Published online Feb 27, 2024. doi: 10.4254/wjh.v16.i2.294
Peer-review started: November 2, 2023
First decision: December 27, 2023
Revised: January 8, 2024
Accepted: February 5, 2024
Article in press: February 5, 2024
Published online: February 27, 2024
Processing time: 116 Days and 21 Hours
Oxidative stress disturbs the balance between the production of reactive oxygen species (ROS) and the detoxification biological process. It plays an important role in the development and progression of many chronic diseases. Upon exposure to oxidative stress or the inducers of ROS, the cellular nucleus undergoes some biological processes via different signaling pathways, such as stress adaption through the forkhead box O signaling pathway, inflammatory response through the IκB kinase/nuclear factor-κB signaling pathway, hypoxic response via the hypoxia-inducible factor/prolyl hydroxylase domain proteins pathway, DNA repair or apoptosis through the p53 signaling pathway, and antioxidant response through the Kelch-like ECH-associated protein 1/nuclear factor E2-related factor 2 signaling pathway. These processes are involved in many diseases. Therefore, oxidative stress has gained more attraction as a targeting process for disease treatment. Meanwhile, anti-oxidative stress agents have been widely explored in pre-clinical trials. However, only limited clinical trials are performed to evaluate the efficacy of anti-oxidative stress agents or antioxidants in diseases. In this letter, we further discuss the current clinical trials related to anti-oxidative stress treat
Core Tip: Oxidative stress disturbs the balance between the production and detoxification of reactive oxygen species, which is implicated in many diseases. Therefore, anti-oxidative stress agents have been widely explored to treat chronic and metabolic diseases. In this letter, we further discuss the current clinical trials related to anti-oxidative stress treatment and summarize current medicines under investigation.
- Citation: Zhang CY, Yang M. Anti-oxidative stress treatment and current clinical trials. World J Hepatol 2024; 16(2): 294-299
- URL: https://www.wjgnet.com/1948-5182/full/v16/i2/294.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i2.294
With great interest, we read a recently published review paper authored by Li et al[1], discussing the progress of using herbal extracts from traditional Chinese medicine as a therapeutic method to treat liver fibrosis via inhibiting oxidative stress.
We agree with the authors that oxidative stress is a critical factor that can be targeted in the treatment of liver fibrosis. Oxidative stress is caused by an imbalance between the production and accumulation of reactive oxygen species (ROS) and the biological system to detoxify ROS products[2]. The accumulation of ROS can cause cell damage through the destruction of proteins and lipids, genetic modification, and disturbance of cellular signaling[2,3]. Therefore, oxidative stress has been recognized as a crucial factor involved in the underlying mechanisms of disease development and progression[4]. In fact, oxidative stress has gained more and more attraction recently due to its important roles in many diseases, such as heart disease[5], cancer[6], hypertension[7], cardiovascular diseases[8], aging[9], neurodegenerative disease[10,11], Alzheimer's disease[12], Parkinson’s disease[13], and metabolic disorders. Moreover, oxidative stress also plays a pivotal role in organ transplantation[14] and infectious diseases[15]. Therefore, anti-oxidative stress as a therapeutic strategy has gained more attention for disease treatment.
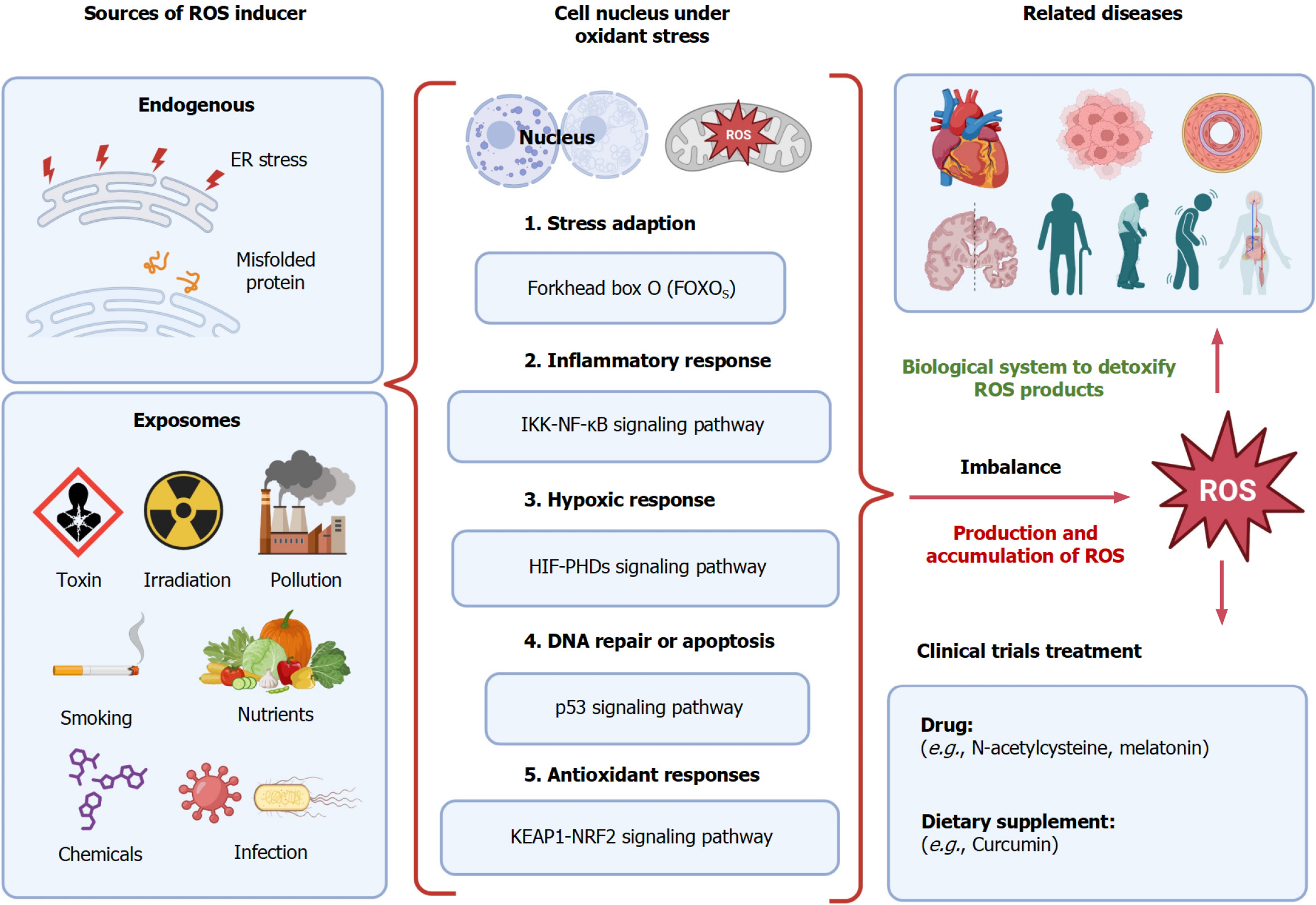
Accumulating studies are performed to decipher the mechanism of oxidative stress in disease. Oxidative stress inducers include endogenous sources and exposomes[16]. Endogenous sources can induce the endoplasmic reticulum stress that may be caused by misfolded proteins, resulting in elevated levels of ROS[17]. The exposomes include but are not limited to toxins, irradiation exposure, air pollution, smoking, nutrients, chemicals, and infection[18]. Upon exposure to oxidant sources, the cellular nucleus undergoes several biological processes (Figure 1), such as stress adaption via the forkhead box signaling pathway[19], inflammatory responses through the nuclear factor (NF)-κB and inhibitor of NF-κB kinase signaling pathway[20,21], hypoxic responses controlled by hypoxia-inducible facto-prolyl hydroxylase domain proteins[22], DNA repair or apoptosis process through the p53 signaling pathway[23], and antioxidant responses through the Kelch-like ECH-associated protein 1 (KEAP1)-transcription factor NF-E2 p45-related factor 2 (NRF2) (KEAP1-NRF2) signaling pathway[24]. The mitochondrion serves as an important organelle to generate ATP as an energy source, and ROS is also produced in this process. The accumulated excessive levels of ROS can result in oxidative stress[25]. Thus, the imbalance of the production of excessive oxidants and antioxidant processes leads to disease development and progression.
Inspired by this published review article, here, we give a further discussion on the current clinical trials that are related to anti-oxidative stress in different diseases using various intervention methods. Currently, two major categories including dietary supplement and drug treatment are used in clinical trials and summarized in this letter (Table 1). The most tested drug in these clinical trials is N-acetylcysteine with application in different diseases, such as cancer (melanoma and leukemia), pulmonary disease, renal disease, liver diseases such as non-alcoholic fatty liver disease, infectious diseases including severe acute respiratory syndrome coronavirus and human immunodeficiency virus infections, obesity, Parkinson's disease, and depressive disorders. The drug melatonin has also been used in many diseases, such as necrotizing enterocolitis, multiple sclerosis, and septic shock. Curcumin is a dietary supplement, which has been tested for renal transplantation disorder, coronary artery disease, metabolic syndrome, kidney disease, and others (Table 1). The data were collected from the website https://clinicaltrials.gov (accessed on October 28, 2023) using the keywords anti-oxidative stress, disease, and treatments such as drugs and nutrients, including ongoing and com
| NCT number | Condition(s) | Category | Intervention(s) | Phase(s) |
| NCT05511766 | Cirrhosis, hepatic encephalopathy | Drug | Allopurinol 300 mg, Atorvastatin 20 mg | 2 and 3 |
| NCT01054768 | Anemia, sickle cell | Drug | Alpha-lipoic acid and acetyl-L-carnitine | 2 |
| NCT05558878 | Diabetic peripheral neuropathy | Drug | Ambroxol oral product | NA |
| NCT00916448 | Endotoxemia, multi-organ dysfunction | Drug | Atazanavir, E. coli endotoxin | NA |
| NCT03820245 | Oxidative stress, atherosclerosis | Dietary | Bixin, norbixin, lycopene | NA |
| NCT05957432 | Helicobacter pylori infection | Drug | Black seed oil, vonoprazan, amoxicillin, clarithromycin | 2 |
| NCT03529396 | Vivax malaria, glucose-6-phosphate dehydrogenase | Drug | Chloroquine, primaquine | 2 |
| NCT03935958 | Disorder in renal transplantation | Dietary | Curcumin | NA |
| NCT04458116 | Coronary artery disease | Dietary | Curcumin | NA |
| NCT03514667 | Metabolic syndrome | Dietary | Nanomicielle curcumin | NA |
| NCT04413266 | Kidney diseases, peritoneal dialysis | Dietary | Curcumin supplementation | NA |
| NCT05966441 | Chemotherapy peripheral neuropathy | Dietary | Curcumin, paclitaxel | 2 |
| NCT06083480 | Osteoarthritis, knee arthroplasty | Drug | GlyNAC (combined glycine and N-acetylcysteine) | 4 |
| NCT01854294 | Amyotrophic lateral sclerosis | Drug | GM604 | 2 |
| NCT01891500 | Persistent fetal circulation syndrome | Drug | Inhaled nitric oxide, nitrogen Gas | 4 |
| NCT05033639 | Necrotizing enterocolitis | Drug | Melatonin 6 mg | 1 and 2 |
| NCT02463318 | Multiple sclerosis | Drug | Melatonin, hydrogen peroxide | NA |
| NCT03557229 | Septic shock | Drug | Melatonin, vitamins C and E, N-acetyl cysteine | 3 |
| NCT02587741 | Diabetic retinopathy | Drug | Metformin, lantus, Novomix30 | 1 |
| NCT01501929 | Hypertension | Drug | Metoprolol succinate, nebivolol | 4 |
| NCT05742698 | Frontotemporal dementia | Drug | Nabilone | 2 |
| NCT02294591 | Bipolar disorder | Drug | N-acetyl cysteine | 2 |
| NCT02972398 | Major depressive disorders | Drug | N-acetyl cysteine | NA |
| NCT01612221 | Risk for melanoma | Drug | N-acetyl cysteine | 2 |
| NCT05611086 | Lymphoblastic leukemia | Drug | N-acetyl cysteine | 4 |
| NCT01501110 | Ischemic heart disease | Drug | N-acetyl cysteine | 4 |
| NCT05460858 | Female infertility, endometrioma | Drug | N-acetyl cysteine | 3 |
| NCT03956888 | Chronic obstructive pulmonary disease | Drug | N-acetyl cysteine | 3 |
| NCT01907061 | Acute renal failure | Drug | N-acetyl cysteine | NA |
| NCT02124525 | Tobacco smoking, inflammation | Drug | N-acetyl cysteine | 3 |
| NCT04792021 | SARS-CoV-2 infection | Drug | N-acetyl cysteine | 3 |
| NCT04154982 | Cardiac arrhythmia | Drug | N-acetyl cysteine | 2 |
| NCT03596125 | Preterm delivery | Drug | N-acetyl cysteine | 2 and 3 |
| NCT04732000 | Surgical recovery | Drug | N-acetyl cysteine | 2 |
| NCT02252341 | Bipolar disorder | Dietary | N-acetyl cysteine | 4 |
| NCT01587001 | Pulmonary sarcoidosis | Dietary | N-acetyl cysteine | NA |
| NCT01962961 | HIV infection, endothelial dysfunction | Dietary | N-acetyl cysteine | 1 and 2 |
| NCT04440280 | Fuchs endothelial corneal dystrophy | Drug | N-acetyl cysteine solution, visine | 2 |
| NCT02117700 | Obesity, NAFLD, cardiovascular disease | Dietary | N-acetyl cysteine 600 mg | 1 and 2 |
| NCT05589584 | Steatosis, non-fatty liver | Drug | N-acetyl cysteine | 3 |
| NCT04459052 | Parkinson disease | Dietary | N-acetyl cysteine, F18 Fluorodopa | 2 |
| NCT01384591 | Aging | Drug | N-acetyl cysteine, losartan | 1 and 2 |
| NCT03056014 | Type 1 diabetes | Drug | N-acetyl cysteine, omega-6 fish oil | 1 |
| NCT04022161 | Cardiovascular, endothelial dysfunction | Drug | Nitrogen gas for inhalation, nitric oxide | 2 |
| NCT03273413 | Autosomal dominant polycystic kidney | Drug | Pravastatin | 4 |
| NCT02161653 | Severe alcoholic hepatitis | Drug | Prednisone, metadoxine, pentoxifylline | 4 |
| NCT05770297 | Endometriosis, dysmenorrhea | Dietary | Propolis | NA |
| NCT05753436 | Diabetes, dyslipidemias, hypertension | Dietary | Puritans pride turmeric curcumin | 2 |
| NCT01663103 | Renal insufficiency, chronic | Drug | Rilonacept | 4 |
| NCT01388478 | Alzheimer's disease | Drug | R-pramipexole | 2 |
| NCT03738176 | Oral lichen planus | Drug | Sesame oil, triamcinolone | 1 |
| NCT03402204 | Ischemic stroke | Drug | Simvastatin 10 mg, simvastatin 40 mg | 3 |
| NCT05145270 | Major depressive disorder | Dietary | Sulforaphane, escitalopram | 4 |
| NCT05149716 | Oxidative stress | Dietary | Taurine | NA |
In summary, oxidative stress is involved in many diseases and functions as a promising target in disease treatment and therapeutic drug screening. More potent antioxidants are expected to be explored to improve treatment outcomes. Meanwhile, the synergistic application of anti-oxidative drugs is an option to improve the therapeutic efficacy of other drugs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ebraheim LLM, Egypt S-Editor: Qu XL L-Editor: Wang TQ P-Editor: Zheng XM
| 1. | Li Z, Zhu JF, Ouyang H. Progress on traditional Chinese medicine in improving hepatic fibrosis through inhibiting oxidative stress. World J Hepatol. 2023;15:1091-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 2. | Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev. 2017;2017:8416763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1959] [Cited by in RCA: 2332] [Article Influence: 291.5] [Reference Citation Analysis (0)] |
| 3. | Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, Rajkovic J, Tsouh Fokou PV, Azzini E, Peluso I, Prakash Mishra A, Nigam M, El Rayess Y, Beyrouthy ME, Polito L, Iriti M, Martins N, Martorell M, Docea AO, Setzer WN, Calina D, Cho WC, Sharifi-Rad J. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front Physiol. 2020;11:694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1271] [Cited by in RCA: 913] [Article Influence: 182.6] [Reference Citation Analysis (0)] |
| 4. | Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 2021;20:689-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1299] [Cited by in RCA: 1452] [Article Influence: 363.0] [Reference Citation Analysis (0)] |
| 5. | Peoples JN, Saraf A, Ghazal N, Pham TT, Kwong JQ. Mitochondrial dysfunction and oxidative stress in heart disease. Exp Mol Med. 2019;51:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 584] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 6. | Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative Stress in Cancer. Cancer Cell. 2020;38:167-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1195] [Cited by in RCA: 1531] [Article Influence: 306.2] [Reference Citation Analysis (0)] |
| 7. | Griendling KK, Camargo LL, Rios FJ, Alves-Lopes R, Montezano AC, Touyz RM. Oxidative Stress and Hypertension. Circ Res. 2021;128:993-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 301] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 8. | Dubois-Deruy E, Peugnet V, Turkieh A, Pinet F. Oxidative Stress in Cardiovascular Diseases. Antioxidants (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 315] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 9. | Luo J, Mills K, le Cessie S, Noordam R, van Heemst D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res Rev. 2020;57:100982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 369] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 10. | Sumien N, Cunningham JT, Davis DL, Engelland R, Fadeyibi O, Farmer GE, Mabry S, Mensah-Kane P, Trinh OTP, Vann PH, Wilson EN, Cunningham RL. Neurodegenerative Disease: Roles for Sex, Hormones, and Oxidative Stress. Endocrinology. 2021;162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 11. | Mangrulkar SV, Wankhede NL, Kale MB, Upaganlawar AB, Taksande BG, Umekar MJ, Anwer MK, Dailah HG, Mohan S, Behl T. Mitochondrial Dysfunction as a Signaling Target for Therapeutic Intervention in Major Neurodegenerative Disease. Neurotox Res. 2023;41:708-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Li S, Xiao J, Huang C, Sun J. Identification and validation of oxidative stress and immune-related hub genes in Alzheimer's disease through bioinformatics analysis. Sci Rep. 2023;13:657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 13. | Hor SL, Teoh SL, Lim WL. Plant Polyphenols as Neuroprotective Agents in Parkinson's Disease Targeting Oxidative Stress. Curr Drug Targets. 2020;21:458-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Carcy R, Cougnon M, Poet M, Durandy M, Sicard A, Counillon L, Blondeau N, Hauet T, Tauc M, F Pisani D. Targeting oxidative stress, a crucial challenge in renal transplantation outcome. Free Radic Biol Med. 2021;169:258-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Foo J, Bellot G, Pervaiz S, Alonso S. Mitochondria-mediated oxidative stress during viral infection. Trends Microbiol. 2022;30:679-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 163] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 16. | Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21:363-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 2846] [Article Influence: 569.2] [Reference Citation Analysis (0)] |
| 17. | Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21:396-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 1005] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 18. | Iakovou E, Kourti M. A Comprehensive Overview of the Complex Role of Oxidative Stress in Aging, The Contributing Environmental Stressors and Emerging Antioxidant Therapeutic Interventions. Front Aging Neurosci. 2022;14:827900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 19. | Rodriguez-Colman MJ, Dansen TB, Burgering BMT. FOXO transcription factors as mediators of stress adaptation. Nat Rev Mol Cell Biol. 2024;25:46-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 20. | Bartolini D, Galli F. The functional interactome of GSTP: A regulatory biomolecular network at the interface with the Nrf2 adaption response to oxidative stress. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1019:29-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Zhang T, Tsutsuki H, Ono K, Akaike T, Sawa T. Antioxidative and anti-inflammatory actions of reactive cysteine persulfides. J Clin Biochem Nutr. 2021;68:5-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Martin-Puig S, Tello D, Aragonés J. Novel perspectives on the PHD-HIF oxygen sensing pathway in cardioprotection mediated by IPC and RIPC. Front Physiol. 2015;6:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Shi T, van Soest DMK, Polderman PE, Burgering BMT, Dansen TB. DNA damage and oxidant stress activate p53 through differential upstream signaling pathways. Free Radic Biol Med. 2021;172:298-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 24. | Parvez S, Long MJC, Poganik JR, Aye Y. Redox Signaling by Reactive Electrophiles and Oxidants. Chem Rev. 2018;118:8798-8888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 231] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 25. | Li X, Fang P, Mai J, Choi ET, Wang H, Yang XF. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J Hematol Oncol. 2013;6:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 453] [Cited by in RCA: 558] [Article Influence: 46.5] [Reference Citation Analysis (0)] |