Published online Aug 27, 2024. doi: 10.4254/wjh.v16.i8.1177
Revised: July 9, 2024
Accepted: July 25, 2024
Published online: August 27, 2024
Processing time: 64 Days and 21.3 Hours
Hepatic ischemia-reperfusion injury (IRI) poses a great challenge in liver surgery and transplantation because of oxidative stress and inflammatory responses. The changes in glutamine synthetase (GS) expression during hepatic IRI remain unclear.
To investigate the dynamic expression of GS during hepatic IRI.
Following hepatic ischemia for 1 h and reperfusion, liver tissue samples were collected at 0.5, 6, and 24 hours postreperfusion for fixation, embedding, section
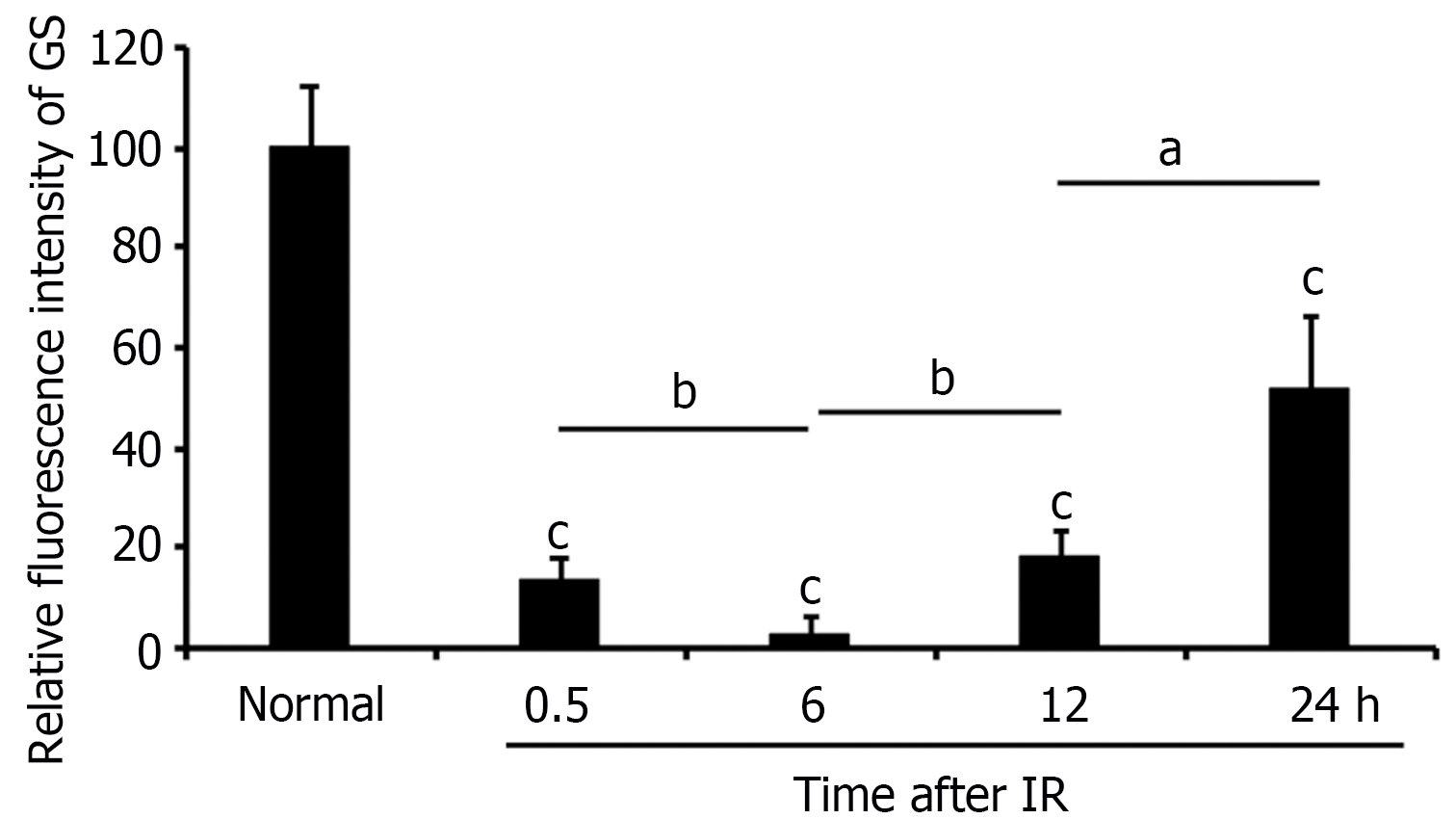
GS expression rapidly decreases in hepatocytes around the central vein after IRI, reaching its lowest point at 6 hours postreperfusion, and then gradually recovers.
GS is highly sensitive to IRI, highlighting its potential role as an indicator of liver injury states and a target for therapeutic intervention.
Core Tip: The role of glutamine synthetase (GS) in hepatic ischemia‒reperfusion injury (IRI), a major challenge in liver surgery and transplantation, was explored in this study. Using a mouse model, dynamic GS expression patterns were observed. The expression of GS, which is typically high around the central vein in normal livers, significantly decreases postreperfusion, which is correlated with liver damage. The re-expression of GS during liver recovery highlights its critical role in cellular defense. These findings suggest that modulating GS expression could be a therapeutic strategy to reduce IRI, improving patient outcomes after liver transplantation.
- Citation: Huang ZH, Dong MQ, Liu FY, Zhou WJ. Dynamics of glutamine synthetase expression in hepatic ischemia-reperfusion injury: Implications for therapeutic interventions. World J Hepatol 2024; 16(8): 1177-1184
- URL: https://www.wjgnet.com/1948-5182/full/v16/i8/1177.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i8.1177
Ischemia-reperfusion injury (IRI) of the liver is a great challenge in various surgical procedures, such as liver tumor resection, liver transplantation, and situations involving hemorrhagic shock with fluid resuscitation. The process of reperfusion following ischemia generates reactive oxygen species (ROS), which play crucial roles in inducing tissue damage and inflammatory responses. These ROS can directly damage cellular components and activate various inflammatory mediators, contributing to overall injury[1-3].
One of the key factors the protect against ROS-induced damage is glutathione (GSH), a vital antioxidant in both rodents and humans. The intravenous administration of GSH during reperfusion has been shown to mitigate reperfusion injury in rat models[4,5]. GSH synthesis relies on three amino acids, glycine, cysteine, and glutamate, with glutamate derived from glutamine (Gln). Reduced levels of Gln are closely linked to decreased intracellular GSH levels[6,7].
Gln is a conditionally essential nutrient that becomes critical during severe injury or illness. It plays a crucial role in tissue metabolism and has been demonstrated to protect against IRI in various tissues, including the liver, by preserving GSH levels[8-13]. Gln synthetase (GS), the enzyme responsible for Gln synthesis, is mainly present in the brain, kidneys, and liver. In the brain, GS is mainly located in astrocytes and is reported to be sensitive to proteasomal degradation in response to oxidative stress[14-17]. This sensitivity suggests that GS could be a key player in the response to oxidative stress during IRI.
In the liver, GS is highly expressed in hepatocytes around the central vein (CV) of liver lobules[18-22]. However, how the expression and distribution of GS change during liver IRI is unclear. In this study, a mouse model was generated to explore the changes in GS expression and distribution during liver IRI. Liver tissues were collected at 0.5, 6, 12, and 24 hours postreperfusion, and GS staining was performed to analyze these changes. Understanding these dynamics could provide insights into the pathological mechanisms of liver IRI and suggest potential therapeutic strategies involving the modulation of GS expression and activity.
All animal experiments were conducted using male mice unless otherwise stated. The mice were maintained on a standard 12-hour light-dark cycle under specific-pathogen-free conditions and had unrestricted access to food and water. The research protocols adhered to the United States Public Health Service Policy on the Use of Laboratory Animals and were approved by the Ethics Committee on the Use and Care of Animals of Southern Medical University.
For the liver I/R model, male C57BL/6J mice aged 6-8 weeks and weighing approximately 20-22 g were anesthetized. The abdominal cavity was then opened, and a vascular clamp was applied to occlude the left liver lobe and the portal triad [hepatic artery, portal vein (PV), and bile duct]. After 1 hour, the clamp was removed, and the incision was sutured. Liver tissue samples were collected at 0.5, 6, 12, and 24 hours postreperfusion for fixation, embedding, sectioning, and staining.
Representative images are displayed with six mice in each group, and the experiment was repeated at least three times to ensure reproducibility and reliability of the results.
