INTRODUCTION
Portal hypertension is the main syndrome characteristic of liver cirrhosis significantly affecting its prognosis. It is defined as a pathological increase in portal pressure, the gold-standard method of clinical evaluation of which is the measurement of the hepatic venous pressure gradient (HVPG). The normal range of HVPG is 1–5 mmHg, whereas a values of ≥ 10 mmHg indicates the presence of clinically significant portal hypertension (CSPH)[1]. The development of CSPH is an important event in the natural history of liver cirrhosis, since it leads to life-threatening complications that decrease the median survival rate to 2-4 years[2].
Transjugular intrahepatic portosystemic shunt (TIPS) is an interventional radiological procedure that significantly reduces portal pressure[3], prevents decompensation and improves survival of cirrhotic patients[4]. During TIPS placement, direct portal pressures are measured and used to calculate the portosystemic pressure gradient (PSPG). The PSPG is the pressure difference between the portal vein and the inferior vena cava (the right atrium). The treatment goal of the TIPS is a PSPG of ≤ 12 mmHg or its reduction by at least 50% of the baseline. Modern guidelines recommend TIPS to cirrhotic patients for preventing recurrent gastroesophageal variceal bleeding with the ineffectiveness of the combined use of non-selective β-blockers and endoscopic band ligation. It is also indicated for acute gastroesophageal variceal bleeding with the ineffectiveness of pharmacotherapy with vasoactive drugs (terlipressin, somatostatin, octreotide) and endoscopic band ligation. In addition, select cirrhotic patients Child-Turcotte-Pugh (CTP) class B (> 7 points) or CTP class C (< 14 points) with active gastroesophageal variceal bleeding at initial esophagogastroduodenoscopy or having the highest risk for rebleeding (HVPG) > 20 mmHg at the time of hemorrhage) can be performed early or pre-emptive TIPS within 72 h of admission (ideally on the first day)[5]. Other indications for its use are refractory ascites and hepatic hydrothorax[6]. The results of the conducted studies indicate the prospects of TIPS in portal vein thrombosis and Budd-Chiari syndrome[7], as well as in cirrhotic patients with CSPH before high-risk surgery[8]. In addition, the possibility of its use in porto-sinusoidal vascular disorders[9] and hepatocellular carcinoma[10] is being discussed.
This editorial describes the milestones to optimize the TIPS technique, focusing on the role of innovative ideas and technical solutions that have made this interventional radiological procedure an important method for the treatment of portal hypertension complications.
INNOVATIVE IDEAS THAT CONTRIBUTE TO THE IMPLEMENTATION OF TIPS IN CLINICAL PRACTICE
The TIPS technique was experimentally developed in the late 1960s at the Department of Diagnostic Radiology at the University of California, Los Angeles. During the research on the possibility of detecting biliary tract obstruction by means of transjugular cholangiography, unintentional hits of a modified Ross needle into the intrahepatic branches of the portal vein suggested a new diagnostic method-transjugular portography, and later-TIPS. To create an intrahepatic portosystemic shunt in experimental animals (5 dogs), after transjugular portography, a channel was formed in the liver parenchyma by Dotter’s coaxial Teflon thin-wall angioplasty catheters of different sizes conducted through the superior vena cava, right atrium, inferior vena cava and left hepatic vein. To keep it open, initially a radiopaque rigid Teflon tube 6-10 cm long with an inner diameter of 6 mm was installed in it, which functioned well, but had the property of shifting cranially into the inferior vena cava, and in one case even into the right atrium[11]. A spring coil tubing coated with silicone-based copolymer with an inner diameter of 6 mm, placed with the distal end in the portal vein and the proximal end in the inferior vena cava, was stable and offered better results. Such a shunt diverted a substantial fraction and sometimes all portal blood into the systemic circulation and remained patent for two weeks. Later, usually at the caudal end of the tubing, where it came in contact with the wall of the portal vein, a blood clot began to form, which eventually led to shunt thrombosis[12]. Thus, these initial experimental studies on dogs, as well as human specimens of normal and cirrhotic liver, established the prospects of TIPS, but the question of the material for creating a shunt remained open.
In the early 1980s, Colapinto et al[13], the Grüntzig dilatation catheter with a balloon at least 9 mm in diameter and 4-6 cm long was used as a shunt. In their research, they placed it in a channel that was formed in the liver parenchyma between the hepatic and portal veins in six patients with decompensated liver cirrhosis, who had massive gastroesophageal variceal bleeding. All of them immediately experienced a decrease in portal pressure by 10-15 mmHg, and angiography performed 12 h after the procedure showed shunt patency, which was also confirmed in three of the four surviving patients six weeks later.
Somewhat later, Palmaz et al[14], in experimental animals (12 dogs) a specially made expandable tubular woven mesh of stainless steel wire was used as a stent to create an intrahepatic portosystemic shunt. It was placed in the channel formed in the liver parenchyma between the anterior surface of the inferior vena cava and the bifurcation of the portal vein, and then expanded by inflating an angioplasty balloon. In eight of the nine surviving dogs, the shunt functioned for nine months after installation. Subsequently, a similar model was used in nine dogs with chronic Portal hypertension induced by intraportal injections of polyvinyl alcohol (Ivalon). A shunt patency rate of 100% was observed up to 48 wk. Low portal pressure and high shunt flow accounted for the good results[15].
In 1989, Richter et al[16] presented the first clinical description of TIPS using two Palmaz stents in cirrhotic patients CTP class C with CSPH. Despite a decrease in PSPG from 38 to 18 mmHg and a significant improvement in the clinical status of the patient, he died on day 11 after the procedure because of sudden onset of acute respiratory distress arising from acute nosocomial fungus and cytomegalovirus infection. The autopsy revealed a fully patent shunt without superficial thrombus. Microscopically, a thin endothelial layer on the inner shunt surface was found to be present. A year later, the same authors published a paper reporting the successful outcome of TIPS using the Palmaz stent in nine cirrhotic patients with CSPH and histories of multiple life-threatening gastroesophageal variceal bleeding[17].
These optimistic results were the impetus for the introduction of TIPS into clinical practice. However, two main TIPS-related problems immediately arose, namely shunt dysfunction and post-TIPS hepatic encephalopathy.
EVOLUTION OF TECHNICAL REFINEMENTS OF TIPS STENTS
TIPS stents should contribute to an effective reduction in portal pressure and have special mechanical properties, including high elasticity, strength to withstand liver stiffness, wear resistance and good biocompatibility to reduce the risk of thrombosis and intimal hyperplasia, which can lead to shunt dysfunction, as well as an optimal diameter to prevent post-TIPS hepatic encephalopathy[18].
The first-generation TIPS stents were mainly represented by bare metal stents (BMSs) made of biomedical metals or alloys. For example, the Palmaz® stainless steel stent (Cordis, Miami, FL, United States), known for its high mechanical strength and corrosion resistance, had insufficient flexibility and its use was accompanied by a high incidence of complications. The nitinol (nickel–titanium alloy) stents such as the Zilver® (Cook Medical, Bloomington, IN, United States), Luminexx® (Bard Inc. New Jersey, United States), Smart Control® (Cordis, Miami, Fl, United States), etc. have shown good biocompatibility and corrosion resistance results, and the unique shape memory and superelasticity properties that allow them to expand themselves have contributed to widespread use in TIPS[19]. Nevertheless, in the era of BMSs, shunt dysfunction was a frequent and dangerous complication of TIPS, which, as a rule, was a consequence of its acute thrombosis, pseudo-intimal hyperplasia as a result of bile leakage from damaged bile ducts into the stent lumen and hyperplasia of the hepatic vein intima[20]. The gradual development of stenosis or occlusion of the shunt reduced the effectiveness of TIPS, largely limiting its use to “rescue therapy” or “bridge” to liver transplantation[21].
To solve this problem, several experimental and clinical studies have focused their efforts on the development of covered stent grafts. Their use should have significantly improved the long-term shunt patency due to the absence of pseudo-intimal hyperplasia. After studying various materials, it turned out that the best results were shown by polytetrafluoroethylene-covered stent grafts (PTFE-SGs)[22]. An important condition for PTFE-SGs installation is to ensure their sufficient length: so that they are located in the right or left branch of the portal vein at a distance of at least 1-2 cm from its bifurcation (uncovered part), passes through the channel formed in the liver parenchyma and then along the hepatic vein to the confluence with the inferior vena cava (covered part). At the same time, the distance of the cranial stent end to the inferior vena cava should be no more than 1 cm[23]. It is also necessary to completely cover the intraparenchymal channel to prevent bile from entering the stent lumen and the development of pseudo-intimal hyperplasia[24].
In the late 1990s, PTFE-SGs, and later extended PTFE-SGs (ePTFE-SGs), with diameters of 8, 10 and 12 mm were introduced into clinical practice and showed a significant advantage over BMSs. This, in particular, was demonstrated in a meta-analysis including of six studies involving 1.275 cirrhotic patients with CSPH (346-TIPS with PTFE-SGs and 929-TIPS with BMSs), in which the use of PTFE-SGs contributed to better primary shunt patency [hazard ratio (HR): 0.28, 95% confidence interval (CI): 0.20-0.35], lower incidence of HE (HR: 0.65, 95%CI: 0.45-0.86) and less mortality (HR: 0.76, 95%CI: 0.58-0.94)[25].
Since the early 2000s, self-expanding ePTFE-SGs have become available. In 2004, W.L. Gore & Associates in Phoenix AZ, United States developed self-expanding nitinol ePTFE-SG VIATORR® specifically for TIPS (Viatorr TIPS Stent-VTS), which was the first to receive approval from the United States Food and Drug Administration. The uncovered part of its self–expanding nitinol skeleton (2 cm) is located in the portal vein, whereas the covered part (5-8 cm) is located in the intraparenchymal channel and hepatic vein. The stent graft is available in diameters of 8, 10, and 12 mm[26]. In a retrospective single-center study by Geeroms et al[27] including 285 cirrhotic patients with CSPH treated with TIPS using VTS stent grafts, the 1-, 2- and 5-year primary shunt patency was 91.5%, 89.2% and 86.2%, respectively, with no new shunt dysfunctions after 5 years' follow-up. Shunt revision was performed more often in ascites patients (P = 0.02). The 1-, 4- and 10-year survival rates were 69.2%, 52.1% and 30.7%, respectively. In a retrospective single-center study by Li et al[28] including 59 cirrhotic patients with CSPH, elective TIPS implantation using VTS stent grafts contributed to a decrease in PSPG from 21 mmHg (interquartile range: 19-25) to 13 mmHg (interquartile range: 10-16). The cumulative rate of overall mortality was 34.2% at five years. The cumulative rates of shunt dysfunction and gastroesophageal variceal rebleeding were 11.0% and 28.3% at five years, respectively. The cumulative four-year post-TIPS hepatic encephalopathy free rate was 48.6%.
As an alternative to VTS stent grafts, non-dedicated ePTFE-SGs like the Fluency® (Angiomed GmbH, a subsidiary of C.R. Bard, Inc.), primarily designed for treating peripheral vascular diseases, were adapted for TIPS. Unlike VTS stent grafts, it is completely covered and does not have an uncovered part on the portal vein side. In a retrospective single-center study by Luo et al[29], including 495 cirrhotic patients with CSPH treated with TIPS using Fluency stent grafts, early procedure-related complications occurred in 67 patients (13.5%). TIPS creation resulted in an immediate decrease in mean PSPG from 23.4 ± 7.1 mmHg to 7.6 ± 3.5 mmHg. The 1- and 3-year primary shunt patency was 93%, and 75.9%, respectively. The 1- and 3-year survival rates were 93.4% and 77.2%, respectively. The 1- and 3-year probability of remaining free of gastroesophageal variceal bleeding rates were 94.2% and 71.4%, respectively.
A meta-analysis of four randomized controlled trials (RCTs) has been performed to compare the outcomes of self-expanding ePTFE-SGs vs BMSs for TIPS. VTS stent grafts alone, Fluency stent grafts alone, and VTS stent grafts plus Fluency stent grafts were employed in one, two, and one RCT, respectively. It was demonstrated that the self-expanding ePTFE-SGs group had significantly higher probabilities of overall survival (HR: 0.67, 95%CI: 0.50-0.90, P = 0.008) and shunt patency (HR: 0.42, 95%CI: 0.29-0.62, P < 0.0001) than the BMSs group. Additionally, the self-expanding ePTFE-SGs group might have a lower risk of post-TIPS hepatic encephalopathy than the BMSs group (HR: 0.70, 95%CI: 0.49-1.00, P = 0.05)[30].
In 2017, a new dedicated ePTFE-SG known as the VIATORR® TIPS Endoprosthesis with Controlled Expansion (VCX) (W.L. Gore & Associates, Phoenix, AZ, United States) was introduced. Its diameter from 8 to 10 mm is adjustable regardless of the possible passive dilation. This makes it possible to accurately calibrate the shunt and monitor the PSPG throughout the entire postoperative period, ensuring a good clinical outcome with a fairly low complication rate[31].
Thus, over the past quarter century, there has been significant progress in the qualitative characteristics of TIPS stents, among which the use of PTFE-SGs has proved to be the most successful. If all the rules of their installation are followed, shunt dysfunction is currently rare. However, the problem of post-TIPS hepatic encephalopathy does not lose its relevance.
TECHNICAL SOLUTIONS AIMED AT PREVENTING POST-TIPS HEPATIC ENCEPHALOPATHY
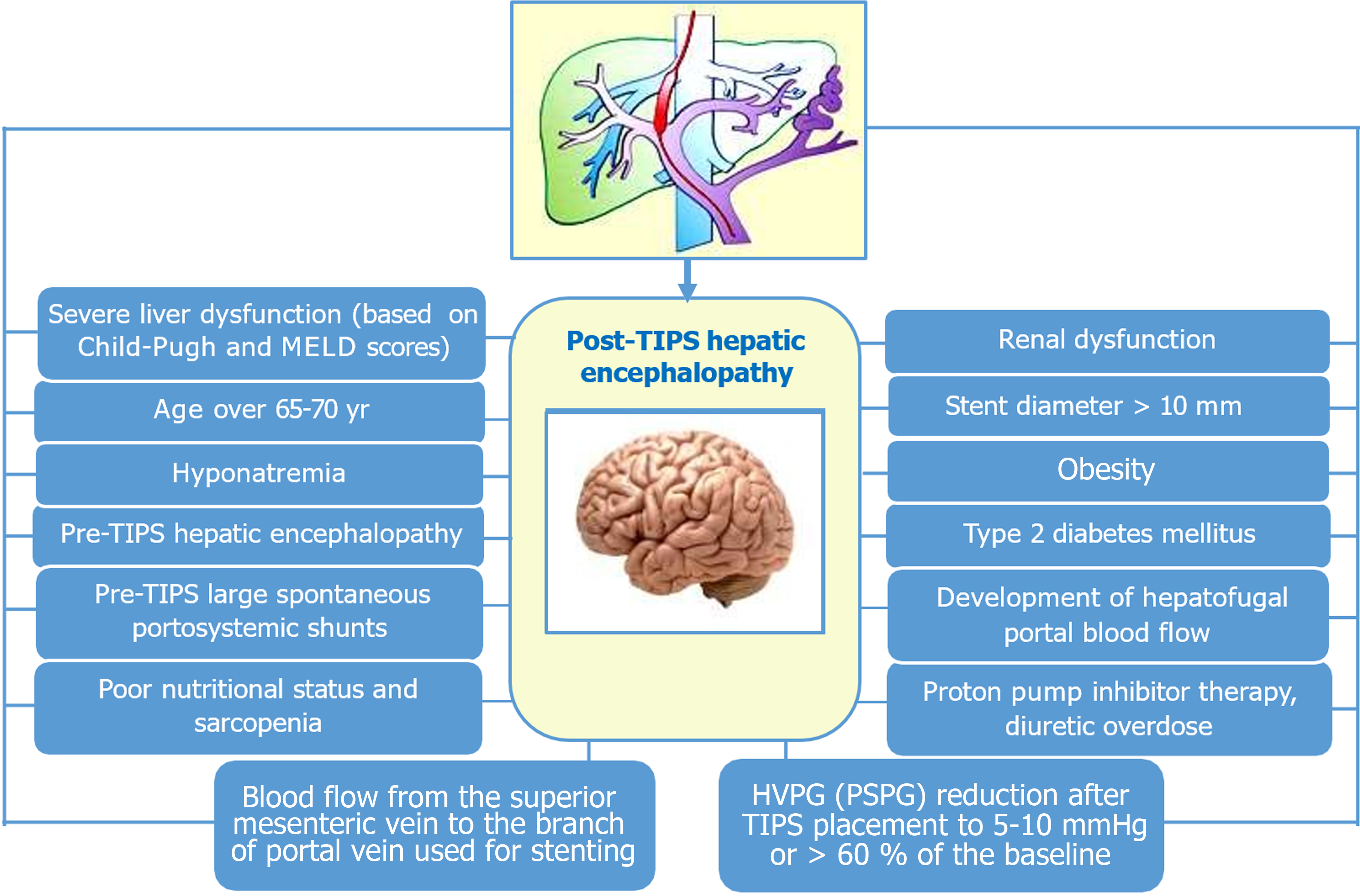
Hepatic encephalopathy is one of the most common complications of TIPS and occurs in 23%-55% of patients during the first year after surgery. Its pathophysiology is difficult and consists of a complex interaction of hyperammonemia and systemic inflammation. Simply put, it is caused by hyperammonemia-related neurotoxicity. In the context of the development of post-TIPS hepatic encephalopathy, two factors must be considered. First of all, this is a “steal” phenomenon, when ammonia-enriched blood from the intestine bypasses the liver and is not included in the urea cycle, which exacerbates hyperammonemia. Secondly, portocaval shunting (including TIPS) leads to hyperactivity of phosphate-activated glutaminase in the intestine, contributing to an increase in gut-derived ammonia[32]. Many predictors of post-TIPS hepatic encephalopathy are well described and presented in Figure 1, and some of them are directly related to the technical characteristics of stents and the method of their placement[33]. This should be taken into account, since routine prophylactic therapy by administration of rifaximin and lactulose may be ineffective in this case[34].
Figure 1 Individual predictors for post-transjugular intrahepatic portosystemic shunt hepatic encephalopathy.
HVPG: Hepatic venous pressure gradient; PSPG: Portosystemic pressure gradient; TIPS: Transjugular intrahepatic portosystemic shunt.
Optimal stent diameter
In this regard, the question of the optimal diameter and degree of expansion of PTFE-SGs for effective reduction in portal pressure without the risk of developing post-TIPS hepatic encephalopathy remains relevant[35]. Obviously, a larger stent diameter leads to a greater reduction in portal pressure. However, the stent diameter does not correlate with PSPG, which cannot be predicted by pre-TIPS hemodynamic variables, but depends on individual conditions[36].
Given the experience of using BMSs, TIPS with 12 mm self-expanding PTFE-SGs were initially employed. However, the high incidence of post-TIPS hepatic encephalopathy forced them to be abandoned[37]. Indeed, in a retrospective single-center study by Habash et al[38] including 360 cirrhotic patients with CSPH treated with TIPS using 12 mm VTS stent grafts, percentage of patients with symptoms of post-TIPS hepatic encephalopathy were 34.4%, 42.9%, and 49.5% at 3, 6, and 12 months, respectively. In a systematic review and meta-analysis including of five RCTs or observational studies involving 489 cirrhotic patients with CSPH treated with TIPS using 8 or 10 mm PTFE-SGs, the 8 mm PTFE-SGs group had higher efficacy regarding one-year or three-year overall survival [odds ratio (OR): 2.88, P = 0.003] and (OR: 1.81, P = 0.04) and lower post-TIPS hepatic encephalopathy (OR: 0.69, P = 0.04) compared with 10 mm PTFE-SGs group. There were no significant differences in gastroesophageal variceal rebleeding rate (OR: 0.80, P = 0.67). However, shunt dysfunction was lower in 10 mm PTFE-SGs group (OR: 2.26, P = 0.003)[39]. In a retrospective single-center study by Kloster et al[40] including 33 cirrhotic patients with CSPH treated with TIPS using 8 mm and 10 mm (85% and 15% patients, respectively) VCX stent grafts, mean final PSPG was 6 mmHg. Cumulative post-TIPS hepatic encephalopathy incidence was 61%. 1-, 3-, 6-, and 12-month post-TIPS hepatic encephalopathy rates were 24%, 30%, 53%, and 61% over 247-d median follow-up.
A significant proportion of patients who develop severe post-TIPS hepatic encephalopathy have a fairly low PSPG (5-10 mmHg). Therefore, in order to achieve good results when choosing the optimal diameter of ePTFE-SGs, it is necessary to keep within a very narrow therapeutic framework of PSPG, namely less than 12 mmHg to control gastroesophageal variceal bleeding and above 10 mmHg for preventing post-TIPS hepatic encephalopathy. In practice, it is difficult to achieve this goal when using self-expanding ePTFE-SGs. Indeed, after the initial ePTFE-SGs expansion to 8 mm and a drop in PSPG, for example, to the required 10 mmHg, it cannot be excluded that further ePTFE-SGs expansion to 10 mm will not lead to a subsequent decrease in PSPG, increasing the risk of developing post-TIPS hepatic encephalopathy[41]. For example, in a retrospective single-center study by Borghol et al[42] including 16 cirrhotic patients with CSPH treated with TIPS using 10 mm VTS stent grafts, that was underdilated (i.e., 8 mm) at the time of stent placement, angiography showed its expansion from 8.96 mm ± 1.12 (SD) to 10 mm ± 1.45 (SD) after 6 months (P = 0.04) with no further significant changes over time after 12 months (10.28 mm ± 1.9 mm), 18 months (9.93 ± 1.51 mm) and 24 months (9.92 ± 0.9 mm) after TIPS. In a prospective study by Pieper et al[43], including 20 cirrhotic patients with CSPH treated with TIPS using VTS stent grafts, two-dimensional (2D) and three-dimensional (3D) ultrasonography showed an expansion of underdilated self-expanding stent grafts from 8 mm to 8.8 ± 0.24 mm (2D) and 8.7 ± 0.27 mm (3D) (P < 0.001) after 1 wk and to 9.4 ± 0.15 mm (2D) and 9.4 ± 0.11 mm (3D) (P < 0.001) 6 wk after TIPS.
Schepis et al[44] performed a prospective, non-randomized study of 42 unselected cirrhotic patients with CSPH treated with TIPS using VTS stent grafts, which received underdilated self-expanding stent-grafts (7 and 6 mm) and 53 patients which received self-expanding stent-grafts of 8 mm or more (controls). Post-TIPS hepatic encephalopathy developed in a significantly lower proportion of patients with underdilated self-expanding stent-grafts (27%) than controls (54%) during the first year after the procedure (P = 0.015), but the proportions of patients with recurrent gastroesophageal variceal bleeding or ascites did not differ significantly between groups. VTS stent grafts dilatation above 6 mm, PSPG below 10 mmHg or a decrease in PSPG after TIPS by more than 50% were independently associated with one-year post-TIPS hepatic encephalopathy. In a prospective case-control study by Praktiknjo et al[45] including 114 cirrhotic patients with CSPH treated with TIPS using 10 mm VCX stent grafts underdilated to 8 mm and 10 mm VTS stent grafts underdilated to 8 mm, VCX stent grafts diameter was 8.0 (7.8-9.2) mm at a median time of 359 (87-450) d, compared with VTS stent grafts at 9.9 (9.7-10.0) mm (P < 0.001). PSPG immediately after TIPS procedure and after 7 d did not change significantly in VCX stent grafts [mean 9.4 (± 0.8) vs 10.4 (± 0.7) mmHg, P = 0.115)]. The lack of passive expansion to nominal diameter of underdilated VCX stent grafts contributed to a reduction in hospital readmissions due to post-TIPS hepatic encephalopathy (23% vs 51% for VCX stent grafts and VTS stent grafts (P < 0.001), respectively.
To date, the question of the optimal diameter and degree of expansion of PTFE-SGs for effective reduction in portal pressure without the risk of developing post-TIPS hepatic encephalopathy remains open. It is obvious that for its prevention, the target reduction in PSPG should be less than was required in the era of BMSs, but there is still not enough concrete data to make scientifically sound decisions.
Choosing a branch of the portal vein for stenting
TIPS stents are usually placed via access from the central part of the right hepatic vein to the right branch of the portal vein. The lower anatomical location and horizontal course of the right branch of the portal vein ensure relatively safe and easy penetration of the needle into its lumen[46]. At the same time, there is a theory that blood from the splenic and superior mesenteric veins in portal hypertension does not mix completely in the main portal vein, but enters the left and right branches of the portal vein separately, that is, blood from the superior mesenteric vein mainly enters the right branch, while blood from the splenic vein mainly enters the left branch. It is assumed that the use of the left branch of the portal vein for stenting is more appropriate, since it can reduce the risk of developing post-TIPS hepatic encephalopathy[47]. In a study by Deng et al[48], including 15 cirrhotic patients with CSPH, blood samples were collected from the left branch, right branch and main trunk of the portal vein during TIPS. The plasma ammonia concentration was 96.4 ± 17.6 mmol/L, 113.5 ± 18.4 mmol/L and 106.9 ± 38.7 mmol/L (P > 0.05), respectively, without any statistically significant differences (P > 0.05). In a retrospective single-center study by Yang et al[49] including 243 cirrhotic patients with CSPH treated with TIPS, it was found that in about three quarters of cases, blood from the splenic and superior mesenteric veins was completely mixed in the intrahepatic portal system under portography, this was accompanied by a comparable risk of post-TIPS hepatic encephalopathy. For the rest of the patients, in about three quarters of the cases, the right branch of the portal vein received blood from the splenic vein, and the left branch of the portal vein received blood from the superior mesenteric vein. If the right branch of the portal vein received blood from the superior mesenteric vein, and the left branch of the portal vein received blood from the splenic vein, this was accompanied by an increased risk of post-TIPS hepatic encephalopathy.
In studies where BMSs was used for TIPS creation the frequency of hepatic encephalopathy in stenting of the left branch of the portal vein was lower than in stenting of the right branch of the portal vein[50-52], however, in the case of using VTS stent grafts, it occurred approximately the same[53]. In a retrospective single-center study by Miraglia et al[54] including 193 cirrhotic patients with CSPH, compared the outcome of TIPS using VCX stent grafts placed in the left branch (37 patients - group 1) and the right branch (156 patients-group 2) of the portal vein. The median follow-up was 9.6 months. PSPG after TIPS was 6.2 ± 2.2 mmHg in group 1 vs 6.3 ± 2.8 mmHg in group 2 (P = 0.839). The stent was dilated to 8-mm in 95% of patients in group 1 vs 77% of patients in group 2 (P = 0.015). The incidence of post-TIPS hepatic encephalopathy was 13% of patients in group 1 and 24% of patients in group 2 (P = 0.177).
The results of these studies do not allow us to draw a definitive conclusion, and further multicenter prospective RCTs are needed to find out whether the occurrence of post-TIPS hepatic encephalopathy may be associated with the choice of a specific branch of the portal vein for stenting.
TRANS-TIPS ANTEGRADE TRANSVENOUS OBLITERATION OF GASTROESOPHAGEAL VARICES
Maintaining hepatofugal blood flow, namely the gastroesophageal pathway of portosystemic shunting, despite a decrease in PSPG after TIPS, is a significant risk factor for gastroesophageal variceal rebleeding. Indeed, the determining factor in formation of gastroesophageal varices is the type of hepatofugal blood flow, and a gastroesophageal pathway of portosystemic shunting is the most important in this situation. The left gastric vein plays the main role in this pathway. It drains blood from both surfaces of the stomach, ascends from right to left along the lesser curvature of the stomach into the lesser omentum to the esophageal hiatus, where it receives esophageal veins. The left gastric vein then turns backward and passes from left to right behind the omental bursa and flows into the portal vein. Anastomoses between the left and right gastric veins and the left and short gastric veins, respectively indicated by terms “coronary vein” and “posterior gastric vein”, have clinical significance only in portal hypertension, because they are involved in the formation of gastroesophageal varices and related with them paraesophageal varices[55]
Trans-TIPS antegrade transvenous obliteration of gastroesophageal varices should be considered as a therapeutic option with high blood flow in them and the presence of large afferent gastric veins or shunts under portography[56]. The procedure can be used as an adjunct to retrograde transvenous obliteration of gastric varices or as an alternative in the absence of gastrorenal shunts. If multiple afferent gastric veins are present, the largest vein (usually the left gastric vein) is left for balloon occlusion, while the small veins are occluded using coils or vascular plugs. The advantage of this approach is that it does not require new access, which minimizes the risk of vascular and biliary injury, while the disadvantages include a time-consuming invasive pathway consists in prolonged and indirect access to afferent gastric veins[57].
A recent systematic review and meta-analysis of 11 studies involving 1,075 cirrhotic patients with CSPH showed that the combination of TIPS with antegrade transvenous obliteration of gastroesophageal varices is accompanied by fewer gastroesophageal variceal rebleeding than after TIPS alone [relative risk (RR): 0.59, 95%CI: 0.43-0.81, P = 0.001], and better results were obtained with the use of PTFE-SGs (RR: 0.56, 95%CI: 0.36-0.86, P = 0.008). At the same time, there were no differences in the frequency of shunt dysfunction (RR: 0.88, 95%CI: 0.64-1.19, P = 0.40), post-TIPS hepatic encephalopathy (RR: 0.84, 95%CI: 0.66-1.06, P = 0.13) and mortality (RR: 0.87, 95%CI: 0.65-1.17, P = 0.34). The authors draw an important conclusion about an individual approach in determining the indications for the combined use of TIPS with antegrade transvenous obliteration of gastroesophageal varices, taking into account the balance of risk and benefit[58].
CONCLUSION
The worldwide experience of using TIPS has made it an important method of treating complications of portal hypertension, which has actually replaced open surgical interventions. This interventional radiological procedure has gone through a complex, almost half-century-long path of evolution from innovative ideas to original technical solutions. The transition from BMSs to ePTFE-SGs made it possible to significantly prevent shunt dysfunction. However, the question of its preferred diameter, which contributes to an optimal reduction of portal pressure without the risk of developing post-TIPS hepatic encephalopathy, remains relevant. Whether the choice of a specific branch of the portal vein for stenting plays a role in its occurrence should be studied in the future. Currently, hepatic encephalopathy is one of the most common complications of TIPS, significantly affecting its effectiveness and prognosis. Careful selection of patients based on cognitive indicators, nutritional status, assessment of liver function, etc., will reduce the incidence of post-TIPS hepatic encephalopathy and improve treatment results. Trans-TIPS antegrade transvenous obliteration of gastroesophageal varices can be considered with high blood flow in them and the presence of large afferent gastric veins as a therapeutic option aimed at preventing gastroesophageal variceal rebleeding. Optimize of TIPS technique has significantly expanded the indications for its use and made it one of the main methods for the treatment of portal hypertension complications. At the same time, there are a number of limitations and unresolved issues that require further RCTs involving a large cohort of patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Russia
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Nassar G, France S-Editor: Fan JR L-Editor: A P-Editor: Cai YX