Published online Jun 27, 2024. doi: 10.4254/wjh.v16.i6.900
Revised: May 5, 2024
Accepted: June 3, 2024
Published online: June 27, 2024
Processing time: 87 Days and 22.2 Hours
Achievement of a ‘clinical cure’ in chronic hepatitis B (CHB) implies sustained virological suppression and immunological control over the infection, which is the ideal treatment goal according to domestic and international CHB management guidelines. Clinical practice has shown encouraging results for specific patient cohorts using tailored treatment regimens. These regimens incorporate either nucleos(t)ide analogs, immunomodulatory agents such as pegylated interferon α, or a strategic combination of both, sequentially or concurrently administered. Despite these advancements in the clinical handling of hepatitis B, achieving a clinical cure remains elusive for a considerable subset of patients due to the number of challenges that preclude the realization of optimal treatment outcomes. These include, but are not limited to, the emergence of antiviral resistance, incomplete immune recovery, and the persistence of covalently closed circular DNA. Moreover, the variance in response to interferon therapy and the lack of definitive biomarkers for treatment cessation also contribute to the complexity of achieving a clinical cure. This article briefly overviews the current research progress and existing issues in pursuing a clinical cure for hepatitis B.
Core Tip: Clinical cure has become an ideal goal pursued by chronic hepatitis B (CHB) patients. To enable more patients to achieve this goal, immunotherapy targets, the development of drugs targeting the viral life cycle, gene editing technologies, and the application of other methods have promoted the achievement of a clinical cure for CHB; however, this topic warrants continuous exploration.
- Citation: Li YP, Liu CR, He L, Dang SS. Hepatitis B cure: Current situation and prospects. World J Hepatol 2024; 16(6): 900-911
- URL: https://www.wjgnet.com/1948-5182/full/v16/i6/900.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i6.900
Chronic hepatitis B (CHB) is an important disease posing a severe threat to human health. According to World Health Organization estimates, nearly 1/3 of the world’s people have been infected with hepatitis B virus (HBV). There are more than 86 million HBV-infected people in China, which ranks first in the world in the number of infected people[1,2]. In recent years, approximately 50%-80% of hepatocellular carcinoma (HCC) cases worldwide have been caused by chronic HBV infection, and more than half of the new HCC cases and deaths worldwide occurred in China[3], specifically more than 84% caused by HBV infection[4]. Moreover, the risk of HCC in HBV-infected patients is 10-30 times that of the uninfected population[5]. Therefore, treating and managing CHB, delaying the progression of liver cirrhosis, and reducing the incidence of HCC are the current clinical priority issues to be addressed[6,7]. Since 2015, the Chinese guidelines for the prevention and treatment of CHB have proposed the idea of pursuing a clinical cure for CHB, and many scholars at home and abroad have started active exploration. Reports on research projects related to clinical cures were readily available and showed promising results. The clinical cure for hepatitis B has also been constantly updated from the 2015 edition to the 2022 edition in China. The clinical cure for CHB has substantially advanced from theoretical to practical. While these results are very encouraging, many patients still do not respond well to treatments, and many difficulties must be overcome to achieve a good treatment effect.
The clinical cure for hepatitis B must start with the antiviral treatment of hepatitis B. As is well known, the antiviral treatment for hepatitis B mainly includes two types of drugs: Nucleos(t)ide analogs (NAs) and interferons. The advent of NAs has brought remarkable changes to the anti-viral treatment of hepatitis B. The use of nucleoside drugs has marked a new approach to the antiviral treatment of hepatitis B, and the treatment of CHB has since entered the era of antiviral treatment. After nearly 30 years of development, current NAs exhibit various advantages, including well-established therapeutic efficacy, low resistance, minimal long-term adverse reactions, and affordable pricing. Most CHB patients can achieve long-term virological suppression during the medication period, thus improving long-term outcomes[8,9]. However, upon summarizing a substantial amount of clinical data on NAs, it was found that their clearance effect on hepatitis B surface antigen (HBsAg) is extremely limited, with an annual clearance rate of < 1%[10,11]. Nevertheless, even with such a low clearance rate, it ignites hope among scholars in the field of liver disease for the clinical cure of hepatitis B. In fact, achieving clearance of covalently closed circular DNA (cccDNA) solely with NAs would require at least 30 to 40 years[12], indicating that patients would need to take NAs for their entire lifetime.
In 2013, Block et al[13] initiated a discussion on the concept of “cure” for CHB, finding that even individuals who spontaneously cleared acute HBV infection were still at a higher risk of death from HCC compared to those who have never been infected with HBV[13]. The term “cure” used alone to describe the treatment outcomes of CHB is limited. Therefore, these scholars also pioneered the “functional cure (apparent virological cure)” concept, which entails clearance of HBsAg and sustained suppression of HBV DNA. Consequently, the types of CHB cures are categorized into complete cure, also known as virological cure, and functional cure, also known as clinical cure or immunological cure[14]. The former type refers to serum HBsAg clearance, intrahepatic and serum HBV DNA clearance (including intrahepatic cccDNA and integrated HBV DNA), and persistent positive serum anti-HBc, with or without developing anti-HBs. The cccDNA structure is stable and persistent, and the HBV genome can be integrated into host DNA. Currently, there is a lack of specific targeted drugs for cccDNA and integrated HBV DNA; therefore, it is challenging to achieve a complete cure. In contrast, a clinical cure is easier to achieve. First-line antiviral drugs such as NAs and interferon both have the potential to achieve clinical cure; thus, clinical cure has gradually become the ideal treatment goal for patients with CHB.
Interferon therapy has been used in the treatment of CHB for over 50 years, during which a substantial body of clinical experience and evidence-based medical data have been accumulated. The initial therapeutic goal of standard interferon-alpha or pegylated interferon alpha (Peg-IFNα) treatment was to achieve a sustained virological response. The primary treatment objectives for HBeAg-positive patients with CHB are HBeAg clearance and serological conversion; for HBeAg-negative patients, the key goal is sustained HBV DNA suppression. The treatment duration for Peg-IFNα, determined by these primary objectives, is 48 wk, followed by a 24-wk observation period post-treatment. In 2016, Marcellin et al[15] reported for the first time research outcomes where the primary goal was HBsAg clearance or serological conversion, showing that 48 wk of Peg-IFNα combined with tenofovir disoproxil fumarate (TDF) treatment could achieve an HBsAg clearance rate of 9.1%[15]. This highlighted the feasibility of aiming for a clinical cure as a treatment goal, garnering significant attention and interest among clinicians. Subsequently, studies with the primary goal of achieving HBsAg clearance or associated serological conversion have gradually increased. An increasing number of studies have focused on Peg-IFNα treatment strategies to enhance clinical cure rates, meeting the crucial requirements of current clinical practice.
The sustained suppression of HBV DNA by NAs has laid the foundation for the clinical cure of CHB. The prudent use of Peg-IFNα on top of NAs therapy can significantly enhance the clinical cure rate of CHB. Interferons exert dual immunomodulatory and antiviral effects through mechanisms such as enhancing immune cell functions, promoting cytokine expression, inducing the production of interferon-stimulated genes (ISGs), and encoding various antiviral proteins via the interferon signaling pathway, which impact crucial biological processes like HBV replication and transcription. Compared to NAs, the course of Peg-IFN therapy is limited rather than long-term, with higher serological responses and more sustained effects. However, Peg-IFN is effective only for a subset of patients and lacks the robust tolerability seen with NAs.
Extensive clinical research has confirmed that combination therapy with NAs and Peg-IFN represents the most promising treatment strategy for achieving clinical cure by integrating potent viral suppression with the host immune response restoration. In clinical practice, the combined NA and Peg-IFN treatment regimens have accumulated numerous successful cases of HBsAg seroconversion and safe discontinuation of NAs, supported by evidence-based medical data. Currently, combined treatment approaches primarily include initial combination therapy and sequential combination therapy, the latter encompassing ‘switch’ strategies (from NAs to Peg-IFN) and ‘add-on’ strategies (NAs in addition to Peg-IFN). In addition, prolonging treatment duration and intermittent therapy are popular research directions for achieving clinical cure in current CHB management.
Combination therapy capitalizes on the different antiviral mechanisms of NAs and Peg-IFNα. Rational concurrent use can restore the host’s immune response against the potent viral suppression, yielding synergistic and complementary effects and making it the most viable available treatment strategy for achieving ideal therapeutic goals[14]. Moreover, numerous clinical studies have confirmed that sequential or combined treatment regimens with NAs and Peg-IFNα achieve favorable outcomes in specific subpopulations that benefit from interferon therapy. Prospective studies of Peg-IFNα treatment in individuals with CHB have shown that the HBsAg clearance rate in patients receiving combined Peg-IFNα and TDF therapy surpasses that in patients treated with either agent alone[16,17].
Sequential therapy: In their study, Huang et al[18] included 43 patients undergoing sequential peg-IFN therapy following NAs. Their results indicated that sequential peg-IFNα treatment for 48 wk achieved a 32.6% HBsAg clearance rate and 27.9% serological conversion rate[18]. In contrast, no cases of HBsAg loss were observed in the NAs monotherapy group. Japanese researchers conducted a study involving 23 patients treated with NAs for over a year who achieved HBeAg clearance and had HBsAg levels < 1500 IU/mL. These patients were additionally treated with peg-IFNα for 48 wk, followed by a follow-up. At 72 wk, a significant increase in efficacy was observed, with a 37.4% HBsAg clearance rate and 29.7% serological conversion rate compared to the group treated with NAs alone[19]. Many other studies, including those conducted by our team, have similarly confirmed that adding peg-IFNα in NAs-treated patients with CHB and HBsAg levels < 1500 IU/mL can significantly increase the HBsAg clearance rate[20]. Overall, for NAs-experienced patients with low HBsAg levels and sustained HBV suppression, sequential or combination therapy of NAs with Peg-IFNα can significantly increase the rates of HBsAg seroclearance and seroconversion (Table 1)[21-35].
| Treatment option | Ref. | Duration of interferon use | HBsAg negative conversion rate (%) |
| “NA plus Peg-IFNα” strategy | Marcellin et al[21], 2016 | 48 wk | 9.1 |
| Hagiwara et al[22], 2018 | 48 wk | 10.4 | |
| Hu et al[23], 2021 | 48 wk | 11.5 | |
| “Switching NA to Peg-IFNα” strategy | Han et al[24], 2016 | 48 wk | 8.5 |
| Wu et al[25], 2019 | 48 wk | 9.3 | |
| Hu et al[26], 2018 | 48 wk | 14.4 | |
| “NA plus Peg-IFNα” strategy | Bourlière et al[27], 2017 | 48 wk | 7.8 |
| Lim et al[28], 2019 | 48 wk | 9.0 | |
| Li et al[29], 2015 | 48 wk | 18.5 | |
| Wu et al[20], 2020 | 72 wk | 37.4 | |
| “Peg-IFNα monotherapy” strategy (IHCs) | Cao et al[30], 2017 | 48 wk | 29.8 |
| Wu et al[31], 2021 | 72 wk | 47.9 | |
| Chen et al[32], 2021 | 48 wk | 55.6 | |
| “Extended treatment” strategy | Hu et al[26], 2018 | 96 wk | 20.7 |
| Li et al[33], 2011 | 81.32 ± 39.36 wk | 21.7 | |
| Yan et al[34], 2018 | 96 wk | 29.0 | |
| “Intermittent treatment” strategy | Li et al[35], 2022 | / | 19.4 |
Extended treatment: In a multicenter clinical study with HBsAg clearance as the primary treatment goal reported by Hu et al[26], patients who had been on NAs treatment for over 2 years and had undergone HBeAg seroconversion were switched to Peg-IFNα therapy for either 48 or 96 wk. The results revealed that the HBsAg clearance rates at 48 wk and 96 wk were 14.4% (22/153) and 20.7% (31/150), respectively. For patients with baseline HBsAg < 1500 IU/mL and HBsAg < 200 IU/mL at 24 wk of treatment, the HBsAg clearance rates at 48 wk and 96 wk were 51.4% and 58.7%, respectively. These results proved that extending the Peg-IFN treatment duration in the NAs-treated population can increase the clinical cure rate.
Intermittent treatment: Extending the course of Peg-IFNα may enhance clinical cure rates; however, this strategy should not be adopted indefinitely. Instead, an extended overall treatment duration with phased intermittent therapy could be more advantageous. Such staged intermittent treatment strategy for long-term antiviral therapy aims to ultimately achieve clinical cure objectives, involving “treat-interrupt-retreat” cycles of Peg-IFNα on top of ongoing NA therapy, which has been clinically proven effective. Long-term treatment with Peg-IFNα may lead to CD8+ T-cell exhaustion, failing to sustain an immunological response. Therefore, pausing Peg-IFNα treatment allows for the recovery of host immune function while maintaining NA therapy, which promotes the rebuilding of specific immunity under sustained HBV DNA control, creating an opportunity for subsequent Peg-IFNα re-administration[36,37].
Therefore, these results are very promising, and they anticipate the definite attainment of a clinical cure for CHB. The table below lists some representative clinical cure studies of different Peg-IFNα-based treatment strategies in recent years (Table 1).
The Asia-Pacific region exhibits a high prevalence of HBV infection, prompting numerous clinical investigations centered on interferon-based therapies within this geographic area. Consequently, an ongoing international debate persists regarding the therapeutic efficacy of interferon in patients with CHB. Notably, various nations in Europe and America have also conducted comprehensive clinical assessments to ascertain the efficacy of interferon-based treatments for CHB patients within their respective populations.
The Hepatitis B Research Network has enrolled adults and children suffering from CHB in multiple medical centers in the United States and Canada and published a series of studies. For instance, investigations led by Perrillo et al[38] examined CHB patients in the immune-tolerant phase positive for the e antigen. Notably, while the proportion of adults and children experiencing alanine aminotransferase (ALT) elevation was comparable following the addition of interferon to NAs, a significantly higher proportion of children achieved a > 1 Log10 IU/mL decline in HBsAg (39% vs 22%, P < 0.05)[38]. Additionally, Terrault et al[39] conducted a randomized (1:1) trial involving the administration of TDF over 192 wk, with or without Peg-IFNα administered during the initial 24 wk. Subsequently, TDF was withdrawn at week 192, followed by a 48-wk off-treatment follow-up period until week 240. Noteworthy findings revealed a higher HBsAg clearance rate in the combination therapy group during the treatment phase. However, by week 240, no statistically significant disparity in HBsAg clearance rates was observed between the two groups, underscoring the nuanced implications of combining Peg-IFNα with TDF therapy and subsequent TDF discontinuation[39].
In conclusion, these studies underscore the complexity and variability of therapeutic responses to interferon-based regimens across diverse patient populations, necessitating further exploration and refinement of treatment strategies in the management of CHB.
Clinical cure problem for dominant population and non-dominant population of interferon treatment: Currently, the overall clinical cure rate achieved by Peg-IFNα in CHB patients is only close to 10%, much higher than the 1% with NAs; however, these results are still unsatisfactory. Studies have found that in some populations, after treatment with Peg-IFNα, the clinical cure rate of CHB patients was significantly higher than that of other CHB patients. The advantageous population for Peg-IFNα treatment mainly refers to patients treated with NAs for > 1 year, with low levels of HBsAg (especially ≤ 1500 IU/mL), negative for HBeAg, and with HBV DNA < 2000 IU/mL. Current studies in China, such as the “Mount Everest Engineering Project” and “Hepatitis B Uncapping Project”, have all been focusing on the Peg-IFNα dominant population, and the clinical cure rate can reach > 30%[20,40]. For patients with inactive HBsAg carrier status (IHC), the Asia-Pacific, European, and American guidelines unanimously advise against treatment[41-43]. In recent years, studies have found that the domestic IHC population has exceeded 30 million, and with age, the IHC population is at higher risk of HBV reactivation, liver cirrhosis, or even HCC. A study from Taiwan that enrolled 1965 IHC patients and followed them up for an average of 11.5 years showed that 16% had HBV reactivation, and for patients with HBV reactivation, the cumulative incidence of liver cirrhosis during the 20 years reached 46%, and the 25-year cumulative incidence of liver cirrhosis reached 15% in all subjects[44]. In addition, previous studies have found that 14%-24% of IHC patients may develop HBeAg-negative CHB, and nearly 20% may reverse to HBeAg-positive CHB; 25%-62% of IHC patients tend to have moderate to severe liver fibrosis, and 3.3%-62% moderate to severe liver inflammation, while approximately 10% may even have liver cirrhosis[45]. Ethnic differences may be the main reason for the widely different study results between China, Europe, and the United States. Consequently, significant changes have occurred in treating IHC patients in recent years. The definition of the IHC population fits with the category of the Peg-IFNα dominant population, so numerous studies have used Peg-IFNα for IHC patients and achieved significant results. Among 142 Peg-IFNα-naive CHB patients with low HBsAg quantification enrolled in our center, the HBsAg clearance rate reached 47.9% after 72 wk of Peg-IFNα use[31]. A meta-analysis revealed that the overall HBsAg clearance rate at 48 wk in the IHC population could reach an encouraging 47%[46], also showing that selecting appropriate CHB patients for Peg-IFNα-containing treatment can maximize the advantage of interferon.
Another issue to be addressed is the clinical pathway for individuals who are not suitable candidates for interferon therapy to achieve clinical cure. Currently, many researchers are trying to broaden the study scope of the clinically cured population in pursuit of the maximum clinical cure for CHB patients. Therefore, many researchers have also begun studying nondominant populations’ clinical cure. Clinical cure projects such as the “Voyage Project,” “Leadership Project,” and “Oasis Project” are also underway among non-ideal populations. For instance, in the “Voyage Project,” > 40% of patients classified as immunotolerant achieved a reduction in HBsAg to < 3000 IU/mL following Peg-IFNα therapy, which suggests that individuals within the interferon non-ideal population may gradually transition to ideal candidates through proactive treatment, thereby presenting an opportunity to attain the higher goal of clinical cure[47]. In addition, although the clinical cure rate of HBeAg-positive treatment-naive CHB patients using PEG-IFNα-based treatment is lower than that of patients with a predominance of NA treatment, the result is significantly better than that of NA monotherapy[48]. Whether there are better treatment strategies to improve the cure rate for these non-ideal populations in clinical practice, such as setting the goal but the course of treatment and the intermittent treatment strategy, is also being actively explored.
Clinical cure of hepatitis B in children: Studies of clinical cure for children CHB patients have also made a great break
Clinical cure of hepatitis B in parturients: HBV-infected parturients are often in a particular immune activation state after delivery. Recently, there has been an increasing number of clinical studies on this group of patients. For example, a domestic study that included HBeAg-positive parturients who received antiviral treatment in the third trimester according to their wishes and for whom the treatment was discontinued immediately after delivery showed that, whether antiviral or not, the ALT level was significantly increased in the postpartum period of pregnancy, and 40% of the parturients had acute inflammation of the liver[55]. Another study that enrolled 45 parturients with CHB showed that 24.4% had an acute increase in ALT and 11.8% had HBeAg clearance; HBeAg-positive parturients may experience immune activation after delivery[56], which may explain the postpartum increase in ALT and the development of hepatitis in the puerpera. With the delivery of the fetus and the release of maternal immunosuppression, CD8+ T cells, their memory T-cell subsets, and effector T-cell subsets in patients with hepatitis activation and the increased expression levels of perforin and granzyme B may have an important role in breaking immune tolerance and causing hepatitis[57]. Thus, the postpartum stage may be a good opportunity to achieve a clinical cure for CHB mothers, and combined with Peg-IFNα treatment, it is expected to further improve the clinical cure rate.
Urgency to explore clinical cure biomarkers: With the expansion of various multicenter clinical studies, the use of Peg-IFNα in the CHB patient population has become more widespread. However, due to the existence of some adverse reactions associated with Peg-IFNα, it is urgent to identify specific indicators to predict the efficacy of Peg-IFNα therapy. On the one hand, this approach can instill more confidence in CHB patients and help identify individuals suitable for Peg-IFNα treatment. On the other hand, it can facilitate timely treatment discontinuation in cases of poor efficacy, thereby maximizing patient benefit. Although HBV DNA can be used as an evaluation indicator for antiviral treatment, it cannot predict HBsAg clearance well. Three early predictive indicators have been identified: Low baseline HBsAg levels, rapid decline in HBsAg at 12/24 wk of treatment, and more than a twofold increase in ALT at 12 wk of treatment. In recent years, various novel serum markers have been used to predict the efficacy of CHB treatment. HBV RNA is transcribed from cccDNA and, to some extent, can reflect the transcriptional activity level of cccDNA within the liver[58]. It has been found that HBV RNA levels were lower in CHB patients who achieved HBeAg clearance, and HBV RNA levels at 12/24 wk could effectively predict HBeAg clearance. A strong positive correlation between HBV RNA and HBeAg has also been established[59]. In current clinical studies on the treatment of CHB, HBV RNA has been identified as a predictive factor for the clinical response to Peg-IFNα treatment at 12 wk[60]. A small number of patients who experience HBsAg clearance may encounter virological relapse after treatment discontinuation, which may be due to the continued production of HBsAg from residual cccDNA. Therefore, HBV RNA might also serve as a biomarker for predicting the timing of discontinuation of Peg-IFNα treatment following therapy.
The drawbacks of Peg-IFN include the necessity for rigorous patient selection based on indications, administration via subcutaneous injection, and the potential for numerous adverse reactions. Due to the risk of liver decompensation, Peg-IFN is contraindicated in patients with decompensated liver cirrhosis, pregnant women, and those with polyethylene glycol interferon intolerance[61]. Some studies suggest that extending the treatment duration beyond 48 wk is beneficial, particularly in HBeAg-negative patients, but this is challenging in practice due to poor patient tolerability[62,63].
Furthermore, long-term use of interferon may lead to many psychiatric issues such as depression, irritability, anxiety, agitation, loss of appetite, fatigue, sleep disturbances, and impaired cognitive function, significantly impacting patients’ quality of life and work capacity[64]. Therefore, a thorough assessment of patients’ mental status is necessary before initiating interferon therapy, further narrowing the target patient population. Interferon-associated depression warrants particular attention in clinical practice, emphasizing the necessity of monitoring the mental health status of patients undergoing interferon therapy.
As research progresses, achieving clinical cure through Peg-IFNα inhibition and clearance of HBV has become closely associated with the antiviral immune response of the host. The indicators with an ability to predict cure can be summarized into three aspects: Virological indicators, biochemical indicators, and immunological indicators. Currently, virological indicators include HBsAg levels and declines, HBeAg, HBV DNA, HBcrAg levels and declines, and baseline HBV RNA levels. Biochemical indicators mainly include elevated ALT levels. Immunological indicators encompass natural killer cells, dendritic cells (DCs), B cells, follicular helper T cells, and T regulatory cells. CHB results from complex interactions between host immunity and viral replication involving innate and adaptive immune cells. The mechanisms involved are intricate. Peg-IFNα has been demonstrated to activate both host innate and adaptive immune responses, which is beneficial for increasing the clearance rate of HBsAg in patients. Scholars have achieved remarkable progress in improving immune responses by combining Peg-IFNα with other immune strategies. Studies on mouse liver cancer models have found that combining Peg-IFNα with PD-1 antibodies can restore or even enhance the cytotoxic effects of CD8+ T cells, demonstrating synergistic anti-tumor effects[65]. Evidence confirmed that PD-1+CXCR5+CD8+ T cells possess the functions of traditional CD8+ T cells and can activate B cells. PD-1 does not exhibit an exhausted phenotype in CXCR5+CD8+ T cells. Furthermore, CXCR5+CD8+ T cells have a particular role in controlling viremia in CHB patients[66]. The above-mentioned immune cell subsets may provide potential immunotherapeutic targets for HBV cure. It is believed that with continuous exploration of the immune mechanisms and the discovery of new immune targets, strategies for CHB cure will become more diverse. In conclusion, numerous factors in clinical settings can influence the response to Peg-IFNα. Identifying the factors influencing the response to Peg-IFNα, especially the impact of host immune mechanisms, will be significant for the clinical application of Peg-IFNα.
In 2019, Gane et al[67] first attempted to use the PD-1 inhibitor nivolumab in HBeAg-negative CHB patients. Their results showed that at 12 wk of treatment, patients receiving nivolumab at 0.3 mg/kg, regardless of whether HBV therapeutic vaccine was added, had an average decrease in HBsAg of 0.3 Log10 IU/mL compared to baseline. At 24 wk of treatment, patients receiving nivolumab at 0.3 mg/kg had an average decrease in HBsAg of 0.48 Log10 IU/mL compared to baseline[67]. This pilot study provided the first evidence of the efficacy and safety of immune checkpoint inhibitors (ICIs) in non-tumor chronic HBV-infected individuals. It also demonstrated that ICIs can restore HBV-specific immune responses in patients with chronic HBV infection. Some studies have suggested that long-term use of IFN may also induce the expression of PD-L1, which could further suppress T cell function[68,69]. Both IFN-α/γ can induce the expression of PD-L1 in liver cancer cells and liver cells. The IFN-α/γ-STAT1-PD-L1 pathway significantly mediates T cell hyporesponsiveness and the inactivation of liver-infiltrating T cells in the hepatic microenvironment. This effect, known as “adaptive resistance,” is a self-protective mechanism for the body. However, this mechanism is not conducive to the clearance of HBV. Therefore, some scholars have proposed the combination of a PD-1 inhibitor and Peg-IFNα to achieve the goal of HBV clearance, relieve immunosuppression, and enhance the scavenging effect of HBsAg. Mouse studies have found that anti-PDL1-IFNα chimeric dimers can enhance the function of DCs, breaking immune tolerance[70]. Combined treatment with the hepatitis B vaccine can induce persistent anti-HBs immune responses in CHB mice, potentially achieving long-term HBsAg clearance. However, there are no clinical studies on combining ICIs with Peg-IFNα in CHB patients. Nevertheless, based on the foundation laid by the above research, it is believed that ICIs combined with Peg-IFNα may achieve more effective clinical cures for CHB, building upon the effects of Peg-IFNα.
Due to the close association between HBV clearance and T cell function, there has been increasing use of T cell engineering and manipulation in the clinical cure of CHB in recent years. One notable approach is the adoptive transfer of engineered T cells. This technique involves obtaining T cells from the patient’s blood, modifying them with HBV-specific receptor gene transfer technology to engineer HBV-specific receptors on the surface of T cells, expanding these engineered T cells in vitro, and then reinfusing them into the patient’s body to exert immunological effects[71]. Currently, there are two main types of engineered T cells used for HBV therapy: Chimeric antigen receptor T cells and T cells modified with specific T cell receptors[72-74]. Studies have found that leukemia patients with concurrent CHB who received bone marrow transplants from donors with HBV-specific T cell responses (vaccinated or spontaneous HBV clearers) experienced HBV clearance[75,76]. Similar studies have detected HBV clearance in recipients with HBV-specific adaptive immune responses after liver transplantation from HBsAg-positive donors, with increased HBV-specific cellular and humoral immune responses[77]. These studies collectively indicate that the adoptive transfer of engineered HBV-specific T cells for immunological restoration therapy could represent a breakthrough in achieving clinical cure in CHB patients.
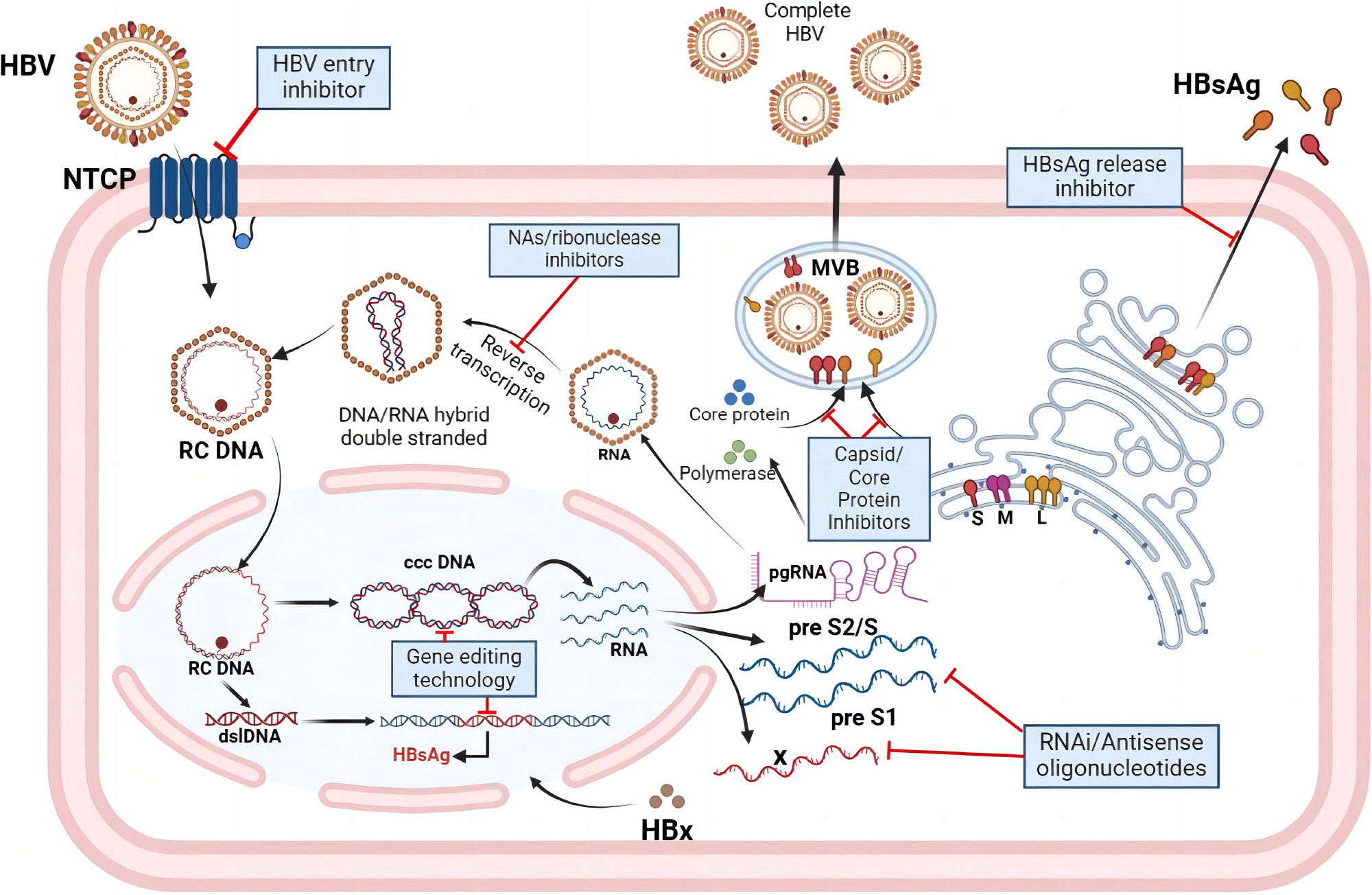
Currently available drugs still cannot completely eliminate cccDNA and integrated host genes containing HBV DNA, while the efficacy of existing antiviral therapies for CHB in clinical use is limited. Therefore, research projects and developing new drugs for HBV treatment are actively underway. With a deeper understanding of the HBV lifecycle, drug development has begun to target the entire process of HBV replication. Anti-HBV drugs currently under development primarily aim to block and inhibit HBV infection and replication in liver cells, exerting antiviral effects by targeting the virus lifecycle. They mainly operate through the following mechanisms: (1) HBV entry inhibitors: Blocking HBV entry into liver cells; (2) Nucleic acid polymers: Inhibiting the production of HBsAg; (3) NAs: Interfering with the DNA polymerase required for HBV DNA replication, commonly used as oral antiviral medications; (4) Post-transcriptional regulation interference: Interfering with or inhibiting HBV RNA, preventing virus translation. Drugs targeting this mechanism mainly include RNA interference and antisense oligonucleotides; (5) Capsid inhibitors: Interfering with the protein capsid of HBV and preventing virus assembly; and (6) HBsAg release inhibitors: Inhibiting the release of HBsAg from infected liver cells (Figure 1).
Peg-IFNα can exert antiviral activity at multiple stages of HBV replication. For example, during the reverse transcription of pgRNA into HBV DNA, Peg-IFNα can enhance the expression of APOBEC3 cytidine deaminase and ISGs, inducing widespread G-to-A mutations and cccDNA degradation, thereby blocking HBV DNA replication and depleting the cccDNA pool[78,79]. It can also induce downregulation of HBV RNA synthesis and promote the degradation of pgRNA[80,81]. These mechanisms indicate that the use of Peg-IFNα can somewhat promote the complete cure of CHB. However, the core of a complete cure for HBV infection lies in eradicating all HBV genes, including intrahepatic cccDNA and integrated HBV DNA. Although Peg-IFNα has some effect on cccDNA, its impact remains limited. In recent years, the development of gene editing technology has shown promising perspectives for the complete cure of HBV. CRISPR (Clustered Regularly Interspaced Short Palindromic Repeat) is a repeat sequence in prokaryotic genomes, primarily functioning to excise viral DNA fragments integrated into prokaryotic genomes through an enzyme called Cas9. Initially discovered in bacteria, CRISPR-Cas9 quickly became a breakthrough gene-editing tool for treating various diseases, including HBV infection. By designing guide RNA complementary to the target DNA sequence, the CRISPR-Cas9 system can selectively and specifically cleave the target DNA genome, resulting in site-specific double-strand breaks. The broken ends induce error-prone non-homologous end-joining, often resulting in frameshift mutations, producing non-functional truncated proteins, and ultimately inactivating the target DNA genome[82,83]. However, before CRISPR-Cas9 technology can be used in clinical applications, potential challenges must be addressed. In the face of these challenges, continuous improvement of CRISPR-Cas9 technology is underway, and utilizing CRISPR-mediated gene editing technology to completely eradicate all HBV genomes to achieve a complete cure may also be a powerful tool in conquering chronic HBV infection.
There are still many urgent problems to be solved regarding CHB clinical cohort studies, basic research, new drug research and development, dominant and nondominant populations, efficacy prediction and evaluation indicators, combined antiviral and immune regulation treatment regimens, and individualized treatment. In the future, a complete cure for hepatitis B is expected to require a combination of various drugs. However, the clinical cure for CHB is an achievable ideal treatment goal that researchers should continue to address.
| 1. | Revill PA, Chisari FV, Block JM, Dandri M, Gehring AJ, Guo H, Hu J, Kramvis A, Lampertico P, Janssen HLA, Levrero M, Li W, Liang TJ, Lim SG, Lu F, Penicaud MC, Tavis JE, Thimme R; Members of the ICE-HBV Working Groups; ICE-HBV Stakeholders Group Chairs; ICE-HBV Senior Advisors, Zoulim F. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol Hepatol. 2019;4:545-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 371] [Article Influence: 61.8] [Reference Citation Analysis (1)] |
| 2. | Nguyen MH, Wong G, Gane E, Kao JH, Dusheiko G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin Microbiol Rev. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 305] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 3. | Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15 Suppl 4:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 734] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 4. | Zhou J, Sun H, Wang Z, Cong W, Zeng M, Zhou W, Bie P, Liu L, Wen T, Kuang M, Han G, Yan Z, Wang M, Liu R, Lu L, Ren Z, Zeng Z, Liang P, Liang C, Chen M, Yan F, Wang W, Hou J, Ji Y, Yun J, Bai X, Cai D, Chen W, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Guo Y, Hua B, Huang X, Jia W, Li Q, Li T, Li X, Li Y, Li Y, Liang J, Ling C, Liu T, Liu X, Lu S, Lv G, Mao Y, Meng Z, Peng T, Ren W, Shi H, Shi G, Shi M, Song T, Tao K, Wang J, Wang K, Wang L, Wang W, Wang X, Wang Z, Xiang B, Xing B, Xu J, Yang J, Yang J, Yang Y, Yang Y, Ye S, Yin Z, Zeng Y, Zhang B, Zhang B, Zhang L, Zhang S, Zhang T, Zhang Y, Zhao M, Zhao Y, Zheng H, Zhou L, Zhu J, Zhu K, Liu R, Shi Y, Xiao Y, Zhang L, Yang C, Wu Z, Dai Z, Chen M, Cai J, Wang W, Cai X, Li Q, Shen F, Qin S, Teng G, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2022 Edition). Liver Cancer. 2023;12:405-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 176] [Reference Citation Analysis (0)] |
| 5. | Franceschi S, Montella M, Polesel J, La Vecchia C, Crispo A, Dal Maso L, Casarin P, Izzo F, Tommasi LG, Chemin I, Trépo C, Crovatto M, Talamini R. Hepatitis viruses, alcohol, and tobacco in the etiology of hepatocellular carcinoma in Italy. Cancer Epidemiol Biomarkers Prev. 2006;15:683-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, Decarli A, Trevisi P, Ribero ML, Martelli C, Porru S, Nardi G. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 441] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 7. | Wild CP, Montesano R. A model of interaction: aflatoxins and hepatitis viruses in liver cancer aetiology and prevention. Cancer Lett. 2009;286:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Cornberg M, Lok AS, Terrault NA, Zoulim F; 2019 EASL-AASLD HBV Treatment Endpoints Conference Faculty. Guidance for design and endpoints of clinical trials in chronic hepatitis B - Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference(‡). J Hepatol. 2020;72:539-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 9. | Chinese Society of Infectious Diseases; Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. The guidelines of prevention and treatment for chronic hepatitis B (2019 version). Zhonghua Gan Zang Bing Za Zhi. 2019;27:938-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 110] [Reference Citation Analysis (0)] |
| 10. | Buti M, Tsai N, Petersen J, Flisiak R, Gurel S, Krastev Z, Aguilar Schall R, Flaherty JF, Martins EB, Charuworn P, Kitrinos KM, Subramanian GM, Gane E, Marcellin P. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci. 2015;60:1457-1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 11. | Papatheodoridis G, Triantos C, Hadziyannis E, Zisimopoulos K, Georgiou A, Voulgaris T, Vlachogiannakos I, Nikolopoulou V, Manolakopoulos S. Serum HBsAg kinetics and usefulness of interferon-inducible protein 10 serum in HBeAg-negative chronic hepatitis B patients treated with tenofovir disoproxil fumarate. J Viral Hepat. 2015;22:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Chevaliez S, Hézode C, Bahrami S, Grare M, Pawlotsky JM. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol. 2013;58:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 13. | Block TM, Gish R, Guo H, Mehta A, Cuconati A, Thomas London W, Guo JT. Chronic hepatitis B: what should be the goal for new therapies? Antiviral Res. 2013;98:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Chinese Society of Infectious Disease Chinese Society of Hepatology; Chinese Medical Association. The expert consensus on clinical cure (functional cure) of chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi. 2019;27:594-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Marcellin P, Ahn SH, Chuang WL, Hui AJ, Tabak F, Mehta R, Petersen J, Lee CM, Ma X, Caruntu FA, Tak WY, Elkhashab M, Lin L, Wu G, Martins EB, Charuworn P, Yee LJ, Lim SG, Foster GR, Fung S, Morano L, Samuel D, Agarwal K, Idilman R, Strasser SI, Buti M, Gaeta GB, Papatheodoridis G, Flisiak R, Chan HL. Predictors of response to tenofovir disoproxil fumarate plus peginterferon alfa-2a combination therapy for chronic hepatitis B. Aliment Pharmacol Ther. 2016;44:957-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Zheng C, Yan H, Zeng J, Cai S, Wu X. Comparison of pegylated interferon monotherapy and de novo pegylated interferon plus tenofovir combination therapy in patients with chronic hepatitis B. Infect Drug Resist. 2019;12:845-854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Ahn SH, Marcellin P, Ma X, Caruntu FA, Tak WY, Elkhashab M, Chuang WL, Tabak F, Mehta R, Petersen J, Guyer W, Jump B, Chan A, Subramanian M, Crans G, Fung S, Buti M, Gaeta GB, Hui AJ, Papatheodoridis G, Flisiak R, Chan HLY. Hepatitis B Surface Antigen Loss with Tenofovir Disoproxil Fumarate Plus Peginterferon Alfa-2a: Week 120 Analysis. Dig Dis Sci. 2018;63:3487-3497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Huang J, Zhang K, Chen W, Liao J, Luo X, Chen R. Switching to PegIFNα-2b leads to HBsAg loss in patients with low HBsAg levels and HBV DNA suppressed by NAs. Sci Rep. 2017;7:13383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Tamai H, Ida Y, Shingaki N, Shimizu R, Fukatsu K, Itonaga M, Yoshida T, Maeda Y, Moribata K, Maekita T, Iguchi M, Kato J, Kitano M. Add-on Pegylated Interferon Alpha-2a Therapy in Chronic Hepatitis B Japanese Patients Treated with Entecavir. Hepat Res Treat. 2017;2017:2093847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Wu FP, Yang Y, Li M, Liu YX, Li YP, Wang WJ, Shi JJ, Zhang X, Jia XL, Dang SS. Add-on pegylated interferon augments hepatitis B surface antigen clearance vs continuous nucleos(t)ide analog monotherapy in Chinese patients with chronic hepatitis B and hepatitis B surface antigen ≤ 1500 IU/mL: An observational study. World J Gastroenterol. 2020;26:1525-1539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak WY, Elkashab M, Chuang WL, Lim SG, Tabak F, Mehta R, Petersen J, Foster GR, Lou L, Martins EB, Dinh P, Lin L, Corsa A, Charuworn P, Subramanian GM, Reiser H, Reesink HW, Fung S, Strasser SI, Trinh H, Buti M, Gaeta GB, Hui AJ, Papatheodoridis G, Flisiak R, Chan HL; Study 149 Investigators. Combination of Tenofovir Disoproxil Fumarate and Peginterferon α-2a Increases Loss of Hepatitis B Surface Antigen in Patients With Chronic Hepatitis B. Gastroenterology. 2016;150:134-144.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 271] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 22. | Hagiwara S, Nishida N, Watanabe T, Ida H, Sakurai T, Ueshima K, Takita M, Komeda Y, Nishijima N, Osaki Y, Kudo M. Sustained antiviral effects and clearance of hepatitis surface antigen after combination therapy with entecavir and pegylated interferon in chronic hepatitis B. Antivir Ther. 2018;23:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Hu C, Song Y, Tang C, Li M, Liu J, Liu J, Liao M, Zhou F, Zhang YY, Zhou Y. Effect of Pegylated Interferon Plus Tenofovir Combination on Higher Hepatitis B Surface Antigen Loss in Treatment-naive Patients With Hepatitis B e Antigen -positive Chronic Hepatitis B: A Real-world Experience. Clin Ther. 2021;43:572-581.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Han M, Jiang J, Hou J, Tan D, Sun Y, Zhao M, Ning Q. Sustained immune control in HBeAg-positive patients who switched from entecavir therapy to pegylated interferon-α2a: 1 year follow-up of the OSST study. Antivir Ther. 2016;21:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Wu D, Wang P, Han M, Chen Y, Chen X, Xia Q, Yan W, Wan X, Zhu C, Xie Q, Jiang J, Wei L, Tan D, Dou X, Yu Y, Hou J, Luo X, Ning Q. Sequential combination therapy with interferon, interleukin-2 and therapeutic vaccine in entecavir-suppressed chronic hepatitis B patients: the Endeavor study. Hepatol Int. 2019;13:573-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Hu P, Shang J, Zhang W, Gong G, Li Y, Chen X, Jiang J, Xie Q, Dou X, Sun Y, Li Y, Liu Y, Liu G, Mao D, Chi X, Tang H, Li X, Xie Y, Chen X, Jiang J, Zhao P, Hou J, Gao Z, Fan H, Ding J, Zhang D, Ren H. HBsAg Loss with Peg-interferon Alfa-2a in Hepatitis B Patients with Partial Response to Nucleos(t)ide Analog: New Switch Study. J Clin Transl Hepatol. 2018;6:25-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 27. | Bourlière M, Rabiega P, Ganne-Carrie N, Serfaty L, Marcellin P, Barthe Y, Thabut D, Guyader D, Hezode C, Picon M, Causse X, Leroy V, Bronowicki JP, Carrieri P, Riachi G, Rosa I, Attali P, Molina JM, Bacq Y, Tran A, Grangé JD, Zoulim F, Fontaine H, Alric L, Bertucci I, Bouvier-Alias M, Carrat F; ANRS HB06 PEGAN Study Group. Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen-negative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: a randomised, controlled, open-label trial. Lancet Gastroenterol Hepatol. 2017;2:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 28. | Lim SG, Yang WL, Chang J, Ngu J, Tan Y, Ahmed T, Dan YY, Lee YM, Lee GH, Tan PS, Phyo WW, Khin LW, Lee C, Tay A, Chan E. Switch or add-on peginterferon to chronic hepatitis B patients already on nucleos(t)ide analogue therapy (swap study): Final results. Hepatology. 2019;70:125A-126A. |
| 29. | Li GJ, Yu YQ, Chen SL, Fan P, Shao LY, Chen JZ, Li CS, Yi B, Chen WC, Xie SY, Mao XN, Zou HH, Zhang WH. Sequential combination therapy with pegylated interferon leads to loss of hepatitis B surface antigen and hepatitis B e antigen (HBeAg) seroconversion in HBeAg-positive chronic hepatitis B patients receiving long-term entecavir treatment. Antimicrob Agents Chemother. 2015;59:4121-4128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Cao Z, Liu Y, Ma L, Lu J, Jin Y, Ren S, He Z, Shen C, Chen X. A potent hepatitis B surface antigen response in subjects with inactive hepatitis B surface antigen carrier treated with pegylated-interferon alpha. Hepatology. 2017;66:1058-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 31. | Wu F, Lu R, Liu Y, Wang Y, Tian Y, Li Y, Li M, Wang W, Zhang X, Jia X, Dang S. Efficacy and safety of peginterferon alpha monotherapy in Chinese inactive chronic hepatitis B virus carriers. Liver Int. 2021;41:2032-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Chen XB, Liu FF, Shu FL, Liu JY, Yang JH. Peginterferon alfa-2b combined with tenofovir disoproxil fumarate induced high clinical cure rate in inactive chronic hepatitis B virus carriers. Clin Res Hepatol Gastroenterol. 2021;45:101723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Li MH, Xie Y, Lu Y, Qiu GH, Liu F, Li XH, Zhao H, Song SJ, Guan XP, Cheng J, Xu DZ. High rates of HBsAg loss and seroconversion result from prolonged course of pegasys treatment. Zhonghua Gan Zang Bing Za Zhi. 2011;19:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Yan YJ, Wang XX, Cao ZH, Lu JF, Jin Y, He ZM, Geng N, Ren S, Ma LN, Chen XY. Low-levels of HBsAg quantification at 48-week in HBeAg-negative chronic hepatitis B patients are the advantageous population for HBsAg clearance. Zhonghua Gan Zang Bing Za Zhi. 2018;26:813-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 35. | Li M, Xie S, Bi X, Sun F, Zeng Z, Deng W, Jiang T, Lin Y, Yang L, Lu Y, Zhang L, Yi W, Xie Y. An optimized mode of interferon intermittent therapy help improve HBsAg disappearance in chronic hepatitis B patients. Front Microbiol. 2022;13:960589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Micco L, Peppa D, Loggi E, Schurich A, Jefferson L, Cursaro C, Panno AM, Bernardi M, Brander C, Bihl F, Andreone P, Maini MK. Differential boosting of innate and adaptive antiviral responses during pegylated-interferon-alpha therapy of chronic hepatitis B. J Hepatol. 2013;58:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 199] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 37. | Thimme R, Dandri M. Dissecting the divergent effects of interferon-alpha on immune cells: time to rethink combination therapy in chronic hepatitis B? J Hepatol. 2013;58:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 38. | Perrillo R, Lin HS, Schwarz KB, Rosenthal P, Lisker-Melman M, Chung RT, Prokunina-Olsson L, Cloherty G, Feld J; Hepatitis B Research Network (HBRN). Changes in serum hepatitis B surface and e antigen, interferon-inducible protein 10, and aminotransferase levels during combination therapy of immune-tolerant chronic hepatitis B. Hepatology. 2022;76:775-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Terrault NA, Lok AS, Wahed AS, Ghany MG, Perrillo RP, Fried MW, Wong DK, Khalili M, Lau DTY, Sterling RK, Di Bisceglie AM, Lisker-Melman M, Cooper SL, Chung RT, Patel K, Roberts LR, Belle SH, Janssen HLA; Hepatitis B Research Network. Randomized Trial of Tenofovir With or Without Peginterferon Alfa Followed by Protocolized Treatment Withdrawal in Adults With Chronic Hepatitis B. Am J Gastroenterol. 2023;118:1214-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Xie C, Xie DY, Fu L, Zhang WH, Wei J, Li GJ, Yang JH, Chen XY, Li JB, Shangn J, Guan YJ, Jiang YF, Guo Y, Zeng YL, Chang JB, Peng YZ, Lin MH, Huang GY, Li J, Gu SW, Geng JW, Gao ZL. Functional cure based on pegylated interferon ɑ-2b therapy in nucleoside analog-suppressed HBeAg negative chronic hepatitis B: a multicenter real-world study (Everest Project in China)-4 years data update. Hepatology. 2022;76:S24-S25. |
| 41. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1942] [Article Influence: 215.8] [Reference Citation Analysis (0)] |
| 42. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2812] [Article Influence: 401.7] [Reference Citation Analysis (0)] |
| 43. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3765] [Article Influence: 470.6] [Reference Citation Analysis (1)] |
| 44. | Chu CM, Liaw YF. Incidence and risk factors of progression to cirrhosis in inactive carriers of hepatitis B virus. Am J Gastroenterol. 2009;104:1693-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 556] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 46. | Song A, Lin X, Lu J, Ren S, Cao Z, Zheng S, Hu Z, Li H, Shen C, Chen X. Pegylated Interferon Treatment for the Effective Clearance of Hepatitis B Surface Antigen in Inactive HBsAg Carriers: A Meta-Analysis. Front Immunol. 2021;12:779347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Zhang Q, Sun F, Yu Y, Sui S, Zhao T, Shang J. The functional cure strategies of patients with high HBsAg levels at baseline with interferon therapy (OASIS Project 1.5 Year Data). The 32nd Conference of the Asian Pacific Association for the Study of the Liver-APASL 2023; 2023 Feb 15-19; Taiwan, China. APASL, 2023: FP13-70. |
| 48. | Li J, Qu L, Sun X, Liu Y, Gong Q, Yu D, Zhang D, Jiang J, Chen J, Wei D, Han Y, Gao Y, Zhang Q, She W, Chen L, Zhang J, Zhang X. Peg-interferon alpha add-on Tenofovir disoproxil fumarate achieved more HBsAg loss in HBeAg-positive chronic hepatitis B naïve patients. J Viral Hepat. 2021;28:1381-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Bortolotti F, Jara P, Barbera C, Gregorio GV, Vegnente A, Zancan L, Hierro L, Crivellaro C, Vergani GM, Iorio R, Pace M, Con P, Gatta A. Long term effect of alpha interferon in children with chronic hepatitis B. Gut. 2000;46:715-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Kansu A, Doğanci T, Akman SA, Artan R, Kuyucu N, Kalayci AG, Dikici B, Dalgiç B, Selimoğlu A, Kasirga E, Ozkan TB, Kuloğlu Z, Aydoğdu S, Boşnak M, Ertekin V, Tanir G, Haspolat K, Girgin N, Yağci RV. Comparison of two different regimens of combined interferon-alpha2a and lamivudine therapy in children with chronic hepatitis B infection. Antivir Ther. 2006;11:255-261. [PubMed] |
| 51. | Yan Y, Allweiss L, Yang D, Kang J, Wang J, Qian X, Zhang T, Liu H, Wang L, Liu S, Sui J, Chen X, Dandri M, Zhao J, Lu F. Down-regulation of cell membrane localized NTCP expression in proliferating hepatocytes prevents hepatitis B virus infection. Emerg Microbes Infect. 2019;8:879-894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 52. | Allweiss L, Volz T, Giersch K, Kah J, Raffa G, Petersen J, Lohse AW, Beninati C, Pollicino T, Urban S, Lütgehetmann M, Dandri M. Proliferation of primary human hepatocytes and prevention of hepatitis B virus reinfection efficiently deplete nuclear cccDNA in vivo. Gut. 2018;67:542-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 53. | Zhang M, Li J, Wang FS. Antiviral therapy and clinical cure for chronic hepatitis B in children: progress and challenges. Zhonghua Gan Zang Bing Za Zhi. 2021;29:1218-1223. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 54. | Zhang M, Li J, Xu Z, Fan P, Dong Y, Wang F, Gao Y, Yan J, Cao L, Ji D, Feng D, Zhong Y, Zhang Y, Hong W, Zhang C, Wang FS. Functional cure is associated with younger age in children undergoing antiviral treatment for active chronic hepatitis B. Hepatol Int. 2024;18:435-448. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 55. | Wang F, Song M, Hu Y, Yang L, Bi X, Lin Y, Jiang T, Deng W, Wang S, Sun F, Zeng Z, Lu Y, Shen G, Liu R, Chang M, Wu S, Gao Y, Hao H, Xu M, Chen X, Hu L, Wan G, Zhang L, Li M, Xie Y. The relation of the frequency and functional molecules expression on plasmacytoid dendritic cells to postpartum hepatitis in women with HBeAg-positive chronic hepatitis B virus infection. Front Immunol. 2022;13:1062123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 56. | Tang Q, Wang C, Liu X, Hu P. Cytokine profiles of chronic HBV infected women during pregnancy and postpartum. Hepatology. 2022;76:S324-S324. |
| 57. | Song A, Liu Y, Cao Z, Lu J, Ren S, Zheng S, Ma L, Hu Z, Lin X, Li H, Zheng Y, Chen X. Clinical Features and T Cell Immune Characteristics of Postpartum Hepatitis Flare in Pregnant Women With HBeAg-Positive Chronic HBV Infection. Front Immunol. 2022;13:881321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Huang H, Wang J, Li W, Chen R, Chen X, Zhang F, Xu D, Lu F. Serum HBV DNA plus RNA shows superiority in reflecting the activity of intrahepatic cccDNA in treatment-naïve HBV-infected individuals. J Clin Virol. 2018;99-100:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 59. | Zhang Q, Huang H, Sun A, Liu C, Wang Z, Shi F, Duan W, Sun X, Wang Q, Sun P, Pu C, Zhang Y. Change of Cytokines in Chronic Hepatitis B Patients and HBeAg are Positively Correlated with HBV RNA, Based on Real-world Study. J Clin Transl Hepatol. 2022;10:390-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Farag MS, van Campenhout MJH, Pfefferkorn M, Fischer J, Deichsel D, Boonstra A, van Vuuren AJ, Ferenci P, Feld JJ, Berg T, Hansen BE, van Bömmel F, Janssen HLA. Hepatitis B Virus RNA as Early Predictor for Response to Pegylated Interferon Alpha in HBeAg-Negative Chronic Hepatitis B. Clin Infect Dis. 2021;72:202-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 61. | Chinese Society of Hepatology; Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2022). Zhonghua Gan Zang Bing Za Zhi. 2022;30:1309-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 62. | Zhu YY, Wu YL, Chen J, Zheng Q, Dong J, Jiang JJ. Prolonged duration of the routine pegylated-interferon alfa-2a therapy produces superior virological response in HBeAg-positive chronic hepatitis B patients: a single-center cohort study. Zhonghua Gan Zang Bing Za Zhi. 2012;20:737-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Cao ZH, Ma LN, Zhang HW, Liu YL, Chen XY. Extended treatment with peginterferon α-2a in combination with lamivudine or adefovir for 96 weeks yields high rates of HBeAg and HBsAg seroconversion. J Dig Dis. 2013;14:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Pinto EF, Andrade C. Interferon-Related Depression: A Primer on Mechanisms, Treatment, and Prevention of a Common Clinical Problem. Curr Neuropharmacol. 2016;14:743-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 65. | Zhu Y, Chen M, Xu D, Li TE, Zhang Z, Li JH, Wang XY, Yang X, Lu L, Jia HL, Dong QZ, Qin LX. The combination of PD-1 blockade with interferon-α has a synergistic effect on hepatocellular carcinoma. Cell Mol Immunol. 2022;19:726-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 66. | Chu F, Li HS, Liu X, Cao J, Ma W, Ma Y, Weng J, Zhu Z, Cheng X, Wang Z, Liu J, Jiang ZY, Luong AU, Peng W, Wang J, Balakrishnan K, Yee C, Dong C, Davis RE, Watowich SS, Neelapu SS. CXCR5(+)CD8(+) T cells are a distinct functional subset with an antitumor activity. Leukemia. 2019;33:2640-2653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 67. | Gane E, Verdon DJ, Brooks AE, Gaggar A, Nguyen AH, Subramanian GM, Schwabe C, Dunbar PR. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J Hepatol. 2019;71:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 68. | Li N, Wang J, Zhang N, Zhuang M, Zong Z, Zou J, Li G, Wang X, Zhou H, Zhang L, Shi Y. Cross-talk between TNF-α and IFN-γ signaling in induction of B7-H1 expression in hepatocellular carcinoma cells. Cancer Immunol Immunother. 2018;67:271-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 69. | Liu L, Hou J, Xu Y, Qin L, Liu W, Zhang H, Li Y, Chen M, Deng M, Zhao B, Hu J, Zheng H, Li C, Meng S. PD-L1 upregulation by IFN-α/γ-mediated Stat1 suppresses anti-HBV T cell response. PLoS One. 2020;15:e0228302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 70. | Meng CY, Sun S, Liang Y, Xu H, Zhang C, Zhang M, Wang FS, Fu YX, Peng H. Engineered anti-PDL1 with IFNα targets both immunoinhibitory and activating signals in the liver to break HBV immune tolerance. Gut. 2023;72:1544-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 71. | Tan AT, Schreiber S. Adoptive T-cell therapy for HBV-associated HCC and HBV infection. Antiviral Res. 2020;176:104748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1877] [Cited by in RCA: 1833] [Article Influence: 183.3] [Reference Citation Analysis (0)] |
| 73. | June CH, Sadelain M. Chimeric Antigen Receptor Therapy. N Engl J Med. 2018;379:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1587] [Article Influence: 226.7] [Reference Citation Analysis (0)] |
| 74. | Labanieh L, Majzner RG, Mackall CL. Programming CAR-T cells to kill cancer. Nat Biomed Eng. 2018;2:377-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 286] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 75. | Ilan Y, Nagler A, Adler R, Naparstek E, Or R, Slavin S, Brautbar C, Shouval D. Adoptive transfer of immunity to hepatitis B virus after T cell-depleted allogeneic bone marrow transplantation. Hepatology. 1993;18:246-252. [PubMed] |
| 76. | Lau GK, Lok AS, Liang RH, Lai CL, Chiu EK, Lau YL, Lam SK. Clearance of hepatitis B surface antigen after bone marrow transplantation: role of adoptive immunity transfer. Hepatology. 1997;25:1497-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 142] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 77. | Loggi E, Bihl F, Chisholm JV 3rd, Biselli M, Bontadini A, Vitale G, Ercolani G, Grazi GL, Pinna AD, Bernardi M, Brander C, Andreone P. Anti-HBs re-seroconversion after liver transplantation in a patient with past HBV infection receiving a HBsAg positive graft. J Hepatol. 2009;50:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Bonvin M, Achermann F, Greeve I, Stroka D, Keogh A, Inderbitzin D, Candinas D, Sommer P, Wain-Hobson S, Vartanian JP, Greeve J. Interferon-inducible expression of APOBEC3 editing enzymes in human hepatocytes and inhibition of hepatitis B virus replication. Hepatology. 2006;43:1364-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 79. | Stadler D, Kächele M, Jones AN, Hess J, Urban C, Schneider J, Xia Y, Oswald A, Nebioglu F, Bester R, Lasitschka F, Ringelhan M, Ko C, Chou WM, Geerlof A, van de Klundert MA, Wettengel JM, Schirmacher P, Heikenwälder M, Schreiner S, Bartenschlager R, Pichlmair A, Sattler M, Unger K, Protzer U. Interferon-induced degradation of the persistent hepatitis B virus cccDNA form depends on ISG20. EMBO Rep. 2021;22:e49568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 80. | Gordien E, Rosmorduc O, Peltekian C, Garreau F, Bréchot C, Kremsdorf D. Inhibition of hepatitis B virus replication by the interferon-inducible MxA protein. J Virol. 2001;75:2684-2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 81. | Wang YX, Niklasch M, Liu T, Wang Y, Shi B, Yuan W, Baumert TF, Yuan Z, Tong S, Nassal M, Wen YM. Interferon-inducible MX2 is a host restriction factor of hepatitis B virus replication. J Hepatol. 2020;72:865-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 82. | Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4367] [Cited by in RCA: 4108] [Article Influence: 373.5] [Reference Citation Analysis (0)] |
| 83. | Kim H, Kim JS. A guide to genome engineering with programmable nucleases. Nat Rev Genet. 2014;15:321-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 812] [Article Influence: 73.8] [Reference Citation Analysis (0)] |