Immunofluorescent staining of mouse liver tissues was performed as previously described[21,22]. In brief, mouse liver tissues were fixed in 10% neutral buffered formalin, cut into 3.5-μm thick sections, and incubated with EDTA (50 ×, Key GENE BioTECH KGIHC002) for 15 minutes at 120 °C. Non-specific binding sites were blocked with goat serum for 1 hour at 37 °C. The sections were then incubated with appropriate primary antibodies overnight at 4 °C, extensively washed in PBS, and subsequently incubated with Alexa 488/594 conjugated secondary antibodies (Cytoskeleton Inc., Denver, United States) for 1 hour. Sections were counterstained with 4,6-diamidino-2-phenylindole, washed, and mounted for observation under a scanning confocal microscope (Olympus, Fluoview FV 1000). The primary antibodies used for immunofluorescence included anti-GS antibody (1: 50, Abcam, ab125724).
Mouse liver tissues were fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin as previously described[21,22]. Sections of 3.5 μm thickness were stained with hematoxylin and eosin (HE).
The GS staining positive area were calculated in six random fields from each section at 200 × magnification using image J software. The experimental data were statistically analyzed by Student’s t-test. All data are presented as mean ± SEM. P < 0.05 was considered statistically significant. In all cases, data from at least three independent experiments was used.
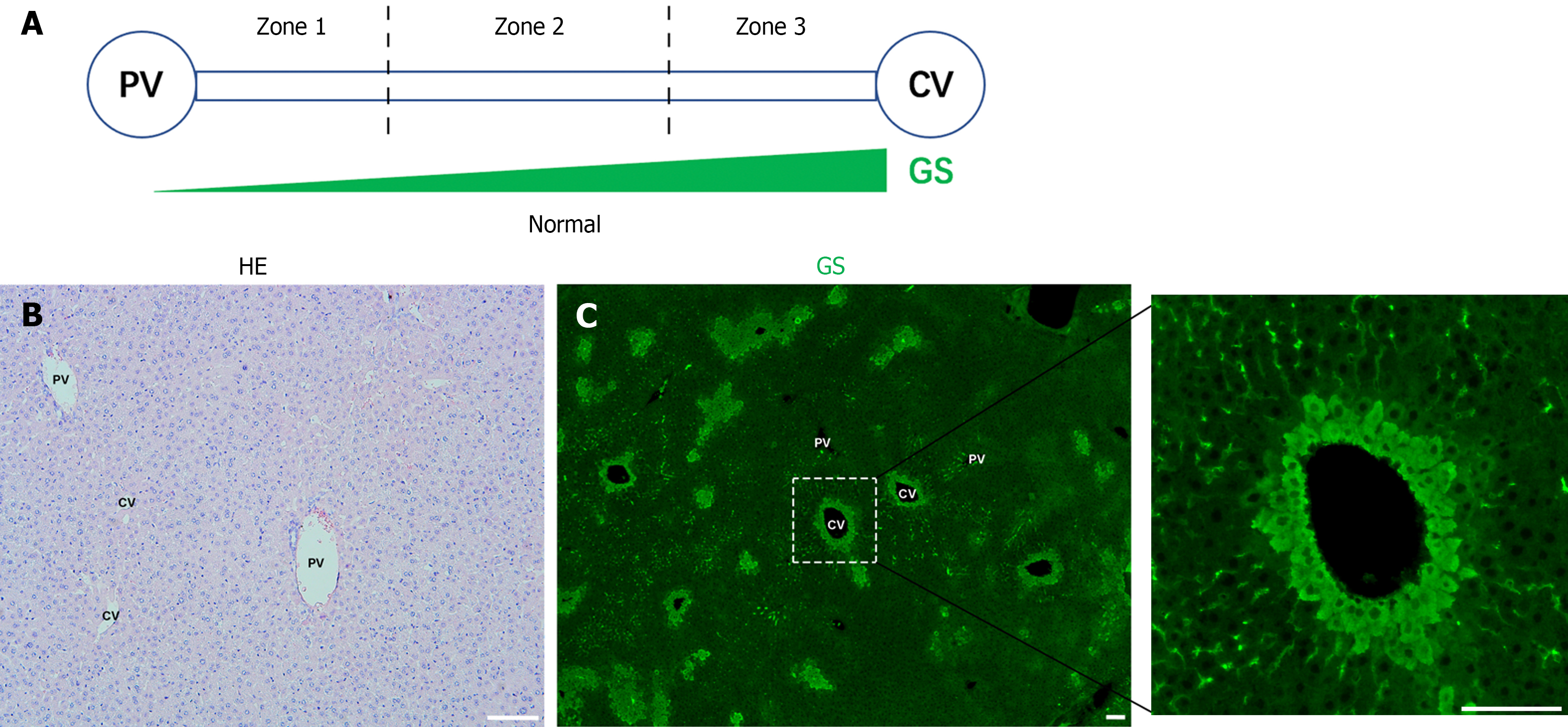
Liver lobules can be divided into three zones from the PV to the CV: Zone 1, Zone 2, and Zone 3 (Figure 1A). Normal liver slides from C57BL/6J were stained with HE (Figure 1B). As reported[17], GS is specifically expressed in hepatocytes around the CV (Figure 1C).
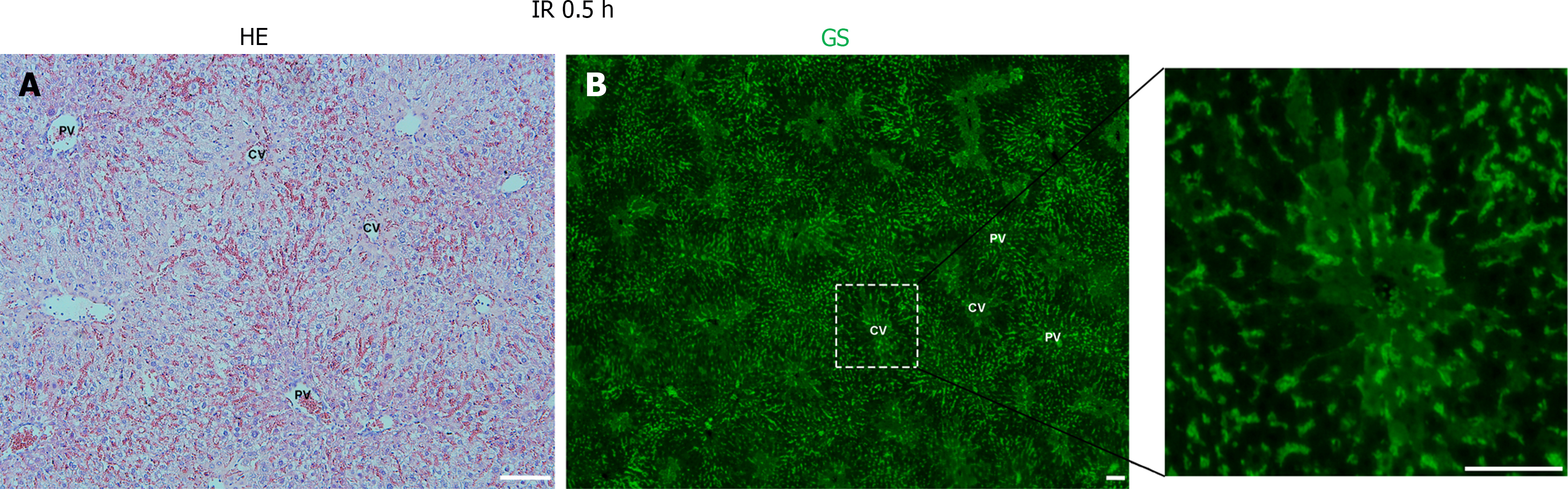
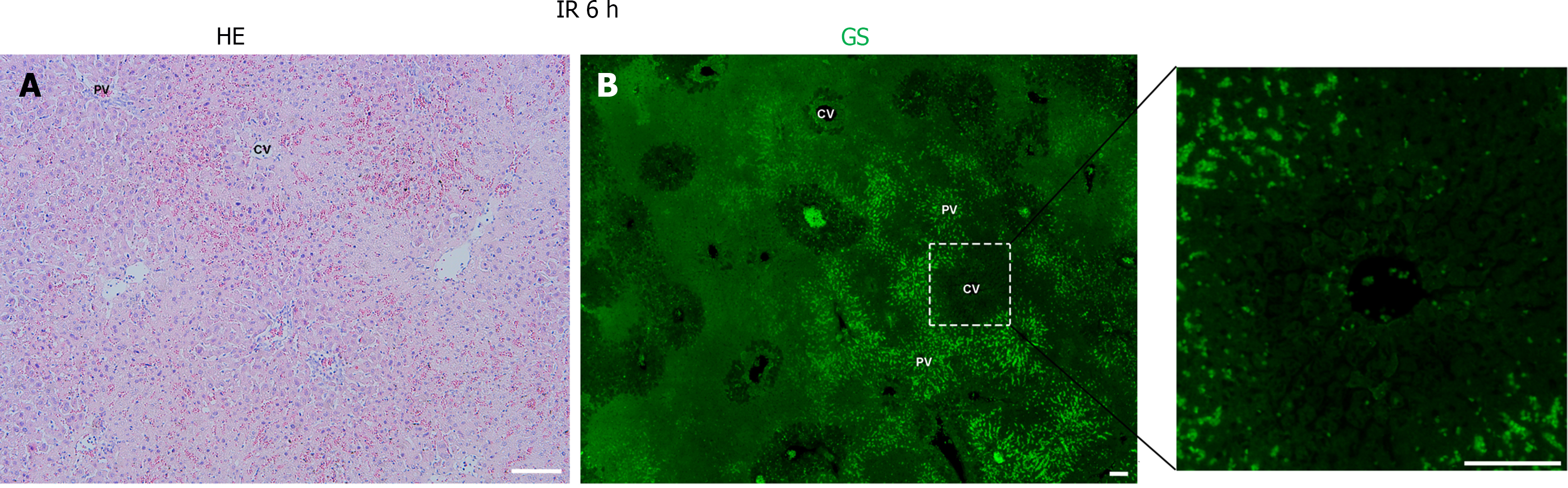
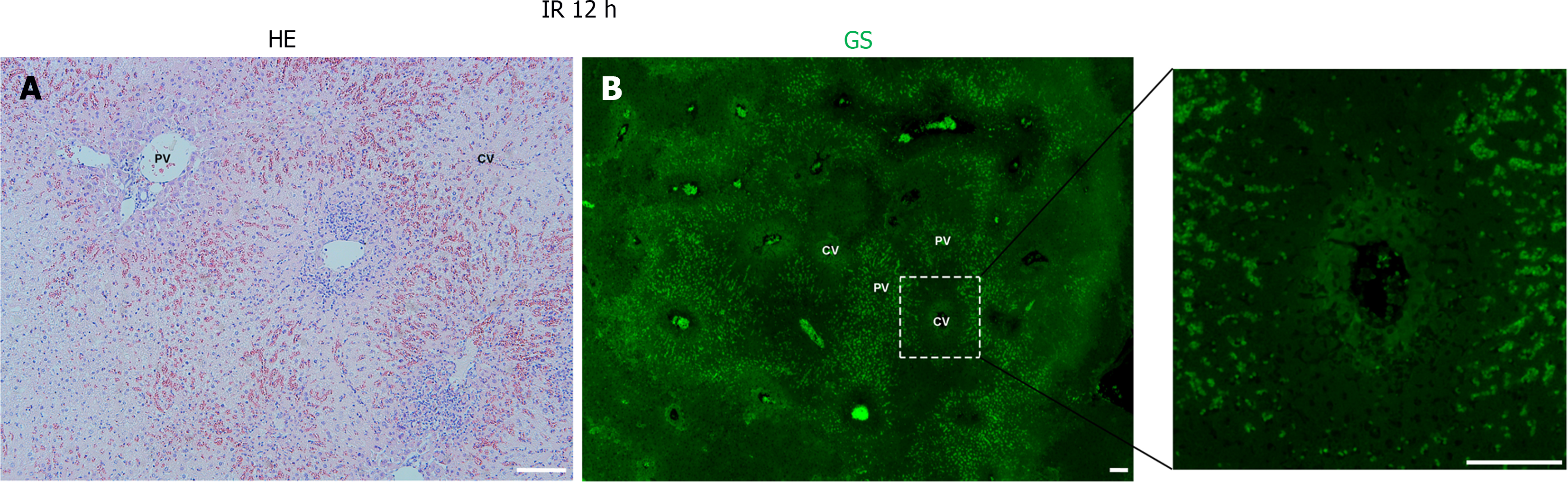
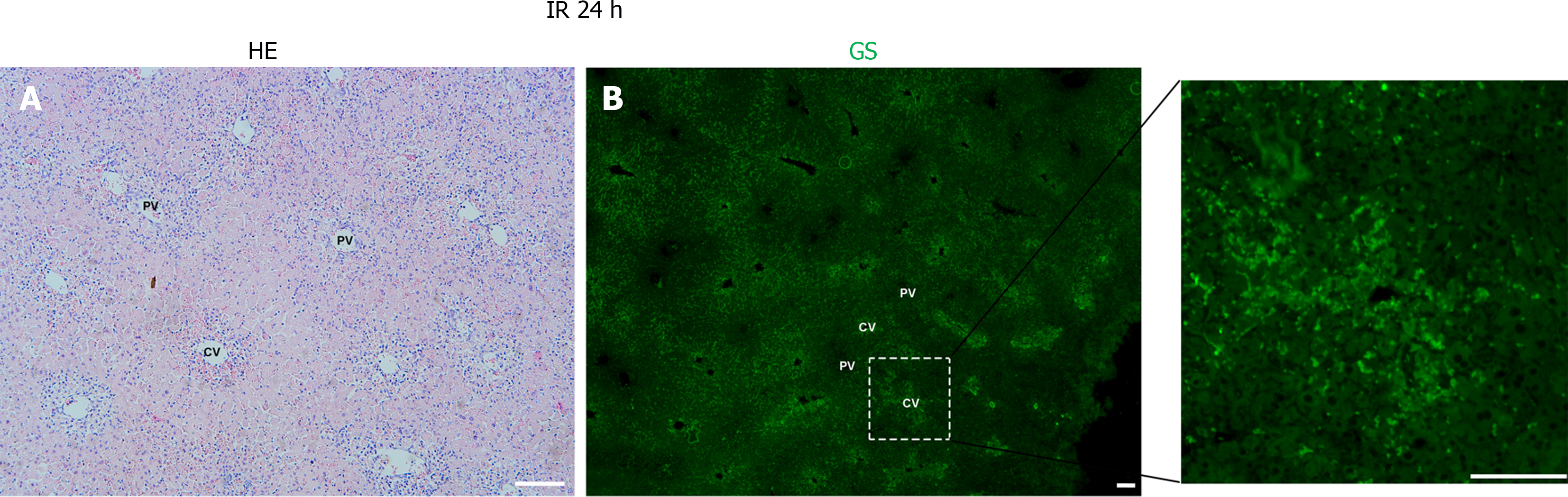
Following hepatic ischemia for 1 hour and reperfusion, 0.5 hours post-reperfusion, HE staining showed hepatocyte damage, with hepatic sinusoids filled with blood cells (Figure 2A). GS staining decreased significantly in hepatocytes around the CV while increased in hepatic sinusoids (Figure 2B). At 6 hours post-reperfusion, HE staining showed extensive necrosis of liver cells near CV (Figure 3A). The GS staining of hepatocytes and hepatic sinusoids near CV almost disappeared, while hepatic sinusoids near PV showed high expression of GS (Figure 3B). At 12 hours post-reperfusion, HE staining showed a large number of necrotic areas and extensive infiltration of inflammatory cells in the liver (Figure 4A). GS staining began to restore weak expression in liver cells near CV (Figure 4B). At 24 hours post-reperfusion, HE staining showed a reduction in the area of liver necrosis, and liver injury began to recover, with still a large number of inflammatory cells infiltrating (Figure 5A). The expression of GS in hepatocytes near CV further increased (Figure 5B), indicating the liver is in a recovery phase. The GS-positive area in the CV region of the liver at different time points after I/R was analyzed using Image J. The results indicate that GS expression rapidly decreases after IRI, reaching its lowest point at 6 hours post-reperfusion, and then gradually recovers (Figure 6).
This study discovered significant changes in the expression and distribution of GS during liver IRI. In normal liver, GS is mainly expressed in hepatocytes around the CV. After IRI, GS expression in hepatocytes near the CV weakens and disappears but re-expresses during liver injury recovery. This indicates that changes in GS expression can indicate different states of liver IRI, suggesting that GS might be an important intervention target for liver IRI.
IRI is a cause of many fatal diseases, such as myocardial infarction and stroke, and limits the therapeutic effects of medical interventions like organ transplantation. It is thus a major factor in the morbidity and mortality of many diseases. Numerous reports indicate that ROS, especially superoxide anion (O2-), are massively produced during I/R, mainly derived from xanthine oxidase in many tissues[23,24]. Therefore, developing treatments to inhibit ROS production or reduce their levels in the body is a rational approach to treating IRI. GSH is crucial for defending against ROS damage. To maintain GSH levels during oxidative stress, Gln supplementation may be a safe and well-tolerated method, administration of Gln is considered helpful for various systemic inflammatory states[25,26].
Gln plays multiple roles, regulating the expression of genes related to metabolism, signal transduction, cell defense, and repair, and activating intracellular signaling pathways. It can also protect GSH to counteract oxidative stress[27,28]. It has been reported that Gln administration can protect the intestine, heart, and skeletal muscle from IRI[8,9,29]. In a liver I/R model, Gln supplementation increased the liver's GSH supply, and Gln pretreatment could protect the liver from damage[30,31].
GS, encoded by the GLUL gene, converts glutamate and ammonia into Gln. GS plays a vital role in regulating Gln levels in the body, especially in the liver, where it is specifically highly expressed in hepatocytes around the CV, responsible for converting glutamate and ammonia into Gln. Despite the significant role of Gln in IRI, the role of GS in IRI has received less attention. This study systematically investigated the changes in GS expression during liver IRI. During the early stage of liver IRI (30 minutes post-reperfusion), GS expression in hepatocytes around the CV significantly decreased, and at 6 hours, its expression was almost undetectable. The rise in GS staining signal in the liver sinusoids is likely due to the high expression of GS in the aggregated blood cells or non-specific staining. During liver injury recovery, GS expression in hepatocytes around the CV gradually returned. This indicates that GS is highly sensitive to liver IRI, consistent with the liver's sensitivity to ischemic effects.
During liver IRI, the potential mechanism underlying the observed GS expression may involve oxidative stress-induced degradation or transcriptional repression of GS in hepatocytes around the CV. ROS, generated during reperfusion, are known to damage cellular components and activate proteolytic pathways, potentially leading to the degradation of GS[14,15]. Additionally, the inflammatory response triggered by IRI could modulate gene expression, resulting in reduced GS levels. The re-emergence of GS expression during the recovery phase suggests a resilience mechanism that restores cellular functions post-injury.
Understanding the differences between mice and humans in GS expression and response to IRI is crucial for translating these findings into clinical practice. When extrapolating to human conditions, it is necessary to detect GS in clinical samples from patients, considering the interspecies differences in GS regulation and metabolic pathways. The supplementation of Gln has been shown to reduce liver IRI in both mouse models and clinical applications[10-13,30,31], suggesting a high potential for targeting GS in clinical settings. Enhancing the stability of GS or promoting its expression could mitigate liver IRI, offering a novel therapeutic approach for patients undergoing liver surgeries, transplants, or experiencing ischemic events. Further research into GS regulation in human tissues and clinical trials will be essential to validate these strategies and their applicability in clinical medicine.
In conclusion, this study provides the first systematic analysis of GS expression changes during liver IRI. It demonstrates that GS expression in hepatocytes around the CV significantly decreases during the early stages of liver IRI and re-emerges during the recovery phase. This suggests that GS is highly sensitive to oxidative stress induced by IRI, high
We thank Central Laboratory, Southern Medical University for providing facilities and technical support.
| 1. | Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, Piña E, Geller DA. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res. 2008;147:153-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 287] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 2. | Elias-Miró M, Jiménez-Castro MB, Rodés J, Peralta C. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radic Res. 2013;47:555-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 3. | Kaltenmeier C, Wang R, Popp B, Geller D, Tohme S, Yazdani HO. Role of Immuno-Inflammatory Signals in Liver Ischemia-Reperfusion Injury. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 4. | Pretzsch E, Nieß H, Khaled NB, Bösch F, Guba M, Werner J, Angele M, Chaudry IH. Molecular Mechanisms of Ischaemia-Reperfusion Injury and Regeneration in the Liver-Shock and Surgery-Associated Changes. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Schauer RJ, Kalmuk S, Gerbes AL, Leiderer R, Meissner H, Schildberg FW, Messmer K, Bilzer M. Intravenous administration of glutathione protects parenchymal and non-parenchymal liver cells against reperfusion injury following rat liver transplantation. World J Gastroenterol. 2004;10:864-870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Matés JM, Pérez-Gómez C, Núñez de Castro I, Asenjo M, Márquez J. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int J Biochem Cell Biol. 2002;34:439-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Liu D, Chen Z. The regulatory effects of glutamine on illness and health. Protein Pept Lett. 2011;18:658-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Harward TR, Coe D, Souba WW, Klingman N, Seeger JM. Glutamine preserves gut glutathione levels during intestinal ischemia/reperfusion. J Surg Res. 1994;56:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Prem JT, Eppinger M, Lemmon G, Miller S, Nolan D, Peoples J. The role of glutamine in skeletal muscle ischemia/reperfusion injury in the rat hind limb model. Am J Surg. 1999;178:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Xu F, Dai CL, Peng SL, Zhao Y, Jia CJ, Xu YQ. Preconditioning with glutamine protects against ischemia/reperfusion-induced hepatic injury in rats with obstructive jaundice. Pharmacology. 2014;93:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Sözen S, Kisakürek M, Yildiz F, Gönültaş M, Dinçel AS. The effects of glutamine on hepatic ischemia reperfusion injury in rats. Hippokratia. 2011;15:161-166. [PubMed] |
| 12. | Zhang SC, Shi Q, Feng YN, Fang J. Tissue-protective effect of glutamine on hepatic ischemia-reperfusion injury via induction of heme oxygenase-1. Pharmacology. 2013;91:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Araújo Júnior RJ, Silva Júnior RG, Vasconcelos MP, Guimarães SB, Vasconcelos PR, Garcia JH. Preconditioning with L-alanyl-glutamine reduces hepatic ischemia-reperfusion injury in rats. Acta Cir Bras. 2011;26 Suppl 1:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Nakamura K, Stadtman ER. Oxidative inactivation of glutamine synthetase subunits. Proc Natl Acad Sci U S A. 1984;81:2011-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Floyd RA. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990;4:2587-2597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 651] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 16. | Oliver CN, Starke-Reed PE, Stadtman ER, Liu GJ, Carney JM, Floyd RA. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci U S A. 1990;87:5144-5147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 514] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 17. | Görg B, Qvartskhava N, Voss P, Grune T, Häussinger D, Schliess F. Reversible inhibition of mammalian glutamine synthetase by tyrosine nitration. FEBS Lett. 2007;581:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Zhou Y, Eid T, Hassel B, Danbolt NC. Novel aspects of glutamine synthetase in ammonia homeostasis. Neurochem Int. 2020;140:104809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Soria LR, Nitzahn M, De Angelis A, Khoja S, Attanasio S, Annunziata P, Palmer DJ, Ng P, Lipshutz GS, Brunetti-Pierri N. Hepatic glutamine synthetase augmentation enhances ammonia detoxification. J Inherit Metab Dis. 2019;42:1128-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Lin Y, Liu Z, Dong M, Zhou W. [An update of understanding of the hepatic vascular system and new research strategies]. Nan Fang Yi Ke Da Xue Xue Bao. 2022;42:1907-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Xu M, Xu HH, Lin Y, Sun X, Wang LJ, Fang ZP, Su XH, Liang XJ, Hu Y, Liu ZM, Cheng Y, Wei Y, Li J, Li L, Liu HJ, Cheng Z, Tang N, Peng C, Li T, Liu T, Qiao L, Wu D, Ding YQ, Zhou WJ. LECT2, a Ligand for Tie1, Plays a Crucial Role in Liver Fibrogenesis. Cell. 2019;178:1478-1492.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 22. | Lin Y, Dong MQ, Liu ZM, Xu M, Huang ZH, Liu HJ, Gao Y, Zhou WJ. A strategy of vascular-targeted therapy for liver fibrosis. Hepatology. 2022;76:660-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 23. | McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3970] [Cited by in RCA: 3779] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 24. | Halliwell B, Gutteridge JM. Free radicals, lipid peroxidation, and cell damage. Lancet. 1984;2:1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Lacey JM, Wilmore DW. Is glutamine a conditionally essential amino acid? Nutr Rev. 1990;48:297-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 535] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 26. | Lochs H, Allison SP, Meier R, Pirlich M, Kondrup J, Schneider S, van den Berghe G, Pichard C. Introductory to the ESPEN Guidelines on Enteral Nutrition: Terminology, definitions and general topics. Clin Nutr. 2006;25:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 270] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 27. | Santora R, Kozar RA. Molecular mechanisms of pharmaconutrients. J Surg Res. 2010;161:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC, Corless M, Newsholme P. Molecular mechanisms of glutamine action. J Cell Physiol. 2005;204:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 317] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 29. | Wischmeyer PE, Jayakar D, Williams U, Singleton KD, Riehm J, Bacha EA, Jeevanandam V, Christians U, Serkova N. Single dose of glutamine enhances myocardial tissue metabolism, glutathione content, and improves myocardial function after ischemia-reperfusion injury. JPEN J Parenter Enteral Nutr. 2003;27:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Hong RW, Rounds JD, Helton WS, Robinson MK, Wilmore DW. Glutamine preserves liver glutathione after lethal hepatic injury. Ann Surg. 1992;215:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 176] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Jia CJ, Dai CL, Zhang X, Cui K, Xu F, Xu YQ. Alanyl-glutamine dipeptide inhibits hepatic ischemia-reperfusion injury in rats. World J Gastroenterol. 2006;12:1373-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |