Published online Apr 27, 2024. doi: 10.4254/wjh.v16.i4.625
Peer-review started: October 17, 2023
First decision: January 2, 2024
Revised: February 23, 2024
Accepted: March 18, 2024
Article in press: March 18, 2024
Published online: April 27, 2024
Processing time: 189 Days and 16.6 Hours
Liver cirrhosis patients admitted to intensive care unit (ICU) have a high mortality rate.
To establish and validate a nomogram for predicting in-hospital mortality of ICU patients with liver cirrhosis.
We extracted demographic, etiological, vital sign, laboratory test, comorbidity, complication, treatment, and severity score data of liver cirrhosis patients from the Medical Information Mart for Intensive Care IV (MIMIC-IV) and electronic ICU (eICU) collaborative research database (eICU-CRD). Predictor selection and model building were based on the MIMIC-IV dataset. The variables selected through least absolute shrinkage and selection operator analysis were further screened through multivariate regression analysis to obtain final predictors. The final predictors were included in the multivariate logistic regression model, which was used to construct a nomogram. Finally, we conducted external validation using the eICU-CRD. The area under the receiver operating characteristic curve (AUC), decision curve, and calibration curve were used to assess the efficacy of the models.
Risk factors, including the mean respiratory rate, mean systolic blood pressure, mean heart rate, white blood cells, international normalized ratio, total bilirubin, age, invasive ventilation, vasopressor use, maximum stage of acute kidney injury, and sequential organ failure assessment score, were included in the multivariate logistic regression. The model achieved AUCs of 0.864 and 0.808 in the MIMIC-IV and eICU-CRD databases, respectively. The calibration curve also confirmed the predictive ability of the model, while the decision curve confirmed its clinical value.
The nomogram has high accuracy in predicting in-hospital mortality. Improving the included predictors may help improve the prognosis of patients.
Core Tip: Liver cirrhosis patients admitted to the intensive care unit have a high mortality rate. In this study, we collected clinical data from patients with liver cirrhosis and constructed a nomogram predictive model that gained high accuracy in predicting in-hospital mortality. The accuracy was also confirmed by external validation, which suggests that the model can help us identify high-risk patients.
- Citation: Tang XW, Ren WS, Huang S, Zou K, Xu H, Shi XM, Zhang W, Shi L, Lü MH. Development and validation of a nomogram for predicting in-hospital mortality of intensive care unit patients with liver cirrhosis. World J Hepatol 2024; 16(4): 625-639
- URL: https://www.wjgnet.com/1948-5182/full/v16/i4/625.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i4.625
Liver cirrhosis is the terminal stage of various chronic liver diseases[1]. In this stage, the liver undergoes diffuse liver fibrosis, and the normal structure is replaced by regenerated nodules[2]. As a global public health problem, the most common cause of liver cirrhosis includes alcohol-related liver disease, nonalcoholic fatty liver disease (NAFLD), and chronic viral hepatitis B and C[1]. In Africa and Asia, the leading cause of liver cirrhosis is chronic viral hepatitis B, while NAFLD has become the main cause of chronic liver disease in Western countries[3,4]. With the control of viral hepatitis and the increase in obesity and metabolic syndrome, NAFLD is likely to become the major cause of liver cirrhosis[5]. Notably, as the 11th leading cause of death and the third most common cause of death among people aged 45-64 years, liver cirrhosis leads to more than one million deaths annually, which accounts for half of all liver disease deaths[6].
Liver cirrhosis can be divided into compensated and decompensated stages depending on the course of the disease. In the compensated phase, the patient is asymptomatic. In contrast, in the decompensated phase, patients suffer from a variety of complications, such as ascites, portal hypertension-related bleeding, nonobstructive jaundice, and hepatic encephalopathy (HE)[1]. Complications are the cause of repeated hospital admissions and seriously affect the quality of life and prognosis of patients[7]. The risk of death in patients with compensated liver cirrhosis is 4.7 times greater than that in the general population, while the risk increases sharply to 9.7 times greater in the decompensated stage[7]. In the decompensated stage, patients often suffer from hepatic and extrahepatic organ failure[1]. This group of patients often requires intensive care support. A meta-analysis highlighted the importance of receiving intensive care support before patients develop excessive extrahepatic failure[8]. The Model for End-stage Liver Disease (MELD), MELD and Sodium, Chronic Liver Failure-Sequential Organ Failure Assessment, and Child-Turcotte-Pugh were used to assess liver disease and determine patient prognosis[9-11]. However, patients with cirrhosis admitted to the intensive care unit (ICU) may have a more complex situation. Therefore, in this study, we constructed a nomogram suitable for liver cirrhosis patients admitted to the ICU, which aims to identify high-risk patients early and administer intervention.
The Medical Information Mart for Intensive Care IV (MIMIC-IV) database is a publicly available and freely accessible database. It was established in 2003 with funding from the National Institutes of Health by the Massachusetts Institute of Technology (MIT) Laboratory of Computational Physiology (LCP) and the Beth Israel Deaconess Medical Center of Harvard Medical School and Philips Healthcare. Clinical data from more than 190000 patients and 450000 hospitalizations are detailed in the MIMIC-IV database. The eICU collaborative research database (eICU-CRD) is a large public database created by the Philips Group in collaboration with the MIT Laboratory of LCP. The eICU-CRD includes patient information from 335 ICU units in 208 hospitals across the United States using a stratified random sample covering more than 200000 patients admitted to ICUs in 2014 and 2015. The above two databases record detailed information on patient demographics, laboratory test results, medication administration, vital signs, surgical operations, diagnosis, etc. All the data in this study were extracted from the MIMIC-IV and eICU-CRD. We completed the Collaborative Institutional Training Initiative Program course and obtained access to the database (Record ID: 52439741).
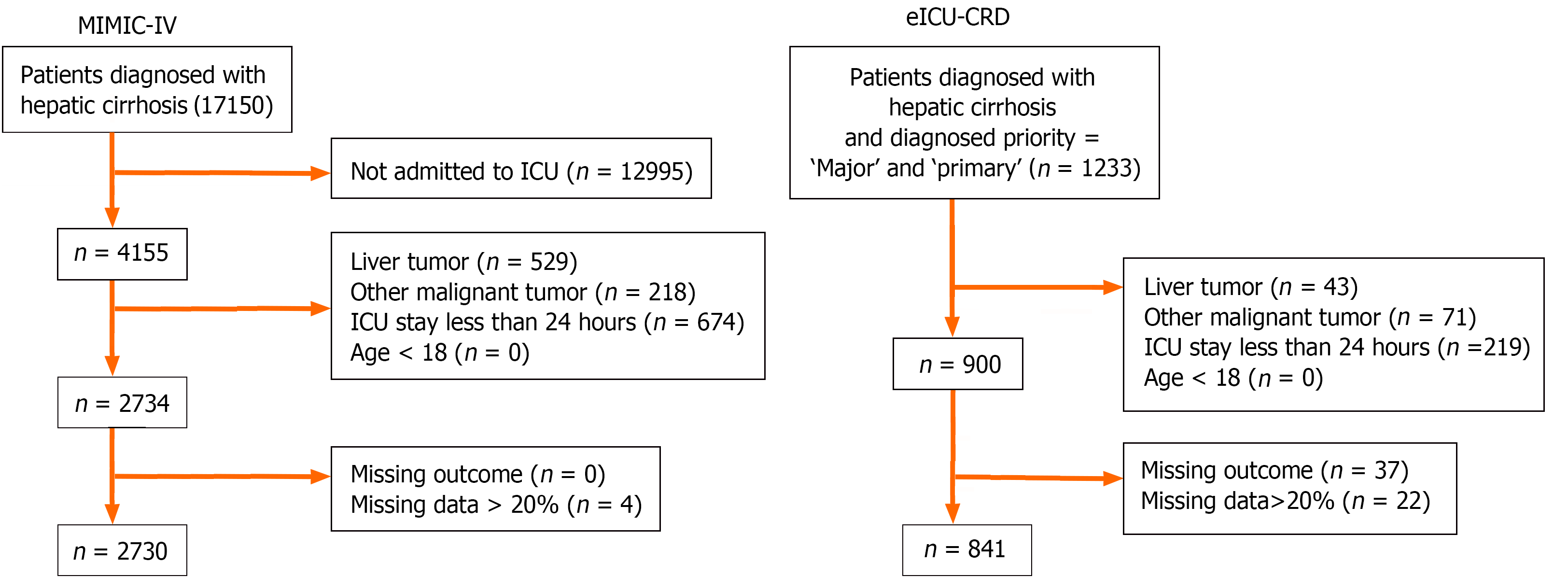
The diagnosis of disease was based on the International Classification of Diseases code. Patients diagnosed with hepatic cirrhosis and admitted to the ICU were enrolled in the study. The following conditions were excluded: (1) had liver cancer or other malignant cancers; (2) were admitted to the ICU less than 24 h; (3) were aged < 18 years; and (4) had missing outcomes or missing data for more than 20% of the patients. Overall, 2730 and 841 patients were enrolled from the MIMIC-IV and eICU-CRD, respectively (Figure 1).
We used the Structured Query Language query tool Navicat Premium to extract the data. The following information of patients were collected: Demographic data (gender, age), etiology, complications [HE, variceal hemorrhage (VH), acute kidney injury (AKI)], comorbidities [chronic obstructive pulmonary disease (COPD), heart failure (HF), myocardial infarct, Rena disease, Diabetes], the first laboratory tests after admitted to ICU [bicarbonate, calcium, chloride, sodium, potassium, blood urea nitrogen (BUN), creatinine, albumin, alanine aminotransferase, aspartate aminotransferase (AST), total bilirubin, international normalized ratio (INR), prothrombin time (PT), hemoglobin, platelets, white blood cells (WBC), red cell distribution width (RDW)], mean vital signs in first day admitted to ICU [heart rate (HR), respiratory rate (RR), systolic blood pressure (SBP), diastolic blood pressure], treatment [invasive ventilation, renal replacement therapy (RRT), vasopressor use] and prognostic scoring system [sequential organ failure assessment (SOFA) and MELD]. The MELD score was calculated as MELD = 9.6 × In (creatinin) + 3.8 × In (total bilirubin) + 11.2 × In (INR) + 6.4 × cause (cholestatic liver disease or alcoholic cirrhosis score is 0; other causes are 1)[12]. To avoid negative numbers in the calculation, if the value of creatinine, total bilirubin or the INR was less than 1, then the value was taken as 1 in the calculation. The diagnosis of AKI met the KDIGO criteria[13]. The official code for the corresponding view is provided (https://github.com/MIT-LCP/mimic-code/). Table 1 shows the baseline data of the patients in the two databases. Table 2 compares the baseline data between the MIMIC-IV and eICU-CRD.
| Variables | MIMIC-IV cohort | P value | eICU cohort | P value | ||||||
| All | Survivors | Non-survivors | All | Survivors | Non-survivors | |||||
| (n = 2730) | 0 (n = 2161) | 1 (n = 569) | (n = 841) | 0 (n = 666) | 1 (n = 175) | |||||
| Demographics | ||||||||||
| Age, median [IQR], year | 59.043 [51.654, 67.468] | 58.865 [51.466, 67.150] | 60.412 [52.537, 69.440] | 0.012 | 56.000 [50.000, 64.000] | 56.000 [50.000, 64.000] | 56.000 [50.000, 64.000] | 0.820 | ||
| Gender, n (%) | Female (0) | 1027 (37.619) | 813 (37.621) | 214 (37.610) | 0.996 | Female (0) | 324 (38.526) | 265 (39.790) | 59 (33.714) | 0.142 |
| Male (1) | 1703 (62.381) | 1348 (62.379) | 355 (62.390) | Male (1) | 517 (61.474) | 401 (60.210) | 116 (66.286) | |||
| Etiology and complications | ||||||||||
| Etiology, n (%) | Alcoholic (0) | 1448 (53.040) | 1129 (52.244) | 319 (56.063) | 0.104 | Alcoholic (0) | 290 (34.483) | 231 (34.685) | 59 (33.714) | 0.81 |
| Others (1) | 1282 (46.960) | 1032 (47.756) | 250 (43.937) | Others (1) | 551 (65.517) | 435 (65.315) | 116 (66.286) | |||
| HE, n (%) | No (0) | 2171 (79.524) | 1752 (81.074) | 419 (73.638) | < 0.001 | No (0) | 605 (71.938) | 503 (75.526) | 102 (58.286) | < 0.001 |
| Yes (1) | 559 (20.476) | 409 (18.926) | 150 (26.362) | Yes (1) | 236 (28.062) | 163 (24.474) | 73 (41.714) | |||
| VH, n (%) | No (0) | 2407 (88.168) | 1896 (87.737) | 511 (89.807) | 0.174 | No (0) | 740 (87.990) | 585 (87.838) | 155 (88.571) | 0.79 |
| Yes (1) | 323 (11.832) | 265 (12.263) | 58 (10.193) | Yes (1) | 101 (12.010) | 81 (12.162) | 20 (11.429) | |||
| AKI_stage_max, n (%) | Without (0) | 646 (23.663) | 625 (28.922) | 21 (3.691) | < 0.001 | Without (0) | 431 (51.249) | 391 (58.709) | 40 (22.857) | < 0.001 |
| Stage Ⅰ (1) | 333 (12.198) | 296 (13.697) | 37 (6.503) | Stage Ⅰ (1) | 164 (19.501) | 114 (17.117) | 50 (28.571) | |||
| Stage Ⅱ (2) | 779 (28.535) | 683 (31.606) | 96 (16.872) | Stage Ⅱ (2) | 29 (3.448) | 23 (3.453) | 6 (3.429) | |||
| Stage Ⅲ (3) | 972 (35.604) | 557 (25.775) | 415 (72.935) | Stage Ⅲ (3) | 217 (25.803) | 138 (20.721) | 79 (45.143) | |||
| Comorbidities | ||||||||||
| Renal_disease, n (%) | No (0) | 2096 (76.777) | 1688 (78.112) | 408 (71.705) | 0.001 | No (0) | 688 (81.807) | 552 (82.883) | 136 (77.714) | 0.115 |
| Yes (1) | 634 (23.223) | 473 (21.888) | 161 (28.295) | Yes (1) | 153 (18.193) | 114 (17.117) | 39 (22.286) | |||
| Diabetes, n (%) | No (0) | 1872 (68.571) | 1479 (68.441) | 393 (69.069) | 0.774 | No (0) | 642 (76.338) | 507 (76.126) | 135 (77.143) | 0.778 |
| Yes (1) | 858 (31.429) | 682 (31.559) | 176 (30.931) | Yes (1) | 199 (23.662) | 159 (23.874) | 40 (22.857) | |||
| COPD, n (%) | No (0) | 2563 (93.883) | 2027 (93.799) | 536 (94.200) | 0.722 | No (0) | 752 (89.417) | 598 (89.790) | 154 (88.000) | 0.493 |
| Yes (1) | 167 (6.117) | 134 (6.201) | 33 (5.800) | Yes (1) | 89 (10.583) | 68 (10.210) | 21 (12.000) | |||
| HF, n (%) | No (0) | 2148 (78.681) | 1724 (79.778) | 424 (74.517) | 0.006 | No (0) | 751 (89.298) | 595 (89.339) | 156 (89.143) | 0.94 |
| Yes (1) | 582 (21.319) | 437 (20.222) | 145 (25.483) | Yes (1) | 90 (10.702) | 71(10.661) | 19(10.857) | |||
| MI, n (%) | No (0) | 2462 (90.183) | 1968 (91.069) | 494 (86.819) | 0.002 | No (0) | 806 (95.838) | 639(95.946) | 167(95.429) | 0.76 |
| Yes (1) | 268 (9.817) | 193 (8.931) | 75 (13.181) | Yes (1) | 35 (4.162) | 27(4.054) | 8(4.571) | |||
| Treatment | ||||||||||
| Vasopressor, n (%) | No (0) | 1569 (57.473) | 1432 (66.266) | 137 (24.077) | < 0.001 | No (0) | 630 (74.911) | 535 (80.330) | 95 (54.286) | < 0.001 |
| Yes (1) | 1161 (42.527) | 729 (33.734) | 432 (75.923) | Yes (1) | 211 (25.089) | 131 (19.670) | 80 (45.714) | |||
| Invasive_ventilation, n (%) | No (0) | 1499 (54.908) | 1333 (61.684) | 166 (29.174) | < 0.001 | No (0) | 651 (77.408) | 538 (80.781) | 113 (64.571) | < 0.001 |
| Yes (1) | 1231 (45.092) | 828 (38.316) | 403 (70.826) | Yes (1) | 190 (22.592) | 128 (19.219) | 62 (35.429) | |||
| RRT, n (%) | No (0) | 2314 (84.762) | 1940 (89.773) | 374 (65.729) | < 0.001 | No (0) | 730 (86.801) | 578 (86.787) | 152 (86.857) | 0.98 |
| Yes (1) | 416 (15.238) | 221 (10.227) | 195 (34.271) | Yes (1) | 111 (13.199) | 88 (13.213) | 23 (13.143) | |||
| Laboratory tests | ||||||||||
| Bicarbonate, median [IQR], mmol/L | 22.000 [19.000, 25.000] | 22.000 [19.000, 25.000] | 20.000 [17.000, 24.000] | < 0.001 | 22.000 [18.000, 25.000] | 22.700 [19.000,26.000] | 21.000 [17.000, 24.000] | < 0.001 | ||
| Calcium, median [IQR], mg/dL | 8.300 [7.700, 8.900] | 8.300 [7.700, 8.800] | 8.300 [7.700, 9.000] | 0.328 | 8.200 [7.700, 8.700] | 8.200 [7.700, 8.700] | 8.200 [7.700, 8.700] | 0.953 | ||
| Chloride, median [IQR], mmol/L | 102.000 [97.000, 107.000] | 103.000 [97.000, 107.000] | 101.000 [95.000, 106.000] | < 0.001 | 102.000 [98.000, 107.000] | 102.000 [98.000, 107.000] | 102.000 [97.000, 108.000] | 0.938 | ||
| Sodium, median [IQR], mmol/L | 137.000 [133.000, 140.000] | 137.000 [133.000, 140.000] | 136.000 [132.000, 140.000] | 0.015 | 136.000 [131.000, 140.000] | 136.000 [131.000, 139.700] | 135.000 [130.000, 140.000] | 0.768 | ||
| Potassium, median [IQR], mmol/L | 4.200 [3.700, 4.800] | 4.200 [3.700, 4.700] | 4.200 [3.700, 4.900] | 0.149 | 4.100 [3.600, 4.600] | 4.020 [3.500, 4.600] | 4.300 [3.800, 4.900] | 0.003 | ||
| BUN, median [IQR], mg/dL | 26.000 [15.000, 45.000] | 24.000 [14.000, 40.000] | 36.000 [20.000, 60.000] | < 0.001 | 25.000 [14.000, 45.000] | 24.000 [13.000, 43.000] | 32.000 [19.000, 54.000] | < 0.001 | ||
| Creatinine, median [IQR], mg/dL | 1.200 [0.800, 2.100] | 1.100 [0.800, 1.800] | 1.800 [1.000, 3.100] | < 0.001 | 1.250 [0.800, 2.200] | 1.100 [0.760, 2.040] | 1.600 [1.100, 2.800] | < 0.001 | ||
| Albumin, median [IQR], g/dL | 3.000 [2.600, 3.400] | 3.000 [2.600, 3.400] | 2.900 [2.400, 3.400] | < 0.001 | 2.500 [2.100, 3.067] | 2.500 [2.100, 3.100] | 2.300 [1.900, 2.800] | < 0.001 | ||
| ALT, median [IQR], IU/L | 31.000 [20.000, 59.500] | 31.000 [20.000, 58.000] | 34.000 [20.000, 65.000] | 0.115 | 36.000 [23.000, 60.000] | 34.000 [23.000, 57.000] | 38.000 [24.000, 70.000] | 0.045 | ||
| AST, median [IQR], IU/L | 63.000 [38.000, 125.000] | 60.000 [37.000, 117.000] | 79.000 [42.000, 149.000] | < 0.001 | 70.000 [43.000, 130.000] | 67.000 [42.000, 118.000] | 86.000 [48.000, 150.000] | 0.004 | ||
| Bilirubin_total, median [IQR], mg/dL | 2.500 [1.100, 6.200] | 2.100 [1.000, 5.000] | 4.800 [1.900, 15.100] | < 0.001 | 3.100 [1.400, 7.000] | 2.800 [1.300, 5.700] | 5.700 [2.400, 14.000] | < 0.001 | ||
| Inr, median [IQR] | 1.600 [1.300, 2.100] | 1.600 [1.300, 2.000] | 2.000 [1.600, 2.700] | < 0.001 | 1.600 [1.300, 2.100] | 1.500 [1.300, 2.000] | 1.900 [1.500, 2.500] | < 0.001 | ||
| Pt, median [IQR], sec | 17.800 [14.600, 22.700] | 17.000 [14.200, 21.100] | 21.850 [17.800, 28.400] | < 0.001 | 18.300 [15.500, 23.400] | 17.800 [15.200, 22.000] | 21.700 [17.400, 27.633] | < 0.001 | ||
| Hemoglobin, median [IQR], g/dL | 9.500 [8.100,11.100] | 9.600 [8.200,11.200] | 9.100 [7.800, 10.600] | < 0.001 | 9.500 [8.000, 11.300] | 9.400 [7.800, 11.300] | 9.600 [8.200, 11.200] | 0.434 | ||
| Platelets, median [IQR], 109/L | 108.000 [68.000, 170.000] | 109.000 [70.000, 171.000] | 100.000 [62.000, 161.000] | 0.012 | 97.000 [63.000, 154.000] | 99.000 [66.000, 155.000] | 89.000 [58.000, 145.000] | 0.094 | ||
| WBC, median [IQR], 109/L | 9.000 [5.800, 13.600] | 8.400 [5.600, 12.700] | 11.500 [7.600, 16.900] | < 0.001 | 9.400 [5.900, 14.300] | 8.700 [5.600, 13.100] | 12.100 [8.400, 17.800] | < 0.001 | ||
| RDW, median [IQR], % | 16.800 [15.100, 18.900] | 16.600 [15.000, 18.700] | 17.800 [15.800, 20.000] | < 0.001 | 17.300 [15.500, 19.600] | 17.100 [15.280, 19.300] | 18.000 [16.400, 20.100] | < 0.001 | ||
| Vital signs | ||||||||||
| HR_mean, median [IQR] | 86.800 [76.237, 98.769] | 85.360 [75.040, 96.875] | 93.304 [80.826, 103.724] | < 0.001 | 89.029 [78.045, 100.000] | 87.105 [76.676, 99.333] | 94.796 [84.556, 102.423] | < 0.001 | ||
| SBP_mean, median [IQR], mmHg | 110.120 [101.694, 122.500] | 112.292 [103.125, 124.917] | 104.828 [97.667, 113.741] | < 0.001 | 108.920 [99.750, 121.000] | 109.963 [100.654, 122.314] | 103.855 [97.103, 115.954] | < 0.001 | ||
| DBP_mean, median [IQR], mmHg | 60.320 [53.963, 68.038] | 61.520 [55.000, 69.080] | 57.095 [50.625, 63.045] | < 0.001 | 59.310 [53.225, 67.000] | 60.231 [53.638, 67.970] | 56.455 [51.579, 63.857] | < 0.001 | ||
| RR_mean, median [IQR] | 18.243 [15.958, 21.200] | 17.872 [15.774, 20.577] | 19.900 [16.846, 23.318] | < 0.001 | 18.640 [16.533, 21.896] | 18.321 [16.277, 20.964] | 20.649 [17.852, 23.911] | < 0.001 | ||
| Prognostic scoring system | ||||||||||
| SOFA, median [IQR] | 8.000 [5.000, 10.000] | 7.000 [5.000, 9.000] | 11.000 [8.000, 14.000] | < 0.001 | 7.000 [5.000, 10.000] | 7.000 [4.000, 9.000] | 9.000 [7.000, 12.000] | < 0.001 | ||
| MELD, median [IQR] | 16.060 [10.225, 23.595] | 14.287 [9.338, 21.346] | 23.674 [16.662, 30.045] | < 0.001 | 17.887 [12.060, 26.087] | 16.699 [10.941, 24.147] | 24.499 [16.194, 32.895] | < 0.001 | ||
| Variables | All | MIMIC | eICU | P value | |
| (n = 3571) | 0 (n = 2730) | 1 (n = 841) | |||
| Hospital_expire_flag, n (%) | 0 | 2827 (79.165) | 2161 (79.158) | 666 (79.191) | 0.983 |
| 1 | 744 (20.835) | 569 (20.842) | 175 (20.809) | ||
| Demographics | |||||
| Age, median [IQR], yr | 58.641 [51.114, 66.693] | 59.043 [51.654, 67.468] | 56.000 [50.000, 64.000] | < 0.001 | |
| Gender, n (%) | Female (0) | 1351 (37.833) | 1027 (37.619) | 324 (38.526) | 0.636 |
| Male (1) | 2220 (62.167) | 1703 (62.381) | 517 (61.474) | ||
| Etiology and complications | |||||
| Etiology, n (%) | Alcoholic (0) | 1738 (48.670) | 1448 (53.040) | 290 (34.483) | < 0.001 |
| Others (1) | 1833 (51.330) | 1282 (46.960) | 551 (65.517) | ||
| HE, n (%) | No (0) | 2776(77.737) | 2171 (79.524) | 605 (71.938) | < 0.001 |
| Yes (1) | 795(22.263) | 559 (20.476) | 236 (28.062) | ||
| VH, n (%) | No (0) | 3147(88.127) | 2407 (88.168) | 740 (87.990) | 0.889 |
| Yes (1) | 424(11.873) | 323 (11.832) | 101 (12.010) | ||
| AKI_stage_max, n (%) | Without (0) | 1077 (30.160) | 646 (23.663) | 431 (51.249) | < 0.001 |
| Stage Ⅰ (1) | 497 (13.918) | 333 (12.198) | 164 (19.501) | ||
| Stage Ⅱ (2) | 808 (22.627) | 779 (28.535) | 29 (3.448) | ||
| Stage Ⅲ (3) | 1189 (33.296) | 972 (35.604) | 217 (25.803) | ||
| Comorbidities | |||||
| Renal_disease, n (%) | No (0) | 2784 (77.961) | 2096 (76.777) | 688 (81.807) | 0.002 |
| Yes (1) | 787 (22.039) | 634 (23.223) | 153 (18.193) | ||
| Diabetes, n (%) | No (0) | 2514 (70.400) | 1872 (68.571) | 642 (76.338) | < 0.001 |
| Yes (1) | 1057 (29.600) | 858 (31.429) | 199 (23.662) | ||
| COPD, n (%) | No (0) | 3315 (92.831) | 2563 (93.883) | 752 (89.417) | < 0.001 |
| Yes (1) | 256 (7.169) | 167 (6.117) | 89 (10.583) | ||
| HF, n (%) | No (0) | 2899 (81.182) | 2148 (78.681) | 751 (89.298) | < 0.001 |
| Yes (1) | 672 (18.818) | 582 (21.319) | 90 (10.702) | ||
| MI, n (%) | No (0) | 3268 (91.515) | 2462 (90.183) | 806 (95.838) | < 0.001 |
| Yes (1) | 303 (8.485) | 268 (9.817) | 35 (4.162) | ||
| Treatment | |||||
| vasopressor, n (%) | No (0) | 2199 (61.579) | 1569 (57.473) | 630 (74.911) | < 0.001 |
| Yes (1) | 1372 (38.421) | 1161 (42.527) | 211 (25.089) | ||
| invasive_ventilation, n (%) | No (0) | 2150 (60.207) | 1499 (54.908) | 651 (77.408) | < 0.001 |
| Yes (1) | 1421 (39.793) | 1231 (45.092) | 190 (22.592) | ||
| RRT, n (%) | No (0) | 3044 (85.242) | 2314 (84.762) | 730 (86.801) | 0.145 |
| Yes (1) | 527 (14.758) | 416 (15.238) | 111 (13.199) | ||
| Laboratory tests | |||||
| Bicarbonate, median [IQR], mmol/L | 22.000 [19.000, 25.000] | 22.000 [19.000, 25.000] | 22.000 [18.000, 25.000] | 0.291 | |
| Calcium, median [IQR], mg/dL | 8.300 [7.700, 8.800] | 8.300 [7.700, 8.900] | 8.200 [7.700, 8.700] | 0.005 | |
| Chloride, median [IQR], mmol/L | 102.000 [97.000, 107.000] | 102.000 [97.000, 107.000] | 102.000 [98.000, 107.000] | 0.108 | |
| Sodium, median [IQR], mmol/L | 137.000 [133.000, 140.000] | 137.000 [133.000, 140.000] | 136.000 [131.000, 140.000] | < 0.001 | |
| Potassium, median [IQR], mmol/L | 4.100 [3.700, 4.700] | 4.200 [3.700, 4.800] | 4.100 [3.600, 4.600] | < 0.001 | |
| BUN, median [IQR], mg/dL | 26.000 [15.000, 45.000] | 26.000 [15.000, 45.000] | 25.000 [14.000, 45.000] | 0.49 | |
| Creatinine, median [IQR], mg/dL | 1.200 [0.800, 2.100] | 1.200 [0.800, 2.100] | 1.250 [0.800, 2.200] | 0.193 | |
| Albumin, median [IQR], g/dL | 2.900 [2.400, 3.350] | 3.000 [2.600, 3.400] | 2.500 [2.100, 3.067] | < 0.001 | |
| ALT, median [IQR], IU/L | 32.000 [20.000, 60.000] | 31.000 [20.000, 59.500] | 36.000 [23.000, 60.000] | 0.001 | |
| AST, median [IQR], IU/L | 65.000 [39.000, 126.000] | 63.000 [38.000, 125.000] | 70.000 [43.000, 130.000] | 0.007 | |
| Bilirubin_total, median [IQR], mg/dL | 2.623 [1.200, 6.400] | 2.500 [1.100, 6.200] | 3.100 [1.400, 7.000] | < 0.001 | |
| INR, median [IQR] | 1.600 [1.300, 2.100] | 1.600 [1.300, 2.100] | 1.600 [1.300, 2.100] | 0.272 | |
| Pt, median [IQR], sec | 18.000 [14.800, 22.900] | 17.800 [14.600, 22.700] | 18.300 [15.500, 23.400] | 0.005 | |
| Hemoglobin, median [IQR], g/dL | 9.500 [8.000, 11.100] | 9.500 [8.100, 11.100] | 9.500 [8.000, 11.300] | 0.88 | |
| Platelets, median [IQR], 109/L | 105.000 [67.000, 166.000] | 108.000 [68.000, 170.000] | 97.000 [63.000, 154.000] | < 0.001 | |
| WBC, median [IQR], 109/L | 9.100 [5.800, 13.700] | 9.000 [5.800, 13.600] | 9.400 [5.900, 14.300] | 0.113 | |
| RDW, median [IQR], % | 17.000 [15.200, 19.100] | 16.800 [15.100, 18.900] | 17.300 [15.500, 19.600] | < 0.001 | |
| Bicarbonate, median [IQR], mmol/L | |||||
| Hr_mean, median [IQR] | 87.182 [76.588, 99.122] | 86.800 [76.237, 98.769] | 89.029 [78.045, 100.000] | 0.002 | |
| SBP_mean, median [IQR], mmHg | 109.842 [101.208, 122.292] | 110.120 [101.694, 122.500] | 108.920 [99.750, 121.000] | 0.002 | |
| Dbp_mean, median [IQR], mmHg | 60.143 [53.750, 67.810] | 60.320 [53.963, 68.038] | 59.310 [53.225, 67.000] | 0.031 | |
| Rr_mean, median [IQR], mmHg | 18.363 [16.047, 21.286] | 18.243 [15.958, 21.200] | 18.640 [16.533, 21.896] | < 0.001 | |
| Prognostic scoring system | |||||
| Meld, median [IQR] | 16.588 [10.602, 24.153] | 16.060 [10.225, 23.595] | 17.887 [12.060, 26.087] | < 0.001 | |
| SOFA, median [IQR] | 7.000 [5.000, 10.000] | 8.000 [5.000, 10.000] | 7.000 [5.000, 10.000] | 0.017 |
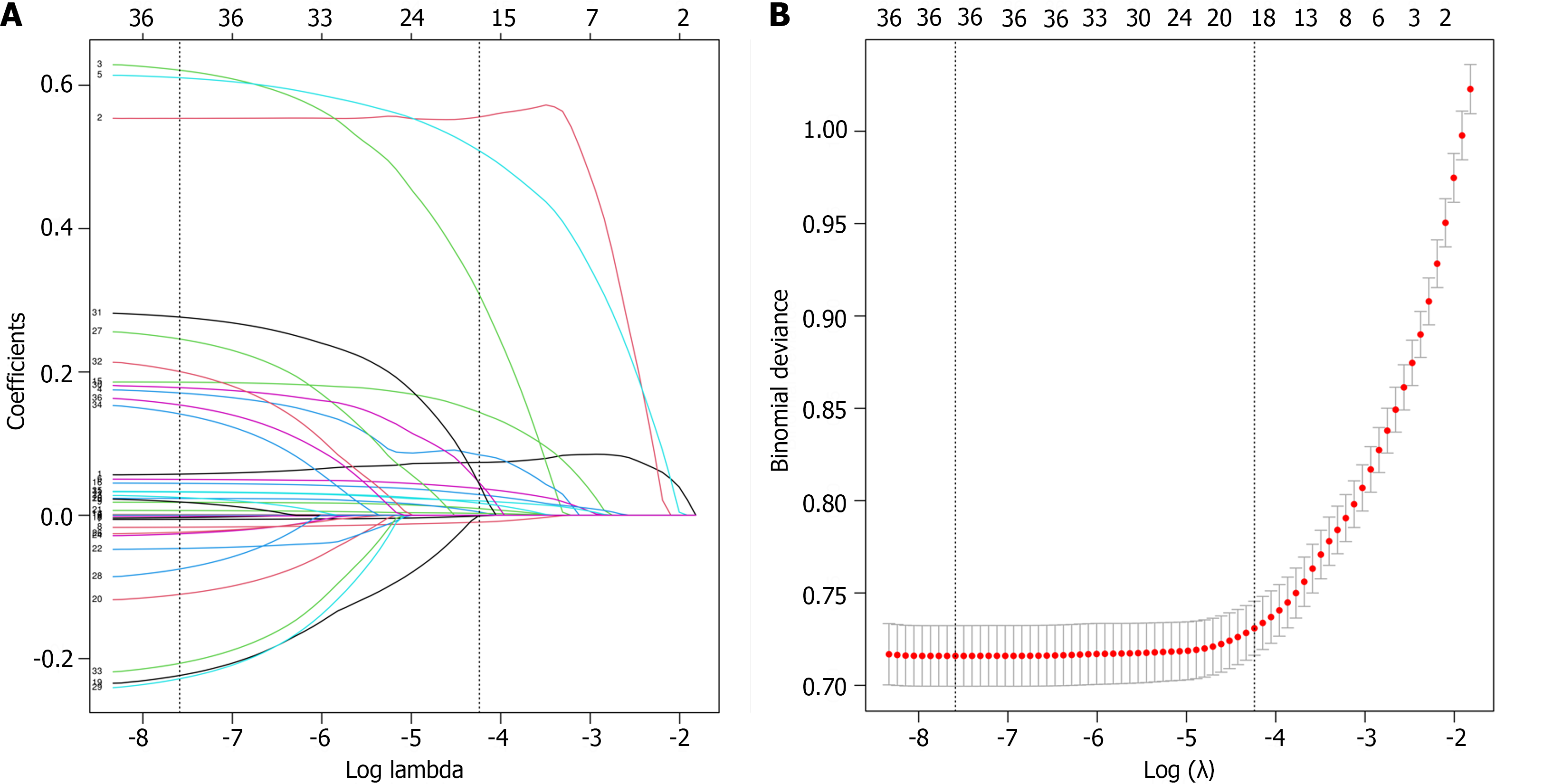
We used least absolute shrinkage and selection operator (LASSO) regression to select the candidate variables (Figure 2). The LASSO algorithm adds a penalty function, which continuously shrinks the coefficients, to achieve the goals of simplifying the model and avoiding collinearity and overfitting. The selected predictors were subjected to multivariate logistic regression. Predictors with P < 0.05 and odds ratios not containing 1 were considered final predictors (Table 3). The final predictors were included in the multivariate logistic regression model, which was used to construct a nomogram.
| Multivariable analysis based on LASSO regression result | Multivariable logistics model | |||||
| Predictor | β | P value | Odds ratio (95%CI) | β | P value | Odds Ratio |
| SOFA | 0.074 | 0 | 1.076 (1.037-1.118) | 0.078 | 0 | 1.082 (1.044-1.121) |
| RR_mean | 0.05 | 0 | 1.052 (1.022-1.082) | 0.054 | 0 | 1.055 (1.026-1.085) |
| DBP_mean | -0.009 | 0.226 | 0.991 (0.976-1.006) | |||
| SBP_mean | -0.014 | 0.006 | 0.986 (0.976-0.996) | -0.019 | 0 | 0.982 (0.973-0.99) |
| HR_mean | 0.02 | 0 | 1.021 (1.012-1.029) | 0.017 | 0 | 1.017 (1.009-1.024) |
| RDW | 0.029 | 0.161 | 1.029 (0.988-1.072) | |||
| WBC | 0.027 | 0 | 1.027 (1.012-1.042) | 0.029 | 0 | 1.029 (1.015-1.044) |
| INR | 0.203 | 0 | 1.226 (1.102-1.366) | 0.207 | 0 | 1.230 (1.106-1.371) |
| Bilirubin_total | 0.043 | 0 | 1.044 (1.029-1.059) | 0.046 | 0 | 1.047 (1.033-1.062) |
| ALT | 0 | 0.029 | 1 (0.999-1) | |||
| BUN | 0.004 | 0.049 | 1.004 (1-1.008) | |||
| Age | 0.033 | 0 | 1.034 (1.022-1.045) | 0.039 | 0 | 1.039 (1.029-1.051) |
| AKI_stage_max 1 | 0.588 | 0.052 | 1.801 (1.002-3.3) | 0.616 | 0.041 | 1.851 (1.031-3.387) |
| AKI_stage_max 2 | 0.683 | 0.01 | 1.981 (1.2-3.398) | 0.709 | 0.007 | 2.031 (1.237-3.472) |
| AKI_stage_max 3 | 1.701 | 0 | 5.48 (3.402-9.231) | 1.746 | 0 | 5.729 (3.585-9.585) |
| RRT1 | 0.002 | 0.987 | 1.002 (0.743-1.35) | |||
| Invasive_ventilation1 | 0.653 | 0 | 1.922 (1.456-2.543) | 0.599 | 0 | 1.820 (1.385-2.397) |
| Vasopressor1 | 0.536 | 0 | 1.709 (1.279-2.288) | 0.541 | 0 | 1.718 (1.291-2.290) |
| MI1 | 0.299 | 0.113 | 1.349 (0.928-1.949) | |||
| HF1 | 0.206 | 0.169 | 1.229 (0.915-1.646) | |||
Continuous variables are expressed as medians with interquartile ranges and were tested using the Mann-Whitney U test. Categorical variables are expressed as counts and percentages and were tested using the chi-square test. For variables missing less than 20% of the data, we used the method of imputation to fill in the missing values.
A total of 2730 and 814 patients were included in this study from the MIMIC-IV and eICU-CRD, respectively. The mortality rates in the MIMIC-IV and eICU-CRD cohorts were 20.842% and 20.809%, respectively. Although the data comes from different database, compared with survival group, the none-survival group have higher incidence of HE, higher stage of AKI, lower level of bicarbonate and albumin, higher level of BUN, creatinine, total bilirubin, AST, INR, PT, WBC and RDW, higher usage of invasive ventilation and vasopressor, higher HR, RR, lower level of blood pressure, and higher score of SOFA, and MELD.
Thirty-six variables were included in the variable screening process. We used LAASO regression to screen variables with the aim of minimizing the occurrence of covariance and overfitting. To simplify the model as much as possible while ensuring model fitting, we identified the variables at one standard deviation from the minimum penalty coefficient (lambda.min). Variables selected by LASSO regression were included in multivariate regression for secondary screening.
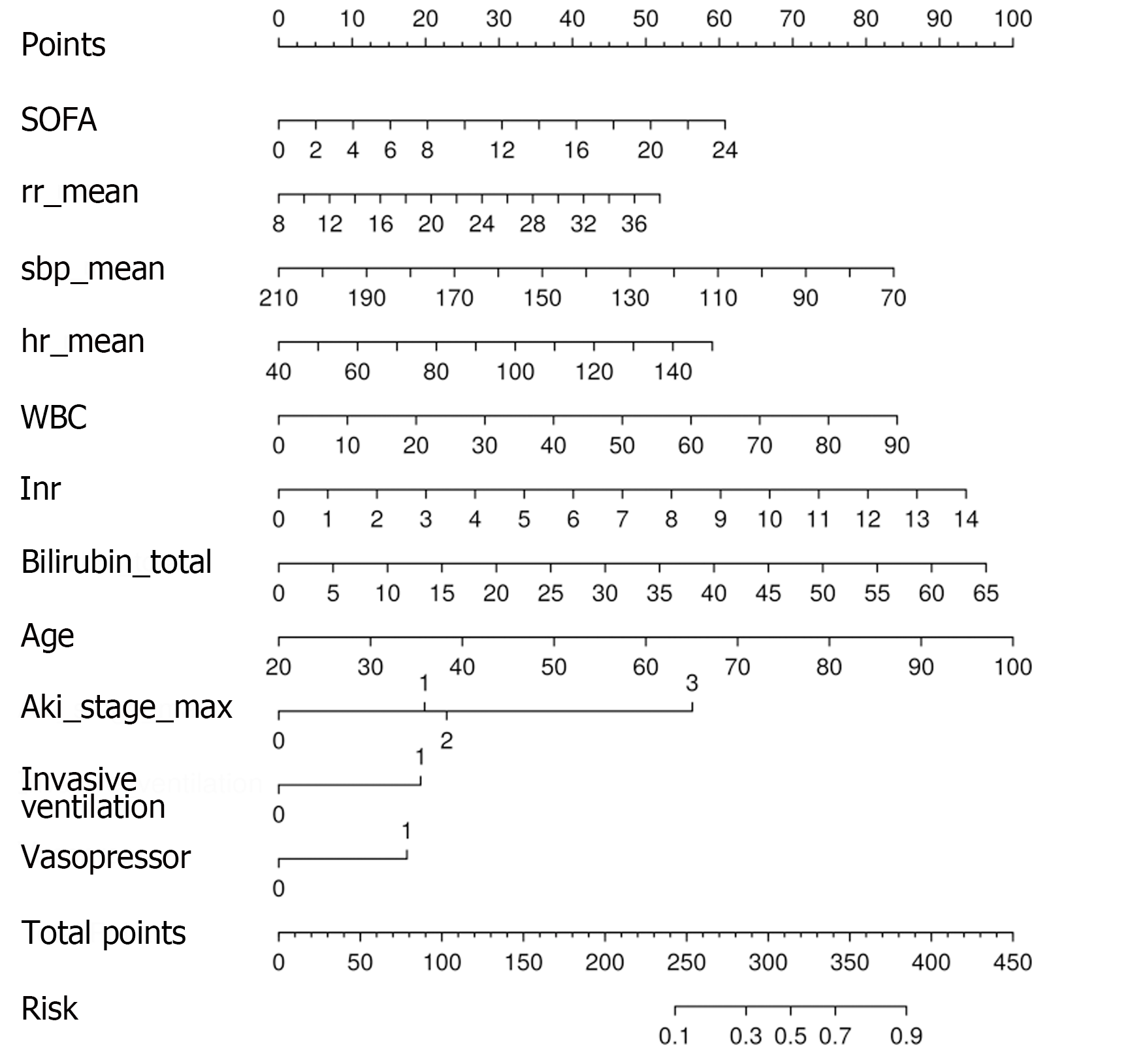
Variables screened by LASSO regression and multivariate regression were used to construct a predictive model. The final model included 11 predictors: SOFA score (OR: 1.082, 95%CI: 1.044-1.121); RR_mean (OR: 1.055, 95%CI: 1.026-1.085); SBP_mean (OR: 0.982, 95%CI: 0.973-0.99); HR_mean (OR: 1.017, 95%CI: 1.009-1.024); WBC (OR: 1.029, 95%CI: 1.015-1.044); INR (OR: 1.230, 95%CI: 1.106-1.371); total bilirubin (OR: 1.047, 95%CI: 1.033-1.062); age (OR: 1.039, 95%CI: 1.029-1.051); invasive_ventilation (OR: 1.82, 95%CI: 1.385-2.397); vasopressor (OR: 1.718, 95%CI: 1.291-2.290); and AKI_stage_max = 1 (OR: 1.851, 95%CI: 1.031-3.387), AKI_stage_max = 2 (OR: 2.031, 95%CI: 1.237-3.472), AKI_stage_max = 3 (OR: 5.729, 95%CI: 3.585-9.585). The nomogram showed the scores of the predictors at different values and risk of death according to the total score (Figure 3).
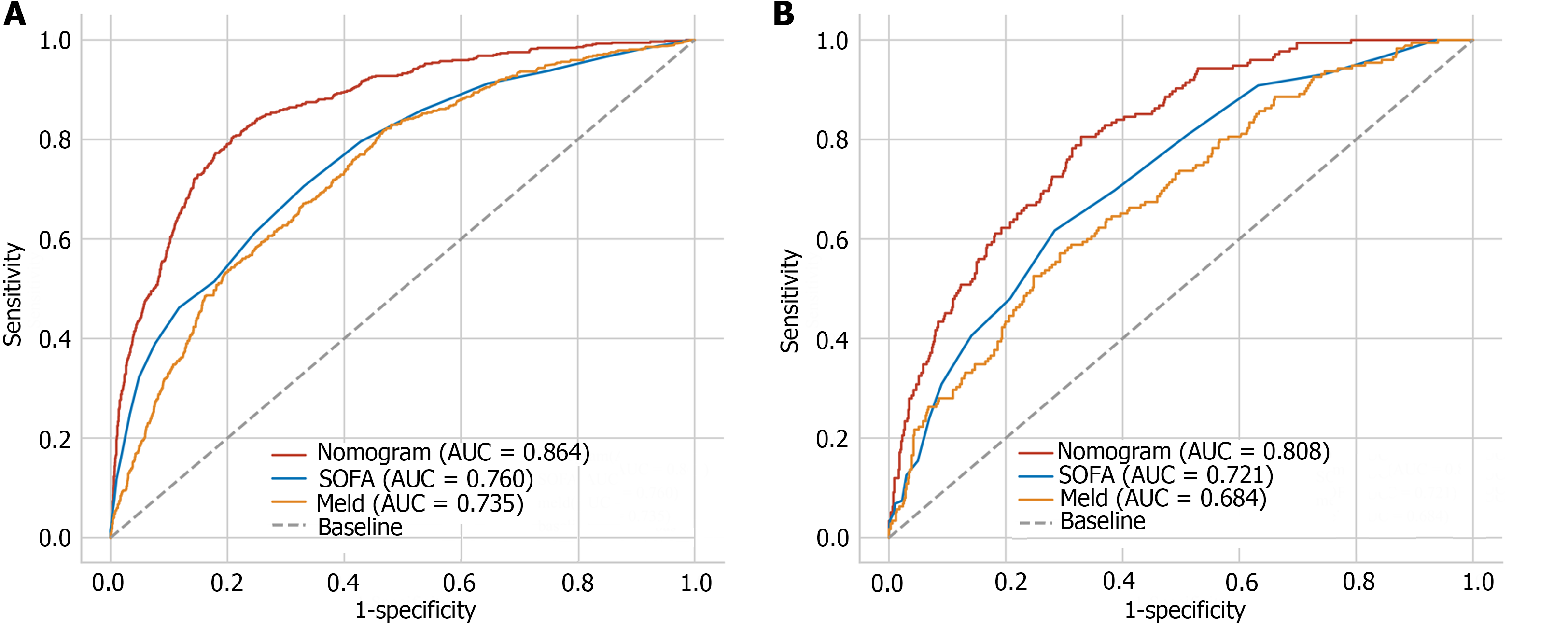
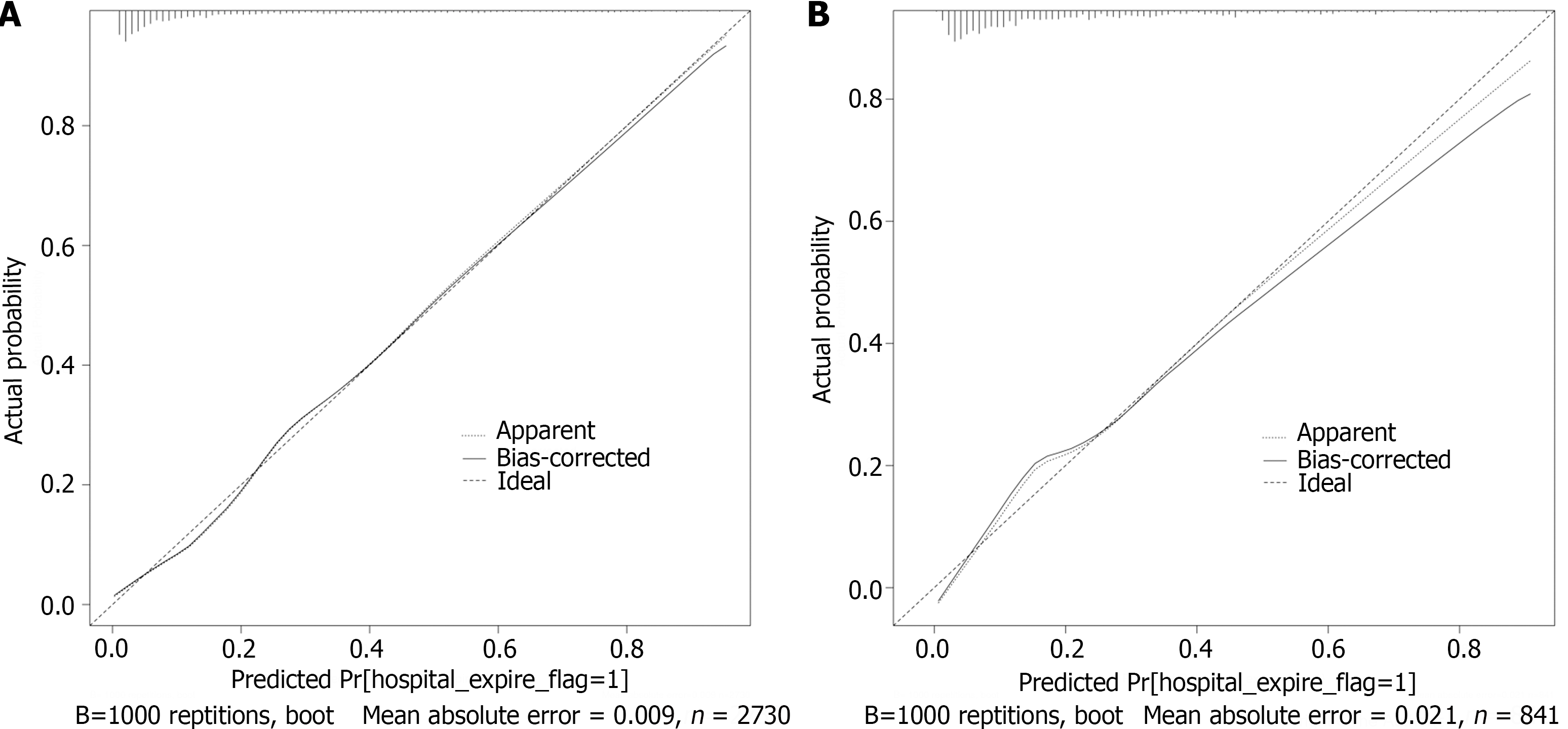
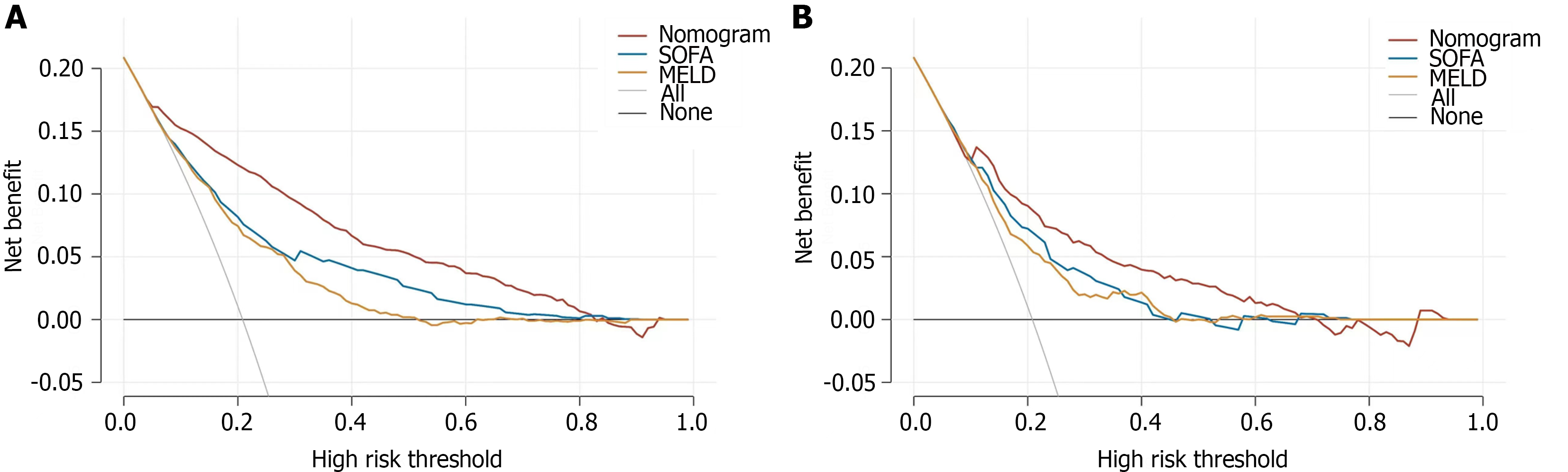
Based on the nomogram scores, we constructed ROC curves (Figure 4). The nomogram model had AUCs of 0.864 and 0.808 in the training and test datasets, respectively. These findings showed that the nomogram has good discrimination ability in the MIMIC-IV and eICU-CRD cohorts. We also compared the nomogram with the traditional prognostic scoring system. The nomogram model outperformed the MELD score and SOFA score in both the training and test sets. The calibration curve showed good agreement between the predicted probability and the actual observation, which also confirmed the predictive ability of the model (Figure 5). We plotted decision curves to demonstrate the value of the clinical application of the model (Figure 6). The model has net benefits at almost the full range of threshold probabilities. Compared to traditional prognostic scoring systems, nomogram-guided clinical interventions also have greater net benefits.
Liver cirrhosis, a global public health problem, is the 11th leading cause of death and the third most common death among people aged 45-64 years[6]. Patients in the decompensated stage of liver cirrhosis develop a variety of complications, often accompanied by hepatic and extrahepatic organ failure[1]. The ICU provides treatment, including respiratory support, circulatory support, RRT and antibiotics, needed by critically ill patients. Timely detection and early intervention for organ failure may improve patient prognosis.
In this study, we developed a nomogram model for predicting in-hospital mortality in patients with liver cirrhosis admitted to the ICU. A total of 11 variables were included in the prediction model after screening. The AUC of the model in the training set (MIMIC-IV) and test set (eICU-CRD) were 0.864 and 0.808, respectively, which indicated that the model had good predictive ability. Recently, a nomogram predictive model was established to predict in-hospital mortality in patients with alcoholic liver cirrhosis based on the MIMIC-III and eICU-CRD[14]. Compared to this study, our study was not limited to patients with alcoholic cirrhosis, and we used the updated MIMIC database MIMIC-IV, which represents a larger sample size. Consistent with their study, our study also concluded that the nomogram had better performance than did the MELD score. In previous studies, the MELD score performed well and outperformed the Child-Pugh score and the Simplified Acute Physiology Score II[15-17]. However, the MELD score did not perform well in our study. Both bilirubin and the INR, as indicators of liver function, reflect the severity of cirrhosis[18]. According to the definition of ACLF developed by the Asian Pacific Association, patients with a serum bilirubin concentration > 5 mg/dL and an INR > 1.5 should be considered for liver failure[19]. As important components of the MELD score, bilirubin concentration and the INR were also included as predictors[20]. According to the multivariate logistic regression analysis, the INR and bilirubin concentration had OR of 1.23 (95%CI: 1.106-1.371) and 1.047 (95%CI: 1.033-1.062), respectively.
The SOFA score assesses illness severity in six organ systems (nervous, respiratory, cardiovascular, renal, liver, and coagulation)[21]. The Sepsis-3 criteria also use the SOFA score to define sepsis[22]. In fact, patients with decompensated cirrhosis are at high risk of bacterial infections and developing sepsis, which greatly increases the mortality rate of liver cirrhosis patients[23,24]. The level of WBC confirmed this. According to both the MIMIC-IV and eICU-CRD, the death group had a greater WBC than the nondeath group. This means that the death group had more severe infections. According to the model, WBC is a risk factor for death, with an OR of 1.029 (95%CI: 1.015-1.044). As prognostic scoring system, both the score of MELD and SOFA in non-death group are higher. In our study, MELD and SOFA scores had close performance and are inferior to nomogram in the MIMIC-IV and eICU-CRD. This may be because the 11-variable nomogram can better reflect the complexity of liver cirrhosis patients admitted to the ICU.
In our study, age was a risk factor for patient death. This may be due to the fact that elderly patients often have a combination of chronic diseases such as hypertension, diabetes mellitus, HF, COPD, etc. For liver cirrhosis patients, older age is associated with a longer disease course and a greater likelihood of entering the decompensated phase of liver cirrhosis. Moreover, circulatory dynamics, immune function and organ function gradually begin to deteriorate as individuals age[25]. This may explain why older patients have a worse prognosis.
Unstable circulatory status is an important reason patients are admitted to the ICU. There is an interaction between heart function and liver function[26]. Hepatic cardiomyopathy has started to receive increased amounts of attention in recent years. Impaired liver function and portal hypertension lead to arterial vasodilatation in patients with cirrhosis, which causes hemodynamic disturbances, including hyperdynamic circulation; increased cardiac output and HR; and impaired myocardial structure and function[27]. Patients suffering from cirrhosis have a weakened immune system, increasing vulnerability to various infections[28]. Severe infection can cause septic shock. Patients with cirrhosis may also develop hypovolemic shock due to VH[29]. Whatever the cause of the shock, the patient is in a critical condition. Patients with shock may have a higher RR and HR and lower pressure and may require vasopressors to maintain pressure. In our study, a higher RR and HR, lower SBP and the use of vasopressors were risk factors for hospital death.
Acute renal failure is a common complication in patients with cirrhosis and is associated with a poorer prognosis and chronic kidney disease[1,30,31]. For patients with liver cirrhosis, prerenal injury, acute tubular necrosis and hepatorenal syndrome are the main causes of AKI[32]. AKI has been reported to occur in 10%-15% of hospitalized patients and more than 50% of ICU patients[33]. In this study, AKI occurred in 70% of the cohort from the MIMIC-IV database and 49% of the cohort from the eICU-CRD. AKI was a significant predictor of hospital mortality in this study. Notably, the mortality group had a greater percentage of patients with stage III AKI in both the MIMIC-IV and the eICU-CRD cohorts. The OR for stage III AKI was as high as 5.729 (95%CI: 3.585-9.585), which was much greater than that for stage I and stage II AKI. Previous studies have also confirmed that a higher AKI stage indicates a worse prognosis[34,35]. Therefore, we should pay attention not only to the occurrence of AKI but also to the stage of AKI. Prevention of AKI development and progression may improve the prognosis of patients with liver cirrhosis.
The need for airway protection due to hepatic coma and respiratory failure resulting from lung infection, pleural effusion, hepatopulmonary syndrome, etc., are the main reasons why liver cirrhosis patients are admitted to the ICU for respiratory support[36,37]. Mechanical ventilation has been demonstrated to be associated with poorer prognosis in several studies[38,39]. Mechanical ventilation (OR: 1.82, 95%CI: 1.385-2.397) was also a risk factor for in-hospital mortality in our study, which is consistent with the findings of previous studies. The length of mechanical ventilation also affects the prognosis of patients. Levesque et al[39] found that the length of ventilation was an independent risk factor for one-year survival [OR: 1.1 (95%CI: 1.0-1.2)]. For patients who are not intubated, aggressive intervention is needed to avoid tracheal intubation. For patients with mechanical ventilation, actively treat the cause of tracheal intubation is needed in order to extubate as early as possible.
Decompensated cirrhosis can affect multiple systems and lead to multiple-organ failure. The prognosis of patients with cirrhosis worsens as the number of organ failures increases[40]. Therefore, cirrhosis is not just a liver disease but also a systemic disease. The complexity of cirrhosis is particularly pronounced in patients admitted to the ICU. Therefore, integrated and comprehensive management is needed for these patients.
There are several limitations of our study. First, several important variables were not included in this study because of the large number of missing data. Second, although external validation was performed for this study, both the training and test sets were from the United States. Therefore, data from other regions are needed to validate the model.
We developed and validated a nomogram model for predicting in-hospital mortality in liver cirrhosis patients admitted to the ICU. The nomogram has high accuracy in predicting hospital mortality. This helps us to identify patients at high risk timely and give intervention actively.
Liver cirrhosis patients in decompensated stage often suffer from hepatic and extrahepatic organ failure and part of them requires intensive care support.
Liver cirrhosis patients admitted to intensive care unit have a high mortality rate.
To identify patients at high risk timely and give intervention actively.
We extracted clinical data of liver cirrhosis patients from the Medical Information Mart for Intensive Care IV and electronic intensive care unit (eICU) collaborative research database. Predictors after selection were used to construct a nomogram prediction model. The efficacy of the model was tested by external validation.
The model gained the area under the receiver operating characteristic curve of 0.864 and 0.808 in the Medical Information Mart for Intensive Care IV and eICU collaborative research respectively. The calibration curve also confirmed the predictive ability of the model, while the decision curve confirmed the clinical use value.
The nomogram model has high accuracy in predicting in-hospital mortality.
The model helps us identify patients at high risk timely and give intervention actively, which may help improve the prognosis of the patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdelmoteleb M, Egypt S-Editor: Zhang L L-Editor: A P-Editor: Cai YX
| 1. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 861] [Article Influence: 215.3] [Reference Citation Analysis (1)] |
| 2. | Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 1172] [Article Influence: 293.0] [Reference Citation Analysis (0)] |
| 3. | Alberts CJ, Clifford GM, Georges D, Negro F, Lesi OA, Hutin YJ, de Martel C. Worldwide prevalence of hepatitis B virus and hepatitis C virus among patients with cirrhosis at country, region, and global levels: a systematic review. Lancet Gastroenterol Hepatol. 2022;7:724-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 115] [Article Influence: 38.3] [Reference Citation Analysis (1)] |
| 4. | Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20:7312-7324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 335] [Cited by in RCA: 407] [Article Influence: 37.0] [Reference Citation Analysis (10)] |
| 5. | GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1158] [Cited by in RCA: 1013] [Article Influence: 202.6] [Reference Citation Analysis (4)] |
| 6. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2296] [Article Influence: 382.7] [Reference Citation Analysis (0)] |
| 7. | Ge PS, Runyon BA. Treatment of Patients with Cirrhosis. N Engl J Med. 2016;375:767-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 234] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 8. | Weil D, Levesque E, McPhail M, Cavallazzi R, Theocharidou E, Cholongitas E, Galbois A, Pan HC, Karvellas CJ, Sauneuf B, Robert R, Fichet J, Piton G, Thevenot T, Capellier G, Di Martino V; METAREACIR Group. Prognosis of cirrhotic patients admitted to intensive care unit: a meta-analysis. Ann Intensive Care. 2017;7:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Kim HY, Kim CW, Kim TY, Song DS, Sinn DH, Yoon EL, Jung YK, Suk KT, Lee SS, Lee CH, Kim TH, Kim JH, Yim HJ, Kim SE, Baik SK, Lee BS, Jang JY, Kim YS, Kim SG, Yang JM, Sohn JH, Lee HJ, Park SH, Choi EH, Kim DJ; Korean Acute-on-Chronic Liver Failure Study Group. Assessment of scoring systems for acute-on-chronic liver failure at predicting short-term mortality in patients with alcoholic hepatitis. World J Gastroenterol. 2016;22:9205-9213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Li J, Liang X, You S, Feng T, Zhou X, Zhu B, Luo J, Xin J, Jiang J, Shi D, Lu Y, Ren K, Wu T, Yang L, Li J, Li T, Cai Q, Sun S, Guo B, Chen J, He L, Li P, Yang H, Hu W, An Z, Jin X, Tian J, Wang B, Chen X, Xin S; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J Hepatol. 2021;75:1104-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 11. | Ruf A, Dirchwolf M, Freeman RB. From Child-Pugh to MELD score and beyond: Taking a walk down memory lane. Ann Hepatol. 2022;27:100535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 12. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3678] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 13. | Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-c184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 3348] [Article Influence: 257.5] [Reference Citation Analysis (0)] |
| 14. | Zheng L, Lu Y, Wu J, Zheng M. Development and validation of a prognostic nomogram model for ICU patients with alcohol-associated cirrhosis. Dig Liver Dis. 2023;55:498-504. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Cavallazzi R, Awe OO, Vasu TS, Hirani A, Vaid U, Leiby BE, Kraft WK, Kane GC. Model for End-Stage Liver Disease score for predicting outcome in critically ill medical patients with liver cirrhosis. J Crit Care. 2012;27:424.e1-424.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Cholongitas E, Senzolo M, Patch D, Kwong K, Nikolopoulou V, Leandro G, Shaw S, Burroughs AK. Risk factors, sequential organ failure assessment and model for end-stage liver disease scores for predicting short term mortality in cirrhotic patients admitted to intensive care unit. Aliment Pharmacol Ther. 2006;23:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 180] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Das V, Boelle PY, Galbois A, Guidet B, Maury E, Carbonell N, Moreau R, Offenstadt G. Cirrhotic patients in the medical intensive care unit: early prognosis and long-term survival. Crit Care Med. 2010;38:2108-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Kim HJ, Lee HW. Important predictor of mortality in patients with end-stage liver disease. Clin Mol Hepatol. 2013;19:105-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 591] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 20. | Kim MS, Kato TS, Farr M, Wu C, Givens RC, Collado E, Mancini DM, Schulze PC. Hepatic dysfunction in ambulatory patients with heart failure: application of the MELD scoring system for outcome prediction. J Am Coll Cardiol. 2013;61:2253-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 21. | Vincent JL, Moreno R. Clinical review: scoring systems in the critically ill. Crit Care. 2010;14:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 392] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 22. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17221] [Article Influence: 1913.4] [Reference Citation Analysis (2)] |
| 23. | Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246-1256, 1256.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 838] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 24. | Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, Bissoli F, Boccia S, Colloredo-Mels G, Corigliano P, Fornaciari G, Marenco G, Pistarà R, Salvagnini M, Sangiovanni A. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 299] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 25. | Kamimura K, Sakamaki A, Kamimura H, Setsu T, Yokoo T, Takamura M, Terai S. Considerations of elderly factors to manage the complication of liver cirrhosis in elderly patients. World J Gastroenterol. 2019;25:1817-1827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Møller S, Bernardi M. Interactions of the heart and the liver. Eur Heart J. 2013;34:2804-2811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 324] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 27. | Møller S, Danielsen KV, Wiese S, Hove JD, Bendtsen F. An update on cirrhotic cardiomyopathy. Expert Rev Gastroenterol Hepatol. 2019;13:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Sehgal R, Kaur N, Maiwall R, Ramakrishna G, Maras JS, Trehanpati N. Plasma Proteomic Analysis Identified Proteins Associated with Faulty Neutrophils Functionality in Decompensated Cirrhosis Patients with Sepsis. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Avadhanam M, Kulkarni AV. Intensive Care Unit Care of a Patient with Cirrhosis. Med Clin North Am. 2023;107:567-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Angeli P, Garcia-Tsao G, Nadim MK, Parikh CR. News in pathophysiology, definition and classification of hepatorenal syndrome: A step beyond the International Club of Ascites (ICA) consensus document. J Hepatol. 2019;71:811-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 31. | Patidar KR, Naved MA, Grama A, Adibuzzaman M, Aziz Ali A, Slaven JE, Desai AP, Ghabril MS, Nephew L, Chalasani N, Orman ES. Acute kidney disease is common and associated with poor outcomes in patients with cirrhosis and acute kidney injury. J Hepatol. 2022;77:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 32. | Asrani SK, Simonetto DA, Kamath PS. Acute-on-Chronic Liver Failure. Clin Gastroenterol Hepatol. 2015;13:2128-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394:1949-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 1171] [Article Influence: 195.2] [Reference Citation Analysis (0)] |
| 34. | Huelin P, Piano S, Solà E, Stanco M, Solé C, Moreira R, Pose E, Fasolato S, Fabrellas N, de Prada G, Pilutti C, Graupera I, Ariza X, Romano A, Elia C, Cárdenas A, Fernández J, Angeli P, Ginès P. Validation of a Staging System for Acute Kidney Injury in Patients With Cirrhosis and Association With Acute-on-Chronic Liver Failure. Clin Gastroenterol Hepatol. 2017;15:438-445.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 35. | Wong F, Reddy KR, Tandon P, O'Leary JG, Garcia-Tsao G, Vargas HE, Lai JC, Biggins SW, Maliakkal B, Fallon M, Subramanian R, Thuluvath P, Kamath PS, Thacker L, Bajaj JS. Progression of Stage 2 and 3 Acute Kidney Injury in Patients With Decompensated Cirrhosis and Ascites. Clin Gastroenterol Hepatol. 2021;19:1661-1669.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Karcz M, Bankey B, Schwaiberger D, Lachmann B, Papadakos PJ. Acute respiratory failure complicating advanced liver disease. Semin Respir Crit Care Med. 2012;33:96-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Saffo S, Garcia-Tsao G. Early mechanical ventilation for grade IV hepatic encephalopathy is associated with increased mortality among patients with cirrhosis: an exploratory study. Acute Crit Care. 2022;37:355-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 38. | Juneja D, Gopal PB, Kapoor D, Raya R, Sathyanarayanan M. Profile and outcome of patients with liver cirrhosis requiring mechanical ventilation. J Intensive Care Med. 2012;27:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Levesque E, Saliba F, Ichaï P, Samuel D. Outcome of patients with cirrhosis requiring mechanical ventilation in ICU. J Hepatol. 2014;60:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 40. | García-Martínez R, Simón-Talero M, Córdoba J. Prognostic assessment in patients with hepatic encephalopathy. Dis Markers. 2011;31:171-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |