Published online Apr 27, 2024. doi: 10.4254/wjh.v16.i4.650
Peer-review started: December 16, 2023
First decision: January 17, 2024
Revised: January 30, 2024
Accepted: March 19, 2024
Article in press: March 19, 2024
Published online: April 27, 2024
Processing time: 130 Days and 5.5 Hours
De novo malignancy is a leading cause of late morbidity and mortality in liver transplant recipients. Cumulative immunosuppression has been shown to contribute to post-transplant malignancy (PTM) risk. There is emerging evidence on the differential carcinogenic risk profile of individual immunosuppressive drugs, independent of the net effect of immunosuppression. Calcineurin inhibit
To investigate the relative carcinogenicity of tacrolimus and MPA in solid organ transplantation.
A literature search was conducted using MEDLINE and Embase databases using the key terms “solid organ transplantation”, “tacrolimus”, “mycophenolic acid”, and “carcinogenicity”, in order to identify relevant articles published in English between 1st January 2002 to 11th August 2022. Related terms, synonyms and explosion of MeSH terms, Boolean operators and truncations were also utilised in the search. Reference lists of retrieved articles were also reviewed to identify any additional articles. Excluding duplicates, abstracts from 1230 records were screened by a single reviewer, whereby 31 records were reviewed in detail. Full-text articles were assessed for eligibility based on pre-specified inclusion and exclusion criteria.
A total of 6 studies were included in this review. All studies were large population registries or cohort studies, which varied in transplant era, type of organ transplanted and immunosuppression protocol used. Overall, there was no clear difference demonstrated between tacrolimus and MPA in de novo PTM risk following solid organ transplantation. Furthermore, no study provided a direct comparison of carcinogenic risk between tacrolimus and MPA monotherapy in solid organ transplantation recipients.
The contrasting carcinogenic risk profiles of tacrolimus and MPA demonstrated in previous experimental studies, and its application in solid organ transplantation, is yet to be confirmed in clinical studies. Thus, the optimal choice of immunosuppression drug to use as maintenance monotherapy in LT recipients is not supported by a strong evidence base and remains unclear.
Core Tip: Cumulative immunosuppression exposure is an important risk factor for the development of post-transplant malignancy. There is emerging evidence on the differential carcinogenic risk profile of individual immunosuppressive drugs, independent of the net immunosuppression effect. This review demonstrates that the evidence on the relative carcinogenicity of tacrolimus and mycophenolic acid, the two agents most commonly used as maintenance monotherapy in liver transplant patients, remains unclear. Further studies are required to determine the clinical relevance of previous experimental findings to enable physicians to tailor immunosuppression regimens to minimize individual malignancy risk in solid organ transplantation.
- Citation: Liu D, Youssef MM, Grace JA, Sinclair M. Relative carcinogenicity of tacrolimus vs mycophenolate after solid organ transplantation and its implications for liver transplant care. World J Hepatol 2024; 16(4): 650-660
- URL: https://www.wjgnet.com/1948-5182/full/v16/i4/650.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i4.650
Liver transplantation (LT) remains the only curative treatment for end-stage liver disease and some cases of hepatocellular carcinoma, with an overall median survival of 20 years[1]. Despite improvements in short-term survival with the decline in rates of rejection and graft failure with the advent of modern immunosuppression regimens, long-term complications including post-transplant malignancy (PTM), have risen. Liver transplant recipients incur a 2- to 3-fold increase in rates of de novo malignancy compared to the general population[2,3]. Indeed, PTM has become a leading cause of late mortality in LT recipients[4,5].
The cumulative exposure to immunosuppression and direct carcinogenicity of individual agents may contribute to the development of PTM[6]. Tacrolimus and mycophenolic acid (MPA) are the most commonly used backbone immunosuppressants post-LT, and are also utilised as maintenance monotherapy in 42% of LT recipients in the United States due to the relatively immune tolerant microenvironment of the liver[7,8]. Experimental data have demonstrated multiple pro-oncogenic effects of tacrolimus, whereas MPA may be protective against tumour growth and progression[9]. This systematic review aims to compare the relative carcinogenicity of tacrolimus and MPA in solid organ transplantation to assist clinicians in making informed decisions regarding choice of immunosuppression regimens for patients.
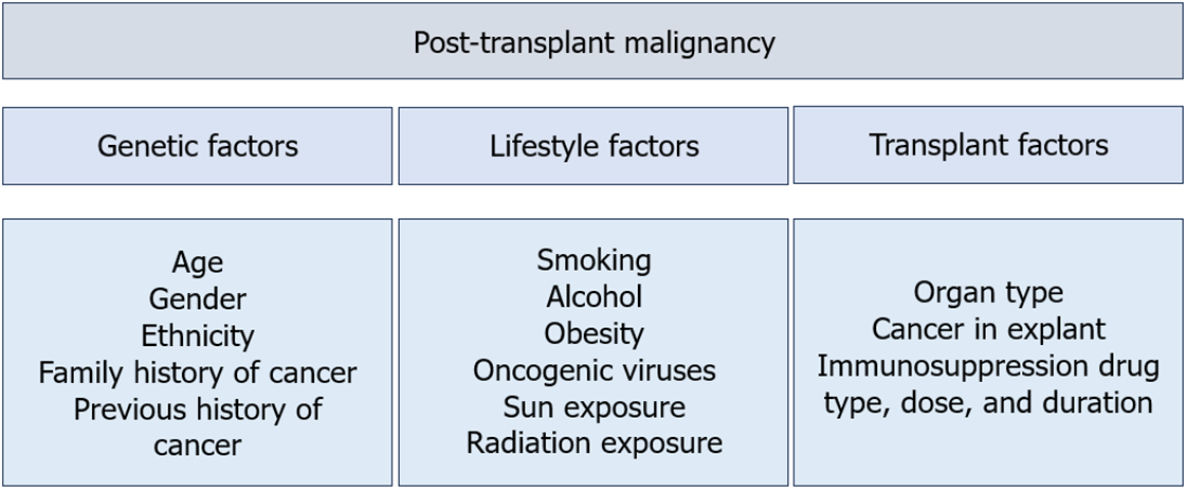
The development of PTM is a consequence of complex interactions between genetic, lifestyle and transplant factors (Figure 1). The central role of the immune system in cancer surveillance is highlighted by the increased malignancy risk that results from congenital and acquired immunodeficiencies, as well as the efficacy of immunotherapy for a growing number of malignancies such as hepatocellular carcinoma, melanoma, and renal cell carcinoma[6].
An intact immune system prevents oncogenesis through 3 main mechanisms. Firstly, the immune system eliminates or suppresses viral infections to prevent virus-induced tumours, as seen in the role of Ebstein Barr virus infections in the development of early post-transplant lymphoproliferative disorder (PTLD)[6,10]. Secondly, inflammation resolution and pathogen elimination prevents the establishment of a pro-inflammatory environment conducive to tumourigenesis[6,10]. Thirdly, cells of the innate and adaptive immune system can identify and eliminate tumour cells based on the expression of tumour-specific antigens and danger signals[6,10]. Chronic immunosuppression exposure disrupts the integrity of cancer immunosurveillance. Furthermore, animal studies have suggested that tumours developing in an immunocompromised host are more immunogenic compared to an immunocompetent host, enabling tumour cells to evade immune recognition and destruction[10]. Unsurprisingly, the incidence of PTM is as high as 20% in solid organ transplant recipien
Individual immunosuppression drugs may also have direct carcinogenic effects, resulting in DNA damage and gene expression changes that promote cancer progression independent of the effects of overall immunosuppression exposure.
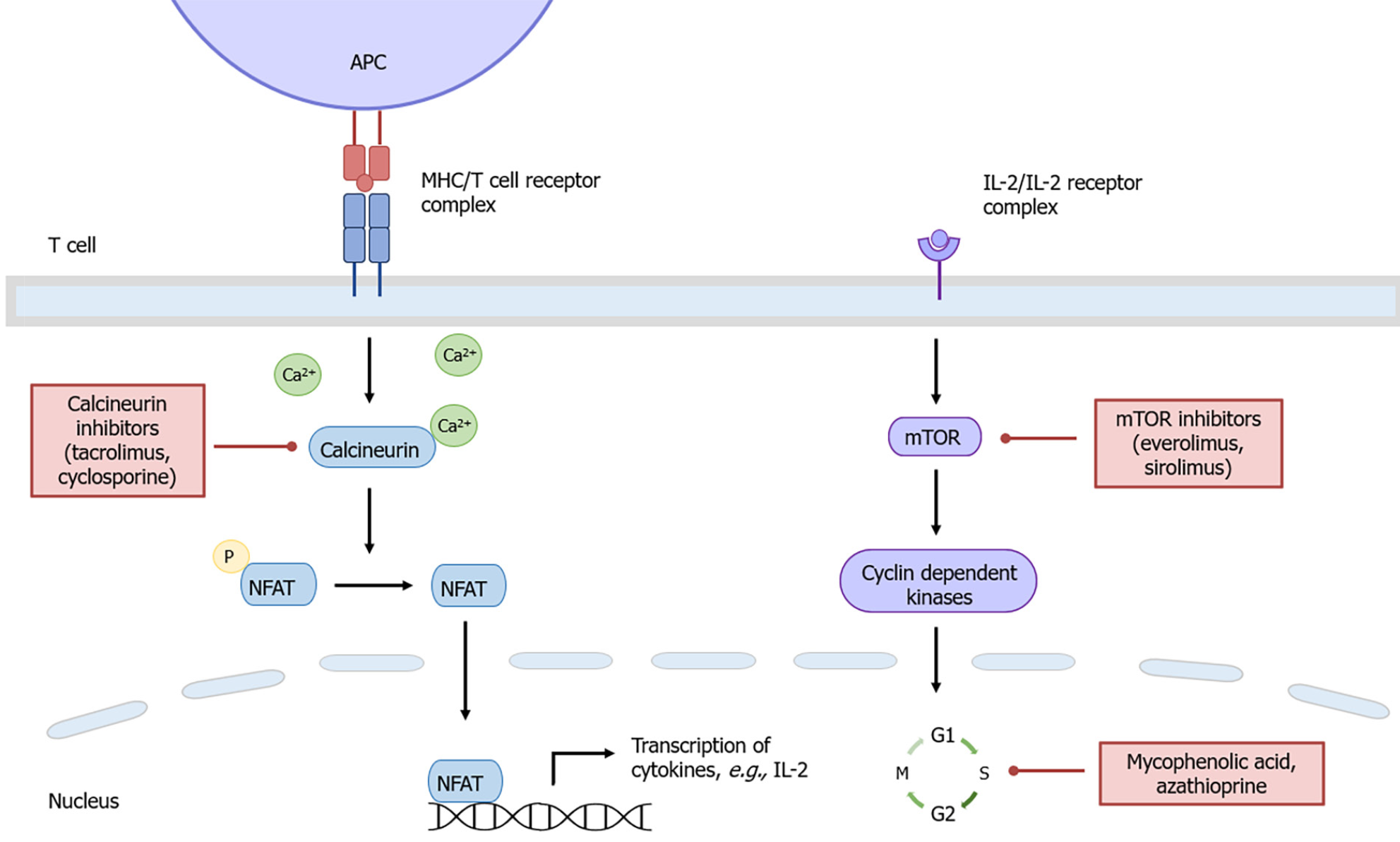
Calcineurin inhibitors (CNI) such as tacrolimus and cyclosporine suppress T cell activation and proliferation by inhibiting interleukin-2 gene transcription (Figure 2)[11]. The reduced rate of cellular rejection and resultant improved graft and patient survival associated with tacrolimus-based immunosuppression has led to tacrolimus being the CNI of choice following solid organ transplantation[12]. However, experimental data suggest tacrolimus may promote cancer progression by creating a tumour-permissive microenvironment independent of its immunosuppressive effects.
Tacrolimus has a dose-dependent effect on the production of transforming growth factor β1 (TGF-β1), a cytokine implicated in tumour growth, metastatic spread and development of biologically aggressive cancers[13,14]. The microenvironment is further altered by TGF-β1 through inhibition of anti-tumour immune responses and promotion of extracellular matrix production and angiogenesis[15].
The direct effect of tacrolimus on tumour angiogenesis is not fully understood and may be tissue-dependent. In vivo studies have demonstrated tacrolimus enhanced lymphangiogenesis and invasion of hepatocellular carcinoma via increased vascular endothelial growth factor (VEGF)-C expression[16]. However, tacrolimus may also hinder angio
Tacrolimus exposure may lead to alterations in gene expression that promote cancer development and progression. Tacrolimus has been found to activate the proto-oncogene, Ras, in human renal epithelial cells and renal cancer cells, contributing to renal cancer development[19]. Notably, the activation of Ras is critical for VEGF over-expression and subsequent angiogenesis[19]. Tacrolimus can also interfere with proline-oxidase and p53-mediated apoptosis, thus promoting tumour growth[20].
Experimental data on cyclosporine has similarly demonstrated its oncogenic effects through the over-expression of TGFβ and VEGF, impaired repair of radiation-induced DNA damage and promotion of apoptosis[21-25]. The shared mechanism of action between tacrolimus and cyclosporine possibly reflects a class-effect of CNIs on malignancy risk.
Mycophenolate mofetil is a key component of backbone immunosuppression following LT, allowing for CNI de-escalation or cessation and minimisation of renal and metabolic dysfunction. The active metabolite, MPA, inhibits inosine monophosphate dehydrogenase (IMPDH) which is a crucial enzyme involved in de novo guanosine nucleotide and DNA synthesis (Figure 2)[26]. This leads to the preferential depletion of lymphocytes due their dependency on de novo purine synthesis[26]. There is currently no experimental data linking MPA to increased carcinogenicity risk independent of its effects associated with overall immunosuppression. On the contrary, MPA has in vitro and in vivo anti-neoplastic properties which may confer a reduced risk of PTM.
MPA has been shown to inhibit the growth of a variety of in vivo tumour cell lines[27-31]. Upregulation of peroxisome proliferative-activated receptor gamma by MPA prevents tumour cell differentiation[32]. Reduced expression of adhesion molecules on lymphocytes and endothelial cells interferes with adhesion receptor-dependent tumour dissemination[33-36]. Furthermore, increased expression of subtypes of adhesion receptors from the β1 integrin family may induce re-differentiation of tumour cells towards a lower invasive phenotype[36]. However, some cancer types have been found to be resistant to the anti-neoplastic properties of MPA[28,37].
The anti-neoplastic properties of MPA may also have a therapeutic potential. The enzyme IMPDH, the target of MPA, is over-expressed in cancer cells[26]. Furthermore, MPA-mediated inhibition of IMPDH has been demonstrated to induce tumour cell apoptosis, however these findings are yet to be confirmed in vivo[38].
Azathioprine is a purine analogue that is incorporated into cellular DNA, where it inhibits purine nucleotide synthesis and interferes with RNA synthesis and metabolism (Figure 2)[15]. It is well known that azathioprine is a risk factor for the development of PTM, in particular, non-melanoma skin cancer. Multiple studies have demonstrated the synergistic effect between ultraviolet A radiation and the azathioprine metabolite, 6-thioguanine, in the generation of mutagenic oxidative DNA damage[39,40]. The carcinogenic effects of azathioprine have limited its use in transplantation in favour of MPA.
Sirolimus and everolimus inhibit mammalian target of rapamycin (mTOR), which subsequently downregulates cyclin-dependent kinases and mRNAs required for cell cycle progression, thus preventing interleukin-2-mediated lymphocyte proliferation (Figure 2)[9]. In vivo studies have shown mTOR inhibitors precipitate tumour cell cycle progression arrest and subsequent apoptosis[41,42]. Impaired VEGF production and signalling also restricts tumour angiogenesis and metastatic spread[43-45]. Interestingly, the simultaneous administration of sirolimus in these models can reverse the pro-angiogenic effects of cyclosporine[43-45]. The potential dual immunosuppressive and anti-neoplastic properties of mTOR inhibitors has led to its increasing utilisation in the transplantation setting.
Multiple population and cohort studies have investigated the role of tacrolimus and MPA in the development of de novo PTM, however a direct causal relationship is difficult to establish. As the two most commonly used drugs for maintenance monotherapy post-LT, the oncogenic risk profile of tacrolimus and MPA warrants further review.
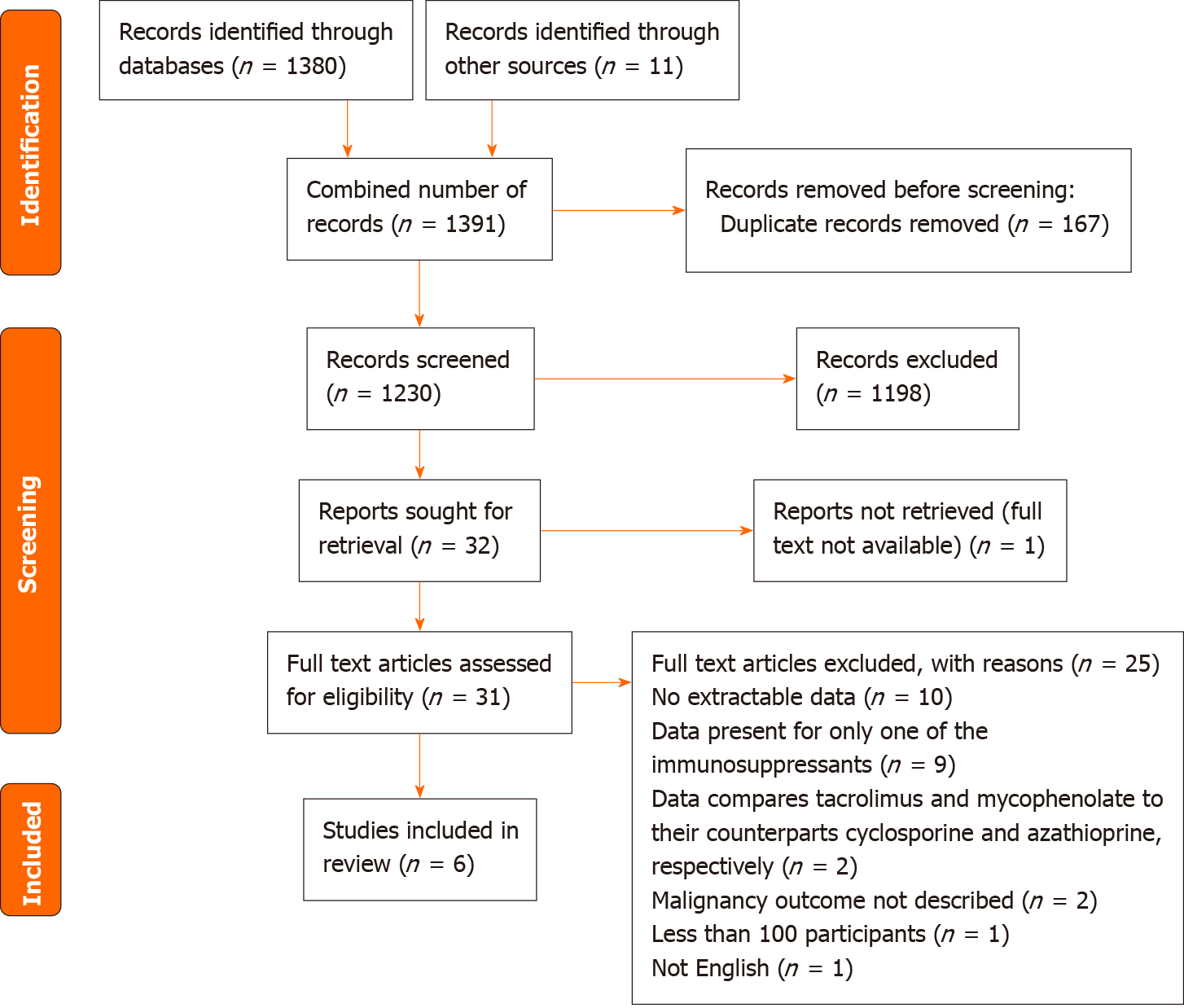
Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were utilised to shortlist relevant articles for this narrative review to minimise bias. A comprehensive literature search was conducted through MEDLINE and Embase electronic databases between 1st January 2002 to 11th August 2022. This time period was selected to include relevant literature since the introduction and clinical use of MPA. The following terms were used, including synonyms and closely related words, as Medical Subject Headings (MeSH) and text words: “Solid Organ Transplantation”, “Tacrolimus”, “Mycophenolic Acid”, and “Carcinogenicity”. Explosion of MeSH terms, Boolean operators and truncations were also utilised throughout the search. Further articles were identified through reference lists of published systematic reviews in the area. Excluding duplicates, abstracts from 1230 records were screened by a single reviewer, whereby 31 records were deemed appropriate for full-text review. Full-text articles were assessed for eligibility based on inclusion and exclusion criteria, leaving 6 studies for inclusion in this review (Table 1, Figure 3).
| Inclusion criteria | Exclusion criteria |
| Involve human solid organ transplant recipients | Presents risk data on only one of the immunosuppressant medications |
| Independent malignancy risk analysis related to both immunosuppressants mycophenolic acid and tacrolimus | Does not specify type of immunosuppression |
| Contains a group of participants exposed to tacrolimus or mycophenolic acid, exclusive of the other | Mean follow up less than one year (given the slow growing nature of malignancy) |
| Greater than 100 participants | Not published in English |
| Greater than 5 cases of malignancy | Full text not available |
| Randomised controlled trials and observational studies | Systematic reviews and meta-analyses |
The 6 studies included in this review are summarised in Table 2. All studies were large population-based registries or cohort studies that analysed PTM risk in the presence or absence of tacrolimus or MPA use. No studies included data on individual drug dosages, plasma levels or duration to assess for cumulative drug exposure. There was heterogeneity amongst the studied populations in type of organ transplanted, transplantation era and immunosuppression regimens used. No studies provided a direct comparative risk of PTM with tacrolimus or MPA monotherapy.
| Ref. | Transplant era | Organ | n | De novo malignancy | Time to malignancy (median) | TAC vs no TAC (OR/HR/IRR) | MPA vs no MPA (OR/HR/IRR) |
| Van Leeuwen et al[50], 2009 | 1982 to 2003 | Kidney | 8162 | 203 lip SCC; 121 lip SCC of the lower vermillion in first transplant) | 6.1 yr | SCC of the lower vermillion of lip during first transplant [IRR: 2.07, 95%CI: 0.45-9.50 (P = 0.35)] | SCC of the lower vermillion of lip during first transplant [IRR: 0.85, 95%CI: 0.28-2.60 (P = 0.77)] |
| Caillard et al[51], 2012 | 1998 to 2007 | Kidney ± pancreas | 21351 | 181 PTLD; 43 graft PTLD | Not specified | Any PTLD [aHR: 0.66, 95%CI: 0.36-1.22 (P = 0.19)]; Graft PTLD [HR: 0.33, 95%CI: 0.16-0.68 | Any PTLD [aHR: 1.22, 95%CI: 0.74-2.02 (P = 0.44)]; Graft PTLD [HR: 0.44, 95%CI: 0.23-0.86 (P = 0.015)a] |
| Hsiao et al[47], 2014 | 2000 to 2008 | Kidney | 642 | 54 non-cutaneous malignancy | 3.9 yr | HR: 1.99, 95%CI: 0.66-6.00 (P = 0.22) | HR: 1.00, 95%CI: 0.40-2.45 (P = 0.99) |
| Coghill et al[49], 2016 | 1995 to 2010 | Kidney ± pancreas, and heart | 2004 | 170 SCC | 9.0 yr | Single SCC (OR: 1.11, 95%CI: 0.48-2.60) | Single SCC (OR: 0.52, 95%CI: 0.32-0.84a) |
| Yeh et al[46], 2020 | 1997 to 2011 | Liver, kidney, and heart | 7852 | 612 cutaneous and non-cutaneous malignancy | Not specified | cHR (heart): 0.6, 95%CI: 0.1-2.7; cHR (kidney): 1.5, 95%CI: 0.8-2.6; aHR (liver): 0.6, 95%CI: 0.2-1.7 | cHR (heart): 1.6, 95%CI: 0.7-3.3; aHR (kidney): 1.5, 95%CI: 1.2-1.8 (P < 0.001)a; cHR (liver): 1.5, 95%CI: 0.9-2.5 |
| Gibson et al[48], 2021 | 2010 to 2018 | Liver, kidney ± pancreas, heart, and lung | 2852 | 242 cutaneous malignancy | 4.7 yr | IRR: 0.83, 95%CI: 0.55-1.25 (P = 0.37) | IRR: 0.78, 95%CI: 0.54-1.12 (P = 0.18) |
A Taiwanese population-based study evaluated risk factors for de novo cutaneous and non-cutaneous malignancy in 7852 liver, heart, and kidney transplant recipients[46]. Among 2127 liver transplant recipients, 111 (5.2%) malignancies were recorded during the mean follow-up period of 4.2 years[46]. Despite the majority of liver transplant recipients using tacrolimus (77.3%) or MPA (99.0%), neither immunosuppressant was associated with PTM risk[46].
Among 687 heart transplant patients, 31 (4.5%) de novo malignancies were reported[46]. Immunosuppression therapy was also not associated with PTM risk in this cohort[46]. However, the smaller number of malignancy outcomes may have contributed to attenuated risk estimates.
De novo malignancy was diagnosed in 470 out of 5038 (9.3%) kidney transplant recipients[46]. The use of MPA was an independent risk factor for PTM in kidney transplant recipients, compared to no MPA use [adjusted hazard ratio (HR): 1.5, 95% confidence interval (95%CI): 1.2-1.8; P < 0.001][46]. MPA exposure was also a risk factor for de novo transitional cell carcinoma (adjusted HR: 1.7, 95%CI: 1.2-2.4; P < 0.01) and renal cell carcinoma (adjusted HR: 1.7, 95%CI: 1.1-2.8; P < 0.05) in a sub-analysis of kidney transplant recipients without hypertension or diabetes as an underlying cause for renal failure[46].
On the contrary, a smaller Taiwanese population cohort study of 642 kidney transplant recipients did not demonstrate an association between MPA or tacrolimus exposure, and the development of 54 (8.4%) de novo malignancies[47]. However, the study’s primary endpoint of hospitalisation due to malignancy as the primary coded diagnosis, likely underestimated the incidence of de novo PTM from the exclusion of malignancies coded as secondary diagnoses or those diagnosed in the community.
Differences in immunosuppression regimens and cumulative exposure to individual drugs may also contribute to the conflicting findings of the aforementioned studies, however this data was not available for analysis. Additionally, lifestyle factors known to influence malignancy risk such as smoking and alcohol consumption, were not included in either study.
Three studies investigated the relationship between immunosuppression and post-transplant cutaneous malignancy.
A population-based study in the United Kingdom investigated the development of post-transplant melanoma and non-melanoma skin cancers in 2852 liver, kidney, pancreas, heart, and lung transplant recipients, compared to 13527 matched controls from the general population[48]. Among 437 liver transplant recipients, 19 (4.3%) skin cancers were diagnosed during the 6.2 year median follow-up period[48]. Liver transplant recipients had the lowest incidence of skin cancer compared to other solid organ transplant recipients [Incidence rate ratio (IRR): 4.34, 95%CI: 2.48-7.58, P = 0.00], possibly reflecting lower immunosuppression requirements and relative immune privilege[48]. Neither tacrolimus nor MPA use was associated with the development of de novo cutaneous malignancy across all solid organ transplantation[48]. However, these findings are limited by small outcome numbers. Additionally, the complex interaction between immunosuppression agents and other risk factors for skin cancer including smoking status and ultraviolet light exposure was not considered.
An American study compared 170 kidney, kidney/pancreas, and heart transplant recipients with de novo cutaneous squamous cell carcinoma (SCC) to 324 matched recipient controls[49]. Risk factors such as smoking status, family history of skin cancer and personal history of pre-cancerous skin lesions were adjusted for, however the cancer group were significantly older than the non-cancer group despite matching. In azathioprine naïve patients, MPA use was associated with lower cutaneous SCC risk, independent of tacrolimus exposure (OR: 0.52, 95%CI: 0.32-0.84)[49]. Current and previous MPA use was also inversely associated with the development of multiple cutaneous SCCs (previous MPA use: OR: 0.53, 95%CI: 0.3-0.94; current MPA use: OR: 0.52, 95%CI: 0.29-0.94)[49]. Conversely, cyclosporine-naïve patients treated with tacrolimus had no significant difference in cutaneous SCC risk compared to no tacrolimus use, when adjusted for MPA exposure[49]. Although the authors considered individual immunosuppression exposure risk in the clinical context of changing multi-drug regimens, this was limited by potential recall bias associated with self-reported questionnaires used to obtain immunosuppression data.
Finally, de novo lip SCC was evaluated in a large Australian and New Zealand registry study of 8162 kidney transplant patients[50]. Mycophenolate use was associated with reduced risk of SCC of the lower vermillion of the lip in univariate (IRR: 0.28, 95%CI: 0.12-0.69, P = 0.006), but not multivariate (IRR: 0.85, 95%CI: 0.28-2.60, P = 0.774) analyses[50]. There was no difference between tacrolimus use vs no use in the risk of lip SCC of the lower vermillion (IRR: 2.07, 95%CI: 0.45-9.50, P = 0.35)[50]. Of note, the study included patients transplanted between 1982 and 2003, with less use of tacrolimus (2/121, 1.7%) and MPA (5/121, 4.1%) during this transplant era, compared to cyclosporine and azathioprine, respectively. This study was likely underpowered to draw conclusions between tacrolimus and MPA exposure and risk of SCC of the lower vermillion of the lip.
A large population registry in France evaluated risk factors for PTLD occurrence in kidney and kidney/pancreas transplant recipients over a 10-year period[51]. Compared to 21170 control kidney transplant recipients, 327 cases of PTLD were recorded and 181 cases were included in the final analysis[51]. Tacrolimus and MPA use were not associated with overall PTLD risk, even when simultaneous kidney pancreas transplant recipients were excluded[51]. However, tacrolimus and MPA were negatively associated with graft site PTLD (tacrolimus: HR: 0.33, 95%CI: 0.16-0.68; MPA: HR: 0.44; 95%CI: 0.23-0.86), which may be attributed to fewer episodes of acute rejection and less immunosuppression exposure in this subgroup[51].
With long-term survival now commonplace following LT, there is an increasing need to improve non-hepatic health to avoid complications including metabolic derangements, renal impairment and de novo malignancy. De novo PTM accounts for approximately 16.4% of late deaths following LT[12,52]. Although immunosuppression exposure is a well-known contributor of PTM risk, there remains uncertainty regarding the carcinogenic effect of specific immunosuppression drugs, alone or in combination. This is the first narrative review that compares the relative carcinogenicity of tacrolimus and MPA in solid organ transplant recipients.
Existing in vitro and in vivo experimental data have portrayed a contrasting carcinogenic risk profile between tacrolimus and MPA. Tacrolimus promotes oncogenesis and tumour growth in its surrounding microenvironment with the activation of proto-oncogenes, production of TGF-β and inhibition of apoptosis[13,19,20]. The data on MPA is limited but suggests possible inhibition of tumour cell differentiation and prevention of vascular spread through alteration of cellular adhesion molecule expression[6]. However, there is currently no human data that directly compares the carcinogenic effects of tacrolimus and MPA in LT or other solid organ transplantation.
This review included a small number of studies that did not demonstrate a clear difference between tacrolimus and MPA in de novo PTM risk following solid organ transplantation. Our findings are in keeping with a recent systematic review and meta-analysis of kidney, liver, heart, and lung transplant recipients, whereby the risk of de novo malignancy did not differ between patients who received MPA and patients who received tacrolimus (OR: 0.88, 95%CI: 0.69-1.14, P = 0.33)[53]. However, the relationship between immunosuppression exposure and de novo PTM risk may vary based on transplant type. In liver transplant recipients, cumulative tacrolimus exposure has been associated with the development of PTM[54,55], although the high tacrolimus doses utilised in these studies are no longer aimed for in routine clinical practice. Furthermore, the conversion from CNI-based immunosuppression to MPA monotherapy post-LT results in either similar or lower rates of PTM[56,57]. Whether the reduction in PTM risk found in these studies is due to the effects of MPA or the reduction in tacrolimus exposure, is unknown. Thus, the differential carcinogenic risk profile of tacrolimus and MPA found in previous experimental studies is yet to be replicated in the clinical setting. Further clarification with large prospective studies is required.
There are inherent practical and financial difficulties in designing studies to compare the relative risk of de novo PTM between tacrolimus and MPA. Large prospective population-based studies of prolonged follow-up duration are required to ensure adequate statistical power. Variables that influence PTM risk such as age, gender, ethnicity, and smoking should be identified. However, there may be unidentifiable confounders that are difficult to capture, owing to the complex interaction between genetic, lifestyle and disease factors in oncogenesis. Population-based registries often rely on International Classification of Diseases coding for data collection, which can lead under-representation of malignancy incidence due to miscoding. Finally, longitudinal recording of drug dose, plasma levels and duration is required to capture changes in immunosuppression regimens frequently seen in routine clinical practice. The accurate calculation of cumulative immunosuppression exposure minimises drug exposure misclassification bias seen in current transplant cohort analyses that presume an unvarying drug regimen.
Immunosuppression minimisation is an important strategy to reduce PTM risk given the limited clinical data surrounding individual agents. There are currently no clear guidelines regarding immunosuppression drug choice to minimise PTM risk following LT. European LT guidelines state CNI-related de novo PTM risk may be due to dosage, and that there is no evidence to suggest MPA contributes to de novo PTM development[58]. In our centre, there is a preference for MPA, alone or in combination with everolimus, due to improved renal outcomes and experimental data suggesting higher PTM risk with tacrolimus. Overall, the choice of immunosuppression needs to be individualised based on recipient characteristics, liver disease aetiology, and alloimmune risk.
Routine cancer surveillance for all transplant recipients is recommended in addition to immunosuppression minimisation. Strict cancer surveillance strategies may lead to earlier cancer detection rates and improved non-cutaneous cancer patient survival in LT recipients[59,60]. As non-melanoma skin cancer is the leading cause of PTM in LT recipients, annual skin examinations by a dermatologist are recommended from 5 years or more after LT[5,61]. Recipients with primary sclerosing cholangitis and inflammatory bowel disease require annual colonoscopies for colorectal cancer surveillance[61]. Age and gender based cancer surveillance for all LT recipients is also recommended.
The clinical relevance of previous experimental studies on the relative carcinogenicity of tacrolimus and MPA, and its application in solid organ transplantation, is yet to be confirmed. Consequently, the choice of immunosuppressive agent to use as maintenance monotherapy in LT patients is not currently supported by a strong evidence base and remains unclear. Further studies are required to enable physicians to tailor immunosuppression regimens to minimise individual malignancy risk.
Many liver transplant (LT) recipients are able to be maintained on long-term immunosuppressive monotherapy, most commonly with either tacrolimus or mycophenolate. In experimental studies, tacrolimus is associated with increased carcinogenicity, whereas mycophenolic acid (MPA) may have anti-neoplastic properties. However, there is minimal clinical data comparing the relative carcinogenicity of tacrolimus and MPA in LT or other solid organ transplant recipients.
Post-transplant malignancy (PTM) is a leading cause of late mortality in LT recipients. Thus, a clinically relevant difference in the carcinogenic risk profile between tacrolimus and MPA will affect the choice of immunosuppressive agent used as maintenance monotherapy in LT patients.
To determine the relative carcinogenicity of tacrolimus and MPA in solid organ transplantation.
A systematic review was conducted using PRISMA guidelines with relevant articles published between 1st January 2002 to 11th August 2022 retrieved from MEDLINE and Embase databases for review.
A total of 6 studies were included in this systematic review, which did not demonstrate a clear difference between tacrolimus and MPA in the development of de novo PTM following solid organ transplantation.
The relative carcinogenicity of tacrolimus and MPA, and its clinical relevance in solid organ transplantation, remains unclear.
This review highlights the need for further large, population-based prospective studies to further assess the carcinogenic profiles of tacrolimus and MPA, to assist physicians in the choice of immunosuppressive agent to use as maintenance monotherapy in LT patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chauhan S, United States S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
| 1. | McCaughan GW, Munn SR. Liver transplantation in Australia and New Zealand. Liver Transpl. 2016;22:830-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Herrero JI. De novo malignancies following liver transplantation: impact and recommendations. Liver Transpl. 2009;15 Suppl 2:S90-S94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Rodríguez-Perálvarez M, De la Mata M, Burroughs AK. Liver transplantation: immunosuppression and oncology. Curr Opin Organ Transplant. 2014;19:253-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Gelson W, Hoare M, Dawwas MF, Vowler S, Gibbs P, Alexander G. The pattern of late mortality in liver transplant recipients in the United Kingdom. Transplantation. 2011;91:1240-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl. 2012;18:1277-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005;80:S254-S264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 412] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 7. | Abrol N, Jadlowiec CC, Taner T. Revisiting the liver's role in transplant alloimmunity. World J Gastroenterol. 2019;25:3123-3135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Wiesner RH, Fung JJ. Present state of immunosuppressive therapy in liver transplant recipients. Liver Transpl. 2011;17 Suppl 3:S1-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Guba M, Graeb C, Jauch KW, Geissler EK. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation. 2004;77:1777-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 271] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 10. | Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4947] [Cited by in RCA: 4527] [Article Influence: 323.4] [Reference Citation Analysis (0)] |
| 11. | Panackel C, Mathew JF, Fawas N M, Jacob M. Immunosuppressive Drugs in Liver Transplant: An Insight. J Clin Exp Hepatol. 2022;12:1557-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Rana A, Ackah RL, Webb GJ, Halazun KJ, Vierling JM, Liu H, Wu MF, Yoeli D, Kueht M, Mindikoglu AL, Sussman NL, Galván NT, Cotton RT, O'Mahony CA, Goss JA. No Gains in Long-term Survival After Liver Transplantation Over the Past Three Decades. Ann Surg. 2019;269:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 13. | Maluccio M, Sharma V, Lagman M, Vyas S, Yang H, Li B, Suthanthiran M. Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Transplantation. 2003;76:597-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Suthanthiran M, Hojo M, Maluccio M, Boffa DJ, Luan FL. Post-transplantation malignancy: a cell autonomous mechanism with implications for therapy. Trans Am Clin Climatol Assoc. 2009;120:369-388. [PubMed] |
| 15. | Gutierrez-Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs. 2007;67:1167-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 284] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 16. | Zhou S, Tan C, Dai Z, Zhu H, Xu M, Zhou Z, Wang W, Zhao Y, Fu X, Zhou J, Fan J. Tacrolimus enhances the invasion potential of hepatocellular carcinoma cells and promotes lymphatic metastasis in a rat model of hepatocellular carcinoma: involvement of vascular endothelial growth factor-C. Transplant Proc. 2011;43:2747-2754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Siamakpour-Reihani S, Caster J, Bandhu Nepal D, Courtwright A, Hilliard E, Usary J, Ketelsen D, Darr D, Shen XJ, Patterson C, Klauber-Demore N. The role of calcineurin/NFAT in SFRP2 induced angiogenesis--a rationale for breast cancer treatment with the calcineurin inhibitor tacrolimus. PLoS One. 2011;6:e20412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Shen Y, Jin R, Liang X, Deng Z, He J, Ding Y, Ding F, Lu L, Liu F, Yang J. Angiogenesis modulation-mediated inhibitory effects of tacrolimus on hypertrophic scar formation. Microvasc Res. 2023;145:104446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Datta D, Contreras AG, Basu A, Dormond O, Flynn E, Briscoe DM, Pal S. Calcineurin inhibitors activate the proto-oncogene Ras and promote protumorigenic signals in renal cancer cells. Cancer Res. 2009;69:8902-8909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Rivera A, Maxwell SA. The p53-induced gene-6 (proline oxidase) mediates apoptosis through a calcineurin-dependent pathway. J Biol Chem. 2005;280:29346-29354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, Shimbo T, Suthanthiran M. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 826] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 22. | Shihab FS, Bennett WM, Isaac J, Yi H, Andoh TF. Nitric oxide modulates vascular endothelial growth factor and receptors in chronic cyclosporine nephrotoxicity. Kidney Int. 2003;63:522-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Herman M, Weinstein T, Korzets A, Chagnac A, Ori Y, Zevin D, Malachi T, Gafter U. Effect of cyclosporin A on DNA repair and cancer incidence in kidney transplant recipients. J Lab Clin Med. 2001;137:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Morisaki T, Matsunaga H, Beppu K, Ihara E, Hirano K, Kanaide H, Mori M, Katano M. A combination of cyclosporin-A (CsA) and interferon-gamma (INF-gamma) induces apoptosis in human gastric carcinoma cells. Anticancer Res. 2000;20:3363-3373. [PubMed] |
| 25. | Nomura T, Yamamoto H, Mimata H, Shitashige M, Shibasaki F, Miyamoto E, Nomura Y. Enhancement by cyclosporin A of taxol-induced apoptosis of human urinary bladder cancer cells. Urol Res. 2002;30:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Kaltenborn A, Schrem H. Mycophenolate mofetil in liver transplantation: a review. Ann Transplant. 2013;18:685-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Williams RH, Lively DH, DeLong DC, Cline JC, Sweeny MJ. Mycophenolic acid: antiviral and antitumor properties. J Antibiot (Tokyo). 1968;21:463-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 81] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Tressler RJ, Garvin LJ, Slate DL. Anti-tumor activity of mycophenolate mofetil against human and mouse tumors in vivo. Int J Cancer. 1994;57:568-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Yokoyama I, Hayashi S, Kobayashi T, Negita M, Yasutomi M, Uchida K, Takagi H. Immunosuppressive drugs and their effect on experimental tumor growth. Transpl Int. 1995;8:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 30. | Sweeney MJ, Gerzon K, Harris PN, Holmes RE, Poore GA, Williams RH. Experimental antitumor activity and preclinical toxicology of mycophenolic acid. Cancer Res. 1972;32:1795-1802. [PubMed] |
| 31. | Koehl GE, Wagner F, Stoeltzing O, Lang SA, Steinbauer M, Schlitt HJ, Geissler EK. Mycophenolate mofetil inhibits tumor growth and angiogenesis in vitro but has variable antitumor effects in vivo, possibly related to bioavailability. Transplantation. 2007;83:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Zheng ZH, Yang Y, Lu XH, Zhang H, Shui XX, Liu C, He XB, Jiang Q, Zhao BH, Si SY. Mycophenolic acid induces adipocyte-like differentiation and reversal of malignancy of breast cancer cells partly through PPARγ. Eur J Pharmacol. 2011;658:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Heemann U, Azuma H, Hamar P, Schmid C, Tilney N, Philipp T. Mycophenolate mofetil inhibits lymphocyte binding and the upregulation of adhesion molecules in acute rejection of rat kidney allografts. Transpl Immunol. 1996;4:64-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Blaheta RA, Leckel K, Wittig B, Zenker D, Oppermann E, Harder S, Scholz M, Weber S, Encke A, Markus BH. Mycophenolate mofetil impairs transendothelial migration of allogeneic CD4 and CD8 T-cells. Transplant Proc. 1999;31:1250-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Leckel K, Beecken WD, Jonas D, Oppermann E, Coman MC, Beck KF, Cinatl J, Hailer NP, Auth MK, Bechstein WO, Shipkova M, Blaheta RA. The immunosuppressive drug mycophenolate mofetil impairs the adhesion capacity of gastrointestinal tumour cells. Clin Exp Immunol. 2003;134:238-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Engl T, Makarević J, Relja B, Natsheh I, Müller I, Beecken WD, Jonas D, Blaheta RA. Mycophenolate mofetil modulates adhesion receptors of the beta1 integrin family on tumor cells: impact on tumor recurrence and malignancy. BMC Cancer. 2005;5:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Dun B, Sharma A, Teng Y, Liu H, Purohit S, Xu H, Zeng L, She JX. Mycophenolic acid inhibits migration and invasion of gastric cancer cells via multiple molecular pathways. PLoS One. 2013;8:e81702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Guidicelli G, Chaigne-Delalande B, Dilhuydy MS, Pinson B, Mahfouf W, Pasquet JM, Mahon FX, Pourquier P, Moreau JF, Legembre P. The necrotic signal induced by mycophenolic acid overcomes apoptosis-resistance in tumor cells. PLoS One. 2009;4:e5493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | O'Donovan P, Perrett CM, Zhang X, Montaner B, Xu YZ, Harwood CA, McGregor JM, Walker SL, Hanaoka F, Karran P. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science. 2005;309:1871-1874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 507] [Cited by in RCA: 435] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 40. | Kelly GE, Meikle W, Sheil AG. Effects of immunosuppressive therapy on the induction of skin tumors by ultraviolet irradiation in hairless mice. Transplantation. 1987;44:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 69] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Majewski M, Korecka M, Joergensen J, Fields L, Kossev P, Schuler W, Shaw L, Wasik MA. Immunosuppressive TOR kinase inhibitor everolimus (RAD) suppresses growth of cells derived from posttransplant lymphoproliferative disorder at allograft-protecting doses. Transplantation. 2003;75:1710-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Nepomuceno RR, Balatoni CE, Natkunam Y, Snow AL, Krams SM, Martinez OM. Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Epstein Barr virus B-cell lymphomas. Cancer Res. 2003;63:4472-4480. [PubMed] |
| 43. | Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1301] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 44. | Luan FL, Ding R, Sharma VK, Chon WJ, Lagman M, Suthanthiran M. Rapamycin is an effective inhibitor of human renal cancer metastasis. Kidney Int. 2003;63:917-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 45. | Boffa DJ, Luan F, Thomas D, Yang H, Sharma VK, Lagman M, Suthanthiran M. Rapamycin inhibits the growth and metastatic progression of non-small cell lung cancer. Clin Cancer Res. 2004;10:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Yeh CC, Khan A, Muo CH, Yang HR, Li PC, Chang CH, Chen TL, Jeng LB, Liao CC. De Novo Malignancy After Heart, Kidney, and Liver Transplant: A Nationwide Study in Taiwan. Exp Clin Transplant. 2020;18:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Hsiao FY, Hsu WW. Epidemiology of post-transplant malignancy in Asian renal transplant recipients: a population-based study. Int Urol Nephrol. 2014;46:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Gibson JAG, Cordaro A, Dobbs TD, Griffiths R, Akbari A, Whitaker S, Hutchings HA, Lyons RA, Whitaker IS. The association between immunosuppression and skin cancer in solid organ transplant recipients: a control-matched cohort study of 2,852 patients. Eur J Dermatol. 2021;31:712-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Coghill AE, Johnson LG, Berg D, Resler AJ, Leca N, Madeleine MM. Immunosuppressive Medications and Squamous Cell Skin Carcinoma: Nested Case-Control Study Within the Skin Cancer after Organ Transplant (SCOT) Cohort. Am J Transplant. 2016;16:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 50. | van Leeuwen MT, Grulich AE, McDonald SP, McCredie MR, Amin J, Stewart JH, Webster AC, Chapman JR, Vajdic CM. Immunosuppression and other risk factors for lip cancer after kidney transplantation. Cancer Epidemiol Biomarkers Prev. 2009;18:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Caillard S, Lamy FX, Quelen C, Dantal J, Lebranchu Y, Lang P, Velten M, Moulin B; French Transplant Centers. Epidemiology of posttransplant lymphoproliferative disorders in adult kidney and kidney pancreas recipients: report of the French registry and analysis of subgroups of lymphomas. Am J Transplant. 2012;12:682-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 52. | Fuochi E, Anastasio L, Lynch EN, Campani C, Dragoni G, Milani S, Galli A, Innocenti T. Main factors influencing long-term outcomes of liver transplantation in 2022. World J Hepatol. 2023;15:321-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 53. | Zwart ES, Yüksel E, Pannekoek A, de Vries R, Mebius RE, Kazemier G. De Novo Carcinoma after Solid Organ Transplantation to Give Insight into Carcinogenesis in General-A Systematic Review and Meta-Analysis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Rodríguez-Perálvarez M, Colmenero J, González A, Gastaca M, Curell A, Caballero-Marcos A, Sánchez-Martínez A, Di Maira T, Herrero JI, Almohalla C, Lorente S, Cuadrado-Lavín A, Pascual S, López-Garrido MÁ, González-Grande R, Gómez-Orellana A, Alejandre R, Zamora-Olaya J, Bernal-Bellido C; Chronic immunosuppression, cancer Spanish consortium. Cumulative exposure to tacrolimus and incidence of cancer after liver transplantation. Am J Transplant. 2022;22:1671-1682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 55. | Carenco C, Assenat E, Faure S, Duny Y, Danan G, Bismuth M, Herrero A, Jung B, Ursic-Bedoya J, Jaber S, Larrey D, Navarro F, Pageaux GP. Tacrolimus and the risk of solid cancers after liver transplant: a dose effect relationship. Am J Transplant. 2015;15:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 56. | Schmeding M, Kiessling A, Neuhaus R, Heidenhain C, Bahra M, Neuhaus P, Neumann UP. Mycophenolate mofetil monotherapy in liver transplantation: 5-year follow-up of a prospective randomized trial. Transplantation. 2011;92:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Aguiar D, Martínez-Urbistondo D, D'Avola D, Iñarrairaegui M, Pardo F, Rotellar F, Sangro B, Quiroga J, Herrero JI. Conversion from Calcineurin Inhibitor-Based Immunosuppression to Mycophenolate Mofetil in Monotherapy Reduces Risk of De Novo Malignancies After Liver Transplantation. Ann Transplant. 2017;22:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 706] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 59. | Finkenstedt A, Graziadei IW, Oberaigner W, Hilbe W, Nachbaur K, Mark W, Margreiter R, Vogel W. Extensive surveillance promotes early diagnosis and improved survival of de novo malignancies in liver transplant recipients. Am J Transplant. 2009;9:2355-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 60. | Herrero JI, Alegre F, Quiroga J, Pardo F, Iñarrairaegui M, Sangro B, Rotellar F, Montiel C, Prieto J. Usefulness of a program of neoplasia surveillance in liver transplantation. A preliminary report. Clin Transplant. 2009;23:532-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, Teperman LW. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19:3-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 360] [Article Influence: 30.0] [Reference Citation Analysis (0)] |