Published online Sep 27, 2024. doi: 10.4254/wjh.v16.i9.1211
Revised: July 31, 2024
Accepted: August 13, 2024
Published online: September 27, 2024
Processing time: 109 Days and 15.8 Hours
Extracellular vesicles (EVs) are small particles released by many cell types in different tissues, including the liver, and transfer specific cargo molecules from originating cells to receptor cells. This process generally culminates in activation of distant cells and inflammation and progression of certain diseases. The global chronic liver disease (CLD) epidemic is estimated at 1.5 billion patients world
Core Tip: Extracellular vesicles are tiny particles released by cells and transport specific molecules from one cell to another, resulting in the sending of a message. Chronic liver diseases are mainly induced by cirrhosis, liver cancer, viral hepatitis, and obesity. Alterations in hepatic lipid metabolism, as fat accumulation in liver cells, can trigger lipotoxicity events that prompt extracellular vesicle release leading to inflammation. In this context, we aimed to provide a comprehensive overview of extracellular vesicles, covering their definition, types, and biogenesis, with emphasis on extracellular vesicles associated with steatosis-related liver disease, diagnosis, treatment, and its possible therapeutic applications.
- Citation: Montoya-Buelna M, Ramirez-Lopez IG, San Juan-Garcia CA, Garcia-Regalado JJ, Millan-Sanchez MS, de la Cruz-Mosso U, Haramati J, Pereira-Suarez AL, Macias-Barragan J. Contribution of extracellular vesicles to steatosis-related liver disease and their therapeutic potential. World J Hepatol 2024; 16(9): 1211-1228
- URL: https://www.wjgnet.com/1948-5182/full/v16/i9/1211.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i9.1211
Extracellular vesicles (EVs) are small membrane vesicles released by cells as a communication mechanism. Due to their features, they can act as vessels for different types of bioactive molecules [DNA, RNA, microRNA (miRNA), proteins, lipids] that mediate cell-to-cell information transmission[1]. This group of vesicles is subclassified as microvesicles, exosomes, apoptotic bodies (ApoBDs), and large oncosomes, according to their biogenesis mechanism, physical properties, and cargo. As will be explored below, recent studies have implicated EVs in the diagnosis, therapy, and prognosis of several diseases.
Chronic liver disease (CLD) is defined as the progressive deterioration of liver functions for more than 6 months of evolution. This impairment derives mainly from conditions such as alcoholic liver disease, chronic viral hepatitis, and nonalcoholic fatty liver disease (NAFLD)[2]. There is a consensus of experts who have proposed renaming NAFLD as “metabolic dysfunction-associated steatotic liver disease”, based on an improved clinical approach to the interplay between metabolic comorbidities associated with liver disease. Although we agree with this idea, this disease is denoted as NAFLD since most of the consulted literature is accessible with the NAFLD nomenclature[3].
The most common molecular harmful events present in CLD are lipotoxicity and oxidative stress, mainly in hepatocytes. Due to the constant damage and even cell death, or as a result of different processes induced by the damage itself, several mediators are released to announce to other cells the altered state encountered in CLD[4]. These mediators include classic cytokines, chemokines, damage-associated molecular patterns, and EVs. There are many reports that show the association of EV proteins and other molecules with CLD development, which will be discussed below in this review.
There exist numerous mechanisms for cell-to-cell communication; one such mechanism is through EVs. In 1946, Chargaff and West[6] described EVs for the first time as small particles in the sediment of plasma supernatant with the capacity to regulate coagulation[5-7]. The term “extracellular vesicles” was first used by Aaronson et al[8] in 1971, who demonstrated their presence and features using electron microscopy. Since then, EV research has grown significantly[8,9].
The structural composition of EVs is complex and variable. They function as vessels surrounded by a membrane that contains molecules. They can carry signals from a donor to a receiving cell, thus establishing a communication system. The concentration and distribution of molecules within the EVs depends on the properties of the donor and receiving cells[10,11]. In addition, the composition can be modified by several factors such as cellular microenvironment, pathologies, wellness conditions, and other environmental factors, enabling the EVs to maintain homeostasis or to inhibit or promote the evolution of diseases[12].
The EV lipidic bilayer membrane consists mainly of complex lipids like phosphatidylserine, glycosphingolipids, sphingomyelin, phosphatidylcholine, phospholipids, and cholesterol. It also contains, in a smaller proportion, proteins and carbohydrates that help maintain its structure and mediate intercellular interactions and components destined for macrophage degradation[13,14].
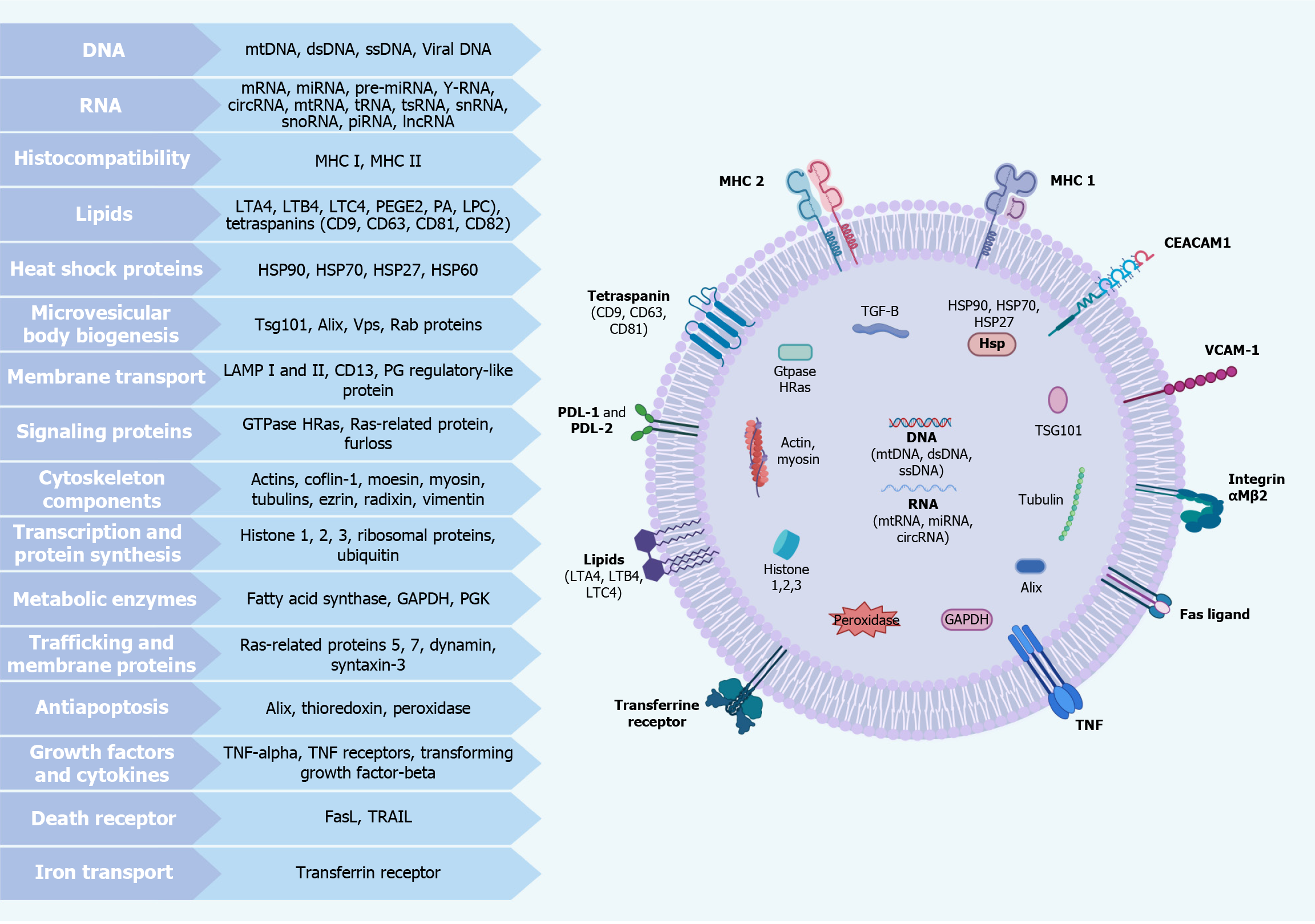
EVs transport and/or contain a wide variety of components, such as organelles, cytokines, enzymes, membrane receptors, signaling factors, amino acids, peptides, and lipids and the precursors involved in their synthesis. The most studied molecules transported by EVs are the nucleic acids derived both from DNA and RNA [miRNA, small interfering RNA, circular RNA (circRNA), and messenger RNA][15-19].
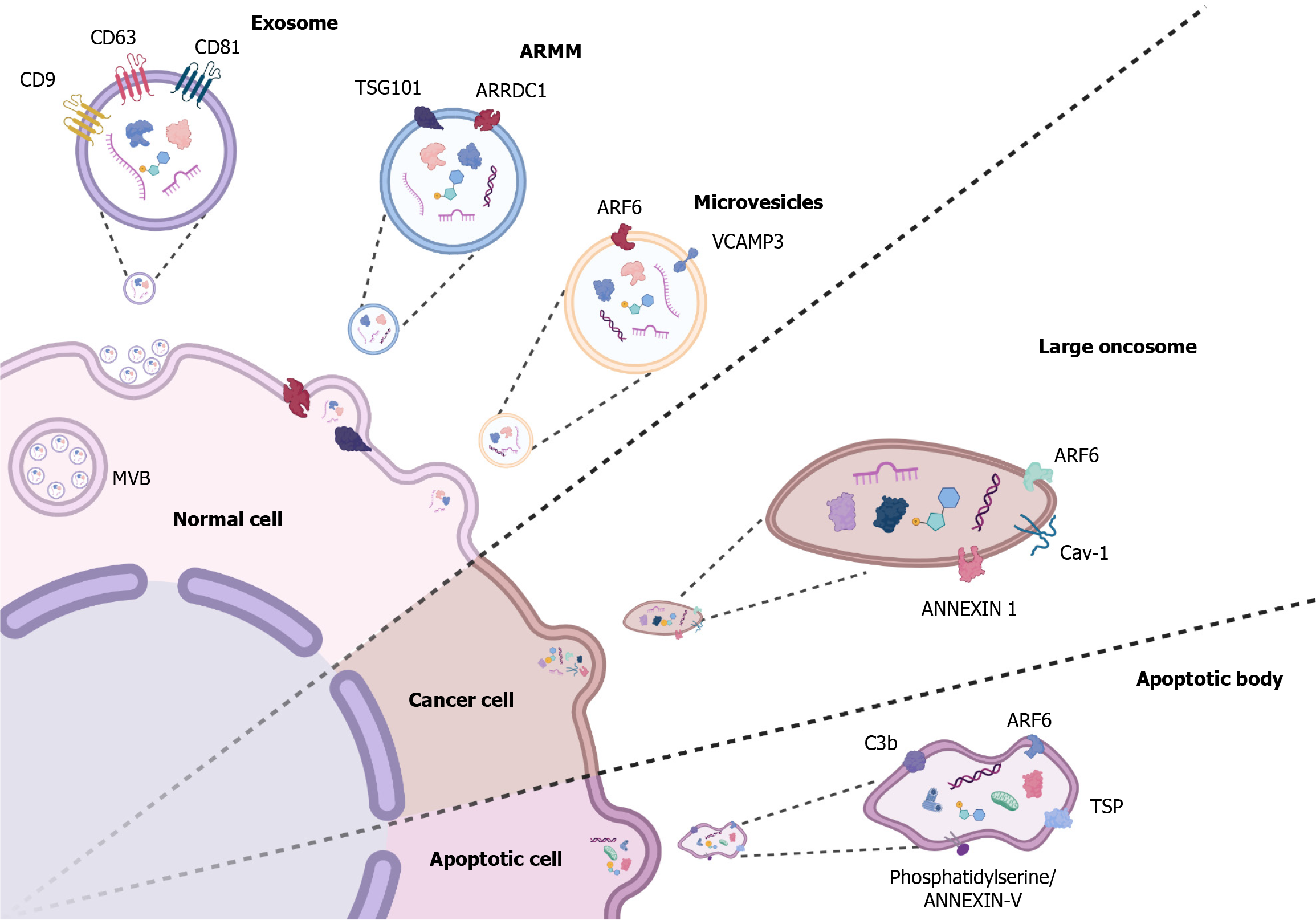
EVs have a heterogeneous origin and formation. They have been reported to originate from the membrane invagination of cytosolic multivesicular bodies (MVBs) or from an apoptotic process. There is a wide variety of EVs with unique size, function, markers, and content, such as exosomes, microvesicles, ApoBDs, large oncosomes, etc. These features are summarized in Table 1[20-25].
| Name | Size | Markers | Characteristics or definition | Content |
| Classical exosomes | 40-150 nm[20] | CD9, CD63, CD81[20,21] | EVs originated in intracellular MVBs containing ILVs released into the extracellular space[20,21] | Proteins, amino acids, metabolites, mRNA, and siRNA[20,21] |
| Non-classical exosomes | 40-150 nm[21] | CD9-, CD63-, CD81-[21] | Exosomes lacking CD9, CD63, and CD81 expression[21] | Not yet determined |
| Microvesicles/ectosomes/microparticles/membrane particles | 50-2000 nm[22] | ARF6, VCAMP3, Annexin A1[21] | EVs originated by budding and detachment of cell membrane[22] | Proteins, amino acids, metabolites, mRNA, siRNA, and DNA[22] |
| ARMM | 40-100 nm[21] | ARRDC1, TSG101[23] | Small microvesicles originated by budding and detachment of cell membrane, regulated by ARRDC1 and TSG101[23] | Proteins, amino acids, metabolites, mRNA, siRNA, and DNA[23] |
| Large oncosomes | 1-10 μm[21] | Myr-Akt1, HB-EGF, Cav-1, ARF6[24] | Atypically large EVs originated by budding and detachment of cell membrane from advanced cancer disease cells[24] | Proteins, enzymes, peptides, miRNA, mRNA, DNA, amino acids, metabolites, and lipids[24] |
| Apoptotic bodies | 50-5000 nm[21] | TSP, C3b, ARF6 ANEXIN V[25] | EVs originated during apoptotic events[25] | DNA, miRNA, RNA, proteins, and lipids[25] |
Exosomes: Exosomes are tiny particles (40-160 nm) that originate from the intraluminal vesicles (ILVs) contained in the intracellular MVBs. Once these MVBs fuse with the cytoplasmic cell membrane and release their contents to the extracellular space, the ILVs are known as exosomes[20].
Although exosome biogenesis has not been fully elucidated, some authors have subdivided the classification of exosome biogenesis into those pathways dependent on the endosomal sorting complex required for transport (ESCRT) and those not dependent on it[26].
It is widely described that the ESCRT-dependent generation of ILVs are loaded with ubiquitinated proteins destined for degradation, but the mechanism by which they are released as exosomes is unclear. Nevertheless, it is known that ESCRT proteins (ESCRT-0, -1, -2, and -3), composed of diverse complexes, produce ILVs and deposit ubiquitinated proteins within them[26,27].
ESCRT-0 (conformed by HRas and STAM 1/2 subcomplexes) recognizes ubiquitinated proteins and recruits them into the endosomal membrane through the interaction of HRas with phosphatidyl inositol 3-phosphate. ESCRT-0 recruits ESCRT-I [conformed by tumor susceptibility gene 101 (TSG101), hVps28, Vps37, and hMvb12 subcomplexes] through interaction with TSG101. After ESCRT-I recruits ESCRT-II (conformed by EAP45, EAP30, and EAP20 subcomplexes), they finally recruit and activate ESCRT-III (conformed by CHMP-6, -4, -3, and -2). This protein senses, stabilizes, and induces the curvature of the budding vesicle to promote its formation by oligomeric assembly. ALG-2-interacting protein X (ALIX) stabilizes the assembly of ESCRT-III oligomers. Once the vesicle forms, ESCRT-III recruits the ATPase vacuolar protein sorting 4 (made up of the SKD1, CHMP5, and LIP5 complexes). This is the only ATPase that participates in ESCRT machinery and disassembles ESCRT-III oligomers. It also returns them into the cytoplasm by an ATP-dependent mechanism[27,28].
ESCRT-III orchestrates protein deubiquitylation through the recruitment of deubiquitinases. However, this is not always an essential step for exosome formation: An exosome study from human urine demonstrated that only 13% of exosome proteins were ubiquitylated with different patterns, suggesting that these proteins could be involved in exosome function and potentially be used as a biomarker and therapeutic target[29]. On the other hand, ALIX can interact directly with ESCRT-III and direct non-ubiquitinated cargo to ILVs, in a mechanism that involves structures of heparan sulphate chains of syndecan that can recruit syntenin-ALIX and support the membrane budding[30-32].
Two ESCRT-independent exosome biogenesis mechanisms have been reported. One involves ceramides and another involves the tetraspanin family. Ceramides are sphingolipids structured by a sphingosine linked to a long-chain fatty acid by an amide. This lipid is located asymmetrically in the membrane, which could affect its fluidity and curvature, taking a spontaneous curvature shape when the ceramide has a specific location. In addition, sphingomyelinases (SMases) can hydrolyze sphingomyelin to produce ceramides. In MVBs, neutral-SMase produce ceramide and induce a spontaneous negative curvature promoting the formation of ILVs[33].
Tetraspanins are a protein superfamily localized in both the cell membrane and endomembrane system of a large variety of cells. They are structured by four transmembrane domains, four to six conserved extracellular cysteine residues, polar residues within the transmembrane domain and distinct palmitoylation sites. Tetraspanins, alongside cholesterol and gangliosides, can be organized in membrane microdomains called tetraspanin-enriched microdomains. These clusters interact with a large variety of transmembrane and cytosolic signaling proteins, permitting their participation in diverse biological processes like cell adhesion, motility, invasion, membrane fusion, signaling, and protein traffic. In addition, tetraspanin-enriched microdomains can induce membrane curvature and interact with cytoskeletal proteins to induce membrane cleavage and budding of ILVs[34].
Exosomes are highly enriched with tetraspanins (7-124-fold greater than the donor cell). Cluster of differentiation (CD) 9, CD63, CD37, CD81, and CD82 tetraspanins are found in higher quantities and may serve as exosome biomarkers. However, other EV subpopulations can also express these tetraspanins, so they should be used cautiously as biomarkers[34].
A less described ESCRT-independent pathway has been recently mentioned. In this pathway, the Rab31 GTPase is recruited into ceramide and cholesterol clusters by flotillins to stimulate membrane budding and epidermal growth factor receptor packaging[35]. Rab27 GTPase controls exosome release by promoting fusion of MVBs with plasma membrane in order to release their contents to the extracellular space (Figure 1)[36].
Exosome protein contents can include the tetraspanin family, integrins, immunoglobulins, receptors, cytoskeleton proteins, proteins related to the ESCRT machinery, heat shock proteins, and proteins involved in vesicle trafficking. The proteins recognized as the main markers are the classical tetraspanins (CD9, CD63, and CD81), ALIX, and TSG101 associated with ESCRT (Figure 1)[20,21]. Nevertheless, the discovery of exosome production in the absence of some of the classical tetraspanins allows for the hypothetical existence of non-classical exosomes that may not present these canonical tetraspanin markers[21,37,38].
Several molecules have been described as exosome components; many of them are involved in their biogenesis. Additionally, exosomes may express bioactive molecules that change according to their host cell type[26,39]. Some of the most important ones are included in Figure 2.
Microvesicles: Microvesicles (also known as microparticles, ectosomes, and membrane particles) are a type of EV with a size range of 50-2000 nm that originate from budding and detachment of the extracellular membrane by exocytosis[40].
Microvesicle biogenesis is less characterized than exosomes, but two ESCRT-dependent mechanisms and a third ESCRT-independent mechanism have been described to explain microvesicle formation. The first involves the ALIX, TSG101, Vps22, Chmp1/3, and vacuolar protein sorting 4 ESCRT complex proteins. A study showed that their absence reduces the secretion of Hedgehog in EVs. Another mechanism consists of the recruitment of the ESCRT subunits TSG101 and VPS4 to the plasma membrane by adapter protein arrestin domain-containing protein 1 (ARRDC1), which promotes the generation of microvesicles called ARRDC1-mediated microvesicles (Figure 1)[23].
The third mechanism is independent of ESCRT and involves the activation of acid sphingomyelinase (A-SMase) through the generation of ceramide in the membrane. In addition to the aforementioned mechanisms, it has also been seen that the small proteins GTPase ADP-rbosylation factor (ARF) 1, ARF6, and RhoA can induce the production of microvesicles (Figure 1)[23]. Likewise, lipids play an important role in microvesicle formation due to the following mechanisms: Phosphatidylinositols recruiting membrane-sculpting proteins and cone-shaped phosphatidylethanolamine inducing membrane curvature[40].
Large oncosomes: Large EVs have been described in various tumors, such as hepatic cancer, prostate cancer, breast cancer, glioblastoma, glioma, pancreatic cancer, colon cancer, melanoma, and leukemia. Large oncosomes (LO) are a type of large EV that come exclusively from cancer cells. LO can be up to a thousand times larger than exosomes (1-10 μm), allowing them to contain an extensive number of molecular compounds derived from tumoral cells and to have a different impact on the tumor microenvironment than exosomes and smaller EVs[24,41].
It is known that LO can originate from non-apoptotic plasma membrane blebbing induced through the inhibition of cytoskeletal regulator diaphanous-related formin-3, by overexpression of oncoproteins (e.g., Myr-Akt1, HB-EGF, and caveolin-1) or by activation of the epidermal growth factor receptor. Nevertheless, LO has not been as widely studied as other EVs[42].
A wide range of molecules have been found in LO, including GTPase ARF6, caveolin-1, metalloproteinases-2 and -9, keratin 18 (cytokeratin type I), glyceraldehyde 3-phosphate dehydrogenase, phosphoglucose isomerase, lactate dehydrogenase B, heat shock 70 kDa protein 5, malate dehydrogenase, aspartate transaminase, glutaminase, caveolin-2, and glutathione S-transferase pi 1 gene (Figure 1)[24].
It has been reported that LOs can use autocrine and paracrine mechanisms to perform their functions, from direct proteolytic activity to the activation of protumorigenic programs into different types of target cells[24]. It has also been reported that LOs originating from an aggressive prostate cancer cell line can express integrin alpha-V on their surface, which can be used to activate AKT and induce both adhesion and invasion of other prostate cancer cells[43].
ApoBD: ApoBDs, also known as apoptosomes, are derived from the division of cellular contents in late-stage apoptosis. Their structure and size (500 nm to 2 µm) are highly variable, and depending on their dimension, they may include large amounts of RNA, proteins, and lipids[25].
Once apoptosis is finished, ApoBDs are released into the extracellular space, where they can be phagocytosed by macrophages, parenchymal cells, or neoplastic cells. Phagocytosis is activated by the identification of ApoBD membrane biomarkers. Annexin V, thrombospondin, and complement component 3b are the most characteristic biomarkers (Figure 1)[22]. ApoBDs are degraded by macrophage phagolysosomes. Some ApoBDs may contain tingible bodies, which are nuclear debris of apoptotic cells[25].
Although their function has not been completely described, it is well-known that ApoBDs are also capable of transporting useful resources to healthy cells; thus, these vesicles do more than participate in phagocytosis and cellular debris degradation. Also, some biomarkers contained in the ApoBD, such as microRNA and DNA, can regulate intercellular communication[25].
In regular conditions, ApoBDs do not release inflammatory cytokines or free cellular constituents to the extracellular space because they are cleared by a fast phagocytic process, avoiding secondary necrosis. Therefore, they have been associated with inflammatory reactions only under pathological circumstances[25].
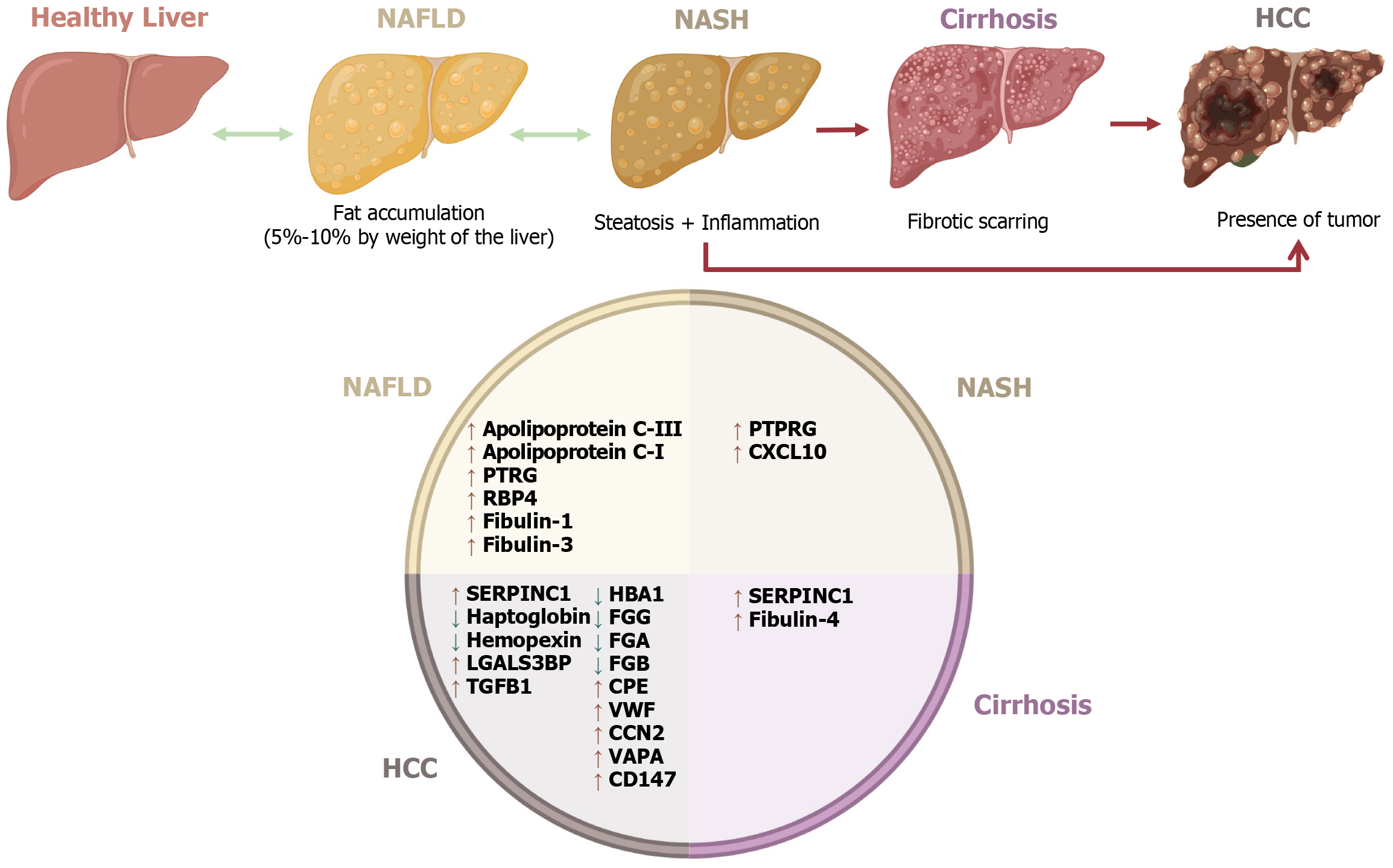
CLD is defined as the progressive deterioration of liver functions for more than 6 months[2]. In 2017, there were an estimated 1.5 billion cases of CLD worldwide[44]. The deterioration of the liver can be produced by alcoholic liver disease, which includes alcohol-fatty liver with or without hepatitis, alcohol hepatitis, and cirrhosis, chronic viral hepatitis (genetic and autoimmune causes), and NAFLD. Some of the patients with NAFLD develop non-alcoholic steatohepatitis (NASH), which leads to cirrhosis and then hepatocellular carcinoma (HCC)[2]. In 2017, Global Health Metrics estimated that the age-standardized prevalence of NAFLD and NASH that leads to cirrhosis or liver cancer is 10935 cases per 100000. However, higher rates were found in North America and the Middle East, corresponding to a higher prevalence of obesity[45].
NAFLD is characterized by a lipidic accumulation in the liver generated by an imbalance in the acquisition and removal of triglycerides[46]. At least one risk factor, including insulin resistance, metabolic syndrome, obesity, dyslipi
Patients with NASH can progress to tissue fibrosis of the liver due to prolonged inflammation, producing cirrhosis. It has been described that 11% of patients with NASH will experience cirrhosis[47]. The progression to severe fibrosis has been associated with age, possibly related to accumulated metabolic alterations in elderly patients[52].
Once cirrhosis is established, the prognosis is unfavorable because of the risk of developing life-threatening comp
Certain proteins carried by different EVs show changes in their expression in different CLD stages; some of them are associated with liver damage progression. Most proteins mentioned in this review show increased levels in different CLD phases, such as apolipoprotein C-III[53], apolipoprotein C-1[53], fibulin-1[54], and fibulin-3[54] in NAFLD, protein tyrosine phosphatase receptor type G in both NAFLD and NASH[55], and fibulin-4 in cirrhosis[56] (Figure 3). Nevertheless, other relevant proteins have shown a particular association with certain types of EVs.
HCC-derived exosomes contain several proteins that are significantly increased when compared with healthy patient exosomes. Some of these proteins are von Willebrand factor, transforming growth factor beta 1, galectin-3-binding protein, serpin family C member 1, hemopexin, haptoglobin, hemoglobin subunit alpha 1, fibrinogen alpha chain, fibrinogen gamma chain, and fibrinogen beta chain. These proteins could be potential biomarkers in HCC diagnosis[57]. Other proteins are increased during HCC, such as carboxypeptidase-E[58] and CCN family member 2[59]. On the other hand, serpin family C member 1 decreases in cirrhosis (Table 2)[57].
| Disease | Protein | Gene name and alias symbols | Role in liver disease | Vesicle source | Expression in disease |
| NAFLD | Apolipoprotein C-III | Apolipoprotein C3 | An increased expression leads to increased steatosis in NAFLD[53] | EVs from human serum[53] | ↑ NAFLD patients[53] |
| Composition: 79 AA | APOC3 | ||||
| MW: 10.85 kDa | Apo-CIII | ||||
| UA: P02656 | ApoC-III | ||||
| HGNC ID: 610 | APOCIII | ||||
| Cytogenetic band: 11q23.3 | Apo-C3 | ||||
| ApoC-3 | |||||
| Apolipoprotein C-I | Apolipoprotein C1 | An increased expression leads to increased steatosis in NAFLD[53] | EVs from human serum[53] | ↑ NAFLD patients[53] | |
| Composition: 83 AA | APOC1 | ||||
| MW: 9.33 kDa | |||||
| UA: P02654 | |||||
| HGNC ID: 607 | |||||
| Cytogenetic band: 19q13.32 | |||||
| Retinol-binding protein 4 | Retinol binding | Enhances the M1-like polarization of Kupffer cells via promoting the activation of NOX2 and NF-κB and ROS accumulation[72] | Serum exosomes from NAFLD patients[72] | ↑ NAFLD patients[72] | |
| Composition: 201 AA | protein 4 | ||||
| MW: 23.01 kDa | |||||
| UA: P02753 | RBP4b | ||||
| HGNC ID: 9922 | |||||
| Cytogenetic band: 10q23.33 | |||||
| Receptor-type tyrosine-protein phosphatase gamma | Protein tyrosine phosphatase receptor type G | Hepatic PTPRG mRNA increase proportionally to the severity of NAFLD[55] | Exosomes from human plasma and murine plasma, serum, and tissue[73] | ↑ of plasmatic approximately 120 kDa protein isoform were associated with the occurrence of liver damage[73] | |
| ↑ NASH[55] | |||||
| Composition: 1445 AA | PTPRG | ||||
| MW: 162.03 kDa | RPTPG | ||||
| UA: P23470 | |||||
| HGNC ID: 9671 | |||||
| Cytogenetic band: 3p14.2 | |||||
| C-X-C motif chemokine 10 | C-X-C motif chemokine ligand 10 | Lipotoxic hepatocyte-derived EVs containing CXCL10 induce macrophage chemotaxis[74,75] | EVs from Mlk3-/- mice[74,75] | ↑ NASH model[74,75] | |
| Composition: 98 AA | |||||
| MW: 10.88 kDa | CXCL10 | ||||
| UA: P02778 | IFI10 | ||||
| HGNC ID: 10637 | IP-10 | ||||
| Cytogenetic band: 4q21.1 | crg-2 | ||||
| mob-1 | |||||
| C7 | |||||
| gIP-10 | |||||
| Fibulin-1 | Fibulin-1 | Correlate with fibrosis stage[54] | EVs from human serum[54] | ↑ NAFLD patients[54] | |
| Composition: 703 AA | |||||
| MW: 77,214 kDa | FBLN1 | ||||
| UA: P23142 | FBLN | ||||
| HGNC ID: 3600 | |||||
| Cytogenetic band: 22q13.31 | |||||
| Fibulin-3 | EGF containing fibulin extracellular matrix protein 1 | Increase with liver fibrosis. Predictor of liver-related events[54] | EVs from human serum[54] | ↑ NAFLD patients[54] | |
| Composition: 493 AA | |||||
| MW: 54.64 | EFEMP1 | ||||
| UA: Q12805 | S1-5 | ||||
| HGNC ID: 3218 | FBLN3 | ||||
| Cytogenetic band: 2p16.1 | MTLV | ||||
| NASH | Antithrombin-III | Serpin family C member 1 | Almost all of the downregulated proteins are produced in the liver[57] | Exosomes from HCC human serum samples[57] | ↑ ATIII in liver cirrhosis and HCC[57] |
| Composition: 464 AA | |||||
| MW: 52.6 kDa | SERPINC1 | ||||
| UA: P01008 | ATIII | ||||
| HGNC ID: 775 | MGC22579 | ||||
| Cytogenetic band: 1q25.1 | |||||
| Von Willebrand factor | Von Willebrand factor | Biomarker of severe liver fibrosis diagnosis and HCC development predictor[76] | Exosomal, from serum samples[57] | ↑ HCC[57] | |
| Composition: 2813 AA | |||||
| MW: 309.26 kDa | VWF | ||||
| UA: P04275 | |||||
| HGNC ID: 12726 | |||||
| Cytogenetic band: 12p13.31 | |||||
| Hemopexin | Hemopexin | ↓ HPX protein develops inflammation and oxidative stress in the liver[77] | Exosomal, from serum samples[57] | ↓ HCC[57] | |
| Composition: 462 AA | |||||
| MW: 51.67 kDa | HPX | ||||
| UA: P02790 | |||||
| HGNC ID: 5171 | |||||
| Cytogenetic band: 11p15.4 | |||||
| Galectin 3 binding protein | Galectin 3 binding protein | Significant biomarker in liver fibrosis, cirrhosis, and HCC in patients with hepatitis C[78] | Exosomes from serum samples[57] | ↑ HCC[57] | |
| Composition: 585 AA | |||||
| MW: 65.33 kDa. | LGALS3BP | ||||
| UA: Q08380 | MAC-2-BP | ||||
| HGNC ID: 6564 | 90K | ||||
| Cytogenetic band: 17q25 | BTBD17B | ||||
| ANGO10B | |||||
| M2BP | |||||
| gp90 | |||||
| CyCAP | |||||
| Cirrhosis | Transforming growth factor beta 1 | Transforming growth factor beta 1 | Promotes HSC activation and ECM production, contributing NAFLD progression[79,80] | Exosomal, from serum samples[57] | ↑ HCC[57] |
| Composition: 390 AA | Hepatoma cell lines, culture media[81] | ↑ Promote tumor metastasis[81] | |||
| MW: 44.32 kDa | TGFB1 | Ascites derived exosomes, from hepatic cirrhosis patients[82] | ↑ Promote cancer[82] | ||
| UA: P01137 | CED | ||||
| HGNC ID: 11766 | TGFbeta | ||||
| Cytogenetic band: 19q13.2 | |||||
| Fibulin-4 | EGF containing fibulin extracellular matrix protein 2 | Increased levels in cirrhosis, correlated with progression of fibrosis[56] | EVs from serum samples of patients with cirrhosis[56] | ↑ Cirrhosis[56] | |
| Composition: 443 AA | |||||
| MW: 49.4 kDa | EFEMP2 | ||||
| UA: O95967 | |||||
| HGNC ID: 3219 | |||||
| Cytogenetic band: 11q13.1 | |||||
| HCC | Haptoglobin | Haptoglobin | Patients with NAFLD with the Hp2-2 genotype had higher BMI, total cholesterol, and ferritin[83] | Exosomal, from serum samples[57] | ↓ HCC[57] |
| Composition: 406AA | |||||
| MW: 45.20 kDa | HP | ||||
| UA: P00738 | |||||
| HGNC ID: 5141 | |||||
| Cytogenetic band: 16q22.2 | |||||
| Hemoglobin subunit alpha | Hemoglobin subunit alpha 1 | Hemoglobin overexpression suppresses oxidative stress[57,84] | Exosomal, from serum samples[57] | ↓ HCC[57] | |
| Composition: 142 AA | Liver biopsies from NASH patients[84] | ↑ NASH[84] | |||
| MW: 15.258 kDa | HBA1 | ||||
| UA: P69905 | HBA-T3 | ||||
| HGNC ID: 4823 | |||||
| Cytogenetic band: 16p13.3 | |||||
| Fibrinogen alpha chain | Fibrinogen alpha chain | α chain fragments rapid alteration in early stages in liver fibrosis[85,86] | Exosomal, from serum samples[57] | ↓ HCC[57] | |
| Composition: 866 AA, | |||||
| MW: 94.97 kDa | FGA | ||||
| UA: P02671 | |||||
| HGNC ID: 3661 | |||||
| Cytogenetic band: 4q31.3 | |||||
| Fibrinogen gamma chain | Fibrinogen gamma chain | Rare cases of hypofibrinogenemia are associated with liver disease[87] | Exosomal, from serum samples[57] | ↓ HCC[57] | |
| Composition: 453 AA | |||||
| MW: 51.51 kDa | FGG | ||||
| UA: P02679 | |||||
| HGNC ID: 3694 | |||||
| Cytogenetic band: 4q32.1 | |||||
| Fibrinogen beta chain | Fibrinogen beta chain | Rarely, hypofibrinogenemia can present with HFSD[88] | Exosomal, from serum samples[57] | ↓ HCC[57] | |
| Composition: 491 AA | |||||
| MW: 55.928 kDa | FGB | ||||
| UA: P02675 | |||||
| HGNC ID: 3662 | |||||
| Cytogenetic band: 4q31.3 | |||||
| Carboxypeptidase E | Carboxypeptidase E | Promotes tumor metastasis and predicts tumor recurrence in early-stage HCC[58] | Exosomes from supernatant culture and human serum[57] | ↑ HCC Promote tumor metastasis[57] | |
| Composition: 476 AA | |||||
| MW: 53.15 kDa | CPE | ||||
| UA: P16870 | |||||
| HGNC ID: 2303 | |||||
| Cytogenetic band: 4q32.3 | |||||
| Vesicle-associated membrane protein-associated protein A | VAMP associated protein A | Facilitates bone-tropic metastasis of HCC by promoting osteoclastogenesis[41] | Large oncosomes from liver cancer mouse model[41] | ↑ HCC[41] | |
| Composition: 249 AA | |||||
| MW: 27.89 kDa | VAPA | ||||
| UA: Q9P0L0 | hVAP-33 | ||||
| HGNC ID: 12648 | VAP-A | ||||
| Cytogenetic band: 18p11.22 | |||||
| Basigin | Basigin (Ok blood group) | Induces angiogenesis by stimulating VEGF production and invasiveness by stimulating MMPs | Microvesicles from SMMC-7721 cell line (Hepatocellular carcinoma)[89] | ↑ HCC in vitro model[89] | |
| Composition: 385 AA | BSG | Promotes the invasion and metastasis of human hepatoma cells by stimulating both tumor cells and peritumoral fibroblasts to produce elevated levels of MMPs[89] | |||
| MW: 42.2 kDa | EMMPRIN | ||||
| UA: P35613 | CD147 | ||||
| HGNC ID: 1116 | EMPRIN | ||||
| Cytogenetic band: 19p13.3 |
It is known that glypican-3 (GPC3) is a reliable immunohistochemical marker for HCC diagnosis (Table 2)[60]. A recent study showed that GPC3 is present exclusively in HCC-derived exosomes, which provides a potential EV-mediated method for HCC early diagnosis and treatment response surveillance[61].
Exosomes promote HCC growth and motility through diverse RNA release mechanisms[62]. Exosomal miR-21/10b stimulates HCC proliferation and metastasis when found in an acidic microenvironment[63]. In addition, miR-92a-2-5p, circRNA-100, 338, and linc00511 facilitate invasiveness and angiogenesis. Furthermore, exosome circRNA-SORE prevents YBX1 degradation, which leads to kinase inhibitor resistance[64-66]. Additionally, tumor cell colonization and extrahepatic metastasis can be induced through EV-Nidogen 1, which promotes premetastatic niche formation[62].
Microvesicles derived from hepatocytes or leuko-endothelial cells show higher plasma levels in patients with severe liver necroinflammatory activity, abundant liver fibrosis, and cirrhosis. For example, in a study of patients with Child-Pugh C without HCC, increased CD31+ and CD41- microvesicle levels were observed. Microvesicle levels over 65 U/L predict 6-month mortality. In addition, increased microvesicle levels were associated with cirrhosis severity[67].
Recent studies show that integrin alpha-V expressed on the LO surface can also interact with VAMP-associated protein A, which is then sorted into its surface. LOs enriched with VAMP-associated protein A facilitate bone-tropic metastasis of HCC by promoting osteoclastogenesis (Table 2)[41].
During hepatic disease, the effectiveness of phagocytic cells is overwhelmed by a substantial number of apoptotic hepatic cells. This results in an inadequate degradation of ApoBDs, which starts a process of autolysis, where the apoptotic cells release their proinflammatory content into the extracellular space[68]. However, there are other proinflammatory mechanisms that influence hepatic injury.
Hepatic tissue-specific macrophages are known as Kupffer cells (KC) and represent the main cells involved in ApoBDs phagocytosis. KC-mediated phagocytosis is related to increased death ligand concentrations, such as TNF-α, FasL, TGF-B, and TNF-related apoptosis-inducing ligand. These death ligands are profibrogenic and produce further hepatocyte apoptosis with ApoBD formation and subsequent KC-mediated phagocytosis, repeating the cycle over and over again[69].
It has been shown that hepatic stellate cells (HSC) can also clear ApoBDs, given that both KC and HSC express phosphatidyl serine receptors, which enables them to recognize apoptotic cells[69]. When KC-mediated phagocytosis is overwhelmed, HSC initiates ApoBDs phagocytosis, which causes HSC to transition from a quiescent to a proliferative, fibrogenic phenotype known as myofibroblasts. These myofibroblasts produce extracellular matrix and scar formation in the liver. NADPH oxidase NOX2 expressed on the HSC membrane has a significant role in this process as it induces reactive oxygen species-mediated collagen production[70,71]. These characteristics are summarized in Table 2[72-91].
Due to their features, EVs are attractive biomarkers, as they offer the specificity of liver biopsy samples and the non-invasiveness of peripheral blood samples[92]. Some reports support this idea. A study in two murine models of NAFLD/NASH found an increase in circulating EVs (microvesicles and exosomes). This increment was time-dependent and consistent with the progressive developmental stages of the disease. The authors also found a significant time-dependent increase in the levels of miR-122 and miR-192 (two miRNAs strongly associated with the liver and NAFLD) contained in these EVs[93]. These two miRNAs (as well as miR-19a and miR-19b, miR-125, and to a lesser extent miR-375) were upregulated in a serum argonaute2-free form in NASH patients in comparison with controls (and miR-122 was upregulated in patients with uncomplicated steatosis)[94]. Also, an association between the increase of miR-122, miR-192, and miR-375 with disease severity was observed, and miR-122 was slightly superior in predicting NASH and fibrosis than the classic markers alanine aminotransferase and aspartate aminotransferase[94].
Another study showed that overexpression of exosomal miR-500 from hepatic macrophages accelerates liver fibrosis by promoting HSC activation in vitro and in vivo[95]. This study also showed that CLD patients presented increasing levels of circulating exosomal miR-500 in accordance with disease stage; in light of this finding, the authors proposed this exosomal miRNA as a biomarker for the progression of liver fibrosis[95].
Patients with NASH (with cirrhosis and pre-cirrhosis) show an increase in circulating EVs compared to healthy individuals[96]. After a proteomic analysis, the authors of that finding proposed seven proteins upregulated in EVs from NASH patients as biomarkers: Von Willebrand factor, Wnt1-inducible signaling pathway protein-1, aminoacyl-tRNA synthetase interacting multifunctional protein 1, IL27RA, ICAM2, IL1β, serine/threonine protein kinase, and repulsive guidance molecule A precursor. Additionally, some of these proteins showed differences between cirrhotic and pre-cirrhotic NASH[96].
The percentage of hepatic exosomes (characterized by the presence of albumin) is increased in patients with NASH compared with patients with NAFLD and healthy subjects[97]. Also, the authors reported that glucose transporter 1 (GLUT1) was significantly higher in exosomes from patients with NASH compared to patients with NAFLD and with healthy subjects. In addition, exosomes and exosomal GLUT1 levels were higher in advanced stages of fibrosis (F2-4) than in early stages (F0-1). This suggests that the content of GLUT1 in exosomes may be an early marker of NAFLD and can be a prognosis tool for the severity of the disease[97].
Lipids contained in EVs have also been pointed out as potential biomarkers. Hepatocytes under lipotoxic stimuli release ceramide-rich EVs, particularly in sphingosine-1-phosphate (S1P), and can activate macrophage chemotaxis, showing that S1P in EVs can be used as a biomarker as well as a potential therapeutic target (e.g., interfering with the signaling axis from S1P in macrophages)[98]. A study in patients with NAFLD and NASH revealed that hepatocyte-derived plasma EV levels decreased significantly after weight loss surgery[99]. Furthermore, pre-surgery EVs were rich in lipids like sphingosine, sphinganine, S1P, and ceramide species, which was correlated with the development of steatosis and inflammation[99]. Altogether, these results suggest the potential of EVs and their cargo molecules as a good non-invasive diagnostic tool for disease progression.
Exosomes also have potential as therapeutic agents for certain liver diseases. Due to their characteristics and cargo, they can be used either as drug delivery tools or as therapy themselves[92].
Recent research has been shedding light on the potential of mesenchymal stem cell (MSC)-EVs to treat liver diseases since they exhibit anti-inflammatory, anti-fibrotic, and regenerative properties, making them effective in treating conditions like liver fibrosis and NAFLD[100].
The intravenous injection of EVs from human liver stem cells as a therapeutic agent in a murine NASH model was shown to recover the expression of several genes involved in fibrosis and inflammation (α-Sma, Col1α1, Tgf-β1; Tnf, IL-1β, Ifn-γ) in the liver of NASH mice. In this study, a reduction of inflammatory cells in the liver, upregulation of IL-10 expression, a significant reduction of alanine aminotransferase in plasma, and reduced fibrosis (but not steatosis) were also observed[101]. Consistent with this result, in a murine in vitro model, the treatment of HSCs with exosomes released by tonsil-derived MSCs induced a reduction in the levels of proteins involved in fibrosis development and promotion (TGF-β, α-SMA, COL1α1, vimentin, and CTGF). These authors concluded that this response was due to the action of the miR-486-5p contained in these EVs[102]. According to a pooled analysis led by Fang et al[103], these nanovesicles can significantly boost liver function and reduce inflammation, offering new hope for treatments. They work by delivering therapeutic molecules directly to liver cells, reducing fibrosis, and promoting healing[103].
Using exosomes derived from human adipose MSCs demonstrated that these vesicles can ameliorate liver fibrosis progression by diminishing the accumulation of lipids, improving the choline-phosphatidylcholine metabolism and attenuating HSC activation[104]. Also, Ganguin et al[105] showed that similar vesicles from LX-2 cells can reverse liver fibrosis effectively, depending on the amount. This promising approach suggests a less invasive alternative to current treatments, opening promising applications in regenerative medicine. As research progresses, the goal is to refine these vesicles for safe and effective treatment in liver conditions[105].
In a high-fat diet mouse model, administration of human umbilical cord MSC-derived exosomes prevented hepatic steatosis. Additionally, in L02 cells, related effects are due to calcium/calmodulin-dependent protein kinase 1 increase by MSC-derived exosomes, which triggered fatty acid β-oxidation elevation and fatty acid synthesis reduction via an enhanced expression of p-AMPK, PPARα, and CPT-1A and the inhibition of mature-SREBP-1C and FASn, respectively[100].
The relationship between EVs, particularly exosomes, and the pathogenesis, diagnosis, and therapy of HCC has been reviewed elsewhere. Briefly, several molecules contained in exosomes, particularly miRNAs, have been found to have potential as biomarkers for liver cancer or to participate in either suppression (e.g., miR-638, miR-326), development (e.g., miR-21, miR-10b, miR-23a/b), or as mechanisms for tumors and metastasis (e.g., miR-1237f, miR-378b), making them potential therapeutic targets for this disease[106-110].
One HCC-specific biomarker, GPC3, was found in exosomes, but not microvesicles, delivered from HCC cells. This proteoglycan, which plays a role in MVB biogenesis and release, accumulates due to the autophagy impairment present in this disease. Therefore, the detection of circulating exosomes enriched with this molecule can be used as a biomarker in patients with CLD before the development of HCC[61].
A recent study with in vitro and in vivo models pointed to exosomal formimidoyltransferase-cyclodeaminase as a potential biomarker for HCC. The high expression of this protein is associated with macrophage infiltration and polarization to the M1 type, suppressing HCC proliferation. This could lead to a better prognosis for HCC patients[111]. On the other hand, a different study showed that miR-200b-3p contained in HCC cell-derived exosomes can facilitate macrophage polarization to the M2 type, accelerating the proliferation and mediating HCC metastasis. Therefore, this exosomal miRNA can be associated with a bad prognosis[112].
Some studies underscored the use of circRNAs present in exosomes to treat HCC. For example, exosomal circ-0051443 was lower in HCC cell lines and patients with HCC compared to normal cells, and its administration to HCC cells had a suppressor effect in cell proliferation and even promoted a certain degree of apoptosis, with a corresponding reduction in tumor size in a murine model[113].
Exosomal circRNAs can also be involved in resistance to therapy, and their blockage leads to a better prognosis. An upregulation of circRNA-SORE (circRNA_104797), transported in exosomes, is critical for sorafenib resistance in certain HCC tumors, and its silencing improves the efficacy of the treatment in vivo[114]. Another study highlighted the importance of exosomal circUHRF1 (hsa_circ_0048677) upregulation in HCC tumors resistant to immunotherapy against PD-1, making these EVs a potential therapeutic target[115].
Apart from their participation in promoting drug resistance, EVs can be used as vessels to deliver molecules aimed for the contrary effect. The silencing of Grp78, a protein that promotes resistance to sorafenib, can be used to overcome this resistance in vitro. This was achieved by modifying bone marrow-derived MSCs to express the small interfering RNA siGRP78 and release it inside exosomes, which were then co-cultured with HepG2 sorafenib-resistant cells. The authors then observed a reduction in proliferation and invasion in the co-cultured cells when treated with the drug[116].
Due to limitations involved in patient staging according to liver disease degree and severity, the majority of the evidence discussed in this review has been obtained by in vitro and in vivo models. It is also not clear which specific cell types secrete distinct EV populations in patients. Thus, it is important to continue investigating and working to understand the role of EVs in the pathology and treatment of CLD.
EVs are key components for cellular communication, particularly for liver cells. This allows the expression of genes and the activation of signaling pathways in the liver microenvironment, which can ultimately impact fibrogenic and inflammatory processes.
Several studies have highlighted the role of EV-carried mediators in the development and progression of CLD as well as the role of specific proteins directly associated with CLD stages. Therefore, it is necessary to continue to investigate EV transported molecules, their presence depending on the CLD stage, and their association with disease features. As evidence continues to mount that EV expression patterns vary at differing stages of CLD, EVs hold great potential to be used as biomarkers, with the finality of improving clinical practices, including non-invasive personalized diagnosis and prognosis, and identifying potential therapeutic targets.
We appreciate the invaluable support of Rita I Gómez-Escamilla for her academic input during the review of the manuscript.
| 1. | Dorairaj V, Sulaiman SA, Abu N, Abdul Murad NA. Extracellular Vesicles in the Development of the Non-Alcoholic Fatty Liver Disease: An Update. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Blundell R, Azzopardi JI. Chronic liver diseases. In: Alavian SM, Nabavi SM, Nabavi SF, Silva AS, editors. Influence of Nutrients, Bioactive Compounds, and Plant Extracts in Liver Diseases. New York: Academic Press, 2021. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966-1986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1212] [Cited by in RCA: 1314] [Article Influence: 657.0] [Reference Citation Analysis (0)] |
| 4. | Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 2916] [Article Influence: 416.6] [Reference Citation Analysis (1)] |
| 5. | Couch Y, Buzàs EI, Di Vizio D, Gho YS, Harrison P, Hill AF, Lötvall J, Raposo G, Stahl PD, Théry C, Witwer KW, Carter DRF. A brief history of nearly EV-erything - The rise and rise of extracellular vesicles. J Extracell Vesicles. 2021;10:e12144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 299] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 6. | CHARGAFF E, WEST R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 417] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Crawford N. The presence of contractile proteins in platelet microparticles isolated from human and animal platelet-free plasma. Br J Haematol. 1971;21:53-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 117] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Aaronson S, Behrens U, Orner R, Haines TH. Ultrastructure of intracellular and extracellular vesicles, membranes, and myelin figures produced by Ochromonas danica. J Ultrastruct Res. 1971;35:418-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Bazzan E, Tinè M, Casara A, Biondini D, Semenzato U, Cocconcelli E, Balestro E, Damin M, Radu CM, Turato G, Baraldo S, Simioni P, Spagnolo P, Saetta M, Cosio MG. Critical Review of the Evolution of Extracellular Vesicles' Knowledge: From 1946 to Today. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 10. | Goldie BJ, Dun MD, Lin M, Smith ND, Verrills NM, Dayas CV, Cairns MJ. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014;42:9195-9208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 234] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 11. | Skotland T, Sandvig K, Llorente A. Lipids in exosomes: Current knowledge and the way forward. Prog Lipid Res. 2017;66:30-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 753] [Article Influence: 94.1] [Reference Citation Analysis (1)] |
| 12. | Yates AG, Pink RC, Erdbrügger U, Siljander PR, Dellar ER, Pantazi P, Akbar N, Cooke WR, Vatish M, Dias-Neto E, Anthony DC, Couch Y. In sickness and in health: The functional role of extracellular vesicles in physiology and pathology in vivo: Part I: Health and Normal Physiology: Part I: Health and Normal Physiology. J Extracell Vesicles. 2022;11:e12151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 13. | Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1394] [Cited by in RCA: 2136] [Article Influence: 356.0] [Reference Citation Analysis (35)] |
| 14. | de Abreu RC, Fernandes H, da Costa Martins PA, Sahoo S, Emanueli C, Ferreira L. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat Rev Cardiol. 2020;17:685-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 300] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 15. | Boilard E. Extracellular vesicles and their content in bioactive lipid mediators: more than a sack of microRNA. J Lipid Res. 2018;59:2037-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 16. | Donoso-Quezada J, Ayala-Mar S, González-Valdez J. The role of lipids in exosome biology and intercellular communication: Function, analytics and applications. Traffic. 2021;22:204-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 191] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 17. | Kim J, Lee SK, Jeong SY, Cho HJ, Park J, Kim TM, Kim S. Cargo proteins in extracellular vesicles: potential for novel therapeutics in non-alcoholic steatohepatitis. J Nanobiotechnology. 2021;19:372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Noren Hooten N, McFarland MH, Freeman DW, Mode NA, Ezike N, Zonderman AB, Evans MK. Association of Extracellular Vesicle Protein Cargo with Race and Clinical Markers of Mortality. Sci Rep. 2019;9:17582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Williams C, Palviainen M, Reichardt NC, Siljander PR, Falcón-Pérez JM. Metabolomics Applied to the Study of Extracellular Vesicles. Metabolites. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6920] [Cited by in RCA: 6558] [Article Influence: 1311.6] [Reference Citation Analysis (0)] |
| 21. | Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ. Reassessment of Exosome Composition. Cell. 2019;177:428-445.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1476] [Cited by in RCA: 2067] [Article Influence: 344.5] [Reference Citation Analysis (1)] |
| 22. | Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 1055] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 23. | Teng F, Fussenegger M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv Sci (Weinh). 2020;8:2003505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 246] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 24. | Ciardiello C, Migliorino R, Leone A, Budillon A. Large extracellular vesicles: Size matters in tumor progression. Cytokine Growth Factor Rev. 2020;51:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 25. | Battistelli M, Falcieri E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 288] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 26. | Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1505] [Cited by in RCA: 1409] [Article Influence: 234.8] [Reference Citation Analysis (0)] |
| 27. | Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 956] [Cited by in RCA: 1170] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 28. | Alonso Y Adell M, Migliano SM, Teis D. ESCRT-III and Vps4: a dynamic multipurpose tool for membrane budding and scission. FEBS J. 2016;283:3288-3302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Huebner AR, Cheng L, Somparn P, Knepper MA, Fenton RA, Pisitkun T. Deubiquitylation of Protein Cargo Is Not an Essential Step in Exosome Formation. Mol Cell Proteomics. 2016;15:1556-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Gurunathan S, Kang MH, Qasim M, Khan K, Kim JH. Biogenesis, Membrane Trafficking, Functions, and Next Generation Nanotherapeutics Medicine of Extracellular Vesicles. Int J Nanomedicine. 2021;16:3357-3383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 31. | Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1382] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 32. | Kim YS, Ahn JS, Kim S, Kim HJ, Kim SH, Kang JS. The potential theragnostic (diagnostic+therapeutic) application of exosomes in diverse biomedical fields. Korean J Physiol Pharmacol. 2018;22:113-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Verderio C, Gabrielli M, Giussani P. Role of sphingolipids in the biogenesis and biological activity of extracellular vesicles. J Lipid Res. 2018;59:1325-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 34. | Andreu Z, Yáñez-Mó M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 697] [Cited by in RCA: 1042] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 35. | Kenific CM, Zhang H, Lyden D. An exosome pathway without an ESCRT. Cell Res. 2021;31:105-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 36. | Xu M, Ji J, Jin D, Wu Y, Wu T, Lin R, Zhu S, Jiang F, Ji Y, Bao B, Li M, Xu W, Xiao M. The biogenesis and secretion of exosomes and multivesicular bodies (MVBs): Intercellular shuttles and implications in human diseases. Genes Dis. 2023;10:1894-1907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 37. | Edgar JR, Eden ER, Futter CE. Hrs- and CD63-dependent competing mechanisms make different sized endosomal intraluminal vesicles. Traffic. 2014;15:197-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 38. | van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 712] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 39. | Wei H, Chen Q, Lin L, Sha C, Li T, Liu Y, Yin X, Xu Y, Chen L, Gao W, Li Y, Zhu X. Regulation of exosome production and cargo sorting. Int J Biol Sci. 2021;17:163-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 251] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 40. | Beer KB, Wehman AM. Mechanisms and functions of extracellular vesicle release in vivo-What we can learn from flies and worms. Cell Adh Migr. 2017;11:135-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Zhang S, Liao X, Chen S, Qian W, Li M, Xu Y, Yang M, Li X, Mo S, Tang M, Wu X, Hu Y, Li Z, Yu R, Abudourousuli A, Song L, Li J. Large Oncosome-Loaded VAPA Promotes Bone-Tropic Metastasis of Hepatocellular Carcinoma Via Formation of Osteoclastic Pre-Metastatic Niche. Adv Sci (Weinh). 2022;9:e2201974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 42. | Minciacchi VR, You S, Spinelli C, Morley S, Zandian M, Aspuria PJ, Cavallini L, Ciardiello C, Reis Sobreiro M, Morello M, Kharmate G, Jang SC, Kim DK, Hosseini-Beheshti E, Tomlinson Guns E, Gleave M, Gho YS, Mathivanan S, Yang W, Freeman MR, Di Vizio D. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget. 2015;6:11327-11341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 303] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 43. | Ciardiello C, Leone A, Lanuti P, Roca MS, Moccia T, Minciacchi VR, Minopoli M, Gigantino V, De Cecio R, Rippa M, Petti L, Capone F, Vitagliano C, Milone MR, Pucci B, Lombardi R, Iannelli F, Di Gennaro E, Bruzzese F, Marchisio M, Carriero MV, Di Vizio D, Budillon A. Large oncosomes overexpressing integrin alpha-V promote prostate cancer adhesion and invasion via AKT activation. J Exp Clin Cancer Res. 2019;38:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 44. | Vento S, Cainelli F. Chronic liver diseases must be reduced worldwide: it is time to act. Lancet Glob Health. 2022;10:e471-e472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 45. | GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789-1858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9354] [Cited by in RCA: 8390] [Article Influence: 1198.6] [Reference Citation Analysis (4)] |
| 46. | Aguilera-Méndez A. [Nonalcoholic hepatic steatosis: a silent disease]. Rev Med Inst Mex Seguro Soc. 2019;56:544-549. [PubMed] |
| 47. | Li B, Zhang C, Zhan YT. Nonalcoholic Fatty Liver Disease Cirrhosis: A Review of Its Epidemiology, Risk Factors, Clinical Presentation, Diagnosis, Management, and Prognosis. Can J Gastroenterol Hepatol. 2018;2018:2784537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 48. | Schuppan D, Schattenberg JM. Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. J Gastroenterol Hepatol. 2013;28 Suppl 1:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 49. | Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1210] [Article Influence: 302.5] [Reference Citation Analysis (0)] |
| 50. | Kořínková L, Pražienková V, Černá L, Karnošová A, Železná B, Kuneš J, Maletínská L. Pathophysiology of NAFLD and NASH in Experimental Models: The Role of Food Intake Regulating Peptides. Front Endocrinol (Lausanne). 2020;11:597583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 51. | Fernando DH, Forbes JM, Angus PW, Herath CB. Development and Progression of Non-Alcoholic Fatty Liver Disease: The Role of Advanced Glycation End Products. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 52. | Negro F. Natural history of NASH and HCC. Liver Int. 2020;40 Suppl 1:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 53. | Nguyen HQ, Lee D, Kim Y, Bang G, Cho K, Lee YS, Yeon JE, Lubman DM, Kim J. Label-free quantitative proteomic analysis of serum extracellular vesicles differentiating patients of alcoholic and nonalcoholic fatty liver diseases. J Proteomics. 2021;245:104278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Sakane S, Hikita H, Shirai K, Sakamoto T, Narumi R, Adachi J, Kakita N, Yamada Y, Toyoda H, Takahashi H, Suda G, Kai M, Tahata Y, Sakamori R, Kumazaki S, Fukumoto K, Myojin Y, Murai K, Kodama T, Tatsumi T, Tomonaga T, Sakamoto N, Morii E, Takehara T. Proteomic analysis of serum extracellular vesicles reveals Fibulin-3 as a new marker predicting liver-related events in MASLD. Hepatol Commun. 2024;8. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 55. | Brenachot X, Ramadori G, Ioris RM, Veyrat-Durebex C, Altirriba J, Aras E, Ljubicic S, Kohno D, Fabbiano S, Clement S, Goossens N, Trajkovski M, Harroch S, Negro F, Coppari R. Hepatic protein tyrosine phosphatase receptor gamma links obesity-induced inflammation to insulin resistance. Nat Commun. 2017;8:1820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 56. | Kumagai M, Tsuchiya A, Yang Y, Takeda N, Natsui K, Natusi Y, Tomiyoshi K, Yamazaki F, Koseki Y, Shinchi H, Imawaka N, Ukekawa R, Nishibu T, Abe H, Sasaki T, Ueda K, Terai S. Fibulin-4 as a potential extracellular vesicle marker of fibrosis in patients with cirrhosis. FEBS Open Bio. 2024;14:1264-1276. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 57. | Zhao L, Shi J, Chang L, Wang Y, Liu S, Li Y, Zhang T, Zuo T, Fu B, Wang G, Ruan Y, Zhang Y, Xu P. Serum-Derived Exosomal Proteins as Potential Candidate Biomarkers for Hepatocellular Carcinoma. ACS Omega. 2021;6:827-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 58. | Hareendran S, Albraidy B, Yang X, Liu A, Breggia A, Chen CC, Loh YP. Exosomal Carboxypeptidase E (CPE) and CPE-shRNA-Loaded Exosomes Regulate Metastatic Phenotype of Tumor Cells. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 59. | Jia Q, Bu Y, Wang Z, Chen B, Zhang Q, Yu S, Liu Q. Maintenance of stemness is associated with the interation of LRP6 and heparin-binding protein CCN2 autocrined by hepatocellular carcinoma. J Exp Clin Cancer Res. 2017;36:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Guo M, Zhang H, Zheng J, Liu Y. Glypican-3: A New Target for Diagnosis and Treatment of Hepatocellular Carcinoma. J Cancer. 2020;11:2008-2021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 61. | Koksal AR, Thevenot P, Aydin Y, Nunez K, Sandow T, Widmer K, Nayak L, Scott J, Delk M, Moehlen MW, Cohen AJ, Dash S. Impaired Autophagy Response in Hepatocellular Carcinomas Enriches Glypican-3 in Exosomes, Not in the Microvesicles. J Hepatocell Carcinoma. 2022;9:959-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Xue T, Yam JWP. Role of Small Extracellular Vesicles in Liver Diseases: Pathogenesis, Diagnosis, and Treatment. J Clin Transl Hepatol. 2022;10:1176-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 63. | Tian XP, Wang CY, Jin XH, Li M, Wang FW, Huang WJ, Yun JP, Xu RH, Cai QQ, Xie D. Acidic Microenvironment Up-Regulates Exosomal miR-21 and miR-10b in Early-Stage Hepatocellular Carcinoma to Promote Cancer Cell Proliferation and Metastasis. Theranostics. 2019;9:1965-1979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 64. | Peng X, Li X, Yang S, Huang M, Wei S, Ma Y, Li Y, Wu B, Jin H, Li B, Tang S, Fan Q, Liu J, Yang L, Li H. LINC00511 drives invasive behavior in hepatocellular carcinoma by regulating exosome secretion and invadopodia formation. J Exp Clin Cancer Res. 2021;40:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 65. | Liu G, Ouyang X, Sun Y, Xiao Y, You B, Gao Y, Yeh S, Li Y, Chang C. The miR-92a-2-5p in exosomes from macrophages increases liver cancer cells invasion via altering the AR/PHLPP/p-AKT/β-catenin signaling. Cell Death Differ. 2020;27:3258-3272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 66. | Huang XY, Huang ZL, Huang J, Xu B, Huang XY, Xu YH, Zhou J, Tang ZY. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J Exp Clin Cancer Res. 2020;39:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 299] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 67. | Payancé A, Silva-Junior G, Bissonnette J, Tanguy M, Pasquet B, Levi C, Roux O, Nekachtali O, Baiges A, Hernández-Gea V, Laouénan C, Lebrec D, Albuquerque M, Paradis V, Moreau R, Valla D, Durand F, Boulanger CM, Garcia-Pagan JC, Rautou PE. Hepatocyte microvesicle levels improve prediction of mortality in patients with cirrhosis. Hepatology. 2018;68:1508-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 68. | Alkhouri N, Carter-Kent C, Feldstein AE. Apoptosis in nonalcoholic fatty liver disease: diagnostic and therapeutic implications. Expert Rev Gastroenterol Hepatol. 2011;5:201-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 69. | Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 352] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 70. | Jiang JX, Venugopal S, Serizawa N, Chen X, Scott F, Li Y, Adamson R, Devaraj S, Shah V, Gershwin ME, Friedman SL, Török NJ. Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo. Gastroenterology. 2010;139:1375-1384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 71. | Takehara T, Tatsumi T, Suzuki T, Rucker EB 3rd, Hennighausen L, Jinushi M, Miyagi T, Kanazawa Y, Hayashi N. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 72. | Yao JM, Ying HZ, Zhang HH, Qiu FS, Wu JQ, Yu CH. Exosomal RBP4 potentiated hepatic lipid accumulation and inflammation in high-fat-diet-fed mice by promoting M1 polarization of Kupffer cells. Free Radic Biol Med. 2023;195:58-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Moratti E, Vezzalini M, Tomasello L, Giavarina D, Sorio C. Identification of protein tyrosine phosphatase receptor gamma extracellular domain (sPTPRG) as a natural soluble protein in plasma. PLoS One. 2015;10:e0119110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 74. | Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, Charlton MR, Shah VH, Malhi H, Gores GJ. Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles From Hepatocytes. Gastroenterology. 2016;150:956-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 392] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 75. | Ibrahim SH, Hirsova P, Tomita K, Bronk SF, Werneburg NW, Harrison SA, Goodfellow VS, Malhi H, Gores GJ. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 2016;63:731-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 76. | Takaya H, Kawaratani H, Tsuji Y, Nakanishi K, Saikawa S, Sato S, Sawada Y, Kaji K, Okura Y, Shimozato N, Kitade M, Akahane T, Moriya K, Namisaki T, Mitoro A, Matsumoto M, Fukui H, Yoshiji H. von Willebrand factor is a useful biomarker for liver fibrosis and prediction of hepatocellular carcinoma development in patients with hepatitis B and C. United European Gastroenterol J. 2018;6:1401-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 77. | Vinchi F, Gastaldi S, Silengo L, Altruda F, Tolosano E. Hemopexin prevents endothelial damage and liver congestion in a mouse model of heme overload. Am J Pathol. 2008;173:289-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 78. | Yang B, Zhang J, Sun L, Huang T, Kong Y, Li L, Sun Z, Yin M, Li X. Mapping Novel Biomarkers of Liver Injury by Tissue Proteomic Analysis. ACS Omega. 2021;6:7127-7138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 79. | Nair B, Nath LR. Inevitable role of TGF-β1 in progression of nonalcoholic fatty liver disease. J Recept Signal Transduct Res. 2020;40:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 80. | Dewidar B, Meyer C, Dooley S, Meindl-Beinker AN. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis-Updated 2019. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 542] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 81. | Qu Z, Feng J, Pan H, Jiang Y, Duan Y, Fa Z. Exosomes derived from HCC cells with different invasion characteristics mediated EMT through TGF-β/Smad signaling pathway. Onco Targets Ther. 2019;12:6897-6905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 82. | Wei M, Yang T, Chen X, Wu Y, Deng X, He W, Yang J, Wang Z. Malignant ascites-derived exosomes promote proliferation and induce carcinoma-associated fibroblasts transition in peritoneal mesothelial cells. Oncotarget. 2017;8:42262-42271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 83. | Zhou J, Liu J, Sheng H, You N, Chen J, Mi X, Yang W, Zang S, Shi J; Chinese NAFLD Clinical Research Network (CNAFLD CRN). Haptoglobin 2-2 Genotype is Associated with More Advanced Disease in Subjects with Non-Alcoholic Steatohepatitis: A Retrospective Study. Adv Ther. 2019;36:880-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Liu W, Baker SS, Baker RD, Nowak NJ, Zhu L. Upregulation of hemoglobin expression by oxidative stress in hepatocytes and its implication in nonalcoholic steatohepatitis. PLoS One. 2011;6:e24363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 85. | Marfà S, Crespo G, Reichenbach V, Forns X, Casals G, Morales-Ruiz M, Navasa M, Jiménez W. Lack of a 5.9 kDa peptide C-terminal fragment of fibrinogen α chain precedes fibrosis progression in patients with liver disease. PLoS One. 2014;9:e109254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 86. | Marfà S, Jimenez W. Fibrinogen α-Chain as a Serum Marker of Liver Disease. In: Patel V, Preedy V, editors. Biomarkers in Disease: Methods, Discoveries and Applications. Dordrecht: Springer, 2017. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 87. | Asselta R, Paraboschi EM, Duga S. Hereditary Hypofibrinogenemia with Hepatic Storage. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 88. | Gu L, Wang B, Liu L, Gan Q, Liu X, Chen L, Chen L. Hepatic fibrinogen storage disease and hypofibrinogenemia caused by fibrinogen Aguadilla mutation: a case report. J Int Med Res. 2020;48:300060519898033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 89. | Zhang W, Zhao P, Xu XL, Cai L, Song ZS, Cao DY, Tao KS, Zhou WP, Chen ZN, Dou KF. Annexin A2 promotes the migration and invasion of human hepatocellular carcinoma cells in vitro by regulating the shedding of CD147-harboring microvesicles from tumor cells. PLoS One. 2013;8:e67268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 90. | UniProt. UniProt consortium. 2024. [cited 3 August 2024]. Available from: https://www.uniprot.org/. |
| 91. | HGNC. HUGO Gene Nomenclature Committee. 2024. [cited 3 August 2024]. Available from: https://www.genenames.org/. |
| 92. | Jiao Y, Xu P, Shi H, Chen D, Shi H. Advances on liver cell-derived exosomes in liver diseases. J Cell Mol Med. 2021;25:15-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 93. | Povero D, Eguchi A, Li H, Johnson CD, Papouchado BG, Wree A, Messer K, Feldstein AE. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS One. 2014;9:e113651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 218] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 94. | Pirola CJ, Fernández Gianotti T, Castaño GO, Mallardi P, San Martino J, Mora Gonzalez Lopez Ledesma M, Flichman D, Mirshahi F, Sanyal AJ, Sookoian S. Circulating microRNA signature in non-alcoholic fatty liver disease: from serum non-coding RNAs to liver histology and disease pathogenesis. Gut. 2015;64:800-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 445] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 95. | Chen L, Huang Y, Duan Z, Huang P, Yao H, Zhou Y, Ji Q, Liu X. Exosomal miR-500 Derived From Lipopolysaccharide-Treated Macrophage Accelerates Liver Fibrosis by Suppressing MFN2. Front Cell Dev Biol. 2021;9:716209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 96. | Povero D, Yamashita H, Ren W, Subramanian MG, Myers RP, Eguchi A, Simonetto DA, Goodman ZD, Harrison SA, Sanyal AJ, Bosch J, Feldstein AE. Characterization and Proteome of Circulating Extracellular Vesicles as Potential Biomarkers for NASH. Hepatol Commun. 2020;4:1263-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 97. | Zhang W, Zhang J, Shi H, Liu F, Yu H, Shi H. Exosome GLUT1 derived from hepatocyte identifies the risk of non-alcoholic steatohepatitis and fibrosis. Hepatol Int. 2023;17:1170-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 98. | Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1α-dependent manner. J Lipid Res. 2016;57:233-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 233] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 99. | Nakao Y, Amrollahi P, Parthasarathy G, Mauer AS, Sehrawat TS, Vanderboom P, Nair KS, Nakao K, Allen AM, Hu TY, Malhi H. Circulating extracellular vesicles are a biomarker for NAFLD resolution and response to weight loss surgery. Nanomedicine. 2021;36:102430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 100. | Yang F, Wu Y, Chen Y, Xi J, Chu Y, Jin J, Yan Y. Human umbilical cord mesenchymal stem cell-derived exosomes ameliorate liver steatosis by promoting fatty acid oxidation and reducing fatty acid synthesis. JHEP Rep. 2023;5:100746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 101. | Bruno S, Pasquino C, Herrera Sanchez MB, Tapparo M, Figliolini F, Grange C, Chiabotto G, Cedrino M, Deregibus MC, Tetta C, Camussi G. HLSC-Derived Extracellular Vesicles Attenuate Liver Fibrosis and Inflammation in a Murine Model of Non-alcoholic Steatohepatitis. Mol Ther. 2020;28:479-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 102. | Kim J, Lee C, Shin Y, Wang S, Han J, Kim M, Kim JM, Shin SC, Lee BJ, Kim TJ, Jung Y. sEVs from tonsil-derived mesenchymal stromal cells alleviate activation of hepatic stellate cells and liver fibrosis through miR-486-5p. Mol Ther. 2021;29:1471-1486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 103. | Fang X, Gao F, Yao Q, Xu H, Yu J, Cao H, Li S. Pooled Analysis of Mesenchymal Stromal Cell-Derived Extracellular Vesicle Therapy for Liver Disease in Preclinical Models. J Pers Med. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 104. | Zhang Z, Shang J, Yang Q, Dai Z, Liang Y, Lai C, Feng T, Zhong D, Zou H, Sun L, Su Y, Yan S, Chen J, Yao Y, Shi Y, Huang X. Exosomes derived from human adipose mesenchymal stem cells ameliorate hepatic fibrosis by inhibiting PI3K/Akt/mTOR pathway and remodeling choline metabolism. J Nanobiotechnology. 2023;21:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 105. | Ganguin AA, Skorup I, Streb S, Othman A, Luciani P. Formation and Investigation of Cell-Derived Nanovesicles as Potential Therapeutics against Chronic Liver Disease. Adv Healthc Mater. 2023;12:e2300811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 106. | Wang H, Lu Z, Zhao X. Tumorigenesis, diagnosis, and therapeutic potential of exosomes in liver cancer. J Hematol Oncol. 2019;12:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 107. | Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2017;14:455-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 219] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 108. | Li S, Chen L. Exosomes in Pathogenesis, Diagnosis, and Treatment of Hepatocellular Carcinoma. Front Oncol. 2022;12:793432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 109. | Wei XC, Liu LJ, Zhu F. Exosomes as potential diagnosis and treatment for liver cancer. World J Gastrointest Oncol. 2022;14:334-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (2)] |
| 110. | Zelli V, Compagnoni C, Capelli R, Corrente A, Di Vito Nolfi M, Zazzeroni F, Alesse E, Tessitore A. Role of exosomal microRNAs in cancer therapy and drug resistance mechanisms: focus on hepatocellular carcinoma. Front Oncol. 2022;12:940056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 111. | Liu Y, Tang Y, Jiang H, Zhang X, Chen X, Guo J, Jin C, Wu M. Exosome-Related FTCD Facilitates M1 Macrophage Polarization and Impacts the Prognosis of Hepatocellular Carcinoma. Biomolecules. 2023;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 112. | Xu Y, Luan G, Liu F, Zhang Y, Li Z, Liu Z, Yang T. Exosomal miR-200b-3p induce macrophage polarization by regulating transcriptional repressor ZEB1 in hepatocellular carcinoma. Hepatol Int. 2023;17:889-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 113. | Chen W, Quan Y, Fan S, Wang H, Liang J, Huang L, Chen L, Liu Q, He P, Ye Y. Exosome-transmitted circular RNA hsa_circ_0051443 suppresses hepatocellular carcinoma progression. Cancer Lett. 2020;475:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 114. | Xu J, Ji L, Liang Y, Wan Z, Zheng W, Song X, Gorshkov K, Sun Q, Lin H, Zheng X, Chen J, Jin RA, Liang X, Cai X. CircRNA-SORE mediates sorafenib resistance in hepatocellular carcinoma by stabilizing YBX1. Signal Transduct Target Ther. 2020;5:298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 308] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 115. | Zhang PF, Gao C, Huang XY, Lu JC, Guo XJ, Shi GM, Cai JB, Ke AW. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol Cancer. 2020;19:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 402] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 116. | Li H, Yang C, Shi Y, Zhao L. Exosomes derived from siRNA against GRP78 modified bone-marrow-derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma. J Nanobiotechnology. 2018;16:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |