Published online May 27, 2024. doi: 10.4254/wjh.v16.i5.688
Revised: February 26, 2024
Accepted: April 7, 2024
Published online: May 27, 2024
Processing time: 166 Days and 19.9 Hours
Non-alcoholic fatty liver disease (NAFLD) encompasses a spectrum of liver disorders of varying severity, ultimately leading to fibrosis. This spectrum primarily consists of NAFL and non-alcoholic steatohepatitis. The pathogenesis of NAFLD is closely associated with disturbances in the gut micr
Core Tip: Non-alcoholic fatty liver disease (NAFLD) is a significant concern within the realm of chronic liver diseases, notably affecting economic health. The disruption of intestinal flora balance by oral bacteria accelerates the progression of NAFLD. Moreover, through the inflamed oral mucosa, these bacteria and their virulence factors may enter the bloodstream, leading to systemic inflammation. Therefore, an innovative therapeutic approach for NAFLD involves strategic adjustments to the microbial balance within the oral cavity and gastrointestinal tract. This review succinctly delineates the roles and mechanisms of oral bacteria in NAFLD, providing a foundational framework for future therapeutic strategies.
- Citation: Mei EH, Yao C, Chen YN, Nan SX, Qi SC. Multifunctional role of oral bacteria in the progression of non-alcoholic fatty liver disease. World J Hepatol 2024; 16(5): 688-702
- URL: https://www.wjgnet.com/1948-5182/full/v16/i5/688.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i5.688
Non-alcoholic fatty liver disease (NAFLD) covers a spectrum of liver disorders, varying in severity from fatty liver to advanced fibrosis, including NAFL and non-alcoholic steatohepatitis (NASH). In the United States, the prevalence of NAFLD was reported at 83.1 million cases in 2015, approximately 25% of the population, and is projected to rise to 100.9 million by 2030[1]. In China, current prevalence rates hover around 30%[2]. Within a timeframe of 2-3 years, 15%-20% of NAFL cases and 10%-20% of NASH cases may advance to cirrhosis[3]. NAFLD is a critical factor in the progression to end-stage liver disease and hepatocellular carcinoma[4], with those affected exhibiting a 65% heightened risk of cardiovascular diseases compared to the general populace[5]. The global recognition of NAFLD as a leading cause of chronic liver disease underscores its escalating prevalence and the significant economic challenge it poses.
The human oral cavity ranks as one of the most microbially diverse regions within the body, harboring around 776 unique bacterial species. According to the Human Oral micro-biome Database (https: www.ehomd.org/), 58% of these species are identified, 16% remain unnamed yet cultivated, and 26% are known solely as uncultivated phylotypes. Disruptions in the balance of the oral microbiota can precipitate a variety of oral health issues, such as periodontitis and dental caries, while also exerting wider systemic impacts. Increasingly, evidence suggests that oral bacteria influence the gut microbial ecology and liver metabolism through both the bloodstream and direct ingestion.
The oral-gut axis demonstrates a substantial correlation with NAFLD, including the translocation of bacteria from the oral cavity to the gastrointestinal tract and the interplay between oral and gut microbiomes[6]. Bacterial migration occurs via three primary pathways: The enteral route, the hematogenous route, and immune cell migration[7]. Notable periodontal pathogens, such as Porphyromonas gingivalis (P. gingivalis), Fusobacterium nucleatum, and Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans), significantly affect the gut microbiota[8-10]. Conversely, by altering the gut ecosystem, oral dysbiosis may intensify chronic liver diseases, such as NAFLD. Furthermore, oral dysbiosis could mirror the intestinal dysbiosis induced by hepatic diseases[11].
This review delves into the interactions between oral bacteria and NAFLD, scrutinizing potential mechanisms and exploring prospective therapeutic strategies for NAFLD.
Among chronic liver diseases globally, NAFLD boasts the highest prevalence, with rates varying from 13.5% in Africa to 31.8% in the Middle East[12]. This variability can be attributed to numerous factors including dietary caloric intake, levels of physical activity, distribution of body fat, socioeconomic status, and genetic factors. Notably, the African-American population exhibits the lowest incidence of NAFLD, while the Hispanic demographic shows a higher prevalence of NASH[13]. A significant correlation exists between NAFLD and metabolic syndrome, with high prevalence rates in individuals manifesting type 2 diabetes, central obesity, dyslipidemia, and metabolic syndrome-affecting 47.3%-63.7% of patients with type 2 diabetes and up to 80% of obese individuals[14,15]. Importantly, NAFLD can also develop in individuals with healthy body mass indices (BMIs), often classified as non-obese or lean NAFLD[16], which typically presents with central obesity or other metabolic risk factors[17].
Day et al[18] initially proposed the “two-hit” theory for the pathogenesis of NAFLD, suggesting a two-step process involving lipid accumulation in hepatocytes in 1998. However, this theory has since been considered oversimplified, with current understanding acknowledging the complexity of NAFLD pathogenesis through multifaceted interactions across various stages. The disease is primarily driven by ectopic fat accumulation due to macrophage infiltration of visceral adipose tissue. Lipid metabolic imbalances lead to the generation of lipotoxic lipids, which trigger cellular stress (oxidative and endoplasmic reticulum stress), inflammasome activation, cellular death, tissue regeneration, and fibrosis[19,20]. Furthermore, NAFLD is associated with metabolic dysregulation and inflammation, influenced by interactions between the liver and both the intestinal and oral microbiota. Epidemiological evidence suggests periodontitis as an independent risk factor for NAFLD progression[21], supported by findings of hepatic lipid deposition in mice with P. gingivalis-induced periodontitis and altered gut microbial compositions in NAFLD patients[22], highlighting a potential pathogenic mechanism of the disease and NAFLD patients have altered gut microbial compositions[23,24].
The diagnosis of NAFLD involves identifying steatosis in the absence of secondary causes such as alcoholic hepatitis, followed by stratifying the risk for NASH and fibrosis[25]. Abdominal ultrasonography, which shows a bright hepatic echo texture and blurred hepatic vasculature[26], is the most commonly used method to detect steatosis. However, its sensitivity for detecting mild steatosis is limited, necessitating additional magnetic resonance imaging evaluations[27]. Risk stratification based on the presence of significant fibrosis is crucial for all NAFLD patients, given its critical role in prognosis. Non-invasive methods include the enhanced liver fibrosis score[28], serum aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio[27], and ultrasonic imaging[29]. While these methods are notable, limitations exist in early diagnosis, making liver biopsy the gold standard for fibrosis assessment in NAFLD patients.
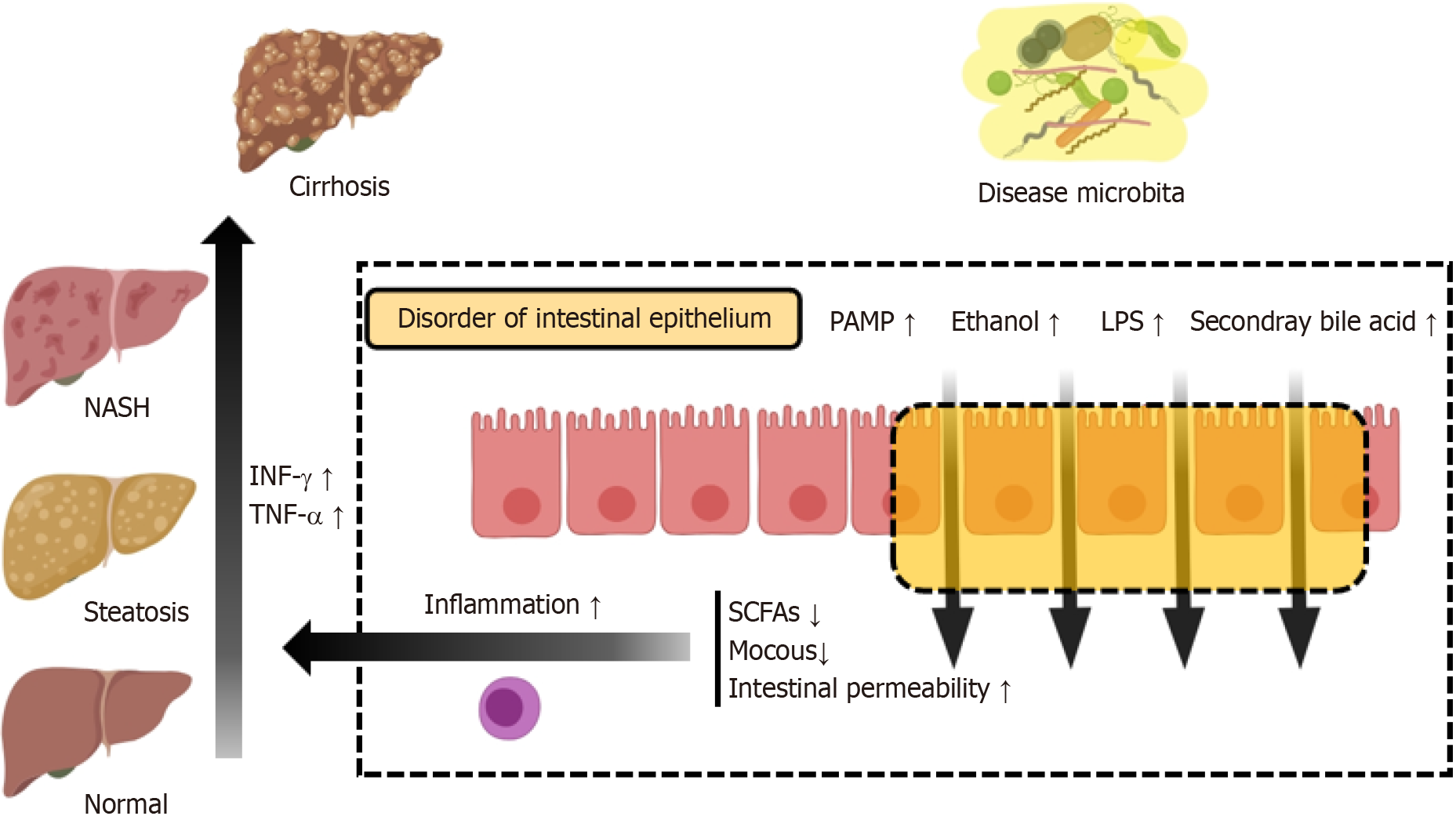
In addition to the skin, the intestinal epithelium constitutes the body’s second largest physical barrier[30]. It functions selectively, facilitating nutrient absorption, obstructing pathogenic invasions, limiting water and electrolyte depletion, and promoting waste elimination[31]. The epithelium, comprising intestinal epithelial cells, features tall villi interspersed with specialized cells such as paneth cells, goblet cells, enteroendocrine cells, and M cells. Paneth cells protect stem cells by releasing antimicrobial peptides (AMPs), including α-defensins, lysozyme C, and phospholipases[32]. Goblet cells are integral to mucin secretion, critical for forming a mucous layer that lubricates and shields epithelial cells, and aids in antigen presentation alongside M cells[33]. Enteroendocrine cells discharge various hormones, and tuft cells specialize in chemosensation[34]. The intestinal epithelium is increasingly recognized as a pivotal element of mucosal immunity, housing about 70% of the body’s lymphocyte population and establishing itself as the largest immune organ[35]. A key feature of intestinal mucosal immunity is the presence of mucosa-associated lymphoid tissue, encapsulated by follicle-associated epithelium, which underpins the intestinal response to external antigens, especially those from ingested bacteria[36]. Ultimately, the barrier function of the intestinal epithelium is multifaceted, encompassing epithelial cells, the mucous layer, the lamina propria, blood circulation, and the microbiota, working collectively to maintain intestinal equilibrium and prevent the entry of harmful substances and microbes.
Dysregulation of symbiotic microbiota and consequent mucosal immune imbalances serve as a common patho
As a critical mucosal barrier, the oral mucosa prevents pathogenic invasions and maintains homeostasis. It is composed of stratified squamous epithelium, divided into masticatory, lining, and specialized mucosae[57]. The masticatory mucosa, found in areas such as the gums and hard palate, is adapted to endure frequent mechanical stress. In contrast, the specialized mucosa, located primarily on the tongue’s dorsal surface, contains nerve endings sensitive to taste and general sensations. The rest of the oral cavity, including the inner surfaces of the lips, cheek mucosa, soft palate, and mouth floor, is lined by mucosa that may be partially keratinized or non-keratinized, depending on the area and its exposure to physical stimuli. Unlike the intestinal epithelium, the oral epithelium generally lacks chemosensory cells, hormone, or mucin-producing cells, and harbors a more diverse microbial community. Oral epithelial cells display unique keratin expression patterns, enhancing immune tolerance in the oral mucosa[58]. Nevertheless, this tolerance is not absolute, as the junctional epithelium and tonsillar crypt epithelium are particularly susceptible sites. The junctional epithelium, connecting teeth and subgingival tissues, is only 3-4 cell layers thick and lies near dental plaque biofilms. The tonsillar crypt epithelium, part of Waldeyer’s ring, contains M cells that present antigens to stimulate adaptive immune responses, thus providing structural pathways for pathogens to invade from the mouth[57,59,60].
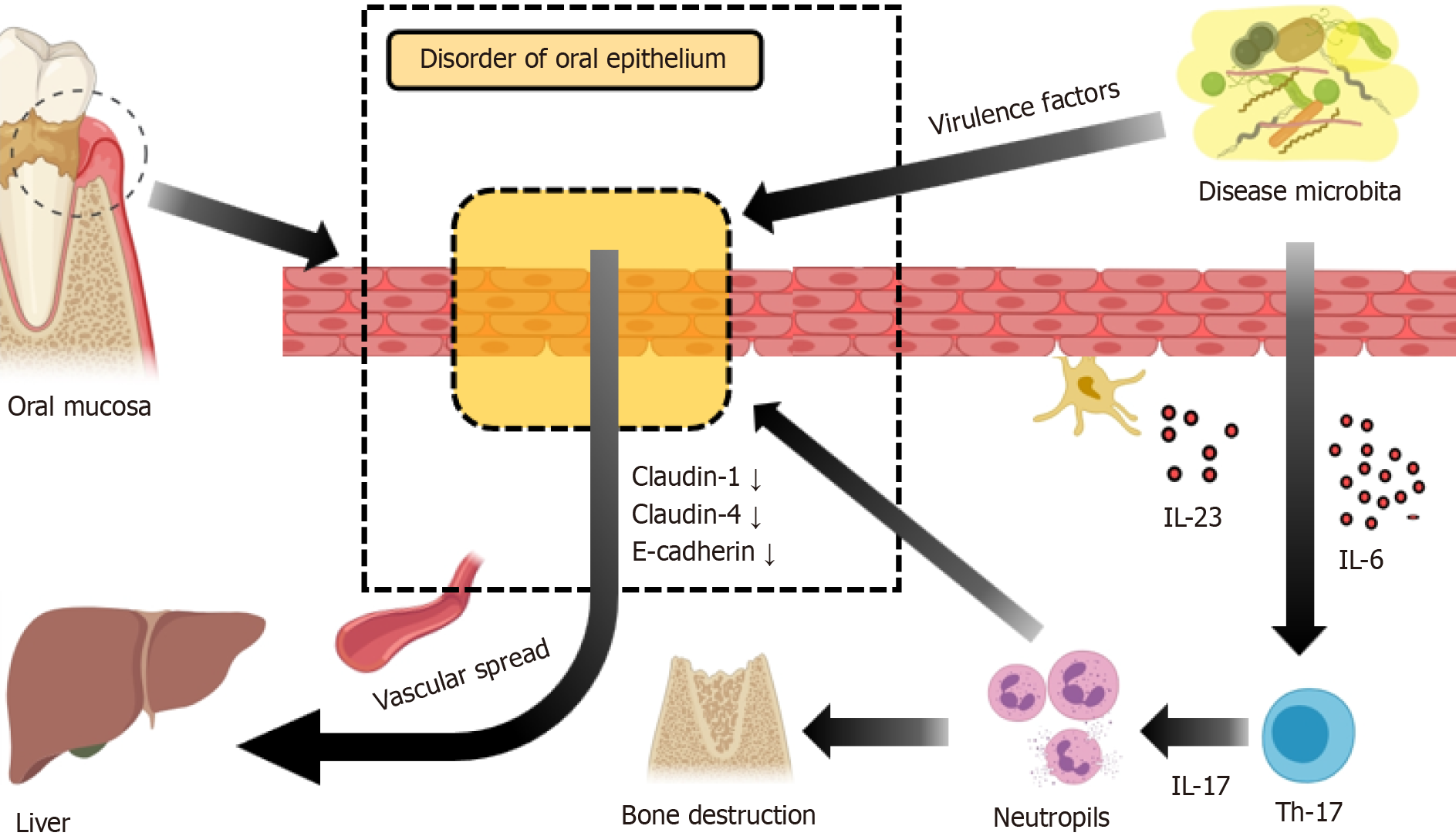
The oral microbiota is diverse and dynamic[61], with complex microbial communities in the mouth influencing the induction, training, and function of mucosal immunity by forming micron-scale microbial habitats and niches[62]. Microbial imbalances are pathogenic factors for common oral diseases like periodontitis[63] and oral candidiasis[64], where the IL-17/Th17-dependent pathway plays a central role in controlling oral mucosal infections and inflammation. Microbial dysbiosis results in the aggregation of IL-6, IL-23-dependent Th17 cells in the gingival sulcus, leading to neutrophil recruitment and subsequent alveolar bone loss[59]. A delicate ecological balance, maintained by the interplay between the microbiota and the immune system, is crucial. Disruption of this balance results in mucosal immune imbalances and pathological changes. Certain periodontal pathogens, including A. actinomycetemcomitans, P. gingivalis, T. forsythia, and T. denticola, can induce inflammatory responses and disrupt intercellular junctions[65,66]. Bacteria possessing virulence factors proliferate in inflamed states and can enter the bloodstream through compromised oral mucosa, thereby exerting deleterious effects on NAFLD (Figure 2).
Epidemiological studies have indicated a connection between periodontitis and NAFLD, with genome-wide association studies suggesting a positive causal relationship between the two conditions[67]. Periodontitis and elevated serum ALT levels were found to be significantly correlated in the study by Furuta et al[68]. In research conducted by Kim et al[69], the fatty liver index and periodontal disease were associated, with a greater correlation observed in females. Furthermore, NAFLD and the number of missing teeth are significantly correlated in males, according to the study by Qiao et al[70].
Periodontitis is a bacterial oral disease in which P. gingivalis, a non-fermentative Gram-negative anaerobic rod, emerges as a crucial periodontal pathogen. A latent connection between P. gingivalis and NAFLD has been discovered. Patients with NAFLD exhibit higher levels of P. gingivalis and its DNA in the oral cavity or liver compared to controls[71,72]. Furthermore, those infected with P. gingivalis and suffering from NASH show more severe fibrosis[71]. Additionally, evidence from numerous in vivo experiments suggests that P. gingivalis infection can promote lipid accumulation, intensify immune responses, and induce insulin resistance, highlighting its significant role in NAFLD/NASH progression[73]. Translocated oral microbes, such as P. gingivalis, disrupt the balance of gut microbiota, which can exacerbate NAFLD through various mechanisms[74-76]. These include altering intestinal permeability, modulating energy absorption evidenced by increased dietary fat intake promoting hepatic fat deposition[41,77-79], regulating bile acid metabolism as seen in changes in bile acid composition due to gut microbiota dysbiosis[80-82], and increasing endogenous ethanol production[56,83].
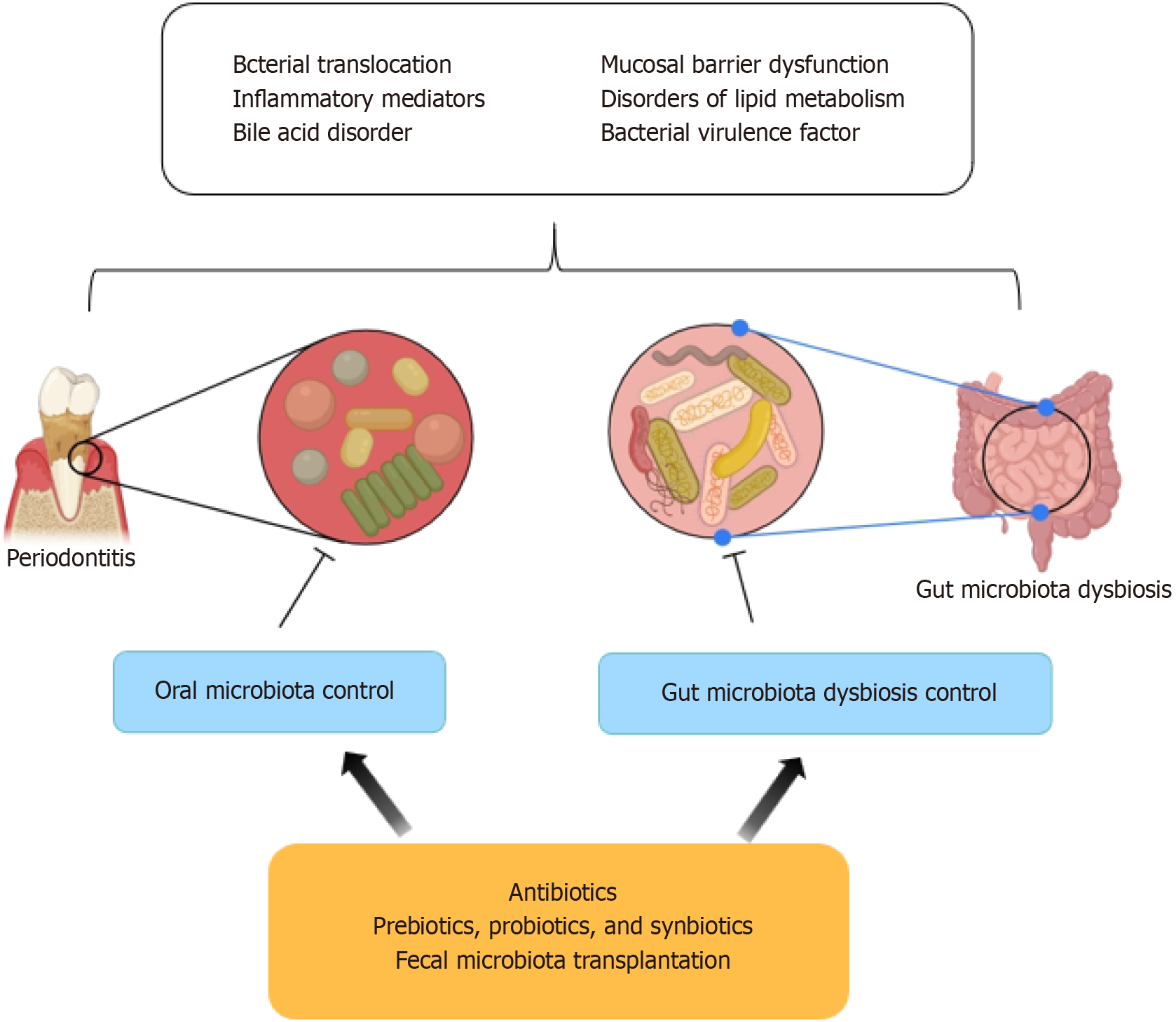
Oral microbiota is implicated in various oral diseases such as periodontitis and dental caries. Recent research has highlighted an increasingly clear link between these microbiota-related oral conditions and NAFLD. The multifaceted interaction among oral microbiota, the intestinal barrier, the immune system, and the liver is susceptible to disruption by environmental and genetic factors, potentially leading to systemic diseases. A significant physiological relationship exists among the oral cavity, intestine, and liver, forming the “oral-gut-liver” axis. In this context, the balance of gut microbiota plays a critical role in NAFLD progression[84]. Additionally, dysbiosis of oral microbiota has been associated with imbalances in gut microbiota[85-87]. The connection between the oral cavity and the liver could be mediated through bacterial translocation, inflammatory responses, bacterial virulence factor toxicity, and disruptions in lipid metabolism (Figures 3 and 4).
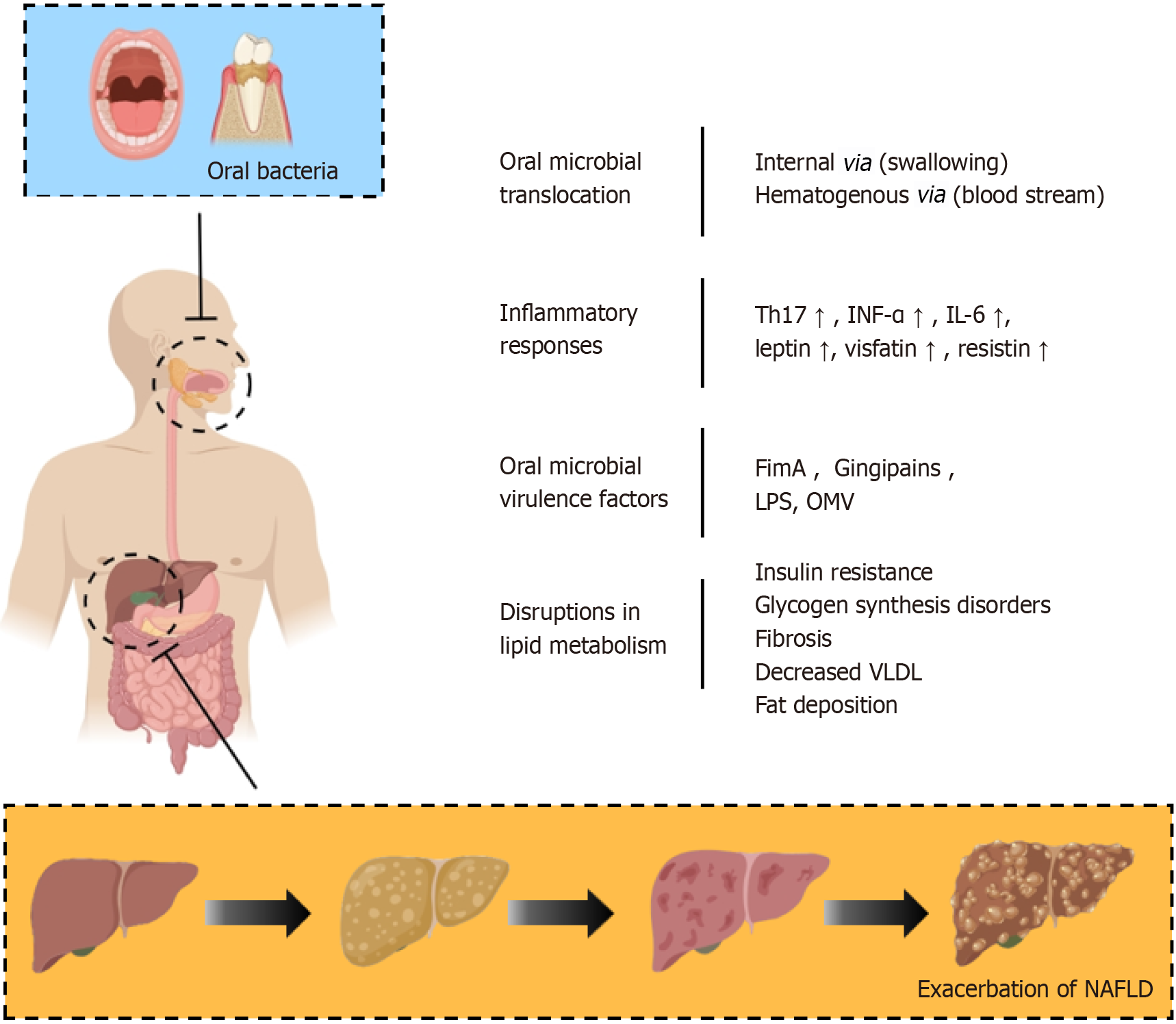
The translocation of oral bacteria to the gut is a pathogenic step in the development of NAFLD, occurring chiefly through hematogenous routes and direct swallowing. Micro-ulcerations within periodontal pockets facilitate systemic bacterial, endotoxin, and inflammatory mediator dissemination, enabling their access to the liver via the hepatic artery. In contrast, healthy gingival epithelium in periodontal tissue acts as a protective barrier against harmful biofilm components[88]. However, inflammation in diseased tissues increases capillary permeability[89], enhances gingival epithelium perm
Another potential pathway for communication between oral bacteria and NAFLD involves the ingestion of oral bacteria via saliva, which may subsequently impact the gut microbiota and, consequently, NAFLD. An individual swallows up to 1.5 liters of saliva daily, containing approximately 1.5 × 1012 oral bacteria[94]. Animal research suggests that consuming oral bacteria associated with periodontal disease, such as P. gingivalis and Actinomyces, can lead to gut microbiota changes. These changes disrupt metabolic pathways related to glucose and lipid metabolism, resulting in insulin resistance and hepatic lipid accumulation[95]. It is important to note that the causal relationship between oral bacterial colonization and the development of intestinal dysbiosis is still debated. Although the acidic gastric environment is fatal for most bacteria, oral microbes are often found in the intestines of healthy individuals; a reduced gut microbiota diversity is observed in patients with periodontal disease[96,97]. Alternatively, some studies suggest that oral bacterial colonization in the gut may depend on the disruption of the intestinal milieu, potentially associated with excessive antibiotic and proton pump inhibitor use, poor dietary choices, and high levels of psychological stress[98,99].
In the oral and intestinal regions, the symbiotic relationship between the immune system, epithelial cells, and microbiota is essential for maintaining tissue function and systemic homeostasis. The immune response to periodontitis and intestinal inflammatory diseases facilitates the growth of pathogenic bacteria. Yao et al[100] identified that Porphyromonas gingivalis disturbs the Th17/Treg cell balance in the liver and spleen, leading to hepatocyte ferroptosis and increased hepatic inflammation, with the nuclear factor κB (NF-κB) signaling pathway playing a critical role[101]. Kitamoto et al[102] found that periodontal disease promotes growth of oral pathogens like Klebsiella and Enterobacter, which can exacerbate intestinal inflammation when they colonize the gut. In mouse models of colitis, heightened intestinal nitrate levels led to a dominance of nitrate-respiring Enterobacteriaceae over anaerobic commensals, exacerbating the disease[103]. Oral microbes are key players in nitrate reduction due to their nitrate reductase activity[104,105]. The TH17 inflammatory pathway also contributes to the progression of intestinal inflammation linked to periodontitis[63,106-108]. Studies have shown that oral TH17 cells can migrate to the gut and stimulate inflammatory reactions there, highlighting the oral-intestinal interconnection in mucosal inflammation[102]. Zhao et al[109] demonstrated that topical administration of AMP Mastoparan X alleviates intestinal inflammation induced by Escherichia coli and helps restore balance to the gut mic
Oral microbiota can induce systemic inflammatory cytokines and oxidative stress, affecting inflammation. In periodontitis, levels of pro-inflammatory cytokines such as tumour necrosis factor alpha (TNF-α) and interleukin (IL)-6[110], as well as serum pro-inflammatory adipokines (e.g., leptin, visfatin, and resistin), are increased[111,112], whereas the anti-inflammatory adipokine adiponectin decreases, potentially exacerbating inflammatory responses and lipid accumulation. Tomofuji et al[113] observed that periodontitis was linked to oxidative DNA damage in the liver. Matthews et al[114] reported that peripheral neutrophils from individuals with chronic periodontitis exhibited an increased in vitro production and release of reactive oxygen species. Önder et al[115] discovered that clinical interventions in periodontal treatment reduced serum reactive oxygen species and lipid peroxide levels in periodontitis patients, indicating that systemic oxidative stress associated with periodontitis may lead to hepatic oxidative damage.
P. gingivalis, a Gram-negative anaerobic bacterium found in the oral cavity, can colonize oral epithelial cells[116]. It possesses numerous virulence factors such as fimbriae, LPS (including LPS-induced endotoxemia), gingipains, and outer membrane vesicles (OMV). These factors are pivotal in the bacterium’s survival, dissemination, and pathogenicity[117,118].
FimA, a specific fimbria found in P. gingivalis, is associated with the onset of NAFLD[119]. FimA can interact with various host receptors, activating adhesion and immune-inflammatory pathways, thereby promoting bacterial colonization and triggering host cell inflammation[120-122]. FimA binds to Toll-like receptor 2 (TLR2), triggering the activation of the NF-κB system and leading to the production of inflammatory cytokines[123]. Furthermore, through its interaction with complement receptor 3, FimA activates the innate immune system, thereby stimulating macrophages/monocytes. This enhances the persistence and survival of P. gingivalis[124].
Gingipains are primary virulence factors linked to the pathogenicity of P. gingivalis[125]. They play a crucial role in biofilm formation and elicit immune-inflammatory responses by activating various immune cellss[124,126]. Gingipains may evade the host’s adaptive immune system by modulating T-cell immunity[127]. Furthermore, gingipains could be implicated in glucose metabolic damage and insulin resistance, meriting additional research.
Biofilms and OMVs represent two crucial structures produced by microbes, which play essential roles in bacterial survival, dissemination, and pathogenicity[128,129]. The OMV of P. gingivalis have the capability to transport virulence factors, including gingipains and LPS. These vesicles can release these virulence factors into the environment, cont
LPS, a component of the outer membrane of Gram-negative bacteria, is vital for microbial pathogenicity[133,134]. Its active component, lipid A, is known for its pyrogenic, pro-inflammatory, and toxic effects on humans and animals. To mitigate these effects, the human innate immune system harbors cells that express LPS receptors, such as TLR4 and CD14, which elicit a strong inflammatory response to LPS[135]. In NAFLD, increased gut-derived endotoxemia, marked by elevated blood LPS levels due to higher gut permeability, is a crucial factor driving disease advance[136-138]. This leads to systemic inflammatory reactions, including raised levels of C-reactive protein, IL-6, and TNF-α[139]. LPS significantly contributes to the activation of host inflammatory responses through Toll-like receptor (TLR) activation[140]. Studies have shown that P. gingivalis LPS’s impact on NAFLD may involve higher expression of TLR2, mRNA levels of inflammasomes, and increased production of pro-inflammatory cytokines[71]. Specifically, in the context of a fatty liver, there is an enhanced sensitivity to LPS, likely due to Kupffer cell proliferation and augmented expression of Toll-like receptors[141,142].
NAFLD is recognized as the hepatic manifestation of metabolic syndrome, characterized by obesity, insulin resistance, hypertension, and dyslipidemia, common to both conditions[143]. Insulin resistance, leading to hepatic fat accumulation, initiates NAFLD, triggering inflammatory responses that may result in liver fibrosis[144]. The oral microbiota contributes to NAFLD progression by promoting insulin resistance, leading to metabolic syndrome. In animal models with ligature-induced periodontitis, increased inflammatory factors, total cholesterol, and triglycerides altered liver glucose and fat metabolism, evidenced by increased liver cell lipid droplets and enlarged mitochondria[145-147]. Obesity and diabetes, components of metabolic syndrome, are significant risk factors for periodontal disease, suggesting NAFLD may indirectly influence periodontal disease pathophysiology through the shared pathway of metabolic syndrome.
The intracellular mechanisms activated by P. gingivalis primarily result in the inhibition of hepatic glycogen synthesis, which leads to fat deposition and the promotion of fibrosis. In the context of hepatic glycogen synthesis, Ishikawa et al[148] documented the internalization of P. gingivalis into HepG2 human liver cells. This process is linked to the inhibition of glycogen synthesis, affecting the phosphorylation of insulin receptor-1, serine/threonine kinase, and glycogen syn
Dietary habits are widely recognized as a crucial factor influencing NAFLD, with the management of caloric intake and the incorporation of appropriate physical activity serving as a key non-pharmacological therapeutic strategy for NAFLD[151]. Recent research has underscored the beneficial effects of addressing periodontal disease in individuals with NAFLD, as demonstrated by the reduction in AST and ALT levels, improvement in serum inflammatory mediators, and decrease in endotoxins[152,153]. The newly established “oral-gut-liver” axis reveals innovative strategies for the prevention and treatment of NAFLD, particularly through the reduction of oral pathogens and correction of gut micr
Owing to periodontal disease’s substantial impact on NAFLD, enhancement of oral hygiene and strategic management of pathogenic oral bacteria represent effective interventions for NAFLD associated with bacterial infection. As noted earlier, P. gingivalis plays an essential role in the interplay between oral bacteria and NAFLD, thus targeting it is an emerging approach. Strategies include employing AMPs to obstruct P. gingivalis adherence to host cells[154], deploying anti-CR3 receptor agents to diminish bacterial attachment[155], utilizing gingipain inhibitors to alleviate periodontitis and related systemic conditions[156], and regulating OMV production to impede biofilm development[133]. However, current research on these agents in the context of NAFLD related to pathogenic oral bacteria like P. gingivalis is preliminary and warrants further investigation.
Numerous methods exist to manipulate the gut microbiota, which include the use of antibiotics, prebiotics, probiotics, or a combination thereof termed synbiotics. These agents can influence the development of NAFLD through anti-inflammatory effects, enhancement of epithelial barrier function, reduction in ethanol production by gut microbiota, and modulation of bile acid and choline metabolism[157-159].
While the widespread use of antibiotics necessitates caution due to their potential to eliminate essential microbial species and foster antibiotic-resistant strains, their impact on NAFLD has been explored in various studies. For instance, alternating the administration of norfloxacin and neomycin has been effective in reducing small intestinal bacterial overgrowth and improving liver function in individuals with cirrhosis[160]. Additionally, long-term oral antibiotic treatment in animal models has been effective in suppressing gut bacteria, lowering portal secondary bile acids, and mitigating hepatic inflammation and fibrosis[161]. Concurrent administration of neomycin, bacitracin, and streptomycin has also been associated with decreases in hepatic triglycerides, lipid accumulation, and serum ceramide production in murine models[162]. Thus, while antibiotics can alter the gut microbiota and potentially slow the progression of liver diseases, their therapeutic use is limited due to the risk of developing antibiotic resistance.
Prebiotics, non-digestible food components, foster the growth of beneficial gut microbiota[163]. They stimulate gut-mediated metabolic alterations, including the reduction of bacterial hepatotoxins, fortification of the intestinal epithelial barrier, and reduction in inflammation, potentially aiding in the mitigation of NAFLD. Prebiotics enhance bacterial synthesis of short-chain fatty acids, encouraging the proliferation of Bifidobacteria and Lactobacilli[164]. Probiotics, consisting of live bacteria or yeast, have been thoroughly researched, with many studies demonstrating their effectiveness in improving NAFLD. For instance, Lactobacillus rhamnosus GG supplementation has been linked with decreased ALT levels and anti-peptidoglycan-polysaccharide antibodies, offering benefits to NAFLD patients[165]. AMPs from Lactobacillus, such as lactococcin, exhibit antibacterial properties against pathogens with a lower propensity for fostering antibiotic resistance[166,167]. Probiotic supplementation may also increase GLP-1 levels, contributing to ameliorations in fatty liver and BMI[168]. Synbiotics, which combine probiotics and prebiotics, are proposed to yield synergistic effects in NAFLD treatment[169].
Fecal microbiota transplantation (FMT) entails transferring fecal matter from a healthy donor to a patient, with the goal of restoring gut microbiota balance. FMT has demonstrated efficacy in combating Clostridium difficile infections and is viewed as potentially applicable to a range of non-gastrointestinal diseases[170].
In summary, a growing compendium of clinical and fundamental research corroborates the association between oral bacteria and NAFLD, particularly among individuals with diabetes and metabolic syndrome. P. gingivalis emerges as a principal agent in fostering hepatic lipid accumulation and inflammation. Oral bacteria precipitate NAFLD via multiple pathways, including bacterial translocation, induction of inflammatory responses, secretion of toxic factors into the bloodstream, and perturbation of liver lipid metabolism. Furthermore, these microorganisms may alter the equilibrium of the gut microbiota through mechanisms such as hematogenous dissemination and direct ingestion. In this context, a dysbiotic gut micro-biome may produce deleterious substances (e.g., LPSs and ethanol), compromising the intestinal barrier and adversely affecting liver health. However, elucidating the precise mechanisms through which oral bacteria impact NAFLD remains challenging, hindered by the complexity of in vitro culturing of oral bacteria and the individual variability in the “oral-gut-liver” axis, influenced by dietary habits. Additionally, the synergistic interactions between oral and gut microbiota and their contribution to insulin resistance in the context of periodontitis, diabetes, metabolic syndrome, and NAFLD warrant further investigation.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade C
P-Reviewer: Herawati F, Indonesia S-Editor: Chen YL L-Editor: A P-Editor: Zhao YQ
| 1. | Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 2914] [Article Influence: 416.3] [Reference Citation Analysis (1)] |
| 2. | Wu Y, Zheng Q, Zou B, Yeo YH, Li X, Li J, Xie X, Feng Y, Stave CD, Zhu Q, Cheung R, Nguyen MH. The epidemiology of NAFLD in Mainland China with analysis by adjusted gross regional domestic product: a meta-analysis. Hepatol Int. 2020;14:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 3. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2357] [Cited by in RCA: 2343] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 4. | Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 586] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 5. | Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1003] [Article Influence: 111.4] [Reference Citation Analysis (0)] |
| 6. | Park SY, Hwang BO, Lim M, Ok SH, Lee SK, Chun KS, Park KK, Hu Y, Chung WY, Song NY. Oral-Gut Microbiome Axis in Gastrointestinal Disease and Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 7. | Tan X, Wang Y, Gong T. The interplay between oral microbiota, gut microbiota and systematic diseases. J Oral Microbiol. 2023;15:2213112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 8. | Haraga H, Sato T, Watanabe K, Hamada N, Tani-Ishii N. Effect of the Progression of Fusobacterium nucleatum-induced Apical Periodontitis on the Gut Microbiota. J Endod. 2022;48:1038-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Fine DH, Patil AG, Velusamy SK. Aggregatibacter actinomycetemcomitans (Aa) Under the Radar: Myths and Misunderstandings of Aa and Its Role in Aggressive Periodontitis. Front Immunol. 2019;10:728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 10. | Arimatsu K, Yamada H, Miyazawa H, Minagawa T, Nakajima M, Ryder MI, Gotoh K, Motooka D, Nakamura S, Iida T, Yamazaki K. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep. 2014;4:4828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 400] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 11. | Nagasaki A, Sakamoto S, Chea C, Ishida E, Furusho H, Fujii M, Takata T, Miyauchi M. Odontogenic infection by Porphyromonas gingivalis exacerbates fibrosis in NASH via hepatic stellate cell activation. Sci Rep. 2020;10:4134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7533] [Article Influence: 837.0] [Reference Citation Analysis (0)] |
| 13. | Bambha K, Belt P, Abraham M, Wilson LA, Pabst M, Ferrell L, Unalp-Arida A, Bass N; Nonalcoholic Steatohepatitis Clinical Research Network Research Group. Ethnicity and nonalcoholic fatty liver disease. Hepatology. 2012;55:769-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1494] [Article Influence: 249.0] [Reference Citation Analysis (0)] |
| 15. | Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 2019;92:82-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 808] [Article Influence: 134.7] [Reference Citation Analysis (0)] |
| 16. | Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, Yang H, Liu C, Kam LY, Tan XXE, Chien N, Trinh S, Henry L, Stave CD, Hosaka T, Cheung RC, Nguyen MH. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:739-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 566] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 17. | Wei JL, Leung JC, Loong TC, Wong GL, Yeung DK, Chan RS, Chan HL, Chim AM, Woo J, Chu WC, Wong VW. Prevalence and Severity of Nonalcoholic Fatty Liver Disease in Non-Obese Patients: A Population Study Using Proton-Magnetic Resonance Spectroscopy. Am J Gastroenterol. 2015;110:1306-14; quiz 1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 241] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 18. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3129] [Article Influence: 115.9] [Reference Citation Analysis (36)] |
| 19. | Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 478] [Article Influence: 39.8] [Reference Citation Analysis (1)] |
| 20. | Sanyal AJ. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2019;16:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 379] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 21. | Akinkugbe AA, Slade GD, Barritt AS, Cole SR, Offenbacher S, Petersmann A, Kocher T, Lerch MM, Mayerle J, Völzke H, Heiss G, Holtfreter B. Periodontitis and Non-alcoholic Fatty Liver Disease, a population-based cohort investigation in the Study of Health in Pomerania. J Clin Periodontol. 2017;44:1077-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Kuraji R, Fujita M, Ito H, Hashimoto S, Numabe Y. Effects of experimental periodontitis on the metabolic system in rats with diet-induced obesity (DIO): an analysis of serum biochemical parameters. Odontology. 2018;106:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 23. | Caussy C, Tripathi A, Humphrey G, Bassirian S, Singh S, Faulkner C, Bettencourt R, Rizo E, Richards L, Xu ZZ, Downes MR, Evans RM, Brenner DA, Sirlin CB, Knight R, Loomba R. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat Commun. 2019;10:1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 238] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 24. | Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK, Jones MB, Sirlin CB, Schnabl B, Brinkac L, Schork N, Chen CH, Brenner DA, Biggs W, Yooseph S, Venter JC, Nelson KE. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017;25:1054-1062.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 752] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 25. | Sinn DH, Kang D, Chang Y, Ryu S, Gu S, Kim H, Seong D, Cho SJ, Yi BK, Park HD, Paik SW, Song YB, Lazo M, Lima JA, Guallar E, Cho J, Gwak GY. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: a retrospective cohort study. Gut. 2017;66:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 26. | Bril F, Ortiz-Lopez C, Lomonaco R, Orsak B, Freckleton M, Chintapalli K, Hardies J, Lai S, Solano F, Tio F, Cusi K. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int. 2015;35:2139-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 27. | Wong VW, Adams LA, de Lédinghen V, Wong GL, Sookoian S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15:461-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 441] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 28. | Anstee QM, Lawitz EJ, Alkhouri N, Wong VW, Romero-Gomez M, Okanoue T, Trauner M, Kersey K, Li G, Han L, Jia C, Wang L, Chen G, Subramanian GM, Myers RP, Djedjos CS, Kohli A, Bzowej N, Younes Z, Sarin S, Shiffman ML, Harrison SA, Afdhal NH, Goodman Z, Younossi ZM. Noninvasive Tests Accurately Identify Advanced Fibrosis due to NASH: Baseline Data From the STELLAR Trials. Hepatology. 2019;70:1521-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 29. | Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, Sy E, Savides MT, Alquiraish MH, Valasek MA, Rizo E, Richards L, Brenner D, Sirlin CB, Loomba R. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:598-607.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 542] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 30. | Gallo RL. Human Skin Is the Largest Epithelial Surface for Interaction with Microbes. J Invest Dermatol. 2017;137:1213-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 31. | Binienda A, Twardowska A, Makaro A, Salaga M. Dietary Carbohydrates and Lipids in the Pathogenesis of Leaky Gut Syndrome: An Overview. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 32. | Shi J. Defensins and Paneth cells in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:1284-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Ma J, Rubin BK, Voynow JA. Mucins, Mucus, and Goblet Cells. Chest. 2018;154:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 276] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 34. | Gribble FM, Reimann F. Enteroendocrine Cells: Chemosensors in the Intestinal Epithelium. Annu Rev Physiol. 2016;78:277-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 424] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 35. | Pabst R, Russell MW, Brandtzaeg P. Tissue distribution of lymphocytes and plasma cells and the role of the gut. Trends Immunol. 2008;29:206-8; author reply 209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Nakamura Y, Kimura S, Hase K. M cell-dependent antigen uptake on follicle-associated epithelium for mucosal immune surveillance. Inflamm Regen. 2018;38:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 37. | Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 743] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 38. | Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 1049] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 39. | Gérard P. Gut microbiota and obesity. Cell Mol Life Sci. 2016;73:147-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 343] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 40. | Rabot S, Membrez M, Bruneau A, Gérard P, Harach T, Moser M, Raymond F, Mansourian R, Chou CJ. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24:4948-4959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 382] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 41. | Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979-984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2086] [Cited by in RCA: 1876] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 42. | Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2376] [Cited by in RCA: 2162] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 43. | Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718-15723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4530] [Cited by in RCA: 4410] [Article Influence: 210.0] [Reference Citation Analysis (4)] |
| 44. | Mazagova M, Wang L, Anfora AT, Wissmueller M, Lesley SA, Miyamoto Y, Eckmann L, Dhungana S, Pathmasiri W, Sumner S, Westwater C, Brenner DA, Schnabl B. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB J. 2015;29:1043-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 45. | Celaj S, Gleeson MW, Deng J, O'Toole GA, Hampton TH, Toft MF, Morrison HG, Sogin ML, Putra J, Suriawinata AA, Gorham JD. The microbiota regulates susceptibility to Fas-mediated acute hepatic injury. Lab Invest. 2014;94:938-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Björkholm B, Bok CM, Lundin A, Rafter J, Hibberd ML, Pettersson S. Intestinal microbiota regulate xenobiotic metabolism in the liver. PLoS One. 2009;4:e6958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 47. | Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, Perlemuter G, Cassard-Doulcier AM, Gérard P. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 715] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 48. | Yari Z, Rahimlou M, Eslamparast T, Ebrahimi-Daryani N, Poustchi H, Hekmatdoost A. Flaxseed supplementation in non-alcoholic fatty liver disease: a pilot randomized, open labeled, controlled study. Int J Food Sci Nutr. 2016;67:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 49. | Rahimlou M, Ahmadnia H, Hekmatdoost A. Dietary supplements and pediatric non-alcoholic fatty liver disease: Present and the future. World J Hepatol. 2015;7:2597-2602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Eslamparast T, Eghtesad S, Poustchi H, Hekmatdoost A. Recent advances in dietary supplementation, in treating non-alcoholic fatty liver disease. World J Hepatol. 2015;7:204-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 51. | Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, McGilvray ID, Allard JP. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 555] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 52. | Vallianou N, Christodoulatos GS, Karampela I, Tsilingiris D, Magkos F, Stratigou T, Kounatidis D, Dalamaga M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives. Biomolecules. 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 53. | Wang B, Jiang X, Cao M, Ge J, Bao Q, Tang L, Chen Y, Li L. Altered Fecal Microbiota Correlates with Liver Biochemistry in Nonobese Patients with Non-alcoholic Fatty Liver Disease. Sci Rep. 2016;6:32002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 271] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 54. | Michail S, Lin M, Frey MR, Fanter R, Paliy O, Hilbush B, Reo NV. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol Ecol. 2015;91:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 55. | Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140:976-986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 544] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 56. | Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1279] [Article Influence: 106.6] [Reference Citation Analysis (1)] |
| 57. | Nanci A. Chapter 12-Oral Mucosa. In: Nanci A. Ten Cate’s Oral Histology. 8th ed. St. Louis (MO): Mosby, 2013: 278-310. [DOI] [Full Text] |
| 58. | Scheuplein RJ, Blank IH. Permeability of the skin. Physiol Rev. 1971;51:702-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1003] [Cited by in RCA: 887] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 59. | Gaffen SL, Moutsopoulos NM. Regulation of host-microbe interactions at oral mucosal barriers by type 17 immunity. Sci Immunol. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 60. | Moutsopoulos NM, Konkel JE. Tissue-Specific Immunity at the Oral Mucosal Barrier. Trends Immunol. 2018;39:276-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 61. | Li D, Wang P, Hu X, Chen F. The gut microbiota: A treasure for human health. Biotechnol Adv. 2016;34:1210-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 62. | Mark Welch JL, Ramírez-Puebla ST, Borisy GG. Oral Microbiome Geography: Micron-Scale Habitat and Niche. Cell Host Microbe. 2020;28:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 150] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 63. | Dutzan N, Kajikawa T, Abusleme L, Greenwell-Wild T, Zuazo CE, Ikeuchi T, Brenchley L, Abe T, Hurabielle C, Martin D, Morell RJ, Freeman AF, Lazarevic V, Trinchieri G, Diaz PI, Holland SM, Belkaid Y, Hajishengallis G, Moutsopoulos NM. A dysbiotic microbiome triggers T(H)17 cells to mediate oral mucosal immunopathology in mice and humans. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 279] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 64. | Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, Bistoni F, Puccetti P, Kastelein RA, Kopf M, Romani L. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695-2706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 436] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 65. | Tatakis DN, Kumar PS. Etiology and pathogenesis of periodontal diseases. Dent Clin North Am. 2005;49:491-516, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 66. | Katz J, Sambandam V, Wu JH, Michalek SM, Balkovetz DF. Characterization of Porphyromonas gingivalis-induced degradation of epithelial cell junctional complexes. Infect Immun. 2000;68:1441-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 161] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 67. | Qiao F, Li X, Liu Y, Zhang S, Liu D, Li C. Periodontitis and NAFLD-related diseases: A bidirectional two-sample Mendelian randomization study. Oral Dis. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 68. | Furuta M, Ekuni D, Yamamoto T, Irie K, Koyama R, Sanbe T, Yamanaka R, Morita M, Kuroki K, Tobe K. Relationship between periodontitis and hepatic abnormalities in young adults. Acta Odontol Scand. 2010;68:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 69. | Kim JY, Lee GN, Song HC, Park YM, Ahn YB, Han K, Ko SH. Association between Fatty Liver Index and Periodontitis: the Korea National Health and Nutrition Examination Survey. Sci Rep. 2020;10:3805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 70. | Qiao F, Fu K, Zhang Q, Liu L, Meng G, Wu H, Xia Y, Bao X, Gu Y, Shi H, Sun S, Wang X, Zhou M, Jia Q, Song K, Niu K. The association between missing teeth and non-alcoholic fatty liver disease in adults. J Clin Periodontol. 2018;45:941-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 71. | Furusho H, Miyauchi M, Hyogo H, Inubushi T, Ao M, Ouhara K, Hisatune J, Kurihara H, Sugai M, Hayes CN, Nakahara T, Aikata H, Takahashi S, Chayama K, Takata T. Dental infection of Porphyromonas gingivalis exacerbates high fat diet-induced steatohepatitis in mice. J Gastroenterol. 2013;48:1259-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 72. | Yoneda M, Naka S, Nakano K, Wada K, Endo H, Mawatari H, Imajo K, Nomura R, Hokamura K, Ono M, Murata S, Tohnai I, Sumida Y, Shima T, Kuboniwa M, Umemura K, Kamisaki Y, Amano A, Okanoue T, Ooshima T, Nakajima A. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012;12:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 73. | Hatasa M, Yoshida S, Takahashi H, Tanaka K, Kubotsu Y, Ohsugi Y, Katagiri T, Iwata T, Katagiri S. Relationship between NAFLD and Periodontal Disease from the View of Clinical and Basic Research, and Immunological Response. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 74. | Luther J, Garber JJ, Khalili H, Dave M, Bale SS, Jindal R, Motola DL, Luther S, Bohr S, Jeoung SW, Deshpande V, Singh G, Turner JR, Yarmush ML, Chung RT, Patel SJ. Hepatic Injury in Nonalcoholic Steatohepatitis Contributes to Altered Intestinal Permeability. Cell Mol Gastroenterol Hepatol. 2015;1:222-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 217] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 75. | Giorgio V, Miele L, Principessa L, Ferretti F, Villa MP, Negro V, Grieco A, Alisi A, Nobili V. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig Liver Dis. 2014;46:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 76. | Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7222] [Cited by in RCA: 6407] [Article Influence: 337.2] [Reference Citation Analysis (0)] |
| 77. | Krajmalnik-Brown R, Ilhan ZE, Kang DW, DiBaise JK. Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract. 2012;27:201-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 570] [Article Influence: 43.8] [Reference Citation Analysis (1)] |
| 78. | Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9796] [Cited by in RCA: 8752] [Article Influence: 460.6] [Reference Citation Analysis (1)] |
| 79. | Lichtman SN, Keku J, Schwab JH, Sartor RB. Hepatic injury associated with small bowel bacterial overgrowth in rats is prevented by metronidazole and tetracycline. Gastroenterology. 1991;100:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 114] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 80. | Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, Kowdley KV, Vincent C, Bodhenheimer HC Jr, Parés A, Trauner M, Marschall HU, Adorini L, Sciacca C, Beecher-Jones T, Castelloe E, Böhm O, Shapiro D. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751-61.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 431] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 81. | Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1678] [Article Influence: 139.8] [Reference Citation Analysis (0)] |
| 82. | de Wit N, Derrien M, Bosch-Vermeulen H, Oosterink E, Keshtkar S, Duval C, de Vogel-van den Bosch J, Kleerebezem M, Müller M, van der Meer R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol Gastrointest Liver Physiol. 2012;303:G589-G599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 318] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 83. | Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology. 2000;119:1340-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 265] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 84. | Chen J, Vitetta L. Gut Microbiota Metabolites in NAFLD Pathogenesis and Therapeutic Implications. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 85. | Wang T, Ishikawa T, Sasaki M, Chiba T. Oral and Gut Microbial Dysbiosis and Non-alcoholic Fatty Liver Disease: The Central Role of Porphyromonas gingivalis. Front Med (Lausanne). 2022;9:822190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 86. | Albuquerque-Souza E, Sahingur SE. Periodontitis, chronic liver diseases, and the emerging oral-gut-liver axis. Periodontol 2000. 2022;89:125-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 87. | Acharya C, Sahingur SE, Bajaj JS. Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI Insight. 2017;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 88. | Tonetti MS, Imboden MA, Gerber L, Lang NP, Laissue J, Mueller C. Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect Immun. 1994;62:4005-4014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 155] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 89. | Kuraji R, Wu YH, Hashimoto S, Mishiro S, Maeda Y, Miyashita Y, Ito H, Miwa Y, Sunohara M, Kapila Y, Numabe Y. Temporal and dynamic changes in gingival blood flow during progression of ligature-induced periodontitis. Oral Dis. 2020;26:1292-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 90. | Horliana AC, Chambrone L, Foz AM, Artese HP, Rabelo Mde S, Pannuti CM, Romito GA. Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: a systematic review. PLoS One. 2014;9:e98271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 91. | Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117:3118-3125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 648] [Cited by in RCA: 549] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 92. | Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 468] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 93. | Takeuchi Y, Umeda M, Ishizuka M, Huang Y, Ishikawa I. Prevalence of periodontopathic bacteria in aggressive periodontitis patients in a Japanese population. J Periodontol. 2003;74:1460-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 94. | Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1304] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 95. | Liu L, Geng Y, Xiong C. Impact of Porphyromonas gingivalis-odontogenic infection on the pathogenesis of non-alcoholic fatty liver disease. Ann Med. 2023;55:2255825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 96. | Schmidt TS, Hayward MR, Coelho LP, Li SS, Costea PI, Voigt AY, Wirbel J, Maistrenko OM, Alves RJ, Bergsten E, de Beaufort C, Sobhani I, Heintz-Buschart A, Sunagawa S, Zeller G, Wilmes P, Bork P. Extensive transmission of microbes along the gastrointestinal tract. Elife. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 365] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 97. | Lourenςo TGB, Spencer SJ, Alm EJ, Colombo APV. Defining the gut microbiota in individuals with periodontal diseases: an exploratory study. J Oral Microbiol. 2018;10:1487741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 98. | Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, Patil KR, Bork P, Typas A. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1160] [Cited by in RCA: 1277] [Article Influence: 182.4] [Reference Citation Analysis (0)] |
| 99. | Zaura E, Brandt BW, Teixeira de Mattos MJ, Buijs MJ, Caspers MP, Rashid MU, Weintraub A, Nord CE, Savell A, Hu Y, Coates AR, Hubank M, Spratt DA, Wilson M, Keijser BJ, Crielaard W. Same Exposure but Two Radically Different Responses to Antibiotics: Resilience of the Salivary Microbiome versus Long-Term Microbial Shifts in Feces. mBio. 2015;6:e01693-e01615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 319] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 100. | Yao C, Lan D, Li X, Wang Y, Qi S, Liu Y. Porphyromonas gingivalis is a risk factor for the development of nonalcoholic fatty liver disease via ferroptosis. Microbes Infect. 2023;25:105040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 101. | Yao C, Lan D, Li X, Wang Y, Qi S. Porphyromonas gingivalis triggers inflammation in hepatocyte depend on ferroptosis via activating the NF-κB signaling pathway. Oral Dis. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 102. | Kitamoto S, Nagao-Kitamoto H, Jiao Y, Gillilland MG 3rd, Hayashi A, Imai J, Sugihara K, Miyoshi M, Brazil JC, Kuffa P, Hill BD, Rizvi SM, Wen F, Bishu S, Inohara N, Eaton KA, Nusrat A, Lei YL, Giannobile WV, Kamada N. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell. 2020;182:447-462.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 414] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 103. | Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, Popova IE, Parikh SJ, Adams LG, Tsolis RM, Stewart VJ, Bäumler AJ. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339:708-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 746] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 104. | Nassar M, Tabib Y, Capucha T, Mizraji G, Nir T, Pevsner-Fischer M, Zilberman-Schapira G, Heyman O, Nussbaum G, Bercovier H, Wilensky A, Elinav E, Burstyn-Cohen T, Hovav AH. GAS6 is a key homeostatic immunological regulator of host-commensal interactions in the oral mucosa. Proc Natl Acad Sci U S A. 2017;114:E337-E346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 105. | Hyde ER, Andrade F, Vaksman Z, Parthasarathy K, Jiang H, Parthasarathy DK, Torregrossa AC, Tribble G, Kaplan HB, Petrosino JF, Bryan NS. Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: implications for nitric oxide homeostasis. PLoS One. 2014;9:e88645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 106. | Pan W, Wang Q, Chen Q. The cytokine network involved in the host immune response to periodontitis. Int J Oral Sci. 2019;11:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 422] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 107. | Friedrich M, Pohin M, Powrie F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity. 2019;50:992-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 510] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 108. | Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017;14:573-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1021] [Cited by in RCA: 1179] [Article Influence: 147.4] [Reference Citation Analysis (0)] |
| 109. | Zhao XQ, Wang L, Zhu CL, Xue XH, Xia XJ, Wu XL, Wu YD, Liu SQ, Zhang GP, Bai YY, Fotina H, Hu JH. Oral Administration of the Antimicrobial Peptide Mastoparan X Alleviates Enterohemorrhagic Escherichia coli-Induced Intestinal Inflammation and Regulates the Gut Microbiota. Probiotics Antimicrob Proteins. 2024;16:138-151. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 110. | Carter-Kent C, Zein NN, Feldstein AE. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: implications for treatment. Am J Gastroenterol. 2008;103:1036-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 111. | Pradeep AR, Raghavendra NM, Prasad MV, Kathariya R, Patel SP, Sharma A. Gingival crevicular fluid and serum visfatin concentration: their relationship in periodontal health and disease. J Periodontol. 2011;82:1314-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 112. | Kardeşler L, Buduneli N, Cetinkalp S, Kinane DF. Adipokines and inflammatory mediators after initial periodontal treatment in patients with type 2 diabetes and chronic periodontitis. J Periodontol. 2010;81:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 113. | Tomofuji T, Ekuni D, Irie K, Azuma T, Tamaki N, Maruyama T, Yamamoto T, Watanabe T, Morita M. Relationships between periodontal inflammation, lipid peroxide and oxidative damage of multiple organs in rats. Biomed Res. 2011;32:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 114. | Matthews JB, Wright HJ, Roberts A, Ling-Mountford N, Cooper PR, Chapple IL. Neutrophil hyper-responsiveness in periodontitis. J Dent Res. 2007;86:718-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 115. | Önder C, Kurgan Ş, Altıngöz SM, Bağış N, Uyanık M, Serdar MA, Kantarcı A, Günhan M. Impact of non-surgical periodontal therapy on saliva and serum levels of markers of oxidative stress. Clin Oral Investig. 2017;21:1961-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 116. | Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16:745-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 949] [Cited by in RCA: 1293] [Article Influence: 184.7] [Reference Citation Analysis (0)] |
| 117. | How KY, Song KP, Chan KG. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front Microbiol. 2016;7:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 475] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 118. | Holt SC, Kesavalu L, Walker S, Genco CA. Virulence factors of Porphyromonas gingivalis. Periodontol 2000. 1999;20:168-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 497] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 119. | Nakahara T, Hyogo H, Ono A, Nagaoki Y, Kawaoka T, Miki D, Tsuge M, Hiraga N, Hayes CN, Hiramatsu A, Imamura M, Kawakami Y, Aikata H, Ochi H, Abe-Chayama H, Furusho H, Shintani T, Kurihara H, Miyauchi M, Takata T, Arihiro K, Chayama K. Involvement of Porphyromonas gingivalis in the progression of non-alcoholic fatty liver disease. J Gastroenterol. 2018;53:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 120. | Dashper SG, Mitchell HL, Seers CA, Gladman SL, Seemann T, Bulach DM, Chandry PS, Cross KJ, Cleal SM, Reynolds EC. Porphyromonas gingivalis Uses Specific Domain Rearrangements and Allelic Exchange to Generate Diversity in Surface Virulence Factors. Front Microbiol. 2017;8:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 121. | Enersen M, Nakano K, Amano A. Porphyromonas gingivalis fimbriae. J Oral Microbiol. 2013;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 122. | Pathirana RD, O'Brien-Simpson NM, Reynolds EC. Host immune responses to Porphyromonas gingivalis antigens. Periodontol 2000. 2010;52:218-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 123. | Zhou Q, Amar S. Identification of signaling pathways in macrophage exposed to Porphyromonas gingivalis or to its purified cell wall components. J Immunol. 2007;179:7777-7790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 124. | Xu W, Zhou W, Wang H, Liang S. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv Protein Chem Struct Biol. 2020;120:45-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 125. | Guo Y, Nguyen KA, Potempa J. Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon's knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000. 2010;54:15-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 263] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 126. | Grenier D, Tanabe S. Porphyromonas gingivalis gingipains trigger a proinflammatory response in human monocyte-derived macrophages through the p38α mitogen-activated protein kinase signal transduction pathway. Toxins (Basel). 2010;2:341-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 127. | Khalaf H, Bengtsson T. Altered T-cell responses by the periodontal pathogen Porphyromonas gingivalis. PLoS One. 2012;7:e45192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 128. | Sharma D, Misba L, Khan AU. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control. 2019;8:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1108] [Cited by in RCA: 900] [Article Influence: 150.0] [Reference Citation Analysis (0)] |
| 129. | Costerton JW, Geesey GG, Cheng KJ. How bacteria stick. Sci Am. 1978;238:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 901] [Cited by in RCA: 671] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 130. | Bai D, Nakao R, Ito A, Uematsu H, Senpuku H. Immunoreactive antigens recognized in serum samples from mice intranasally immunized with Porphyromonas gingivalis outer membrane vesicles. Pathog Dis. 2015;73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 131. | Nakao R, Takashiba S, Kosono S, Yoshida M, Watanabe H, Ohnishi M, Senpuku H. Effect of Porphyromonas gingivalis outer membrane vesicles on gingipain-mediated detachment of cultured oral epithelial cells and immune responses. Microbes Infect. 2014;16:6-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 132. | Seyama M, Yoshida K, Fujiwara N, Ono K, Eguchi T, Kawai H, Guo J, Weng Y, Haoze Y, Uchibe K, Ikegame M, Sasaki A, Nagatsuka H, Okamoto K, Okamura H, Ozaki K. Outer membrane vesicles of Porphyromonas gingivalis attenuate insulin sensitivity by delivering gingipains to the liver. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 133. | Płóciennikowska A, Hromada-Judycka A, Borzęcka K, Kwiatkowska K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2015;72:557-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 365] [Cited by in RCA: 573] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 134. | Darveau RP, Arbabi S, Garcia I, Bainbridge B, Maier RV. Porphyromonas gingivalis lipopolysaccharide is both agonist and antagonist for p38 mitogen-activated protein kinase activation. Infect Immun. 2002;70:1867-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 135. | Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 1436] [Article Influence: 179.5] [Reference Citation Analysis (0)] |
| 136. | Harte AL, da Silva NF, Creely SJ, McGee KC, Billyard T, Youssef-Elabd EM, Tripathi G, Ashour E, Abdalla MS, Sharada HM, Amin AI, Burt AD, Kumar S, Day CP, McTernan PG. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond). 2010;7:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 294] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 137. | Farhadi A, Gundlapalli S, Shaikh M, Frantzides C, Harrell L, Kwasny MM, Keshavarzian A. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int. 2008;28:1026-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 138. | Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 629] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 139. | Masi S, Gkranias N, Li K, Salpea KD, Parkar M, Orlandi M, Suvan JE, Eng HL, Taddei S, Patel K, Darbar U, Donos N, Deanfield JE, Hurel S, Humphries SE, D'Aiuto F. Association between short leukocyte telomere length, endotoxemia, and severe periodontitis in people with diabetes: a cross-sectional survey. Diabetes Care. 2014;37:1140-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 140. | Li Y, Shi Z, Radauer-Preiml I, Andosch A, Casals E, Luetz-Meindl U, Cobaleda M, Lin Z, Jaberi-Douraki M, Italiani P, Horejs-Hoeck J, Himly M, Monteiro-Riviere NA, Duschl A, Puntes VF, Boraschi D. Bacterial endotoxin (lipopolysaccharide) binds to the surface of gold nanoparticles, interferes with biocorona formation and induces human monocyte inflammatory activation. Nanotoxicology. 2017;11:1157-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 141. | Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 743] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 142. | Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 560] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 143. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2611] [Article Influence: 200.8] [Reference Citation Analysis (1)] |
| 144. | López-Suárez A, Guerrero JM, Elvira-González J, Beltrán-Robles M, Cañas-Hormigo F, Bascuñana-Quirell A. Nonalcoholic fatty liver disease is associated with blood pressure in hypertensive and nonhypertensive individuals from the general population with normal levels of alanine aminotransferase. Eur J Gastroenterol Hepatol. 2011;23:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 145. | Vasconcelos ACCG, Vasconcelos DFP, Pereira da Silva FR, de Carvalho França LF, Alves EHP, Lenardo DD, Dos Santos Pessoa L, Novaes PD, Luiz Dos Reis Barbosa A, Mani A, Mariano FS, Medeiros JR, de Oliveira AP. Periodontitis causes abnormalities in the liver of rats. J Periodontol. 2019;90:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 146. | Pessoa LS, Pereira-da Silva FR, Alves EH, França LF, di Lenardo D, Carvalho JS, Martins VB, Sousa FB, Drumond KO, Medeiros JV, de Oliveira JS, Vasconcelos DF. One or two ligatures inducing periodontitis are sufficient to cause fatty liver. Med Oral Patol Oral Cir Bucal. 2018;23:e269-e276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 147. | Vasconcelos DF, Pereira da Silva FR, Pinto ME, Santana LA, Souza IG, Miranda de Souza LK, Oliveira NC, Ventura CA, Novaes PD, Barbosa AL, Medeiros JR, Mikolasevic I, Mani A, Soares de Oliveira J. Decrease of Pericytes is Associated With Liver Disease Caused by Ligature-Induced Periodontitis in Rats. J Periodontol. 2017;88:e49-e57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 148. | Ishikawa M, Yoshida K, Okamura H, Ochiai K, Takamura H, Fujiwara N, Ozaki K. Oral Porphyromonas gingivalis translocates to the liver and regulates hepatic glycogen synthesis through the Akt/GSK-3β signaling pathway. Biochim Biophys Acta. 2013;1832:2035-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 149. | Takamura H, Yoshida K, Okamura H, Fujiwara N, Ozaki K. Porphyromonas gingivalis attenuates the insulin-induced phosphorylation and translocation of forkhead box protein O1 in human hepatocytes. Arch Oral Biol. 2016;69:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 150. | Zaitsu Y, Iwatake M, Sato K, Tsukuba T. Lipid droplets affect elimination of Porphyromonas gingivalis in HepG2 cells by altering the autophagy-lysosome system. Microbes Infect. 2016;18:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 151. | Kim YJ, Kim BK, Park SJ, Kim JH. Impact of Fusobacterium nucleatum in the gastrointestinal tract on natural killer cells. World J Gastroenterol. 2021;27:4879-4889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 152. | Kamata Y, Kessoku T, Shimizu T, Kobayashi T, Kurihashi T, Sato S, Kuraji S, Aoyama N, Iwasaki T, Takashiba S, Hamada N, Kodama T, Tamura T, Ino S, Higurashi T, Taguri M, Yamanaka T, Yoneda M, Usuda H, Wada K, Nakajima A, Minabe M. Efficacy and safety of PERIOdontal treatment versus usual care for Nonalcoholic liver disease: protocol of the PERION multicenter, two-arm, open-label, randomized trial. Trials. 2020;21:291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 153. | Bajaj JS, Matin P, White MB, Fagan A, Deeb JG, Acharya C, Dalmet SS, Sikaroodi M, Gillevet PM, Sahingur SE. Periodontal therapy favorably modulates the oral-gut-hepatic axis in cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2018;315:G824-G837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 154. | Jeong SH, Nam Y, Jung H, Kim J, Rim YA, Park N, Lee K, Choi S, Jang Y, Kim Y, Moon JH, Jung SM, Park SH, Ju JH. Author Correction: Interrupting oral infection of Porphyromonas gingivalis with anti-FimA antibody attenuates bacterial dissemination to the arthritic joint and improves experimental arthritis. Exp Mol Med. 2018;50:1-2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 155. | Cai J, Chen J, Guo H, Pan Y, Zhang Y, Zhao W, Li X, Li Y. Recombinant fimbriae protein of Porphyromonas gingivalis induces an inflammatory response via the TLR4/NFκB signaling pathway in human peripheral blood mononuclear cells. Int J Mol Med. 2019;43:1430-1440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 156. | Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U, Raha D, Griffin C, Holsinger LJ, Arastu-Kapur S, Kaba S, Lee A, Ryder MI, Potempa B, Mydel P, Hellvard A, Adamowicz K, Hasturk H, Walker GD, Reynolds EC, Faull RLM, Curtis MA, Dragunow M, Potempa J. Porphyromonas gingivalis in Alzheimer's disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5:eaau3333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 882] [Cited by in RCA: 1228] [Article Influence: 204.7] [Reference Citation Analysis (0)] |
| 157. | Tarantino G, Finelli C. Systematic review on intervention with prebiotics/probiotics in patients with obesity-related nonalcoholic fatty liver disease. Future Microbiol. 2015;10:889-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 158. | Ferolla SM, Armiliato GN, Couto CA, Ferrari TC. Probiotics as a complementary therapeutic approach in nonalcoholic fatty liver disease. World J Hepatol. 2015;7:559-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 159. | Shen W, Gaskins HR, McIntosh MK. Influence of dietary fat on intestinal microbes, inflammation, barrier function and metabolic outcomes. J Nutr Biochem. 2014;25:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 160. | Janssen AWF, Houben T, Katiraei S, Dijk W, Boutens L, van der Bolt N, Wang Z, Brown JM, Hazen SL, Mandard S, Shiri-Sverdlov R, Kuipers F, Willems van Dijk K, Vervoort J, Stienstra R, Hooiveld GJEJ, Kersten S. Modulation of the gut microbiota impacts nonalcoholic fatty liver disease: a potential role for bile acids. J Lipid Res. 2017;58:1399-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 161. | Gillespie JJ, Wattam AR, Cammer SA, Gabbard JL, Shukla MP, Dalay O, Driscoll T, Hix D, Mane SP, Mao C, Nordberg EK, Scott M, Schulman JR, Snyder EE, Sullivan DE, Wang C, Warren A, Williams KP, Xue T, Yoo HS, Zhang C, Zhang Y, Will R, Kenyon RW, Sobral BW. PATRIC: the comprehensive bacterial bioinformatics resource with a focus on human pathogenic species. Infect Immun. 2011;79:4286-4298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 240] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 162. | Bergheim I, Weber S, Vos M, Krämer S, Volynets V, Kaserouni S, McClain CJ, Bischoff SC. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 401] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 163. | Roberfroid M. Prebiotics: the concept revisited. J Nutr. 2007;137:830S-837S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 764] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 164. | Macfarlane S, Macfarlane GT, Cummings JH. Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther. 2006;24:701-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 371] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 165. | Vajro P, Mandato C, Licenziati MR, Franzese A, Vitale DF, Lenta S, Caropreso M, Vallone G, Meli R. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2011;52:740-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 166. | Asaduzzaman SM, Nagao J, Iida H, Zendo T, Nakayama J, Sonomoto K. Nukacin ISK-1, a bacteriostatic lantibiotic. Antimicrob Agents Chemother. 2009;53:3595-3598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 167. | Cotter PD, Hill C, Ross RP. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 2005;3:777-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1429] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 168. | Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39:1276-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 169. | Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014;99:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 170. | Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 473] [Article Influence: 39.4] [Reference Citation Analysis (1)] |