Published online Jun 27, 2024. doi: 10.4254/wjh.v16.i6.871
Revised: April 23, 2024
Accepted: April 29, 2024
Published online: June 27, 2024
Processing time: 123 Days and 19.1 Hours
Sarcopenia and metabolic dysfunction associated steatotic liver disease (MASLD) are closely intertwined. Sarcopenia, traditionally a disease of the older adult and chronic disease population, has been closely studied as one of the pathophy
Core Tip: Sarcopenia and metabolic dysfunction associated steatotic liver disease (MASLD) share a bidirectional relationship along the liver-muscle axis. With similar pathophysiology and shared risk factors, MASLD is a risk factor for sarcopenia, and vice versa. Early identification and adequate diagnosis are important. However, lack of consensus definition made it difficult to research outcomes. With the recently updated consensus definition for MASLD, researchers may now better identify proper cohorts for study. Consensus on gold standard techniques and muscle mass cutoffs to define sarcopenia are still needed. Future research may identify potential therapeutic targets along the shared liver-muscle axis that would improve outcomes for both sarcopenia and MASLD.
- Citation: Wong R, Yuan LY. Sarcopenia and metabolic dysfunction associated steatotic liver disease: Time to address both. World J Hepatol 2024; 16(6): 871-877
- URL: https://www.wjgnet.com/1948-5182/full/v16/i6/871.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i6.871
With increasing incidence of metabolic dysfunction associated steatotic liver disease (MASLD) and the increasing age of the population, sarcopenia has been closely studied as one of the pathophysiologic conditions at play in the development and progression of MASLD. A comprehensive review published by Viswanath et al[1] closely examines the relationship between these two diagnoses. It highlights numerous shared risk factors, such as elevated lipid profile, physical inactivity, and diabetes mellitus, that suggest a bidirectional relationship between MASLD and sarcopenia. It also proposes pathogenic pathways along the liver-muscle axis that may serve as potential therapeutic targets for further research, such as insulin resistance, hormonal changes, and reduced levels of myokines.
However, for many years, lack of consensus definitions for MASLD and sarcopenia has made it challenging to research their relationship, their outcomes, and to develop targeted therapies. Prior to 2020, the spectrum of steatotic liver disease had been under the dichotomy of “nonalcoholic” and “alcoholic” fatty liver disease. Consensus groups began to meet to develop new nomenclature that would not rely on exclusionary confounder terms and use of stigmatizing language. There were many pros and cons to shifting from a binary, more widely familiar, potentially stigmatizing nomenclature to a more comprehensive, inclusive, less stigmatizing nomenclature to diagnose steatotic liver disease[2]. Ultimately in December 2023, the global Nonalcoholic Fatty Liver Disease Nomenclature Consensus Group published a new nomenclature that better defined criteria for steatotic liver disease, including MASLD, to facilitate diagnose and awareness of the disease[3]. MASLD has many shared diagnostic criteria with diabetes and cardiovascular disease so this new consensus definition better captures the metabolic component in an affirmative nonstigmatising way.
Sarcopenia is classically defined as the loss of skeletal muscle mass and muscle function, which can be part of the physiologic aging process, but can also be accelerated by malnutrition, low physical activity, or inflammatory conditions[4,5]. It is associated with negative outcomes including frailty, chronic disease, limited mobility, and premature mortality[4]. Just as MASLD is expected to increase in parallel to the rising prevalence of obesity globally, sarcopenia is expected to increase as the older adult population grows. Given the chronicity of MASLD as a chronic inflammatory liver disease, researchers have been interested in the impact of MASLD on sarcopenia, and vice versa.
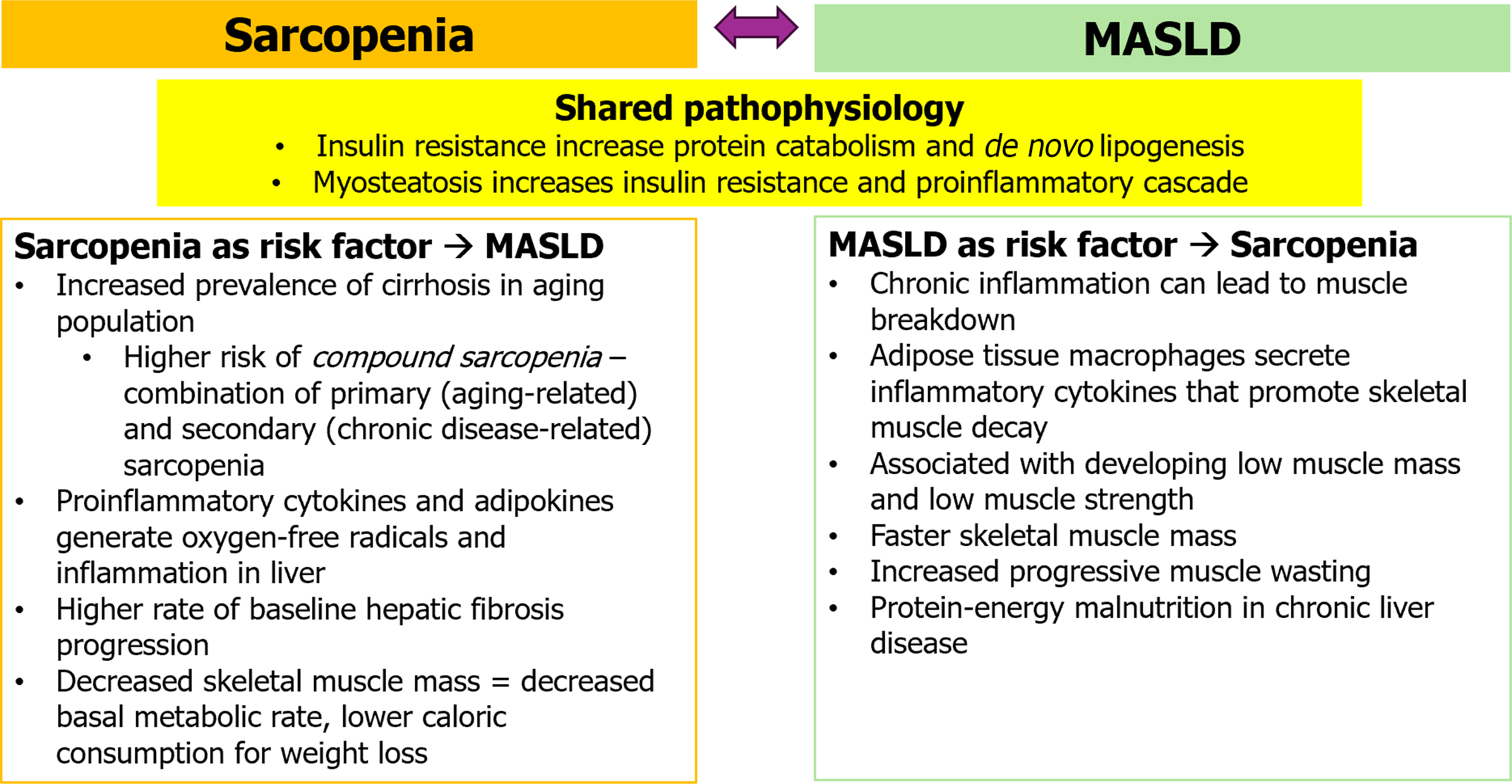
Both MASLD and sarcopenia are closely intertwined, as Viswanath et al[1] summarizes. Pathophysiologically, insulin resistance is believed to play a common role in the development of both diseases. Insulin typically targets the skeletal muscle and can promote muscle protein synthesis while inhibiting muscle protein catabolism[6]. Therefore, insulin resistance can induce muscle attenuation leading to sarcopenia by decreasing protein synthesis in skeletal muscle, increasing protein catabolism, or stimulating skeletal muscle autophagy[7]. Insulin resistance in adipose tissue increases de novo lipogenesis and increases release of free fatty acids that generate hepatic fat accumulation and a proinflammatory environment: A critical pathogenesis pathway for MASLD[8]. Myosteatosis, infiltration of fat into skeletal muscle, on the other hand, increases in insulin resistance and has been associated with early metabolic-associated steatohepatitis (MASH)prior to the onset of sarcopenia[9]. Fat accumulation in muscle tissue also promotes a proinflammatory cascade and oxidative stress, leading to impaired insulin signaling and muscle atrophy. The decreased muscle mass promotes insulin resistance, further exacerbating muscle atrophy. In investigating the muscle-liver-adipose tissue pathway, it remains a question to which disease ultimately comes first: Whether sarcopenia is a consequence of MASLD or a part of the disease natural history, as summarized in Figure 1.
Sarcopenia is an independent risk factor for steatohepatitis and fibrosis[10,11]. One group measured the appendicular skeletal muscle mass in a biopsy-proven MASLD cohort and found that the prevalence of significant fibrosis (> or = F2) was higher in patients with sarcopenia than in those without[12]. Patients with sarcopenia at baseline have a higher rate of hepatic fibrosis progression[13]. Conversely, an increase in skeletal muscle mass has been shown to exert a positive effect in slowing down the progression of MASLD[14]. This effect may be due to increase in skeletal muscle mass causing elevated basal metabolic rate and improvement of insulin resistance[15]. Another study found that low skeletal muscle mass index and central obesity were associated with increased risk of MASLD and cardiovascular disease[16]. The proposed pathophysiological mechanism is through hormonal and cytokine changes found in sarcopenia. Intramuscular lipid accumulation can cause release of proinflammatory cytokines and adipokines that generate oxygen-free radicals and aggravate inflammation in the liver[12,17]. Among older adults with cirrhosis, the combination of primary (aging-related) and secondary (chronic disease-related) sarcopenia, also called compound sarcopenia, is associated with higher odds of mortality and worse outcomes than those without compound sarcopenia[18].
There are different types of sarcopenia. Primary sarcopenia is caused solely by aging. Secondary sarcopenia is caused by other factors, such as chronic disease, physical inactivity, or poor or malnutrition. MASLD is a chronic liver disease that can cause chronic inflammation, that can lead to muscle breakdown, and the development of sarcopenia[19]. Adipose tissue macrophages are thought to secrete inflammatory cytokines, which then promote protein decay in skeletal muscle[5]. The presence of MASLD has been associated with increased risk of developing low muscle mass and low muscle strength, with greater impact on low muscle strength than on low muscle mass[20]. One cohort of 52815 patients found that patients with MASLD had faster skeletal muscle mass loss than those without MASLD[21]. There is also increased risk of progressive muscle wasting in patients with MASH even before cirrhosis, and that this sarcopenia worsens with progression to cirrhosis[22]. Patients with advanced cirrhosis also suffer from protein-energy malnutrition, which contributes to risk of developing sarcopenia[23]. Overall, sarcopenia in patients with cirrhosis is a poor prognostic risk factor for cirrhosis complications and higher mortality[24,25]. Studies have shown that sarcopenia can independently increase overall mortality and cardiac mortality in patients with MASLD[26,27].
A significant challenge to further clarifying research in the relationship between sarcopenia and MASLD has been finding a consensus for the definition of sarcopenia[28,29]. There are various consensus groups who have published different operational cutoffs for low muscle mass, low muscle strength, and low physical performance, making it difficult to study relevant outcomes. One of the first sarcopenia definitions developed by the European Working Group on Sarcopenia (EWGSOP) recently revised their clinical algorithm to combine low muscle mass and low muscle strength to confirm diagnosis, with physical performance as a marker of severity[30]. This update was done to promote early detection of treatment of sarcopenia in hopes of preventing or delaying adverse health outcomes. However, there remains limited agreement between cutoff measures for each component, as well as diagnostic variation on how to best measure skeletal muscle mass. Computed tomography and magnetic resonance imaging can evaluate for whole-body muscle, but no standardized imaging protocol exists, and image acquisition settings may vary between studies[31,32]. Dual-energy X-ray absorptiometry can assess lean muscle mass, particular appendicular skeletal muscle mass, but may have limited availability. Bioelectrical impedance analysis is noninvasive, convenient to use, but may not be as high yield for skeletal muscle mass, as it measures different body compartments, with limitations in obese or cachectic patients from body mass disproportion[33]. Future studies will be needed to assess criterion validity in order to find the best consensus definition for sarcopenia in both clinical and research settings.
Given the close relationship between MASLD and sarcopenia, a multipronged approach to management is important. The mainstay therapy for MASLD is weight loss, which can be achieved through diet and regular physical activity, and improve hepatic steatosis and liver stiffness[34-36]. The treatment for sarcopenia similarly involves nutritional supplementation and regular physical activity to prevent deterioration of muscle mass, maintain muscle strength, and mitigate mobility disability[37,38]. Both MASLD and sarcopenia can benefit from physical activity. However, its impact may be limited in later stages of disease due to increased frailty[39]. One retrospective study found that moderate to vigorous physical activity (exercise average 4-5 times/wk, 555 min/wk) was associated with less risk for sarcopenia based on the measurement of higher skeletal muscle index and hand grip strength[40]. Prospective studies investigating the direct benefit of physical activity on progressive frailty, decline in muscle function, or muscle mass in the late stages of MASLD and sarcopenia are needed in the future.
Based on current pathophysiological knowledge, potential therapy targets along the liver-muscle axis may include testosterone supplementation, strength resistance training, and high protein diet. Testosterone is an anabolic hormone that helps maintain muscle mass and function, and can decrease with normal aging[41]. Testosterone can induce muscle fiber hypertrophy and may be protective against skeletal muscle catabolism by suppressing accumulation of inflammatory transcription factors[42]. There is also an association between MASLD and lower testosterone, as men with MASLD on average have lower testosterone than men without MASLD[43]. Current studies have suggested that testosterone can have a dose-dependent effect to achieve clinically significant gains in muscle mass without adversely affecting cardiovascular risk, but it varies by subject, dosage, and route of administration[41,44,45]. Many current studies were done in healthy young men with low to low-normal testosterone levels, so testosterone may not have as similar effect in frail older adults or patients with chronic illness and warrants further investigation for sarcopenia-directed therapy.
Resistance strength training has been suggested as an alternative therapy target. Physiologically, heavy resistance exercise can trigger the release of various anabolic hormones, including testosterone[46]. Resistance training typically involves repeated, systematic, regular exercises aimed at improving a patient’s physical ability and muscle strength, with both upper and lower body exercises[47-49]. However, resources and engagement may be limited for patients in the community, with a large degree of variability in the implementation of physical activity programs, making it difficult to maintain any muscle mass or strength gains. Further high-quality clinical trials are needed to develop optimal resistant training parameters.
While optimal diets for MASLD are still being explored, high protein intake (1.0-1.2 g/kg/d) has been recommended by the European Society for Clinical Nutrition and Metabolism (ESPEN) for older adults to potentially counteract sarcopenia[50]. Aging patients typically develop anabolic resistance, requiring greater amounts of protein to stimulate the same response of muscle protein synthesis compared to younger adults. Therefore, it has been suggested that if anabolic resistance can be overcome by diet, muscle atrophy may be prevented. However, studies have shown variable effect based on type of protein (plant vs animal), with some even suggesting that higher protein intake is associated with sarcopenia rather than protective[51,52]. One longitudinal study showed that patients had increased risk of sarcopenia despite adequate protein intake[53]. High protein diet also has detrimental effects to other diseases that affect older adults, including coronary artery disease, bone and calcium metabolism, and cancer, so must be implemented with caution[54]. Additional research is needed to explore the effect of different protein sources and clarify optimal protein intake requirements.
Despite ongoing research on the promising effects of these therapies, further research is needed to validate these therapeutic interventions and their long-term effects on sarcopenia and MASLD.
Both sarcopenia and MASLD are associated with significant health risks, as pointed out by Viswanath et al[1] in their review. Therefore, early identification and adequate diagnosis are important. The recently updated consensus definition for MASLD may allow researchers to better identify proper cohorts for study, while gold standard techniques to measure muscle mass are needed in consensus. Given similar pathophysiology and shared risk factors, future research may optimize treatment strategies to target both of these complex diagnoses to maximize the outcome.
| 1. | Viswanath A, Fouda S, Fernandez CJ, Pappachan JM. Metabolic-associated fatty liver disease and sarcopenia: A double whammy. World J Hepatol. 2024;16:152-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 2. | Wong R, Fortune BE. The Shift from nonalcoholic fatty liver disease to metabolic dysfunction-associated fatty liver disease. Clin Liver Dis (Hoboken). 2022;20:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 3. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1295] [Article Influence: 647.5] [Reference Citation Analysis (1)] |
| 4. | Therakomen V, Petchlorlian A, Lakananurak N. Prevalence and risk factors of primary sarcopenia in community-dwelling outpatient elderly: a cross-sectional study. Sci Rep. 2020;10:19551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 5. | Hong SH, Choi KM. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 204] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 6. | Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, Volpi E. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes. 2007;56:1615-1622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Liu ZJ, Zhu CF. Causal relationship between insulin resistance and sarcopenia. Diabetol Metab Syndr. 2023;15:46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 8. | Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753-4761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 642] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 9. | Nachit M, De Rudder M, Thissen JP, Schakman O, Bouzin C, Horsmans Y, Vande Velde G, Leclercq IA. Myosteatosis rather than sarcopenia associates with non-alcoholic steatohepatitis in non-alcoholic fatty liver disease preclinical models. J Cachexia Sarcopenia Muscle. 2021;12:144-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Wijarnpreecha K, Kim D, Raymond P, Scribani M, Ahmed A. Associations between sarcopenia and nonalcoholic fatty liver disease and advanced fibrosis in the USA. Eur J Gastroenterol Hepatol. 2019;31:1121-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Hsieh YC, Joo SK, Koo BK, Lin HC, Kim W. Muscle alterations are independently associated with significant fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2021;41:494-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, Lee KL, Kim W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 340] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 13. | Jo IH, Song DS, Chang UI, Yang JM. Change in skeletal muscle mass is associated with hepatic steatosis in nonalcoholic fatty liver disease. Sci Rep. 2023;13:6920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 14. | Kim G, Lee SE, Lee YB, Jun JE, Ahn J, Bae JC, Jin SM, Hur KY, Jee JH, Lee MK, Kim JH. Relationship Between Relative Skeletal Muscle Mass and Nonalcoholic Fatty Liver Disease: A 7-Year Longitudinal Study. Hepatology. 2018;68:1755-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 15. | Evans PL, McMillin SL, Weyrauch LA, Witczak CA. Regulation of Skeletal Muscle Glucose Transport and Glucose Metabolism by Exercise Training. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 16. | Kouvari M, Polyzos SA, Chrysohoou C, Skoumas J, Pitsavos CS, Panagiotakos DB, Mantzoros CS. Skeletal muscle mass and abdominal obesity are independent predictors of hepatic steatosis and interact to predict ten-year cardiovascular disease incidence: Data from the ATTICA cohort study. Clin Nutr. 2022;41:1281-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Joo SK, Kim W. Interaction between sarcopenia and nonalcoholic fatty liver disease. Clin Mol Hepatol. 2023;29:S68-S78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 18. | Welch N, Attaway A, Bellar A, Alkhafaji H, Vural A, Dasarathy S. Compound Sarcopenia in Hospitalized Patients with Cirrhosis Worsens Outcomes with Increasing Age. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Iwaki M, Kobayashi T, Nogami A, Saito S, Nakajima A, Yoneda M. Impact of Sarcopenia on Non-Alcoholic Fatty Liver Disease. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 20. | Roh E, Hwang SY, Yoo HJ, Baik SH, Lee JH, Son SJ, Kim HJ, Park YS, Lee SG, Cho BL, Jang HC, Kim BJ, Kim M, Won CW, Choi KM. Impact of non-alcoholic fatty liver disease on the risk of sarcopenia: a nationwide multicenter prospective study. Hepatol Int. 2022;16:545-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | Sinn DH, Kang D, Kang M, Guallar E, Hong YS, Lee KH, Park J, Cho J, Gwak GY. Nonalcoholic fatty liver disease and accelerated loss of skeletal muscle mass: A longitudinal cohort study. Hepatology. 2022;76:1746-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 22. | Issa D, Alkhouri N, Tsien C, Shah S, Lopez R, McCullough A, Dasarathy S. Presence of sarcopenia (muscle wasting) in patients with nonalcoholic steatohepatitis. Hepatology. 2014;60:428-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Kumar R, Prakash SS, Priyadarshi RN, Anand U. Sarcopenia in Chronic Liver Disease: A Metabolic Perspective. J Clin Transl Hepatol. 2022;10:1213-1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS One. 2017;12:e0186990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 244] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 25. | Kim D, Wijarnpreecha K, Sandhu KK, Cholankeril G, Ahmed A. Sarcopenia in nonalcoholic fatty liver disease and all-cause and cause-specific mortality in the United States. Liver Int. 2021;41:1832-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 26. | Golabi P, Gerber L, Paik JM, Deshpande R, de Avila L, Younossi ZM. Contribution of sarcopenia and physical inactivity to mortality in people with non-alcoholic fatty liver disease. JHEP Rep. 2020;2:100171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 27. | Sun X, Liu Z, Chen F, Du T. Sarcopenia modifies the associations of nonalcoholic fatty liver disease with all-cause and cardiovascular mortality among older adults. Sci Rep. 2021;11:15647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Coletta G, Phillips SM. An elusive consensus definition of sarcopenia impedes research and clinical treatment: A narrative review. Ageing Res Rev. 2023;86:101883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 101] [Reference Citation Analysis (0)] |
| 29. | Mayhew AJ, Raina P. Sarcopenia: new definitions, same limitations. Age Ageing. 2019;48:613-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6646] [Cited by in RCA: 7782] [Article Influence: 1297.0] [Reference Citation Analysis (1)] |
| 31. | Engelke K, Museyko O, Wang L, Laredo JD. Quantitative analysis of skeletal muscle by computed tomography imaging-State of the art. J Orthop Translat. 2018;15:91-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 32. | Lee K, Shin Y, Huh J, Sung YS, Lee IS, Yoon KH, Kim KW. Recent Issues on Body Composition Imaging for Sarcopenia Evaluation. Korean J Radiol. 2019;20:205-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 33. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6987] [Cited by in RCA: 8454] [Article Influence: 563.6] [Reference Citation Analysis (0)] |
| 34. | Koutoukidis DA, Koshiaris C, Henry JA, Noreik M, Morris E, Manoharan I, Tudor K, Bodenham E, Dunnigan A, Jebb SA, Aveyard P. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism. 2021;115:154455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 35. | Kumar S, Wong R, Newberry C, Yeung M, Peña JM, Sharaiha RZ. Multidisciplinary Clinic Models: A Paradigm of Care for Management of NAFLD. Hepatology. 2021;74:3472-3478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 36. | Oh S, So R, Shida T, Matsuo T, Kim B, Akiyama K, Isobe T, Okamoto Y, Tanaka K, Shoda J. High-Intensity Aerobic Exercise Improves Both Hepatic Fat Content and Stiffness in Sedentary Obese Men with Nonalcoholic Fatty Liver Disease. Sci Rep. 2017;7:43029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 37. | Robinson S, Granic A, Cruz-Jentoft AJ, Sayer AA. The role of nutrition in the prevention of sarcopenia. Am J Clin Nutr. 2023;118:852-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 38. | Angulo J, El Assar M, Álvarez-Bustos A, Rodríguez-Mañas L. Physical activity and exercise: Strategies to manage frailty. Redox Biol. 2020;35:101513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 383] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 39. | Lai JC, Tandon P, Bernal W, Tapper EB, Ekong U, Dasarathy S, Carey EJ. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1611-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 394] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 40. | Seo JH, Lee Y. Association of physical activity with sarcopenia evaluated based on muscle mass and strength in older adults: 2008-2011 and 2014 - 2018 Korea National Health and Nutrition Examination Surveys. BMC Geriatr. 2022;22:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 41. | Bhasin S. Testosterone supplementation for aging-associated sarcopenia. J Gerontol A Biol Sci Med Sci. 2003;58:1002-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Urban RJ, Dillon EL, Choudhary S, Zhao Y, Horstman AM, Tilton RG, Sheffield-Moore M. Translational studies in older men using testosterone to treat sarcopenia. Trans Am Clin Climatol Assoc. 2014;125:27-42; discussion 42. [PubMed] |
| 43. | Hutchison AL, Tavaglione F, Romeo S, Charlton M. Endocrine aspects of metabolic dysfunction-associated steatotic liver disease (MASLD): Beyond insulin resistance. J Hepatol. 2023;79:1524-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 95] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 44. | Parahiba SM, Ribeiro ÉCT, Corrêa C, Bieger P, Perry IS, Souza GC. Effect of testosterone supplementation on sarcopenic components in middle-aged and elderly men: A systematic review and meta-analysis. Exp Gerontol. 2020;142:111106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Shin MJ, Jeon YK, Kim IJ. Testosterone and Sarcopenia. World J Mens Health. 2018;36:192-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 46. | Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM. Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports Med. 2010;40:1037-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 289] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 47. | Hurst C, Robinson SM, Witham MD, Dodds RM, Granic A, Buckland C, De Biase S, Finnegan S, Rochester L, Skelton DA, Sayer AA. Resistance exercise as a treatment for sarcopenia: prescription and delivery. Age Ageing. 2022;51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 139] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 48. | Law TD, Clark LA, Clark BC. Resistance Exercise to Prevent and Manage Sarcopenia and Dynapenia. Annu Rev Gerontol Geriatr. 2016;36:205-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 49. | Zhao H, Cheng R, Song G, Teng J, Shen S, Fu X, Yan Y, Liu C. The Effect of Resistance Training on the Rehabilitation of Elderly Patients with Sarcopenia: A Meta-Analysis. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 50. | Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznariç Z, Nair KS, Singer P, Teta D, Tipton K, Calder PC. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 1055] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 51. | Coelho-Junior HJ, Calvani R, Azzolino D, Picca A, Tosato M, Landi F, Cesari M, Marzetti E. Protein Intake and Sarcopenia in Older Adults: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 52. | Ni Lochlainn M, Bowyer RCE, Welch AA, Whelan K, Steves CJ. Higher dietary protein intake is associated with sarcopenia in older British twins. Age Ageing. 2023;52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 53. | Granic A, Mendonça N, Sayer AA, Hill TR, Davies K, Siervo M, Mathers JC, Jagger C. Effects of dietary patterns and low protein intake on sarcopenia risk in the very old: The Newcastle 85+ study. Clin Nutr. 2020;39:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 54. | Delimaris I. Adverse Effects Associated with Protein Intake above the Recommended Dietary Allowance for Adults. ISRN Nutr. 2013;2013:126929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |