INTRODUCTION
The health burden of hepatitis B
As per the estimates of the Polaris Observatory Collaborators, chronic hepatitis B afflicts 292 million people (3.9% of the world's population) with an additional 58 million having occult infection in 2018[1], which reduced to 257.5 million in 2022[2]. As per the 2022 World Health Organization (WHO) data, 254 million people were living with chronic hepatitis B virus (HBV) infection with an annual 1.2 million new infections and 1.1 million deaths, mostly from cirrhosis and primary hepatocellular carcinoma (HCC)[3]. The epidemiology is variable with regions of high (Western Pacific, South East Asia and Africa), intermediate (Eastern Europe and the Mediterranean Basin) and low (Western Europe and North America) endemicity. There is an increased mobility of the world population from areas of high endemicity to those of low or intermediate endemicity which affects the epidemiology e.g. in the United States, 59% of all HBV patients were of Asian origin and 15% of African origin in 2018[4]. HBV disproportionately affects economically disadvantaged countries, mostly in Africa, the Western Pacific, Latin America and south-east Asia, where approximately 96% of the global burden reside[5,6] and bear the greatest HBV-related mortality burden. In these areas, 50% of the pool is contributed by mother-to-child transmission (MTCT) as the prevalence in pregnant women parallels that in the general population[7]. A recent meta-analysis showed that the global pooled prevalence of hepatitis B surface antigen (HBsAg) in pregnant women was 4.8% (3.8%-5.8%), being higher in countries in the low [6.6% (5.4%-7.9%)] and lower-middle [4.9% (3.8%-6.1%)] income group[8]. Here the tribal/indigenous populations are forced to live closely in poor hygienic conditions which maintain the pool through MTCT, horizontal transmission in toddlers, tribal practices, and are also the ones least likely to be linked to care. The disease is also transmitted by blood/body fluids and reuse of contaminated sharp instruments; hence, it is prevalent among those involved in unsafe sexual practices (those with multiple sex partners, sexually transmitted diseases and men having sex with men), who receive recurrent blood transfusions (hematological disorders, hemodialysis), intravenous drug abusers, unsafe injections in healthcare settings, and tattooing or sharing razors in salons. Other high risk groups are prison or institutional inmates and health care workers. Those exposed to infection or quiescent carriers may have HBV reactivation when they undergo immunosuppression (organ transplants, rheumatological disorders, cancer chemotherapy, immunotherapy) and those who are immunosuppressed (chronic kidney disease, diabetes mellitus, human immunodeficiency virus infection, cancers) have a higher chance of acquiring the infection. As the longevity of the world population increases, such new cases will also add to the pool and mandate treatment. The disease trajectory runs non-linearly through different phases with uncertain time and sequence of inter-category shift depending on multiple host and viral factors. Those acquiring the disease in early childhood are e antigen positive with high HBV DNA load and are at highest risk of progressing to cirrhosis and HCC. Some spontaneously seroconvert to e antigen negative in early adulthood and they can become chronic carriers or develop e negative hepatitis depending on HBV DNA levels. They also carry a risk of disease progression although less than e positive individuals. Without treatment, the cumulative incidence of cirrhosis over 5 years is around 10%-20% of whom 2%-5% develop liver cancer each year[9]. Hence the disease burden is substantial.
WHY THE NEED FOR NEW DRUGS?
There are presently no curative therapies for HBV to tackle this high burden. Functional cure is defined as undetectable HBsAg and HBV DNA below the lower limit of detection in peripheral blood which is sustained after a finite period of therapy with or without development of the surface antibody (anti-HBs). The currently approved therapy of HBV entails only two drug groups, the interferons and their pegylated variety (PEGIFN) and nucleos(t)ide analogues (NA) which are ineffective in permanent, large scale viral eradication as per the above criteria. PEGIFN therapy, though finite, is costly and has multiple serious adverse effects and contraindications with a sustained HBV DNA negativity/e antigen seroconversion rate of about 25% each, but negligible HBsAg loss. NA therapy is at best viral suppressive even on long-term and does not eliminate the virus completely. Both show high relapse rates on withdrawal and their combination as sequential/add on therapy does not provide appreciable additive benefit due to variable response depending on HBV genotype, the phase of infection and immune characteristics of the patient. Furthermore, the rate of HBsAg loss after withdrawal of long-term NA therapy is much lower in Asians compared to Caucasians on follow-up[10], which limits their global applicability as a cure strategy.
However, the second-generation NAs, entecavir and tenofovir, can effectively suppress the virus on long-term therapy, which significantly halts viral transmission and prolongs life by reducing the rate of liver disease progression and the risk of HCC. These are available as affordable generics in most countries, need once daily oral administration, have a good safety profile and the risk of drug resistance is < 1% even after prolonged (≥ 10 years) continuous therapy. Thus, they are presently regarded as the standard of care (SOC) for HBV. However in the longrun, there still remains the risk of drug non-compliance (especially where out-of-pocket spending on drugs is needed) and the adverse effects on kidney and bone with tenofovir.
The available vaccine against HBV is highly efficacious in disease prevention, is inexpensive and has been around since 1986 (with quality improvements in subsequent years). It has been introduced for infants by 190 WHO Member States in their national immunization programmes with 113 introducing the birth dose (within the first 24 hours of life) by the end of 2022[11]. In 2016, the WHO set an ambitious target for global eradication of HBV by 2030 by reducing new transmission by 90% (90% diagnosis, 80% treatment and 65% reduction in consequences) through wider vaccination coverage[12]. It was estimated that after introduction of universal infant immunization, it would take 20-40 years to impact the rate of liver cirrhosis and cancer[13]. In the last 8 years there has been significant improvement with global coverage of 3 doses of HBV vaccine for infants reaching 85%, but that of the birth dose remains at 46%[14], being as high as 80% in the WHO Western Pacific Region, but as low as 18% in the WHO African Region[11]. Vaccine hesitancy and low availability are the causes. Screening of all pregnant women for HBV can substantially reduce transmission (by tenofovir prophylaxis in pregnant women with high viral loads), but the rate is dismally low especially in endemic areas where it is required the most[15]. Screening of donated blood for HBV has improved to 97% but remains below the 100% target set for 2030 and unsafe injections still account for 5% of those administered in healthcare settings[14].
Treatment of the final complications of chronic hepatitis B (liver cirrhosis and HCC) also entails significant health care costs in addition to the treatment of the disease itself. It is estimated that 55% of mortality due to HCC and 45% of deaths from liver dysfunction are attributable to HBV infection[16]. The HBV-related liver disease and HCC occurrence are likely to be accelerated by the rising global crisis of the metabolic syndrome and hazardous alcohol consumption.
Those infected chronically with HBV also suffer negative impacts on their quality of life, employment and personal finances and in 2016, the estimated age-adjusted disability-adjusted life years (DALY) lost globally due to HBV was 5160000[17]. In 2019, it resulted in 18.2 million (95% UI 15.9-20.6) global DALYs (0.7% of total global DALYs) with HBV-related cirrhosis, and liver cancer contributing 59.2% (53.8-64.2), and 31.9% (27.8-36.4) to DALYs, respectively[18]. The broader impact of HBV on society and the economy is often overlooked in budget allocation by governments and other private funders in lieu of the long (apparently healthy) disease duration that precedes end-stage complications[19]. A cost benefit analysis of reaching the WHO target by 2030, 2040, or 2050 using a dynamic mathematical model of HBV transmission, disease progression, and mortality in the six WHO regions, revealed that without increased intervention coverage, HBV-related mortality would cost US$784.35 (731.63-798.33) billion globally in lost productivity over 2022-50. Achieving targets by 2030 would avert 25.64 (17.39-34.55) million infections and 8.63 (7.12-9.74) million hepatitis B-attributable deaths over 2022-50. This achievement would incur an incremental cost of $2934.55 (2778.55-3173.52) per DALY averted by 2050 under a health systems perspective, but would be cost-saving with a net economic benefit of $99.03 (78.66-108.96) billion by 2050 from a societal perspective. These findings highlight HBV as an underappreciated cause of economic burden and investment toward elimination will probably yield substantial returns[20].
Thus, considering all the above factors, it is clear that the health care burden of HBV is significant in the long-term and vaccination will not lead to complete elimination of the pool in the foreseeable future. This along with the target set by the WHO has led to recent renewed interest in novel therapies with an enhanced aim of functional cure in the path to the final target of sterilizing cure i.e. eradicating the covalently closed circular (ccc) DNA from the liver.
HBV LIFE CYCLE AND TARGET OF NEWER DRUGS
Establishment of chronic HBV infection in humans involves a dynamic interplay of viral and host factors of which the most important is a very high replication rate of the virus (trillion copies/day), resulting in high viral load as well as subviral particles (HBsAg) in blood which overwhelms and exhausts the T cells (prime eliminator of HBV) resulting in T cell suppression by various mechanisms such as induction of immune tolerance, expression of immune checkpoint inhibitors, and increased apoptosis. The high blood HBsAg level is maintained by the cccDNA (which is in constant supply from intracellular recycling of nucleocapsids as well as incoming virions) and integrated viral genome within hepatocytes. New drug therapies or their combinations therefore aim to: (1) Clear HBsAg in blood [equivalent to undetectable HBV DNA (complete virus) or surface antigen (HBsAg viral proteins) in blood] with or without development of anti-HBs; and (2) Boost HBV directed T cell responses by direct stimulation and removing inhibition.
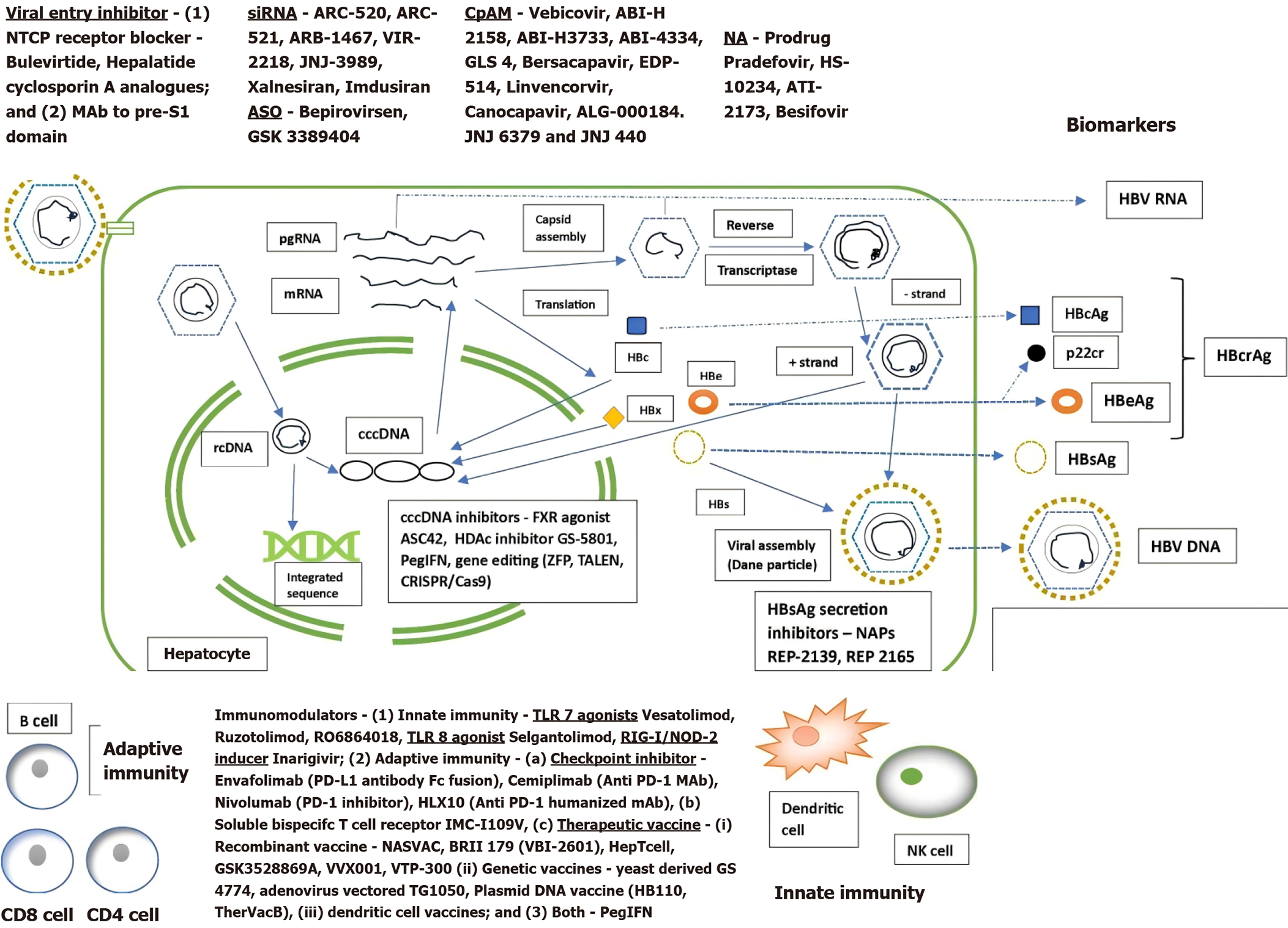
The HBV life cycle and the targets of newer drug action are shown in Figure 1. The only two currently approved groups of drugs for treatment of HBV are PEGIFN and NA. The mechanism of action of PEGIFN involves both the above mechanisms. It induces specific genes via the JAK STAT pathway that interfere with multiple steps of the HBV life cycle (entry, uncoating, transcription, translation and assembly of the nucleocapsid). It also augments cell-mediated immunity and NK cells (response depends on viral genotype). Its effect on transcription is partly mediated by epigenetic modification of cccDNA and it can also degrade cccDNA via APOBEC3s (viral editing enzyme) and accelerate decay of the viral nucleocapsid. This added effect on cccDNA (the ultimate target for sterilizing cure) gives it a distinct edge over all the other drugs presently available (or under trial) for the treatment of HBV. Many ongoing trials use a combination of PEGIFN with other drugs. Some improvement has been made with regard to existing therapy optimizations, including a better understanding of sequential or combination use of PEGIFN, NA and other molecules. Its use with NA as add on/switch after NA withdrawal has resulted in a HBsAg loss of 8%-13%[9] in selected groups of patients.
Figure 1 Hepatitis B virus life cycle and novel drug targets.
NTCP: Sodium taurocholate co-transporting polypeptide; rcDNA: Relaxed circular DNA; cccDNA: Covalently closed circular DNA; CRISPR: Clustered regularly interspaced short palindromic repeats; Cas9: CRISPR-associated protein 9; mRNA: Messenger RNA; pgRNA: Pregenomic RNA; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B envelope antigen; HBcAg: Hepatitis B core antigen; HBcrAg: Hepatitis B core related antigen; TALEN: Transcription Activator-like Effector Nucleases; ZFP: Zinc-Finger Proteins; CpAM: Capsid assembly modulators; siRNA: Short interfering RNA; ASO: Antisense oligonucleotides; NAP: Nucleic acid polymer; HBx: Hepatitis B X protein; HBs: Hepatitis B S protein; HDAc: Histone deacetylase; FXR: Farnesoid X receptor; PegIFN: Pegylated interferon; NA: Nucleotide analogue.
Although NAs are unable to eradicate cccDNA and hence effect sterilizing cure of HBV, they are highly effective in complete viral suppression on long-term therapy which significantly decreases viral transmission and reduces liver-related complications. Thus, they set a high bar for other newer drugs to emulate their inclusion in the treatment armamentarium of HBV.
Newer therapies under trial are summarized in Figure 1 according to their site of action in the HBV life cycle and have been extensively reviewed elsewhere[16,21-25]. The small interfering RNA (siRNA), nucleic acid polymers (NAP), antisense oligonucleotides (ASO) and immunomodulators are subcutaneous or intravenous preparations, while capsid assembly modulators (CpAM) are oral drugs. Most are in phase I/II trials and have shown only modest, unsustained reductions in HBsAg level when used singly or in combination with major concerns like potential toxicity of the delivery platform and the risk of off-target toxicity. A few have performed better when used in combination with PEGIFN (or other immunomodulators) and NAs as dual or triple therapy (concurrent or sequential), but a high proportion of patients experienced viral rebound off-therapy. All such trials enrolled small patient numbers and responses were mainly observed in those with low baseline HBsAg levels (immune control phase).
The best results in preliminary trials have been observed with the following agents: A triple combination of PEGIFN + NA + REP 2139/2165 (NAP) resulted in the highest rate of HBsAg loss reported to date with finite therapy (60% loss, 35% persisted after 48 weeks of follow-up)[26]. Another study of PEGIFN + VIR-2218 (siRNA) combination resulted in a decline in HBsAg levels to < 10 IU/mL after 24 weeks in 54.5% of patients compared to siRNA alone (13.3%)[27]. A phase III study of PEGIFN + Bulevirtide (entry inhibitor) in patients with HBV + HDV coinfection showed a 46.7% decline in HBsAg level while clearing HDV infection[28]. Bulevirtide has been approved for therapy of HDV but its effect on only HBV awaits further confirmation. Another triple combination of PEGIFN + xalnesiran (siRNA) + NA resulted in HBsAg loss of 23% compared to ruzotolimod (TLR7 agonist) + xalnesiran and NA (12% HBsAg loss) and only xalniseran + NA (3%-7% HBsAg loss), after 24 weeks of post-treatment follow-up[29]. A sequential combination in HBeAg-positive patients involving JNJ-3989 (siRNA) and NA with/without JNJ-6379 (CpAM) followed by PEGIFN-α resulted in HBsAg loss of 16% at 24 weeks follow-up[30]. Similarly sequential NA + bepirovirsen (ASO) followed by PEGIFN-α resulted in a HBsAg loss rate of 53%, 65%, and 24% at the end of PEGIFN-α therapy, bepirovirsen therapy, and at 24 weeks follow-up, respectively, in the 24-week bepirovirsen group, and 47%, 59%, and 41%, respectively, in the 12-week bepirovirsen group, but PEGIFN-α therapy resulted in only minimal incremental HBsAg loss and inability to prevent HBsAg relapse[31]. Another study involving bepirovirsen + NA resulted in HBsAg loss of 25% at 24 weeks but only 9%-10% after 24 weeks follow-up[32]. A 24-week trial of therapeutic vaccine BRII-179 + PEGIFN resulted in 25% HBsAg loss after 12 weeks post-treatment compared to 14% in those who received placebo/+PEGIFN[33].
The disadvantage of PEGIFN regimens is the protracted 24-48 weeks course which raises concern about the side effects and shorter duration of therapy needs to be investigated (such as targeting HBsAg level of < 1000 IU at 3 months). Also, studies demonstrating HBsAg loss of 14% with PEGIFN monotherapy in the control arm[33] and 15.9% with PEGIFN initiated at the time of clinical relapse after NA withdrawal for 48 weeks[34] raise concern as to the true contribution of the experimental molecules. In addition such therapies are likely to be very costly.
BOTTLENECKS IN THE IMPLEMENTATION OF NOVEL THERAPIES
In 2016, modelling estimates suggested that globally only 10% of all people living with HBV were diagnosed and only 5%-17% eligible individuals (according to international guidelines) were receiving treatment[1], which marginally increased to 14% and 7.6%, respectively, in 2022 with almost no progress in the regions with the highest prevalence[2]. As per the WHO, although more than 50% of all HBV patients will need treatment according to setting and eligibility criteria, only 13% were aware of their status and 3% were on treatment in 2022. Only 3% of pregnant mothers with a high HBV viral load received antiviral treatment to reduce MTCT[35]. Unless more patients are linked to care, even the newer therapies will have few takers and such a costly endeavour will fail in its aim. To this end, active participation of community and government is needed. There has not been a political commitment to raise community awareness against HBV. This coupled with a disproportionate burden of the disease in low-income countries have resulted in lack of community activism demanding investment in HBV which have led to poor political engagement and insufficient funding in HBV elimination[19].
Although multiple treatment modes are being explored as shown above, going by its present trajectory, a panacea is unlikely to be in the offing. Combinations are more likely to be of benefit but it will skyrocket the cost of therapy making it unaffordable to the most eligible patients. Although of finite therapy duration, the resistance and adverse effect profile of newer drugs remain largely unexplored, but the check point inhibitors are already known to induce autoimmune diseases including hepatitis. Discovering an effective treatment is always a lengthy, time-consuming process having to pass through multiple bottlenecks. Chronic HBV infection is genetically diverse due to the poor proof-reading activity of the viral polymerase and rapid turnover of cccDNA. Thousands of co-existing genetic (both new and escape) variants evolve within a host under selection pressure which greatly hampers the development of any new drug, therapeutic vaccines, and even the effectivity of birth dose vaccination.
Achievement of functional cure will also require sustained inhibition of HBsAg production from both cccDNA and integrated HBV DNA in addition to complete suppression of HBV DNA replication and simultaneous restoration of HBV specific immune response to maintain immune control. HBsAg concentrations differ between HBV genotypes and the integrated viral genome is its predominant source among HBeAg-negative patients in whom, therefore, it does not reflect the true intrahepatic cccDNA burden. Thus, measurement of drug effectivity by decreased level/absence of HBsAg in serum is not a good marker for drug effectivity in all patients and long-term vigilance is needed for the development of occult infection or reappearance of HBsAg until a better marker of the cccDNA pool and activity is found (like HBCrAg or HBV RNA). Apart from the drugs under investigation, other cccDNA suppression mechanisms that may be targeted are inhibition of the multiple steps in its formation from rcDNA, preventing the nuclear import of relaxed circular (rcDNA) by capsid-targeting drugs, silencing its transcriptional activity by inducing the host cell’s epigenetic machinery or by blocking the de-silencing activity of HBV X protein, and degrading the existing cccDNA directly using genome editing with designer nucleases or indirectly through immune-mediated mechanisms (e.g. APOBEC enzymes)[36,37]. As most of the newer experimental drugs aiming at “functional cure” do not eliminate the episomal cccDNA and the HBV DNA sequences integrated in the host genome, gene editing technologies aiming at simultaneous elimination of both hold the most promise for sterilizing cure e.g. zinc-finger proteins, transcription activator-like effector nucleases, and the clustered regularly interspaced palindromic repeat-Cas system (CRISPR/Cas9)[37].
Apart from the biological challenges, HBV cure strategies must demonstrate higher efficacy and comparable safety to the present SOC i.e. long-term NA therapy. They should maintain long-term suppression of the virus and reduce the risk of liver disease progression and HCC even when treatment is stopped after finite duration of therapy rather than many years. This together with the availability of a highly effective low-cost vaccine set a high bar for new therapies to show incremental value.
CONCLUSION
PEGIFN and NA, the only two currently approved therapies for HBV infection are ineffective in terms of permanent viral eradication as per the definition of functional cure, yet NA is presently the SOC due to its effective long-term viral suppression halting viral transmission and liver disease progression. Going by the preliminary results from ongoing trials of novel therapies, while cautious optimism is necessary, it is unlikely to impact the existing HBV burden soon or even in the next several years. The virus has lived within humans for eons and has evolved several complex protective mechanisms against multiple layers of host defences that involves dynamic interaction with the host which makes it difficult to find an easy shot clearing agent or targeting of a mechanism. Already a number of drugs have not seen the light of day after phase I-II trials. The ideal combination is still a far cry as it has to perform better both efficacy-wise (including cost-benefit ratio) and by adverse events compared to the high bar set by the SOC of NAs in addition to its practicality of use (cost, duration and route of administration, number of drugs) and other patient factors (phase of infection and stage of liver disease etc). This further stretches the long and tenuous novel drug development process. Given the slow rate of progress in diagnostics and treatment, present emphasis should be on increasing diagnosis and linkage to care (by coordinated action of community, government and agencies to develop sufficient resources for large-scale testing and treatment) and non-pharmacological preventive measures (health education, blood screening, safe sexual practices, using sterilized needles for injection), vaccination and routine HBV screening (and treatment if needed) of all pregnant women to prevent MTCT.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Indian Society of Gastroenterology, No. LM1012.
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report’s classification
Scientific Quality: Grade C, Grade D
Novelty: Grade C, Grade C
Creativity or Innovation: Grade B, Grade C
Scientific Significance: Grade B, Grade C
P-Reviewer: Ulasoglu C S-Editor: Qu XL L-Editor: Webster JR P-Editor: Zheng XM