Published online Jul 27, 2024. doi: 10.4254/wjh.v16.i7.1009
Revised: June 3, 2024
Accepted: June 27, 2024
Published online: July 27, 2024
Processing time: 137 Days and 2 Hours
Both tenofovir alafenamide (TAF) and tenofovir disoproxil fumarate (TDF) are the first-line treatments for chronic hepatitis B (CHB). We have showed switching from TDF to TAF for 96 weeks resulted in further alanine aminotransferase (ALT) improvement, but data remain lacking on the long-term benefits of TDF switching to TAF on hepatic fibrosis.
To assess the benefits of TDF switching to TAF for 3 years on ALT, aspartate aminotransferase (AST), and hepatic fibrosis improvement in patients with CHB.
A single center retrospective study on 53 patients with CHB who were initially treated with TDF, then switched to TAF to determine dynamic patterns of ALT, AST, AST to platelet ratio index (APRI), fibrosis-4 (FIB-4) scores, and shear wave elastography (SWE) reading improvement at switching week 144, and the associated factors.
The mean age was 55 (28-80); 45.3%, males; 15.1%, clinical cirrhosis; mean baseline ALT, 24.8; AST, 25.7 U/L; APRI, 0.37; and FIB-4, 1.66. After 144 weeks TDF switching to TAF, mean ALT and AST were reduced to 19.7 and 21, respectively. From baseline to switching week 144, the rates of ALT and AST < 35 (male)/25 (female) and < 30 (male)/19 (female) were persistently increased; hepatic fibrosis was also improved by APRI < 0.5, from 79.2% to 96.2%; FIB-4 < 1.45, from 52.8% to 58.5%, respectively; mean APRI was reduced to 0.27; FIB-4, to 1.38; and mean SWE reading, from 7.05 to 6.30 kPa after a mean of 109 weeks switching. The renal function was stable and the frequency of patients with glomerular filtration rate > 60 mL/min was increased from 86.5% at baseline to 88.2% at switching week 144.
Our data confirmed that switching from TDF to TAF for 3 years results in not only persistent ALT/AST improvement, but also hepatic fibrosis improvement by APRI, FIB-4 scores, as well as SWE reading, the important clinical benefits of long-term hepatitis B virus antiviral treatment with TAF.
Core Tip: Tenofovir disoproxil fumarate (TDF), entecavir, and tenofovir alafenamide (TAF) have been used as first-line therapy for chronic hepatitis B. In this study, we assessed the effect of TDF switching to TAF for 3 years (144 weeks) on dynamic changes of alanine aminotransferase (ALT), aspartate aminotransferase (AST), AST to platelet ratio index (APRI), fibrosis-4 (FIB-4) scores and shear wave elastography (SWE) reading. Our study demonstrated that switching from TDF to TAF for 3 years results in persistent mean ALT and AST reduction with high rate of normalization, and also hepatic fibrosis improvement assessed by mean APRI and FIB-4 scores, as well as SWE reading.
- Citation: Huynh T, Bui DM, Zhou TX, Hu KQ. Improvement of hepatic fibrosis after tenofovir disoproxil fumarate switching to tenofovir alafenamide for three years. World J Hepatol 2024; 16(7): 1009-1017
- URL: https://www.wjgnet.com/1948-5182/full/v16/i7/1009.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i7.1009
Hepatitis B virus (HBV) infection is a major global health concern that involves the risk of cirrhosis and hepatocellular carcinoma (HCC). World Health Organization estimates that approximately 296 million people were living with chronic HBV infection in 2019, with approximately 1.5 million people become newly infected each year. In 2019, chronic hepatitis B (CHB) resulted in an estimated 820000 deaths, mostly from cirrhosis and HCC[1]. Antiviral therapy with nucleos(t)ide analogues (NAs) is currently the main treatment option that has significantly improved the outcomes in patients with CHB. The NAs, tenofovir disoproxil fumarate (TDF), approved in 2008, and entecavir (ETV), approved in 2005, have been recommended by international guidelines and used as first-line therapy for CHB. Prolonged treatment with these NAs has been associated with reduction in progression to cirrhosis, lower risk of HCC, and reversal of hepatic decom
Stage of liver fibrosis is very important to determine severity and prognosis, and prioritize for treatment in CHB patients. Previously, the only method of staging fibrosis was liver biopsy, which has been the gold standard[7]. In addition to staging fibrosis, it can grade necrosis, and inflammatory activities. However, there are limitations of this procedure, including cost, risk of serious complications, sampling errors, and observer technical differences[8]. Various non-invasive methods ranging from serum markers to imaging techniques have been developed over the past decade to stage liver fibrosis. Several scoring systems, such as aspartate aminotransferase (AST) to platelet ratio index (APRI) and fibrosis index based on four factors (Fibrosis-4 index, FIB-4) have been widely validated in large cohort studies[9,10]. Liver stiffness measurement (LSM) is another option to stage liver fibrosis noninvasively, including transient elastography (TE) and shear wave elastography (SWE)[11,12].
Prior studies have demonstrated the benefits of switching from TDF to TAF, including better renal profile and bone safety[13-16]. Previously, we have showed switching from TDF to TAF treatment for 96 weeks resulted in alanine aminotransferase (ALT) improvement, but data remain lacking on its long-term effect on biochemical changes and hepatic fibrosis[17]. The present study assessed the effect of TDF switching to TAF for 3 years (144 weeks) on dynamic changes of ALT, AST, APRI, FIB-4 scores and SWE reading, and the associated factors.
This was a single-center retrospective study. Institutional Review Board approval was obtained, and informed consent was waived. Patients with CHB who were initially treated with TDF, then switched to TAF in the Liver Clinic at UCI Medical Center were assessed and enrolled if they met the inclusion criteria. Inclusion criteria included patients who had CHB and ruled out other chronic liver diseases, were treated with TDF then completed the switching to TAF and had regular follow-up for 144 weeks. Exclusion criteria included patients with treatment course less than 144 weeks or missing lab data during the switching treatment and follow-up.
Of the 60 charts of patients reviewed from 12/2016 to 09/2021, 7 patients were excluded from the study due to missing lab data during the switching, or lack of 144 weeks post switching follow-up. Consequently, 53 patients met the inclusion criteria and were included in the present study.
Baseline data collection included age, gender, ethnicity, body mass index (BMI), diagnosis of cirrhosis, spleen size, HBV genotype, hepatitis B e antigen (HBeAg) and e antibody (anti-HBe) test results. The diagnosis of clinical cirrhosis was made based on radiographic, histologic findings, or endoscopic finding of esophageal/gastric varices. Radiographic findings included presence of nodular liver, splenomegaly (> 12 cm), and /or ascites. Histologic findings included presence of stage 3-4 fibrosis. Baseline and follow-up lab data included levels of creatinine, complete blood count (white blood cells, hemoglobin, and platelets), ALT, AST were collected at switching week 24, 48, 96, and 144. ALT and AST were quantified using UV/NADH-Rate method with reference range 7-40 U/L on the Beckman Coulter AU analyzer. Both APRI and FIB-4 scores were calculated at baseline and at switching week 144. SWE was performed at baseline and during follow-up after TAF switching.
Statistical analyses were performed using the Statistical Package for the Social Science software (SPSS, version 25, Chicago, IL, United States). Categorical data were presented as percentages, and continuous data were expressed as the mean and standard deviation. Data values were compared using Pearson χ2 test to evaluate the association between different variables of biochemical and clinical response at the certain switching time points and the improvement of ALT/AST, APRI, FIB-4, and SWE reading. All tests for significance were two-tailed and P < 0.05 was considered statistically significant.
The demographic characteristics of the study population are summarized in Table 1. The mean age of the cohort was 55 (28-80) years; 35 (66%) patients, age > 50 year-old; 24 (45.3%) patients were male. Among the 53 patients, 51 (96.2%), 1 (1.9%), and 1 (1.9%) were Asian, Hispanic, and other races, respectively. Seventeen (32.1%) patients had BMI ≥ 25 kg/m2. Clinical cirrhosis was diagnosed in 8 (15.1%) patients. Four (7.7%) patients had spleen size > 12 cm. In 24 patients with identified HBV genotype, 2 (8.3%) patients had genotype A; 15 (62.5%), genotype B; 6 (25%), genotype C; and 1 (4.2%), genotype D. Fifteen (28. 3%) patients were HBeAg-positive.
| Characteristics | n (%) or range |
| Mean age (years) | 55 (28-80) |
| Age > 50 year-old | 35 (66) |
| Male:Female | 24:29 (45.3:54.7) |
| Ethnicity | |
| Asian | 51 (96.2) |
| Hispanic | 1 (1.9) |
| Other | 1 (1.9) |
| BMI ≥ 25 kg/m2 | 17 (32.1) |
| Clinical cirrhosis | 8 (15.1) |
| Spleen size > 12 cm | 4 (7.7) |
| HBV genotype | |
| A | 2/24 (8.3) |
| B | 15/24 (62.5) |
| C | 6/24 (25) |
| D | 1/24 (4.2) |
| HBeAg + | |
| Yes No | 15 (28.3) 38 (71.7) |
| Mean ALT (U/L) | 24.8 (7-108) |
| Mean AST (U/L) | 25.7 (15-89) |
| Mean creatinine (mg/dL) | 0.86 (0.5–1.7) |
| Mean APRI score | 0.37 (0.13-0.92) |
| Mean FIB-4 score | 1.66 (0.49-5.33) |
| Mean SWE score (kPa) | 7.05 (4-20.9) |
| Platelets ≤ 120 × 109/L | 6 (11.3) |
Baseline laboratory variables were shown in Table 1. Mean serum ALT was 24.8 (7-108) U/L; mean serum AST, 25.7 (15-89) U/L; mean APRI score, 0.37 (0.13–0.92); and mean FIB-4 score, 1.66 (0.49–5.33). In 53 patients, 79.2% had APRI < 0.5; 52.8%, FIB-4 < 1.45. Six of 53 (11.3%) patients had platelets ≤ 120 × 109/L. At baseline, 73.6% of patients had ALT < 35 (males)/25 (female) U/L (35/25); 77.4%, AST < 35/25; 67.9%, both ALT/AST < 35/25; 54.7%, ALT < 30 (males)/19 (female) U/L (30/19); 47.2%, AST < 30/19; and 39.6%, both ALT/AST < 30/19. Mean serum creatinine was 0.86 (0.5-1.7) mg/dL, and 86.5% of patients had glomerular filtration rate (GFR) > 60 mL/min.
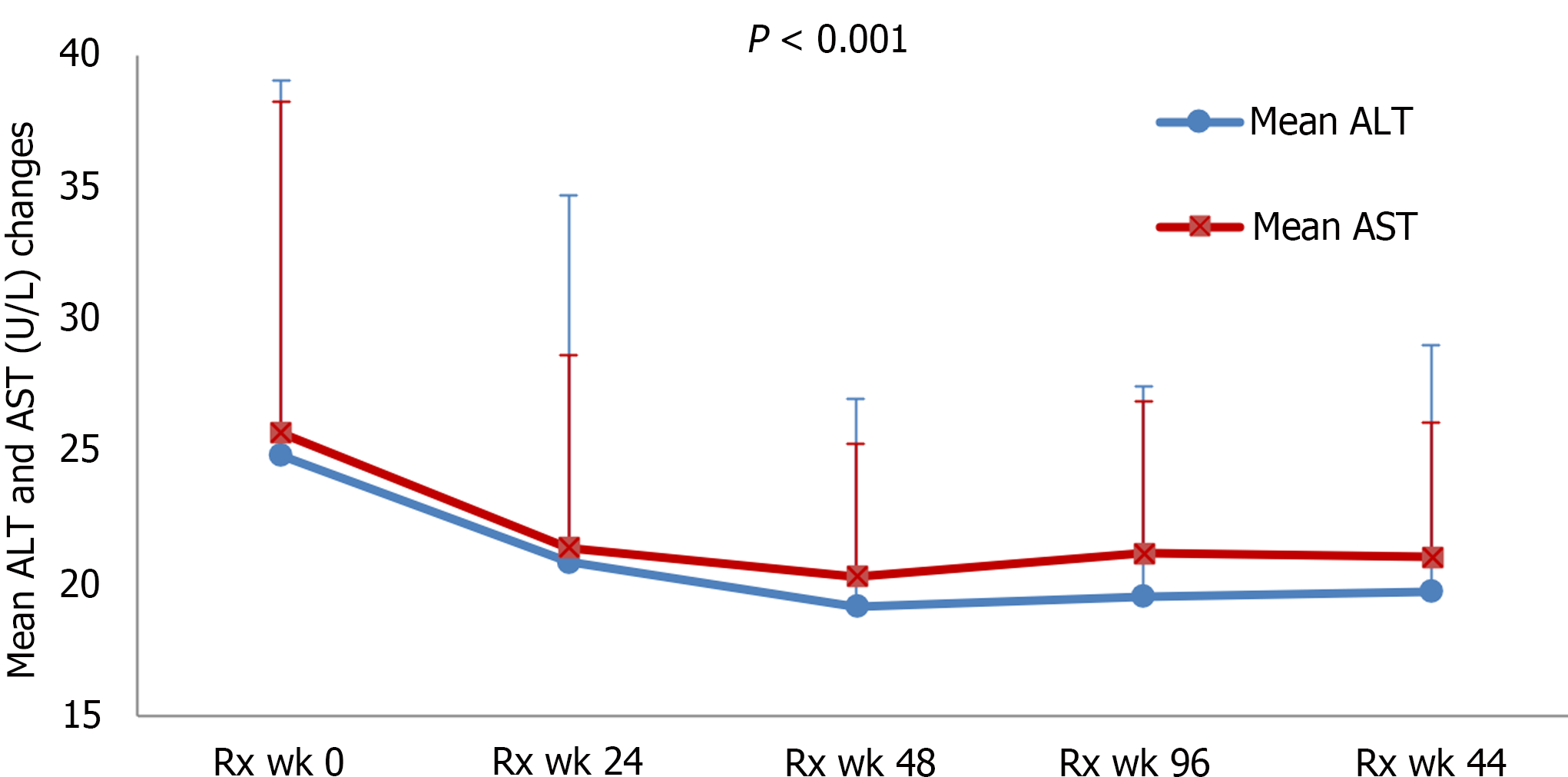
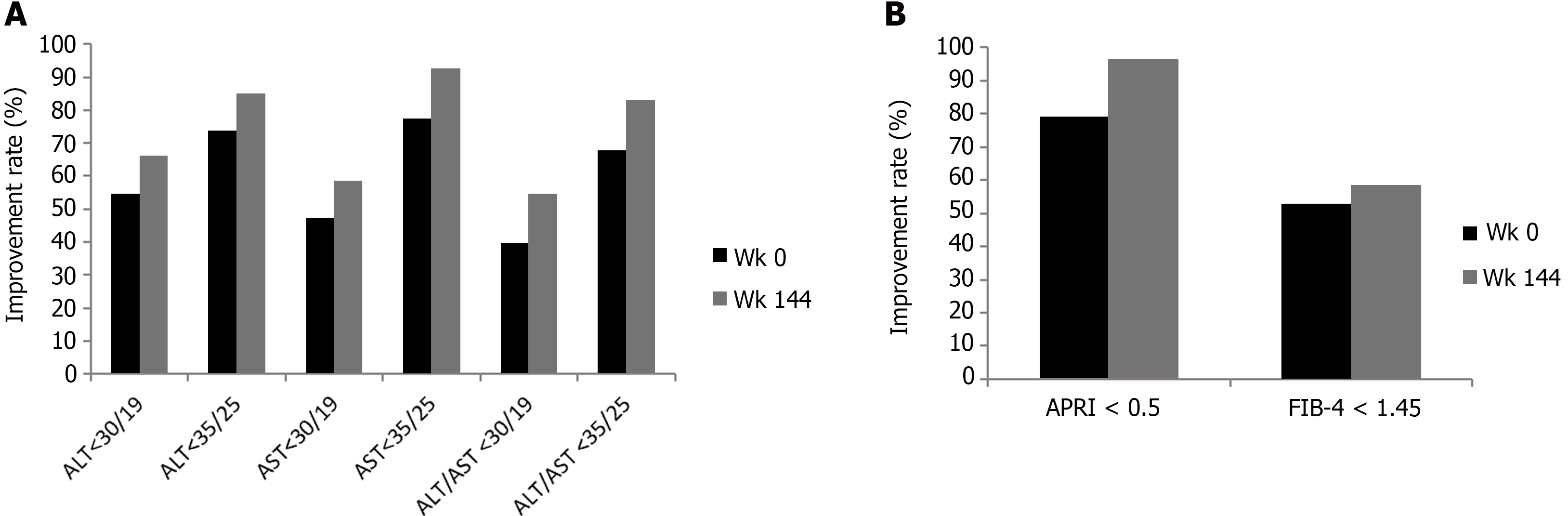
Our previous study and other studies have showed the improvement of ALT at post-switching week 96[17,18]. In the present study, we further assessed the biochemical changes after switching to beyond 96 weeks. In our study cohort, both ALT and AST normalization and the improvement rates were persistent after switching for 96 weeks and up to 144 weeks. As shown in Figure 1, the means (ranges) of ALT and AST were all improved persistently after switching. The mean ALT was reduced to 20.8 (8-106), 19.1 (7-40), 19.5 (9-42), and 19.7 (8-42), P < 0.001; mean AST was reduced to 21.4 (13-59), 20.3 (14-38), 21.2 (13-41), and 21 (13-39), P < 0.001, at switching week 24, 48, 96, and 144, respectively. As shown in Figure 2A, the improvement rates to ALT < 35/25 was increased from 73.6% to 84.9%; AST < 35/25, from 77.4% to 92.5%; and both ALT/AST < 35/25, from 67.9% to 83% at baseline to switching week 144, respectively. Additionally, as shown in Figure 2A, the improvement rates to ALT < 30/19 was increased from 54.7% to 66%; AST < 30/19, from 47.2% to 58.5%; and both ALT/AST < 30/19, from 39.6% to 54.7% at baseline to switching week 144, respectively.
We then assessed different variables associated with ALT/AST improvement. As shown in Table 2, univariate analysis showed that improvement of the ALT to < 30/19 at switching week 144 was significantly associated with male gender (P = 0.016), ALT < 30/19 (P = 0.024), APRI < 0.5 (P = 0.027), and ALT < 35/25 at treatment (Rx) week 24 (P = 0.008), but not with age > 50 year-old (P = 0.945), BMI > 25 kg/m2 (P = 0.888), clinical cirrhosis (P = 0.299), pre-Rx spleen size > 12 cm (P = 0.442), platelet < 120 × 109/L (P = 0.078), AST < 30/19 (P = 0.123), FIB-4 < 1.45 (P = 0.399), and AST < 35/25 at Rx week 24 (P = 0.071).
| ALT < 30/19 | APRI < 0.5 | FIB-4 < 1.45 | |||||||
| Yes | No | P value | Yes | No | P value | Yes | No | P value | |
| Gender (male) | 20/24 (83.3) | 15/29 (51.7) | 0.016 | 23/24 (95.8) | 28/29 (96.6) | 0.891 | 16/24 (66.7) | 15/29 (51.7) | 0.272 |
| Age > 50 years | 23/35 (65.7) | 12/18 (66.7) | 0.945 | 34/35 (97.1) | 17/18 (94.4) | 0.625 | 17/18 (94.4) | 14/35 (40) | 0.001 |
| BMI > 25 kg/m2 | 11/17 (64.7) | 24/36 (66.7) | 0.888 | 35/36 (97.2) | 16/17 (94.1) | 0.58 | 16/17 (94.1) | 15/36 (41.7) | 0.001 |
| Cirrhosis | 4/8 (50) | 31/45 (68.9) | 0.299 | 6/8 (75) | 45/45 (100) | 0.001 | 2/8 (25) | 29/45 (64.4) | 0.037 |
| Pre-Rx spleen size > 12 cm | 2/4 (50) | 33/48 (68.8) | 0.442 | 2/4 (50) | 48/48 (100) | 0.001 | 1/4 (25) | 30/48 (62.5) | 0.142 |
| Platelets < 120 × 109/L Rx wk 24 | 1/4 (25) | 33/46 (71.7) | 0.078 | 2/4 (50) | 46/46 (100) | 0.001 | 1/4 (25) | 29/46 (63) | 0.221 |
| APRI < 0.5 at Rx wk 24 | 33/45 (73.3) | 1/5 (20) | 0.027 | 45/45 (100) | 3/5 (60) | 0.001 | 29/45 (64.4) | 1/5 (20) | 0.106 |
| FIB-4 < 1.45 at Rx wk 24 | 22/31 (71) | 12/19 (63.1) | 0.399 | 31/31 (100) | 17/19 (89.5) | 0.156 | 27/31 (87.1) | 3/19 (15.8) | 0.001 |
| ALT < 30/19 U/L at Rx wk 24 | 28/37 (75.7) | 7/16 (43.8) | 0.024 | 36/37 (97.2) | 15/16 (93.8) | 0.534 | 20/37 (54) | 11/16 (68.8) | 0.319 |
| AST < 30/19 U/L at Rx wk 24 | 25/34 (73.5) | 10/19 (52.6) | 0.123 | 34/34 (100) | 17/19 (89.5) | 0.054 | 23/34 (67.6) | 8/19 (42.1) | 0.07 |
| ALT < 35/25 at Rx wk 24 | 33/45 (73.3) | 2/8 (25) | 0.008 | 44/45 (97.8) | 7/8 (87.5) | 0.16 | 25/45 (55.6) | 6/8 (75) | 0.304 |
| AST < 35/25 at Rx wk 24 | 34/49 (69.4) | 1/4 (25) | 0.071 | 48/49 (97.9) | 3/4 (75) | 0.021 | 28/49 (57.1) | 3/4 (75) | 0.486 |
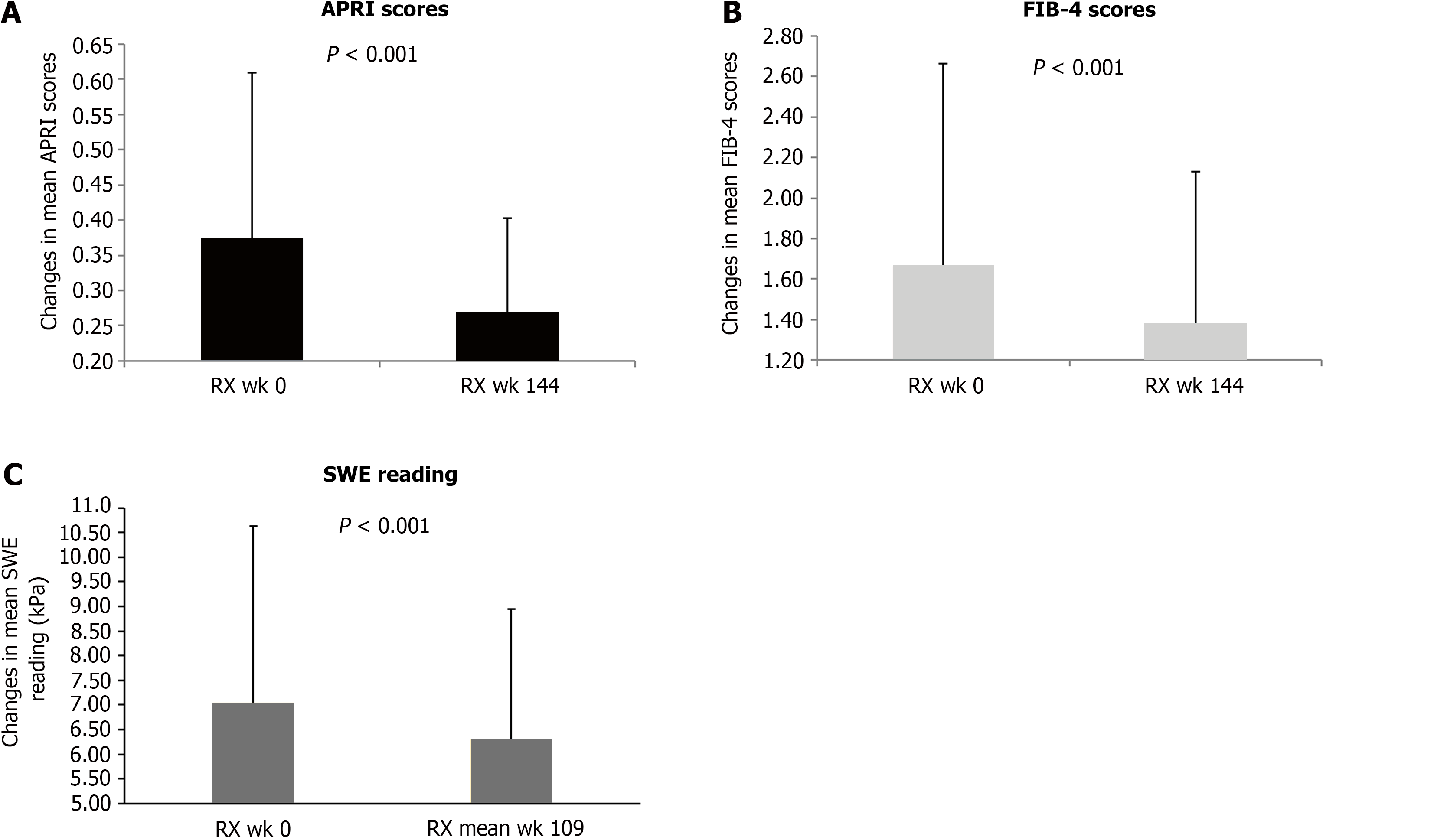
After TDF switching to TAF, the means (ranges) of APRI, and FIB-4 were all improved persistently. The mean APRI was reduced from 0.37 (0.13-0.92) to 0.27 (0.11-0.51), P < 0.001 (Figure 3A); the mean FIB-4, from 1.66 (0.49-5.33) to 1.38 (0.39-2.76), P < 0.001 (Figure 3B) at switching week 144. The rate of APRI score improvement to < 0.5 was increased from 79.2% at baseline to 96.2%, and the rate of FIB-4 score improvement to < 1.45, from 52.8% at baseline to 58.5% at switching week 144 (Figure 2B).
Having demonstrated the significant improvement of APRI and FIB-4 scores, we then assessed different variables associated with the improvement. As shown in Table 2, univariate analysis showed that APRI improvement to < 0.5 at switching week 144 was significantly associated with absence of clinical cirrhosis (P = 0.001), pre-Rx spleen size < 12 cm
We then assessed the improvement of liver fibrosis after TDF switching to TAF by SWE reading. In our study, the reference values of SWE were fibrosis stage 0-1, if the reading < 7.1 kPa; stage 2, if the reading ≥ 7.1 and < 8.7 kPa; stage 3, if the reading ≥ 8.7 and < 10.4 kPa; and stage 4, if the reading ≥ 10.4 kPa[19-21]. In 42 cases with follow-up SWE, the overall improvement of liver fibrosis by SWE reading was 95% and the mean SWE reading was improved from 7.05 to 6.30 kPa after a mean of 109 weeks (range 21–196) switching (Figure 3C). Compared to baseline SWE reading, the rate to fibrosis stage 0-1 was increased from 64% to 86%. The stage 2 fibrosis cases were reduced from 16% to 4%; stage 3 fibrosis cases, from 8% to 2%; and stage 4 fibrosis cases, from 12% to 8% at Rx week 144, respectively. Overall, 95.3% had fibrosis improvement at switching Rx week 144.
Previous studies have showed switching from TDF to TAF results in improvement of renal function[13,14]. In our study, the mean serum creatinine was stable at 0.88 (0.5-1.8) mg/dL from baseline to switching week 144, and the rate of patients with GFR > 60 mL/min was improved from 86.5% at baseline to 88.2% at switching week 144 (P < 0.001).
To our knowledge, few real-world studies have thus far reported the benefits of switching from TDF to TAF for up to week 96[17,18], but there is limited data on long-term benefit of this switching to beyond 96 weeks, including fibrosis improvement. Our study evaluated the clinical benefits after 144 weeks of TDF switching to TAF by assessing the changes in biochemical markers ALT and AST, non-invasive fibrosis score APRI and FIB-4, and LSM by SWE.
ALT normalization under antiviral treatment has been associated with a decrease in viral replication, tissue damage and necroinflammation. A significant reduction of liver enzymes was observed after switching from TDF to TAF for 6 months[22]. Our previous and other studies showed that switching from TDF to TAF resulted in high rates of ALT and AST improvement at switching week 96[17,18,23]. The present study further assessed the benefit of switching from TDF to TAF for 144 weeks. We demonstrated that the mean ALT was further reduced from 24.8 to 19.6 and 19.7 U/L, and the mean AST was also reduced from 25.7 to 21.2 and 21 U/L from baseline to week 96 and week 144 switching, respectively. Our study showed further persistent improvement rate of both ALT/AST to < 35/25 from baseline to post-switching week 144. These results were not only consistent with Toyoda et al[23] study which showed great improvement of ALT/AST < 35/25 at switching week 96, but also demonstrated further persistent improvement from week 96 to week 144 after the switching. In addition, we found the improvement rates of ALT, AST, and both ALT/AST to < 30/19 were also persistently increased from baseline to post-switching week 144. Our data demonstrated the additional benefit of ALT/AST improvement by both < 35/25 and < 30/19 criteria with overall 21% reduction in mean ALT and 18% reduction in mean AST, and improved rates of both ALT and AST normalization, after TDF to TAF switching for 144 weeks.
Our study showed that ALT and AST improvement to < 30 (U/L) for men and < 19 (U/L) for women of at switching week 144 was significantly associated with male gender, ALT < 30/19 and < 35/25, APRI < 0.5 at 24 week switching, but not age, BMI, cirrhosis, pre-Rx spleen size > 12 cm, platelet < 120 × 109/L, AST < 30/19, and < 35/25 at 24 week switching. Our real world data confirmed results from the published studies which show TAF results in continued improvement not only viral suppression, but also ALT and AST normalization[14,16], a unique benefit of long-term TAF treatment.
Besides ALT/AST normalization, another goal of HBV treatment is to slow down the progression of liver fibrosis and even to achieve resolution of liver fibrosis[24,25]. Our previous study showed that HCV direct acting antiviral treatment resulted in highly durable improvement rates of ALT and AST, APRI and FIB-4 scores in HCV patients[26]. Liu et al[27] reported a significant decrease of both APRI and FIB-4 after 5 years of treatment with ETV in HBeAg-negative CHB patients. There were limited studies on the effect of switching TDF to TAF on the improvement of APRI and FIB-4 scores. Our study demonstrated the mean APRI was significantly reduced from 0.37 to 0.27 and the improvement rate of APRI to < 0.5 was increased from 79.2% to 96.2%, equal to 27% reduction in mean APRI and 1.2-times improvement rate of APRI to < 0.5. Likewise, we also found that the mean FIB-4 score was significantly reduced from 1.66 to 1.38 and the improvement rate of FIB-4 to < 1.45 was increased from 52.8% to 58.5%, equal to 16.9% reduction in mean FIB-4, and 1.1-times improvement rate of FIB-4 to < 1.45 after the switching to TAF for 144 weeks. Univariate analysis showed that the improvement of APRI to < 0.5 and FIB-4 to < 1.45 was both significantly associated with absence of clinical cirrhosis. Our study demonstrated switching from TDF to TAF for 144 weeks resulted in hepatic fibrosis regression in CHB patients with persistent improvement by APRI and FIB-4 scores, another important benefit of such treatment switch. Additionally, our data provided supportive evidence of clinical application of both APRI and FIB-4 scores in assessing the effectiveness of HBV antiviral treatment.
LSM, including TE and SWE, has become a standard clinical practice for liver fibrosis assessment. To further determine the long-term clinical benefit of TDF switching to TAF in liver fibrosis improvement as indicated by APRI and FIB-4 scores, we assessed if 144 weeks switching could also impact SWE reading. We found that after switching from TDF to TAF for a mean of 109 weeks, the mean SWE reading was improved from 7.05 to 6.30 kPa, equal to 10.6% score improvement, and the rate of fibrosis stage 0-1 was increased from 64% to 86%, equal to 34.4% improvement. Together with APRI and FIB-4 scores improvement, the above SWE results further confirmed clinical benefits and superiority in liver fibrosis improvement after switching from TDF to TAF for 144 weeks. These benefits might be due to TAF has greater stability in plasma than TDF that allows more efficient uptake by hepatocytes and higher circulating intra-hepatocytic concentration of active metabolites allowing more prominent liver targeting ability[5,6]. In addition, TAF enters hepatocytes by passive diffusion and efficiently taken up by hepatocytes and then hydrolyze to form tenofovir which undergoes phosphorylation to active metabolite, tenofovir diphosphate, provides more persistent intracellular level of tenofovir diphosphate, a potent inhibitor of HBV replication[28,29] and results in more reduced hepatic inflammation and liver fibrosis.
In the present study, our results also showed the renal benefits after switching from TDF to TAF. After 144 weeks of switching, the renal function was stable and even further improved as indicated by stable mean serum creatinine and increased rate of GFR > 60 mL/min from 86.5% at baseline to 88.2%. Our data was consistent with previous studies on the renal benefits of TAF switching[13,14].
Some limitations should be noted in our study. First, this was a single-center retrospective study with small sample size, especially the patients with advanced liver fibrosis. Secondly, we did not have follow-up liver biopsy data to reference the non-invasive fibrosis test results. However, it is the first study to further assess the long-term benefits of switching TDF to TAF on biochemical changes of ALT, AST, APRI, FIB-4 scores and SWE reading. Future studies with larger sample size are warranted to confirm our findings.
In summary, the present study demonstrated that switching from TDF to TAF for 3 years results in not only persistent ALT and AST improvement, but also hepatic fibrosis improvement assessed by APRI and FIB-4 scores, as well as SWE reading, the important clinical benefits of long-term HBV antiviral treatment with TAF.
| 1. | World Health Organization. Hepatitis B. [accessed 2022 Nov 2]. Available from: https://www.who.int/news-room/fact-sheetsdetail/hepatitis-b. |
| 2. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2827] [Article Influence: 403.9] [Reference Citation Analysis (0)] |
| 3. | Papatheodoridis GV, Idilman R, Dalekos GN, Buti M, Chi H, van Boemmel F, Calleja JL, Sypsa V, Goulis J, Manolakopoulos S, Loglio A, Siakavellas S, Keskın O, Gatselis N, Hansen BE, Lehretz M, de la Revilla J, Savvidou S, Kourikou A, Vlachogiannakos I, Galanis K, Yurdaydin C, Berg T, Colombo M, Esteban R, Janssen HLA, Lampertico P. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology. 2017;66:1444-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 226] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 4. | Singal AK, Fontana RJ. Meta-analysis: oral anti-viral agents in adults with decompensated hepatitis B virus cirrhosis. Aliment Pharmacol Ther. 2012;35:674-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Murakami E, Wang T, Park Y, Hao J, Lepist EI, Babusis D, Ray AS. Implications of efficient hepatic delivery by tenofovir alafenamide (GS-7340) for hepatitis B virus therapy. Antimicrob Agents Chemother. 2015;59:3563-3569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 6. | Agarwal K, Fung SK, Nguyen TT, Cheng W, Sicard E, Ryder SD, Flaherty JF, Lawson E, Zhao S, Subramanian GM, McHutchison JG, Gane EJ, Foster GR. Twenty-eight day safety, antiviral activity, and pharmacokinetics of tenofovir alafenamide for treatment of chronic hepatitis B infection. J Hepatol. 2015;62:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 7. | Chapman T, Dubinsky T, Barr RG. Ultrasound Elastography of the Liver: What the Clinician Needs to Know. J Ultrasound Med. 2017;36:1293-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. 2008;28:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Li J, Gordon SC, Rupp LB, Zhang T, Boscarino JA, Vijayadeva V, Schmidt MA, Lu M; Chronic Hepatitis Cohort Study (CHeCS) Investigators. The validity of serum markers for fibrosis staging in chronic hepatitis B and C. J Viral Hepat. 2014;21:930-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Teshale E, Lu M, Rupp LB, Holmberg SD, Moorman AC, Spradling P, Vijayadeva V, Boscarino JA, Schmidt MA, Gordon SC; CHeCS Investigators. APRI and FIB-4 are good predictors of the stage of liver fibrosis in chronic hepatitis B: the Chronic Hepatitis Cohort Study (CHeCS). J Viral Hepat. 2014;21:917-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 1933] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 12. | Herrmann E, de Lédinghen V, Cassinotto C, Chu WC, Leung VY, Ferraioli G, Filice C, Castera L, Vilgrain V, Ronot M, Dumortier J, Guibal A, Pol S, Trebicka J, Jansen C, Strassburg C, Zheng R, Zheng J, Francque S, Vanwolleghem T, Vonghia L, Manesis EK, Zoumpoulis P, Sporea I, Thiele M, Krag A, Cohen-Bacrie C, Criton A, Gay J, Deffieux T, Friedrich-Rust M. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology. 2018;67:260-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 341] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 13. | Fong TL, Lee BT, Tien A, Chang M, Lim C, Ahn A, Bae HS. Improvement of bone mineral density and markers of proximal renal tubular function in chronic hepatitis B patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. J Viral Hepat. 2019;26:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Farag MS, Fung S, Tam E, Doucette K, Wong A, Ramji A, Conway B, Cooper C, Tsoi K, Wong P, Sebastiani G, Brahmania M, Haylock-Jacobs S, Coffin CS, Hansen BE, Janssen HLA. Effectiveness and Renal Safety of Tenofovir Alafenamide Fumarate among Chronic Hepatitis B Patients: Real-World Study. J Viral Hepat. 2021;28:942-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Lim YS, Seto WK, Kurosaki M, Fung S, Kao JH, Hou J, Gordon SC, Flaherty JF, Yee LJ, Zhao Y, Agarwal K, Lampertico P. Review article: switching patients with chronic hepatitis B to tenofovir alafenamide-a review of current data. Aliment Pharmacol Ther. 2022;55:921-943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Su PY, Su WW, Hsu YC, Huang SP, Yen HH. Real-world experience of switching from tenofovir disoproxil fumarate to tenofovir alafenamide in patients with chronic hepatitis B: a retrospective study. PeerJ. 2021;9:e12527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Huynh T, Hu KQ. Tenofovir disoproxil fumarate switching to tenofovir alafenamide for 2 years resulted in both ALT and AST, and APRI score improvement in patients with chronic hepatitis B. Hepatology. 2020;72:497A. |
| 18. | Liang LY, Yip TC, Lai JC, Lam AS, Tse YK, Hui VW, Chan HL, Wong VW, Wong GL. Tenofovir alafenamide is associated with improved alanine aminotransferase and renal safety compared to tenofovir disoproxil fumarate. J Med Virol. 2022;94:4440-4448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Thiele M, Detlefsen S, Sevelsted Møller L, Madsen BS, Fuglsang Hansen J, Fialla AD, Trebicka J, Krag A. Transient and 2-Dimensional Shear-Wave Elastography Provide Comparable Assessment of Alcoholic Liver Fibrosis and Cirrhosis. Gastroenterology. 2016;150:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 20. | Friedrich-Rust M, Lupsor M, de Knegt R, Dries V, Buggisch P, Gebel M, Maier B, Herrmann E, Sagir A, Zachoval R, Shi Y, Schneider MD, Badea R, Rifai K, Poynard T, Zeuzem S, Sarrazin C. Point Shear Wave Elastography by Acoustic Radiation Force Impulse Quantification in Comparison to Transient Elastography for the Noninvasive Assessment of Liver Fibrosis in Chronic Hepatitis C: A Prospective International Multicenter Study. Ultraschall Med. 2015;36:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Ferraioli G, Tinelli C, Lissandrin R, Zicchetti M, Dal Bello B, Filice G, Filice C. Point shear wave elastography method for assessing liver stiffness. World J Gastroenterol. 2014;20:4787-4796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 116] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Squillace N, Ricci E, Menzaghi B, De Socio GV, Passerini S, Martinelli C, Mameli MS, Maggi P, Falasca K, Cordier L, Celesia BM, Salomoni E, Di Biagio A, Pellicanò GF, Bonfanti P; CISAI Study Group. The Effect of Switching from Tenofovir Disoproxil Fumarate (TDF) to Tenofovir Alafenamide (TAF) on Liver Enzymes, Glucose, and Lipid Profile. Drug Des Devel Ther. 2020;14:5515-5520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Toyoda H, Leong J, Landis C, Atsukawa M, Watanabe T, Huang DQ, Liu J, Quek SXZ, Ishikawa T, Arai T, Yokohama K, Chuma M, Takaguchi K, Uojima H, Senoo T, Dang H, Maeda M, Hoang J, Le RH, Yasuda S, Thin KN, Tran S, Chien N, Henry L, Asai A, Fukunishi S, Cheung R, Lim SG, Trinh HN, Nguyen MH. Treatment and Renal Outcomes Up to 96 Weeks After Tenofovir Alafenamide Switch From Tenofovir Disoproxil Fumarate in Routine Practice. Hepatology. 2021;74:656-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Udompap P, Sukonrut K, Suvannarerg V, Pongpaibul A, Charatcharoenwitthaya P. Prospective comparison of transient elastography, point shear wave elastography, APRI and FIB-4 for staging liver fibrosis in chronic viral hepatitis. J Viral Hepat. 2020;27:437-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Roade L, Riveiro-Barciela M, Esteban R, Buti M. Long-term efficacy and safety of nucleos(t)ides analogues in patients with chronic hepatitis B. Ther Adv Infect Dis. 2021;8:2049936120985954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Huynh T, Ma S, Hu KQ. HCV direct acting antiviral treatment leads to highly durable rates of ALT and AST lower than 30/19 criteria and improved APRI and FIB-4 scores. Hepatol Commun. 2022;6:3496-3504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 27. | Liu R, Guo J, Lu Y, Zhang L, Shen G, Wu S, Chang M, Hu L, Hao H, Li M, Xie Y. Changes in APRI and FIB-4 in HBeAg-negative treatment-naive chronic hepatitis B patients with significant liver histological lesions receiving 5-year entecavir therapy. Clin Exp Med. 2019;19:309-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Ogawa E, Furusyo N, Nguyen MH. Tenofovir alafenamide in the treatment of chronic hepatitis B: design, development, and place in therapy. Drug Des Devel Ther. 2017;11:3197-3204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Byrne R, Carey I, Agarwal K. Tenofovir alafenamide in the treatment of chronic hepatitis B virus infection: rationale and clinical trial evidence. Therap Adv Gastroenterol. 2018;11:1756284818786108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |