TO THE EDITOR
Hepatitis B viral infection is a significant threat to global health despite advancement in vaccination strategies and new anti-viral treatments[1]. This double stranded DNA virus, belonging to Hepadnaviridae family infects hepatocytes by transporting into the nucleus where it persists as an episome which is also known as covalently closed circular DNA, hence serving as a template for continuous viral replication[2]. The persistent inflammation due to chronic hepatitis B virus (HBV) infection ultimately leads to fibrosis, cirrhosis and hepatocellular carcinoma (HCC). Metabolic dysfunction associated steatotic liver disease (MASLD) is another global health problem due to rising incidence of obesity[3]. Based on the widely accepted nomenclature emerged in 2023 MASLD is defined as “steatotic liver disease (SLD) diagnosed via liver biopsy or imaging plus any one of five parameters of metabolic syndrome”. The five parameters of metabolic syndrome includes body mass index ≥ 25 kg/m2 (≥ 23 kg/m2 in Asian) or waist circumference > 94 cm in men, > 80 cm in women, fasting serum glucose ≥ 5.6 mmol/L or 2 hours post-load glucose level ≥ 7.8 mmol/L or glycated hemoglobin ≥ 5.7% or on specific drug treatment, blood pressure ≥ 17.3/11.3 Kpa or specific drug treatment, plasma triglycerides ≥ 1.7 mmol/L or specific drug treatment, plasma high-density lipoprotein cholesterol ≤ 1.0 mmol/L for men and ≤ 1.3 mmol/L for women or specific drug treatment[4]. Hitting the liver hard, the coexistence of both enemies is not rare and the prevalence of concomitant HBV infection and MASLD ranges from 14%-70%[5]. Considering the significant consequences of both HBV and MASLD when affecting liver alone, it is imperative to understand the complex relationship between concomitant HBV infection and MASLD and its impact on liver.
PATHOGENESIS OF CO-COMITANT HBV INFECTION AND MASLD
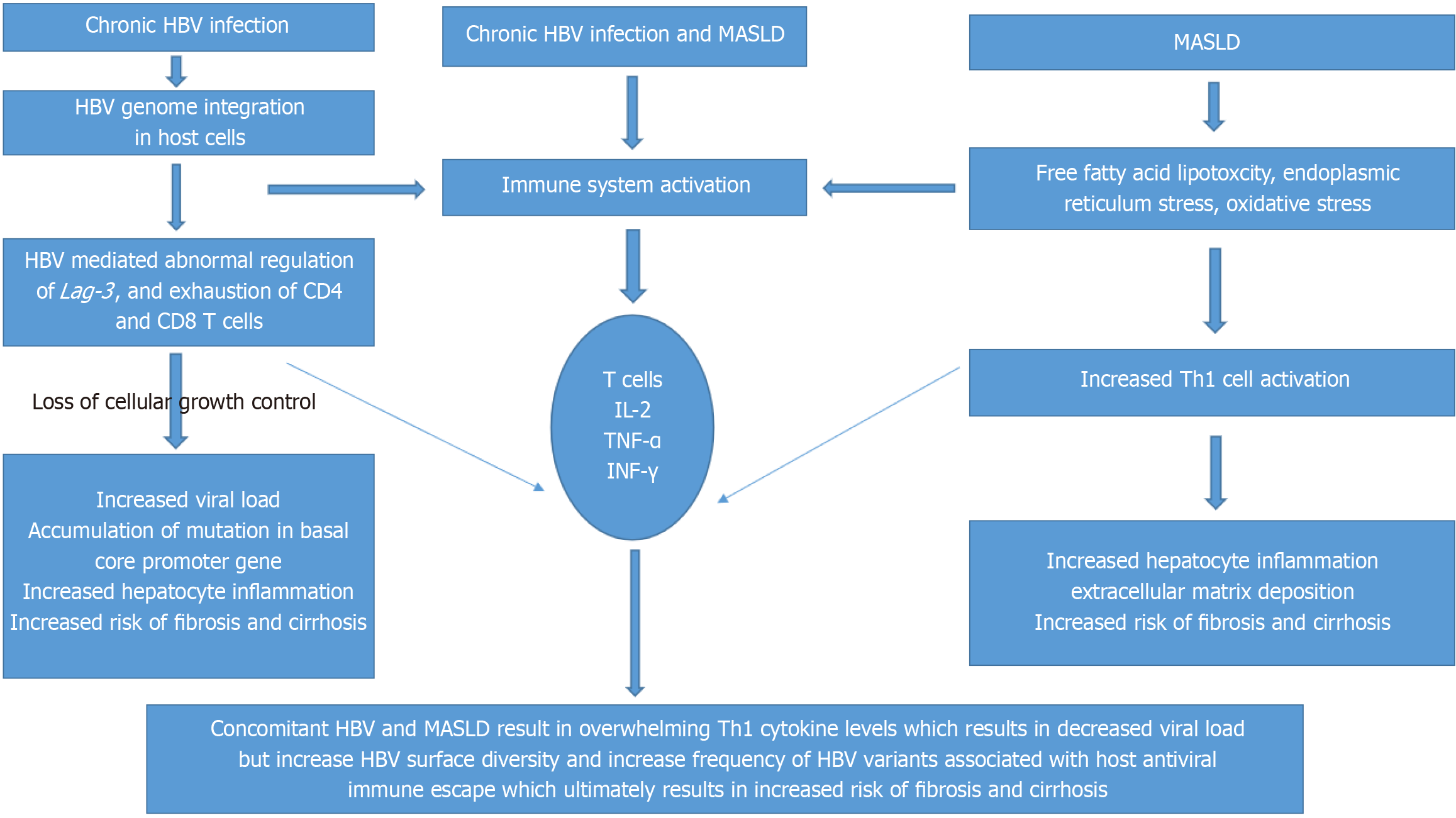
Liver is an immune privilege organ due to its unique blood supply and the composition of its cells, including Kupffer cells, natural killer cells, CD4 T cells and CD8 T cells. Hepatic steatosis suppresses secretion of hepatitis B surface antigen (HepBsAg) at the cellular level by inducing endoplasmic reticulum stress. Stearic acid and oleic acid, which induced lipid accumulation in HepG2.2.15 cells, significantly inhibited secretion of HBV DNA and HepBsAg[6]. One meta-analysis analyzed 28648 patients from 54 studies and found that that there is inverse co relation between hepatic steatosis and HBeAg (odds ratio = 0.82; 95% confidence interval: 0.75-0.91)[7]. Adiponectin, which suppresses hepatic steatosis and promotes DNA replication, plays a crucial role in the inverse relationship[8]. A study using HepG2-HBV-stable cells found that adiponectin levels increased in patients with high viral replication[9]. On the other hand, loss of HepBsAg is associated with interaction between HBV and host immune system. Obesity and steaotic liver disease activates CD8T cells which may potentially involve in loss of HepBsAg cumulatively causing hepatocyte inflammation, warrants further investigation and research[10]. One prospective, cohort study, explains concomitant HBV infection and MASLD can result in increased pro-inflammatory cytokine levels which can result in lower HBV markers (P < 0.01), but results in increased HBV surface diversity (P = 0.02) and greater frequency of HBV variants associated with host antiviral immune escape, potentially impacting liver disease progression, ultimately resulting in the development of HCC (Figure 1)[11].
Figure 1 Pathogenesis of chronic hepatitis B virus infection alone, metabolic dysfunction associated steatotic liver disease alone and concomitant chronic hepatitis B virus infection and metabolic dysfunction associated steatotic liver disease.
HBV: Hepatitis B virus; Lag-3: Lymphocyte activation gene 3; MASLD: Metabolic dysfunction associated steatotic liver disease; IL-2: Interleukin-2; TNF-α: Tumor necrosis factor alpha; IFN-γ: Interferon gamma; Th1: Type-1 helper.
UNCERTAIN IMPACT OF CONCOMITANT HBV INFECTION AND MASLD ON LIVER
The influence of MASLD on degree of liver fibrosis varies among individuals due to the presence of different cardiometabolic risk factors with diabetes being the most important independent predictor of liver fibrosis[12]. Presence of insulin resistance with obesity has a synergistic effect on progression of liver fibrosis[12]. The impact of concomitant chronic HBV infection along with MASLD on progression of liver fibrosis is unknown[9]. One retrospective cohort study by Lin et al[13] evaluated 1613 chronic hepatitis B (CHB) patients over a period of 5.02 years among which 483 have hepatic steatosis diagnosed via liver biopsy and abdominal imaging were found to have increased risk of HCC irrespective of their overweight and number of cardiometabolic risk factors (P < 0.001)[13]. It is well known that cardiovascular disease is the most common cause of mortality in patients with MASLD[4]. Another recent study prospectively evaluated 336866 patients over a mean follow up of 15 years with MASLD with concomitant CHB or chronic hepatitis C (CHC) showed increased cumulative risk of cirrhosis or HCC (P < 0.001) hazard ratios with 95% confidence intervals for HCC were 8.81 (7.83-9.92) for non-SLD with CHB or CHC, 1.52 (1.32-1.74) for MASLD without CHB or CHC, and 8.86 (7.76-10.12) for MASLD with CHB or CHC, compared with non-SLD without CHB or CHC. Among patients with CHB or CHC who received treatment during follow up, MASLD was associated with increased risk of HCC or cirrhosis underscoring the need to prioritizing treatment before addressing MASLD[3]. Additionally CHB in patients with MAFLD had been found to be associated with increase liver fibrosis but decrease risk of atherosclerosis which is attributable to the effects of inverse co-relationship between high viral load and degree of steatosis, which gives insight to clinicians for aggressive management that CHB and MASLD as evident by the study have synergistic effects on progression of liver disease along with reversing lipid lowering effects when treating patients with antivirals also needing lipid lowering therapies[14]. In contrary hepatic steatosis with concomitant hepatitis C virus infection have increased risk of liver fibrosis but has no effect on risk of cardiovascular disease[15]. One study by Huang et al[16] consecutively recruited 4084 treatment naive HepBeAg negative patients over a period of 15 years and concluded that hepatic steatosis facilitates HepBsAg seroclearance and seroconversion[16]. It is well known that loss of HepBsAg is associated with less severe liver related outcomes such as the development of HCC. These findings raise a clinical dilemma in managing CHB patients with concomitant MASLD, whether correction of MASLD benefits CHB patients or not[16]. Despite potential facilitation of seroclearance and seroconversion of HepBsAg, long term outcome of metabolic dysfunction on liver and other organs should not be ignored[16]. The relationship between CHB and MASLD had been evaluated in a study published in recently[17]. This retrospective analysis included 18980 participants after excluding those patients with alcohol consumption and CHC to minimize their interaction with CHB and MASLD[17]. The prevalence of CHB carriers was 11.7% and MASLD was 40.3% in the study arm. Comparison of clinical characteristics was made among HBV carriers, resolved HBV infection and healthy control group which showed that no clinical difference was found between resolved HBV infection and healthy control group[17]. However, HBV carriers exhibited better lipid profiles but higher severity of liver fibrosis than healthy control group. Comparison of clinical characteristics was made among dual etiology group (chronic HBV infection with MASLD) with MASLD alone group and HBV alone group. Among MASLD patients, dual etiology group displayed a higher liver inflammation and fibrosis indices compared to those with MASLD alone. Among patients with HBV infection, dual etiology patients displayed severe liver inflammation than HBV alone group. This study included many patients which increases its credibility. Moreover, this study included comprehensive association of all metabolic risk factors[17]. Though this study clearly explains complex interaction between CHB and MASLD, but further research is needed to explain the complex relationship in long term prognosis and clinical outcome of these patients[17].
CONCLUSION
Inverse co-relationship between DNA viral replication and hepatic steatosis though complex yet to administer due to rising pandemic is crucial. HBV genetic variants can emerge during concomitant HBV infection and MASLD which can modify disease severity and cancer risk. The study recently published by Wang et al[17] comprehensively evaluated the clinical characteristics of patients between concomitant HBV infection and MASLD and successfully demonstrated the association between metabolic risk factors. However, further studies are needed to validate the underlying mechanisms to determine long term risks of clinical events especially the development of HCC which is the leading cause of heightened mortality. Aiming at optimizing holistic health care approach and clinical strategies in managing patients with dual etiology to minimize progression into chronic liver disease that needs to be investigated.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Pakistan
Peer-review report’s classification
Scientific Quality: Grade B, Grade B, Grade C
Novelty: Grade B, Grade B, Grade B
Creativity or Innovation: Grade B, Grade B, Grade C
Scientific Significance: Grade B, Grade B, Grade B
P-Reviewer: Koganti SB; Ning ZX S-Editor: Bai Y L-Editor: A P-Editor: Zhao YQ