||||
| HbA1c, median (IQR), mmol/L | 36 (6) | 36 (6) | 38 (5) | < 0.05 |
| HbA1c ≥ 48 mmol/L | 6 (4) | 0 | 6 (12) | < 0.05a |
| Fasting glucose, median (IQR), mmol/L | 4.9 (0.8) | 4.9 (0.6) | 5.1 (0.8) | < 0.05 |
| Fasting insulin pmol/L | 65 (58) | 66 (53) | 61 (79) | 0.73 |
| 2-hour glucose, median (IQR), mmol/L | 6.8 (2.2) | 6.8 (1.8) | 7.1 (3.8) | 0.63 |
| OGTT 2h glucose ≥ 11.1 | 17 (12) | 8 (8) | 9 (18) | 0.10 |
| OGTT glucose AUC, median (IQR), mmol/L/minute | 899 (242) | 879 (233) | 905 (365) | 0.53 |
| OGTT Insulin AUC, median (IQR), pmol/L/minute | 56295 (49073) | 66311 (54818) | 45952 (33450) | < 0.05 |
| Matsuda Index, median (IQR) | 3.7 (3.5) | 3.6 (2.8) | 3.8 (4.5) | 0.57 |
| HOMA-IR, median (IQR) | 1.2 (1.1) | 1.3 (1.0) | 1.2 (1.5) | 0.81 |
| Known cardiometabolic co-morbidity | ||||
| Hypercholesterolemia | 18 (12) | 9 (9) | 7 (15) | 0.40 |
| Hypertension | 16 (11) | 11 (11) | 7 (15) | 0.60 |
| Type 2 diabetes | 6 (4) | 0 (0) | 6 (12) | < 0.05a |
| Current medication | ||||
| Lipid-lowering medicine | 14 (10) | 8 (8) | 6 (12) | 0.55 |
| Antihypertensive medication | 19 (13) | 12 (12) | 7 (14) | 0.79 |
| Anti-diabetic medicine | 6 (4) | 1 (1) | 5 (10) | < 0.05 |
| Antiviral medicine | 23 (16) | 22 (22) | 1 (2) | < 0.05a |
| Collectively assessed cardiometabolic co-morbidity | ||||
| Obesity | 112 (76) | 75 (77) | 37 (76) | 1.00 |
| MASLD | 69 (47) | 44 (45) | 25 (51) | 0.48 |
| Hypercholesterolemia | 65 (44) | 37 (38) | 28 (57) | < 0.05 |
| Hypertension | 42 (29) | 25 (26) | 17 (35) | 0.25 |
| Type 2 diabetes | 17 (12) | 8 (8) | 9 (18) | 0.10 |
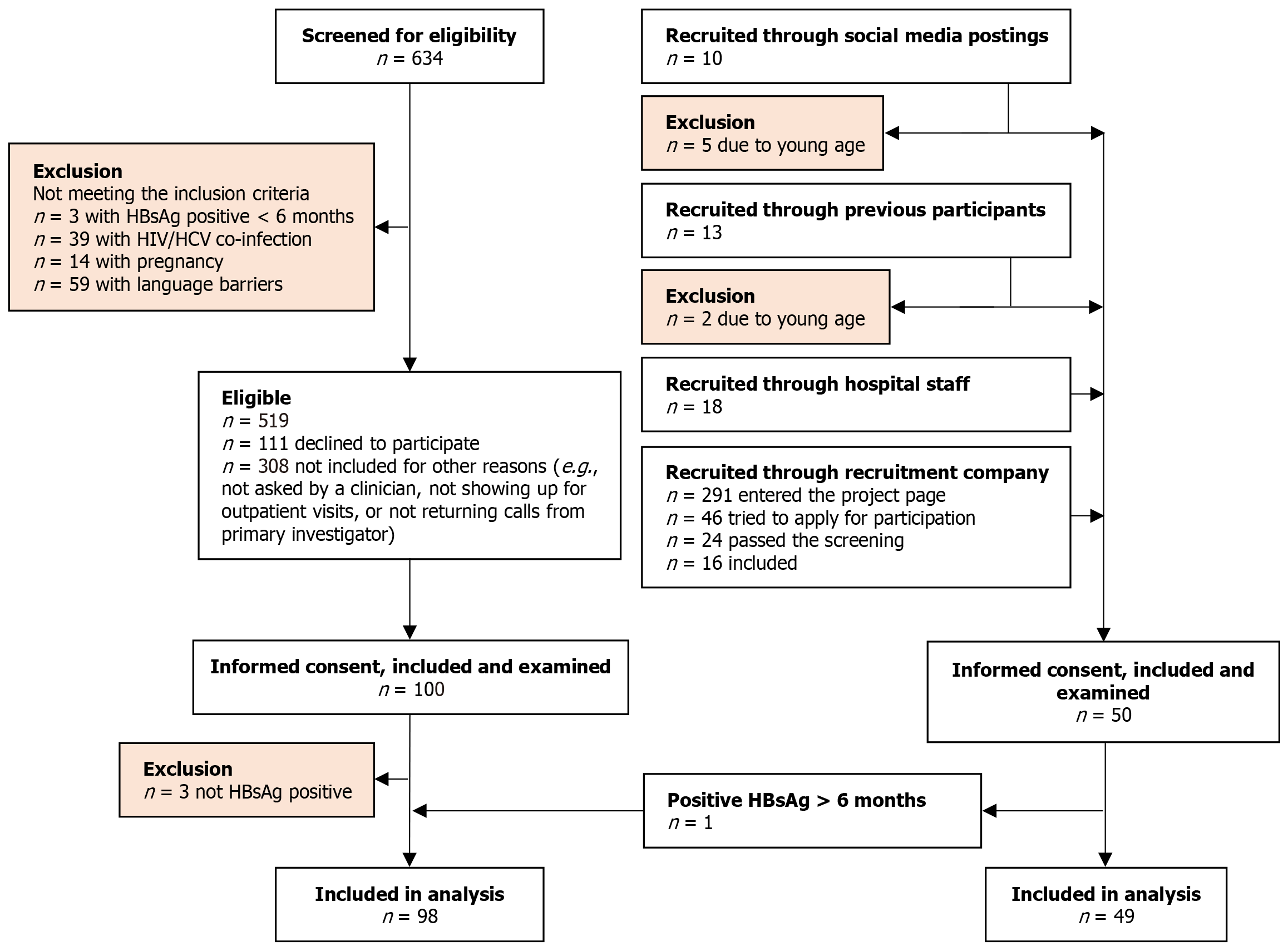
The participants were recruited between March 2022 and December 2023. We included 100 patients with CHB and 50 matched individuals from the comparison group but had to exclude three patients with CHB due to loss of HBsAg; one individual from the comparison group, who was diagnosed with CHB due to positive HBsAg > 6 months, was switched to the CHB group, resulting in 98 patients with CHB and 49 persons in the comparison group (Figure 1). Planned matching of the comparison group for age, sex, BMI, and country-of-birth was successful (Table 1). The level of education did not differ significantly between the groups. More patients with CHB were married or had partners. No participants reported excessive alcohol consumption. Fewer patients with CHB reported current alcohol consumption. There were no differences in smoking or drug use between the groups (Table 1).
The groups were similar in body weight, WHR, total body fat content, total lean body mass, and abdominal obesity (Table 2).
We found a tendency towards increased ALT and TE-score in patients with CHB (Table 2). Eight persons from the comparison group showed previous infection with HBV by positive anti-HBc, and 27 (55%) in the comparison group had protective anti-HBs, indicating a sufficient vaccination response or prior infection. Five (5%) patients with CHB were HBeAg positive, and one (1%) had positive anti-HDV. We found no differences in FIB-4 scores or CAP (Table 2). One participant from the comparison group did not have a FibroScan; therefore, one CAP and one TE score were missing.
We found no statistically significant differences between the groups' measured lipid profiles or blood pressure (Table 2).
None of the patients with CHB had elevated HbA1c levels (> 48 mmol/L) compared to six participants in the comparison group. We found tendencies of lower fasting glucose, lower measured HbA1c and number of individuals having 2-hour post-OGTT glucose values > 11.1 mmol/L in the CHB patients. However, a tendency towards a higher insulin AUC was observed in patients with CHB. We found no difference in the Matsuda index or HOMA-IR (Table 2). Three (0.4%) of plasma glucose samples, 21 (2.8%) of the plasma insulin samples, and two (1.4%) of the HbA1c samples were hemolyzed or lost during transfer between the departments.
We found no significant differences in the known diagnoses of hypercholesterolemia or hypertension. However, no patients with CHB were diagnosed with T2D compared to six (12%) in the comparison group. Patients with CHB used less anti-diabetic medication and more antiviral medication. We also found no significant differences in the use of lipid-lowering (statins) or antihypertensive medications (Table 2).
In the patients with CHB, we found a collectively assessed prevalence of 77% being obese, 45% having MASLD, 38% having hypercholesterolemia, 26% having hypertension, and 8% having T2D. There were no significant differences between the groups; however, there was a tendency towards lower hypercholesterolemia in patients with CHB (Table 2).
Patients with CHB had lower self-assessed sitting time than those in the comparison group, a tendency toward lower VO2max, and a tendency toward lower self-assessed MVPA. There was no difference in the self-assessed walking activity. The activity monitors showed no differences between the groups in MVPA or sedentary time (Table 3). Nine activity monitor measurements were lost or fell off the participants: Six in the CHB group and three in the comparison group.
| | All participants | Chronic hepatitis B | Comparison group | P value |
| Participants, n | 147 | 98 | 49 | |
| VO2max, mean (± SD), mL/kg/minute | 28.0 (± 7.7) | 26.9 (± 7.5) | 30.2 (± 7.6) | < 0.05 |
| Self-assessed physical activity | ||||
| MVPA, median (IQR), minute/week | 120 (353) | 68 (262) | 188 (406) | < 0.05 |
| Walking activity, mean (± SD), minute/week | 243 (± 548) | 290 (± 538) | 180 (± 364) | 0.10 |
| Sitting time, mean (± SD), minute/week | 2100 (± 2047) | 1680 (± 1470) | 2625 (± 1680) | < 0.05a |
| Measured physical activity | ||||
| MVPA, median (IQR), minute/week | 388 (230) | 357 (257) | 424 (204) | 0.23 |
| Walking activity, mean (± SD), minute/week | 624 (± 232) | 628 (± 248) | 615 (± 201) | 0.76 |
| Sitting time, mean (± SD), minute/week | 3471 (± 981) | 3536 (± 992) | 3345 (± 957) | 0.27 |
| Sedentary time, median (IQR), minute/week | 8399 (656) | 8449 (735) | 8393 (567) | 0.65 |
In a sub-analysis of the patients with CHB treated with antiviral medicine compared with untreated, we found that patients with CHB on antiviral treatment had a tendency of higher WHR mean 0.91 (± 0.06) vs 0.86 (± 0.09) in the untreated group, a tendency of lower levels of cholesterol of median 4.3 mmol/L (IQR: 0.8) vs 4.9 mmol/L (IQR: 1.1) in the untreated group, a tendency of lower levels of HDL cholesterol of median 1.2 mmol/L (IQR: 0.4) vs 1.3 mmol/L (IQR: 0.6), and significantly lower HBV DNA of median 0 IU/mL (IQR: 7.8) compared to 852 IU/mL (IQR: 3747), respectively. We found no differences in BMI, indices of glucose metabolism, triglycerides, LDL cholesterol, ALT, HBeAg, Fib-4 score, TE score, CAP, or VO2max (Supplementary Table 1).
In this baseline assessment of the prospective FitLiver Cohort Study, which included patients with CHB and a matched comparison group, we found that matching the two groups according to sex, age, BMI, and country-of-birth reflected a similar prevalence of cardiometabolic co-morbidities. However, we found that known diagnosis of T2D, and the number of persons with increased HbA1c levels was higher in the comparison group. We found tendencies toward a lower prevalence of collectively assessed hypercholesterolemia in patients with CHB compared to the comparison group. Furthermore, we found no differences in the collectively assessed obesity, MASLD, or hypertension. Individuals from the comparison group had a higher self-assessed sitting time, a tendency toward higher MVPA, and a tendency toward higher VO2max compared to patients with CHB.
Owing to the matching of BMI in this study, it is not surprising that body anthropometrics were similar between the groups. Both groups were, on average, overweight with a BMI > 25, which is similar to the general Danish population, in which approximately 50% were assessed to have a BMI > 25[30]. The collective assessment of obesity, including increased WHR and BMI, further increased the percentage to 76% in both groups, indicating a risk of future cardiometabolic co-morbidities[31].
By investigating liver parameters, we found a tendency of increased ALT and TE-scores in patients with CHB, assessed to indicate inflammation and fibrosis in the liver, which is less surprising both due to chronic inflammation in the liver caused by HBV and due to the combination of CHB, obesity, hyperglycaemia, and dyslipidemia, which have been shown to increase ALT levels in patients with CHB[32]. Relatively few of the patients with CHB were HBeAg positive. Only 1% were anti-HDV positive (no measured HDV-RNA), indicating a generally less active viral infection, also shown by the modest median of 310 IU/mL in HBV DNA, which is probably also associated with the 22% of patients with CHB who received antiviral treatment suppressing viral load. The suggestion of less active viral replication was further confirmed by the median ALT, FIB-4, and TE scores being within normal ranges. The finding of previous HBV infection among individuals in the comparison group of 16% shows that this group of people has a higher exposure to HBV compared to the general Danish population (estimated prevalence of 3%)[33]. However, only 55% had protective antibodies indicating positive vaccination status or cleared previous infection protecting against future HBV infection among the comparison group. Since vaccination against HBV is not part of the general Danish vaccination program, this might indicate a need to improve the vaccination status among populations at a higher risk of HBV exposure. Hepatic steatosis, indicated by CAP > 248, showed a prevalence of 50% in all participants, with a tendency of less (48%) in the patients with CHB compared to the comparison group (53%), and a MASLD prevalence of 47% in all participants which is higher than in a recent American assessment of MASLD prevalence of 32.45%[34]. Furthermore, the previous term, non-alcoholic fatty liver disease, was assessed to have a global prevalence of 29.8%, whereas European countries had an estimated prevalence of 31%[35]. This indicates that both of our study participant groups had an increased prevalence of MASLD compared to the general population.
Hypercholesterolemia can be defined in several ways. In a study from United States, the prevalence of hypercholeste
The prevalence of collectively assessed hypertension in the patients with CHB was 26% and 35% in the comparison group. A Danish assessment of hypertension by essential screening showed a prevalence of 26%[38] which is similar to our findings.
The finding of no diagnosed T2D in the patients with CHB was surprising, since a previous nationwide Danish registry study found a T2D prevalence of 4% among patients with CHB, with a median age of 36 years[14]. Our study collectively assessed T2D by including elevated HbA1c, increased 2-hour glucose levels after an OGTT, or documented diagnosis and treatment with antidiabetic medication. In Denmark, HbA1c is commonly used to diagnose T2D; surprisingly, we found that all patients with CHB had levels below 48 mmol/L. However, HbA1c levels may not be as reliable in certain populations, such as those with hemoglobinopathies, iron deficiency due to anemia, and specific ethnic backgrounds[39]. A higher insulin AUC in patients with CHB suggests an increased insulin requirement to maintain normal blood glucose levels, possibly indicating increased insulin resistance in patients with CHB. However, we found no difference in HOMA-IR. The collectively assessed T2D in both the patients with CHB, and the comparison group was higher than the estimated prevalence of T2D in Denmark at 5.5%[40].
Our study found low VO2max values in both groups (27 mL/kg/minute in patients with CHB and 30 mL/kg/minute in the comparison group) when compared with a cross-sectional German study investigating normal VO2max values at a similar age. They found a mean VO2max of 35 mL/kg/minute in men and 29 mL/kg/minute in women[41]. The World Health Organization recommends 150-300 minutes of moderate-intensity, 75-150 minutes of vigorous-intensity physical activity, or some equivalent combination of moderate-intensity and vigorous-intensity aerobic physical activity per week[42]. In a Korean cohort following 9727 patients with CHB starting antiviral treatment, they found that patients who were physically active for more than 150 minutes of moderate-intensity aerobic exercise each week (assessed by self-assessed physical activity questionnaires) had a lower risk of HCC[43]. Our study found that self-assessed MVPA in both groups was lower than recommended. However, interestingly, the measured MVPA seemed to comply with the recommen
The comparison of treated and untreated patients with CHB showed significant differences only in viral load, which was expected. However, there were tendencies of lower total cholesterol and HDL cholesterol, which have been found previously[44] in patients with CHB treated with antiviral medicine.
A strength of this study is the combination of clinical history and clinical and biochemical findings, including OGTT, to better describe the prevalence of cardiometabolic comorbidities among patients with CHB. Matching with the comparison group enabled the elimination of known confounding factors when comparing the groups prospectively. However, the study was limited by selection or volunteer bias, as participants who willingly agreed to participate may have different characteristics from those of the target population. The suggestion that the included patients with CHB were healthier than the general CHB population in Denmark can be supported by the fact that none of the CHB patients had been diagnosed with T2D and had low alcohol use, which probably also reflects the recommendation of low alcohol use when having CHB[13]. Selection- or volunteer bias can result in the inclusion of a healthier population in research studies, as the examinations require time, ability, and willingness to undergo extensive health examinations[45].
In this baseline assessment of the FitLiver Cohort Study, we found that the patients with CHB and the comparison group were well-matched and had a similar prevalence of cardiometabolic comorbidities. Furthermore, both groups had a low mean VO2max, indicating lower physical fitness, and the patients with CHB had a shorter sitting time than those in the comparison group.
We humbly thank all participants involved in the study for their time and participation. At the Centre for Physical Activity Research, Copenhagen University Hospital, Rigshospitalet, we thank the staff, especially Katja Kofoed, Emil Nelander Schmidt, and Jonas Hvidemose, for assisting in examining participants. We thank the staff at the Department of Infectious Diseases, Copenhagen University Hospital, Hvidovre, especially the project nurses Louise Riger Jensen and Signe Villadsen for assisting with the recruitment and transient elastography of the participants. We thank The Danish National Institute of Public Health, the University of Southern Denmark, and TrygFonden for lending us activity trackers for this project.
| 1. | GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:796-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 417] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 2. | Si J, Yu C, Guo Y, Bian Z, Meng R, Yang L, Chen Y, Jin J, Liu J, Guo Z, Chen J, Chen Z, Lv J, Li L; China Kadoorie Biobank Collaborative Group. Chronic hepatitis B virus infection and total and cause-specific mortality: a prospective cohort study of 0.5 million people. BMJ Open. 2019;9:e027696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62:956-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 408] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 4. | Su TH, Hu TH, Chen CY, Huang YH, Chuang WL, Lin CC, Wang CC, Su WW, Chen MY, Peng CY, Chien RN, Huang YW, Wang HY, Lin CL, Yang SS, Chen TM, Mo LR, Hsu SJ, Tseng KC, Hsieh TY, Suk FM, Hu CT, Bair MJ, Liang CC, Lei YC, Tseng TC, Chen CL, Kao JH; C-TEAM study group and the Taiwan Liver Diseases Consortium. Four-year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. 2016;36:1755-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 5. | Chen YJ, Chen KW, Shih YL, Su FY, Lin YP, Meng FC, Lin F, Yu YS, Han CL, Wang CH, Lin JW, Hsieh TY, Li YH, Lin GM. Chronic hepatitis B, nonalcoholic steatohepatitis and physical fitness of military males: CHIEF study. World J Gastroenterol. 2017;23:4587-4594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Yi YH, Kim YJ, Lee SY, Cho BM, Cho YH, Lee JG. Health behaviors of Korean adults with hepatitis B: Findings of the 2016 Korean National Health and Nutrition Examination Survey. World J Gastroenterol. 2018;24:3163-3170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Cefalu WT, Bray GA, Home PD, Garvey WT, Klein S, Pi-Sunyer FX, Hu FB, Raz I, Van Gaal L, Wolfe BM, Ryan DH. Advances in the Science, Treatment, and Prevention of the Disease of Obesity: Reflections From a Diabetes Care Editors' Expert Forum. Diabetes Care. 2015;38:1567-1582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 8. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1725] [Cited by in RCA: 1745] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 9. | Oh H, Jun DW, Lee IH, Ahn HJ, Kim BO, Jung S, Nguyen MH. Increasing comorbidities in a South Korea insured population-based cohort of patients with chronic hepatitis B. Aliment Pharmacol Ther. 2020;52:371-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Yu MW, Lin CL, Liu CJ, Yang SH, Tseng YL, Wu CF. Influence of Metabolic Risk Factors on Risk of Hepatocellular Carcinoma and Liver-Related Death in Men With Chronic Hepatitis B: A Large Cohort Study. Gastroenterology. 2017;153:1006-1017.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | Wang CS, Yao WJ, Chang TT, Wang ST, Chou P. The impact of type 2 diabetes on the development of hepatocellular carcinoma in different viral hepatitis statuses. Cancer Epidemiol Biomarkers Prev. 2009;18:2054-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Raffetti E, Fattovich G, Donato F. Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: a systematic review and meta-analysis. Liver Int. 2016;36:1239-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 13. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3804] [Article Influence: 475.5] [Reference Citation Analysis (1)] |
| 14. | Bollerup S, Hallager S, Engsig F, Mocroft A, Krarup H, Madsen LG, Thielsen P, Balslev U, Mens H, Barfod TS, Clausen MR, Hobolth L, Laursen AL, Tarp B, Roege BT, Hansen JB, Mygind L, Christensen PB, Gerstoft J, Weis N. Mortality and cause of death in persons with chronic hepatitis B virus infection versus healthy persons from the general population in Denmark. J Viral Hepat. 2022;29:727-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Jespersen S, Plomgaard P, Madsbad S, Hansen AE, Bandholm T, Pedersen BK, Ritz C, Weis N, Krogh-Madsen R. Effect of aerobic exercise training on the fat fraction of the liver in persons with chronic hepatitis B and hepatic steatosis: Trial protocol for a randomized controlled intervention trial- The FitLiver study. Trials. 2023;24:398. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC, Ferraioli G, Chan WK, Wong VW, Myers RP, Chayama K, Friedrich-Rust M, Beaugrand M, Shen F, Hiriart JB, Sarin SK, Badea R, Jung KS, Marcellin P, Filice C, Mahadeva S, Wong GL, Crotty P, Masaki K, Bojunga J, Bedossa P, Keim V, Wiegand J. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 843] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 17. | Knopp JL, Holder-Pearson L, Chase JG. Insulin Units and Conversion Factors: A Story of Truth, Boots, and Faster Half-Truths. J Diabetes Sci Technol. 2019;13:597-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 18. | Radcliffe Department of Medicine. HOMA2 calculator. [cited 13 September 2024]. Available from: https://www.rdm.ox.ac.uk/about/our-clinical-facilities-and-units/DTU/software/homa. |
| 19. | World Health Organization (WHO). Waist circumference and waist–hip ratio: report of a WHO expert consultation, Geneva, 8–11 Dec 2008. [cited 13 September 2024]. Available from: https://iris.who.int/bitstream/handle/10665/44583/9789241501491_eng.pdf?sequence=1. |
| 20. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Narro GEC, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Ann Hepatol. 2024;29:101133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 325] [Article Influence: 325.0] [Reference Citation Analysis (0)] |
| 21. | Nantsupawat N, Booncharoen A, Wisetborisut A, Jiraporncharoen W, Pinyopornpanish K, Chutarattanakul L, Angkurawaranon C. Appropriate Total cholesterol cut-offs for detection of abnormal LDL cholesterol and non-HDL cholesterol among low cardiovascular risk population. Lipids Health Dis. 2019;18:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, Wainford RD, Williams B, Schutte AE. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 2103] [Article Influence: 420.6] [Reference Citation Analysis (0)] |
| 23. | Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, Muiesan ML, Tsioufis K, Agabiti-Rosei E, Algharably EAE, Azizi M, Benetos A, Borghi C, Hitij JB, Cifkova R, Coca A, Cornelissen V, Cruickshank JK, Cunha PG, Danser AHJ, Pinho RM, Delles C, Dominiczak AF, Dorobantu M, Doumas M, Fernández-Alfonso MS, Halimi JM, Járai Z, Jelaković B, Jordan J, Kuznetsova T, Laurent S, Lovic D, Lurbe E, Mahfoud F, Manolis A, Miglinas M, Narkiewicz K, Niiranen T, Palatini P, Parati G, Pathak A, Persu A, Polonia J, Redon J, Sarafidis P, Schmieder R, Spronck B, Stabouli S, Stergiou G, Taddei S, Thomopoulos C, Tomaszewski M, Van de Borne P, Wanner C, Weber T, Williams B, Zhang ZY, Kjeldsen SE. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens. 2023;41:1874-2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 1355] [Article Influence: 677.5] [Reference Citation Analysis (0)] |
| 24. | International Diabetes Federation. IDF Clinical Practice Recommendations for Managing Type 2 Diabetes in Primary Care. 2017. [cited 3 September 2024]. Available from: https://idf.org/media/uploads/2023/05/attachments-63.pdf. |
| 25. | Rasmussen IE, Løk M, Durrer CG, Foged F, Schelde VG, Budde JB, Rasmussen RS, Høvighoff EF, Rasmussen V, Lyngbæk M, Jønck S, Krogh-Madsen R, Lindegaard B, Jørgensen PG, Køber L, Vejlstrup N, Klarlund Pedersen B, Ried-Larsen M, Lund MAV, Christensen RH, Berg RMG. Impact of high-intensity interval training on cardiac structure and function after COVID-19: an investigator-blinded randomized controlled trial. J Appl Physiol (1985). 2023;135:421-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 26. | Thomsen SN, Simonsen C, Christensen JF. Postoperative exercise training in patients with colorectal liver metastases undergoing surgery (ELMA): a protocol for a randomized controlled trial. 2023 Preprint. Available from: OSF Preprints. [DOI] [Full Text] |
| 27. | Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377-381. [PubMed] |
| 28. | Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2524] [Cited by in RCA: 2781] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 29. | Brønd JC. PhysAccel package. [cited 13 September 2024]. Available from: https://github.com/jbrond/PhysAccel. |
| 30. | The Danish Health Authority. Danskernes sundhed – Den Nationale Sundhedsprofl 2021. 2022. [cited 13 September 2024]. Available from: https://www.sst.dk/-/media/Udgivelser/2022/Sundhedsprofil/Sundhedsprofilen.ashx. |
| 31. | Valenzuela PL, Carrera-Bastos P, Castillo-García A, Lieberman DE, Santos-Lozano A, Lucia A. Obesity and the risk of cardiometabolic diseases. Nat Rev Cardiol. 2023;20:475-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 200] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 32. | Wang YY, Lin SY, Sheu WH, Liu PH, Tung KC. Obesity and diabetic hyperglycemia were associated with serum alanine aminotransferase activity in patients with hepatitis B infection. Metabolism. 2010;59:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Bollerup S, Wessman M, Hansen JF, Nielsen S, Hay G, Cowan S, Krarup H, Omland L, Jepsen P, Weis N, Christensen PB. Increasing prevalence of chronic hepatitis B virus infection and low linkage to care in Denmark on 31 December 2016 - an update based on nationwide registers. Infect Dis (Lond). 2023;55:17-26. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Kalligeros M, Vassilopoulos A, Vassilopoulos S, Victor DW, Mylonakis E, Noureddin M. Prevalence of Steatotic Liver Disease (MASLD, MetALD, and ALD) in the United States: NHANES 2017-2020. Clin Gastroenterol Hepatol. 2024;22:1330-1332.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 89] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 35. | Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, Ye Q, Huang DQ, Zhao C, Zhang J, Liu C, Chang N, Xing F, Yan S, Wan ZH, Tang NSY, Mayumi M, Liu X, Liu C, Rui F, Yang H, Yang Y, Jin R, Le RHX, Xu Y, Le DM, Barnett S, Stave CD, Cheung R, Zhu Q, Nguyen MH. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:2809-2817.e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 401] [Article Influence: 133.7] [Reference Citation Analysis (2)] |
| 36. | Wong ND, Lopez V, Tang S, Williams GR. Prevalence, treatment, and control of combined hypertension and hypercholesterolemia in the United States. Am J Cardiol. 2006;98:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Blacher J, Gabet A, Vallée A, Ferrières J, Bruckert E, Farnier M, Olié V. Prevalence and management of hypercholesterolemia in France, the Esteban observational study. Medicine (Baltimore). 2020;99:e23445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Kronborg CN, Hallas J, Jacobsen IA. Prevalence, awareness, and control of arterial hypertension in Denmark. J Am Soc Hypertens. 2009;3:19-24.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Malkani S, DeSilva T. Controversies on how diabetes is diagnosed. Curr Opin Endocrinol Diabetes Obes. 2012;19:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Carstensen B, Rønn PF, Jørgensen ME. Prevalence, incidence and mortality of type 1 and type 2 diabetes in Denmark 1996-2016. BMJ Open Diabetes Res Care. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 41. | Rapp D, Scharhag J, Wagenpfeil S, Scholl J. Reference values for peak oxygen uptake: cross-sectional analysis of cycle ergometry-based cardiopulmonary exercise tests of 10 090 adult German volunteers from the Prevention First Registry. BMJ Open. 2018;8:e018697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 42. | Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, Carty C, Chaput JP, Chastin S, Chou R, Dempsey PC, DiPietro L, Ekelund U, Firth J, Friedenreich CM, Garcia L, Gichu M, Jago R, Katzmarzyk PT, Lambert E, Leitzmann M, Milton K, Ortega FB, Ranasinghe C, Stamatakis E, Tiedemann A, Troiano RP, van der Ploeg HP, Wari V, Willumsen JF. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451-1462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4541] [Cited by in RCA: 5403] [Article Influence: 1080.6] [Reference Citation Analysis (1)] |
| 43. | Chun HS, Park S, Lee M, Cho Y, Kim HS, Choe AR, Kim HY, Yoo K, Kim TH. Association of Physical Activity with the Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Yakut A, Aladag M. The relationship between the use of nucleos(t)ide analogs and metabolic parameters in patients with chronic hepatitis B. Eur Rev Med Pharmacol Sci. 2023;27:9315-9323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (2)] |
| 45. | Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1531] [Cited by in RCA: 1886] [Article Influence: 94.3] [Reference Citation Analysis (0)] |