Published online Feb 27, 2024. doi: 10.4254/wjh.v16.i2.264
Peer-review started: October 18, 2023
First decision: December 26, 2023
Revised: January 9, 2024
Accepted: February 1, 2024
Article in press: February 1, 2024
Published online: February 27, 2024
Processing time: 132 Days and 2.2 Hours
Liver fibrosis is a formidable global medical challenge, with no effective clinical treatment currently available. Yinhuang granule (YHG) is a proprietary Chinese medicine comprising Scutellariae Radix and Lonicerae Japonicae Flos. It is frequently used for upper respiratory tract infections, pharyngitis, as well as acute and chronic tonsillitis.
To investigate the potential of YHG in alleviating carbon tetrachloride (CCl4)-induced liver fibrosis in mice.
To induce a hepatic fibrosis model in mice, this study involved intraperitoneal injections of 2 mL/kg of CCl4 twice a week for 4 wk. Meanwhile, liver fibrosis mice in the low dose of YHG (0.4 g/kg) and high dose of YHG (0.8 g/kg) groups were orally administered YHG once a day for 4 wk. Serum alanine/aspartate aminotransferase (ALT/AST) activity and liver hydroxyproline content were detected. Sirius red and Masson's trichrome staining assay were conducted. Real-time polymerase chain reaction, western-blot and enzyme-linked immunosorbent assay were conducted. Liver glutathione content, superoxide dismutase activity level, reactive oxygen species and protein carbonylation amount were detected.
The administration of YHG ameliorated hepatocellular injury in CCl4-treated mice, as reflected by decreased serum ALT/AST activity and improved liver histological evaluation. YHG also attenuated liver fibrosis, evident through reduced liver hydroxyproline content, improvements in Sirius red and Masson's trichrome staining, and lowered serum hyaluronic acid levels. Furthermore, YHG hindered the activation of hepatic stellate cells (HSCs) and ameliorated oxidative stress injury and inflammation in liver from CCl4-treated mice. YHG prompted the nuclear accumulation of nuclear factor erythroid 2-related factor 2 (Nrf2) and upregulated the expression of Nrf2-dependent downstream antioxidant genes. In addition, YHG promoted mitochondrial biogenesis in liver from CCl4-treated mice, as demonstrated by increased liver adenosine triphosphate content, mitochondrial DNA levels, and the expression of peroxisome proliferator-activated receptor gamma coactivator 1 alpha and nuclear respiratory factor 1.
YHG effectively attenuates CCl4-induced liver fibrosis in mice by inhibiting the activation of HSCs, reducing inflammation, alleviating liver oxidative stress damage through Nrf2 activation, and promoting liver mitochondrial biogenesis.
Core Tip: Yinhuang granule (YHG), a Chinese patent medicine comprising Scutellariae Radix and Lonicerae Japonicae Flos, is traditionally employed for the management of tonsillitis, pharyngitis, as well as upper respiratory tract infections in clinical practice. Here, our study found that YHG effectively alleviated liver fibrosis in carbon tetrachloride-treated mice through various mechanisms, including the inhibition of hepatic stellate cells activation, reduction of inflammation, alleviation of liver oxidative stress damage by prompting nuclear factor erythroid 2-related factor 2 activation, and promotion of liver mitochondrial biogenesis. These findings substantiate the potential clinical use of YHG as a therapy for liver fibrosis.
- Citation: Ouyang H, Miao H, Li Z, Wu D, Gao SC, Dai YY, Gao XD, Chai HS, Hu WY, Zhu JF. Yinhuang granule alleviates carbon tetrachloride-induced liver fibrosis in mice and its mechanism. World J Hepatol 2024; 16(2): 264-278
- URL: https://www.wjgnet.com/1948-5182/full/v16/i2/264.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i2.264
Liver fibrosis is a complex process of continuous hepatic injury and subsequent tissue repair in response to various types of chronic liver insults, resulting in the pathological accumulation of extracellular matrix (ECM) components within the hepatic microenvironment[1,2]. In the absence of timely intervention, the relentless cycle of liver injury and futile regeneration persists, ultimately leading to the gradual progression of liver fibrosis into advanced cirrhosis and the potential development of hepatocellular carcinoma[1,2]. Notably, liver fibrosis can arise from diverse etiologies, encompassing viral hepatitis, alcoholic liver disease, non-alcoholic steatohepatitis, cholestasis, autoimmune hepatitis, etc[3,4]. Epidemiological data suggest that liver fibrosis affects approximately 18.0%-27.0% of individuals afflicted by various chronic liver diseases[5]. Indeed, liver fibrosis represents a significant global health concern, underscored by the current absence of an efficacious pharmaceutical intervention in clinical practice.
A plethora of studies have underscored the pivotal role of hepatic stellate cells (HSCs) activation in the progression of liver fibrosis[1,6,7]. Activated HSCs manifest an exuberant production of diverse ECMs including fibronectin, proteo
With the continuous deepening of research, there is increasing evidence that numerous traditional Chinese patent medicines, natural products and ingredients have demonstrated efficacy in effectively ameliorating liver injury and treating liver dieaseas[12-19]. Yinhuang granule (YHG) is a Chinese patent medicine comprising Scutellariae Radix and Lonicerae Japonicae Flos. Previous study has demonstrated the potential hepatoprotective effects of the individual components of YHG, with the water extract of Lonicerae Japonicae Flos ameliorating liver fibrosis in CCl4-treated mice, and the methanol extract of Scutellariae Radix inhibiting liver fibrosis induced by bile duct ligation or CCl4 in rats[17,18]. Additionally, baicalin and chlorogenic acid, the primary bioactive compounds within YHG, have also exhibited pro
YHG was provided by Prof. Lili Ji, Institute of Chinese Medicine, Shanghai University of Traditional Chinese Medicine. The reagents used in this study are listed in Table 1.
| Reagents | Company |
| Anti-Lamin B1 | Hangzhou Hua-An Biotechnology Co., Ltd. (Hangzhou, China) |
| Anti-α-SMA | Cell Signaling Technology (Danvers, MA, United States) |
| Anti-NRF1 | Cell Signaling Technology (Danvers, MA, United States) |
| Anti-β-actin | Cell Signaling Technology (Danvers, MA, United States) |
| Anti-Nrf2 | Gene Tex Inc. (Alton Parkway lrvine, CA, United States) |
| Anti-GCLC | Santa Cruz (Santa Cruz, CA, United States) |
| Anti-NQO1 | Abways Technology, Inc. (Shanghai, China) |
| Anti-GCLM | Abways Technology, Inc. (Shanghai, China) |
| Anti-PGC1 | Abcam (Shanghai, China) |
| Kits for detecting ALT/AST activity | Nanjing Jiancheng Bioengineering Institute (Nanjing, China) |
| Kits for detecting liver hydroxyproline content | Nanjing Jiancheng Bioengineering Institute (Nanjing, China) |
| Kits for detecting hepatic GSH | Nanjing Jiancheng Bioengineering Institute (Nanjing, China) |
| Kits for detecting protein carbonylation amount | Nanjing Jiancheng Bioengineering Institute (Nanjing, China) |
| Kits for detecting SOD activity | Nanjing Jiancheng Bioengineering Institute (Nanjing, China) |
| ATP content | Beyotime Biotech (Shanghai, China) |
| ELISA kits | RapidBio (West Hills, CA, United States) |
| mitochondrial DNA extraction | Sangon Biotech (Shanghai, China) |
| NE-PER nuclear and cytoplasmic extraction reagents | Thermo Fisher Scientific (Waltham, MA, United States) |
| BCA Protein Assay Kits | Thermo Fisher Scientific (Waltham, MA, United States) |
| PrimeScript Master Mix | Takara (Shiga, Japan) |
| SYBR Premix Ex Taq | Takara (Shiga, Japan) |
| Trizol reagent | Life Technology (Carlsbad, CA, United States) |
| 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA) | Life Technology (Carlsbad, CA, United States) |
SPF male C57BL/6 mice (20 ± 2 g), obtained from the Shanghai Experimental Animal Center of Chinese Academy of Sciences, were kept at a controlled environment, and received humane care following the institutional animal care guidelines approved by the Experimental Animal Ethical Committee of Shanghai University of Traditional Chinese Medicine (Approval No. PZSHUTCM190912010).
Mice were divided into 5 groups (n = 6 per group) including control group, CCl4 model group, CCl4+YHG (0.4 g/kg) group, CCl4+YHG (0.8 g/kg) group, YHG (0.8 g/kg) group. YHG (dissolved in 0.5% CMC-Na solution) was orally administered to mice every day, and CCl4 (mixed 1:3 in olive oil, 2 mL/kg) was i.p. injected into mice twice a week for a total of 4 wk. The selection of the CCl4 dose followed a previous study[24]. Following the treatment period, the mice were euthanized, and samples were collected for subsequent analysis.
Liver samples were sectioned and stained with H&E, Sirius red and Masson's trichrome for histological evaluation of liver injury and hepatic collagen deposition.
We performed these experiments following the manufacturer’s instructions.
Hepatic reactive oxygen species (ROS) level was measured previously described[25].
Mitochondrial DNA was extracted following the manufacturer’s instruction.
Real-time polymerase chain reaction was performed as previously described[25]. The primer sequences are shown in Table 2.
| Target | Primer | Sequence (5'-3') |
| Col1a1 | FP | TGACTGGAAGAGCGGAGAGT |
| RP | GACGGCTGAGTAGGGAACAC | |
| Col3a1 | FP | ATGGGTTTCCCTGGTCCTAA |
| RP | TGCCTTGTAATCCTTGTGGA | |
| Fn1 | FP | AGAACCAGAGGAGGCACAAG |
| RP | CCGTGTAAGGGTCAAAGCAT | |
| Tgfb1 | FP | TGCCCTCTACAACCAACACA |
| RP | GTTGGACAACTGCTCCACCT | |
| Acta2 | FP | GGGAGTAATGGTTGGAATGG |
| RP | GGTGATGATGCCGTGTTCTA | |
| TNFα | FP | AGGCACTCCCCCAAAAGAT |
| RP | CAGTAGACAGAAGAGCGTGGTG | |
| IL-1β | FP | AGTTGACGGACCCCAAAAG |
| RP | CTTCTCCACAGCCACAATGA | |
| IL-6 | FP | ACAAAGCCAGAGTCCTTCAGAGAG |
| RP | TTGGATGGTCTTGGTCCTTAGCC | |
| iNOS | FP | CAGGCGGTGCCTATGTCTC |
| RP | CAGCTGGGCTGTACAAACCTT | |
| Nqo1 | FP | CTCGTGGAGACGCTTTACAT |
| RP | CGTTTCTTCCATCCTTCCAG | |
| Gclc | FP | CGGAGGAACGATGTCTGAGT |
| RP | CTGGGGAATGAAGTGATGGT | |
| Gclm | FP | CAATGACCCGAAAGAACTGC |
| RP | CAATGACCCGAAAGAACTGC | |
| Actin | FP | TTCGTTGCCGGTCCACACCC |
| RP | GCTTTGCACATGCCGGAGCC | |
| 18s | FP | CGCGGTTCTATTTTGTTGGT |
| RP | AGTCGGCATCGTTTATGGTC | |
| ND1 | FP | CTAGCAGAAACAAACCGGGC |
| RP | CCGGCTGCGTATTCTACGTT |
Western-blot was detected as previously described[25]. The quantification of protein bands was standardized by calculating the average ratio of integrated optical density. Internal controls such as β-actin or Lamin B1 expression were used for normalization, and further standardized to the control group.
The data is presented as the mean ± SEM. Group differences were assessed using non-parametric one-way The Analysis of Variance (ANOVA), followed by the least significant difference post hoc test when ANOVA indicated a significant F-value and homogeneity of variance. In cases where homogeneity of variance was not met, the Mann-Whitney U non-parametric ANOVA was employed. Statistical significance was set at P < 0.05.
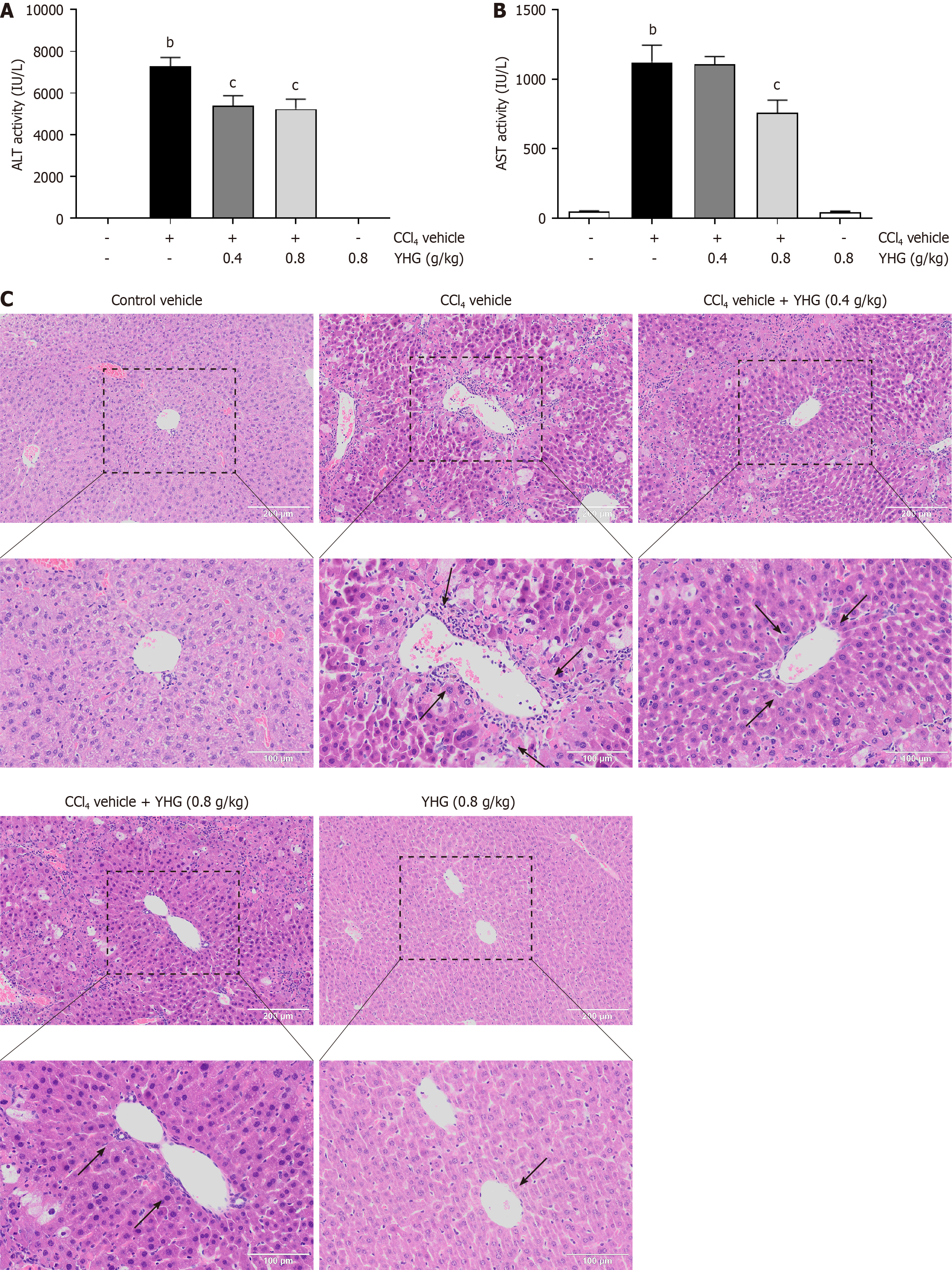
As depicted in Figure 1A, YHG (0.4, 0.8 g/kg) effectively decreased the elevated serum alanine aminotransferase (ALT) activity in CCl4-treated mice. Furthermore, YHG at a dosage of 0.8 g/kg also effectively decreased the elevated serum aspartate aminotransferase (AST) activity in CCl4-treated mice (Figure 1B). Notably, YHG (0.8 g/kg) did not exert any impact on ALT or AST activity alone (Figure 1A and B). Evaluation of liver histology unveiled that CCl4 administration induced obvious liver injury in mice, which was characterized by immune cell infiltration, as well as hepatocyte swelling and necrosis (Figure 1C). However, YHG (0.4, 0.8 g/kg) effectively alleviated these pathological changes.
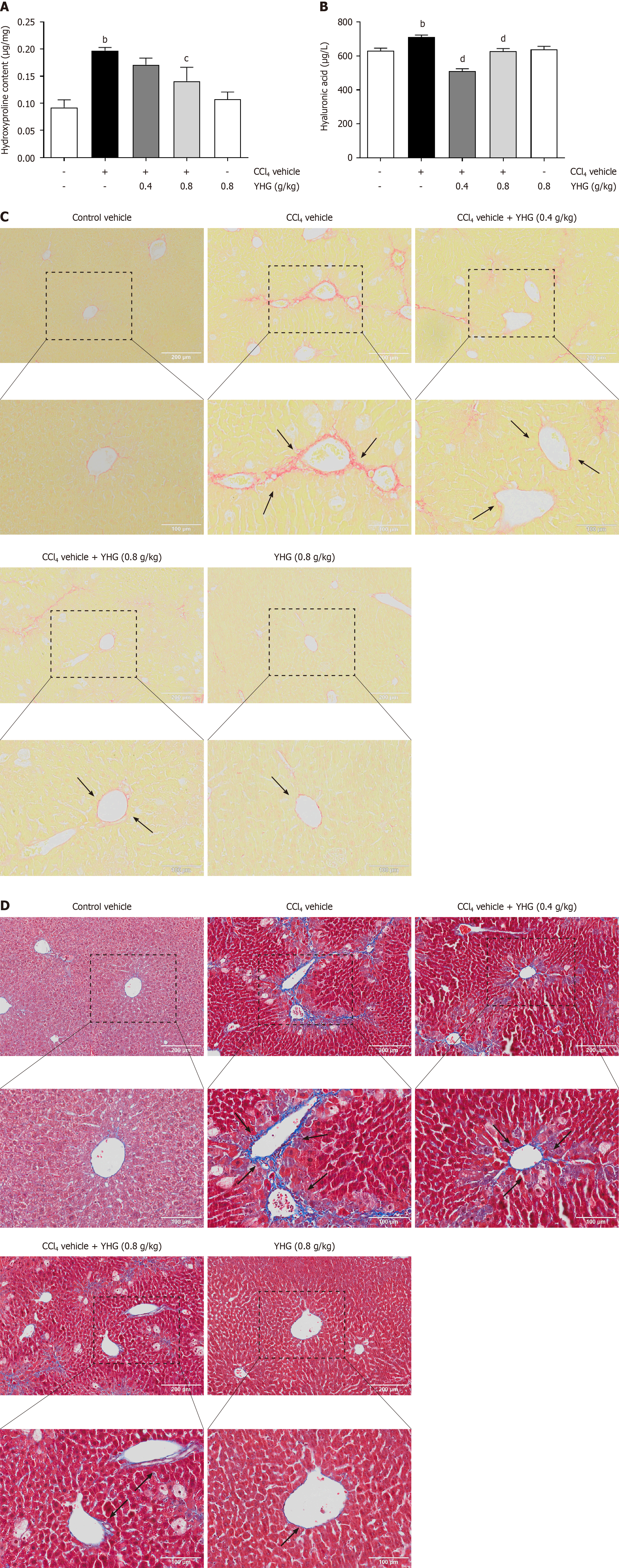
As shown in Figure 2A, YHG (0.8 g/kg) decreased the increased hydroxyproline content in liver of CCl4-treated mice. Additionally, YHG (0.4, 0.8 g/kg) significantly reduced the increased serum hyaluronic acid levels induced by CCl4 (Figure 2B). YHG (0.8 g/kg) alone did not affect liver hydroxyproline content or serum hyaluronic acid levels (Figure 2A and B). Furthermore, as depicted in Figure 2C and D, the treatment with YHG (0.4, 0.8 g/kg) effectively decreased hepatic collagen deposition in CCl4-treated mice. It’s worth noting that YHG (0.8 g/kg) alone did not induce any significant changes in the staining patterns, as demonstrated by Masson's trichrome staining and Sirius red staining.
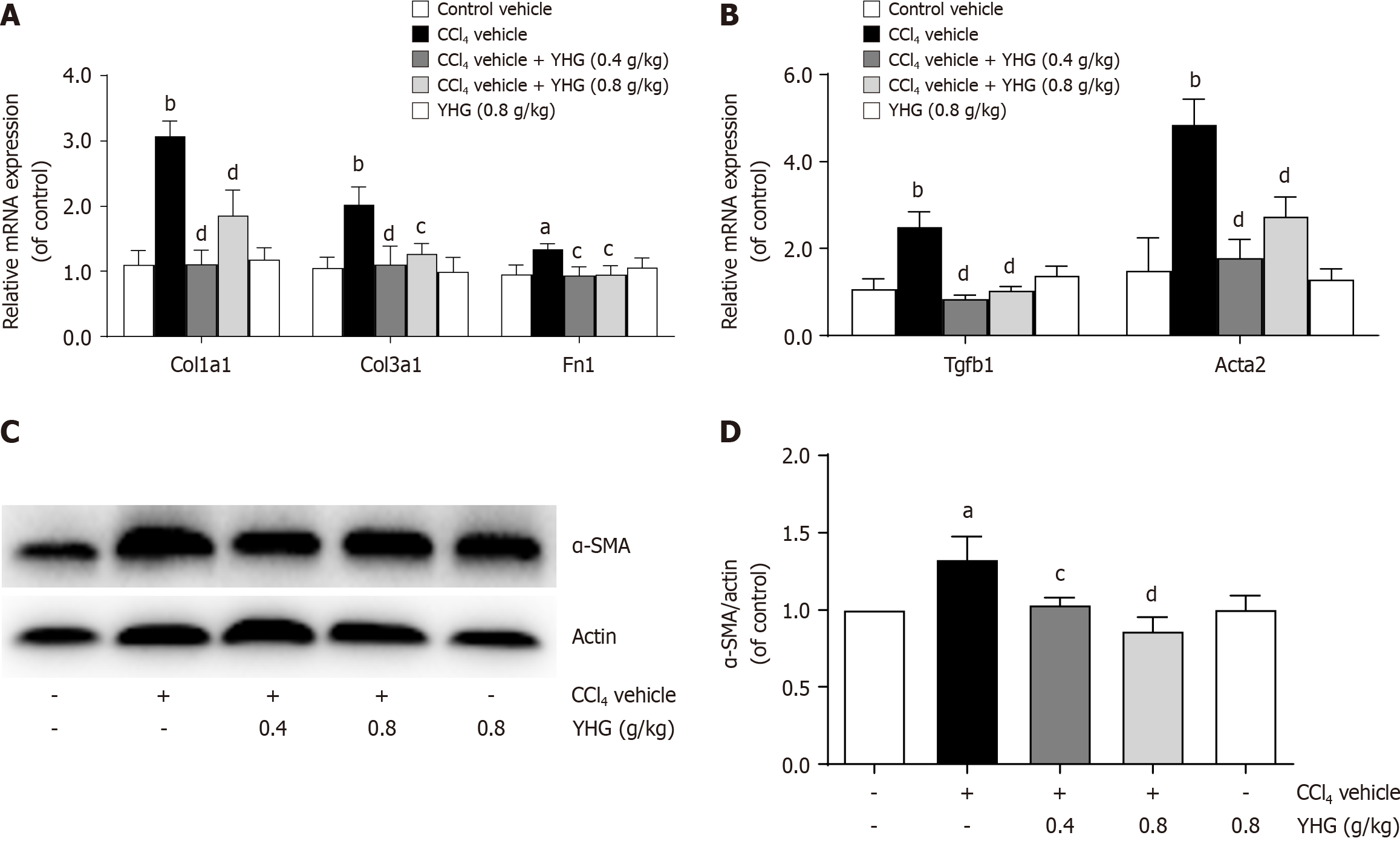
Figure 3A illustrated that YHG (0.4, 0.8 g/kg) significantly reduced the enhanced hepatic of Col1a1, Col3a1, and fibronectin (Fn1) mRNA expression in CCl4-treated mice. Additionally, YHG (0.4, 0.8 g/kg) significantly attenuated the increased hepatic mRNA expression of transforming growth factor (TGF)-β in CCl4-induced mice (Figure 3B). The typical biomarker for HSCs activation, alpha-smooth muscle actin (α-SMA), showed reduced hepatic mRNA and protein expression upon treatment with YHG (0.4, 0.8 g/kg) in CCl4-treated mice (Figure 3B-D).
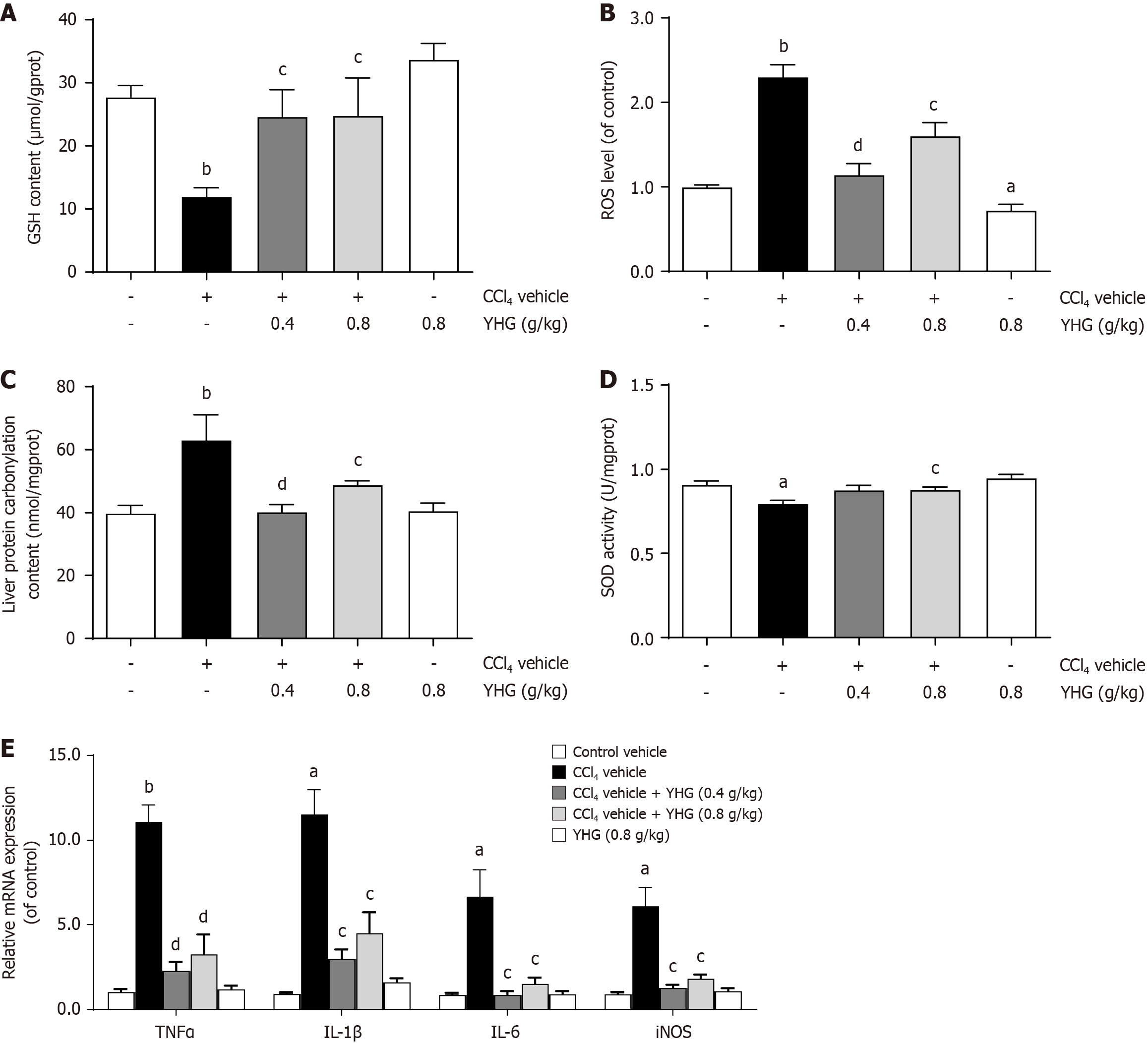
As demonstrated in Figure 4A, CCl4 caused a decline in hepatic glutathione (GSH) content in mice, which was reversed by YHG (0.4, 0.8 g/kg). Furthermore, as depicted in Figure 4B and C, YHG (0.4, 0.8 g/kg) effectively reduced the increased levels of hepatic ROS and liver protein carbonylation in CCl4-induced mice. Moreover, CCl4 decreased hepatic superoxide dismutase (SOD) activity in mice, which was restored by YHG (0.8 g/kg) (Figure 4D). Additionally, Figure 4E shows that YHG (0.4, 0.8 g/kg) suppressed the hepatic mRNA expression of tumour necrosis factor alpha (TNFα), interleukin (IL)-1β, IL-6, and inducible nitric oxide synthase (iNOS) in mice treated with CCl4.
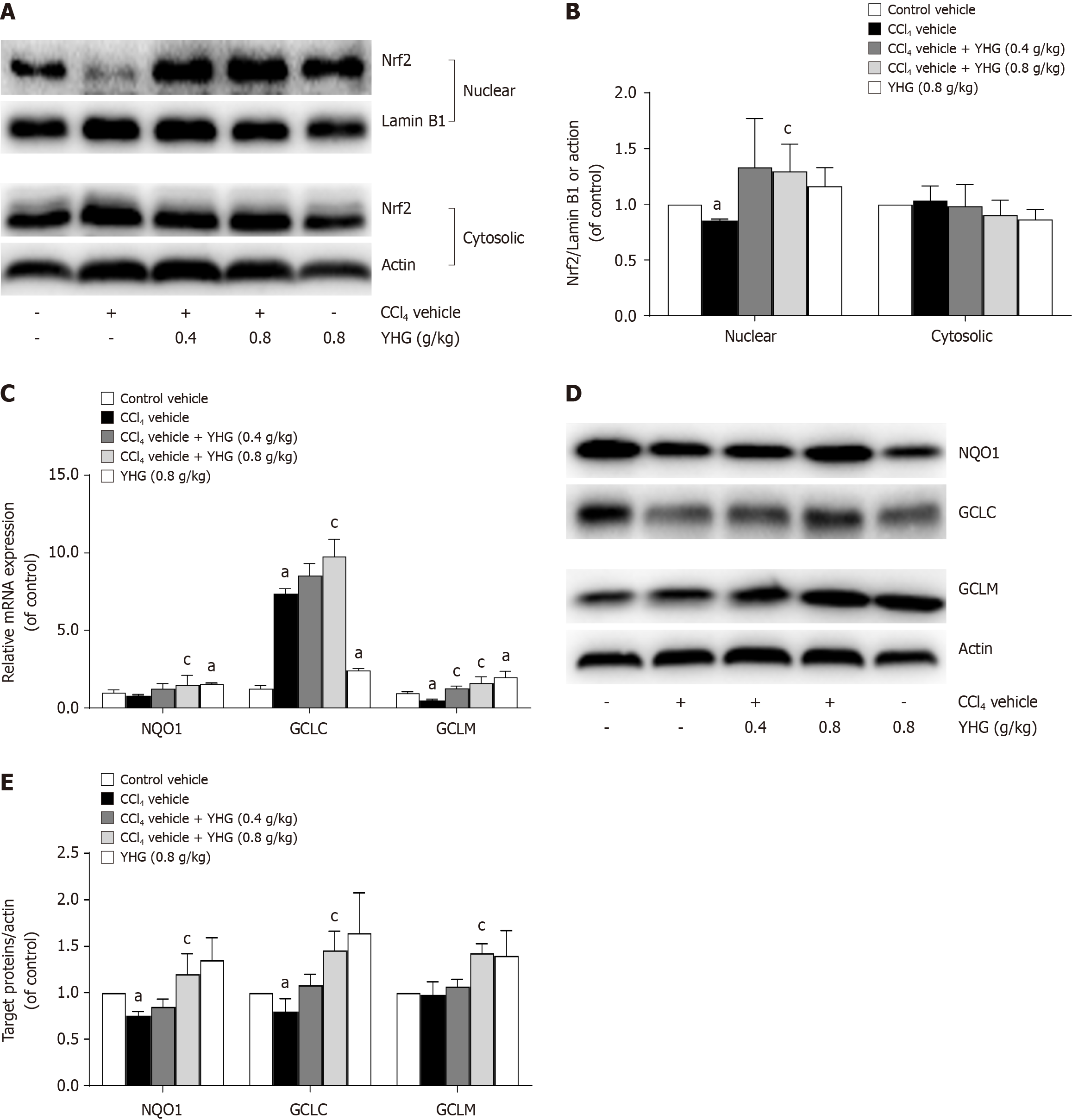
As demonstrated in Figure 5A and B, YHG (0.8 g/kg) promoted the nuclear accumulation of nuclear factor erythroid 2-related factor 2 (Nrf2) in livers from mice exposed to CCl4. Additionally, YHG (0.8 g/kg) increased hepatic mRNA expression of glutamate-cysteine ligase (GCLC), modifier subunit of glutamate-cysteine ligase (GCLM) and NAD(P)H:quinone oxidoreductase-1 (NQO1). Furthermore, YHG (0.4 g/kg) also elevated mRNA expression of GCLM in livers of mice exposed to CCl4 (Figure 5C). Notably, YHG (0.8 g/kg) increased the hepatic protein expression of GCLC, GCLM, and NQO1 in livers of mice exposed to CCl4 (Figure 5D and E).
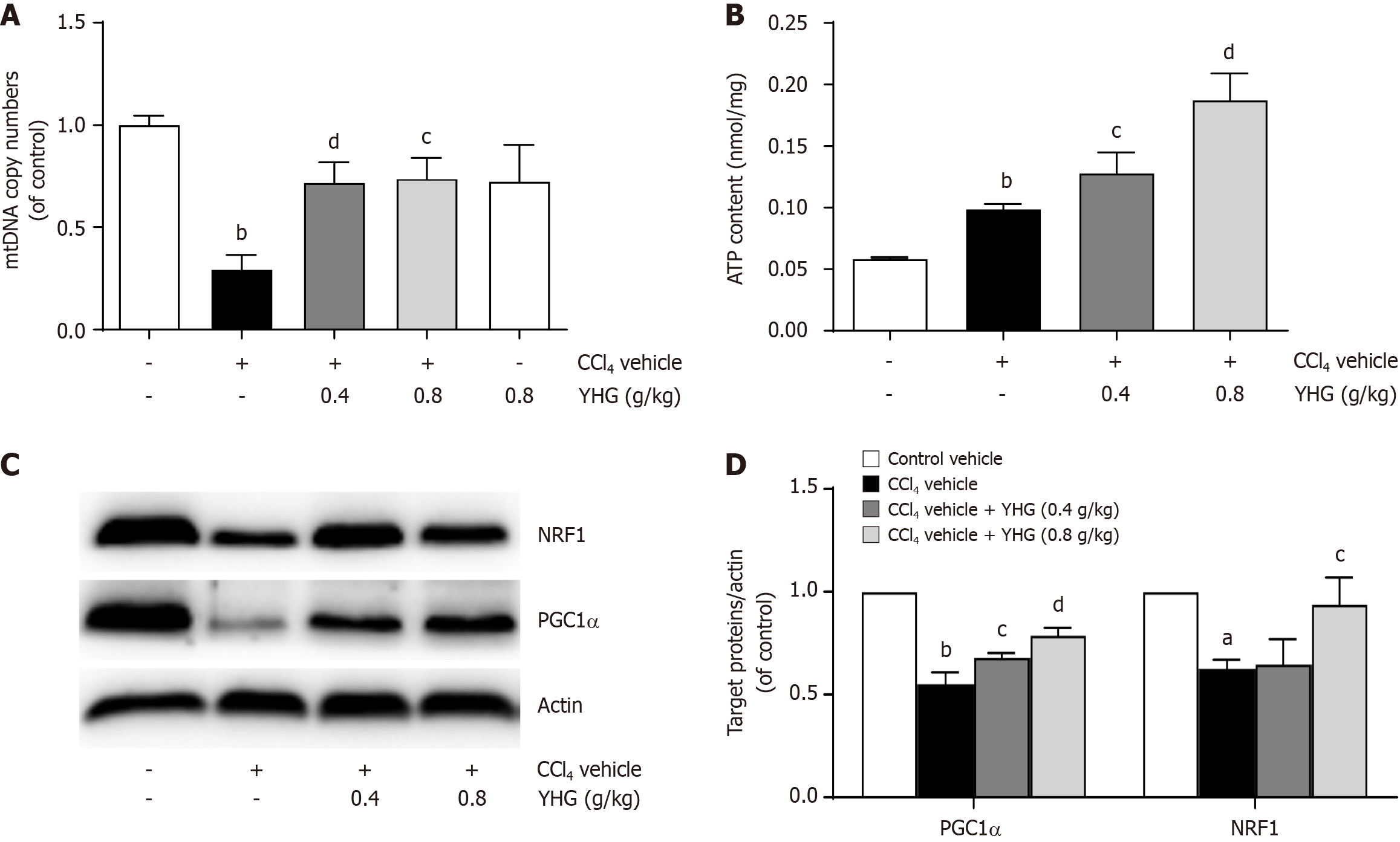
As depicted in Figure 6A, YHG (0.4, 0.8 g/kg) obviously elevated the decreased expression of hepatic mitochondrial DNA (mtDNA) copy in liver from mice exposed to CCl4. Additionally, YHG (0.4, 0.8 g/kg) significantly increased adenosine triphosphate (ATP) content in liver from CCl4-treated mice (Figure 6B). Furthermore, YHG (0.4, 0.8 g/kg) elevated the reduced hepatic expression of peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PGC1α) protein, while YHG (0.8 g/kg) enhanced the decreased expression of nuclear respiratory factor1 (NRF1) protein in livers from CCl4-induced mice (Figure 6C and D).
YHG has excellent anti-inflammatory capacity and is generally used in clinic for clearing hotness and wind, and pharyngeal detoxification. In this study, YHG was demonstrated to alleviate hepatocellular injury, hepatic collagen deposition, and inflammation in CCl4-treated mice. It also showed inhibitory effects on HSCs, as evidenced by the reduction in the elevated hepatic expression of α-SMA, a key indicating HSCs transdifferentiation and activation[26]. The enhanced expression of ECM components including Col1a1, Col3a1, and Fn1 in the livers of CCl4-treated mice was decreased by YHG. Furthermore, YHG reduced the elevated expression of TGFβ, a predominant pro-fibrogenic molecule[27], in the livers of CCl4-treated mice. These findings collectively highlight the immense potential of YHG in the clinical treatment of liver fibrosis.
Recent studies have discovered novel pathways and signals that play significant roles in regulating the activation of HSCs during the progression of liver fibrosis, including oxidative stress and inflammatory responses[28]. Oxidative stress is characterized by an imbalance between the production of ROS and the antioxidant system's ability to scavenge these harmful molecules. Free radicals generated during oxidative stress have been shown to induce the activation and proliferation of HSCs[29,30]. In this study, YHG was found to reduce the elevated hepatic levels of ROS and protein carbonylation, as well as restore the diminished hepatic GSH content and SOD activity in mice treated with CCl4. Furthermore, YHG was found to reduce the elevated hepatic expression of pro-inflammatory cytokines such as TNFα, IL-1β, IL-6, and iNOS. These findings collectively suggest that YHG has the ability to alleviate hepatic oxidative stress injury and inflammatory response in CCl4-treated mice, which may contribute to its potential in alleviating CCl4-induced liver fibrosis in mice.
Nrf2 serves as the principal transcription factor that plays a crucial role in regulating the expression of various downstream antioxidant enzymes and cytoprotective genes[31]. Numerous studies have demonstrated that enhancing Nrf2 activation to combat liver oxidative stress injury is crucial for alleviating liver fibrosis, as observed with various natural compounds such as schisandrin B, asiatic acid, Xiaochaihutang, stevia, tanshinol, and hyperoside[32-37]. In CCl4-treated mouse livers, the nuclear accumulation of Nrf2 was decreased, but YHG was able to rescue this reduction. GCLC, GCLM, and NQO1 are known as downstream antioxidant enzymes regulated by Nrf2[38]. The elevated hepatic expre
Mitochondria play a core role in the production of energy and cellular metabolism, and their dysfunction can lead to a range of health issues. To maintain mitochondrial health and overall cellular function, a balance between mitochondrial turnover, fission and fusion processes, and the promotion of mitochondrial biogenesis is indeed crucial. Mitochondrial biogenesis involves the generation of the new mitochondria to replace damaged ones and maintain cellular energy production. This process helps ensure that cells have a healthy population of mitochondria and can effectively meet their energy demands[39]. Recent studies have shown that inducing mitochondrial biogenesis is beneficial in alleviating liver fibrosis in rats with secondary biliary cirrhosis or treated with carbon tetrachloride[40,41], as well as in mice with diet-induced obesity and non-alcoholic steatohepatitis[42]. Additionally, resveratrol has been reported to induce HSCs death through apoptosis, autophagy/mitophagy, and mitochondrial biogenesis[43]. The transcription of mtDNA holds a pivotal role in the process of mitochondrial biogenesis, and PGC1α and NRF1 tightly regulate this mechanism[39,44]. Furthermore, Nrf2 not only assumes a central role in protecting against oxidative stress injury but also enhances the structural and functional integrity of mitochondria under stress conditions[45]. It has been reported that Nrf2 enhances the expression of NRF1 by binding to its promoter sites[46]. In this study, YHG was found to enhance hepatic ATP levels, increase the reduced mtDNA content, and improve the decreased expression of PGC1α and NRF1 in CCl4-treated mice. These findings imply that YHG promotes mitochondrial biogenesis in CCl4-induced liver fibrosis in mice, which contributes to its protective effects against liver fibrosis.
YHG effectively alleviated liver fibrosis induced by CCl4 in mice via various mechanisms, including the inhibition of HSCs activation, reduction of inflammation, alleviation of liver oxidative stress damage by promoting Nrf2 activation, and promotion of liver mitochondrial biogenesis. These findings suggest that YHG has immense promise for clinical utilization in the management of liver fibrosis.
Liver fibrosis is a formidable global medical challenge, with no effective clinical treatment currently available. Yinhuang granule (YHG) is a proprietary Chinese medicine comprising Scutellariae Radix and Lonicerae Japonicae Flos. However, its pharmacological mechanism is still unclear.
To investigate the potential of YHG in alleviating liver fibrosis in mice.
To investigate the potential of YHG against liver fibrosis in mice through in vivo and in vitro experiments.
Liver fibrosis model mice were generated by intraperitoneal injections of 2 mL/kg of carbon tetrachloride (CCl4) twice a week for 4 wk. Liver fibrosis mice in the low dose of YHG (0.4 g/kg) and high dose of YHG (0.8 g/kg) groups were orally administered YHG once a day for 4 wk. Serum alanine/aspartate aminotransferase activity and liver hydroxyproline content were detected. Sirius red and Masson's trichrome staining assay were conducted. Real-time polymerase chain reaction, western-blot and enzyme-linked immunosorbent assay were conducted. Liver glutathione content, superoxide dismutase activity level, reactive oxygen species and protein carbonylation amount were detected.
YHG ameliorated hepatocellular injury and liver fibrosis in CCl4-treated mice. YHG inhibited hepatic stellate cells (HSCs) activation, alleviated oxidative stress, inhibited inflammation, and promoted mitochondrial biogenesis.
YHG effectively attenuates CCl4-induced liver fibrosis in mice by inhibiting the activation of HSCs, reducing inflammation, alleviating liver oxidative stress damage through Nrf2 activation, and promoting liver mitochondrial biogenesis.
Further investigation into the mechanism of YHG against liver fibrosis is necessary.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Selamoglu Z, Turkey S-Editor: Liu JH L-Editor: A P-Editor: Zheng XM
| 1. | Jiao J, Friedman SL, Aloman C. Hepatic fibrosis. Curr Opin Gastroenterol. 2009;25:223-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 2. | Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1380] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 3. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4123] [Article Influence: 206.2] [Reference Citation Analysis (3)] |
| 4. | Mormone E, George J, Nieto N. Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches. Chem Biol Interact. 2011;193:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 5. | Ginès P, Graupera I, Lammert F, Angeli P, Caballeria L, Krag A, Guha IN, Murad SD, Castera L. Screening for liver fibrosis in the general population: a call for action. Lancet Gastroenterol Hepatol. 2016;1:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 6. | Li D, Friedman SL. Liver fibrogenesis and the role of hepatic stellate cells: new insights and prospects for therapy. J Gastroenterol Hepatol. 1999;14:618-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 271] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Elpek GÖ. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update. World J Gastroenterol. 2014;20:7260-7276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 289] [Cited by in RCA: 293] [Article Influence: 26.6] [Reference Citation Analysis (4)] |
| 8. | Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577-594.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 628] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 9. | Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1005] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 10. | Li S, Hong M, Tan HY, Wang N, Feng Y. Insights into the Role and Interdependence of Oxidative Stress and Inflammation in Liver Diseases. Oxid Med Cell Longev. 2016;2016:4234061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 236] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 11. | Luangmonkong T, Suriguga S, Mutsaers HAM, Groothuis GMM, Olinga P, Boersema M. Targeting Oxidative Stress for the Treatment of Liver Fibrosis. Rev Physiol Biochem Pharmacol. 2018;175:71-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 12. | Li Z, Zhu JF, Ouyang H. Progress on traditional Chinese medicine in improving hepatic fibrosis through inhibiting oxidative stress. World J Hepatol. 2023;15:1091-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 13. | Li Z, Zhu J, Ouyang H. Research progress of traditional Chinese medicine in improving hepatic fibrosis based on inhibiting pathological angiogenesis. Front Pharmacol. 2023;14:1303012. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Talas ZS, Bayraktar N, Ozdemir I, Gok Y, Yilmaz I. The effects of synthetic organoselenium compounds on nitric oxide in DMBA-induced rat liver. J Environ Biol. 2009;30:591-593. [PubMed] [DOI] [Full Text] |
| 15. | Selamoglu ZS, Ozdemir I, Ciftci O, Gulhan MF, Savci A. Antioxidant Effect of Ethanolic Extract of Propolis in Liver of L-NAME Treated Rats. Adv Clin Exp Med. 2015;24:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Erdemli ME, Ekhteiari Salmas R, Durdagi S, Akgul H, Demirkol M, Aksungur Z, Selamoglu Z. Biochemical changes induced by grape seed extract and low level laser therapy administration during intraoral wound healing in rat liver: an experimental and in silico study. J Biomol Struct Dyn. 2018;36:993-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Miao H, Zhang Y, Huang Z, Lu B, Ji L. Lonicera japonica Attenuates Carbon Tetrachloride-Induced Liver Fibrosis in Mice: Molecular Mechanisms of Action. Am J Chin Med. 2019;47:351-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Nan JX, Park EJ, Kim YC, Ko G, Sohn DH. Scutellaria baicalensis inhibits liver fibrosis induced by bile duct ligation or carbon tetrachloride in rats. J Pharm Pharmacol. 2002;54:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Shi H, Dong L, Bai Y, Zhao J, Zhang Y, Zhang L. Chlorogenic acid against carbon tetrachloride-induced liver fibrosis in rats. Eur J Pharmacol. 2009;623:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Shi H, Dong L, Jiang J, Zhao J, Zhao G, Dang X, Lu X, Jia M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology. 2013;303:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 21. | Shi H, Shi A, Dong L, Lu X, Wang Y, Zhao J, Dai F, Guo X. Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin Nutr. 2016;35:1366-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 22. | Qiao H, Han H, Hong D, Ren Z, Chen Y, Zhou C. Protective effects of baicalin on carbon tetrachloride induced liver injury by activating PPARγ and inhibiting TGFβ1. Pharm Biol. 2011;49:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Yang F, Luo L, Zhu ZD, Zhou X, Wang Y, Xue J, Zhang J, Cai X, Chen ZL, Ma Q, Chen YF, Wang YJ, Luo YY, Liu P, Zhao L. Chlorogenic Acid Inhibits Liver Fibrosis by Blocking the miR-21-Regulated TGF-β1/Smad7 Signaling Pathway in Vitro and in Vivo. Front Pharmacol. 2017;8:929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 24. | Zhang Y, Miao H, Yan H, Sheng Y, Ji L. Hepatoprotective effect of Forsythiae Fructus water extract against carbon tetrachloride-induced liver fibrosis in mice. J Ethnopharmacol. 2018;218:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Ouyang H, Du A, Zhou L, Zhang T, Lu B, Wang Z, Ji L. Chlorogenic acid improves diabetic retinopathy by alleviating blood-retinal-barrier dysfunction via inducing Nrf2 activation. Phytother Res. 2022;36:1386-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S73-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 27. | Yoshida K, Matsuzaki K. Differential Regulation of TGF-β/Smad Signaling in Hepatic Stellate Cells between Acute and Chronic Liver Injuries. Front Physiol. 2012;3:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1221] [Cited by in RCA: 2001] [Article Influence: 250.1] [Reference Citation Analysis (0)] |
| 29. | Sánchez-Valle V, Chávez-Tapia NC, Uribe M, Méndez-Sánchez N. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr Med Chem. 2012;19:4850-4860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 415] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 30. | Ezhilarasan D. Oxidative stress is bane in chronic liver diseases: Clinical and experimental perspective. Arab J Gastroenterol. 2018;19:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 31. | Xu D, Xu M, Jeong S, Qian Y, Wu H, Xia Q, Kong X. The Role of Nrf2 in Liver Disease: Novel Molecular Mechanisms and Therapeutic Approaches. Front Pharmacol. 2018;9:1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 32. | Chen Q, Zhang H, Cao Y, Li Y, Sun S, Zhang J, Zhang G. Schisandrin B attenuates CCl(4)-induced liver fibrosis in rats by regulation of Nrf2-ARE and TGF-β/Smad signaling pathways. Drug Des Devel Ther. 2017;11:2179-2191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 33. | Li J, Hu R, Xu S, Li Y, Qin Y, Wu Q, Xiao Z. Xiaochaihutang attenuates liver fibrosis by activation of Nrf2 pathway in rats. Biomed Pharmacother. 2017;96:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Zou L, Chen S, Li L, Wu T. The protective effect of hyperoside on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. Exp Toxicol Pathol. 2017;69:451-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Fan J, Chen Q, Wei L, Zhou X, Wang R, Zhang H. Asiatic acid ameliorates CCl(4)-induced liver fibrosis in rats: involvement of Nrf2/ARE, NF-κB/IκBα, and JAK1/STAT3 signaling pathways. Drug Des Devel Ther. 2018;12:3595-3605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Ramos-Tovar E, Hernández-Aquino E, Casas-Grajales S, Buendia-Montaño LD, Galindo-Gómez S, Camacho J, Tsutsumi V, Muriel P. Stevia Prevents Acute and Chronic Liver Injury Induced by Carbon Tetrachloride by Blocking Oxidative Stress through Nrf2 Upregulation. Oxid Med Cell Longev. 2018;2018:3823426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Wang R, Wang J, Song F, Li S, Yuan Y. Tanshinol ameliorates CCl(4)-induced liver fibrosis in rats through the regulation of Nrf2/HO-1 and NF-κB/IκBα signaling pathway. Drug Des Devel Ther. 2018;12:1281-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 38. | Zhang H, Zhou L, Yuen J, Birkner N, Leppert V, O'Day PA, Forman HJ. Delayed Nrf2-regulated antioxidant gene induction in response to silica nanoparticles. Free Radic Biol Med. 2017;108:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Whitaker RM, Corum D, Beeson CC, Schnellmann RG. Mitochondrial Biogenesis as a Pharmacological Target: A New Approach to Acute and Chronic Diseases. Annu Rev Pharmacol Toxicol. 2016;56:229-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 40. | Serviddio G, Bellanti F, Stanca E, Lunetti P, Blonda M, Tamborra R, Siculella L, Vendemiale G, Capobianco L, Giudetti AM. Silybin exerts antioxidant effects and induces mitochondrial biogenesis in liver of rat with secondary biliary cirrhosis. Free Radic Biol Med. 2014;73:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Kang JW, Hong JM, Lee SM. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J Pineal Res. 2016;60:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 42. | Kim B, Farruggia C, Ku CS, Pham TX, Yang Y, Bae M, Wegner CJ, Farrell NJ, Harness E, Park YK, Koo SI, Lee JY. Astaxanthin inhibits inflammation and fibrosis in the liver and adipose tissue of mouse models of diet-induced obesity and nonalcoholic steatohepatitis. J Nutr Biochem. 2017;43:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 43. | Meira Martins LA, Vieira MQ, Ilha M, de Vasconcelos M, Biehl HB, Lima DB, Schein V, Barbé-Tuana F, Borojevic R, Guma FC. The interplay between apoptosis, mitophagy and mitochondrial biogenesis induced by resveratrol can determine activated hepatic stellate cells death or survival. Cell Biochem Biophys. 2015;71:657-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 44. | Viña J, Gomez-Cabrera MC, Borras C, Froio T, Sanchis-Gomar F, Martinez-Bello VE, Pallardo FV. Mitochondrial biogenesis in exercise and in ageing. Adv Drug Deliv Rev. 2009;61:1369-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 45. | Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med. 2015;88:179-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 651] [Cited by in RCA: 736] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 46. | Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 474] [Article Influence: 27.9] [Reference Citation Analysis (0)] |