Published online May 27, 2024. doi: 10.4254/wjh.v16.i5.832
Revised: February 2, 2024
Accepted: March 5, 2024
Published online: May 27, 2024
Processing time: 163 Days and 6.3 Hours
Metabolic-dysfunction associated steatotic liver disease (MASLD) is a hepatic manifestation of metabolic syndrome. Studies suggest ornithine aspartate (LOLA) as drug therapy.
To analyze the influence of LOLA intake on gut microbiota using a nutritional model of MASLD.
Adult male Sprague Dawley rats were randomized into three groups: Control (10 rats fed with a standard diet), MASLD (10 rats fed with a high-fat and choline-deficient diet), and LOLA (10 rats receiving 200 mg/kg/d LOLA, after the 16th week receiving high-fat and choline-deficient diet). After 28 wk of the experiment, animals were euthanized, and feces present in the intestine were collected. Following fecal DNA extraction, the V4 region of the 16S rRNA gene was amplified followed by sequencing in an Ion S5™ system.
Alpha and beta diversity metrics were comparable between MASLD and LOLA. 3 OTUs were differentially abundant between MASLD and LOLA, which belong to the species Helicobacter rodentium, Parabacteroides goldsteinii, and Parabacteroides distasonis. The functional prediction provided two different metabolic profiles between MASLD and LOLA. The 9 pathways differentially abundant in MASLD are related to a change in energy source, adenosine/purine nucleotides degradation as well as guanosine and adenosine deoxyribonucleotides biosynthesis. The 14 pathways differentially abundant in LOLA are associated with four major metabolic functions primarily influenced by L-aspartate, including tricarboxylic acid cycle pathways, purine/guanosine nucleotides biosynthesis, pyrimidine ribonucleotides biosynthesis and salvage as well as lipid IVA biosynthesis.
Although LOLA had no influence on alpha and beta diversity in this nutritional model of MASLD, it was associated with changes in specific gut microbes and their related metabolic pathways.
Core Tip: In this nutritional model of metabolic dysfunction-associated steatotic liver disease, ornithine aspartate administration did not exhibit any impact on the alpha and beta diversity of the gut microbiota. Nonetheless, notable distinctions were noted among the cohorts concerning Parabacteroides goldsteinii and Parabacteroides distasonis, both of which pose as promising prospects for advanced probiotics owing to their advantageous implications in mitigating inflammation and addressing obesity. The variances observed in bacterial composition and metabolic profiles were directly associated with the presence of L-aspartate.
- Citation: Lange EC, Rampelotto PH, Longo L, de Freitas LBR, Uribe-Cruz C, Alvares-da-Silva MR. Ornithine aspartate effects on bacterial composition and metabolic pathways in a rat model of steatotic liver disease. World J Hepatol 2024; 16(5): 832-842
- URL: https://www.wjgnet.com/1948-5182/full/v16/i5/832.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i5.832
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a general term for a hepatic manifestation of metabolic syndrome, with a complex pathophysiology. The disease is usually silent and asymptomatic, manifesting in more advanced and irreversible stages of liver damage[1,2].
Currently, there is no specific drug therapy approved for MASLD. Several studies are investigating promising drugs, like peroxisome proliferator-activated receptors (PPARs) and glucagon-like peptide 1 (GLP-1) agonists[3] and have recently been suggested that ornithine aspartate (LOLA) can be useful[4]. LOLA improves the capacity of the circulating ammonia reduction system through the dissociation and absorption of its amino acids[5]. The relationship between gut microbiota and amino acids occurs in a bidirectional way; while bacteria play a role in the metabolism and synthesis of amino acids, amino acids contribute to survival and homeostasis of the microbiota[6,7]. In addition, due to the close relationship between the gut and liver, dysbiosis (i.e., the imbalance of the gut microbiota) has been linked to the progression of MASLD, and a large body of evidence suggests that microbiota modulation may be an important target in the treatment of MASLD[8].
Here, we further explored the influence of LOLA intake on the gut microbiota and related metabolic pathways using an experimental model of MASLD, providing evidence on the molecular mechanisms of how LOLA induces changes in probiotic microbes and their metabolism.
Thirty adult (60 d old) male Sprague Dawley rats were used. The animals were randomized by weight, and two rats were housed in each polypropylene cage with sawdust-covered floors. They were kept in a controlled temperature environment (22 ± 2 °C), and a 12-hour light/dark cycle. The methods can be accessed in detail in the article recently published by the group[9].
The animals were randomized into three groups, control (10 rats fed with a standard diet), MASLD (10 rats fed with a high-fat and choline-deficient diet; HFCD), and LOLA (10 rats receiving 200 mg/kg/d LOLA, after the 16th week receiving HFCD diet)[9-11]. The animals in the control and MASLD groups were weighed weekly throughout the experiment to track body weight, whereas those in the LOLA group were initially weighed weekly until week 16 and then twice a week thereafter to facilitate adjustments in LOLA doses. After 28 wk of the experiment, all animals were euthanized. The livers were collected aseptically, and their weights were measured. This biological material was stored for the quantitative assessment of fat deposits, as described below. Additionally, feces present in the intestine were collected aseptically, frozen in liquid nitrogen, and stored in a -80ºC freezer until the proposed evaluation.
All the animals who received HFCD presented induced MASLD, and no animal died during the experimental period. All experimental procedures were approved by the Ethics Committee for the Use of Animals of Hospital de Clínicas de Porto Alegre (Protocol number: 2020-0037). The procedures for the use of scientific animals were conducted following the Guide for the Care and Use of Laboratory Animals, 8th ed[12].
To analyze hepatic lipid content, the previously frozen liver tissue samples were thawed on ice and homogenized in phosphate buffer saline (20 mg of tissue/mL). Using this homogenate, the concentrations of triglycerides, total cholesterol, and total lipid accumulation were evaluated. Hepatic triglycerides and total cholesterol were assayed enzymatically using colorimetry (Labtest Diagnóstica SA, Brazil). The total lipid concentration was determined using the modified protocol of Gómez-Lechón et al[13]. Briefly, liver tissue was homogenized in phosphate buffer saline and incubated with 1 μL of Nile Red solution (1 mg/mL in acetone) at 37 °C for 15 min. Fluorescence was measured at 488 nm excitation and 550 nm emission wavelengths (SpectraMax M3). The values obtained were normalized to the total protein content of the homogenate[14]. The results are expressed as fluorescence/μg protein. All analyses were performed in duplicate.
The genomic material was obtained from approximately 200 mg of the fecal sample with an appropriate commercial kit (QIAmp DNA Stool Mini kit, Qiagen, Hilden, Germany) following the manufacturer's instructions. At the end of the DNA extraction, an average of 5-10 μg of genomic material was obtained. The hypervariable V4 region from the rRNA gene was amplified through PCR using genomic DNA (approximately 50 ng per reaction) and the following primer pair: 515F (5'-GTGCCAGCMGCCGCGGTAA-3') and 806R (5'-GGACTACHVGGGTWTCTAAT-3'). To pool different samples in the same reaction, we used the primer-fusion method, and each sample had a distinct barcode attached to the corresponding PCR product. The amplification was performed using Platinum™ PCR SuperMix High Fidelity (Invitro-gen, Carlsbad, CA, United States). PCR was conducted under the following conditions: 95 ºC for 600 s and thereafter 30 cycles of 95 ºC for 30 s, 57 ºC for 30 s, and 72 ºC for 60 s with a final extension of 72 ºC for 60 s. The products were verified through electrophoresis in an agarose gel, purified with AMPure XP PCR Purification Kit (Beckman Coulter, Brea, CA, United States), quantified using Qubit™ dsDNA HS Assay Kit (Invitrogen, Carlsbad, CA, United States), and subjected to emulsion PCR using Ion Chef™ System (Thermo Fisher Scientific, Waltham, MA, United States). After, the resulting enriched beads were sequenced in Ion S5™ System (Thermo Fisher Scientific, Waltham, MA, United States) using Ion 510™ Chip Kit (Thermo Fisher Scientific, Waltham, MA, United States).
The sequence data exported from the Ion S5™ System was processed using a custom pipeline in Mothur v.1.47.0[15]. Initially, sequences were depleted of barcodes and primers (where no mismatch was allowed), and then a quality filter was applied to eliminate low-quality reads. Quality control was conducted by trimming the low-quality reads, those with incorrect length, those containing an ambiguous base, or containing homopolymers longer than 6 bp. All potentially chimeric sequences were identified and removed using VSEARCH[16]. In addition, singletons were removed to avoid potentially spurious sequences derived from PCR or sequencing errors.
After these initial quality filtering and trimming steps, the remaining sequences were clustered into operational taxonomic units (OTUs) based on a 99% identity level and were classified against the SILVA v138 reference database at 97% similarity. Sequences that could not be classified (i.e., “unknown” sequences), as well as sequences identified as eukaryotes, mitochondria, and chloroplasts, were removed before further analysis. After all filtering steps, the resulting OTUs table was composed of 754725 sequences, with an average of 27952 sequences per sample. The resulting OTUs table was rarefied to the smallest library size (i.e., 17109). Subsequent analyses of the sequence dataset were performed in R v. 4.0.0 using vegan, phyloseq, ggplot2, and Microbi-omeAnalystR packages.
Alpha diversity was assessed using the number of observed taxa, ACE, and Shannon index. Statistical confidence was accessed using the multivariate Kruskal-Wallis test. For the overall comparison of significant differences among bacterial communities (i.e., beta diversity), principal coordinates analysis (PCoA) was performed. A matrix using the Bray–Curtis dissimilarity metric was calculated for each pair of samples. To achieve statistical confidence for the sample grouping observed by PCoA, the ANOSIM multivariate test was performed on the distance matrix. To compare additional differences among the microbial communities, clustering methods based on Bray–Curtis dissimilarity were performed. The results of hierarchical clustering were visualized using dendrograms. Venn dendrogram was generated using InteractiVenn[17].
To identify differentially abundant taxa at the OTU level, the linear discriminant effect size (Lefse) method was performed[18]. The algorithm performs a nonparametric factorial Kruskal–Wallis sum rank test and linear discriminant analysis (LDA) to determine statistically significant different features among taxa and estimates the effect size of the difference. Differences were considered significant for a logarithmic LDA score threshold of ± 2.0 and a P-value < 0.05. To further refine the taxonomic classification of the differentially abundant OTUs down to the species level, we blasted the reference sequence of the OTUs against the NCBI 16S rRNA database by using BLAST.
Metabolic pathways were predicted using the OTU sequences (at 99% similarity) and their abundance in PICRUSt2[19]. The functional annotation of PICRUSt2 predictions was obtained based on the MetaCyc database. MetaCyc pathway abundances are calculated in PICRUSt2 through structured mappings of EC gene families to pathways. Differentially abundant pathways were determined using Lefse. Differences were considered significant for a logarithmic LDA score threshold of ± 4.0 and a P value < 0.05.
The general characteristics of the experimental model developed regarding body weight gains of the rats, liver weight, as well the concentrations of triglycerides, total cholesterol, and total lipid accumulation in the liver, are available in Table 1.
| Variables | Control | MASLD | LOLA | P value |
| Body weight (g) | 180.9 ± 37.4a | 325.1 ± 92.2c | 310 ± 56.1c | < 0.001 |
| Fresh liver weight (g) | 17.2 ± 2.4a | 30.8 ± 4.3c | 27.3 ± 2.6c | < 0.001 |
| Triglycerides (mg/dL) | 20.52 ± 3.4a | 94.51 ± 30.4c | 90.94 ± 33.2c | < 0.001 |
| Total cholesterol (mg/dL) | 3.17 ± 1.9a | 45.57 ± 8.4c | 39.88 ± 13.8c | < 0.001 |
| Total lipid accumulation (fluorecence/μg protein) | 160.3 ± 32.2a | 2921 ± 769.9c | 2123 ± 576c | < 0.001 |
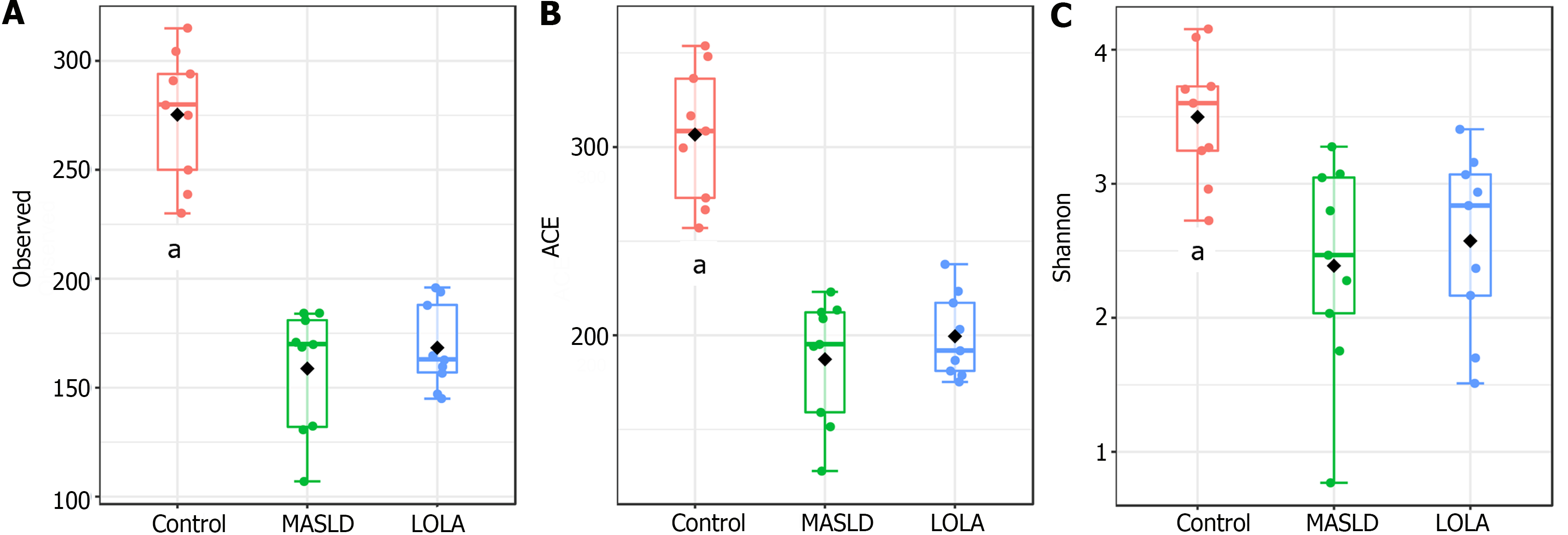
The number of observed taxa, ACE, and Shannon indexes were used to compare the alpha diversity among the three groups, i.e., control, MASLD, and LOLA (Figure 1). Significant differences were observed when control was compared to MASLD and LOLA (P = 0.0001, P = 0.0001, and P = 0.005 for observed taxa, ACE, and Shannon, respectively). However, no difference was observed between MASLD and LOLA (P = 0.6, P = 0.9, and P = 0.6 for observed taxa, ACE, and Shannon, respectively).
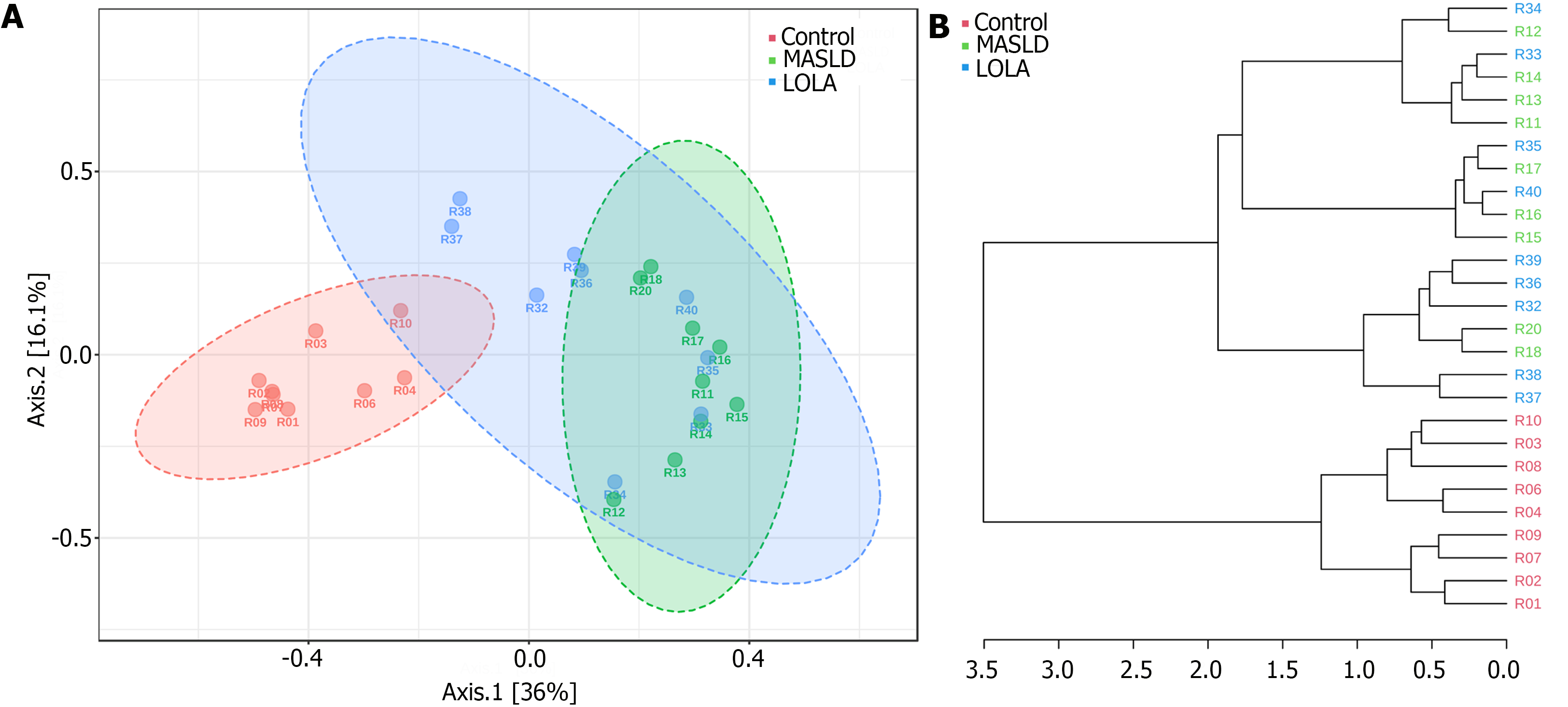
PCoA analysis and dendrogram clustering (based on the Bray–Curtis metric) was used to determine the clustering of samples and to better understand similarities and differences among the bacterial community structures (Figure 2). Significant differences were observed when control was compared to MASLD and LOLA, which was confirmed by ANOSIM (P = 0.001). However, no difference was observed between MASLD and LOLA groups (ANOSIM, P = 0.1).
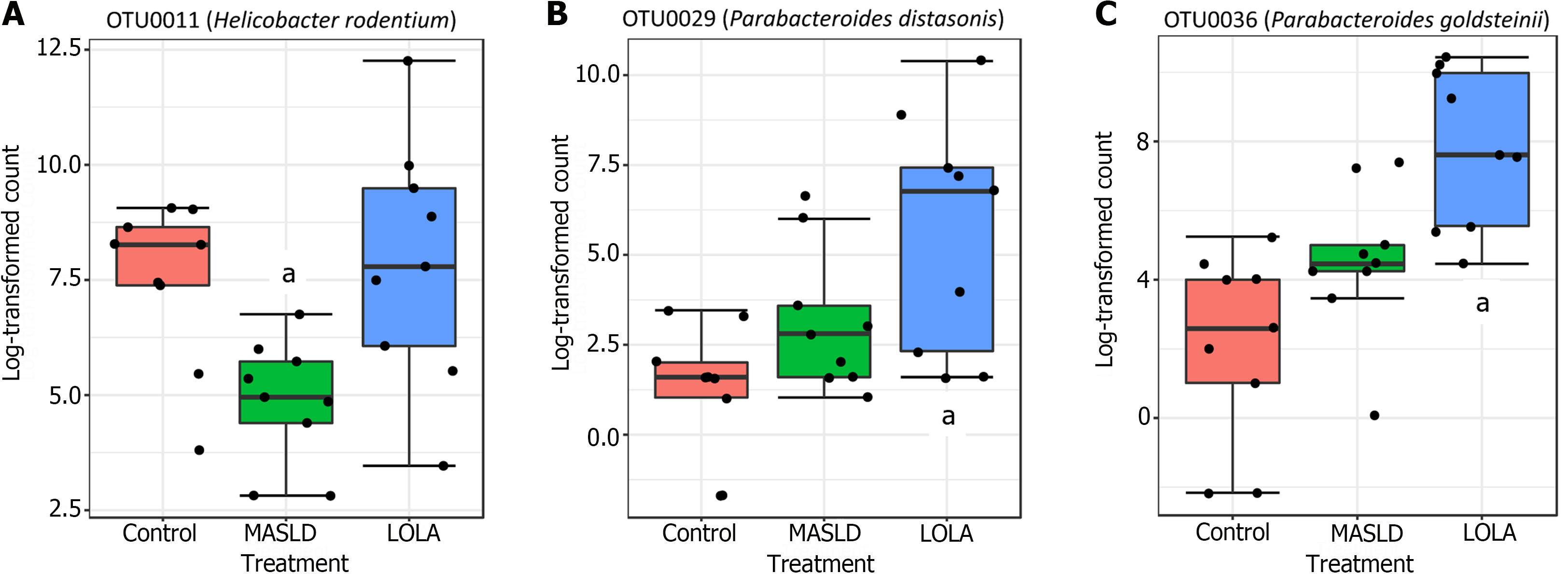
Differentially abundant microbiota: In total, 22 OTUs were differentially abundant among groups, which belong to 12 genera/families according to the SILVA rRNA database. Among these 22 OTUs, only 3 differed between LOLA and MASLD (Helicobacter rodentium, Parabacteroides goldsteinii, and Parabacteroides distasonis) (Figure 3). The other OTUs showed differential abundance relative to the control group: 10 OTUs increased at MASLD and LOLA (Romboutsia ilealis, Bacteroides vulgatus, Lachnospiraceae_unclassified (2 OTUs), Tyzzerella nexilis, Faecalimonas umbilicata, Peptococcaceae uncultured, Blautia pseudococcoides or Blautia hominis, Ruthenibacterium lactatiformans, and Anaerotignum faecicola) (Supplementary Figure 1 and 9) OTUs decreased at MASLD and LOLA (Lactobacillus gasseri, Lachnospiraceae_unclassified, Lachnospiraceae_NK4A136_group (5 OTUs), and Muribaculaceae_ge) (Supplementary Figure 2).
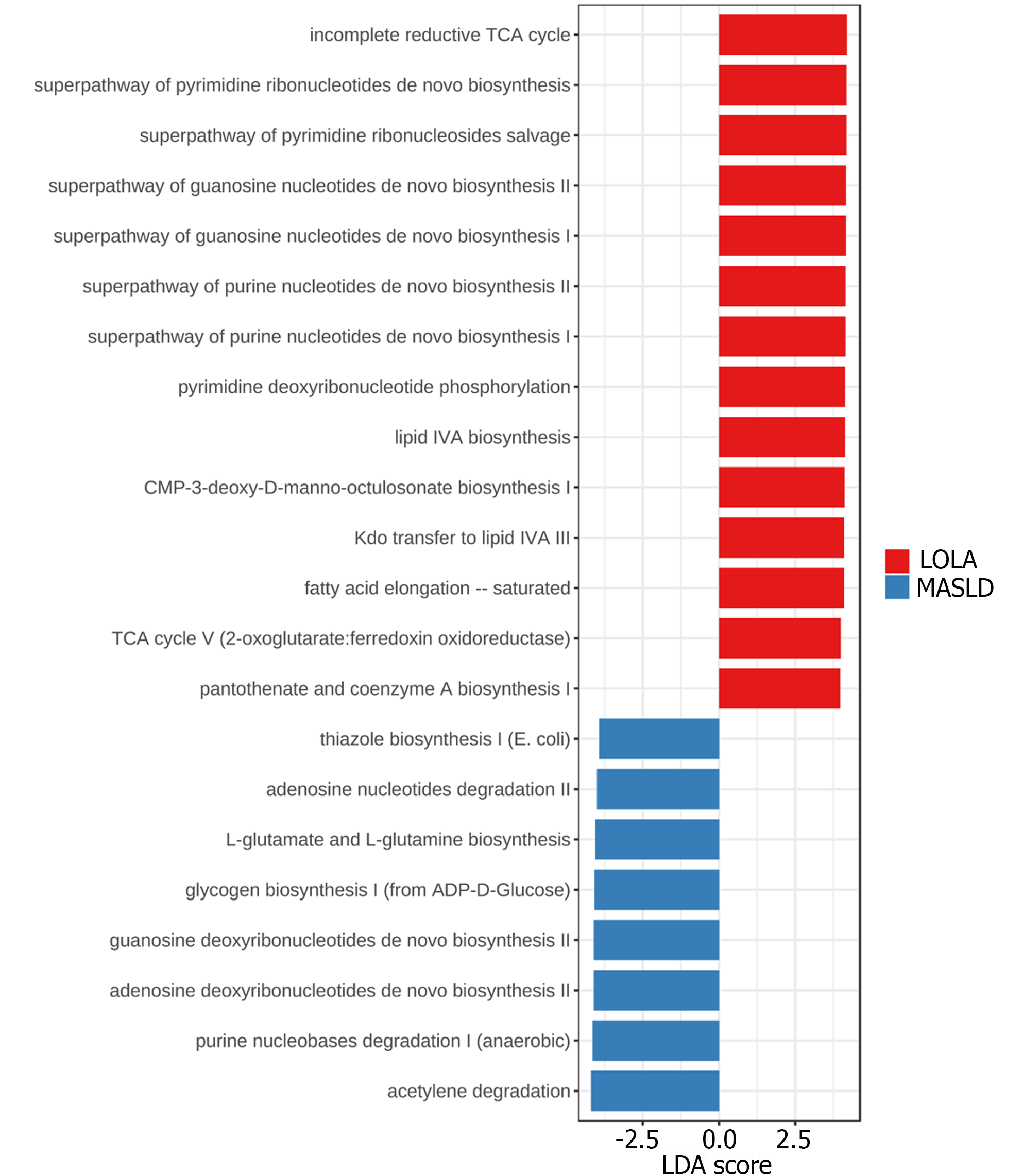
The metabolic prediction was calculated using PICRUSt2 and the MetaCyc database. In total, 22 differentially abundant microbial metabolic pathways were identified between MASLD and LOLA (Figure 4).
The 8 pathways differentially abundant in MASLD are related to a change in energy source, adenosine, and purine nucleotides degradation, as well as guanosine and adenosine deoxyribonucleotides biosynthesis. The 14 pathways differentially abundant in LOLA are related to four major metabolic functions, including tricarboxylic acid cycle pathways, purine/guanosine nucleotides biosynthesis, pyrimidine ribonucleotides biosynthesis and salvage as well as lipid IVA biosynthesis (Supplementary Figures 3 and 4).
In addition, the lipid IVA biosynthesis pathways were restored to a level like control (Supplementary Figure 4B), while TCA cycle (Supplementary Figure 3A) and nucleotides (Supplementary Figure 3B), and ribonucleotides biosynthesis (Supplementary Figure 4A) pathways did not present differences between MASLD and control, meaning these pathways increased in LOLA do not worsen the conditions when MASLD was induced.
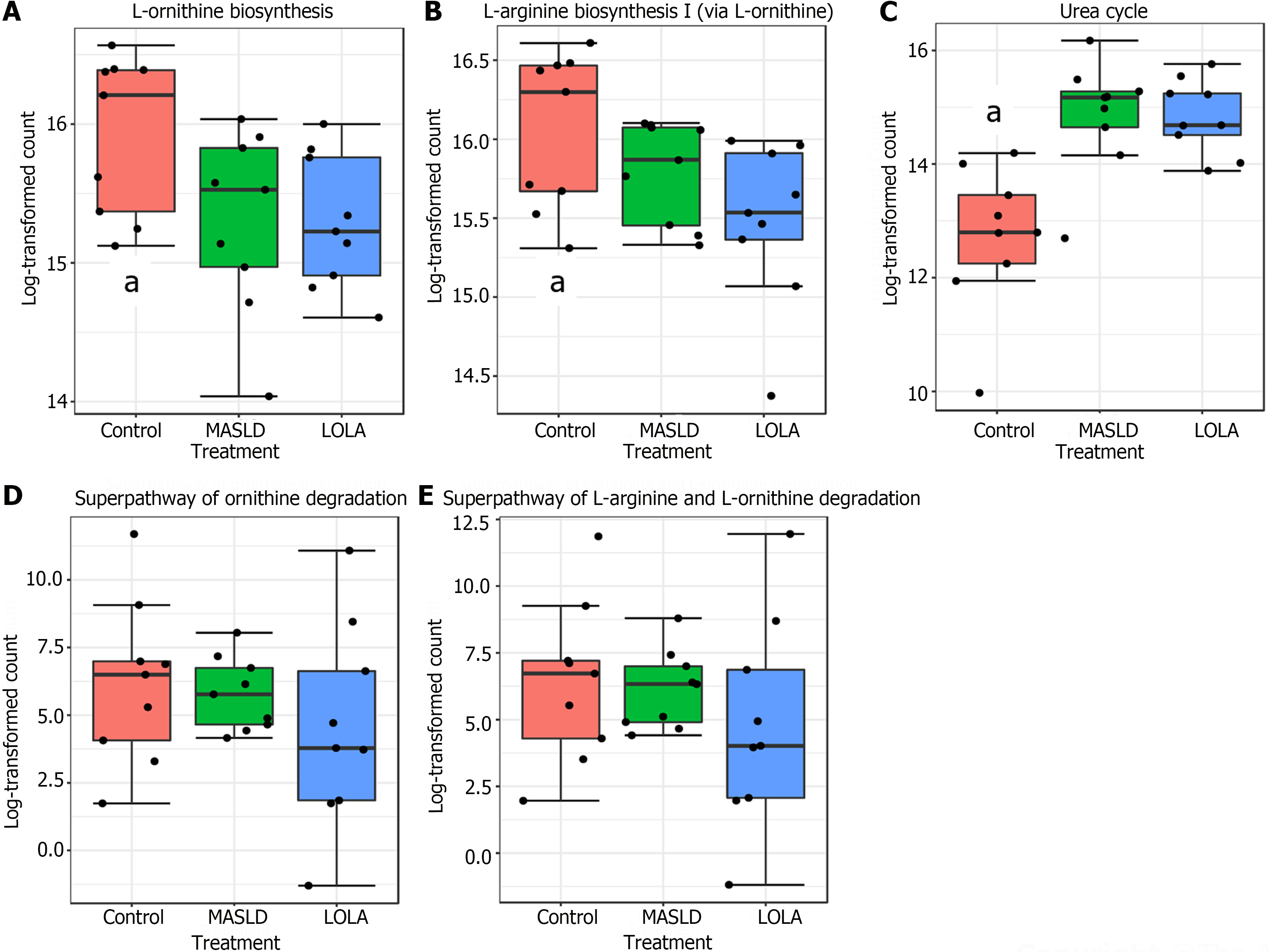
Worth of note, all pathways related to L-ornithine did not present significant differences when comparing LOLA and MASLD (Figure 5). Of special interest, the urea cycle was increased in MASLD when compared to control, and treatment with LOLA was not able to restore its levels.
In this study, we demonstrated that LOLA may induce changes in bacterial composition and metabolic pathways in a nutritional rat model of MASLD, that mirrors human disease. It is noteworthy that in a previous study conducted by our research team using this experimental model, histopathological analysis revealed that subjects treated with HFCD exhibited a predominant occurrence of microvesicular steatosis, in conjunction with moderate macrovesicular steatosis. These findings were accompanied by minimal signs of inflammatory activity and occasional fibrosis. Furthermore, improvements were observed in liver disease parameters (biochemistry, collagen deposition, miRNAs) and cardiovascular health (systemic inflammation and cardiomyocyte integrity)[9-11].
Butterworth and Canbay suggest that LOLA can induce beneficial effects on MASLD based not only on decreasing ammonia, but also through its transformation in L-glutamine, L-arginine, and glutathione. However, as the evidence is scarce, LOLA is not used in MASLD[4,20]. So far, only a recent work analyzed microbiota changes at LOLA treatment. Horvath et al[21], 2022, evaluated the microbiota composition of 15 cirrhotic patients treated or not treated with LOLA. The authors reported no differences in alpha and beta diversities, but significant differential abundances in two genera (i.e., Flavonifractor and Oscilospira) were found in patients treated with LOLA. It is important to emphasize that cirrhosis itself can induce several important changes in gut microbiota[22,23]. Our study also did not show any difference in alpha and beta diversities comparing LOLA to MASLD.
However, in the present study, 22 OTUs were differentially abundant among groups; among them, only 3 differed between LOLA and MASLD, namely: Helicobacter rodentium, Parabacteroides goldsteinii, and Parabacteroides distasonis. The other OTUs showed an increase (10 OTUs) or decrease (9 OTUs) in control when compared to both intervention groups (MASLD and LOLA), but no significant differentiation was observed between MASLD and LOLA. Among the 3 OTUs that differed between MASLD and LOLA, the development of the disease only caused a reduction in the relative abundance of Helicobacter rodentium, a normal colonizer of the gastrointestinal tract of rats, while LOLA intervention restored its abundance to the control levels. A possible hypothesis is that Helicobacter rodentium could be benefited from the presence of macromolecules, such as amino acids, as a source of nitrogen for metabolic pathways, increasing its abundance with LOLA intervention and restoring it to control levels[24]. Interestingly, Parabacteroides goldsteinii and Parabacteroides distasonis are two species recently identified as candidates for next-generation probiotics due to its beneficial effects in preventing and treating inflammation and obesity[25,26]. The mechanisms by which these probiotics act may be related to a reduction in adipocyte hypertrophy, recovery of gene expression involved in lipid transport, lipogenesis, and β-oxidation in the liver in addition to maintaining intestinal integrity, inducing thermogenesis, and reducing endotoxemia and inflammation, thus preserving intestinal homeostasis and improve gut barrier functions in vivo[27]. Taken together, the findings suggest that LOLA may act in MASLD by secondary mechanisms, affecting specific probiotic microbes and attenuating inflammation and fat accumulation.
The functional prediction provided two different metabolic profiles between MASLD and LOLA. In MASLD, the change in energy source is evidenced by the acetylene degradation and glycogen biosynthesis, which indicates that acetylene is been used as a source of carbon and energy (characteristic of anaerobes) while glucose metabolism has been suppressed and stored in glycogen. Thiazole biosynthesis, which synthesizes the precursor of thiamine (vitamin B1), is related to the storage of glucose in glycogen because thiamine is able to decrease hyperglycemia and increase the glycogen content[28]. The nucleotide degradation and deoxyribonucleotide biosynthesis is another interesting metabolic feature of MASLD involving nucleotide recycling because deoxyribonucleotides are obtained by the reduction of ribose already incorporated into nucleotides. On this matter, the L-glutamate and L-glutamine biosynthesis pathway may have an important role as L-glutamate is at the crossroads of amino acid metabolism[29], where it can donate its amino group for new amino acid synthesis or can lose the amino group via deamination to 2-oxoglutarate.
Altogether, our results indicate that the bacterial and metabolic differences in LOLA treatment are related to L-aspartate, while no differences in microbial pathways related to L-ornithine were observed when comparing LOLA and MASLD. This contrasting effect of LOLA components on the gut microbiota may be explained by the pharmacokinetic and pharmacodynamic properties of the drug[30]. As orally administered, LOLA promptly dissociates into its component amino acids L-ornithine and L-aspartate in the duodenum, which are absorbed from the small intestine by active transport across the brush border of the intestinal epithelium. Most of the aspartate is metabolized in the intestine, and the remaining serves primarily for glutamine synthesis in the liver, muscles, and brain[31]. On the other hand, L-ornithine may be quickly absorbed by the epithelium cells and, after reaching the portal blood, it is directly taken up by the periportal hepatocytes of the liver and metabolized by the mitochondria where it serves as an intermediary in the urea cycle and is an activator of carbamyl-phosphate synthetase, the rate-limiting enzyme of urea synthesis[32]. In summary, this means that, after being absorbed, L-aspartate is reabsorbed and available in the gut where it can also be used by some microorganisms, while L-ornithine is quickly absorbed and taken up to the liver. This fast absorption of L-ornithine does not leave time for microbial usage, which also explains why LOLA does not restore the elevated gut microbial urea cycle metabolism observed in MASLD.
In conclusion, although LOLA had no influence on alpha and beta diversity in this nutritional model of MASLD, it was associated with changes in specific probiotic members of the gut microbiota and their related metabolic pathways. In addition, our results indicate that the bacterial and metabolic differences in LOLA treatment are related to L-aspartate, while no differences in microbial pathways related to L-ornithine were observed when comparing LOLA and MASLD. Finally, the composition and functional prediction of the microbiota observed in our work could be complemented with metagenomics sequencing and metabolomics analysis in future studies.
There are scarce evidence of ornithine aspartate (LOLA) usefulness in metabolic-dysfunction associated steatotic liver disease (MASLD). Since LOLA comes into direct contact with the intestinal tract upon oral administration, it could interact with the intestinal microbiota. This study analyzed LOLA’s influence on gut microbiota (GM) in an experimental model of MASLD.
Considering GM emerging role in the development of MASLD and the significant role of amino acids in the protection and function of the intestinal barrier, we decided to investigate LOLA’s role on GM for a better understanding of the factors and molecular mechanisms involved in the pathophysiology of the disease.
To evaluate the diversity, structure and composition of the intestinal microbiota and identify which microbiota taxa are related to MASLD and LOLA, as well as identify and analyze the predicted metabolic pathways of the microbiota in an experimental model of MASLD with or without LOLA treatment.
Adult male Sprague Dawley rats were randomized into three groups: Control (10 rats fed with a standard diet), MASLD (10 rats fed with a high-fat and choline-deficient diet), and LOLA (10 rats receiving 200 mg/kg/d LOLA, after the 16th week receiving high-fat and choline-deficient diet). After 28 wk of the experiment, animals were euthanized and feces present in the intestine were collected. After fecal DNA extraction, the V4 region of the 16S rRNA gene was amplified followed by sequencing in an Ion S5™ system.
Alpha and beta diversity metrics were comparable between MASLD and LOLA. 3 OTUs were differentially abundant between MASLD and LOLA, which belong to the species Helicobacter rodentium, Parabacteroides goldsteinii, and Parabacteroides distasonis. The functional prediction provided two different metabolic profiles between MASLD and LOLA. The 9 pathways differentially abundant in MASLD are related to a change in energy source, adenosine/purine nucleotides degradation as well as guanosine and adenosine deoxyribonucleotides biosynthesis. The 14 pathways differentially abundant in LOLA are associated with four major metabolic functions primarily influenced by L-aspartate, including tricarboxylic acid cycle pathways, purine/guanosine nucleotides biosynthesis, pyrimidine ribonucleotides biosynthesis and salvage as well as lipid IVA biosynthesis.
Although LOLA had no influence on alpha and beta diversity in this nutritional model of MASLD, it was associated with changes in specific gut microbes and their related metabolic pathways.
Metabolomic and metagenomic sequencing analyzes in future studies, as well as a detailed evaluation of the probiotics found and their prophylactic use in MASLD are needed.
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
| 1. | Chao CY, Battat R, Al Khoury A, Restellini S, Sebastiani G, Bessissow T. Co-existence of non-alcoholic fatty liver disease and inflammatory bowel disease: A review article. World J Gastroenterol. 2016;22:7727-7734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (34)] |
| 2. | Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, Kashyap S, Mechanick JI, Mouzaki M, Nadolsky K, Rinella ME, Vos MB, Younossi Z. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28:528-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 574] [Article Influence: 191.3] [Reference Citation Analysis (1)] |
| 3. | Fraile JM, Palliyil S, Barelle C, Porter AJ, Kovaleva M. Non-Alcoholic Steatohepatitis (NASH) - A Review of a Crowded Clinical Landscape, Driven by a Complex Disease. Drug Des Devel Ther. 2021;15:3997-4009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 4. | Butterworth RF, Canbay A. Hepatoprotection by L-Ornithine L-Aspartate in Non-Alcoholic Fatty Liver Disease. Dig Dis. 2019;37:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Blanco Vela CI, Poo Ramírez JL. Efficacy of oral L-ornithine L-aspartate in cirrhotic patients with hyperammonemic hepatic encephalopathy. Ann Hepatol. 2011;10 Suppl 2:S55-S59. [PubMed] |
| 6. | Ma N, Ma X. Dietary Amino Acids and the Gut-Microbiome-Immune Axis: Physiological Metabolism and Therapeutic Prospects. Compr Rev Food Sci Food Saf. 2019;18:221-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 7. | Lin R, Liu W, Piao M, Zhu H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids. 2017;49:2083-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 224] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 8. | Houghton D, Stewart CJ, Day CP, Trenell M. Gut Microbiota and Lifestyle Interventions in NAFLD. Int J Mol Sci. 2016;17:447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Longo L, Tonin Ferrari J, Rampelotto PH, Hirata Dellavia G, Pasqualotto A, P Oliveira C, Thadeu Schmidt Cerski C, Reverbel da Silveira T, Uribe-Cruz C, Álvares-da-Silva MR. Gut Dysbiosis and Increased Intestinal Permeability Drive microRNAs, NLRP-3 Inflammasome and Liver Fibrosis in a Nutritional Model of Non-Alcoholic Steatohepatitis in Adult Male Sprague Dawley Rats. Clin Exp Gastroenterol. 2020;13:351-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Longo L, Rampelotto PH, Filippi-Chiela E, de Souza VEG, Salvati F, Cerski CT, da Silveira TR, Oliveira CP, Uribe-Cruz C, Álvares-da-Silva MR. Gut dysbiosis and systemic inflammation promote cardiomyocyte abnormalities in an experimental model of steatohepatitis. World J Hepatol. 2021;13:2052-2070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | de Freitas LBR, Longo L, Filippi-Chiela E, de Souza VEG, Behrens L, Pereira MHM, Leonhard LC, Zanettini G, Pinzon CE, Luchese E, Lima GJSP, Cerski CT, Uribe-Cruz C, Álvares-da-Silva MR. Ornithine Aspartate and Vitamin-E Combination Has Beneficial Effects on Cardiovascular Risk Factors in an Animal Model of Nonalcoholic Fatty Liver Disease in Rats. Biomolecules. 2022;12:1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | National Research Council. Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press. 2011. [DOI] [Full Text] |
| 13. | Gómez-Lechón MJ, Donato MT, Martínez-Romero A, Jiménez N, Castell JV, O'Connor JE. A human hepatocellular in vitro model to investigate steatosis. Chem Biol Interact. 2007;165:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 429] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 14. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8015] [Cited by in RCA: 40252] [Article Influence: 821.5] [Reference Citation Analysis (0)] |
| 15. | Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537-7541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14372] [Cited by in RCA: 13807] [Article Influence: 862.9] [Reference Citation Analysis (0)] |
| 16. | Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016;4:e2584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4320] [Cited by in RCA: 5589] [Article Influence: 621.0] [Reference Citation Analysis (0)] |
| 17. | Heberle H, Meirelles GV, da Silva FR, Telles GP, Minghim R. InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinformatics. 2015;16:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 998] [Cited by in RCA: 1563] [Article Influence: 156.3] [Reference Citation Analysis (0)] |
| 18. | Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11842] [Cited by in RCA: 10325] [Article Influence: 737.5] [Reference Citation Analysis (0)] |
| 19. | Douglas GM, Maffei VJ, Zaneveld JR, Yurgel SN, Brown JR, Taylor CM, Huttenhower C, Langille MGI. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;38:685-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3069] [Cited by in RCA: 3147] [Article Influence: 629.4] [Reference Citation Analysis (0)] |
| 20. | Canbay A, Sowa JP. L-Ornithine L-Aspartate (LOLA) as a Novel Approach for Therapy of Non-alcoholic Fatty Liver Disease. Drugs. 2019;79:39-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Horvath A, Traub J, Aliwa B, Bourgeois B, Madl T, Stadlbauer V. Oral Intake of L-Ornithine-L-Aspartate Is Associated with Distinct Microbiome and Metabolome Changes in Cirrhosis. Nutrients. 2022;14:748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 22. | Solé C, Guilly S, Da Silva K, Llopis M, Le-Chatelier E, Huelin P, Carol M, Moreira R, Fabrellas N, De Prada G, Napoleone L, Graupera I, Pose E, Juanola A, Borruel N, Berland M, Toapanta D, Casellas F, Guarner F, Doré J, Solà E, Ehrlich SD, Ginès P. Alterations in Gut Microbiome in Cirrhosis as Assessed by Quantitative Metagenomics: Relationship With Acute-on-Chronic Liver Failure and Prognosis. Gastroenterology. 2021;160:206-218.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 23. | Álvares-da-Silva MR, Oliveira CP, Fagan A, Longo L, Thoen RU, Yoshimura Zitelli PM, Tanaka Ferreira RM, Mcgeorge S, Shamsaddini A, Farias AQ, Sikaroodi M, Gillevet PM, Bajaj JS. Interaction of Microbiome, Diet, and Hospitalizations Between Brazilian and American Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2022;20:930-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Hussain A, Tabrez E, Peela J, Honnavar P Dr, Tabrez SSM. Vitamin C: A Preventative, Therapeutic Agent Against Helicobacter pylori. Cureus. 2018;10:e3062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Wu TR, Lin CS, Chang CJ, Lin TL, Martel J, Ko YF, Ojcius DM, Lu CC, Young JD, Lai HC. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut. 2019;68:248-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 548] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 26. | Lai CH, Lin TL, Huang MZ, Li SW, Wu HY, Chiu YF, Yang CY, Chiu CH, Lai HC. Gut Commensal Parabacteroides goldsteinii MTS01 Alters Gut Microbiota Composition and Reduces Cholesterol to Mitigate Helicobacter pylori-Induced Pathogenesis. Front Immunol. 2022;13:916848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Chang CJ, Lin TL, Tsai YL, Wu TR, Lai WF, Lu CC, Lai HC. Next generation probiotics in disease amelioration. J Food Drug Anal. 2019;27:615-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 211] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 28. | Kalyesubula M, Mopuri R, Asiku J, Rosov A, Yosefi S, Edery N, Bocobza S, Moallem U, Dvir H. High-dose vitamin B1 therapy prevents the development of experimental fatty liver driven by overnutrition. Dis Model Mech. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Newsholme P, Lima MM, Procopio J, Pithon-Curi TC, Doi SQ, Bazotte RB, Curi R. Glutamine and glutamate as vital metabolites. Braz J Med Biol Res. 2003;36:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 253] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 30. | Kircheis G, Lüth S. Pharmacokinetic and Pharmacodynamic Properties of L-Ornithine L-Aspartate (LOLA) in Hepatic Encephalopathy. Drugs. 2019;79:23-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Butterworth RF, McPhail MJW. L-Ornithine L-Aspartate (LOLA) for Hepatic Encephalopathy in Cirrhosis: Results of Randomized Controlled Trials and Meta-Analyses. Drugs. 2019;79:31-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Jain A, Sharma BC, Mahajan B, Srivastava S, Kumar A, Sachdeva S, Sonika U, Dalal A. L-ornithine L-aspartate in acute treatment of severe hepatic encephalopathy: A double-blind randomized controlled trial. Hepatology. 2022;75:1194-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |