Published online Feb 27, 2024. doi: 10.4254/wjh.v16.i2.286
Peer-review started: November 30, 2023
First decision: December 8, 2023
Revised: December 22, 2023
Accepted: January 16, 2024
Article in press: January 16, 2024
Published online: February 27, 2024
Processing time: 88 Days and 21.3 Hours
Chronic hepatitis C virus (HCV) infection is a major global health concern that leads to liver fibrosis, cirrhosis, and cancer. Regimens containing direct-acting antivirals (DAAs) have become the mainstay of HCV treatment, achieving a high sustained virological response (SVR) with minimal adverse events.
A 74-year-old woman with chronic HCV infection was treated with the DAAs ledipasvir, and sofosbuvir for 12 wk and achieved SVR. Twenty-four weeks after treatment completion, the liver enzyme and serum IgG levels increased, and antinuclear antibody became positive without HCV viremia, suggesting the development of autoimmune hepatitis (AIH). After liver biopsy indicated AIH, a definite AIH diagnosis was made and prednisolone was initiated. The treatment was effective, and the liver enzyme and serum IgG levels normalized. However, multiple strictures of the intrahepatic and extrahepatic bile ducts with dilatation of the peripheral bile ducts appeared on magnetic resonance cholangiopancreatography after 3 years of achieving SVR, which were consistent with primary sclerosing cholangitis.
The potential risk of developing autoimmune liver diseases after DAA treatment should be considered.
Core Tip: Direct-acting antivirals (DAAs) for chronic hepatitis C virus (HCV) infection are widely used as a safe and effective treatment intervention. Chronic HCV infection alters the innate and adaptive immune responses, both functionally and phenotypically. Rapid viral clearance following DAAs treatment restores adaptive immune function. Herein, we report a rare case of autoimmune hepatitis and primary sclerosing cholangitis that developed after DAAs treatment for HCV. The potential risk of developing autoimmune liver diseases after DAAs treatment owing to the restoration of host immunity following rapid viral clearance should be considered.
- Citation: Morihisa Y, Chung H, Towatari S, Yamashita D, Inokuma T. Autoimmune hepatitis and primary sclerosing cholangitis after direct-acting antiviral treatment for hepatitis C virus: A case report. World J Hepatol 2024; 16(2): 286-293
- URL: https://www.wjgnet.com/1948-5182/full/v16/i2/286.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i2.286
Hepatitis C virus (HCV) infection is a growing international concern because of its substantial morbidity and mortality. HCV has a worldwide prevalence of 0.7%, infecting over 56.8 million people, with approximately 1.5 million new infections every year[1]. Most patients (50%-90%) develop chronic infections and chronic liver diseases, such as cirrhosis, liver failure, and hepatocellular carcinoma[2]. Pegylated interferon alpha and ribavirin administration was the basis of the antiviral therapy for HCV; however, the frequent side effects and poor treatment outcomes were problematic[3-5]. In 2013, the first interferon-free treatment regimen was approved for the treatment of chronic hepatitis C (CHC) and several other direct-acting antivirals (DAAs) have been developed since. Such DAA regimens present excellent safety profiles and high response rates, which exceed 97% not only in clinical trials, but also in real-world clinical settings. As a result, most patients with CHC have achieved sustained virological response (SVR)[6,7].
Recently, various studies have focused on the functional changes of the immune system induced by the rapid viral clearance of DAAs after chronic HCV infections, and some reports have demonstrated the recovery of innate and adaptive immune responses after the SVR[8]. Interestingly, some case reports have described patients who developed autoimmune hepatitis (AIH) after DAA treatment for HCV, suggesting that the recovery of host immunity is associated with the development of autoimmune liver disorders.
Herein, we report a rare case of a woman with CHC who developed AIH and primary sclerosing cholangitis (PSC) after antiviral therapy with DAA.
A 74-year-old woman visited our department for the treatment of HCV infection.
She was administered a 12-wk combination regimen of sofosbuvir (SOF) and ledipasvir (LDV) and achieved SVR. Twenty-four weeks after treatment completion, the liver enzyme levels increased.
She had a history of ectopic pregnancy and had received a blood transfusion 50 years before.
She had no remarkable family history or history of autoimmune diseases. She had a history of smoking and consumed 350 mL of beer once a week.
She denied having fever or chills, malaise, or fatigue but presented discrete weight loss.
Laboratory examinations showed an undetectable serum HCV RNA; however, serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were elevated at 335 U/L and 329 U/L, respectively (Table 1). The total bilirubin level was not increased, and prothrombin time was not prolonged. The serum IgG level was increased at 3481 mg/dL. The antinuclear antibody (ANA) and antismooth muscle antibody (ASMA) titers were 1:80 in a homogeneous pattern and 1:640, respectively, whereas the antimitochondrial antibody (AMA) was negative. Notably, ANA and ASMA were negative before the start of the DAA regimen, yet the titers of both antibodies gradually increased during and after treatment (Table 2).
| Blood test | Value | Unit |
| Hematology | ||
| WBC | 59.00 | × 102/μL |
| RBC | 400.00 | × 104/μL |
| Hb | 12.30 | g/dL |
| Ht | 37.30 | % |
| PLT | 13.30 | × 104/μL |
| PT (%) | 81.90 | % |
| Serum chemistry | ||
| TP | 9.70 | g/dL |
| ALB | 3.60 | g/dL |
| T-Bil | 1.00 | mg/dL |
| AST | 418.00 | U/L |
| ALT | 366.00 | U/L |
| ALP | 371.00 | U/L |
| γ-GT | 102.00 | U/L |
| LD | 388.00 | U/L |
| BUN | 13.40 | mg/dL |
| Cre | 0.65 | mg/dL |
| CRP | 0.02 | mg/dL |
| IgA | 605.00 | mg/dL |
| IgM | 795.00 | mg/dL |
| IgG | 3481.00 | mg/dL |
| ANA | 80 | |
| ASMA | 640 | |
| AMA | < 20 | |
| HAV-IgM | Negative | |
| HEV-IgA | Negative | |
| HBs-Ag | Negative | |
| HBs-Ab | Negative | |
| HBc-Ab | Positive | |
| HBV-DNA | < 2.1 | Logcopy/mL |
| HCV-Ab | Positive | |
| HCV-RNA | < 1.2 | LogIU/mL |
| CMV-IgM | Negative | |
| CMV-IgG | Positive | |
| VCA-IgM | Negative | |
| VCA-IgG | Positive | |
| EBNA | Positive | |
| HLA | DR4 |
| Blood test | ANA | ASMA |
| Before DAAs (SOF/LDV) therapy | < 20 | < 20 |
| After DAAs (SOF/LDV) therapy | 40 | 20 |
| SVR 12 | 40 | 160 |
| SVR 24 | 80 | 640 |
Contrast-enhanced computed tomography revealed no abnormal findings at the time of exacerbation. Liver biopsy examination showed inflammatory cell infiltration mainly composed of lymphocytes and plasma cells in the portal and lobular areas and severe interface hepatitis with rosette formation (Figure 1A and B).
The definite diagnosis of AIH was made according to the International Diagnostic Criteria and Simplified Criteria for AIH, based on laboratory tests and liver biopsy findings, with scores of 18 and 8 points, respectively.
After the AIH diagnosis, a daily 20 mg (0.5 mg/kg) dose of prednisolone was started. Serum AST, ALT, and IgG levels decreased significantly and reached normal limits (Figure 2). Daily 600 mg ursodeoxycholic acid (UDCA) doses were administered along with tapering of the prednisolone dose. Remission of AIH and normalization of serum AST, ALT, and IgG levels were maintained with daily doses of prednisolone (2.5 mg) and UDCA (600 mg).
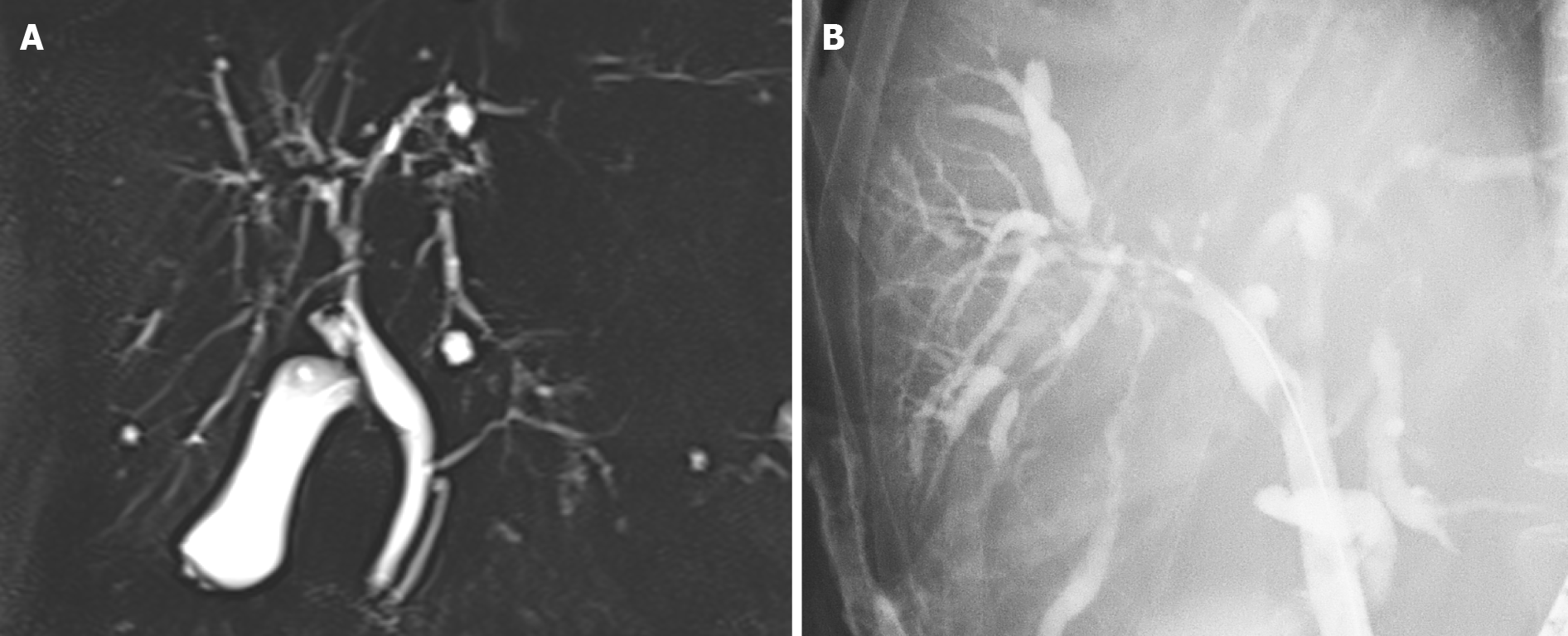
The patient was still receiving treatment with 2.5 mg prednisolone and 600 mg UDCA three years after achieving SVR. Although laboratory examinations showed no elevation of serum liver enzymes, IgG, or IgG 4, magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography revealed diffuse stenosis extending from the intrahepatic bile duct to the common hepatic duct, and dilation of the peripheral bile duct (Figure 3). A second liver biopsy was performed, which showed improvement of the interface hepatitis, yet massive infiltration of lymphocytes and plasma cells and proliferation of interlobular bile ducts in the portal triad area (Figure 1C and D). She was diagnosed with PSC based on the 2016 diagnostic criteria.
Most cases of HCV infection do not present spontaneous remission and evolve to chronic viral hepatitis, which can lead to cirrhosis and hepatocellular carcinoma. Persistent HCV infection alters innate and adaptive immune responses functionally and phenotypically, including natural killer (NK) cell dysfunction and reduced NK cell diversity[9], viral escape mutation[10], HCV-specific CD8 T cell exhaustion, increased regulatory CD4 T cells (Treg), and deletion of HCV-specific CD4 T cells[11-13]. T cell exhaustion occurs due to ongoing antigen stimulation and is characterized by the loss of effector functions and increased expression of inhibitory markers[9,14,15]. Recently, DAAs have enabled almost all patients to completely eliminate HCV and achieve SVR. The effect of DAAs on rapid viral clearance is being investigated worldwide. Several reports have revealed that DAA therapy rapidly restores some adaptive immune functions, such as relative Treg reduction and HCV-specific T-cell function recovery[16,17]. In patients with AIH, a low number of func
Mucosal-associated invariant T (MAIT) cells have recently gathered attention as possible factors associated with autoimmune disease[19]. MAIT cells are an innate-like T cell subset that comprises 5%-10% peripheral T cells and approximately 12%-50% of T cells in the liver and gastrointestinal tract[20,21]. The dominance of MAIT cells in the liver indicates their potential essential role in the pathogenesis of chronic HCV infection[22]. Among patients infected with HCV, the number and function of intrahepatic and peripheral MAIT cells were significantly reduced compared to those in healthy controls[23]. Additionally, impaired peripheral MAIT cells do not recover after successful antiviral therapy; in contrast, the number of MAIT cells in the liver increased after therapy[22]. In multiple sclerosis, an autoimmune disease, MAIT cells are reportedly reduced in the peripheral blood and can be detected in most of the cerebrospinal fluid[24]. Therefore, activation of MAIT cells in lesions may facilitate inflammation and fibrosis in autoimmune diseases.
Notably, human leukocyte antigen (HLA)-DR4 was positive in our case. Classical (type 1) AIH is strongly associated with the HLA-DR3 (HLA-DRB1*03) and HLA-DR4 (HLA-DRB1*04), whereas the type 2 disease is associated with the HLA-DRB1*07 and HLA-DRB1*03[25,26]. In Japan, where HLA-DR3 is rare, AIH is primarily associated with the HLA-DR4 serotype[27]. Genetic and environmental factors are involved in the development of AIH[28]. In our case, HLA-DR4 as a genetic factor and various levels of immune activation associated with the elimination of HCV due to DAA treatment as an environmental factor likely induced an immune response to liver autoantigens, leading to AIH onset. Further studies are required to elucidate these underlying mechanisms.
Only four cases of AIH that developed after DAA treatment have been reported in the English literature, including our case[29-31] (Table 3). All identified patients were females, with a median age of 76 years. HLA-DR4 was positive in our case; however, such was not identified in the other cases. Three cases had HCV genotype Ib and one had serotype I. Only one case had a coexisting autoimmune disease, namely, idiopathic thrombocytopenic purpura with CHC infection[31]. Although reports of HCV and AIH overlap exist, the four cases have no findings that suggested AIH before DAA treatment initiation. In our case, ANA and ASMA were negative before the start of the DAA regimen, and the titers of both antibodies gradually increased during and after treatment. Therefore, the DAA treatment is thought to have triggered the development of AIH. Three cases were treated with SOF/LDV[30,31], and one case was treated with elbasvir and grazoprevir[29]. Laboratory examinations showed an increase in ANA in all cases and an increase in ASMA in two cases[30]. IgG levels were elevated in three cases[29,30]. All patients underwent liver biopsy, which revealed interface hepatitis and infiltration of various inflammatory cells, including plasma cells, in the portal zonal areas. Serological and histological findings suggested the development of AIH. All patients received prednisolone, which led to improvements in serum AST, ALT, and IgG levels. Furthermore, serum HCV RNA was continuously undetectable in all cases. Only in our case, PSC was diagnosed 3 years after prednisolone treatment. Despite the small number of cases, immunosuppressive therapy is likely to be effective when AIH develops after HCV treatment.
| Ref. | Age | Sex | HLA-DR4 | Autoimmune disease | Genotype | DAA | Onset | Treatment | Follow up |
| Matsumoto et al[29], 2018 | 81 | Female | Negative | None | 1 (serotype) | EBR/GZR | 2 months | PSL | Improvement |
| Covini et al[31], 2018 | 72 | Female | NA | ITP | 1b | SOF/LDV | 2 wk | PSL | Improvement |
| Montón et al[30], 2020 | 72 | Female | NA | None | 1b | SOF/LDV | 3 yr | PSL | Improvement |
| Our case | 80 | Female | Positive | None | 1b | SOF/LDV | 9 months | PSL + UDCA | Development of PSC |
Our case suggested that AIH and PSC development may be attributed to the rapid changes in immune function induced by DAA treatment. However, it was not possible to directly elucidate the mechanism underlying the association in this report. Therefore, accumulation of similar cases to clarify the mechanism is required.
Studies on antiviral therapy for HCV, highlighting the safety and effectiveness of DAA regimens, have reported sporadic episodes of adverse effects associated with immune dysregulation. We encountered a rare case of AIH with PSC that developed after DAA treatment. The potential risk of developing autoimmune liver diseases after DAA treatment owing to the restoration of host immunity associated with rapid viral clearance should be considered. Further studies are necessary to clarify the frequency and mechanism of autoimmune liver diseases following DAA treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mahmoud MZ, Saudi Arabia S-Editor: Chen YL L-Editor: A P-Editor: Yuan YY
| 1. | Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7:396-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 359] [Article Influence: 119.7] [Reference Citation Analysis (1)] |
| 2. | European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1281] [Cited by in RCA: 1212] [Article Influence: 173.1] [Reference Citation Analysis (0)] |
| 3. | Pawlotsky JM, Feld JJ, Zeuzem S, Hoofnagle JH. From non-A, non-B hepatitis to hepatitis C virus cure. J Hepatol. 2015;62:S87-S99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 245] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 4. | Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 491] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 5. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 919] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 6. | Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, George J, Dore GJ. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 381] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 7. | Herzer K, Gerken G, Kroy D, Tacke F, Plewe J, Eurich D, Spengler U, Strassburg CP, Welker MW, Pischke S, Sterneck M, Mehrabi A, Weiss KH, Herber A, Berg T, Zimmermann T, Galle PR, Heinzow H, Schmidt H, Markova A, Serfert Y, Manns MP, Zeuzem S, Wedemeyer H. Impact of direct-acting antiviral therapy on the need for liver transplantation related to hepatitis C in Germany. J Hepatol. 2018;69:982-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Wedemeyer H, Khera T, Strunz B, Björkström NK. Reversal of Immunity After Clearance of Chronic HCV Infection-All Reset? Front Immunol. 2020;11:571166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Björkström NK, Strunz B, Ljunggren HG. Natural killer cells in antiviral immunity. Nat Rev Immunol. 2022;22:112-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 285] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 10. | Salimi Alizei E, Hofmann M, Thimme R, Neumann-Haefelin C. Mutational escape from cellular immunity in viral hepatitis: variations on a theme. Curr Opin Virol. 2021;50:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Shoukry NH. Hepatitis C Vaccines, Antibodies, and T Cells. Front Immunol. 2018;9:1480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Shuai Z, Leung MW, He X, Zhang W, Yang G, Leung PS, Eric Gershwin M. Adaptive immunity in the liver. Cell Mol Immunol. 2016;13:354-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Kubes P, Jenne C. Immune Responses in the Liver. Annu Rev Immunol. 2018;36:247-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 601] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 14. | Utzschneider DT, Legat A, Fuertes Marraco SA, Carrié L, Luescher I, Speiser DE, Zehn D. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat Immunol. 2013;14:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 225] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 15. | Zheng K, Zheng X, Yang W. The Role of Metabolic Dysfunction in T-Cell Exhaustion During Chronic Viral Infection. Front Immunol. 2022;13:843242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Luxenburger H, Neumann-Haefelin C, Thimme R, Boettler T. HCV-Specific T Cell Responses During and After Chronic HCV Infection. Viruses. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Ghosh A, Romani S, Kottilil S, Poonia B. Lymphocyte Landscape after Chronic Hepatitis C Virus (HCV) Cure: The New Normal. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Lapierre P, Lamarre A. Regulatory T Cells in Autoimmune and Viral Chronic Hepatitis. J Immunol Res. 2015;2015:479703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Toubal A, Nel I, Lotersztajn S, Lehuen A. Mucosal-associated invariant T cells and disease. Nat Rev Immunol. 2019;19:643-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 20. | Franciszkiewicz K, Salou M, Legoux F, Zhou Q, Cui Y, Bessoles S, Lantz O. MHC class I-related molecule, MR1, and mucosal-associated invariant T cells. Immunol Rev. 2016;272:120-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 591] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 22. | Bolte FJ, O'Keefe AC, Webb LM, Serti E, Rivera E, Liang TJ, Ghany M, Rehermann B. Intra-Hepatic Depletion of Mucosal-Associated Invariant T Cells in Hepatitis C Virus-Induced Liver Inflammation. Gastroenterology. 2017;153:1392-1403.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 23. | Beudeker BJB, van Oord GW, Arends JE, Schulze Zur Wiesch J, van der Heide MS, de Knegt RJ, Verbon A, Boonstra A, Claassen MAA. Mucosal-associated invariant T-cell frequency and function in blood and liver of HCV mono- and HCV/HIV co-infected patients with advanced fibrosis. Liver Int. 2018;38:458-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Miyazaki Y, Miyake S, Chiba A, Lantz O, Yamamura T. Mucosal-associated invariant T cells regulate Th1 response in multiple sclerosis. Int Immunol. 2011;23:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 25. | Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Autoimmmune hepatitis. Cell Mol Immunol. 2022;19:158-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 26. | Bittencourt PL, Goldberg AC, Cançado EL, Porta G, Laudanna AA, Kalil J. Different HLA profiles confer susceptibility to autoimmune hepatitis type 1 and 2. Am J Gastroenterol. 1998;93:1394-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Furumoto Y, Asano T, Sugita T, Abe H, Chuganji Y, Fujiki K, Sakata A, Aizawa Y. Evaluation of the role of HLA-DR antigens in Japanese type 1 autoimmune hepatitis. BMC Gastroenterol. 2015;15:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Chen J, Eslick GD, Weltman M. Systematic review with meta-analysis: clinical manifestations and management of autoimmune hepatitis in the elderly. Aliment Pharmacol Ther. 2014;39:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Matsumoto K, Kikuchi K, Kajiyama Y, Takano Y, Mabuchi M, Doi S, Sato K, Miyakawa H, Yasuda I. Development of Autoimmune Hepatitis during Direct-acting Antiviral Therapy for Chronic Hepatitis C Virus Infection. Intern Med. 2018;57:2669-2673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Montón C, Escudero MªD, Pascual I. The development of type-1 autoimmune hepatitis after chronic hepatitis C (HCV) clearance by direct-acting antivirals (DAA). Rev Esp Enferm Dig. 2020;112:664-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Covini G, Bredi E, Badalamenti S, Roncalli M, Aghemo A, Colombo M. Autoimmune Hepatitis During Ledipasvir/Sofosbuvir Treatment of Hepatitis C: A Case Report. Hepatol Commun. 2018;2:1179-1183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |