Published online Mar 27, 2024. doi: 10.4254/wjh.v16.i3.418
Peer-review started: December 6, 2023
First decision: December 19, 2023
Revised: January 2, 2024
Accepted: February 8, 2024
Article in press: February 8, 2024
Published online: March 27, 2024
Processing time: 107 Days and 13.9 Hours
Bacterial infections (BI) negatively affect the natural course of cirrhosis. The most frequent BI are urinary tract infections (UTI), pneumonia, and spontaneous-bacterial peritonitis (SBP).
To assess the relevance of bacterial infections beyond the commonly recognized types in patients with cirrhosis and to investigate their relationship with other clinical variables.
We retrospectively analyzed patients with cirrhosis and BI treated between 2015 and 2018 at our tertiary care center. BIs were classified as typical and atypical, and clinical as well as laboratory parameters were compared between the two groups.
In a cohort of 488 patients with cirrhosis, we identified 225 typical BI (95 UTI, 73 SBP, 72 pulmonary infections) and 74 atypical BIs, predominantly cholangitis and soft tissue infections (21 each), followed by intra-abdominal BIs (n = 9), cholecystitis (n = 6), head/throat BIs (n = 6), osteoarticular BIs (n = 5), and endocarditis (n = 3). We did not observe differences concerning age, sex, or etiology of cirrhosis in patients with typical vs atypical BI. Atypical BIs were more common in patients with more advanced cirrhosis, as evidenced by Model of End Stage Liver Disease (15.1 ± 7.4 vs 12.9 ± 5.1; P = 0.005) and Child-Pugh scores (8.6 ± 2.5 vs 8.0 ± 2; P = 0.05).
Atypical BIs in cirrhosis patients exhibit a distinct spectrum and are associated with more advanced stages of the disease. Hence, the work-up of cirrhosis patients with suspected BI requires detailed work-up to elucidate whether typical BI can be identified.
Core Tip: Bacterial infections (BI) affect the natural course of liver cirrhosis and can trigger decompensation or death. The most frequent BI in cirrhosis (urinary tract infections, pneumonia or spontaneous-bacterial peritonitis) were retrospectively compared to infections at other body sites, which are thought to be less frequently affected (so-called “atypical BI”). When comparing typical/atypical BI, no differences in age, sex, or etiology of cirrhosis were found. Notably, for atypical BI, the stage of cirrhosis was less advanced, as expressed by laboratory parameters and clinical scores (e.g. Model of End Stage Liver Disease - and Child-Pugh-Score).
- Citation: Schneitler S, Schneider C, Casper M, Lammert F, Krawczyk M, Becker SL, Reichert MC. Retrospective study of the incidence, risk factors, treatment outcomes of bacterial infections at uncommon sites in cirrhotic patients. World J Hepatol 2024; 16(3): 418-427
- URL: https://www.wjgnet.com/1948-5182/full/v16/i3/418.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i3.418
Bacterial infections (BI) significantly affect the natural history of cirrhosis and may lead to a dramatic increase in mortality of infected patients[1-3]. Furthermore, BI are the most common event causing hepatic decompensation[4]. The more severe course of BI is attributed to the acquired immunodeficiency of patients with cirrhosis, the increased bacterial translocation from the intestinal tract, and the consequences of portal hypertension. The most common BI in cirrhosis include urinary tract infection (UTI), pneumonia, and spontaneous-bacterial peritonitis (SBP)[3]. Whereas infections at other body sites also occur relatively frequently in patients with cirrhosis (herein further called “atypical BI”), these have been investigated far less in-depth, in particular due to the lack of sufficiently large cohorts of patients with these specific BI in the setting of cirrhosis.
Accurate microbiological diagnostics are essential for targeted antibiotic therapy. This is often challenging in patients with cirrhosis, as invasive collecting of samples (e.g. ascites, or sputum) is not always feasible. Commonly, empirical antibiotic therapy is insufficient. Indeed, Lameirão Gomes et al[5] showed in a retrospective analysis that in only 60% of cases, empirical therapy was adequate against the infection-causing pathogens.
Here, we aimed to specifically compare the clinical and microbiological characteristics of patients with cirrhosis and typical BI (pneumonia, UTI and SBP) as compared to atypical BI, by exploiting a large database[6] (INCA database) of patients with BI and cirrhosis.
This analysis was carried out as sub-study of the INCA trial, the study protocol of which has been published[6]. The study analyzed data from inpatients with cirrhosis and BI who received treatment at Saarland University Medical Center in Homburg, Southwest Germany, between January 1, 2015, and December 31, 2018. All hospitalized patients with cirrhosis were considered for inclusion. Patients with severe comorbidities such as end-stage heart failure, HIV infection and non-resectable cancer (except hepatocellular carcinoma Barcelona Lever Clinic Classification stages A-C), as well as patients in whom a BI could not be confirmed were excluded. Cirrhosis was defined by (1) biopsy; (2) a combination of clinical, laboratory, ultrasound and endoscopic findings; or (3) transient elastography > 13.0 kPa[7]. In patients with transient elastography < 19.7 kPa, diagnosis of cirrhosis was additionally confirmed by (1) or (2). Results pertaining to different disease aspects of this cohort have been reported previously[7]. Overall, 488 patients with cirrhosis and BI requiring antibiotic therapy were finally included. BI were categorized applying stringent criteria (Supplementary Table 1). The electronic medical records were reviewed for clinical data, and further information regarding medication use (such as antibiotic therapy, beta-blocker, lactulose, statins) and laboratory parameters at the time of inclusion were recorded. The use of long-term antibiotics (prescribed for prophylaxis of SBP or for recurrent hepatic encephalopathy) was also documented.
All atypical BI cases were analyzed using the microbiological databases HyBASE® (epiNET AG, Germany) and M/Lab (Dorner, Germany) at Saarland University Medical Center. The diagnostics carried out during the event period, the main detected pathogens, and the related antibiotic therapy were recorded. Of note, all microbiological diagnostic procedures such as Gram staining, culture techniques and identification methods were performed using standard operating procedures. Species identification of culture-grown bacterial colonies was carried out using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS, Bruker, Germany). Subsequently, the pathogens were grouped into Gram-positive and Gram-negative pathogens. In addition, the available antibiograms were interpreted with respect to resistance behavior using the multi-drug resistance (MDR) classification by Magiorakos et al[8]. The antibiotic therapy was categorized into the following antibiotic classes: Penicillins, cephalosporins, carbapenems, quinolones, macrolides, glycopeptides, linezolid, metronidazole, and others. In addition, the assessment included the administration of monotherapy and combination therapies, the length of therapy given, and the effectiveness of empirical therapy.
All variables are described as proportions, means with standard deviations, or medians with interquartile ranges (IQR). The univariate analysis was performed with chi2-square test, t-test, or Mann-Whitney U test, according to the distribution of the test variable. The statistical analyses were performed with SPSS 22.0 (SPSS, Munich, Germany). Two-sided P values < 0.05 were regarded as significant.
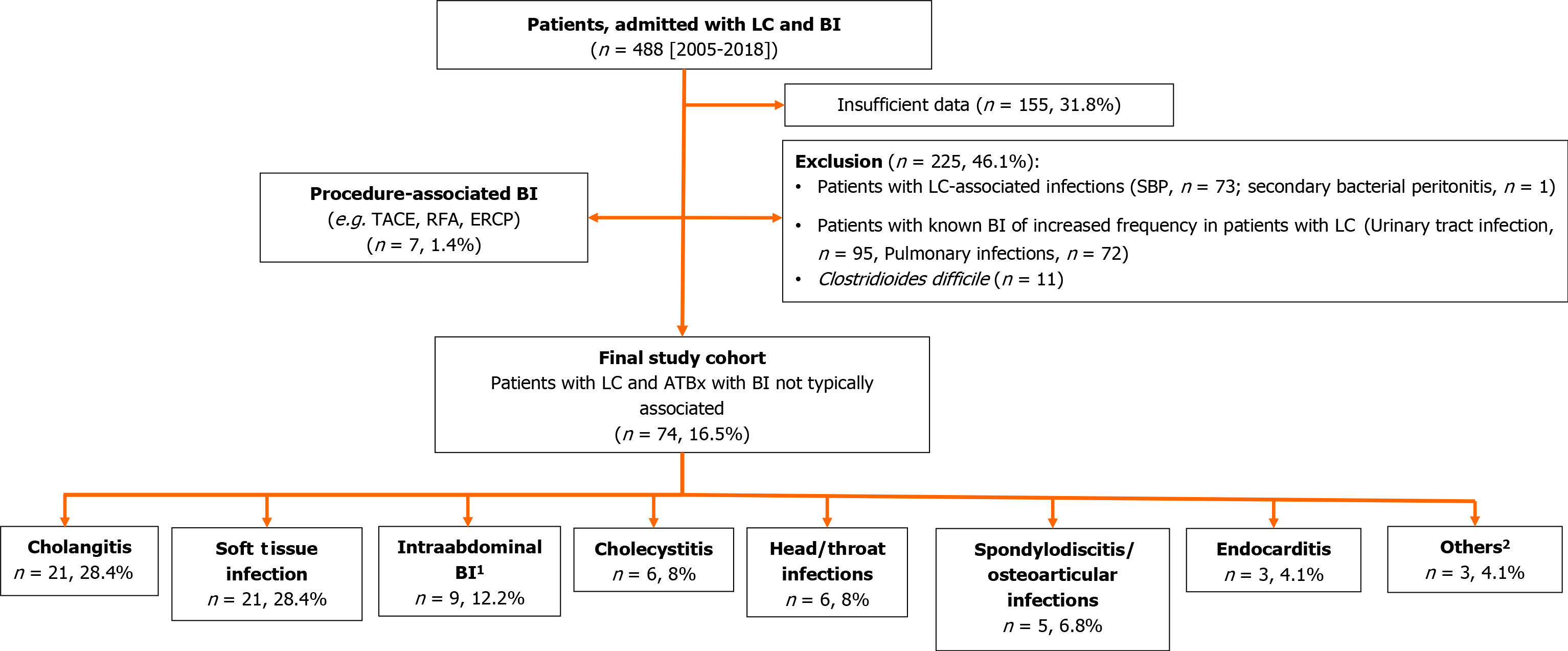
Overall, the retrospective search of the electronic data records of hospitalized patients with cirrhosis yielded 1128 patients with cirrhosis. Among them, 488 (43.3%) patients were treated with antibiotics due to BI. Figure 1 illustrates the workflow for the inclusion of patients into the study cohort. Tables 1 and 2 summarizes the detailed baseline and specific characteristics of these patients.
| Atypical BI (n = 74) | Common BI (n = 225) | No BI (n = 640) | P value1 | P value2 | P value3 | |
| Sex (female) | 20 (27.0) | 80 (35.6) | 218 (34.1) | 0.20 | 0.243 | 0.88 |
| Age (yr) | 61.14 ± 12.61 | 61.34 ± 11.95 | 60.45 ± 10.76 | 0.901 | 0.61 | |
| Diabetes (yes) | 28 (37.8) | 69 (30.7) | 199 (31.1) | 0.26 | 0.238 | 0.706 |
| Etiology of cirrhosis | ||||||
| Alcoholic | 35 (47.3) | 147 (65.3) | 317 (49.5) | |||
| Hepatitis C | 2 (2.7) | 29 (12.9) | 101 (15.8) | |||
| Hepatitis B | 1 (1.4) | 3 (1.3) | 21 (3.3) | |||
| NASH | 5 (6.8) | 7 (3.1) | 60 (9.4) | |||
| Cryptogenic | 6 (8.1) | 19 (8.4) | 72 (11.3) | |||
| PSC | 7 (9.5) | 2 (0.9) | 6 (0.9) | |||
| Others | 11 (14.9) | 10 (4.4) | 48 (7.5) | |||
| Hemochromatosis | 3 (4.1) | 1 (0.4) | 10 (1.6) | |||
| PBC | 4 (5.4) | 2 (0.9) | 5 (0.8) | |||
| Medication | ||||||
| Beta blocker | 33 (45.8) | 115 (51.8) | 297 (47.1) | 0.42 | 0.901 | 0.359 |
| Long term ATBx | 13 (18.1) | 74 (33.1) | 73 (11.6) | 0.017 | 0.128 | < 0.001 |
| Lactulose | 24 (33.3) | 108 (48.6) | 196 (31.1) | 0.029 | 0.689 | < 0.001 |
| PPI | 49 (68.1) | 183 (82.4) | 419 (66.3) | 0.013 | 0.794 | < 0.001 |
| Laboratory parameters | ||||||
| Serum sodium (mmol/L) | 137.62 ± 4.04 | 136.56 ± 5.03 | 138.10 ± 4.61 | 0.10 | 0.346 | < 0.001 |
| Creatinine (mg/dL) | 1.14 ± 0.60 | 1.38 ± 1.17 | 1.029 ± 0.52 | 0.018 | 0.106 | < 0.001 |
| Total bilirubin (mg/dL) | 2.83 ± 4.00 | 3.73 ± 5.88 | 2.21 ± 3.96 | 0.14 | 0.205 | < 0.001 |
| Albumin (g/dL) | 33.24 ± 6.73 | 32.95 ± 6.91 | 36.64 ± 7.14 | 0.75 | < 0.001 | < 0.001 |
| Hemoglobin (g/dL) | 11.98 ± 2.21 | 11.19 ± 2.34 | 12.66 ± 2.48 | 0.009 | 0.025 | < 0.001 |
| INR | 1.27 ± 0.34 | 1.37 ± 58 | 1.25 ± 0.32 | 0.15 | 0.639 | 0.001 |
| ASAT | 108.69 ± 257.76 | 80.55 ± 104.89 | 84.75 ± 185.15 | 0.31 | 0.475 | 0.930 |
| ALAT | 71.46 ± 219.92 | 53.63 ± 121.43 | 69.08 ± 164.11 | 0.39 | 0.911 | 0.327 |
| Platelets | 164.93 ± 110.00 | 150.74 ± 88.46 | 150.27 ± 79.27 | 0.26 | 0.151 | 0.527 |
| MELD | 12.86 ± 5.13 | 15.10 ± 7.44 | 11.60 ± 5.13 | 0.005 | 0.049 | < 0.001 |
| CPS | 7.99 ± 2.15 | 8.61 ± 2.50 | 7.19 ± 5.44 | 0.05 | 0.003 | < 0.001 |
| Fibroscan (kPa) | 41.96 ± 21.94 | 46 ± 21.90 | 37.06 ± 21.44 | 0.22 | 0.106 | < 0.001 |
| Atypical BI (n = 74) | |
| Outcome | |
| Dead within 30 d | 7 (9.5) |
| Sepsis | 9 (12.7) |
| Laboratory parameters (at BI) | |
| Serum sodium (mmol/L) | 137 ± 5.5 |
| Creatinine (mg/dL) | 1.13 ± 13.5 |
| Total bilirubin (mg/dL) | 1.9 ± 6.67 |
| Albumin (g/dL) | 30.0 ± 6.25 |
| Hemoglobin (g/dL) | 11.8 ± 3.00 |
| INR | 1.20 ± 0.48 |
| ASAT (U/l) | 67.0 ± 42.47 |
| ALAT (U/l) | 44 ± 30.92 |
| Platelets | 154 ± 103 |
| MELD | 14.5 ± 6.23 |
| CPS | 8 ± 1.86 |
| WBC (×109) | 8.2 ± 5.05 |
| CRP (mg/dL) | 43.1 ± 61.77 |
The patients were predominantly men (n = 322, 66.1%). The median age was 61 [Range 26-92, (IQR 54-68)], and the predominant etiology of cirrhosis was alcohol-associated (n = 259, 53.1%). Most patients were in Child-Pugh stage (CPS) B. Figure 1 shows the distribution of the BI. In general, patients with BI were in an advanced stage of cirrhosis, as reflected by lower serum sodium and albumin concentrations as well as hemoglobin levels and higher creatinine, bilirubin and international normalized ratio, as compared to patients with cirrhosis and no BI. No differences were found concerning the presence of age, sex, or diabetes.
Concerning the common BI, 95 urinary tract infections, 73 SBP, 72 pulmonary infections, and 11 Clostridioides difficile infections were recorded. The most frequently atypical BI were soft-tissue infections (n = 21), bacterial cholangitis (n = 21), and intra-abdominal BI (n = 9) (Figure 1). Regardless of Gram classification, cholangitis (n = 21, 28.4% each) and soft tissue infections (n = 21, 28.4%) were the most common atypical BI presentations. These were followed by intra-abdominal infections, including cholecystitis (n = 15, 19%). Among neck and head infections, peritonsillar abscesses and parotitis were equally common (2 each).
The most frequent bacterial detections for atypical BI were detected in the Gram negative (n = 20; most frequently Escherichia coli (E. coli), Pseudomonas spp.) spectrum, e.g. being responsible for 8 out of 20 cholangitis cases and 6 out of 20 soft tissue infections. Most MDR detections were Gram-negative (8/20), and Escherichia coli (E. coli) (6/8) was the most frequently detected pathogen (Table 3).
| Pathogen | Organs frequently affected (n) | % MDR1 |
| Gram positive (n = 17) | ||
| Staphylococcus aureus (n = 10) | Soft tissue infection[3], abscess[3], discitis/osteomyelitis[2], endocarditis[2] | 1/10 |
| Streptococcus spp. (n = 4) | Cholangitis/cholecystitis[1], endocarditis[1], meningitis[1], epididymitis[1] | NU |
| Enterococcus faecium (n = 3) | Cholangitis/cholecystitis[3] | NA |
| Gram negative (n = 20) | ||
| Escherichia coli (n = 7) | Cholangitis[5], soft tissue infection[2] | 6/7 |
| Klebsiella spp. (n = 3) | Cholangitis[1], soft tissue infection[1], appendicitis[1] | 1/3 |
| Enterobacter spp. (n = 2) | Cholangitis[1], periprothetic infection of hip joint[1] | 1/2 |
| Pseudomonas spp. (n = 4) | Soft tissue infection[2], cholangitis[1], abscess[1] | 0/4 |
| Campylobacter spp. (n = 3) | Colitis[3] | 0/3 |
| Acinetobacter baumanii (n = 1) | Soft tissue infection[1] | 0/1 |
A total of 70 cases (94.6%) were treated with empirical antibiotic therapy, with penicillin predominating (Table 4), followed equally by cephalosporins and metronidazole (19.2% each). Metronidazole was always used as a combination partner, with cephalosporin being the most frequently used combination (11.0%). The administered antibiotic therapy was most common targeted against Gram-positive pathogens (35.6%) and frequently administered over a period of up to two weeks (38.4%). Looking at the efficiency of empirical antibiotic therapy in terms of microbiological detection, the most common problem was that sufficient microbiological tests were not performed, and hence no microbiological analysis was performed (32.9%) (Table 4).
| Variable | Number (n = 73)1 |
| Empirical antibiotic treatment | |
| Monotherapy | 40 (54.8) |
| Combination therapy with > 2 antibiotics (n) | 24 (32.9) |
| Combination therapy with > 3 antibiotics (n) | 6 (8.2) |
| Unspecific antibiotic information | 3 (4,1) |
| Antibiotic classes1 | |
| Penicillins | 25 (34.2) |
| Cephalosporins | 14 (19.2) |
| Metronidazole | 14 (19.2) |
| Carbapenems | 13 (17.8) |
| Other | 13 (17.8) |
| Quinolones | 11 (15.1) |
| Glycopeptides | 6 (8,2) |
| Not assessable | 4 (5.5) |
| Most frequent antibiotic combinations | |
| Cephalosporins with Metronidazole | 8 (11) |
| Carbapenems with others | 4 (5.5) |
| Quinolones with Metronidazole | 3 (4.1) |
| Coverage | |
| Gram positive | 26 (35.6) |
| Gram negative | 17 (23.3) |
| Gram positive and negative | 12 (16.4) |
| Gram negative and anaerobic | 12 (16.4) |
| Non-rankable/gram positive. Negative and anaerobic | 6 (8.2) |
| Duration of therapy | |
| One week | 18 (24.7) |
| Up to two weeks | 28 (38.4) |
| More than two weeks | 10 (13.7) |
| No data | 18 (24.7) |
| Efficacy of empirical antibiotic therapy | |
| No sufficient data | 17 (23.3) |
| No resistance to antibiotics being used | 19 (26) |
| Change in multi-resistant germ under antibiotic therapy | 2 (2.7) |
| Antibiotic therapy not adequate | 7 (9.6) |
| No germ detection with adequate diagnostics. Effectiveness of antibiotic therapy cannot be assessed | 5 (6.8) |
| No germ detection in the absence of microbiological diagnostics | 24 (32.9) |
When comparing patients with common vs atypical BI, the stage of cirrhosis in patients with atypical BI was less advanced, as reflected by lower creatinine levels (1.14 ± 0.60 vs 1.38 ± 1.17; P = 0.018) as well as CPS (7.99 ± 2.15 vs 8.61 ± 2.50; P = 0.05) and Model of End Stage Liver Disease (MELD) scores (12.9 ± 5.1 vs 15.1 ± 7.44; P = 0.005). No differences were found with respect to sex or diabetes. Long-term antibiotics (P = 0.002), lactulose (P = 0.03) and proton pump inhibitors (P = 0.013) were prescribed more frequently for patients with common BI.
Bacterial Infections remain a major contributor to morbidity in patients with liver cirrhosis, but data on less frequently occurring infections are scarce. In this retrospective analysis we compared less frequent BI (termed “atypical BI”), such as soft tissue infections, and found them to be present in a relevant proportion of BI in patients with cirrhosis. Our cohort of patients resembled a typical cohort of patients with cirrhosis in Western countries with respect to age, etiology of cirrhosis (predominantly alcoholic), and sex (predominantly male patients). Notably, the stage of cirrhosis in patients with atypical BI was less advanced. The typical BI frequently observed in cirrhosis were associated with liver function. We also confirmed previous observations that BI occurred more commonly in patients with advanced stage of cirrhosis, as expressed by higher MELD score and CPS[9,10].
Of note, the definition of atypical BI is not consistent in the literature. Even though pneumonia, UTI and SBP are consistently reported as common BI, discrepancies exist for other infections, in particular cellulitis. For example, in their recent analysis, Fricker et al[11] subsumed cellulitis as atypical BI. Other study groups e.g. Jalan et al[12] included cellulitis among the more frequent BI. Additionally, the localization of skin- and soft tissue BI is usually not further specified. Compared to typical BI, cellulitis is often a purely clinical diagnosis without a confirmatory laboratory method, making it much more difficult to classify and this may be one of the reasons why the definition and classification in the literature varies. Due to the clinically frequent presence of peripheral edema with dysfunction of the skin barrier, skin and soft tissue infections of the lower limb are more likely to occur in cirrhotics and should therefore be given more attention as a potential typical focus of infection.
Multidrug resistance is an increasingly important issue[13]. The range here is wide, from 29% Extended Spectrum Beta Lactamase-producing Enterobacterales in Korea to rather Gram-positive problems, with 9% vancomycin-resistant enterococci in the United States[14,15]. Fricker et al[11] reported an antibiotic resistance in 38% of cases, but did not specify how resistance was defined and which antibiotic classes were considered. Jalan et al[12] also discuss that depending on the geographical region, multidrug-resistant bacterial infections have become more frequent. In our analysis, we were able to show that when a pathogen was detected, resistance tended to occur in the Gram-negative range and one major pathogen was E. coli. In our study, not many multi-resistant pathogens were detected. However, it must be considered that only the cases with microbiological pathogen identification were considered. Internationally, gram-negative pathogens predominate in infections of liver cirrhotic patients, whereby no distinction is made between typical and atypical infections. Our data showed an empirically more frequent antibiotic coverage in the gram-positive spectrum with, however, more frequent detection of a gram-negative infection. Hillert et al[16] found, that a gram-positive pathogen was detected in 54% of cases, with the most common single pathogen detection being E. coli. Hillert et al[16] inclusion criterion was the presence of ascites.
Our data indicate that the general recommendations for antibiotic therapy can also be followed for atypical BI in cirrhotics and that empirical antibiotic therapy should be based on the localization of the clinical infection focus. Despite immunosuppression and multiple contacts in the health care system, broader antibiotic coverage is not empirically necessary, especially not for multidrug-resistant pathogens. In addition to the clinical localization, the presence of a long-term antibiotic therapy must also be included in the consideration of antibiotics therapy in cirrhotics and need further studies.
To our knowledge, there is no study evaluating how microbiological diagnostics and long-term use of antibiotics in liver cirrhosis patients influence infections and whether previous long-term antibiotics should be included in empirical treatment decisions.
A limiting factor in this data collection is the retrospective method, which makes it difficult to objectively assess appropriate microbiological diagnostics and the resulting decisions. Furthermore, the inclusion of many centers to collect sufficient case numbers and other experiences would certainly be useful to avoid monocentric aspects.
Cirrhosis is expected to further increase worldwide in the coming years, e.g. due to the increase in non-alcoholic steatohepatitis[17,18]. BI remain a major cause of morbidity and mortality in these patients. The relevance of a correct adequately chosen antibiotic in face of an increasing antimicrobial resistance rate worldwide is paramount[19]. Out data shows that atypical BI in patients with cirrhosis have different characteristics. With an increasing degree of liver failure, the severity and the spectrum of BI change. Prospective multicentric studies are needed to improve our understanding of an optimal diagnostic and therapeutic management of these disease entities in patients with liver cirrhosis. Further research is also warranted to identify whether infections at atypical body sites and more common sites differ depending on the causative bacterial species.
Typical infections in patients with liver cirrhosis have standardized diagnostic algorithms and are therefore recognized and treated quickly. Clinically, however, unusual infections are also more frequent in patients with cirrhosis. These are not included in guidelines and are therefore often not adequately addressed in diagnostic and therapeutic algorithms.
The study aimed to analyze a cirrhosis cohort for typical and atypical infections. The aim is to derive improved diagnostic and therapeutic algorithms from these analyses in the future.
The main aim is to identify the most common pathogens for atypical infections and their resistance patterns in relation to the stage of liver cirrhosis. Algorithms for the improved detection of infections, including atypical situations, can then be developed.
For the analysis, data were analyzed in relation to the research question in a cirrhosis cohort.
The cohort showed that atypical infections are not so rare overall and should be clinically investigated more frequently in order to initiate the correct diagnosis and treatment. It was also shown that the pathogen spectrum recorded did not always correspond correctly with the empirical therapy, and that microbiological diagnostics are therefore particularly relevant in this patient population.
We were able to show that the stage of cirrhosis is associated with a change in infections and that this needs to be taken into account. The relevance of these findings must be considered in the light of the increasing role of liver disease and its sequelae in the global burden of disease.
Confirmation of these results in larger multicenter studies and development of corresponding algorithms.
We would like to thank all patients who participated in the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wan X, China S-Editor: Liu JH L-Editor: A P-Editor: Guo X
| 1. | Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56 Suppl 1:S1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 2. | Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246-1256, 1256.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 836] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 3. | Piano S, Tonon M, Angeli P. Changes in the epidemiology and management of bacterial infections in cirrhosis. Clin Mol Hepatol. 2021;27:437-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, Giovo I, Uschner FE, Jansen C, Jimenez C, Mookerjee R, Gustot T, Albillos A, Bañares R, Jarcuska P, Steib C, Reiberger T, Acevedo J, Gatti P, Shawcross DL, Zeuzem S, Zipprich A, Piano S, Berg T, Bruns T, Danielsen KV, Coenraad M, Merli M, Stauber R, Zoller H, Ramos JP, Solé C, Soriano G, de Gottardi A, Gronbaek H, Saliba F, Trautwein C, Kani HT, Francque S, Ryder S, Nahon P, Romero-Gomez M, Van Vlierberghe H, Francoz C, Manns M, Garcia-Lopez E, Tufoni M, Amoros A, Pavesi M, Sanchez C, Praktiknjo M, Curto A, Pitarch C, Putignano A, Moreno E, Bernal W, Aguilar F, Clària J, Ponzo P, Vitalis Z, Zaccherini G, Balogh B, Gerbes A, Vargas V, Alessandria C, Bernardi M, Ginès P, Moreau R, Angeli P, Jalan R, Arroyo V; PREDICT STUDY group of the EASL-CLIF CONSORTIUM. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74:1097-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 5. | Lameirão Gomes C, Violante Silva R, Carrola P, Presa J. Bacterial Infections in Patients with Liver Cirrhosis in an Internal Medicine Department. GE Port J Gastroenterol. 2019;26:324-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Casper M, Mengel M, Fuhrmann C, Herrmann E, Appenrodt B, Schiedermaier P, Reichert M, Bruns T, Engelmann C, Grünhage F, Lammert F; INCA trial group. The INCA trial (Impact of NOD2 genotype-guided antibiotic prevention on survival in patients with liver Cirrhosis and Ascites): study protocol for a randomized controlled trial. Trials. 2015;16:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Reichert MC, Ripoll C, Casper M, Greinert R, Vandieken E, Grünhage F, Appenrodt B, Zipprich A, Lammert F. Common NOD2 Risk Variants as Major Susceptibility Factors for Bacterial Infections in Compensated Cirrhosis. Clin Transl Gastroenterol. 2019;10:e00002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6072] [Cited by in RCA: 8746] [Article Influence: 624.7] [Reference Citation Analysis (0)] |
| 9. | European Association for the Study of the Liver. Corrigendum to "EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis" [J Hepatol 69 (2018) 406-460]. J Hepatol. 2018;69:1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 10. | Van der Merwe S, Chokshi S, Bernsmeier C, Albillos A. The multifactorial mechanisms of bacterial infection in decompensated cirrhosis. J Hepatol. 2021;75 Suppl 1:S82-S100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 11. | Fricker ZP, Mukthinuthalapati VVPK, Akinyeye S, Chalasani N, Attar BM, Balakrishnan M, Ghabril M, Long MT. MELD-Na Is More Strongly Associated with Risk of Infection and Outcomes Than Other Characteristics of Patients with Cirrhosis. Dig Dis Sci. 2021;66:247-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R, Albillos A, Lammert F, Wilmer A, Mookerjee R, Vila J, Garcia-Martinez R, Wendon J, Such J, Cordoba J, Sanyal A, Garcia-Tsao G, Arroyo V, Burroughs A, Ginès P. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 642] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 13. | Piano S, Singh V, Caraceni P, Maiwall R, Alessandria C, Fernandez J, Soares EC, Kim DJ, Kim SE, Marino M, Vorobioff J, Barea RCR, Merli M, Elkrief L, Vargas V, Krag A, Singh SP, Lesmana LA, Toledo C, Marciano S, Verhelst X, Wong F, Intagliata N, Rabinowich L, Colombato L, Kim SG, Gerbes A, Durand F, Roblero JP, Bhamidimarri KR, Boyer TD, Maevskaya M, Fassio E, Kim HS, Hwang JS, Gines P, Gadano A, Sarin SK, Angeli P; International Club of Ascites Global Study Group. Epidemiology and Effects of Bacterial Infections in Patients With Cirrhosis Worldwide. Gastroenterology. 2019;156:1368-1380.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 328] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 14. | Song JY, Jung SJ, Park CW, Sohn JW, Kim WJ, Kim MJ, Cheong HJ. Prognostic significance of infection acquisition sites in spontaneous bacterial peritonitis: nosocomial versus community acquired. J Korean Med Sci. 2006;21:666-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Tandon P, Delisle A, Topal JE, Garcia-Tsao G. High prevalence of antibiotic-resistant bacterial infections among patients with cirrhosis at a US liver center. Clin Gastroenterol Hepatol. 2012;10:1291-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Hillert A, Schultalbers M, Tergast TL, Vonberg RP, Rademacher J, Wedemeyer H, Cornberg M, Ziesing S, Maasoumy B, Höner Zu Siederdissen C. Antimicrobial resistance in patients with decompensated liver cirrhosis and bacterial infections in a tertiary center in Northern Germany. BMC Gastroenterol. 2021;21:296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2286] [Article Influence: 381.0] [Reference Citation Analysis (0)] |
| 18. | Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, Sheron N; EASL HEPAHEALTH Steering Committee. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69:718-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 475] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 19. | Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8908] [Cited by in RCA: 7297] [Article Influence: 2432.3] [Reference Citation Analysis (0)] |