Published online Mar 27, 2024. doi: 10.4254/wjh.v16.i3.379
Peer-review started: October 19, 2023
First decision: December 26, 2023
Revised: January 17, 2024
Accepted: February 26, 2024
Article in press: February 26, 2024
Published online: March 27, 2024
Processing time: 153 Days and 23.6 Hours
Due to development of an immune-dysregulated phenotype, advanced liver disease in all forms predisposes patients to sepsis acquisition, including by opportunistic pathogens such as fungi. Little data exists on fungal infection within a medical intensive liver unit (MILU), particularly in relation to acute on chronic liver failure.
To investigate the impact of fungal infections among critically ill patients with advanced liver disease, and compare outcomes to those of patients with bacterial infections.
From our prospective registry of MILU patients from 2018-2022, we included 27 patients with culture-positive fungal infections and 183 with bacterial infections. We compared outcomes between patients admitted to the MILU with fungal infections to bacterial counterparts. Data was extracted through chart review.
All fungal infections were due to Candida species, and were most frequently blood isolates. Mortality among patients with fungal infections was significantly worse relative to the bacterial cohort (93% vs 52%, P < 0.001). The majority of the fungal cohort developed grade 2 or 3 acute on chronic liver failure (ACLF) (90% vs 64%, P = 0.02). Patients in the fungal cohort had increased use of vasopressors (96% vs 70%, P = 0.04), mechanical ventilation (96% vs 65%, P < 0.001), and dialysis due to acute kidney injury (78% vs 52%, P = 0.014). On MILU admission, the fungal cohort had significantly higher Acute Physiology and Chronic Health Evaluation (108 vs 91, P = 0.003), Acute Physiology Score (86 vs 65, P = 0.003), and Model for End-Stage Liver Disease-Sodium scores (86 vs 65, P = 0.041). There was no significant difference in the rate of central line use preceding culture (52% vs 40%, P = 0.2). Patients with fungal infection had higher rate of transplant hold placement, and lower rates of transplant; however, differences did not achieve statistical significance.
Mortality was worse among patients with fungal infections, likely attributable to severe ACLF development. Prospective studies examining empiric antifungals in severe ACLF and associations between fungal infections and transplant outcomes are critical.
Core Tip: In the critical care setting, patients with advanced liver disease who develop fungal infections have significantly higher mortality than those who develop bacterial infections. These patients require greater support with vasopressors, mechanical ventilation, and dialysis than their counterparts with bacterial infections. Patients who developed fungal infections appeared more acutely ill on admission to the intensive care unit, with higher Acute Physiology and Chronic Health Evaluation, Acute Physiology Score, and Model for End-Stage Liver Disease scores. In such patients, fungal infection development is closely associated with development of severe acute-on-chronic liver failure. Further work elucidating this relationship will allow for better prognostication and development of predictors for acute on chronic liver failure in this population.
- Citation: Khan S, Hong H, Bass S, Wang Y, Wang XF, Sims OT, Koval CE, Kapoor A, Lindenmeyer CC. Comparison of fungal vs bacterial infections in the medical intensive liver unit: Cause or corollary for high mortality? World J Hepatol 2024; 16(3): 379-392
- URL: https://www.wjgnet.com/1948-5182/full/v16/i3/379.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i3.379
Advanced liver disease predisposes patients to acquisition of infections. This vulnerability is best described in cirrhosis, through development of a cirrhosis-associated immune dysfunction (CAID). Intestinal dysbiosis and disruption of the gut barrier leads to gut inflammation, causing portal and systemic inflammation in cirrhosis patients[1]. Despite persistent immune activation[2-5], converse immunodeficiency develops due to immune exhaustion and senescence in advanced cirrhosis[6]. Immune dysfunction through impaired phagocytosis, complement deficiency, and Kupffer cell disruption mediates this vulnerability to invasive fungal infections[7-9]. Vulnerability due to immune dysfunction is further com
This theorized immunodeficient phenotype also predisposes patients to other types of opportunistic pathogens[14,16], including fungal infections. The existing literature on ACLF has predominantly focused on bacterial infections due to their prevalence as the primary triggers of ACLF in Western countries[18]. Invasive fungal infections, however, are emerging as under-recognized significant causes of mortality, particularly in the critical care setting[7,11,16,19]. Recent studies have demonstrated an association between fungal infections with the development of severe ACLF, increased rate of intensive care admission among infected patients, and higher mortality[15,19], when compared with bacterial infections.
Due to this significant impact, there has been a growing interest in further characterizing the impact of fungal in
The Cleveland Clinic MILU is a multi-disciplinary care setting designed for daily co-management of patients by hepatology and critical care teams, with a special focus on bridging critically ill patients to transplant. We designed a cohort study comparing patients with fungal and bacterial infections, who were admitted to our MILU between January 2018 to September 2022. To identify a study sample of patients with culture-confirmed infections, we queried our prospectively-curated, longitudinal MILU database for patients with positive cultures. Diagnostic criteria for infections were: positive blood cultures/cultures from sterile sites in combination with clinical symptoms of infection, which were usually treated with antimicrobials in consultation with our infectious disease department[22]. Fungal infections were deemed present if fungi were isolated from blood (candidemia) or other sterile sites (peritoneal fluid), or urine in certain cases. Positive cultures from urinary sources were included as infection if there were clinically associated symptoms and were treated with targeted antifungal agents. One case of tracheitis was included following isolation from tracheal biopsy due to complicated wound infection at a tracheostomy site. For patients with multiple positive sites of fungal culture including blood and non-sterile sites, infection was classified as fungemia. Among patients with bacterial isolates, 15 patients had 2 separate culture-positive instances of infection within the same MILU stay. In such cases, the second instance of infection was used in the mortality analysis. All infection parameters were defined in consultation with our transplant infectious disease department. Multi-drug resistant organisms (MDRO) were defined using previously established guidelines for each isolated organism: resistance to two or more classes of antibiotics for the majority of bacterial pathogens; and resistance to two or more classes of antifungals for fungal pathogens[23-27].
Furthermore, patients were included if they had clinically significant advanced liver disease, as defined by the presence of cirrhosis, acute liver failure, severe alcohol-associated hepatitis, or severe acute liver injury. Cirrhosis was defined either as biopsy-proven bridging fibrosis of the liver or as a composite of clinical signs, laboratory tests, endo
The primary outcome of interest for this study was mortality from time of onset of infection, which was determined by the date of a positive culture. Mortality was compared between patients with fungal and bacterial infections in the MILU.
Secondary outcomes of interest included need for cardiopulmonary support, development of acute kidney injury requiring dialysis, transplant evaluation endpoints and length of stay. Three separate lengths of stay were compared: total stay from hospital admission to discharge/death, time from intensive care unit (ICU) admission to ICU discharge and time from hospital admission to ICU discharge. Outcomes were compared between fungal and bacterial cohorts. Comparisons were also conducted on characteristics of acute illness including labs at infection, illness severity scoring and severity of ACLF, if applicable, at the time of culture. Finally, pre-infection predisposing variables were analyzed for differences between bacterial and fungal cohorts, including circulatory failure requiring hemodynamic support, prior antimicrobial use, and admission scores of illness severity.
All variables and outcomes were collected through chart extraction. Patients were identified from our longitudinal, prospective registry of all admissions to the MILU, and eligible cases were extracted from the electronic medical record based on culture positivity. ACLF was defined as suggested by the chronic liver failure consortium (CLIF-C OFs), graded by the number and severity of organ failures after an initial insult[30,31]. Infections were considered to have precipitated ACLF if the date of culture was prior to or on the day of syndrome development. Furthermore, grading of ACLF was done at the time of positive culture. Labs of interest at time points of infection were taken within 3 d prior to or after the date of culture, if unavailable at the date of culture. Stress dose steroid use preceding infection was defined as steroid dosing equivalent to 50 mg of hydrocortisone every 8 h, used for at least 3 d in the preceding 3 months from date of positive culture. MDROs were defined using pre-established criteria by an international expert proposal for interim standard definitions for acquired resistance[23,24,26,27]. Elucidation of epidemiology of fungal infection and colonization within our unit to inform antimicrobial protocols was done using individual culture data.
Measures of central tendency (means and standard deviations for normally distributed continuous variables, medians and quartiles for non-normally distributed continuous variables) and frequency distributions were used to characterize the sample. Comparisons between fungal and bacterial cohorts were done using Wilcoxon rank sum and Welch’s two-sample t-tests for continuous variables. Pearson’s chi-square and Fischer’s exact tests were used for comparison of categorical variables. A Kaplan-Meier curve was constructed to compare survival from ICU admission. All statistical analyses were conducted using R 4.0.5. Core Team (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2018. URL http://www.R-project.org/). P values < 0.05 were considered statistically significant. All statistical analyses were conducted in partnership with biostatisticians from our institution’s department of quantitative health sciences.
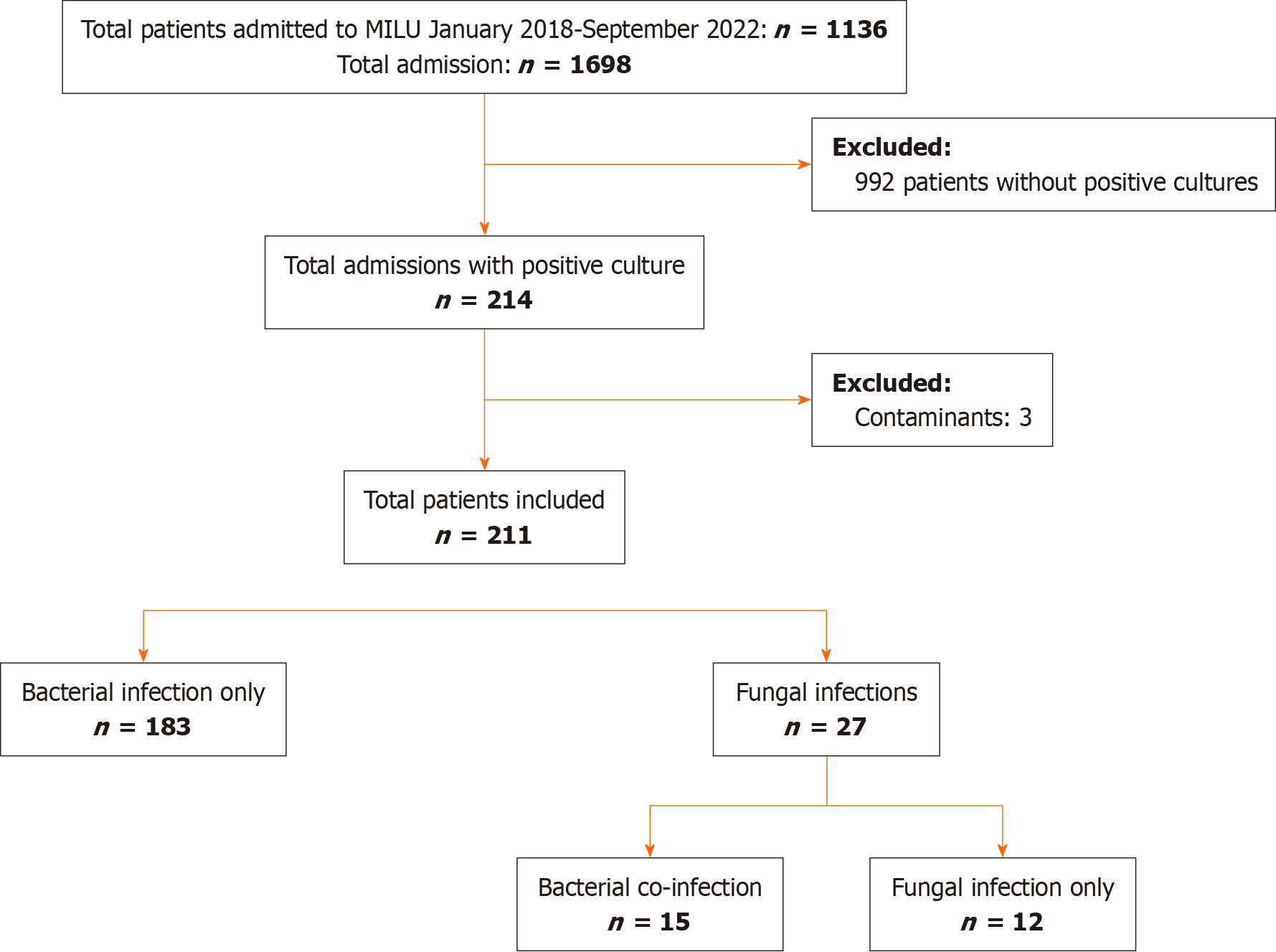
From 2018-2022, 1136 individual patients were treated in the MILU, accounting for 1698 admissions. Of these, we isolated 214 unique patients with positive microbial cultures. Of this population, we further excluded 3 cases with positive cultures as these were clinically treated as contaminants (Figure 1).
Twenty-seven patients with positive fungal cultures, and 183 with bacterial infections were included in our analysis. Ten patients in the fungal cohort had bacterial co-infections. Of the bacterial cohort, 15 patients had 2 instances of separate infections within the same MILU stay. The last infection prior to discharge or death was utilized for analysis in these cases.
There were no differences in baseline demographics of age, race, or sex between the 2 cohorts (Table 1). Both cohorts also had similar Charlson Comorbidity Scores. The fungal and bacterial cohorts had similar proportions of patients admitted with cirrhosis, alcohol associated hepatitis, acute liver failure and severe acute liver injury. Viral hepatitis due to hepatitis B and C infection was more commonly the etiology of liver disease among patients with fungal infections, but other etiologies were similar between cohorts. Patients with fungal infections had higher rates of hepatorenal syndrome. One case of alcohol-associated hepatitis occurred without underlying cirrhosis in the bacterial cohort, while all other cases occurred with comorbid cirrhosis.
| Characteristic | Bacteria, n = 1831 | Fungal, n = 271 | P value2 |
| Age | 60 (50, 66) | 58 (46, 66) | 0.3 |
| Sex | |||
| Female | 73 (40) | 12 (44) | |
| Male | 110 (60) | 15 (56) | |
| Race | |||
| American Indian/Alaska Native | 1 (0.5) | 0 (0) | |
| Asian | 1 (0.5) | 0 (0) | |
| Black | 27 (15) | 6 (22) | |
| Declined | 1 (0.5) | 2 (7.4) | |
| Multiracial/cultural | 7 (3.8) | 1 (3.7) | |
| Unavailable | 7 (3.8) | 1 (3.7) | |
| White | 139 (76) | 17 (63) | |
| Charlson Comorbidity Score | 6.00 (5.00, 7.00) | 6.00 (4.00, 7.00) | 0.3 |
| Hepatocellular carcinoma | 18 (9.8) | 1 (3.7) | 0.5 |
| Principal liver diagnosis | |||
| Acute liver failure | 10 (5) | 2 (7.4) | > 0.9 |
| Cirrhosis | 141 (78) | 21 (78) | > 0.9 |
| Alcohol-associated hepatitis (comorbid3) | 16 (9.3) | 6 (22) | 0.093 |
| Acute severe liver injury/other4 | 34 (17) | 4 (14.6) | > 0.9 |
| Etiology of viral disease | |||
| Viral hepatitis | 24 (13) | 9 (33) | 0.016 |
| Alcohol-associated | 75 (41) | 13 (48) | 0.6 |
| Autoimmune | 8 (4.4) | 1 (3.7) | > 0.9 |
| NASH | 44 (24) | 5 (19) | 0.7 |
| Primary biliary cholangitis | 4 (2.2) | 1 (3.7) | > 0.9 |
| Primary sclerosing cholangitis | 17 (9.3) | 1 (3.7) | 0.5 |
| Other | 34 (19) | 6 (22) | 0.9 |
| Toxins | 4 (2.2) | 0 (0) | > 0.9 |
| Ischemic injury | 5 (2.7) | 1 (3.7) | > 0.9 |
| Cryptogenic | 16 (8.7) | 1 (3.7) | 0.6 |
| Decompensation defining events | |||
| Ascites | 144 (79) | 23 (85) | 0.6 |
| Hepatic encephalopathy | 138 (75) | 25 (93) | 0.08 |
| Hepatorenal syndrome | 63 (34) | 16 (59) | 0.023 |
| EV history/variceal bleeding | 106 (58) | 16 (59) | > 0.9 |
| HPS | 2 (1.1) | 0 (0) | > 0.9 |
| PoPHTN | 6 (3.3) | 0 (0) | 0.7 |
| Hepatic hydrothorax | 27 (15) | 3 (11) | 0.8 |
| SBP | 45 (25) | 12 (44) | 0.053 |
| Coagulopathy | 122 (67) | 21 (78) | 0.3 |
| Thrombocytopenia | 107 (58) | 20 (74) | 0.2 |
Among the fungal cohort, 33% of patients also suffered surgical illnesses including small bowel obstruction, cholecystitis, colitis and abdominal fistula, during their ICU stay. Of those with isolated fungal infection, 71% received 5 d of antibiotic therapy prior to initiation of antifungal treatment.
All isolated fungal infections were Candida infections (Table 2). Candida glabrata was the most common isolated fungus, followed by Candida albicans. Isolates were most frequently from blood, followed by ascites and urine. We isolated one case of secondary peritonitis and one case of tracheitis.
| Organism | Urinary source | Bacteremia | Spontaneous peritonitis | Secondary peritonitis | Tracheitis | Total per organism |
| Candida glabrata | - | 6 | 2 | 1 | - | 9 |
| Candida albicans | 1 | 4 | 2 | - | 1 | 8 |
| Candida krusei | - | 2 | - | - | - | 2 |
| Candida dubliniensis | 1 | 3 | 2 | - | - | 6 |
| Candida (other) | 2 | 2 | - | - | - | 4 |
| Total per source | 4 | 17 | 6 | 1 | 1 |
Among 183 patients with bacterial infections, 45 (24.5%) had co-infections with multiple bacterial isolates and 15 (8.1%) patients had 2 separate instances of bacterial infection during their MILU stay. Blood was the most frequently isolated source (Appendix). Spontaneous bacterial peritonitis, and respiratory and urinary tract infections were the most common sources of gram-positive infections following bacteremia. There were 117 g-positive cultures, of which the most common organism was Enterococcus faecium, followed by methicillin-resistant Staphylococcus aureus. There were 126 g-negative isolates, and the majority were caused by Escherichia coli, followed by Klebsiella species.
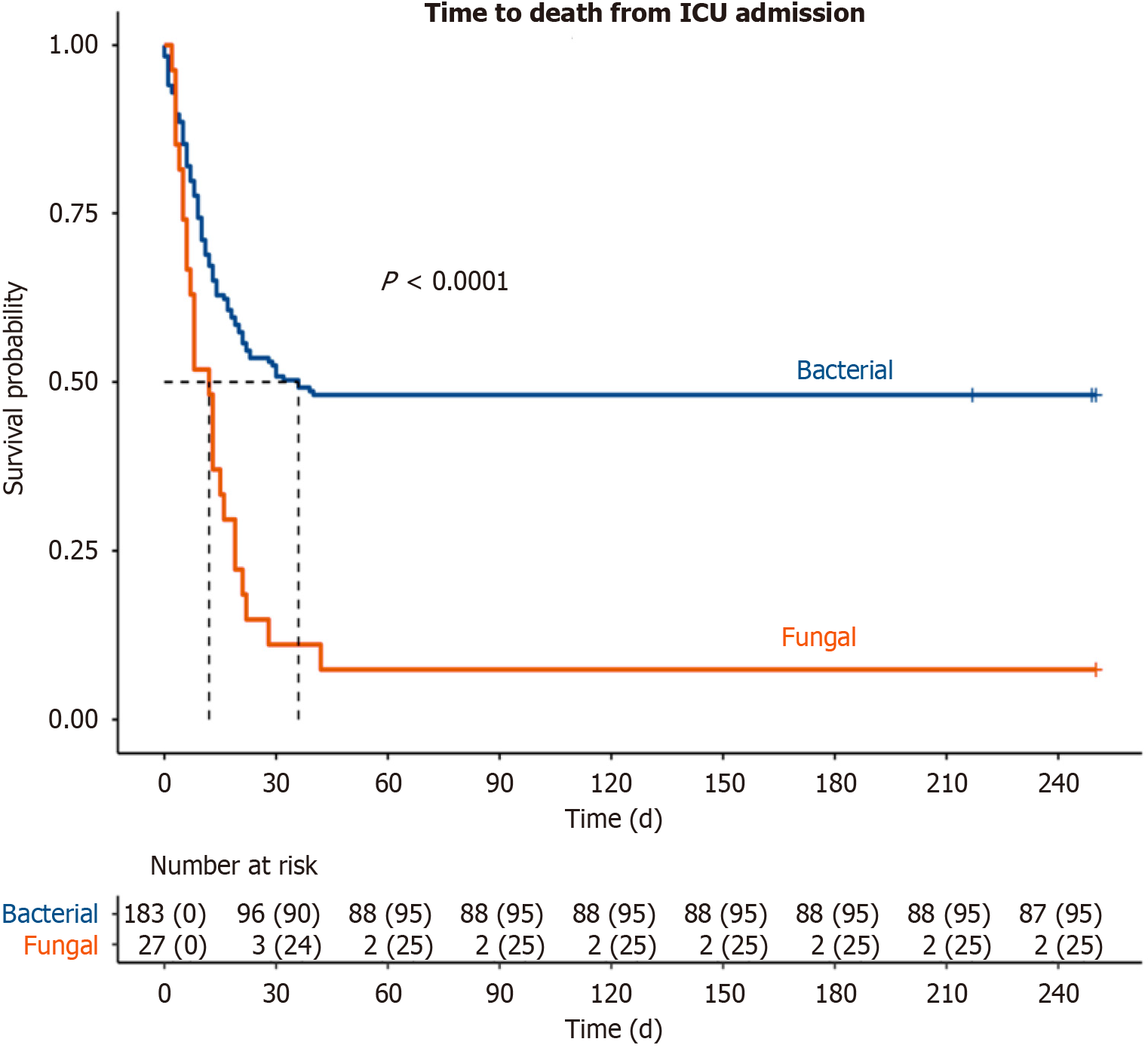
The mortality rate among patients with fungal infections was significantly higher than those with bacterial infections (93% vs 52%, P < 0.001, Figure 2). Median survival among the fungal cohort was 12 d relative to 31 d in the bacterial cohort (Figure 2). The majority of patients with fungal infections had severe ACLF, defined as ACLF grade 2 or higher (90% vs 64%, P = 0.02, Table 3), and either died or transitioned to hospice during their MILU stay (93% vs 52%, P < 0.0001). One patient with fungal infection had decompensated cirrhosis without ACLF, while 34 patients in the bacterial cohort had decompensated cirrhosis alone. Significantly higher proportions of those in the fungal cohort required vasopressor support (96% vs 70%, P = 0.04), mechanical ventilation (96% vs 65%, P < 0.001), and dialysis initiation due to acute kidney injury (78% vs 52%, P = 0.014). There were no differences in indication for intubation, MILU length of stay (LOS) or overall hospital LOS. However, those in the fungal cohort had longer hospital LOS prior to MILU admission (8 d vs 0 d, P = 0.046).
| Characteristic | Bacteria, n = 1831 | Fungal, n = 271 | P value2 |
| Intensive care outcomes | |||
| ACLF grade | 0.017 | ||
| < 2 | 50 (36) | 2 (9.5) | |
| ≥ 2 | 90 (64) | 19 (90) | |
| Death during admission or hospice | 95 (52) | 25 (93) | < 0.001 |
| Vasopressor requirement | 129 (70) | 26 (96) | 0.004 |
| Mechanical ventilation | 118 (65) | 26 (96) | < 0.001 |
| Indication for intubation | |||
| Airway protection | 93 (79) | 22 (85) | 0.6 |
| Respiratory failure | 25 (21) | 4 (15) | |
| Dialysis due to acute kidney injury | 95 (52) | 21 (78) | 0.014 |
| ICU LOS (d) | 5 (2, 10) | 6 (4, 16) | 0.063 |
| Hospital LOS (d) | 16 (7, 28) | 17 (12, 30) | 0.3 |
| Hosp admit to ICU (d) | 0 (0, 6) | 8 (0, 13) | 0.046 |
| Transplant-related outcomes | |||
| Evaluated for transplant | 107 (58) | 13 (48) | 0.3 |
| Listed | 57 (53) | 4 (31) | 0.13 |
| Organ listed | 0.3 | ||
| Liver | 44 (79) | 3 (60) | |
| Liver and kidney | 12 (21) | 2 (40) | |
| Hold placed | 32 (57) | 4 (100) | 0.14 |
| Transplant occurred | 28 (50) | 0 (0) | 0.056 |
There were no differences between fungal and bacterial cohorts in rate of transplant evaluation initiation (48% vs 58%, P = 0.3) or rate of listing (31% vs 51%, P = 0.13). Of those patients who were listed, all patients with fungal infection were subsequently placed on hold, and no patients with fungal infections received a transplant. Patients with fungal infection had higher rate of hold placement (100% vs 57%, P = 0.14), and lower rates of transplant compared to bacterial counterparts (0% vs 50%, 0 = 0.056); however, these differences did not achieve statistical significance.
At the time of positive culture, fungal and bacterial cohorts had similar rates of infection with MDROs (37% vs 50%, P = 0.2) and Child-Pugh scores (11 vs 11, P = 0.064) (Table 4). Patients in the fungal cohort had higher Model for End-Stage Liver Disease-Sodium (MELD-Na) (33 vs 28, P = 0.017) and CLIF scores (13 vs 11, P < 0.001). Albumin, lactate, leukocyte count, and C-reactive protein were not significantly different between cohorts.
| Characteristic | Bacteria, n = 1831 | Fungal, n = 271 | P value2 |
| MDRO | 90 (50) | 10 (37) | 0.2 |
| MELD-Na (time of positive culture) | 28 (22, 33) | 33 (25, 38) | 0.017 |
| Child-Pugh score (time of positive culture) | 11.00 (9.00, 12.00) | 11.00 (10.00, 13.00) | 0.064 |
| CLIF-C score (time of positive culture) | 11.00 (9.00, 13.00) | 13.00 (12.00, 14.50) | < 0.001 |
| Lab values of interest at time of culture | |||
| Leukocyte count | 13 (7, 19) | 16 (11, 18) | > 0.9 |
| C-reactive protein | 6 (3, 12) | 5 (4, 8) | 0.057 |
| Albumin | 2.70 (2.20, 3.30) | 3.10 (2.70, 3.40) | 0.086 |
| Bilirubin | 6 (2, 13) | 13 (4, 23) | 0.035 |
| Lactate | 2.9 (1.9, 5.2) | 4.4 (2.2, 7.3) | 0.3 |
| International normalized ratio | 1.80 (1.40, 2.10) | 2.05 (1.78, 3.00) | 0.046 |
At the time of MILU admission, patients with fungal infection had significantly higher Acute Physiology and Chronic Health Evaluation (108 vs 91, P = 0.003), Acute Physiology Score (86 vs 65, P = 0.003), and MELD-Na scores (86 vs 65, P = 0.041) (Table 5). There was no significant difference in the rate of central line use in 48 h preceding positive culture in fungal patients (52% vs 40%, P = 0.2). Prior infection or colonization with MDRO was more common in the fungal cohort (41% vs 21%, P = 0.027). Foley catheter use within 48 h preceding infection was less common among the fungal cohort relative to the bacterial cohort (19% vs 44%, P = 0.013). There were no significant differences in preceding stress dose steroid use, screening MRSA nasal swab, regular large volume paracentesis requirement prior to admission (defined as at least monthly paracenteses within the preceding six months of admission), outpatient immunosuppression use, prior antibiotic exposure, prior antifungal exposure, or SARS-CoV-2 infection within preceding 30 d (Table 5).
| Characteristic | Bacteria, n = 1831 | Fungal, n = 271 | P value2 |
| APACHE III Score | 91 (71, 112) | 108 (96, 121) | 0.003 |
| Acute Physiology Score | 65 (50, 90) | 86 (75, 108) | 0.003 |
| MELD-Na (admission) | 29 (23, 35) | 32 (28, 38) | 0.041 |
| Stress dose steroid use in past 3 months | 29 (16) | 6 (22) | 0.4 |
| Foley in past 48 h | 80 (44) | 5 (19) | 0.013 |
| Central line in past 48 h | 73 (40) | 14 (52) | 0.2 |
| Positive MRSA nasal swab | 15 (8.2) | 5 (19) | 0.15 |
| Prior MDRO infection/colonization | 39 (21) | 11 (41) | 0.027 |
| Regular LVP | 72 (39) | 14 (52) | 0.2 |
| Immunosuppressive medications (at time of admission) | 40 (22) | 6 (22) | > 0.9 |
| Charlson Comorbidity Score | 6.00 (5.00, 7.00) | 6.00 (4.00, 7.00) | 0.5 |
| Prior antibiotic classes exposed | 4.00 (3.00, 5.00) | 4.00 (3.00, 5.00) | 0.9 |
| Prior antifungal classes exposed | 0.11 | ||
| 0 | 104 (57) | 10 (37) | |
| 1 | 58 (32) | 14 (52) | |
| 2 | 16 (8.8) | 2 (7.4) | |
| 3 | 3 (1.7) | 1 (3.7) | |
| COVID within 30 d prior | 9 (4.9) | 2 (7.4) | 0.6 |
While bacterial infections have been recognized as a major cause of mortality among patients with advanced liver disease, especially as the most common trigger for ACLF, outcomes of fungal infections have not been as well studied. Our study is among the few to examine survival in this population and is among the first to compare outcomes of fungal and bacterial infections in the intensive care setting. Our findings demonstrate survival reductions are associated with fungal infections among patients with advanced liver disease who are receiving care in intensive care units such as the MILU. Further, our findings suggest the need for future work, such as exploration of predictors of poor outcomes to elucidate indications for palliative care, and implications for transplant.
The stark difference in mortality among fungal and bacterial cohorts is the most notable finding of our study. As bacterial infections are common and confer a 4-fold increase in mortality, several studies have examined factors associated with infection acquisition, outcomes, and prevention strategies[13,32-35]. Our findings highlight, comparative to bacterial infections that fungal infections are associated with worse survival, as 93% of patients in our fungal cohort died or transitioned to hospice care. This may be attributable in part to development of ACLF, as the majority of patients with fungal infections had severe ACLF relative to bacterial counterparts. It is clear from our results that fungal infection is likely associated with ACLF severity; however, we were unable to run predictive models given the respective aspect of our study design. Our findings affirm the need for future work to further elucidate associations, and the potential benefits of empiric or prophylactic fungal coverage.
Furthermore, patients with fungal infection had severely reduced rates of transplant. Half of listed patients with bacterial infections received liver transplantation, whereas no patients with fungal infections received liver tran
While prior studies have reported relatively lower rates of fungal infection, our study found prevalence of fungal infection among all culture-positive patients in the ICU to be 12.9%, or 10.9% when including only sterile source isolates. This is higher than previously postulated estimates ranging between 2%-7% among hospitalized patients with cirrhosis[15,43], suggesting that fungal infection may be more common specifically in the intensive care setting. The incidence of invasive candidiasis in non-selected patients in the ICU has been reported to be between 1%-2%, and on the rise[44]. Our estimation of prevalence may be subject to bias, however, due to the limited size of our fungal cohort. Nevertheless, underestimation of prevalence of invasive fungal infections has been suggested in the past due to dependence of prior estimates on performance of specific fungal cultures. To enable early recognition, interest in non-culture based diagnostic tools is growing, though current clinical use remains limited[45]. Additionally, similar to our findings, Candida infection has been associated with prolonged antibiotic administration prior to diagnosis of Candidemia[46], potentially leading to under-diagnosis. Further epidemiologic characterizations of patients with advanced liver disease is thus crucial, as inadequate antimicrobial coverage is associated with increased mortality[15,34]. Our findings demonstrate this, as Candida glabrata was the most commonly isolated species, in keeping with recent trends towards the rising prevalence of non-albicans species[44]. While echinocandins have been recommended by the Infectious Disease Society of America for empiric antifungal therapy[47], recommendations differ for C. glabrata depending on susceptibility due to resistance.
In addition to empiric therapy, the severe mortality associated with fungal infections raises the importance of early risk stratification for potential prophylactic therapy. Markers of utility may be MELD-Na or ACLF grade cutoffs; MELD-Na has previously been shown to be predictive of fungal infection development[48]. In our study, MELD-Na was higher both at MILU admission and at time of infection among the fungal cohort. A prior study has also raised the possibility of prophylaxis in waitlisted patients with severe ACLF[49]. Our findings on pre-infection variables of interest may represent useful targets of future work to identify appropriate indications for prophylactic antifungals. In our cohort, fungal infections were associated with a higher rate of prior multi-drug resistant colonization and infection. Prior bacterial infection has been established as a risk factor for subsequent fungal infection development[19,43,50], and may represent an important indication to explore for prophylactic antifungal therapy in critically-ill liver patients. Additionally, bilirubin and INR were significantly higher among patients with fungal infections, though this may represent collinearity with the MELD-Na score. Further studies investigating these markers for independent inclusion in predictive models would be valuable. A prolonged hospital stay prior to ICU admission in the fungal cohort compared to the bacterial cohort may also suggest an increased rate of nosocomial infections in this population. Invasive fungal infections have been reported as important causes of healthcare-acquired infections, and may warrant further study in this setting[46].
Parallel to the need for early aggressive treatment among patients with fungal infections is also the need to develop prognostication tools to guide goals of care. The concepts of futility and palliative care in severe ACLF are rising due to the associated reductions in quality of life beyond that associated with decompensated cirrhosis alone. In our study, despite their poor survival, the fungal cohort had similar lengths of stay in the intensive care unit compared to bacterial counterparts, with higher rates of vasopressor support, mechanical intubation, and dialysis initiation. Furthermore, prior work has shown that survival with Candida infection despite timely administration of antifungals is poor[51]. Our findings suggest the importance of exploring the potential prognostic role of positive fungal culture in severe ACLF, to better inform advanced care planning, improve end of life quality, and reduce psychosocial patient and family burden.
Several factors set our study apart from others. We provide data from a large population of unique MILU patients and used data from a prospectively maintained database over the course of four years. Within the unit, all patients are daily co-managed by hepatologists and intensivists, ensuring multi-disciplinary comprehensive care. We report on a critically ill, unique population of patients with complex pathologies seeking care at a quaternary center. We are also one of few studies to provide granular data on fungal infections in the critical care setting, and to comment on interplay with ACLF. An important limitation of some prior studies has been the use of population-based databases[37].
With these strengths, our study had some notable limitations. Despite data extracted from a prospective registry, our population had a limited sample size, and for this reason our study was limited in its ability to construct predictive models. Though being a quaternary referral center provides complexity and allows study of a critically ill population, data on patients’ pre-care from prior hospital admissions is at times unavailable; this may have impacted our comparison of pre-infection variables. Finally, due to our requirement for culture positivity, our study did not include patients who may otherwise meet criteria for infection despite lack of microbiological isolation. Selection by culture may allow bias towards selection of a population with higher illness severity; however, our study aimed to investigate infections in this cohort of critically ill patients, and culture positivity is crucial for differentiating infection from other acute states of decompensation/inflammation.
Our findings demonstrate that fungal infection is associated with severe ACLF and marked increase in mortality among critically ill patients with advanced liver disease. We highlight the poor outcomes in this population despite aggressive supportive care and efforts towards stabilization for transplant evaluation. Future multi-center prospective studies are necessary to predict infection and prognosticate trajectory of care.
Advanced liver disease predisposes critically ill patients to the development of fungal infections. While bacterial infections have been well-studied as the most common cause of acute-on-chronic liver failure and associated mortality, fungal infections have been relatively under-studied in the intensive care setting.
Infections increase mortality four-fold among critically ill liver patients, but few studies have compared predictors and outcomes of fungal infections to bacterial infections in this population.
We compared outcomes of fungal and bacterial infections among critically ill patients who were admitted to our unique medical intensive liver unit (MILU) from 2018-2022. We also conducted a comprehensive comparison of predictors and illness severity scores between these cohorts. Finally, we characterized microbiologic epidemiology of infections within our unit.
Patients were identified for inclusion from a prospectively-curated database of all admissions to our MILU during the study period. Infections were defined based on culture positivity and clinical presentation. Data on outcomes and predictors of interest were collected manually through chart review.
We found that fungal infections among our patients were all caused by Candida species and were most frequently blood isolates. Mortality was significantly worse among the fungal cohort relative to patients with bacterial infections, as the majority of these patients died or transitioned to hospice during the intensive care unit (ICU) stay. The majority of patients in the fungal cohort developed severe acute on chronic liver failure, and they had higher need for vasopressors, mechanical ventilation and acute kidney injury. Further, patients who developed fungal infections were sicker on admission to the unit. Patients with fungal infection had higher rate of transplant hold placement, and lower rates of transplant; however, differences did not achieve statistical significance.
Fungal infection is a poor prognostic marker for patients with advanced liver disease in the critical care setting, and it is associated with significantly worse mortality than bacterial infection. This may be in large part due to development of severe acute on chronic liver failure. Patients who developed fungal infections had higher Model for End-Stage Liver Disease-Sodium, Acute Physiology and Chronic Health Evaluation, and Acute Physiology Score scores on admission to the ICU.
We believe our work highlights the importance of a need for future studies to investigate associations between fungal infections and acute on chronic liver failure. Furthermore, research efforts examining prognostic markers, potential indications for prophylactic/empiric antifungal use, and transplant outcomes would be equally important and informative for clinical practice.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology & hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liang X, China S-Editor: Gong ZM L-Editor: A P-Editor: Zheng XM
| 1. | Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 560] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 2. | Doi H, Iyer TK, Carpenter E, Li H, Chang KM, Vonderheide RH, Kaplan DE. Dysfunctional B-cell activation in cirrhosis resulting from hepatitis C infection associated with disappearance of CD27-positive B-cell population. Hepatology. 2012;55:709-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Girón JA, Alvarez-Mon M, Menéndez-Caro JL, Abreu L, Albillos A, Manzano L, Durántez A. Increased spontaneous and lymphokine-conditioned IgA and IgG synthesis by B cells from alcoholic cirrhotic patients. Hepatology. 1992;16:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Tritto G, Bechlis Z, Stadlbauer V, Davies N, Francés R, Shah N, Mookerjee RP, Such J, Jalan R. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. J Hepatol. 2011;55:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Manigold T, Böcker U, Hanck C, Gundt J, Traber P, Antoni C, Rossol S. Differential expression of toll-like receptors 2 and 4 in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2003;15:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 851] [Article Influence: 77.4] [Reference Citation Analysis (1)] |
| 7. | Rolando N, Harvey F, Brahm J, Philpott-Howard J, Alexander G, Casewell M, Fagan E, Williams R. Fungal infection: a common, unrecognised complication of acute liver failure. J Hepatol. 1991;12:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 126] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Vergis N, Khamri W, Beale K, Sadiq F, Aletrari MO, Moore C, Atkinson SR, Bernsmeier C, Possamai LA, Petts G, Ryan JM, Abeles RD, James S, Foxton M, Hogan B, Foster GR, O'Brien AJ, Ma Y, Shawcross DL, Wendon JA, Antoniades CG, Thursz MR. Defective monocyte oxidative burst predicts infection in alcoholic hepatitis and is associated with reduced expression of NADPH oxidase. Gut. 2017;66:519-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Lopes ME, Nakagaki BN, Mattos MS, Campolina-Silva GH, Meira RO, Paixão PHM, Oliveira AG, Faustino LD, Gonçalves R, Menezes GB. Susceptibility to Infections During Acute Liver Injury Depends on Transient Disruption of Liver Macrophage Niche. Front Immunol. 2022;13:892114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Triger DR, Goepel JR, Slater DN, Underwood JC. Systemic candidiasis complicating acute hepatic failure in patients treated with cimetidine. Lancet. 1981;2:837-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Lahmer T, Messer M, Schwerdtfeger C, Rasch S, Lee M, Saugel B, Schmid RM, Huber W. Invasive mycosis in medical intensive care unit patients with severe alcoholic hepatitis. Mycopathologia. 2014;177:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Gustot T, Maillart E, Bocci M, Surin R, Trépo E, Degré D, Lucidi V, Taccone FS, Delforge ML, Vincent JL, Donckier V, Jacobs F, Moreno C. Invasive aspergillosis in patients with severe alcoholic hepatitis. J Hepatol. 2014;60:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246-1256, 1256.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 838] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 14. | Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death: analysis of the National Hospital Discharge Survey. Chest. 2003;124:1016-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, Reverter E, Martínez J, Saliba F, Jalan R, Welzel T, Pavesi M, Hernández-Tejero M, Ginès P, Arroyo V; European Foundation for the Study of Chronic Liver Failure. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67:1870-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 400] [Article Influence: 57.1] [Reference Citation Analysis (2)] |
| 16. | Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, Bissoli F, Boccia S, Colloredo-Mels G, Corigliano P, Fornaciari G, Marenco G, Pistarà R, Salvagnini M, Sangiovanni A. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 299] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 17. | Mücke MM, Rumyantseva T, Mücke VT, Schwarzkopf K, Joshi S, Kempf VAJ, Welsch C, Zeuzem S, Lange CM. Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality. Liver Int. 2018;38:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 441] [Article Influence: 55.1] [Reference Citation Analysis (1)] |
| 19. | Bajaj JS, Reddy RK, Tandon P, Wong F, Kamath PS, Biggins SW, Garcia-Tsao G, Fallon M, Maliakkal B, Lai J, Vargas HE, Subramanian RM, Thuluvath P, Thacker LR, OʼLeary JG. Prediction of Fungal Infection Development and Their Impact on Survival Using the NACSELD Cohort. Am J Gastroenterol. 2018;113:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 20. | Bucsics T, Schwabl P, Mandorfer M, Peck-Radosavljevic M. Prognosis of cirrhotic patients with fungiascites and spontaneous fungal peritonitis (SFP). J Hepatol. 2016;64:1452-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Alexopoulou A, Vasilieva L, Agiasotelli D, Dourakis SP. Fungal infections in patients with cirrhosis. J Hepatol. 2015;63:1043-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Nanchal R, Subramanian R, Karvellas CJ, Hollenberg SM, Peppard WJ, Singbartl K, Truwit J, Al-Khafaji AH, Killian AJ, Alquraini M, Alshammari K, Alshamsi F, Belley-Cote E, Cartin-Ceba R, Dionne JC, Galusca DM, Huang DT, Hyzy RC, Junek M, Kandiah P, Kumar G, Morgan RL, Morris PE, Olson JC, Sieracki R, Steadman R, Taylor B, Alhazzani W. Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU: Cardiovascular, Endocrine, Hematologic, Pulmonary, and Renal Considerations. Crit Care Med. 2020;48:e173-e191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 23. | Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6072] [Cited by in RCA: 8778] [Article Influence: 627.0] [Reference Citation Analysis (0)] |
| 24. | Hahn WO, Werth BJ, Butler-Wu SM, Rakita RM. Multidrug-Resistant Corynebacterium striatum Associated with Increased Use of Parenteral Antimicrobial Drugs. Emerg Infect Dis. 2016;22:1908-1914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Arendrup MC, Patterson TF. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J Infect Dis. 2017;216:S445-S451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 442] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 26. | Brooke JS. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev. 2012;25:2-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 943] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 27. | Richter SS, Heilmann KP, Dohrn CL, Riahi F, Beekmann SE, Doern GV. Changing epidemiology of antimicrobial-resistant Streptococcus pneumoniae in the United States, 2004-2005. Clin Infect Dis. 2009;48:e23-e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology. 2012;55:965-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 355] [Article Influence: 27.3] [Reference Citation Analysis (35)] |
| 29. | Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology. 2020;71:306-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 570] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 30. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2173] [Article Influence: 181.1] [Reference Citation Analysis (5)] |
| 31. | Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P, Hopf C, Alessandria C, Rodriguez E, Solis-Muñoz P, Laleman W, Trebicka J, Zeuzem S, Gustot T, Mookerjee R, Elkrief L, Soriano G, Cordoba J, Morando F, Gerbes A, Agarwal B, Samuel D, Bernardi M, Arroyo V; CANONIC study investigators of the EASL-CLIF Consortium. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 735] [Article Influence: 66.8] [Reference Citation Analysis (1)] |
| 32. | Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R, Albillos A, Lammert F, Wilmer A, Mookerjee R, Vila J, Garcia-Martinez R, Wendon J, Such J, Cordoba J, Sanyal A, Garcia-Tsao G, Arroyo V, Burroughs A, Ginès P. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 644] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 33. | Fernández J, Prado V, Trebicka J, Amoros A, Gustot T, Wiest R, Deulofeu C, Garcia E, Acevedo J, Fuhrmann V, Durand F, Sánchez C, Papp M, Caraceni P, Vargas V, Bañares R, Piano S, Janicko M, Albillos A, Alessandria C, Soriano G, Welzel TM, Laleman W, Gerbes A, De Gottardi A, Merli M, Coenraad M, Saliba F, Pavesi M, Jalan R, Ginès P, Angeli P, Arroyo V; European Foundation for the Study of Chronic Liver Failure (EF-Clif). Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol. 2019;70:398-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 247] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 34. | Arabi YM, Dara SI, Memish Z, Al Abdulkareem A, Tamim HM, Al-Shirawi N, Parrillo JE, Dodek P, Lapinsky S, Feinstein D, Wood G, Dial S, Zanotti S, Kumar A; Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Antimicrobial therapeutic determinants of outcomes from septic shock among patients with cirrhosis. Hepatology. 2012;56:2305-2315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 35. | Kim M, Cardoso FS, Pawlowski A, Wunderink R, Ladner DP, Abraldes JG, Karvellas CJ. The impact of multidrug-resistant microorganisms on critically ill patients with cirrhosis in the intensive care unit: a cohort study. Hepatol Commun. 2023;7:e0038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 36. | Belli LS, Duvoux C, Artzner T, Bernal W, Conti S, Cortesi PA, Sacleux SC, Pageaux GP, Radenne S, Trebicka J, Fernandez J, Perricone G, Piano S, Nadalin S, Morelli MC, Martini S, Polak WG, Zieniewicz K, Toso C, Berenguer M, Iegri C, Invernizzi F, Volpes R, Karam V, Adam R, Faitot F, Rabinovich L, Saliba F, Meunier L, Lesurtel M, Uschner FE, Fondevila C, Michard B, Coilly A, Meszaros M, Poinsot D, Schnitzbauer A, De Carlis LG, Fumagalli R, Angeli P, Arroyo V, Jalan R; ELITA/EF-CLIF working group. Liver transplantation for patients with acute-on-chronic liver failure (ACLF) in Europe: Results of the ELITA/EF-CLIF collaborative study (ECLIS). J Hepatol. 2021;75:610-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 37. | Sundaram V, Jalan R, Wu T, Volk ML, Asrani SK, Klein AS, Wong RJ. Factors Associated with Survival of Patients With Severe Acute-On-Chronic Liver Failure Before and After Liver Transplantation. Gastroenterology. 2019;156:1381-1391.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 249] [Article Influence: 41.5] [Reference Citation Analysis (1)] |
| 38. | Li X, Zhang L, Pu C, Tang S. Liver transplantation in Acute-on-Chronic liver failure: Timing of transplantation and selection of patient population. Front Med (Lausanne). 2022;9:1030336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 39. | Artzner T, Michard B, Weiss E, Barbier L, Noorah Z, Merle JC, Paugam-Burtz C, Francoz C, Durand F, Soubrane O, Pirani T, Theocharidou E, O'Grady J, Bernal W, Heaton N, Salamé E, Bucur P, Barraud H, Lefebvre F, Serfaty L, Besch C, Bachellier P, Schneider F, Levesque E, Faitot F. Liver transplantation for critically ill cirrhotic patients: Stratifying utility based on pretransplant factors. Am J Transplant. 2020;20:2437-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 40. | Sundaram V, Mahmud N, Perricone G, Katarey D, Wong RJ, Karvellas CJ, Fortune BE, Rahimi RS, Maddur H, Jou JH, Kriss M, Stein LL, Lee M, Jalan R; Multi-Organ Dysfunction, Evaluation for Liver Transplantation (MODEL) Consortium. Longterm Outcomes of Patients Undergoing Liver Transplantation for Acute-on-Chronic Liver Failure. Liver Transpl. 2020;26:1594-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 41. | Yang M, Peng B, Zhuang Q, Li J, Liu H, Cheng K, Ming Y. Models to predict the short-term survival of acute-on-chronic liver failure patients following liver transplantation. BMC Gastroenterol. 2022;22:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Artru F, Louvet A, Ruiz I, Levesque E, Labreuche J, Ursic-Bedoya J, Lassailly G, Dharancy S, Boleslawski E, Lebuffe G, Kipnis E, Ichai P, Coilly A, De Martin E, Antonini TM, Vibert E, Jaber S, Herrerro A, Samuel D, Duhamel A, Pageaux GP, Mathurin P, Saliba F. Liver transplantation in the most severely ill cirrhotic patients: A multicenter study in acute-on-chronic liver failure grade 3. J Hepatol. 2017;67:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 287] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 43. | Piano S, Singh V, Caraceni P, Maiwall R, Alessandria C, Fernandez J, Soares EC, Kim DJ, Kim SE, Marino M, Vorobioff J, Barea RCR, Merli M, Elkrief L, Vargas V, Krag A, Singh SP, Lesmana LA, Toledo C, Marciano S, Verhelst X, Wong F, Intagliata N, Rabinowich L, Colombato L, Kim SG, Gerbes A, Durand F, Roblero JP, Bhamidimarri KR, Boyer TD, Maevskaya M, Fassio E, Kim HS, Hwang JS, Gines P, Gadano A, Sarin SK, Angeli P; International Club of Ascites Global Study Group. Epidemiology and Effects of Bacterial Infections in Patients With Cirrhosis Worldwide. Gastroenterology. 2019;156:1368-1380.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 331] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 44. | Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis. 2003;3:685-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 584] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 45. | Monday LM, Parraga Acosta T, Alangaden G. T2Candida for the Diagnosis and Management of Invasive Candida Infections. J Fungi (Basel). 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 46. | Bartoletti M, Giannella M, Caraceni P, Domenicali M, Ambretti S, Tedeschi S, Verucchi G, Badia L, Lewis RE, Bernardi M, Viale P. Epidemiology and outcomes of bloodstream infection in patients with cirrhosis. J Hepatol. 2014;61:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 47. | Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1860] [Cited by in RCA: 2224] [Article Influence: 247.1] [Reference Citation Analysis (1)] |
| 48. | Habib S, Yarlagadda S, Carreon TA, Schader LM, Hsu CH. Fungal Infection in Acutely Decompensated Cirrhosis Patients: Value of Model for End-Stage Liver Disease Score. Gastroenterology Res. 2020;13:199-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 49. | Fernández J, Piano S, Bartoletti M, Wey EQ. Management of bacterial and fungal infections in cirrhosis: The MDRO challenge. J Hepatol. 2021;75 Suppl 1:S101-S117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 50. | Bajaj JS, O'Leary JG, Reddy KR, Wong F, Biggins SW, Patton H, Fallon MB, Garcia-Tsao G, Maliakkal B, Malik R, Subramanian RM, Thacker LR, Kamath PS; North American Consortium For The Study Of End-Stage Liver Disease (NACSELD). Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 438] [Article Influence: 39.8] [Reference Citation Analysis (1)] |
| 51. | Grim SA, Berger K, Teng C, Gupta S, Layden JE, Janda WM, Clark NM. Timing of susceptibility-based antifungal drug administration in patients with Candida bloodstream infection: correlation with outcomes. J Antimicrob Chemother. 2012;67:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |