Published online Jan 27, 2025. doi: 10.4254/wjh.v17.i1.101212
Revised: October 27, 2024
Accepted: November 20, 2024
Published online: January 27, 2025
Processing time: 120 Days and 14.9 Hours
Chronic liver disease is a growing global health problem, leading to hepatic decompensation characterized by an array of clinical and biochemical complications. Several scoring systems have been introduced in assessing the severity of hepatic decompensation with the most frequent ones are Child-Pugh score, model of end-stage liver disease (MELD) score, and MELD-Na score. Anemia is frequently observed in cirrhotic patients and is linked to worsened clinical outcomes. Although studies have explored anemia in liver disease, few have investigated the correlation of hemoglobin level with the severity of hepatic decompensation.
To determine the relationship between hemoglobin levels and the severity of decompensated liver disease and comparing the strength of this correlation using the Child-Pugh, MELD, and MELD-Na scores.
This cross-sectional study was conducted at a tertiary care hospital with 652 decompensated liver disease patients enrolled in the study. Data was collected on demographics, clinical history, and laboratory findings, including hemoglobin levels, bilirubin, albumin, prothrombin time (international normalized ratio), sodium, and creatinine. The Child-Pugh, MELD, and MELD-Na scores were calculated. Statistical analysis was performed using Statistical Package for the Social Sciences version 26, and correlations between hemoglobin levels and severity scores were assessed using Spearman's correlation coefficient.
The study included 405 males (62.1%) and 247 females (37.9%) with an average age of 58.8 years. Significant inverse correlations were found between hemoglobin levels and Child-Pugh, MELD, and MELD-Na scores (P < 0.01), with the MELD scoring system being the strongest correlator among all. One-way analysis of variance revealed significant differences in hemoglobin levels across the severity groups of each scoring system (P = 0.001). Tukey's post hoc analysis confirmed significant internal differences among each severity group.
Understanding the correlation between hemoglobin and liver disease severity can improve patient management by offering insights into prognosis and guiding treatment decisions.
Core Tip: This study investigates the correlation between hemoglobin levels and disease severity in patients with decompensated liver disease. A cohort of 652 patients was assessed using Child-Pugh, model of end-stage liver disease (MELD), and MELD-Na scores to measure disease severity and its association with hemoglobin levels. The analysis revealed a strong inverse correlation, with lower hemoglobin levels associated with higher disease severity. Among the scoring systems, MELD and MELD-Na showed the most significant associations. These findings suggest that hemoglobin levels could serve as a valuable marker in assessing disease progression, potentially improving clinical management for patients with decompensated liver disease.
- Citation: Ullah H, Huma S, Yasin G, Ashraf M, Tahir N, Tahir Uddin Q, Shabana H, A R Hussein M, Shalaby A, Mossaad Alsayyad M, Said A, Farahat A, Hamed HI, Ayoub HSA, Imam MS, Elmahdi E. Comparison of different severity scores in correlating hemoglobin levels with the severity of hepatic decompensation: An observational study. World J Hepatol 2025; 17(1): 101212
- URL: https://www.wjgnet.com/1948-5182/full/v17/i1/101212.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i1.101212
Chronic liver disease, or liver cirrhosis, has become one of the most alarming health issues worldwide, especially due to the increasing incidence of obesity and non-alcoholic liver disease. Cirrhosis liver is a condition in which normal liver parenchyma is replaced by fibrotic tissue and bands with regenerating nodules, disrupting the normal architecture, and thus compromising all types of liver functions. In the earlier stages, there may not be any clinical or biochemical abnormalities in liver cirrhosis, and it is simply termed compensated cirrhosis[1].
Decompensated liver disease represents an advanced stage of liver cirrhosis, characterized by the manifestation of significant clinical symptoms and biochemical changes[2]. These manifestations include jaundice, bruising with pro
Several factors have been identified to worsen hepatic decompensation, including systemic or local infections (in
Based on these factors, scoring systems have been developed to assess the severity of liver decompensation. The most common ones are the Child-Pugh score, the model of end-stage liver disease (MELD) score, and its updated form with the sodium, the MELD-Na score[5]. The Child-Pugh score uses bilirubin, albumin, PT, ascites, and hepatic encephalopathy as measures of the severity of hepatic decompensation[6,7]. Table 1 shows the interpretation of the Child-Pugh score.
| Variable | 1 point | 2 points | 3 points | Interpretation |
| Serum albumin (mg/dL) | > 3.5 | 2.8–3.5 | < 2.8 | Sum of points: 5–6 = A; 7–9 = B; > 9 = C |
| Serum bilirubin (mg/dL) | < 2 | 2–3 | > 3 | |
| International normalized ratio | < 1.7 | 1.7–2.3 | > 2.3 | |
| Ascites | None | Mild | Marked | |
| Encephalopathy | None | Grade 1 and 2 | Grade 3 and 4 |
The MELD, is another scoring system for assessing the severity of chronic liver disease, initially developed to predict mortality after the TIPS procedure, and was also found beneficial for the severity of hepatic dysfunction and prioritization for liver transplant[8,9]. Later, the MELD-Na score was updated by incorporating serum sodium levels, thus using creatinine, bilirubin, INR, and serum sodium to assess the severity of decompensated cirrhosis[10].
Table 2 shows the interpretation of the MELD score.
| Model of end-stage liver disease score | 3 months mortality (percentage) |
| 9 or less | 1.9 |
| 10–19 | 6 |
| 20–29 | 19.6 |
| 30–39 | 52.6 |
| 40 or above | 71.3 |
Anemia is associated with chronic liver disease in several studies, and its severity often correlates with the progression of hepatic dysfunction[11]. The presence of anemia in decompensated liver disease exacerbates the overall clinical condition as it can precipitate hepatic encephalopathy and worsen the hemodynamic status, which is already com
While numerous studies have explored the individual and collective impacts of anemia and liver disease, the specific relationship between hemoglobin levels and the severity of decompensated liver disease has not been thoroughly investigated. For instance, Özatli et al[15] discussed the pathophysiology of anemia in liver disease. Still, they did not correlate hemoglobin levels with the severity of hepatic decompensation based on the Child-Pugh score and MELD-Na score. Similarly, Manrai et al[12] examined the prevalence and mechanisms of anemia in cirrhosis without delving into its relationship with liver disease severity.
Given the significant impact of anemia on clinical outcomes in liver disease patients, understanding the correlation of hemoglobin with the severity of hepatic decompensation can provide valuable insights into patient management and prognosis. This relationship can potentially serve as a marker for disease severity and help guide therapeutic inter
The main objective of this study is to determine the correlation between hemoglobin levels and the severity of decompensated liver disease based on the Child-Pugh score, MELD score, and MELD-Na score and compare the re
This cross-sectional analytical study was done at the Department of Gastroenterology, Hayatabad Medical Complex, Peshawar which is a tertiary care hospital, over the period of two years (February 2022 to February 2024). The study protocol was reviewed and approved by the Institutional Review Board of Hayatabad Medical Complex, Peshawar, No. 597/HEC/BPSC/2021 dated February 2, 2022. A total of 652 patients with chronic liver disease were included in the study. The inclusion criteria were patients of 15 years and above with decompensated liver disease, characterized by clinical manifestations such as ascites, jaundice, hepatic encephalopathy, or variceal hemorrhage.
Patients with acute liver failure (n = 23), patients with coexisting hematological disorders (n = 4) or other malignancy (hepatoma n = 11), patients who had received blood transfusions within the last three months prior to enrollment (n = 9) and pregnant women (n = 5) were excluded from the study. The reason behind the exclusion is, these factors can overestimate or underestimate the hemoglobin level, like hematological disorders, pregnancy, malignancy like hepatoma and prior blood transfusions, or overestimate the severity of hepatic decompensation like acute liver failure and malignancy like hepatoma, without affecting the hemoglobin levels significantly or even causing rise in the hemoglobin level despite of hepatic decompensation. Written informed consent was obtained from all participants before enrollment. The study adhered to the principles of the Declaration of Helsinki.
All the participants were subjected to a thorough history, followed by clinical examination and desired investigations. The following information was recorded for each patient.
Demographic details: Age, gender.
Clinical history and examination: Etiology of liver disease, risk factors for hepatic decompensation, clinical features of hepatic decompensation, presence of comorbid conditions.
Laboratory data: Full blood count (hemoglobin levels), serum bilirubin, serum albumin, PT (or INR), serum electrolytes (e.g., sodium), liver function test and renal function test (e.g., creatinine).
The Child-Pugh score was calculated using serum bilirubin (mg/dL), serum albumin (g/dL), INR, level of ascites (none, mild, moderate to severe based on clinical and ultrasonological findings), and hepatic encephalopathy (none, grade 1-2, grade 3-4). The participants were then classified as Child-Pugh classes A (5-6 points), B (7-9 points), and C (10-15 points) based on the severity.
MELD-Na score was calculated using the mentioned formula and severity was determined based on the scores mentioned in Table 2.
Hemoglobin levels were measured using standard laboratory techniques using an automated hematology analyzer.
Data were analyzed using Statistical Package for the Social Sciences software version 26 (IBM Corp., Armonk, NY, United States). Continuous variables were expressed as mean ± SD or median (interquartile range) as appropriate. Categorical variables were presented as frequencies and percentages.
The primary outcome was the correlation between hemoglobin levels and the Child-Pugh score, MELD score, and MELD-Na score. Spearman correlation coefficients were calculated based on the distribution of the data to quantify and compare the strength of the correlation of hemoglobin levels with the Child-Pugh class, MELD scores, and MELD-Na scores. Secondary analyses included comparisons of hemoglobin levels across the different Child-Pugh, MELD, and MELD-Na classes using the analysis of variance (ANOVA) test. The significance of variation in the hemoglobin level among different severity groups was calculated by Tukey’s post hoc analysis. A P value of < 0.05 was considered statistically significant.
A total of 652 patients with both genders having chronic liver disease due to any cause, admitted to the hospital were included in the study. Table 3 highlights the central tendency (mean) and variability (range) of different variables within the study population including Child-Pugh score, MELD score, and MELD-Na score.
| Variable | Number | Mean (SD) | Percentage | Minimum | Maximum |
| Age | - | 58.8 (17.4) | - | 15 | 90 |
| Gender | Male = 405, female = 247 | - | 62.1, 37.9 | - | - |
| Serum sodium (mEq/L) | - | 128.8 (6.2) | - | 120 | 149 |
| Serum bilirubin (mg/dL) | - | 5.6 (5.0) | - | 0.8 | 25.3 |
| Serum albumin (mg/dL) | - | 3.0 (0.9) | - | 1.2 | 4.9 |
| Serum creatinine (mg/dL) | - | 2.1 (1.1) | - | 0.6 | 5.0 |
| International normalized ratio | - | 1.9 (0.7) | - | 1.1 | 6.4 |
| Hemoglobin (g/dL) | - | 11.1 (2.1) | - | 5.3 | 17.2 |
| Child-Pugh score | - | 10.5 (2.6) | - | 6 | 15 |
| MELD score | - | 23 (9) | - | 5 | 48 |
| MELD-Na score | - | 27 (9) | - | 1 | 49 |
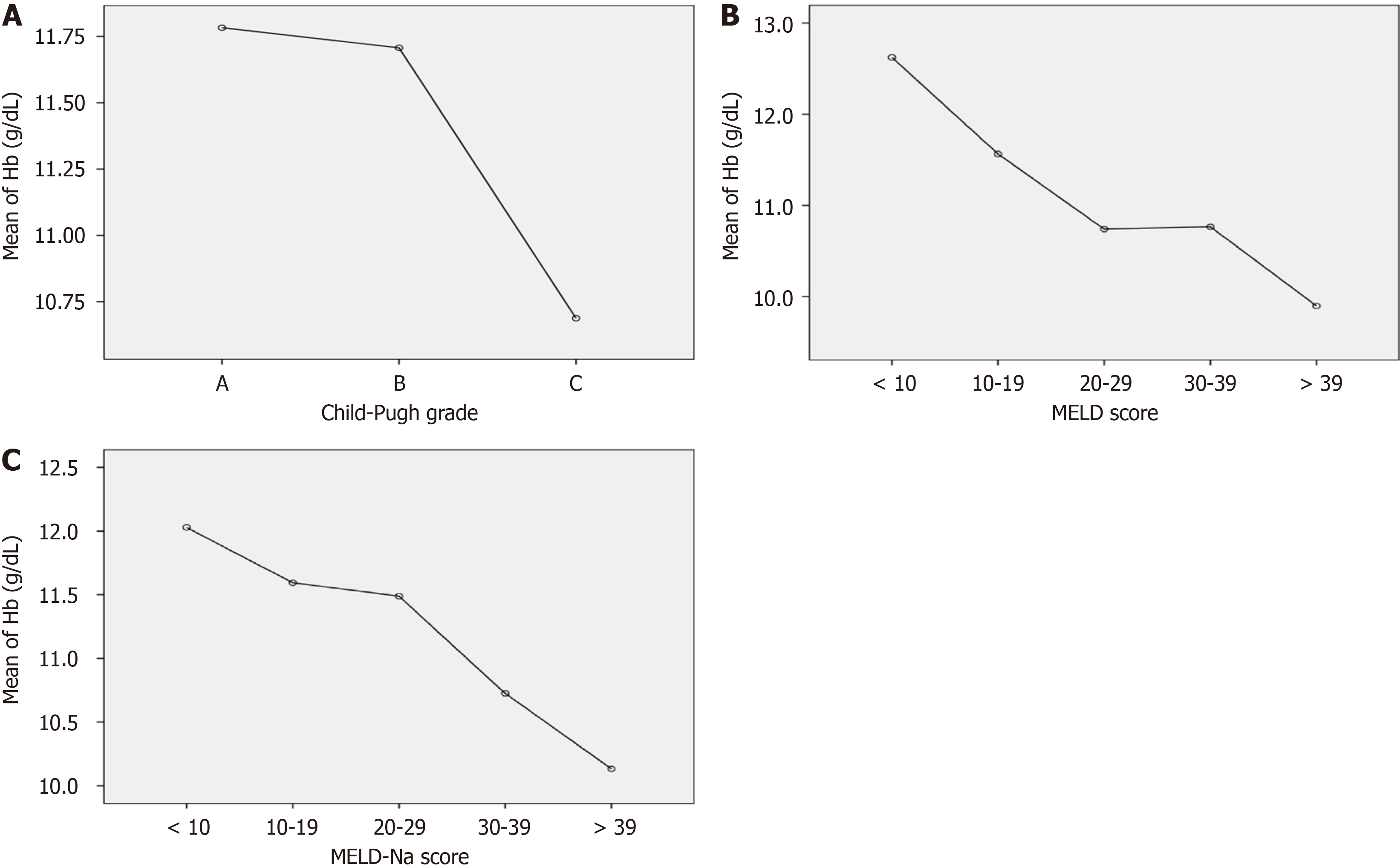
One-way ANOVA comparing the mean hemoglobin values among different Child-Pugh classes showed a significant variance (F = 20.01, P = 0.001) among the hemoglobin values of the three Child-Pugh classes, as shown in Table 4.
| Child-Pugh class | Number of patients | Mean hemoglobin (g/dL) | SD (g/dL) | Analysis of variance (F value) | P value |
| A | 41 | 11.8 | 2.1 | 20.011 | 0.001 |
| B | 211 | 11.7 | 1.9 | ||
| C | 400 | 10.6 | 2.1 | ||
| Total | 652 | 11.1 | 2.1 |
Tukey’s post hoc shows a significant difference between the mean hemoglobin of Child-Pugh class A and C, and B and C (P = 0.001) as plotted in Figure 1A.
Table 5 summarizes the distribution of patients into 5 groups based on different ranges of MELD scores showing their mean (SD) hemoglobin values. One-way ANOVA showed significant variance (F = 13.04, P = 0.001) in the hemoglobin values among the different ranges of MELD score.
| Group | Model of end-stage liver disease score range | Number of patients | Mean hemoglobin level (g/dL) | SD (g/dL) | Analysis of variance (F value) | P value |
| 1 | < 10 | 33 | 12.6 | 1.8 | 13.042 | 0.001 |
| 2 | 10–19 | 236 | 11.5 | 2.0 | ||
| 3 | 20–29 | 165 | 10.7 | 2.0 | ||
| 4 | 30–39 | 181 | 10.7 | 1.9 | ||
| 5 | > 39 | 37 | 9.8 | 1.9 | ||
| Total | 652 | 11.1 | 2.0 |
Tukey’s post hoc test shows a significant difference (P < 0.001) among the hemoglobin of each group except between groups 1 and 2 (P = 0.06), shown in Figure 1B.
Table 6 summarizes the distribution of patients into 5 groups based on different ranges of MELD-Na scores showing their mean (SD) hemoglobin values. One-way ANOVA showed significant variance (F = 9.6, P = 0.001) in the hemoglobin values among the different ranges of MELD-Na score.
| Group | Model of end-stage liver disease-Na score range | Number of patients | Mean hemoglobin level (g/dL) | SD (g/dL) | Analysis of variance (F value) | P value |
| 1 | < 10 | 22 | 12.0 | 2.1 | 9.6 | .001 |
| 2 | 10-19 | 119 | 11.5 | 2.0 | ||
| 3 | 20-29 | 192 | 11.4 | 2.1 | ||
| 4 | 30–39 | 274 | 10.7 | 1.9 | ||
| 5 | > 39 | 45 | 10.1 | 1.9 | ||
| Total | 652 | 11.1 | 2.0 |
Tukey’s post hoc test shows a significant difference (P < 0.001) among the mean hemoglobin of each group except groups 1 and 2 (P = 0.9), 1 and 3 (P = 0.8), and 2 and 3 (P = 0.9), shown in Figure 1C.
Pearson correlation coefficient shows that there is a significant inverse between hemoglobin and Child-Pugh score, hemoglobin and MELD score, and hemoglobin and MELD-Na score, as shown in Table 7.
| Correlation (Pearson) | R value | P value |
| Hemoglobin vs Child-Pugh | -0.21 | < 0.01 |
| Hemoglobin vs MELD | -0.26 | < 0.01 |
| Hemoglobin vs MELD-Na | -0.25 | < 0.01 |
The multinominal logistic regression analysis showed that by each 1 g/dL fall in the hemoglobin level, the likelihood of going from group 1severity of MELD and MELD-Na score (0–9) to group 2 (10–19), increases by 22.6% (P = 0.019), from group 2 to group 3 (20–29 score) by 19.8% (P < 0.001), from group 3 to the group 4 (30–39 score) by 3.3% (P = 0.5), and from group 4 to the group 5 (score > 40) by 16.7% (P < 0.001).
Our study shows a significant inverse correlation between hemoglobin levels and the severity of chronic liver disease assessed by the Child-Pugh score, MELD score, and MELD-Na score. Anemia is one of the frequent findings in liver cirrhosis and is multifactorial, including malnutrition, malabsorption, chronic inflammatory states, bleeding from the gastrointestinal tract, bone marrow suppression, and splenic sequestrations[13]. In this study, when we correlated the hemoglobin levels with Child-Pugh classes, we found a significant negative correlation between the hemoglobin levels and the severity of chronic liver disease based on Child-Pugh severity classes, with Pearson “r” value of -0.21 (P < 0.01). While comparing the mean hemoglobin values among the different Child-Pugh severity classes (A to C), there was a significant difference in hemoglobin values between class A and C, and between B and C. However, the difference between classes A and B was not significant, as indicated by Tukey’s post hoc test. From this result, we can conclude that a fall in hemoglobin levels is more pronounced as the chronic liver disease increases in severity.
While investigating the relationship of anemia with the severity of liver disease in transplant patients, Collas et al[16] reached similar conclusions, stating that anemia becomes more pronounced as liver disease progresses in transplant patients. Similarly, Garcia-Tsao et al[17] relate anemia with the progression of liver disease.
Our findings have demonstrated a significant negative correlation between hemoglobin and MELD score, with “r” value = -0.26 (P < 0.01), showing patients with MELD score less than 10 are having mean (SD) hemoglobin of 12.6 (1.8 g/dL) g/dL, as compared to the patients having MELD score of 40 and above, for whom the mean (SD) hemoglobin was 9.8 (1.9 g/dL) g/dL. This indicates a significant fall in hemoglobin value with the rise in hepatic decompensation. These findings are consistent with the previous research narrating the association between anemia and liver cirrhosis[18]. Although previous research has proposed a relationship between anemia and the severity of hepatic dysfunction, the exact relation of fall in the hemoglobin level to the hepatic decompensation based on the severity scoring systems was lacking. In our study, multinominal logistic regression analysis has shown the progression in the severity of hepatic decompensation with each g/dL fall in hemoglobin level. The statistically insignificant odds of progression of 3.3% from group 3 to group 4 MELD score by 1 mg/dL fall in the hemoglobin (P = 0.5) strengthens our narrative as the mean hemoglobin of both these groups is the same, i.e., 10.7 mg/dL.
MELD-Na score, an updated form of MELD score with an additional factor i.e. serum sodium, also showed a significant inverse relation between hemoglobin and severity of hepatic decompensation (“r” = -0.25, P < 0.01) like MELD score.
In this study, we also compared the strength of the correlation of hemoglobin level to the severity of hepatic de
There are conflicting studies regarding the prognostic value of the Child-Pugh score, MELD score, and MELD-Na score, with some studies showing the Child-Pugh score as superior, some concluding MELD and MELD-Na as superior, while some showing equivocal results[19-21]. Teng et al[22] concluded the Child-Pugh score as a better predictor of the severity of hepatic decompensation as compared to the MELD score after gastric variceal bleed, while Peng et al[20] found no significant difference between the two scores with similar end-point. Similarly in another study Sempere et al[23] found the MELD score to be a better severity predictor than the Child-Pugh score.
Our finding regarding the stronger correlation of hemoglobin with MELD and MELD-Na scores than with the Child-Pugh score is not unexpected. Literature suggests that the MELD score and MELD-Na score take into account more objective and laboratory-based parameters like serum creatinine, and serum sodium, which are directly related to renal dysfunction. Renal impairment is one of the major causes and precipitant of anemia in chronic liver disease as inferred by several researchers[24,25]. Similarly, serum sodium (as in the MELD-Na score) has also been studied as a potentiating factor of anemia and decreasing hemoglobin levels. Mansoor et al[26] in their study conclude that hyponatremia ad
Our study highlights the prognostic yield of hemoglobin value in patients with chronic liver disease as predicted by Child-Pugh, MELD, and MELD-Na scores. Based on this fact, it is very important to regularly monitor the hemoglobin levels in patients with liver cirrhosis, particularly those with higher Child-Pugh, MELD, or MELD-Na scores. This can also guide the clinicians to take prompt measures to treat anemia while managing other severity predictors like ele
The main strength of our study is the larger sample size, which increases the statistical power and reliability of the results. The simple and straightforward methodology will help the study's replicability and minimize bias. To the best of our knowledge and the literature, none of the studies calculated the odds of falling into a more severe MELD group by each mh/dL fall in the hemoglobin level.
The major limitation of the study is that it is done in a single center, which may limit its generalizability. However, this limitation has been addressed by taking a larger sample size.
This study has concluded a significant inverse relation between the hemoglobin level and severity of hepatic decom
The authors would like to thank the deanship of scientific research at Shaqra University for supporting this work.
| 1. | Sharma A, Nagalli S. Chronic Liver Disease. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2024. [PubMed] |
| 2. | Nusrat S, Khan MS, Fazili J, Madhoun MF. Cirrhosis and its complications: evidence based treatment. World J Gastroenterol. 2014;20:5442-5460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 128] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (7)] |
| 3. | D’amico G, Perricone G. Prediction of Decompensation in Patients with Compensated Cirrhosis: Does Etiology Matter? Curr Hepatol Rep. 2019;18:144-156. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2170] [Article Influence: 180.8] [Reference Citation Analysis (5)] |
| 5. | Peng Y, Qi X, Guo X. Child-Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Medicine (Baltimore). 2016;95:e2877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 343] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 6. | Cholongitas E, Papatheodoridis GV, Vangeli M, Terreni N, Patch D, Burroughs AK. "Systematic review: The model for end-stage liver disease--should it replace Child–Pugh's classification for assessing prognosis in cirrhosis?". Aliment Pharmacol Ther. 2005;22:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 293] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 7. | Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2004;42:S100-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 426] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 8. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2069] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 9. | Kamath PS, Kim WR; Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1228] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 10. | Godfrey EL, Kueht ML, Rana A, Awad S. MELD-Na (the new MELD) and peri-operative outcomes in emergency surgery. Am J Surg. 2018;216:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Scheiner B, Semmler G, Maurer F, Schwabl P, Bucsics TA, Paternostro R, Bauer D, Simbrunner B, Trauner M, Mandorfer M, Reiberger T. Prevalence of and risk factors for anaemia in patients with advanced chronic liver disease. Liver Int. 2020;40:194-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (4)] |
| 12. | Manrai M, Dawra S, Kapoor R, Srivastava S, Singh A. Anemia in cirrhosis: An underestimated entity. World J Clin Cases. 2022;10:777-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (10)] |
| 13. | Gonzalez-Casas R, Jones EA, Moreno-Otero R. Spectrum of anemia associated with chronic liver disease. World J Gastroenterol. 2009;15:4653-4658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Bothou C, Rüschenbaum S, Kubesch A, Quenstedt L, Schwarzkopf K, Welsch C, Zeuzem S, Welzel TM, Lange CM. Anemia and Systemic Inflammation Rather than Arterial Circulatory Dysfunction Predict Decompensation of Liver Cirrhosis. J Clin Med. 2020;9:1263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Özatli D, Köksal AS, Haznedaroglu IC, Simsek H, Karakus S, Büyükasik Y, Kosar A, Özcebe O, Dündar S. Erythrocytes: Anemias in Chronic Liver Diseases. Hematology. 2000;5:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Collas O, Robertson FP, Fuller BJ, Davidson BR. Anaemia in patients with chronic liver disease and its association with morbidity and mortality following liver transplantation. Int J Surg. 2018;53:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Garcia-Tsao G, Bosch J, Groszmann RJ. Portal hypertension and variceal bleeding--unresolved issues. Summary of an American Association for the study of liver diseases and European Association for the study of the liver single-topic conference. Hepatology. 2008;47:1764-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 18. | Singh S, Manrai M, V S P, Kumar D, Srivastava S, Pathak B. Association of liver cirrhosis severity with anemia: does it matter? Ann Gastroenterol. 2020;33:272-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Chawla YK, Kashinath RC, Duseja A, Dhiman RK. Predicting Mortality Across a Broad Spectrum of Liver Disease-An Assessment of Model for End-Stage Liver Disease (MELD), Child-Turcotte-Pugh (CTP), and Creatinine-Modified CTP Scores. J Clin Exp Hepatol. 2011;1:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Peng Y, Qi X, Dai J, Li H, Guo X. Child-Pugh versus MELD score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis. Int J Clin Exp Med. 2015;8:751-757. [PubMed] |
| 21. | Chaurasia RK, Pradhan B, Chaudhary S, Jha SM. Child-Turcotte-Pugh versus model for end stage liver disease score for predicting survival in hospitalized patients with decompensated cirrhosis. J Nepal Health Res Counc. 2013;11:9-16. [PubMed] |
| 22. | Teng W, Chen WT, Ho YP, Jeng WJ, Huang CH, Chen YC, Lin SM, Chiu CT, Lin CY, Sheen IS. Predictors of mortality within 6 weeks after treatment of gastric variceal bleeding in cirrhotic patients. Medicine (Baltimore). 2014;93:e321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Sempere L, Palazón JM, Sánchez-Payá J, Pascual S, de Madaria E, Poveda MJ, Carnicer F, Zapater P, Pérez-Mateo M. Assessing the short- and long-term prognosis of patients with cirrhosis and acute variceal bleeding. Rev Esp Enferm Dig. 2009;101:236-248. [PubMed] |
| 24. | Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Prim Care. 2008;35:329-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 25. | Kumar R, Priyadarshi RN, Anand U. Chronic renal dysfunction in cirrhosis: A new frontier in hepatology. World J Gastroenterol. 2021;27:990-1005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (3)] |
| 26. | Mansoor F, Bai P, Kaur N, Sultan S, Sharma S, Dilip A, Kammawal Y, Shahid S, Rizwan A. Evaluation of Serum Electrolyte Levels in Patients With Anemia. Cureus. 2021;13:e18417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Rafiq M, Arooj A, Fayyaz N, Samad A, Bashir S. Evaluation of serum electrolyte levels in iron deficiency anemia patients. TPMJ. 2021;28:691-696. |
| 28. | Durand F, Valla D. Assessment of prognosis of cirrhosis. Semin Liver Dis. 2008;28:110-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 29. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R; United Network for Organ Sharing Liver Disease Severity Score Committee. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 1864] [Article Influence: 84.7] [Reference Citation Analysis (0)] |