Published online Jan 27, 2025. doi: 10.4254/wjh.v17.i1.96105
Revised: October 29, 2024
Accepted: November 19, 2024
Published online: January 27, 2025
Processing time: 249 Days and 23 Hours
Liver fibrosis and cirrhosis are global medical challenges that require safe and effective treatments. In the past two decades, there has been a surge in research on stem cell therapy for liver fibrosis and cirrhosis. This study aimed to conduct a comprehensive analysis of the research hotspots and trends in this field through bibliometrics.
To conduct a bibliometric analysis on hotspots and trends in stem cell therapy for treatment of liver fibrosis and cirrhosis.
Publications on stem cell therapy for liver fibrosis and cirrhosis were retrieved from the Web of Science Core Collection database. The distribution and collaboration among literature, authors, countries, and institutions were analyzed vi
As of September 20, 2024, a total of 1935 documents were retrieved dating from 2004 to 2024, with 1186 strongly relevant publications obtained after screening. China, the United States, and Japan were the major contributors in this field. Cairo University, Zhejiang University and Yamaguchi University were the major institution in this field. The journal Stem Cell Research & Therapy published the most papers. There were 686 authors, with Shuji Terai, Isao Sakaida, Soon Koo Baik, and Lanjuan Li publishing the most papers. The research focused on alcoholic cirrhosis and nonalcoholic fatty liver disease. The emerging areas of interest were extracellular vesicles, exosomes, and their enriched microRNAs. The field is experiencing rapid growth due to the changing research trends and increasing literature.
These findings provide a thorough overview of stem cell therapy in the field of liver fibrosis and cirrhosis.
Core Tip: Liver fibrosis and cirrhosis are global challenges that require effective treatments. This study presented a bibliometric analysis of publications on stem cell therapy for liver fibrosis and cirrhosis from the last 20 years that were retrieved from the Web of Science Core Collection database. China was the productive country. Cairo University was the major institution. Shuji Terai was the most prolific author. Stem Cell Research & Therapy was the leading journal publishing the most papers. The text presented an overview of several emerging areas of interest, including alcoholic cirrhosis, nonalcoholic fatty liver disease, extracellular vesicles, exosomes, and inflammation.
- Citation: Zhu WY, Li X, Xie JL, Lu Q, Ma YJ, Zhu ZJ, Liu J. Hotspots and trends in stem cell therapy for liver fibrosis and cirrhosis: A bibliometric analysis. World J Hepatol 2025; 17(1): 96105
- URL: https://www.wjgnet.com/1948-5182/full/v17/i1/96105.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i1.96105
Liver fibrosis and cirrhosis are global health challenges. Liver fibrosis occurs as a chronic wound healing response to liver injury from various causes such as viral hepatitis, alcoholic liver disease, drug-induced liver injury, and metabolic-related liver diseases. Cirrhosis represents the advanced stage of liver fibrosis and is characterized by distorted liver structure, liver dysfunction, and multiple life-threatening complications[1]. Overall, 10% to 20% of patients with the most common chronic liver diseases, including chronic viral hepatitis, alcoholic liver disease, and nonalcoholic fatty liver disease (NAFLD), will develop cirrhosis in 10-20 years. Research to alleviate liver fibrosis and prevent its progression to cirrhosis holds significant clinical value.
Stem cells are undifferentiated cells that can self-renew and differentiate into different tissues. Mesenchymal stem cells (MSCs) are adult pluripotent stem cells with extensive clinical applications[2]. MSCs can be classified into umbilical cord (UC)-MSC, adipose-derived (AD)-MSC, bone marrow (BM)-MSC, and other types based on their sources. In recent years, stem cell therapy for liver fibrosis and cirrhosis has attracted significant attention. As a result, there is a growing number of publications on this topic. Therefore, it is necessary to utilize bibliometric methods to analyze the research status, hotspots, and frontiers of stem cell therapy for liver fibrosis and cirrhosis.
This study summarized the current knowledge on stem cell therapy for liver fibrosis and cirrhosis using bibliometric methods. It aimed to answer questions about the current understanding, latest trends, and therapeutic potential of stem cells in treating liver fibrosis and cirrhosis. Although stem cells have demonstrated potential in preclinical and clinical trials, large-scale clinical trials have been limited due to various factors. These factors include the complexity of etiology, the diversity of cell sources, the quantity of cells, potential tumorigenicity, and ethical issues. The findings from this study provide an overview of the current research status in this field.
We retrieved and exported relevant articles from the Web of Science (WoS) Core Collection on September 20, 2024. The literature had been published between 2004 and 2024. All authors agreed on the search strategy described below: [TS = (therapy OR treatment OR treat OR therapia OR therapeutics OR therapeusis OR cure OR remedy OR handling OR management)] AND [TS = (“cirrhosis” OR “liver fibrosis” OR “liver cirrhosis” OR “hepatofibrosis” OR “hepatocirrhosis” OR “hepatic fibrosis” OR “hepatic cirrhosis” OR “cirrhosis, liver” OR “fibrosis, liver” OR “cirrhosis, hepatic” OR “fibrosis, hepatic”)] AND [TS = (“mesenchymal stem cell*” OR “mesenchymal stromal cell*” OR “bone marrow stromal stem cell*” OR “mesenchymal stem cell*” OR “bone marrow stromal cell*” OR “mesenchymal progenitor cell*” OR “Wharton* Jelly Cell*” OR “stem cell*” OR “progenitor cell*”)]. A total of 1935 publications were retrieved. Following the screening process, non-English literature was excluded, and only articles or review articles were retained, resulting in 1800 valid publications. All records and references were then exported. To facilitate further analysis, 614 irrelevant publications were screened out by two-person back-to-back screening. Subsequently, 1186 highly relevant publications were further studied.
We analyzed the number and trend of publications using Microsoft Excel. We analyzed the number of papers, centrality, and collaboration relationships among authors, countries, and institutions using CiteSpace, Bibliometrix R-package, VOSviewer and Pajek. We also studied the keywords, clusters, and citation bursts and generated a visualized co-oc
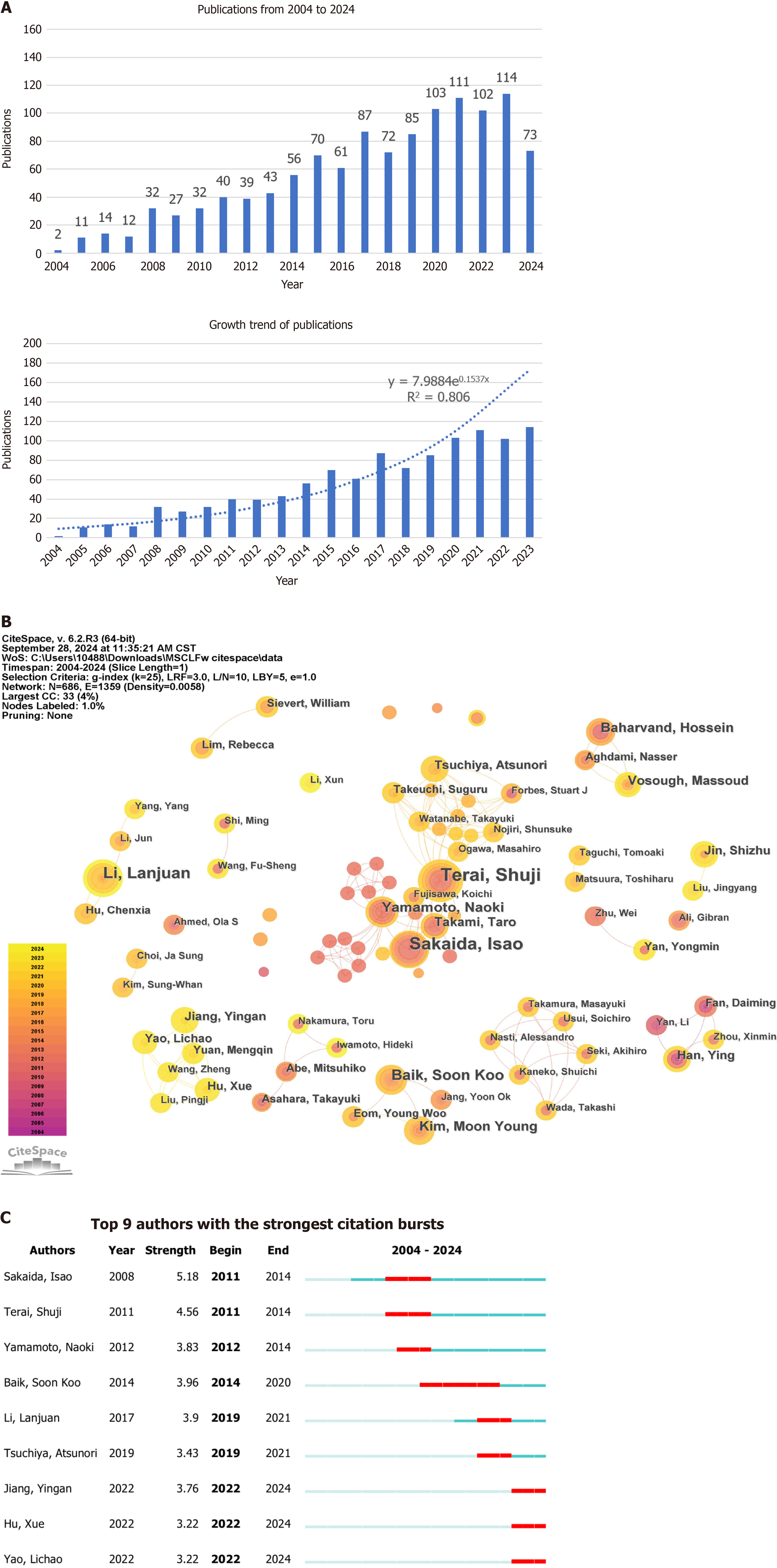
As shown in Figure 1, this study analyzed 1186 articles on stem cell application in treating liver fibrosis and cirrhosis. Figure 2A shows the year, number, and growth trend of the publications. The first article was published on July 15, 2004. Since then, the number of annual publications has steadily increased from 2 in 2004 to 114 in 2023. In the 1st 9 months of 2024, 73 articles were published. Since the data for 2024 is incomplete, we conducted an exponential analysis on publication counts from 2004 to 2023. The independent variable was the publication year (x), and the dependent variable was the annual number of literature articles (y). The model demonstrated a strong fit with the data (R² = 0.8803). Based on the exponential curve equation y = 7.9884e0.1537x, a clear trend of increasing literature counts year by year was found. This provided further evidence of the significant growth and development in research on the use of stem cells in the treatment of liver fibrosis and cirrhosis.
A total of 686 authors participated in research on stem cell therapy for liver fibrosis and cirrhosis. The network density was 0.0058, indicating weak collaborative relationships among authors (Figure 2B). Table 1 shows the most prolific authors: Shuji Terai (with 22 articles and an H-index of 17); Isao Sakaida (with 19 articles and an H-index of 14); Lanjuan Li (with 15 articles and an H-index of 11); and Soon Koo Baik (with 12 articles and an H-index of 11). Figure 2C illustrates authors with the strongest citation bursts, with nine authors exhibiting sustained bursts for at least 2 years. Isao Sakaida and Shuji Terai began their bursts in 2011, Yingan Jiang, Xue Hu, and Lichao Yao have sustained their bursts to the present.
| Rank | Author | Articles | H-index | Total citations |
| 1 | Shuji Terai | 22 | 17 | 668 |
| 2 | Isao Sakaida | 19 | 14 | 388 |
| 3 | Lanjuan Li | 15 | 11 | 370 |
| 4 | Soon Koo Baik | 12 | 11 | 845 |
| 5 | Naoki Yamamoto | 10 | 7 | 195 |
| 6 | Hossein Baharvand | 9 | 9 | 643 |
| 7 | Moon Young Kim | 9 | 9 | 520 |
| 8 | Taro Takami | 8 | 7 | 174 |
| 9 | Massoud Vosough | 8 | 5 | 140 |
| 10 | Yingan Jiang | 7 | 3 | 27 |
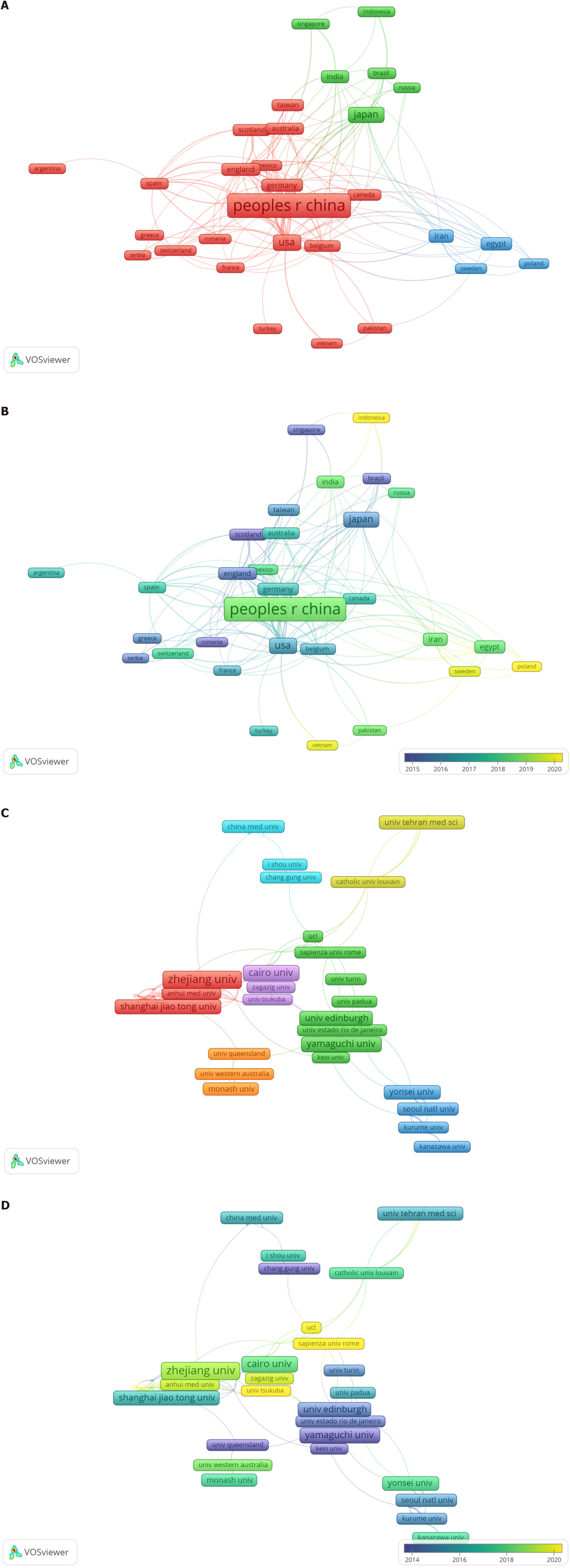
A total of 391 institutions from 63 countries/regions have conducted research on stem cell therapy for liver fibrosis and cirrhosis. The top 10 productive countries and institutions are listed in Table 2. China led with 463 publications, followed by the United States with 152 publications. Other productive countries included Japan, South Korea, Egypt, Italy, and Iran. Japan, Egypt, and Italy have numerous research achievements and demonstrate high centrality, indicating the importance of their research. In 63 countries, 34 countries published five or more articles. Figure 3 depicts the collaboration among countries and regions. We conducted a co-authorship analysis of all publications, and these 34 countries formed 7 clusters. England, United States, and Japan have made significant contributions to the early development of research in this field. South Korea, China, Egypt, and Iran have been more involved in research in this field since 2018.
| Rank | Country | Articles | Centrality | Institution | Articles | Centrality |
| 1 | China | 463 | 0.06 | Cairo University | 63 | 0.13 |
| 2 | United States | 152 | 0.06 | Zhejiang University | 43 | 0.08 |
| 3 | Japan | 122 | 0.08 | Yamaguchi University | 25 | 0.02 |
| 4 | South Korea | 76 | 0.06 | Air Force Military Medical University | 20 | 0 |
| 5 | Egypt | 63 | 0.11 | Sun Yat-sen University | 20 | 0.02 |
| 6 | Italy | 59 | 0.24 | Capital Medical University | 18 | 0.02 |
| 7 | Iran | 57 | 0 | Chinese Academy of Sciences | 17 | 0.05 |
| 8 | India | 50 | 0.58 | Academic Center for Education, Culture & Research | 17 | 0.04 |
| 9 | England | 43 | 0.56 | Chinese Academy of Medical Sciences - Peking Union Medical College | 16 | 0.04 |
| 10 | Germany | 41 | 0.47 | University of London | 15 | 0.31 |
A total of 391 institutions globally have conducted research on this topic. The data showed that Cairo University ranked first with 63 publications, followed by Zhejiang University (43) and Yamaguchi University (25) (Table 2). To further investigate the collaboration between research institutions, we conducted a co-authorship analysis of all publications. Figure 3 shows that 120 institutions have published at least 5 papers. Institutions such as the University of Queensland, University of Pittsburgh, Yamaguchi University, and Tokai University have made significant contributions to the early development of this field. Institutions like Cairo University, Zhejiang University, University College London, and Sapienza University of Rome have been more involved in research related to this field since 2018. Cairo University has achieved significant progress in the research of stem cell therapy for liver fibrosis and cirrhosis, led by Salama, Medhat, Elzainy, Zekri, Bahnassy, and Hamman, in collaboration with the Egyptian Knowledge Bank. Keywords fre
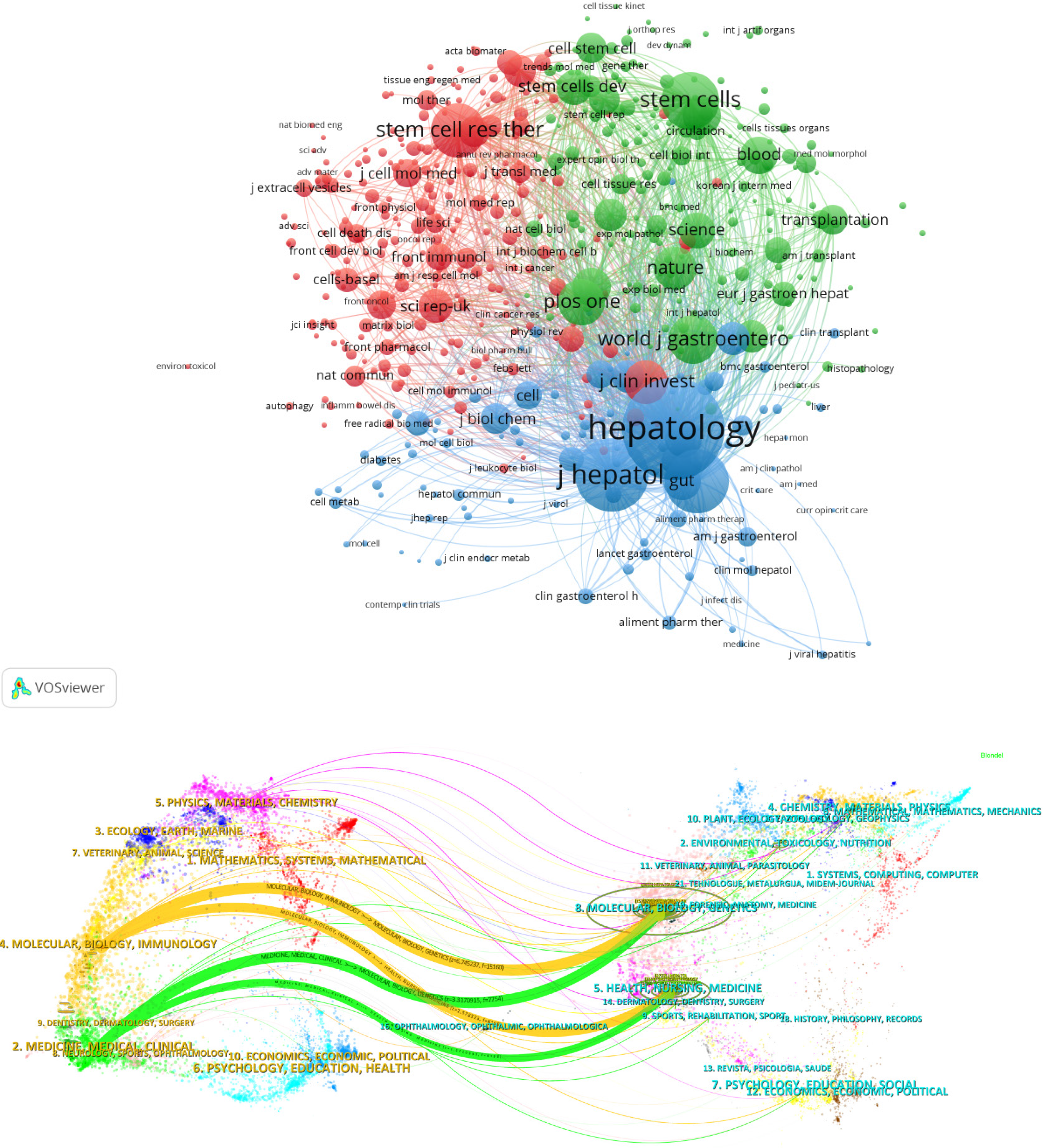
A dual-map overlay of journals can visualize the intersection and integration between disciplines, aiding researchers in predicting trends and forecasting future research directions. The left side of the map displays the journals that published the cited literature, while the right side shows the journals that cited the literature. The curves connecting the two sides represent the citation paths[7]. The orange path in Figure 4 indicated that journals published in the field of molecular/biology/immunology are often influenced by journals in the fields of molecular/biology/genetics (z = 6.745237, f = 15160) and health/nursing/medicine (z = 2.578323, f = 6158). The green path demonstrated that journals published in the medicine/medical/clinical fields were also commonly influenced by journals in molecular/biology/genetics (z = 3.3170915, f = 7754) and health/nursing/medicine (z = 1.6710632, f = 4198). The dual-map overlay of journals indicated a shift in research trends towards immunology-related areas and clinical applications.
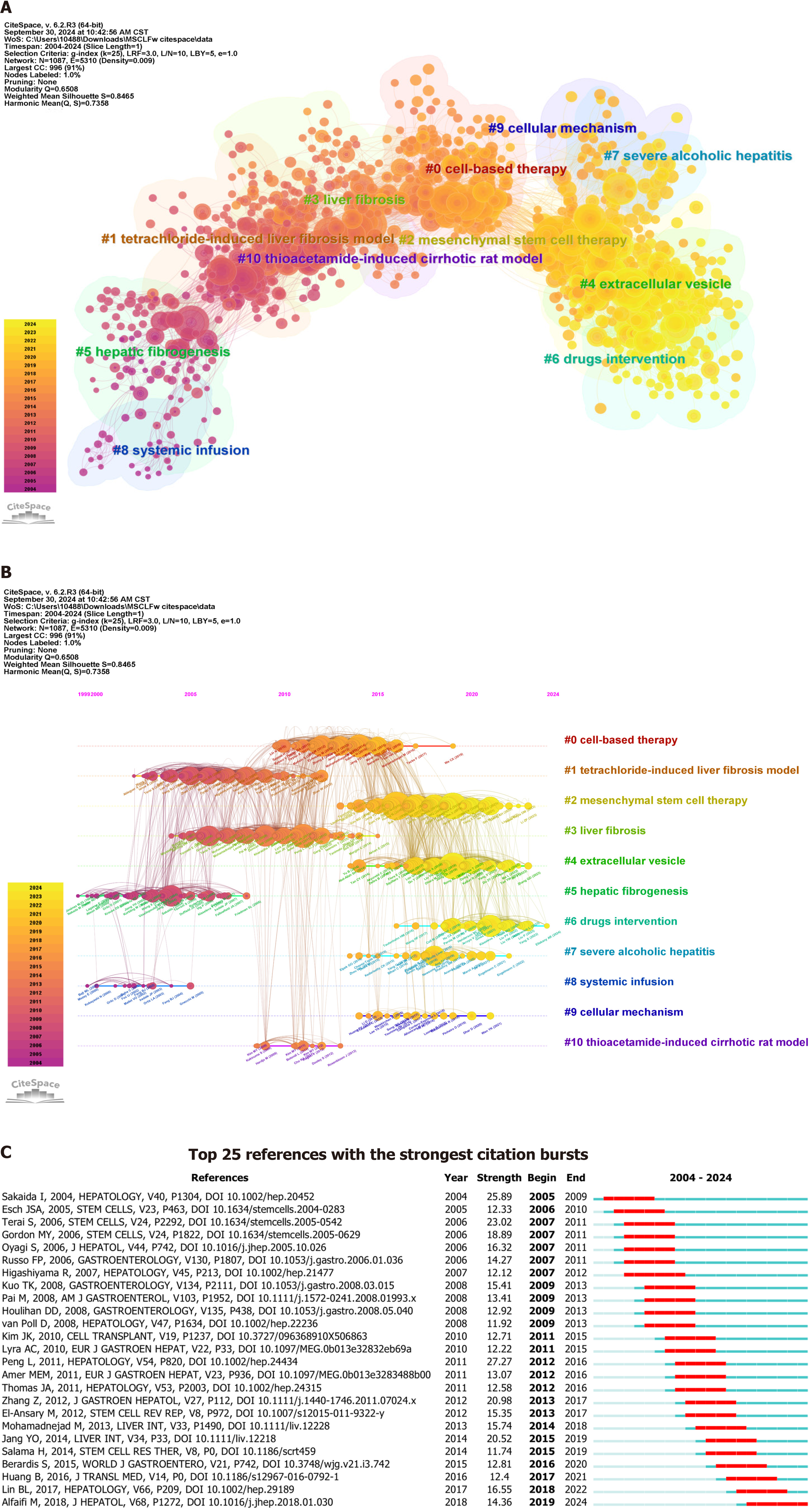
The co-citation analysis evaluated the correlation between publications by constructing a co-citation network. This method helps explore the development and evolution trends in a research field. In this study, the data were divided by year, and the references of the top 50 citing articles were extracted. The resulting co-citation network cluster consisted of 1087 cited articles and 5310 links. Figure 5A shows the clustering of references into 11 groups based on their citation patterns. The modularity Q was 0.6508, indicating a clear clustering division. These clusters were closely connected, reflecting similarity in research content. The size of the nodes represented the citation count, while a purple ring indicated higher betweenness centrality. Table 3 presents the 11 largest clusters, ranked by size. These clusters demonstrated high homogeneity, with Silhouette Coefficient all greater than 0.7. The cluster labels were derived from the noun phrases in the titles of the citing documents[8].
| Cluster ID | Size | Silhouette | Mean year | Label (LLR) |
| 0 | 172 | 0.825 | 2013 | Cell-based therapy |
| 1 | 164 | 0.833 | 2007 | Tetrachloride-induced liver fibrosis model |
| 2 | 162 | 0.743 | 2017 | Mesenchymal stem cell therapy |
| 3 | 132 | 0.792 | 2009 | Liver fibrosis |
| 4 | 119 | 0.902 | 2018 | Extracellular vesicle |
| 5 | 95 | 0.937 | 2003 | Hepatic fibrogenesis |
| 6 | 69 | 0.896 | 2021 | Drugs intervention |
| 7 | 23 | 0.983 | 2017 | Severe alcoholic hepatitis |
| 8 | 22 | 0.991 | 2002 | Systemic infusion |
| 9 | 18 | 0.967 | 2017 | Cellular mechanism |
| 10 | 14 | 0.977 | 2010 | Thioacetamide-induced cirrhotic rat model |
The largest cluster in the network was 0 cell-based therapy, which consisted of 172 co-cited references (Table 3 and Figure 5B). The top-cited article was ‘Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: A pilot study’ by Jang et al[9]. The most cited references in this cluster also included ‘Human umbilical cord mesenchymal stem cells improve liver function and ascites in decom
Cluster 4 (extracellular vesicles) and 6 (drug intervention) are newly emerged clusters.
The main citing article in Cluster 4 was ‘Innovative preconditioning strategies for improving the therapeutic efficacy of extracellular vesicles derived from mesenchymal stem cells in gastrointestinal diseases’ by Didamoony et al[11]. The most frequently cited reference was ‘Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway’ by Rong et al[12].
The main citing article in Cluster 6 was ‘Progress of mesenchymal stem cells (MSCs) & MSC-exosomes combined with drugs intervention in liver fibrosis’ by Xu et al[13]. The most frequently cited reference was ‘Molecular and cellular mechanisms of liver fibrosis and its regression’ by Kisseleva and Brenner[14].
Clusters 4 (extracellular vesicles) and 6 (drug intervention) both focus on exploring the potential, mechanisms, and clinical outlooks of MSCs and their derived extracellular vehicles (EVs) in treating liver fibrosis and cirrhosis, as well as the effects of combining MSCs/MSC-derived exosomes with drug therapy on liver fibrosis. Recent studies have in
We analyzed the citation bursts of co-cited references and found that the top 25 references with the strongest citation bursts all had prolonged periods of burst activity (Figure 5C). Among them, the paper published by Rong et al[12] titled ‘Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway’ has continued to be cited up to the present.
Keywords are important in academic papers. The co-occurrence of keywords refers to the situation where two or more keywords appear together in the same document. By analyzing these co-occurrence relationships, we can understand the connections between keywords and trends in the evolution of the field. This helps us identify the hotspots and frontiers in the field. Keyword clusters was created using CiteSpace, VOSviewer and Pajek (Figure 6). The top 20 keywords with the highest frequency are listed in Table 4.
| Rank | Count | High frequency keyword | Year | Centrality |
| 1 | 411 | Mesenchymal stromal cell | 2004 | 0.04 |
| 2 | 368 | Liver fibrosis | 2004 | 0.04 |
| 3 | 360 | Cirrhosis | 2005 | 0 |
| 4 | 322 | Transplantation | 2005 | 0.04 |
| 5 | 270 | Stem cell | 2005 | 0.01 |
| 6 | 211 | Differentiation | 2004 | 0.1 |
| 7 | 190 | Hepatic stellate cell | 2006 | 0.01 |
| 8 | 184 | Stromal cells | 2008 | 0 |
| 9 | 179 | Bone marrow | 2005 | 0 |
| 10 | 176 | Therapy | 2004 | 0.13 |
| 11 | 164 | Fibrosis | 2008 | 0 |
| 12 | 159 | Expression | 2004 | 0.06 |
| 13 | 146 | Regeneration | 2004 | 0.04 |
| 14 | 135 | Cell therapy | 2006 | 0.02 |
| 15 | 122 | In vitro | 2006 | 0.09 |
| 16 | 117 | Mouse | 2006 | 0 |
| 17 | 110 | Progenitor cells | 2006 | 0.01 |
| 18 | 106 | Injury | 2011 | 0 |
| 19 | 104 | Disease | 2006 | 0.05 |
| 20 | 102 | Hepatocytes | 2004 | 0.09 |
The core research object in this field was mesenchymal stromal cell, which was the most frequent keyword with a frequency of 411, followed by liver fibrosis (368) and cirrhosis (360). The keywords transplantation (322), stem cell (270), and differentiation (211) suggest that researchers are investigating various therapeutic strategies for cirrhosis and liver fibrosis disease, beyond mesenchymal stromal cells. The differentiation potential of different cell types has also been given significant attention. The hepatic stellate cell (190) is the main source of myofibroblasts, a crucial cell in liver fi
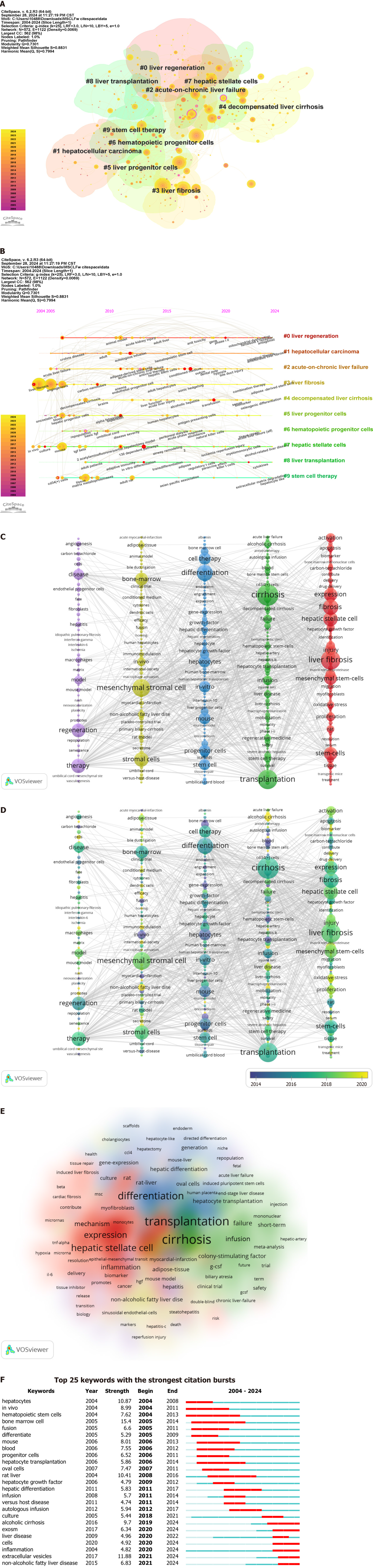
Keyword clustering connects similar topics in network groups. We conducted keyword clustering analysis using the Log-Likelihood Ratio algorithm in Citespace. The resulting network comprises 572 nodes and 1122 connecting lines, encompassing 10 major clusters numbered from 0 and arranged in descending order of size. The modularity of the network is 0.7301, exceeding the significance threshold of 0.3, indicating clear keyword clustering. The average silhouette coefficient of 0.8898 confirms the validity of the clusters, demonstrating high intra-cluster similarity and significant inter-cluster difference (Figure 6A). We also extracted the timeline of co-occurrence keyword network related to stem cell therapy for liver fibrosis and cirrhosis, showing the changes of keywords in each cluster and the burst keywords (Figure 6B).
We conducted cluster analysis on keywords using the association strength method in VOSviewer, resulting in five clusters. Figure 6C visualizes the keyword network, where the size of nodes reflects keyword frequency. A total of 381 keywords that appeared at least 5 times were grouped into these five clusters. Closely related keywords were clustered together. The first cluster, shown in red, focuses on the causes and mechanisms of liver fibrosis, including ‘carbon-tetrachloride’, ‘oxidative stress’, and ‘hepatic stellate cell’. The second cluster, in green, emphasizes clinical treatment and research on cirrhosis, with keywords such as ‘cirrhosis’, ‘transplantation’, ‘hepatocyte transplantation’, ‘survival’, ‘mortality’, ‘safety’, and ‘phase I-II’. The third cluster, in blue, centers on cell therapy, with keywords like ‘cell therapy’, ‘differentiation’, ‘hepatocytes’, and ‘liver regeneration’. The fourth cluster, in yellow, focuses on basic research on mesenchymal stromal cells, mainly involving ‘mesenchymal stromal cell’, ‘bone-marrow’, ‘immunomodulation’, and ‘conditioned medium’. The fifth cluster, in purple, covers the role of mesenchymal stromal cells in promoting liver regeneration, including ‘regeneration’, ‘angiogenesis’, and ‘macrophages’. Figure 6D visualizes the temporal overlap of keywords, with early keywords shown in purple-blue and recent ones in yellow. Early research primarily focused on ‘gene-expression’, ‘hepatocyte transplantation’, and ‘bone-marrow cells’. Recent research has concentrated on topics such as ‘alcoholic cirrhosis’, ‘non-alcoholic fatty liver disease’, ‘nanoparticles’, ‘immunomodulation’, ‘exosomes’, and ‘extracellular vesicles’. Figure 6E also displays the Density visualization of keywords, aiding in understanding the overall structure of the map.
Keywords with the strongest citation bursts often reflect the research hotspots and frontiers in a field over a certain period. We identified the top 25 keywords with high citation rates in this field, with their burst durations lasting for at least 5 years (Figure 6F). Certain keywords, such as hematopoietic stem cells (2004-2013) and bone marrow cell (2005-2014) have experienced a decade-long burst. Other keywords, such as hepatocyte transplantation (2006-2014), rat liver (2008-2016), in vivo (2004-2011), and mouse (2004-2011), emerged early and persisted. This suggests a focus on animal experiments in the early stages of research. More recently, exosome, inflammation, EVs, and NAFLD have gained significant attention. These topics continue to be hotspots of research, attracting numerous researchers. In terms of burst strength, bone marrow cell (15.4) exhibited the strongest burst, followed by EVs (11.88), hepatocytes (10.87), rat liver (10.41), and alcoholic cirrhosis (9.7). EVs had a strong citation burst that has persisted, making it one of the most popular research hotspots in this field.
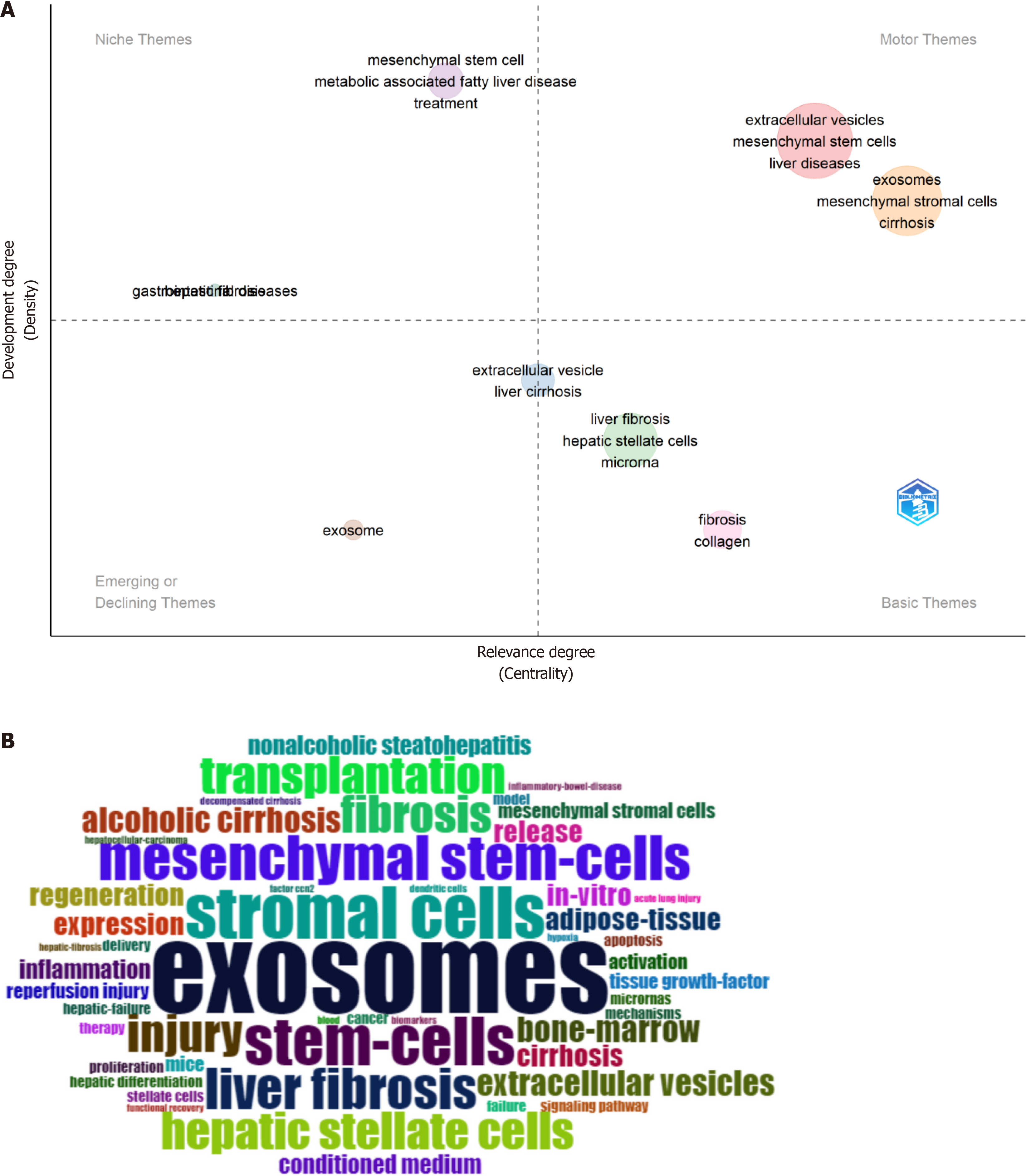
By constructing a thematic map (Figure 7A), we analyzed the interconnections and trends of various research theme. Based on their attention and research intensity, these themes were divided into four categories. Notably, motor themes garnered significant attention and activity, encompassing keywords such as regeneration, biomarkers, cell-free therapy, drug delivery systems, human amnion epithelial cells, macrophages, secretome, immunomodulation, and inflammation. The word cloud in Figure 7B showcases frequent keywords in MSC-EVs research, highlighting key areas of focus within this subdomain.
This study visualized the research trends in stem cell therapy for the treatment of liver fibrosis or cirrhosis from 2004 to 2024, with the aim of providing practical and instructive information for new researchers entering the field.
The top 10 countries published a total of 1126 articles, accounting for 94.9% of all articles. China had the most publications, followed by the United States and Japan, with all three countries publishing over 100 articles. Moreover, eight out of the top ten institutions were situated in Asia. This highlighted the substantial contributions of Asian research institutions to the research on stem cell therapy for liver fibrosis or cirrhosis, which is also linked to the etiology and epidemiological distribution of liver cirrhosis. Hepatitis B is the major cause of liver cirrhosis in China, Korea, and Iran, whereas in the United States, Japan, and Egypt, it is hepatitis C. Alcohol is the main etiology of liver cirrhosis in European countries like the United Kingdom[16]. The incidence of NAFLD-related cirrhosis and alcoholic cirrhosis is increasing due to changes in the etiology of liver cirrhosis, which is attracting more attention and is expected to drive overall research improvement.
Researchers can increase their chances of publishing their manuscripts by targeting journals with a high volume of articles in their field. The following journals have a substantial number of publications: Stem Cell Research & Therapy [75 articles, impact factor (IF) 7.1]; Hepatology (28 articles, IF 12.9]; Journal of Hepatology (14 articles, IF 26.8); and Gastroenterology (10 articles, IF 25.7).
Co-citation of references indicates a close connection between publications, and studying this network helps un
In 2004, Sakaida et al[17] conducted a study using a mouse model of liver fibrosis induced by CCl4. They transplanted BM cells by injecting them into the mice’s tail veins. The results showed that the cells migrated to the fibrotic site, promoted fibrolysis, and effectively reduced liver fibrosis. That study was an important milestone in stem cell research for liver fibrosis and cirrhosis.
The clinical trial conducted by Terai et al[18] initially investigated the safety and efficacy of autologous BM cell infusion. Subsequently, Peng et al[19] demonstrated that BM-MSCs were safe and could improve overall survival in patients with hepatitis B virus-induced cirrhosis complicated with liver failure or hepatitis B virus-related acute-on-chronic liver failure[20]. Suk et al[21] and Jang et al[9] also explored the efficacy of BM-MSCs on alcoholic cirrhosis through two clinical trials. Furthermore, Alfaifi et al[22] reviewed the efficacy, safety, transplantation route, and strategies to enhance the therapeutic efficacy of MSCs in the treatment of liver fibrosis.
Zhang et al[10] demonstrated that UC-MSCs improved liver function and reduced ascites in patients with deco
Keyword analysis holds significant importance in identifying research hotspots, tracking research trends, and discovering emerging topics. This study revealed that alcoholic cirrhosis and NAFLD have become prominent causes of liver disease in recent years. Additionally, exosomes and EVs have become popular keywords for treatment approaches. These changes offer valuable guidance for future research directions.
Exploring the differences in stem cell therapy for liver fibrosis caused by different etiologies is crucial in guiding clinical practice. Currently, there is no research that systematically compares the differences in treatment outcomes for liver cirrhosis caused by different etiologies, such as hepatitis B, alcoholic cirrhosis, and NAFLD, due to their geo
Stem cells have shown promising results in the treatment of hepatitis B-related cirrhosis. Both autologous and allogeneic BM-MSC and UC-MSC transplantation have been proven safe for cirrhosis caused by chronic hepatitis B. Transplantation has improved liver function and synthetic capacity and reduced severe infections[19,20,24].
The study by Zhang et al[10] demonstrated the safety and efficacy of UC-MSCs in the treatment of chronic hepatitis B-related decompensated cirrhosis. Furthermore, that study also revealed that UC-MSCs can reduce the concentrations of fibrotic markers (i.e., laminin, hyaluronic acid, PIIINP, and type IV collagen) at the 24th and 48th week. Additionally, at week 48, the UC-MSC treatment group had significantly higher levels of hepatocyte growth factor compared to the control group, while the level of transforming growth factor β did not change significantly.
Significant progress has also been made in stem cell therapy for hepatitis C-related cirrhosis. Clinical trials have shown that BM-MSCs can effectively improve the synthetic function of the liver, reduce the level of liver enzyme and bilirubin, and lower the Model for end-stage liver disease score[25,26]. Salama et al[3] treated 90 patients with end-stage liver disease using granulocyte colony-stimulating factor combined with autologous CD34 (+) and CD133 (+) stem cells, which normalized the liver enzymes and improved the synthetic function of the liver. They also used granulocyte colony-stimulating factor combined with autologous MSCs to treat hepatitis C-related end-stage liver disease, resulting in a significant reduction in liver fibrosis markers PIIICP and PIIINP. This suggested that MSCs can treat liver fibrosis by downregulating collagen matrix formation[27].
For patients with primary biliary cirrhosis, Wang et al[28] investigated the safety and therapeutic efficacy of UC-MSCs in those who exhibited an incomplete response to ursodeoxycholic acid. UC-MSC treatment led to substantial symptom relief, including fatigue and pruritus. Serum alkaline phosphatase and gamma-glutamyl transferase levels significantly decreased after treatment, and the Mayo risk score remained stable.
In recent years, there has been a significant increase in the incidence of alcoholic cirrhosis. Pai et al[29] treated 9 patients with alcoholic cirrhosis using autologous CD34+ adult progenitor cells. All treated patients showed good tolerance and marked biochemical improvement. Child-Pugh scores decreased in 7 patients, and ascites resolved in 5 patients. In a European multicenter phase II study, treatment with allogeneic liver-derived progenitor cells showed significant improvement in systemic inflammatory markers and clinical indicators for liver function in patients with alcoholic cirrhosis[30]. Two studies showed that autologous BM-MSC transplantation significantly alleviated liver fibrosis and clinical parameters in patients with alcoholic cirrhosis[10,21]. Subsequently, the researchers used cDNA microarrays to analyze the correlation between therapeutic effects and gene expression. High initial Laennec scores and performance of BM-MSC transplantation were predictive factors for responders. Olfactory receptor 2 L8, microRNA (miRNA) 4520-2, and chloride intracellular channel protein 3 genes were upregulated among the responders. Eleven pathways, including CD36, retinol-binding protein 4, and inositol phosphate, were reported as possibly negatively correlated with the the
Nonalcoholic steatohepatitis poses a significant global health concern due to the increased risk of liver fibrosis, cirrhosis, and liver cancer. Urgent intervention is needed to address this issue. Stem cell therapy is the primary focus of research for treating liver fibrosis in NAFLD, although clinical trial results are pending. Animal studies have shown that AD-MSCs and human amnion epithelial cells can improve liver function, reduce fibrosis, and suppress inflammation in mice with NAFLD cirrhosis[32-34].
Researchers have discovered that soluble factors from human amnion epithelial cells can also contribute to the improvement of liver fibrosis[33]. EVs have gained increasing interest to researchers in recent years[34]. Multiple studies show that small EVs derived from MSCs can reduce the NAFLD activity score in animal models[35,36]. These EVs also alleviate liver fibrosis and collagen deposition by regulating lipid homeostasis[36,37], inhibiting the activation of hepatic stellate cells, and correcting choline metabolism disorders[38].
In an in vitro liver fibrosis model, exosomes derived from UC-MSCs effectively suppressed the expression of transforming growth factor β1, interleukin 1β, and interleukin 6. It also inhibited LX2 activation, reducing the production of extracellular matrix proteins (COL I and α-SMA). In addition, it lowered alanine aminotransferase and aspartate aminotransferase levels, increased albumin levels, and decreased the production of reactive oxygen species[39]. Studies suggest that miRNAs against transforming growth factor may play a vital role in reducing liver fibrosis. One specific miRNA, miR-627-5p, derived from exosomes of human UC-MSCs, was shown to improve glucose and lipid metabolism in rats by suppressing FTO expression. This led to reduced liver injury and a delay in the progression of NAFLD[40]. MiR-223-3p in AD-MSC-EVs inhibited E2F1 transcription factor, regulated liver fibrosis[41], and delayed NAFLD pro
Research on stem cell therapy to treat liver cirrhosis has shown promising results. Most studies have focused on MSCs sourced from various tissues. Cell-free therapies like MSC-EVs, exosomes, and miRNAs enriched in MSC-EVs are also being studied for liver fibrosis and cirrhosis treatment. More research and clinical trials are needed to understand their therapeutic mechanisms and improve treatment effectiveness.
This study also has some limitations. Network analysis based on the WoS database may not fully capture overall trends. Other databases lack comprehensive bibliometric analysis data, and varying export formats hinder literature consolidation, potentially causing omissions. Future improvements in methods are planned.
This study presented the first bibliometric analysis of stem cell therapy for liver fibrosis and cirrhosis. The findings identified prominent studies, countries, institutions, journals, and authors. The study provided useful information on the future research direction of stem cells in liver fibrosis and cirrhosis, helping researchers understand the development, hotspots, trends, and frontiers of stem cell therapy.
| 1. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [PubMed] [DOI] [Full Text] |
| 2. | Tan F, Li X, Wang Z, Li J, Shahzad K, Zheng J. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther. 2024;9:17. [PubMed] [DOI] [Full Text] |
| 3. | Salama H, Zekri AR, Bahnassy AA, Medhat E, Halim HA, Ahmed OS, Mohamed G, Al Alim SA, Sherif GM. Autologous CD34+ and CD133+ stem cells transplantation in patients with end stage liver disease. World J Gastroenterol. 2010;16:5297-5305. [PubMed] [DOI] [Full Text] |
| 4. | Salama H, Zekri AR, Zern M, Bahnassy A, Loutfy S, Shalaby S, Vigen C, Burke W, Mostafa M, Medhat E, Alfi O, Huttinger E. Autologous hematopoietic stem cell transplantation in 48 patients with end-stage chronic liver diseases. Cell Transplant. 2010;19:1475-1486. [PubMed] [DOI] [Full Text] |
| 5. | Didamoony MA, Atwa AM, Ahmed LA. Modulatory effect of rupatadine on mesenchymal stem cell-derived exosomes in hepatic fibrosis in rats: A potential role for miR-200a. Life Sci. 2023;324:121710. [PubMed] [DOI] [Full Text] |
| 6. | Elzainy A, El Sadik A, Altowayan WM. Comparison between the Regenerative and Therapeutic Impacts of Bone Marrow Mesenchymal Stem Cells and Adipose Mesenchymal Stem Cells Pre-Treated with Melatonin on Liver Fibrosis. Biomolecules. 2024;14:297. [PubMed] [DOI] [Full Text] |
| 7. | Chen C, Dubin R, Kim MC. Emerging trends and new developments in regenerative medicine: a scientometric update (2000 - 2014). Expert Opin Biol Ther. 2014;14:1295-1317. [PubMed] [DOI] [Full Text] |
| 8. | Chen C, Ibekwe-Sanjuan F, Hou J. The structure and dynamics of cocitation clusters: A multiple-perspective cocitation analysis. J Am Soc Inf Sci. 2010;61:1386-1409. [DOI] [Full Text] |
| 9. | Jang YO, Kim YJ, Baik SK, Kim MY, Eom YW, Cho MY, Park HJ, Park SY, Kim BR, Kim JW, Soo Kim H, Kwon SO, Choi EH, Kim YM. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int. 2014;34:33-41. [PubMed] [DOI] [Full Text] |
| 10. | Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J, Chen L, Lv S, Li Y, Yu S, Geng H, Jin L, Lau GK, Wang FS. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27 Suppl 2:112-120. [PubMed] [DOI] [Full Text] |
| 11. | Didamoony MA, Soubh AA, Atwa AM, Ahmed LA. Innovative preconditioning strategies for improving the therapeutic efficacy of extracellular vesicles derived from mesenchymal stem cells in gastrointestinal diseases. Inflammopharmacology. 2023;31:2973-2993. [PubMed] [DOI] [Full Text] |
| 12. | Rong X, Liu J, Yao X, Jiang T, Wang Y, Xie F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther. 2019;10:98. [PubMed] [DOI] [Full Text] |
| 13. | Xu Y, Zhou X, Wang X, Jin Y, Zhou L, Ye J. Progress of mesenchymal stem cells (MSCs) & MSC-Exosomes combined with drugs intervention in liver fibrosis. Biomed Pharmacother. 2024;176:116848. [PubMed] [DOI] [Full Text] |
| 14. | Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151-166. [PubMed] [DOI] [Full Text] |
| 15. | Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199-210. [PubMed] [DOI] [Full Text] |
| 16. | Huang DQ, Terrault NA, Tacke F, Gluud LL, Arrese M, Bugianesi E, Loomba R. Global epidemiology of cirrhosis - aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023;20:388-398. [PubMed] [DOI] [Full Text] |
| 17. | Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304-1311. [PubMed] [DOI] [Full Text] |
| 18. | Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, Yokoyama Y, Uchida K, Yamasaki T, Fujii Y, Okita K, Sakaida I. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292-2298. [PubMed] [DOI] [Full Text] |
| 19. | Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, Zheng YB, Gao ZL. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820-828. [PubMed] [DOI] [Full Text] |
| 20. | Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, Chen XY, Liu QL, Peng L, Li JG, Mei YY, Weng WZ, Peng YW, Cao HJ, Xie JQ, Xie SB, Xiang AP, Gao ZL. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. 2017;66:209-219. [PubMed] [DOI] [Full Text] |
| 21. | Suk KT, Yoon JH, Kim MY, Kim CW, Kim JK, Park H, Hwang SG, Kim DJ, Lee BS, Lee SH, Kim HS, Jang JY, Lee CH, Kim BS, Jang YO, Cho MY, Jung ES, Kim YM, Bae SH, Baik SK. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology. 2016;64:2185-2197. [PubMed] [DOI] [Full Text] |
| 22. | Alfaifi M, Eom YW, Newsome PN, Baik SK. Mesenchymal stromal cell therapy for liver diseases. J Hepatol. 2018;68:1272-1285. [PubMed] [DOI] [Full Text] |
| 23. | Mohamadnejad M, Alimoghaddam K, Bagheri M, Ashrafi M, Abdollahzadeh L, Akhlaghpoor S, Bashtar M, Ghavamzadeh A, Malekzadeh R. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013;33:1490-1496. [PubMed] [DOI] [Full Text] |
| 24. | Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z, Zhang A, Shi J, Chen L, Lv S, He W, Geng H, Jin L, Liu Z, Wang FS. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725-731. [PubMed] [DOI] [Full Text] |
| 25. | Amin MA, Sabry D, Rashed LA, Aref WM, el-Ghobary MA, Farhan MS, Fouad HA, Youssef YA. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin Transplant. 2013;27:607-612. [PubMed] [DOI] [Full Text] |
| 26. | El-Ansary M, Abdel-Aziz I, Mogawer S, Abdel-Hamid S, Hammam O, Teaema S, Wahdan M. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev Rep. 2012;8:972-981. [PubMed] [DOI] [Full Text] |
| 27. | Salama H, Zekri AR, Medhat E, Al Alim SA, Ahmed OS, Bahnassy AA, Lotfy MM, Ahmed R, Musa S. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther. 2014;5:70. [PubMed] [DOI] [Full Text] |
| 28. | Wang L, Li J, Liu H, Li Y, Fu J, Sun Y, Xu R, Lin H, Wang S, Lv S, Chen L, Zou Z, Li B, Shi M, Zhang Z, Wang FS. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28 Suppl 1:85-92. [PubMed] [DOI] [Full Text] |
| 29. | Pai M, Zacharoulis D, Milicevic MN, Helmy S, Jiao LR, Levicar N, Tait P, Scott M, Marley SB, Jestice K, Glibetic M, Bansi D, Khan SA, Kyriakou D, Rountas C, Thillainayagam A, Nicholls JP, Jensen S, Apperley JF, Gordon MY, Habib NA. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103:1952-1958. [PubMed] [DOI] [Full Text] |
| 30. | Nevens F, Gustot T, Laterre PF, Lasser LL, Haralampiev LE, Vargas V, Lyubomirova D, Albillos A, Najimi M, Michel S, Stoykov I, Gordillo N, Vainilovich Y, Barthel V, Clerget-Chossat N, Sokal EM. A phase II study of human allogeneic liver-derived progenitor cell therapy for acute-on-chronic liver failure and acute decompensation. JHEP Rep. 2021;3:100291. [PubMed] [DOI] [Full Text] |
| 31. | Gupta H, Youn GS, Han SH, Shin MJ, Yoon SJ, Han DH, Lee NY, Kim DJ, Baik SK, Suk KT. Response-Related Factors of Bone Marrow-Derived Mesenchymal Stem Cells Transplantation in Patients with Alcoholic Cirrhosis. J Clin Med. 2019;8. [PubMed] [DOI] [Full Text] |
| 32. | Seki A, Sakai Y, Komura T, Nasti A, Yoshida K, Higashimoto M, Honda M, Usui S, Takamura M, Takamura T, Ochiya T, Furuichi K, Wada T, Kaneko S. Adipose tissue-derived stem cells as a regenerative therapy for a mouse steatohepatitis-induced cirrhosis model. Hepatology. 2013;58:1133-1142. [PubMed] [DOI] [Full Text] |
| 33. | Kuk N, Hodge A, Sun Y, Correia J, Alhomrani M, Samuel C, Moore G, Lim R, Sievert W. Human amnion epithelial cells and their soluble factors reduce liver fibrosis in murine non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2019;34:1441-1449. [PubMed] [DOI] [Full Text] |
| 34. | Watanabe T, Tsuchiya A, Takeuchi S, Nojiri S, Yoshida T, Ogawa M, Itoh M, Takamura M, Suganami T, Ogawa Y, Terai S. Development of a non-alcoholic steatohepatitis model with rapid accumulation of fibrosis, and its treatment using mesenchymal stem cells and their small extracellular vesicles. Regen Ther. 2020;14:252-261. [PubMed] [DOI] [Full Text] |
| 35. | Zhang B, Zhang B, Lai RC, Sim WK, Lam KP, Lim SK. MSC-sEV Treatment Polarizes Pro-Fibrotic M2 Macrophages without Exacerbating Liver Fibrosis in NASH. Int J Mol Sci. 2023;24:8092. [PubMed] [DOI] [Full Text] |
| 36. | Yang F, Wu Y, Chen Y, Xi J, Chu Y, Jin J, Yan Y. Human umbilical cord mesenchymal stem cell-derived exosomes ameliorate liver steatosis by promoting fatty acid oxidation and reducing fatty acid synthesis. JHEP Rep. 2023;5:100746. [PubMed] [DOI] [Full Text] |
| 37. | Hu J, Li S, Zhong X, Wei Y, Sun Q, Zhong L. Human umbilical cord mesenchymal stem cells attenuate diet-induced obesity and NASH-related fibrosis in mice. Heliyon. 2024;10:e25460. [PubMed] [DOI] [Full Text] |
| 38. | Zhang Z, Shang J, Yang Q, Dai Z, Liang Y, Lai C, Feng T, Zhong D, Zou H, Sun L, Su Y, Yan S, Chen J, Yao Y, Shi Y, Huang X. Exosomes derived from human adipose mesenchymal stem cells ameliorate hepatic fibrosis by inhibiting PI3K/Akt/mTOR pathway and remodeling choline metabolism. J Nanobiotechnology. 2023;21:29. [PubMed] [DOI] [Full Text] |
| 39. | Sani F, Soufi Zomorrod M, Azarpira N, Soleimani M. The Effect of Mesenchymal Stem Cell-Derived Exosomes and miR17-5p Inhibitor on Multicellular Liver Fibrosis Microtissues. Stem Cells Int. 2023;2023:8836452. [PubMed] [DOI] [Full Text] |
| 40. | Cheng L, Yu P, Li F, Jiang X, Jiao X, Shen Y, Lai X. Human umbilical cord-derived mesenchymal stem cell-exosomal miR-627-5p ameliorates non-alcoholic fatty liver disease by repressing FTO expression. Hum Cell. 2021;34:1697-1708. [PubMed] [DOI] [Full Text] |
| 41. | Zhang Y, Xu N, Xu J, Kong B, Copple B, Guo GL, Wang L. E2F1 is a novel fibrogenic gene that regulates cholestatic liver fibrosis through the Egr-1/SHP/EID1 network. Hepatology. 2014;60:919-930. [PubMed] [DOI] [Full Text] |