Published online Sep 27, 2024. doi: 10.4254/wjh.v16.i9.1269
Revised: June 16, 2024
Accepted: June 27, 2024
Published online: September 27, 2024
Processing time: 206 Days and 19 Hours
Survival in patients with autoimmune liver disease overlap syndromes (AILDOS) compared to those with single autoimmune liver disease is unclear.
To investigate the survival of patients with AILDOS and assess the accuracy of non-invasive serum models for predicting liver-related death.
Patients with AILDOS were defined as either autoimmune hepatitis and primary biliary cholangitis overlap (AIH-PBC) or autoimmune hepatitis and primary sclerosing cholangitis overlap (AIH-PSC) and were identified from three tertiary centres for this cohort study. Liver-related death or transplantation (liver-related mortality) was determined using a population-based data linkage system. Prognostic scores for liver-related death were compared for accuracy [including liver outcome score (LOS), Hepascore, Mayo Score, model for end-stage liver disease (MELD) score and MELD incorporated with serum sodium (MELD-Na) score].
Twenty-two AILDOS patients were followed for a median of 3.1 years (range, 0.35-7.7). Fourteen were female, the median age was 46.7 years (range, 17.8 to 82.1) and median Hepascore was 1 (range, 0.07-1). At five years post enrolment, 57% of patients remained free from liver-related mortality (74% AIH-PBC, 27% AIH-PSC). There was no significant difference in survival between AIH-PBC and AIH-PSC. LOS was a significant predictor of liver-related mortality (P < 0.05) in patients with AIH-PBC (n = 14) but not AIH-PSC (n = 8). A LOS cut-point of 6 discriminated liver-related mortality in AIH-PBC patients (P = 0.012, log-rank test, 100% sensitivity, 77.8% specificity) (Harrell's C-statistic 0.867). The MELD score, MELD-Na score and Mayo Score were not predictive of liver-related mortality in any group.
Survival in the rare, AILDOS is unclear. The current study supports the LOS as a predictor of liver-related mor
Core Tip: The rare, autoimmune liver disease overlap syndromes (AILDOS), currently have no predictors of survival. AILDOS can be further classified into Autoimmune Hepatitis and Primary Biliary Cholangitis Overlap (AIH-PBC) and Autoimmune Hepatitis and Primary Sclerosing Cholangitis Overlap. Liver-related mortality was defined as liver-related death or liver transplantation. This study validates the liver outcome score as a predictive model of liver-mortality in AIH-PBC patients. The model for end-stage liver disease (MELD) score, MELD incorporated with serum sodium score and Mayo score were not predictive of liver-related mortality in any group.
- Citation: Jayabalan D, Huang Y, Calzadilla-Bertot L, Janjua M, de Boer B, Joseph J, Cheng W, Hazeldine S, Smith BW, MacQuillan GC, Wallace MC, Garas G, Adams LA, Jeffrey GP. Predictors of survival in autoimmune liver disease overlap syndromes. World J Hepatol 2024; 16(9): 1269-1277
- URL: https://www.wjgnet.com/1948-5182/full/v16/i9/1269.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i9.1269
Autoimmune liver disease (AILD) consists of autoimmune hepatitis (AIH), primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC)[1]. The AILD overlap syndromes (AILDOS), however, do not conform to classic diagnostic categories. Instead, they are characterized by overlapping features of AIH and PBC and AIH and PSC[1]. AILDOS present with overlapping symptoms, clinical findings, biochemistry, immunological findings and histology of the individual AILDs[2,3]. The reported incidence of AILD is 1-2 per 100000 population per year for each individual disease[4]. AILDOS are far less prevalent conditions and as low as 7% of patients with PBC and 8% of patients with PSC have features that overlap with AIH[5]. There is little reported data on the clinical outcome of AILDOS due to their rarity, however one study found the 5-year risk of liver-related death was 15.5%[6] and another reported an 85.7% 5-year risk of liver-related death in AIH and PSC Overlap (AIH-PSC)[1,7,8].
Non-invasive measures of liver fibrosis and predicted survival are used to stage the severity of liver disease and assess the risk of liver-related death or liver transplantation and are recommended by all International Liver disease associations to optimise the management and outcomes of patients with chronic liver disease[9]. The liver outcome score (LOS) [in
Patients with AILDOS who had a Hepascore performed from 2004 to 2015 and were part of the Hepascore and Clinical Outcome (HACO) cohort, a state-wide cohort of Australian patients assessed for chronic liver disease[13]. A diagnosis of AILDOS was defined as a diagnosis of AIH and PBC or AIH and PSC using the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) code K75.4 and K74.3 (AIH-PBC) or K75.4 and K83.0 (AIH-PSC). Exclusion criteria was liver transplantation before the start of the study. The International AIH Group criteria, the European Association of Study of the Liver clinical practice guidelines and the American College of Gastroenterology clinical guidelines were used for the diagnosis of AIH, PBC and PSC respectively[14-16]. AIH-PBC and AIH-PSC diag
The first Hepascore test was used as the date of enrolment in the study and the date of diagnosis, and all biochemical data was collected within 6 months of the Hepascore test.
Age, sex and Hepascore were obtained from the West Australian PathWest database. Endpoints extracted from the Western Australia Data Linkage Unit (WADLU) included all-cause death, liver-related death, and liver transplantation. WADLU is a validated population-based data linkage system that links multiple health-related datasets including the state cancer register, the state hospital morbidity database, and the state mortality records. The hospital morbidity data system has 100% coverage of data for public and private hospital admissions in Western Australia. ICD-10 classification codes were used to record the diagnosis at hospital admission and the cause of death. All patient data was deidentified before analysis. Liver-related death was defined as from variceal bleeding, hepatocellular carcinoma or liver failure (ICD-10 codes of I98.3, C22.0 and K72.0 respectively), and death in which liver disease was the major contributing factor. The primary endpoint used in the study was liver-related mortality (liver-related death or liver transplantation). This study was approved by Sir Charles Gairdner Hospital Human Research Ethics Committee, the Data linkage unit, and the Western Australia Department of Health Human Research Ethics Committee.
Additional blood test results were retrospectively extracted, if they were available, from Western Australian health data base. These tests included white cell count, hemoglobin (Hb), platelets, international normalized ratio, albumin, alanine aminotransferase, alkaline phosphatase (ALP), aspartate aminotransferase (AST), ferritin and transferrin, serum antimitochondrial antibody (AMA), including the AMA-M2 subtype, smooth muscle antibody (SMA), SMA vascular glomerular tubular pattern, liver kidney microsomal type 1 antibody and perinuclear antineutrophil cytoplasmic antibody.
The LOS for survival was calculated using the following formula: -0.1792 × albumin (g/L) + 0.0042 × GGT (U/L) + 0.0041 × HA (μg/L) + 0.0377 × age + 0.4492 (if sex = male) + 8[10]. This simple serum liver panel model has been validated in predicting liver-related mortality in patients with CHC infection with a LOS ≥ 5.5 being classified as high risk for liver-related mortality[10]. The MELD score, the MELD-Na score and the ALP/AST ratio were calculated for all patients[11]. The Mayo Score for PSC is a model that predicts overall survival in PSC patients. It uses age, bilirubin, AST, albumin and variceal bleeding history, and was calculated for all patients in the current study[12].
Categorical variables were expressed as an absolute count and percentage, and continuous variables were expressed as a median and range. Fisher’s exact test was used to compare categorical variables and the Mann Whitney U test was used to compare continuous variables. Kaplan-Meier survival curves of AILDOS, AIH-PBC and AIH-PSC were calculated. Schoenfeld residuals was used to test the proportional hazards assumption. The association between variables and liver-related mortality was calculated using Cox regression and the hazard ratio with a 95%CI[17]. Univariate variables with P < 0.1 were included in stepwise backward conditional selection for multivariate analysis, of which a significance level was set as P < 0.05. Components of the Hepascore, LOS, MELD and Mayo Score were excluded from regression analysis. Harrell’s C-statistic was used to evaluate the predictive ability of models[18]. Receiver operating characteristic curve analysis was used to predict liver-related mortality, using the Youden Index to define cut points. Kaplan-Meier survival curves and the log-rank test were additionally used to compare liver-related mortality. STATA IC18 (Stata Corporation, College Station, TX, United States) was used for all statistical analysis performed and analysis was reviewed by an expert in biomedical statistics (Luis Calzadilla-Bertot).
The study was approved by Sir Charles Gairdner Hospital Human Research Ethics Committee (No. RGS0000001775).
The general patient characteristics at baseline are reported in Table 1. A total of 22 AILDOS patients were included in the final analysis, of which 14 patients had AIH-PBC and 8 patients had AIH-PSC. The median age for AILDOS patients was 46.7 years with 36.4% of patients being male. AIH-PBC patients were significantly older than the AIH-PSC patients, with median ages of 55.8 and 23.3 years respectively (P = 0.008). AIH-PBC patients had a higher LOS than AIH-PSC, with median scores of 5.98 and 4.18 respectively. Additionally, AIH-PBC patients had significantly higher ferritin (P = 0.0272) than AIH-PSC, with means of 176 and 32.5 respectively. There were no other statistically significant differences between the AIH-PBC and AIH-PSC groups.
| Characteristics | AILDOS (n = 22) (all patients) | AIH-PBC (n = 14) | AIH-PSC (n = 8) | P value |
| Age, median (range) years | 46.7 (17.8-82.1) | 55.8 (26.9-82.1) | 23.3 (17.8-62.0) | 0.008b |
| Male | 8 (36.4) | 5 (35.7) | 3 (37.5) | 0.935 |
| Hepascore, median (range) | 1 (0.07-1) | 1 (0.07-1) | 0.91 (0.12-1) | 0.628 |
| LOS, median (range) | 5.75 (1.46-13.7) | 5.98 (2.41-13.65) | 4.18 (1.46-6.49) | 0.143 |
| MELD, median (range) | 8 (6-12) | 8.5 (6-12) | 6 (6-11) | 0.427 |
| MELD-Na, median (range) | 10 (6-14) | 9.5 (6-14) | 10 (6-13) | 0.966 |
| Mayo Score, median (range) | 0.76 (-1.28-2.92) | 0.79 (-0.50-2.92) | 0.1 (-1.28-1.9) | 0.256 |
| Variceal bleeding history | 2 (9.1) | 1 (7.1) | 1 (12.5) | 1.000 |
| WCC, median (range) | 5.20 (2.43-7.94) | 5.4 (4.4-7.94) | 3.2 (2.43-6.8) | 0.052 |
| Hb low | 5 (25) | 2 (15.4) | 3 (42.9) | 0.290 |
| Platelet, median (range) | (35-514) | 173 (69-514) | 153 (35-388) | 0.843 |
| INR, median (range) | 1 (0.9-1.3) | 1.05 (0.9-1.2) | 1 (0.9-1.3) | 0.794 |
| Bilirubin, median (range) | 17 (3-62) | 17.8 (7-62) | 13 (3-56) | 0.561 |
| ALT, median (range) | 67.5 (14-677) | 72 (14-677) | 55 (24-137) | 0.250 |
| ALP, median (range) | 146 (20-1200) | 156 (20-1200) | 136 (67-546) | 0.968 |
| AST, median (range) | 74 (24-303) | 76 (24-303) | 55 (38-102) | 0.533 |
| ALP/AST ratio, median (range) | 2.03 (0.625-7.64) | 2.05 (0.625-7.64) | 1.78 (1.40-2.21) | 0.610 |
| GGT, median (range) | 165 (13-1838) | 177 (12-1838) | 73.5 (24-637) | 0.275 |
| Albumin, median (range) | 39 (31-43) | 39 (32-42) | 40 (31-43) | 0.810 |
| Ferritin, median (range) | 85 (7-913) | 176 (45-913) | 32.5 (7-79) | 0.027a |
| Transferrin, median (range) | 31.5 (23-42) | 31.5 (23-42) | 36 (30-41) | 0.443 |
| AMA | 6 (35.3) | 6 (54.5) | 0 (0) | 0.043a |
| AMA-M2 | 7 (100) | 7 (100) | 0 (0) | 1.000 |
| SMA | 10 (52.6) | 5 (41.7) | 5 (71.4) | 0.350 |
| SMA-VGT | 3 (15.8) | 3 (25) | 1 (14.3) | 1.000 |
| LKM-1 | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| pANCA | 4 (21.1) | 2 (18.2) | 2 (25) | 1.000 |
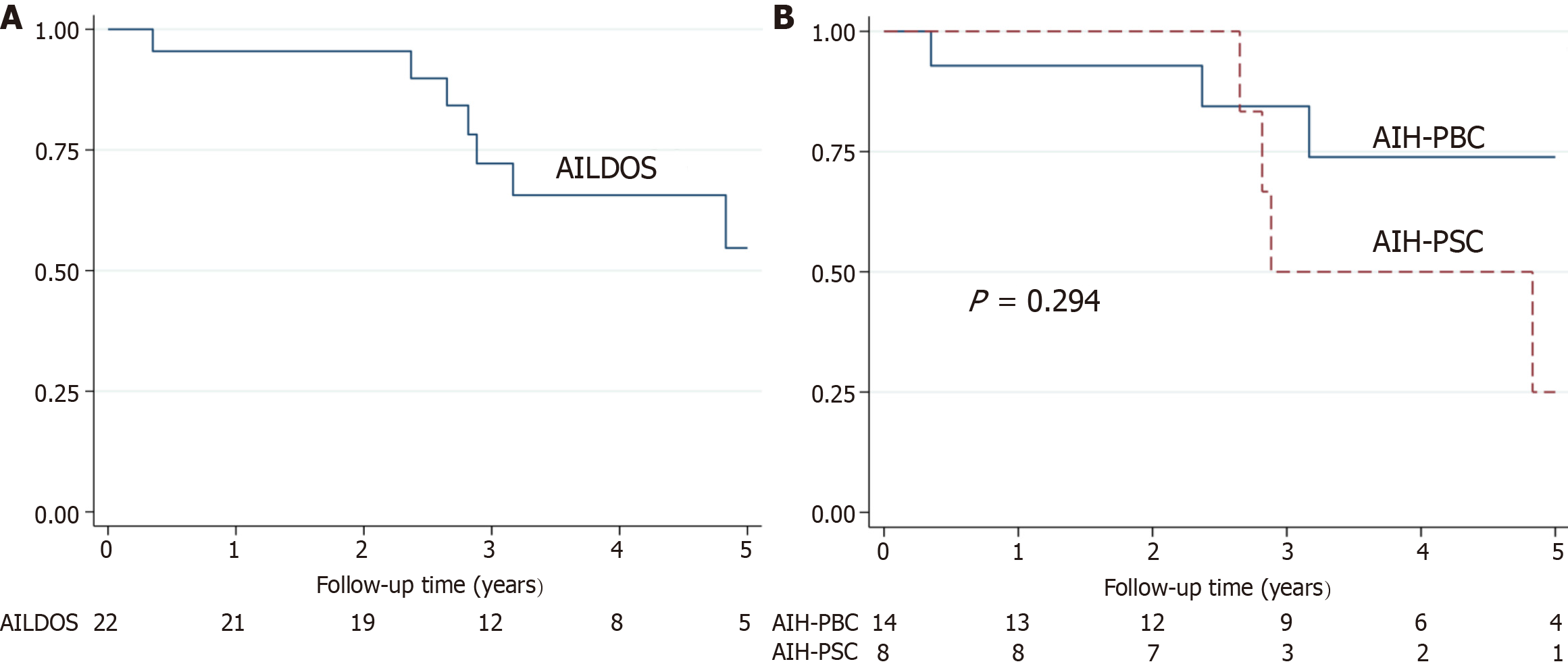
For patients with AILDOS, the median follow-up time was 3.1 years (range, 0.35-7.7 years). Eight patients had a liver-related mortality endpoint during the study and of these, three were liver-related deaths and five received a liver transplantation (Figure 1). At five years post enrolment, 57% of patients remained free from liver-related mortality. Univariate analysis found that the LOS and a low Hb were significant predictors of liver-related mortality (P < 0.05). Hepascore and Mayo Score were not significantly associated with liver-related mortality, but were retained for multiva
| AILDOS (all patients) | AIH-PBC | AIH-PSC | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| LOS | 1.37 (1.03, 1.82) | 0.030 | 1.56 (1.02, 2.38) | 0.040 | NS | |
| Hb low | 5.20 (1.16, 23.44) | 0.032 | 10.82 (0.97, 120.93) | 0.053 | NS | |
| Hepascore | 240.5 (0.42, 136411) | 0.090 | NS | NS | ||
| Mayo score | 2.25 (0.94, 5.39) | 0.068 | NS | NS | ||
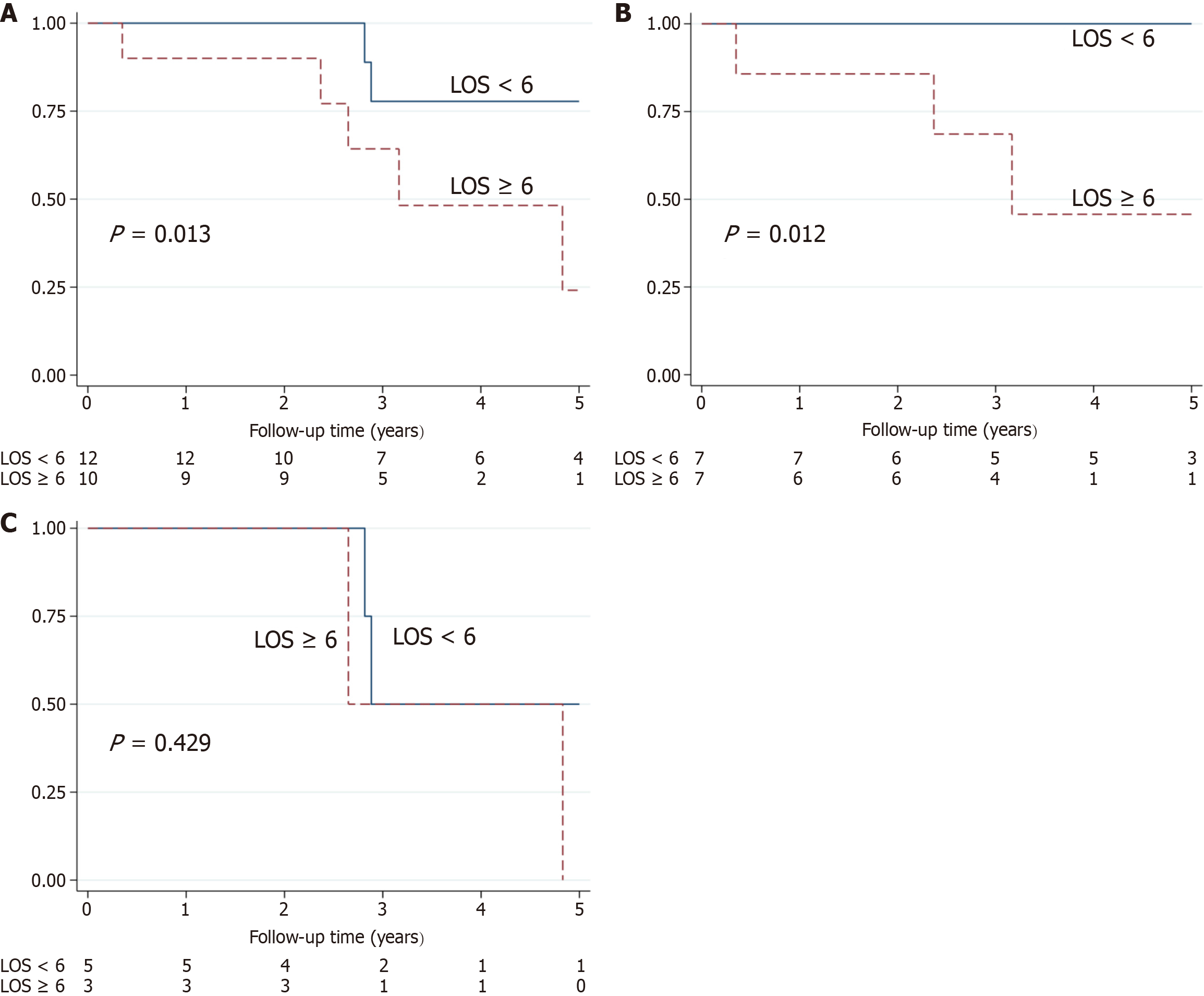
Subgroup analysis was performed on the AIH-PBC and AIH-PSC patient groups. The median follow-up time for AIH-PBC was 3.33 years (range: 0.35-7.7 years). Three deaths occurred, all of which were liver-related deaths, and one patient had a liver transplantation (Figure 1). At five years post enrolment, 74% of patients remained free from liver-related mortality. Univariate analysis of AIH-PBC patients showed that the LOS was a significant predictor of liver-related mortality (P < 0.05). Low Hb was retained for the multivariate analysis (P = 0.052; Table 2). Cox regression showed that the LOS was a significant predictor of liver-related mortality (P < 0.05; Table 3) and the Harrell’s C-Statistic was 0.867. A LOS cut point of 6 yielded a sensitivity of 100% and a specificity 77.8% for the prediction of liver-related mortality in AIH-PBC patients. A cut point of 6 significantly discriminated liver-related mortality in AIH-PBC patients (P = 0.012, log-rank test; Figure 2).
For patients with AIH-PSC, the median follow-up time was 2.85 years (range, 1.8-6.9 years). No deaths occurred and four patients received a liver transplantation (Figure 1). At five years post enrolment, 27% of patients remained free from liver-related mortality. No significant difference was identified between liver-related mortality of the AIH-PBC and AIH-PSC patient groups (P = 0.294, log-rank test). Univariate analysis of AIH-PSC showed no significant predictors of liver-related mortality (Table 2). A cut point of 6 failed to discriminate liver-related mortality in AIH-PSC patients (P = 0.429, log-rank test; Figure 2). The
In multivariate analysis, the MELD score, MELD-Na score and Mayo Score were not predictive of liver-related mortality in all AILDOS patients, AIH-PBC patients or in AIH-PSC patients (P > 0.05; Supplementary Table 1).
The current study is the first to identify a predictive model for liver-related mortality in AIH-PBC. This is one of the largest studies that has evaluated prognostic factors affecting liver-related clinical outcomes for patients with AILDOS, and as such yields novel findings. Individuals with AIH-PBC have poorer long-term outcomes when compared to patients with either AIH alone[19,20] or PBC alone[21,22], and some studies have demonstrated worse outcomes in those diagnosed with AIH-PSC when compared to patients with AIH alone or PSC alone[7,23,24].
Base line characteristics were similar between AIH-PBC and AIH-PSC. AIH-PBC patients were significantly older (55.8 years vs 23.3 years, P = 0.008), explained by PBC diagnosis being more prevalent in older patients when compared to PSC[25,26]. AIH-PBC patients also had significantly higher ferritin (P = 0.0272) than AIH-PSC. Ferritin is a known marker of hepatic necro-inflammation, but there is limited literature as to the role of ferritin in AILD diagnosis[27].
The primary finding of the current study is that LOS is a significant predictor of liver-related mortality in AIH-PBC patients (P < 0.05). Despite retention in multivariate analysis, the Hepascore is not a significant predictor of liver-related mortality in those diagnosed with AILDOS. Using a LOS cut point of 6, a significant difference between liver-related mortality was identified in AIH-PBC patients (P = 0.012) and in AILDOS patients (P = 0.013). The Harrell’s C-statistic was 0.867 and 0.730 for AIH-PBC and AILDOS patients respectively. No significant associations were identified for the AIH-PSC group.
Similarly, in CHC patients, LOS is significantly more accurate than the Hepascore at predicting liver-related death (P = 0.0009)[10]. Given the high Hepascore values in all patient groups at baseline (median of 1), suggestive of cirrhosis, the expectation is poor outcomes for all patients. Despite this, the ability of LOS to effectively stratify patients by liver-related mortality reflects that of use of LOS as a prognostic tool in those diagnosed with AIH-PBC will improve clinical care and their long-term outcomes.
The current study interestingly revealed a lack of significance of MELD and MELD-Na as predictors of liver-related mortality in AILDOS and AIH-PBC. Another study presented MELD approaching significance as a predictor of impaired overall survival in AILDOS patients (P = 0.05), but the study was limited by a small sample size of five patients in the entire AILDOS cohort, whilst the current study has twenty two patients[28]. The historical application of MELD and MELD-Na of predicting survival in cirrhotic patients undergoing transjugular intrahepatic portosystemic shunt pro
An additional finding of the current study was that in individuals with AILDOS, a low Hb is a significant predictive factor of poorer liver-related mortality (P = 0.041). Anemia, characterized by low Hb, is an established extrahepatic manifestation seen in approximately 75% of individuals with advanced liver disease[30]. In cirrhotic patients particularly, the etiology of anemia is complex and multifactorial, and various mechanisms are thought to be involved, including hepcidin metabolism, hemolysis, alcohol toxicity, splenomegaly, and chronic blood loss into the gastrointestinal tract[30,31]. In the current cohort, only two patients (9%) had a variceal bleeding history. Cirrhosis status was unable to be retrospectively obtained. The symptoms and complications of anemia increase cardiovascular morbidity and mortality, impair cognition and decrease health-related quality of life[32]. Anemia negatively impacts liver-related mortality in those diagnosed with AILDOS through the multifactorial mechanisms described.
There are currently no predictive models for liver-related mortality in AIH-PBC. With the progressive implementation of non-invasive fibrosis markers into liver disease patients routine care, using LOS to risk stratify AIH-PBC patients and to serve as surrogate endpoints in clinical trials is essential to optimize treatment, and in turn, liver-related outcomes for these patients. Having been demonstrated a predictor of survival in both AIH-PBC and CHC, further research into the application of LOS in other hepatic conditions is essential.
Limitations of the current study include a relatively small sample size, its retrospective nature, the lack of availability of treatment data, and the inclusion bias associated with having at least one Hepascore test performed being a requirement for entry into the HACO cohort. A relatively small sample size is expected given the low incidence of AILDOS[5]. This attracts attention to the paucity in the literature regarding the exact definition of AILDOS for which there is no precise consensus, as the pathogenesis of AILDOS is not understood. An established, stringent diagnostic criteria is warranted to facilitate collation of data between centres, enabling larger sample sizes for analysis. Biopsy evidence of cirrhosis was unable to be retrospectively obtained, and should be considered when drawing conclusions regarding predictive score accuracy in AILDOS. A larger sample size would be required for further trials to exclude cirrhosis from analysis.
In summary, the LOS has been demonstrated to be a predictor of liver-related mortality in patients with AIH-PBC. This is a novel study as no previous predictors of survival in AILDOS patients have been validated. Further, large-volume studies regarding the factors affecting prognosis for AILDOS and investigating LOS as a predictive model are crucial to ensure the best outcomes for patients with this rare condition.
| 1. | Silveira MG, Lindor KD. Overlap syndromes with autoimmune hepatitis in chronic cholestatic liver diseases. Expert Rev Gastroenterol Hepatol. 2007;1:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Freedman BL, Danford CJ, Patwardhan V, Bonder A. Treatment of Overlap Syndromes in Autoimmune Liver Disease: A Systematic Review and Meta-Analysis. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Alghamdi W, Qumosani K. A195 triple overlap syndrome? a rare case of AIH, PBC and PSC overlap. J Can Assoc Gastroenterol. 2018;1:287-287. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Jepsen P, Grønbæk L, Vilstrup H. Worldwide Incidence of Autoimmune Liver Disease. Dig Dis. 2015;33 Suppl 2:2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Czaja AJ. The overlap syndromes of autoimmune hepatitis. Dig Dis Sci. 2013;58:326-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Huang Y, Joseph J, de Boer WB, Cheng W, Adams LA, MacQuillan G, Garas G, Raftopoulos S, Jeffrey GP. Long-term Liver-related Outcomes of Patients With Chronic Liver Diseases in Australia. Clin Gastroenterol Hepatol. 2020;18:496-504.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Al-Chalabi T, Portmann BC, Bernal W, McFarlane IG, Heneghan MA. Autoimmune hepatitis overlap syndromes: an evaluation of treatment response, long-term outcome and survival. Aliment Pharmacol Ther. 2008;28:209-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Lüth S, Kanzler S, Frenzel C, Kasper HU, Dienes HP, Schramm C, Galle PR, Herkel J, Lohse AW. Characteristics and long-term prognosis of the autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome. J Clin Gastroenterol. 2009;43:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Di Martino V, Weil D, Cervoni JP, Thevenot T. New prognostic markers in liver cirrhosis. World J Hepatol. 2015;7:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Huang Y, Adams LA, MacQuillan G, Speers D, Joseph J, Bulsara MK, Jeffrey GP. Serum models accurately predict liver-related clinical outcomes in chronic hepatitis C. J Gastroenterol Hepatol. 2016;31:1736-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Kim WR, Therneau TM, Wiesner RH, Poterucha JJ, Benson JT, Malinchoc M, LaRusso NF, Lindor KD, Dickson ER. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc. 2000;75:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, Kench J, Farrell G, McCaughan GW, Jeffrey GP. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 392] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 14. | Lindor KD, Kowdley KV, Harrison ME; American College of Gastroenterology. ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am J Gastroenterol. 2015;110:646-59; quiz 660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 337] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 15. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 904] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 16. | Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis: Current Status and Future Directions. Gut Liver. 2016;10:177-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 17. | Jepsen P, Vilstrup H, Andersen PK. The clinical course of cirrhosis: The importance of multistate models and competing risks analysis. Hepatology. 2015;62:292-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 18. | Antolini L, Boracchi P, Biganzoli E. A time-dependent discrimination index for survival data. Stat Med. 2005;24:3927-3944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Martínez Casas OY, Díaz Ramírez GS, Marín Zuluaga JI, Santos Ó, Muñoz Maya O, Donado Gómez JH, Restrepo Gutiérrez JC. Autoimmune hepatitis - primary biliary cholangitis overlap syndrome. Long-term outcomes of a retrospective cohort in a university hospital. Gastroenterol Hepatol. 2018;41:544-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Park Y, Cho Y, Cho EJ, Kim YJ. Retrospective analysis of autoimmune hepatitis-primary biliary cirrhosis overlap syndrome in Korea: characteristics, treatments, and outcomes. Clin Mol Hepatol. 2015;21:150-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Poupon R, Chazouilleres O, Corpechot C, Chrétien Y. Development of autoimmune hepatitis in patients with typical primary biliary cirrhosis. Hepatology. 2006;44:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Yang F, Wang Q, Wang Z, Miao Q, Xiao X, Tang R, Chen X, Bian Z, Zhang H, Yang Y, Sheng L, Fang J, Qiu D, Krawitt EL, Gershwin ME, Ma X. The Natural History and Prognosis of Primary Biliary Cirrhosis with Clinical Features of Autoimmune Hepatitis. Clin Rev Allergy Immunol. 2016;50:114-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Czaja AJ. Frequency and nature of the variant syndromes of autoimmune liver disease. Hepatology. 1998;28:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 210] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Lian M, Li B, Xiao X, Yang Y, Jiang P, Yan L, Sun C, Zhang J, Wei Y, Li Y, Chen W, Jiang X, Miao Q, Chen X, Qiu D, Sheng L, Hua J, Tang R, Wang Q, Eric Gershwin M, Ma X. Comparative clinical characteristics and natural history of three variants of sclerosing cholangitis: IgG4-related SC, PSC/AIH and PSC alone. Autoimmun Rev. 2017;16:875-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Rupp C, Rössler A, Zhou T, Rauber C, Friedrich K, Wannhoff A, Weiss KH, Sauer P, Schirmacher P, Süsal C, Stremmel W, Gotthardt DN. Impact of age at diagnosis on disease progression in patients with primary sclerosing cholangitis. United European Gastroenterol J. 2018;6:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Hirschfield GM, Dyson JK, Alexander GJM, Chapman MH, Collier J, Hübscher S, Patanwala I, Pereira SP, Thain C, Thorburn D, Tiniakos D, Walmsley M, Webster G, Jones DEJ. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut. 2018;67:1568-1594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 220] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 27. | Maiwall R, Kumar S, Chaudhary AK, Maras J, Wani Z, Kumar C, Rastogi A, Bihari C, Vashisht C, Sarin SK. Serum ferritin predicts early mortality in patients with decompensated cirrhosis. J Hepatol. 2014;61:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Hoffmann K, Hinz U, Hillebrand N, Ganten T, Gotthardt D, Longerich T, Schirmacher P, Schemmer P. The MELD score predicts the short-term and overall survival after liver transplantation in patients with primary sclerosing cholangitis or autoimmune liver diseases. Langenbecks Arch Surg. 2014;399:1001-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Ruf A, Dirchwolf M, Freeman RB. From Child-Pugh to MELD score and beyond: Taking a walk down memory lane. Ann Hepatol. 2022;27:100535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 30. | Gkamprela E, Deutsch M, Pectasides D. Iron deficiency anemia in chronic liver disease: etiopathogenesis, diagnosis and treatment. Ann Gastroenterol. 2017;30:405-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Gonzalez-Casas R, Jones EA, Moreno-Otero R. Spectrum of anemia associated with chronic liver disease. World J Gastroenterol. 2009;15:4653-4658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 32. | Les I, Doval E, Flavià M, Jacas C, Cárdenas G, Esteban R, Guardia J, Córdoba J. Quality of life in cirrhosis is related to potentially treatable factors. Eur J Gastroenterol Hepatol. 2010;22:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |