Published online Feb 27, 2024. doi: 10.4254/wjh.v16.i2.251
Peer-review started: November 11, 2023
First decision: December 14, 2023
Revised: December 24, 2023
Accepted: January 15, 2024
Article in press: January 15, 2024
Published online: February 27, 2024
Processing time: 107 Days and 21.8 Hours
The increased expression of G3BP1 was positively correlated with the prognosis of liver failure.
To investigate the effect of G3BP1 on the prognosis of acute liver failure (ALF) and acute-on-chronic liver failure (ACLF) after the treatment of artificial liver support system (ALSS).
A total of 244 patients with ALF and ACLF were enrolled in this study. The levels of G3BP1 on admission and at discharge were detected. The validation set of 514 patients was collected to verify the predicted effect of G3BP1 and the viability of prognosis.
This study was shown that lactate dehydrogenase (LDH), alpha-fetoprotein (AFP) and prothrombin time were closely related to the prognosis of patients. After the ALSS treatment, the patient’ amount of decreased G3BP1 index in difference of G3BP1 between the value of discharge and admission (difG3BP1) < 0 group had a nearly 10-fold increased risk of progression compared with the amount of increased G3BP1 index. The subgroup analysis showed that the difG3BP1 < 0 group had a higher risk of progression, regardless of model for end-stage liver disease high-risk or low-risk group. At the same time, compared with the inflammatory marks [tumor necrosis factor-α, interleukin (IL)-1β and IL-18], G3BP1 had higher discrimination and was more stable in the model analysis and validation set. When combined with AFP and LDH, concordance index was respectively 0.84 and 0.8 in training and validation cohorts.
This study indicated that G3BP1 could predict the prognosis of ALF or ACLF patients treated with ALSS. The combination of G3BP1, AFP and LDH could accurately evaluate the disease condition and predict the clinical endpoint of patients.
Core Tip: This study retrospectively analyzed the clinical characteristics and laboratory indicators of acute liver failure and acute-on-chronic liver failure patients treated with artificial liver support system (ALSS). It was found that G3BP1, alpha-fetoprotein, lactate dehydrogenase, tumor necrosis factor-α, and interleukin-1β were independent risk factors. G3BP1 could effectively predict liver failure, which has great value to timely provide liver transplantation opportunity for patients who have failed for drug and ALSS treatment.
- Citation: Li WY, Wang LW, Dong J, Wang Y. Evaluation of G3BP1 in the prognosis of acute and acute-on-chronic liver failure after the treatment of artificial liver support system. World J Hepatol 2024; 16(2): 251-263
- URL: https://www.wjgnet.com/1948-5182/full/v16/i2/251.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i2.251
Acute liver failure (ALF) and acute on chronic liver failure (ACLF) are the most common causes of liver disease death[1,2]. In a short period of time, ALF patients may have extensive hepatocytes necrosis and severe liver function damage, accompanied by hepatic encephalopathy (HE), hepatorenal syndrome (HRS), hepatopulmonary syndrome (HPS), etc[3]. ACLF is caused by acute triggers such as bacterial and viral infections, alcoholic hepatitis, and surgery based on chronic liver disease. Acute decompensation of liver function failure or combined extrahepatic organ failure can occur in a short period of time[4]. The significant characteristics of ALF and ACLF are rapid progression of the disease, which can lead to rapid occurrence of extrahepatic organ failure, manifested as liver coma or hepatorenal syndrome, leading to high mortality rate and extremely poor prognosis. At present, clinical treatment methods mainly include comprehensive drug therapy, artificial liver support therapy, and liver transplantation[5,6]. Among them, liver transplantation is currently considered the most effective method to treat patients with ALF and ACLF. However, due to limited donors, high surgical costs, unpredictable postoperative complications, and the need for long-term use of immunosuppressants after liver transplantation, liver transplantation is still not widely used in clinical practice[7]. Therefore, early judgment of prognosis and timely intervention during the progression of ALF and ACLF are of great significance to improving survival rate.
In recent years, the Chinese Group on the Study of Severe Hepatitis B (COSSH), the European Association for the Study of the Liver-Chronic Liver Failure Consortium (CLIF-C), and Asian-Pacific Association for the Study of the Liver ACLF Research Consortium (AARC) have established COSSH ACLF score[8], CLIF-C ACLF score[9], CLIF Consortium Organ Failure score[9], and AARC ACLF score[10], which can accurately evaluate the condition and prognosis of ACLF patients. Artificial liver support system (ALSS). As an effective treatment, ALSS can reduce bilirubin in a short term, improve inflammatory storm, regulate immunity, which has been widely used in the treatment of ALF and ACLF[11]. However, there is no clinical predict model could evaluate the prognosis of patients with ALF and ACLF treated with ALSS.
Studies have shown that G3BP1 is a key factor in the assembly of stress granules (SGs)[12,13]. Double-stranded RNA will trigger excessive inflammation and apoptosis, if the SGs was without nucleators G3BP1[14]. Our previous study found that the arginine-glycine-glycine and RNA-recognition module protein domains of G3BP1 could bind to the nuclear localization sequence of p53. Subsequently, the process of p53 entering the nucleus was inhibited, and p53 could not bind to the promoter region of SLC7A11, thus inhibiting the ferroptosis of hepatocytesduring ALF[15]. G3BP1-mediated SGs could inhibit hepatocyte apoptosis by inhibiting hypoxia-inducible factor 1α-endoplasmic reticulum stress pathway and improve the damage of hepatocytes caused by ischemia and hypoxia during ALF[16]. The above studies showed that the G3BP1 could inhibit inflammatory response, and the increased expression of G3BP1 was positively correlated with the prognosis of liver failure. However, there is no correlation between G3BP1 and the prognosis for the liver failure patients treated by ALSS. The purpose of this study was to verify the predictive efficacy of G3BP1 in patients with ALF and ACLF treated with ALSS, and to provide reference for the clinical development of a new prediction model.
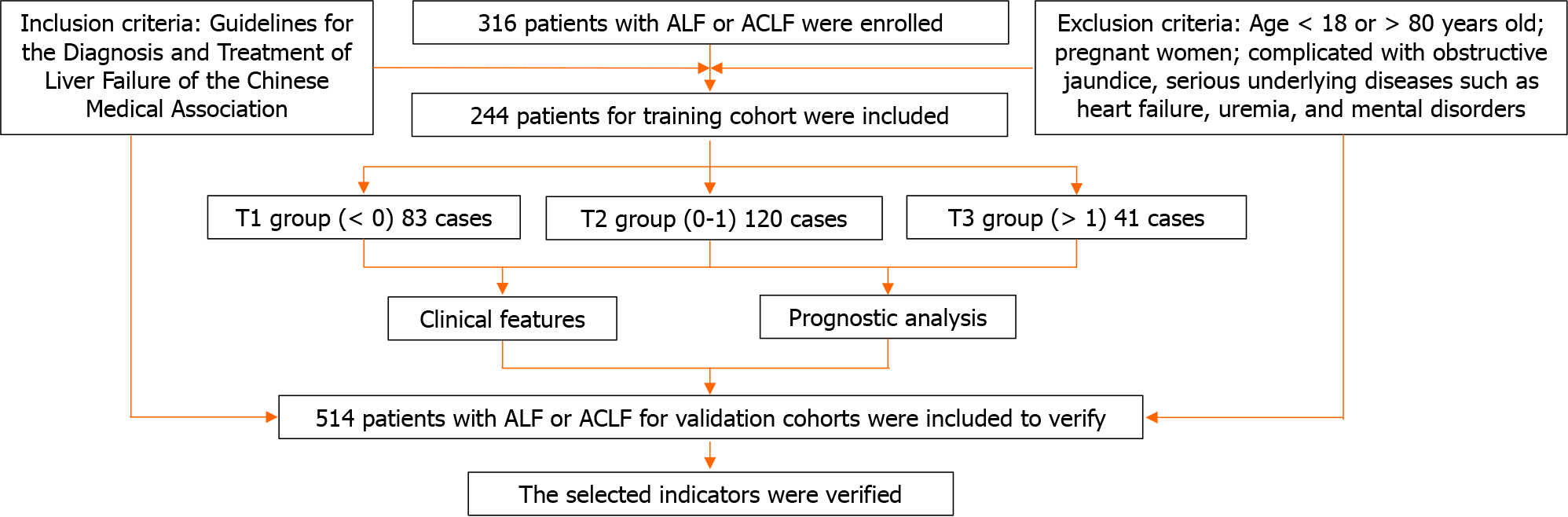
A total of 316 ALF and ACLF patients from January 2018 to December 2022 were selected who underwent ALSS in the Department of Infectious Diseases, Renmin Hospital of Wuhan University. These patients were conducted for a retrospective study, which was approved by the Institutional Review Board of Renmin Hospital of Wuhan University (No. WDRY2022-K212).
The diagnostic criteria for ACLF were in accordance with the Guidelines for the Diagnosis and Treatment of Liver Failure of the Chinese Medical Association (CMA)[17]. The syndrome of liver failure manifested by acute jaundice and coagulation dysfunction caused by various causes based on chronic liver disease. It could be complicated by HE, ascites, electrolyte disturbance, infection, HRS, HPS and other complications, as well as extrahepatic organ failure. Jaundice deepened rapidly and serum total bilirubin (TBIL) ≥ 10 × upper limit of normal value (ULN) or increased ≥ 17.1 μmol/L daily. There were signs of bleeding, with prothrombin activity (PTA) ≤ 40% [or international normalized ratio (INR) ≥ 1.5].
The diagnostic criteria of ALF were similarly in accordance with the Guidelines for the Diagnosis and Treatment of Liver Failure of the CMA[17]. Based on the absence of chronic liver disease, the patients who had developed liver encephalopathy of grade II or within 2 wk, had the following manifestations: (1) Extreme fatigue accompanied by severe gastrointestinal symptoms; (2) Progressive jaundice in a short period of time, serum TBIL ≥ 10 × ULN or daily increase ≥ 17.1 μmol/L; (3) Bleeding tendency, PTA ≤ 40%, or INR ≥ 1.5; and (4) Progressive shrinkage of the liver.
Patients who met any of the following criteria would be excluded: (1) Age < 18 years old or > 80 years old; (2) Pregnant women; and (3) Combined obstructive jaundice, severe underlying diseases such as heart failure, uremia, and mental illness.
As shown in Figure 1, a total of 316 patients with ALF and ACLF were admitted according to the diagnostic criteria. A total of 72 patients were excluded according to the exclusion criteria, and 244 patients were finally included in this study. Mean follow-up was 24 months. The clinical data of patients included in the final analysis were complete. Standard descriptive statistics were used to describe the baseline characteristics of the entire cohort, stratified according to G3BP1 Levels. The patients with the difference of G3BP1 between the value of discharge and admission (difG3BP1) was less than 0, more than 0 and less than 1, more than 1 were respectively divided into T1 group, T2 group and T3 group. The training set was 244. There were respectively 8312041 patients in the T1, T2, and T3 group.
Figure 2 showed the overall distribution of difG3BP1. The value of difG3BP1 was mainly concentrated between -1.0 and 1.5. The largest number of individuals between 0.5 and 1.0, accounting for 70 individuals. In our previous study, it was confirmed that promoting G3BP1 expression could improve hepatocyte injury during ALF by inhibiting ferroptosis[15]. Promoting the expression of G3BP1-mediated SGs could alleviate hepatocyte damage caused by hypoxia during ALF[16]. The increased expression of G3BP1 was positively correlated with the prognosis of liver failure. Therefore, the difG3BP1 value was less than 0, implying a worse prognosis for liver failure. The difG3BP1 value was more than 0, indicating a better prognosis. When observing the difG3BP1 value, it was found that most difG3BP1 values were between 0-1. When the value was greater than 1, the prognosis of patients was generally better. Therefore, we further divided the three group patients with the changed value of difG3BP1 was less than 0, greater than 0 and less than 1, greater than 1. The relative explanation has been added in the manuscript.
General information such as gender, age, family history, and admission number were recorded on the first day of admission. The patient was specifically asked whether had a personal history of diabetes and hepatotoxic medications. Complete blood routine [white blood cell (WBC), neutrophil percent (N%), platelet (PLT)], inflammatory indicators [serum amyloid A protein (SAA), C-reactive protein (CRP), procalcitonin (PCT), albumin (ALB)], coagulation function [PTA, prothrombin time (PT), INR], liver function [alanine aminotransferase (ALT), aspartate transaminase (AST), TBIL, direct bilirubin, lactate dehydrogenase (LDH)], renal function [(creatinine, blood urea nitrogen, glomeruar filtration rate (GFR)], inflammatory factors [interlenkin (IL)-1β, IL-18, tumor necrosis factor-α (TNF-α)], alpha-fetoprotein (AFP), blood type and other indicators were detected. Because the patient might be infected by hepatitis B virus (HBV) or hepatitis E virus (HEV), it was also necessary to detect the levels of HBV-DNA and HEV-IgM. According to the patients’ condition, patients’ needs and examination results, the intravenous catheterization method and ALSS mode were subsequently determined. Before the end of treatment, blood routine, inflammatory indicators, ALB, coagulation function, liver function, LDH, renal function, inflammatory factors, AFP, and other indicators were re-examined. The patients were followed up regularly to record their survival time after discharge.
The ALF and ACLF patients were treated with liver protection to relieve jaundice, promoting liver cell regeneration, and anti-infection. The chronic hepatitis B patients were given antiviral therapy. The cirrhosis with ascites patients were given albumin supplementation, diuresis, and abdominal puncture and drainage if necessary. ALSS was performed after signing the consent form for blood transfusion and informed consent for blood purification. Venous access was established by femoral vein puncture and indwelling double lumen catheter. Routine anti-allergic treatment was performed before operation. All patients were treated with Jianfan DX-10 blood purification machine (Zhuhai, China).
For plasma exchange (PE) treatment mode, the pipe and plasma separator were prepared before starting the machine. Plasma exchange alone was selected after self-testing. Then, the arterial end pipeline, venous end pipeline, slurry pipeline and filtrate pipeline were installed respectively, connected to the plasma separator, and finally installed the return blood rehydration pipe. The pipeline was flushed after installation. The target volume of treatment was set to 2500-3000 mL. The blood pump knob (the flow rate was 60-80 mL/min was used to drain blood. After introducing the blood into the intravenous pot, the blood pump speed was increased to the required flow rate (100-150 mL/min). The filtration pump/blood pump (FP/BP) ratio was set at 0.30, the return pump/FP ratio at 1.0, the flow rate of the SP heparin pump at 1-2 mL/h, and the temperature at 36.5 °C. Subsequently, the patients were treated with mechanical ventilation and blood return. After completing the blood return process, the machine would be disempowered.
For dual plasma molecular adsorption system (DPMAS) treatment mode, a plasma separator, BS330 bilirubin adsorption column and HA330-Ⅱ hemoperfusion apparatus were prepared before starting the machine. The plasma adsorption mode was selected after boot-up self-test. Then the line was installed as PE mode. After pre-flushing, the treatment parameters were set, the target dose was set as 5000 mL, the FP/BP ratio was set at 0.30, the flow rate of heparin pump was 1-2 mL/h, and the temperature was set as 36.5 °C. At the end of the treatment, the pipeline was removed, the blood return process was completed, and the machine would be disempowered.
For half-dose PE combined with DPMAS mode, a plasma separator, BS330 bilirubin adsorption column, and HA330-Ⅱ hemoperfusion apparatus were required for preparation. It was carried out in the order of self-test, selection of plasma adsorption mode, installation of pipelines, and pre-flushing. For parameter settings, the therapeutic target volume was 5000 mL, the FP/BP ratio was set as 0.30, the heparin pump flow rate was 1-2 mL/h, and the temperature was 36.5 °C. After treatment, the machine was switched to PE treatment mode. The machine was turned off at last, the pipeline was removed, and the blood return process was completed.
Referring to the previous methods[16,18], the level of G3BP1 in plasma was detected by enzyme linked immunosorbent assay (ELISA) kit, which was purchased from Ruifen Biotechnology Co., LTD. (Shanghai, China). The anticoagulant tube was used to collect blood samples at admission and discharge. Then all the specimens were sent to the central laboratory of Renmin Hospital of Wuhan University for testing by specialized technicians, who were blind to the clinical data. The blood samples were placed in a cryogenic centrifuge at 3000 revolutions per minute (rpm), 4 for 15 min. The blood supernatant after centrifugation was the plasma sample, and all samples were stored in the refrigerator at -80 °C for subsequent detection. All procedures were performed on ice to prevent protein degradation in the plasma. The sensitivity and stability of the kit were tested by the pre-experiment. The ELISA experimental procedures after centrifugation were performed according to the kit instructions. The optical density (OD) value of each sample was detected by a multifunctional microplate reader purchased from PerkinElmer (Cat. No. EnSight, United States). Each sample was tested by three times, and the average OD value was calculated for statistics.
In our previous study, it was found that the inflammatory factors IL-1β, IL-18 and TNF-α were closely related to the occurrence and development of liver failure. The levels of IL-1β and IL-18, the markers of pyroptosis, are positively correlated with the level of liver inflammation in HBV-related patients[19]. The TNF-α/HMGB1 inflammatory pathway can regulate the pyroptosis level in patients with liver failure complicated with hepatorenal syndrome[20]. Therefore, these inflammatory factors were used to predict the prognosis of patients with G3BP1-mediated ALF. However, in order to be more consistent with the actual clinical indicators, the commonly used clinical indicators were selected through random forest and combined these inflammatory factors to predict the prognosis of patients. The importance evaluation index of model prediction effect was ranked in descending order.
Univariate and multivariate Cox proportional hazards models were used to detect G3BP1, clinical biomarkers (LDH, AFP, PT, etc.), and inflammatory factors (TNF-α, IL-1β and IL-18) for the risk of progression after ALSS treatment. For the models, we treated biomarkers as continuous traits (log-transformed) or as 3-level categorical variables (low, medium, and high) defined according to the tertials of the biomarker level. Adjusted hazard ratio (HR) and 95%CI were obtained for each biomarker. We developed a reference Cox proportional hazards model for progression after ALSS treatment of liver failure in the training cohort and tested whether the inclusion of biomarker levels further improved risk prediction.
For continuous variables, random forest success rate curve model of R software was used to select the survival prognosis factors of patients in the training set. Factors of P < 0.05 were selected as independent prognostic factors. Stepwise Cox regression was used to evaluate the number of variables with the concordance index (C-index) value to obtain the best C-index value with fewer prognostic factors. The HR and 95%CI were estimated by calculating the β coefficient of the predictors and included in the Cox proportional hazard model. The 48-month survival curve was plotted to predict the patients’ survival.
Referring to the previous methods[19,20], IBM SPSS 25.0 statistical software was used to analyze the data. Normally and non-normally distributed continuous variables were respectively expressed as mean ± SD and median (interquartile range). Categorical variables were recorded as frequencies and percentages. Missing values were retained as indeter
As shown in Table 1, the average age of all patients was 51.9 ± 13.2 years. 204 (83.6%) patients were male. 20 patients were treated with PE combined with DPMAS. 36 (14.75%) patients had a family history of liver disease. 33 (13.52%) patients had a history of diabetes. There were no significant differences in age (P = 0.477), number of male patients (P = 0.45), number of patients treated with PE combined with DPMAS (P = 0.938), number of patients with family history of liver disease (P = 0.763), and number of patients with history of diabetes (P = 0.373) among the three groups. Among the detection indicators, AFP (P = 0.024), WBC (P = 0.002), N% (P = 0.004), PTA (P = 0.001), PT (P < 0.001), INR (P < 0.001), TBIL (P = 0.024), LDH (P < 0.001), PLT (P = 0.046). There was no significant difference in SAA (P = 0.385), CRP (P = 0.096), PCT (P = 0.13), ALB (P = 0.799), ALT (P = 0.162), AST (P = 0.244) and GFR (P = 0.104). The number of patients with ALF and ACLF respectively accounted for 21.72% and 78.28% of the total number of patients. The number of ALF patients in T1, T2, and T3 groups was respectively accounted for 26.41%, 54.72%, and 18.87% of the total number of ALF patients. Each group of ACLF patients was accounted for 36.13%, 47.64%, and 16.23% of the total number of ACLF patients.
| Variable1 | DifG3BP1 in training cohort | Validation cohorts | ||||
| Overall | T1 (< 0) | T2 (0-1) | T3 (> 1) | P value2 | ||
| Patients (n) | 244 | 83 | 120 | 41 | 514 | |
| Age (yr), mean ± SD | 51.9 ± 13.2 | 53.20 ± 12.08 | 50.91 ± 14.07 | 52.10 ± 13.10 | 0.477 | 54.35 ± 15.6 |
| Men, n (%) | 204 (83.6) | 68 (81.9) | 99 (82.5) | 37 (90.2) | 0.45 | 426 (82.8) |
| AFP, mean ± SD | 108.93 ± 170.12 | 68.65 ± 119.17 | 124.85 ± 180.26 | 143.86 ± 211.32 | 0.024 | 106.94 ± 114.56 |
| WBC, mean ± SD | 7.19 ± 3.54 | 8.28 ± 4.26 | 6.61 ± 2.97 | 6.70 ± 3.00 | 0.002 | 7.43 ± 3.65 |
| N%, mean ± SD | 69.38 ± 11.85 | 72.79 ± 10.60 | 67.33 ± 12.39 | 68.50 ± 11.35 | 0.004 | 71.34 ± 12.38 |
| SAA, mean ± SD | 9.33 ± 14.10 | 10.27 ± 13.57 | 9.61 ± 15.09 | 6.63 ± 11.99 | 0.385 | 9.56 ± 13.87 |
| CRP, mean ± SD | 14.03 ± 20.31 | 17.94 ± 23.64 | 12.09 ± 17.93 | 11.78 ± 18.84 | 0.096 | 13.67 ± 18.48 |
| PCT, mean ± SD | 0.62 ± 0.75 | 0.76 ± 0.92 | 0.55 ± 0.68 | 0.56 ± 0.54 | 0.13 | 0.65 ± 0.71 |
| ALB (g/L), mean ± SD | 31.23 ± 4.00 | 30.99 ± 3.53 | 31.36 ± 4.43 | 31.31 ± 3.61 | 0.799 | 32.56 ± 4.15 |
| PTA (%), mean ± SD | 43.69 ± 20.01 | 37.24 ± 15.90 | 46.35 ± 21.99 | 48.97 ± 18.40 | 0.001 | 42.17 ± 21.03 |
| PT, mean ± SD | 20.56 ± 6.21 | 23.13 ± 7.09 | 19.41 ± 5.41 | 18.74 ± 4.85 | < 0.001 | 19.15 ± 6.12 |
| INR, mean ± SD | 1.83 ± 0.59 | 2.07 ± 0.70 | 1.72 ± 0.48 | 1.67 ± 0.47 | < 0.001 | 1.87 ± 0.61 |
| ALT, mean ± SD | 276.93 ± 546.24 | 185.54 ± 266.86 | 332.65 ± 701.28 | 298.85 ± 420.08 | 0.162 | 295.13 ± 537.87 |
| TBIL, mean ± SD | 322.21 ± 131.10 | 353.56 ± 150.78 | 303.54 ± 122.07 | 313.37 ± 101.51 | 0.024 | 317.81 ± 145.11 |
| AST, mean ± SD | 247.66 ± 468.92 | 179.95 ± 188.58 | 292.26 ± 623.86 | 254.20 ± 299.16 | 0.244 | 238.98 ± 464.17 |
| LDH, mean ± SD | 273.07 ± 82.84 | 302.18 ± 70.80 | 250.64 ± 84.55 | 279.78 ± 82.64 | < 0.001 | 286.46 ± 86.78 |
| GFR, mean ± SD | 101.69 ± 25.05 | 97.36 ± 27.28 | 104.94 ± 24.51 | 100.96 ± 20.71 | 0.104 | 102.54 ± 26.33 |
| PLT, mean ± SD | 111.18 ± 67.19 | 96.40 ± 66.11 | 118.06 ± 64.91 | 120.95 ± 72.38 | 0.046 | 106.54 ± 71.77 |
| Family history of liver disease, n (%)3 | 36 (14.75) | 14 (16.9) | 17 (14.2) | 5 (12.2) | 0.763 | 78 (15.2) |
| Treatment method = PE + DPMAS (%) | 20 (8.20) | 7 (8.6) | 6 (7.3) | 7 (8.6) | 0.938 | 46 (8.9) |
| LOS time (months), mean ± SD | 35.55 (20.63) | 34.36 (19.78) | 36.51 (22.33) | 35.75 (19.84) | 0.797 | 35.78 (21.34) |
| Diabetes, n (%) | 33 (13.52) | 11 (13.6) | 8 (9.8) | 14 (17.3) | 0.373 | 69 |
| ALF, n (%) | 53 (21.72) | 14 (26.41) | 29 (54.72) | 10 (18.87) | 111 (21.60) | |
| ACLF, n (%) | 191 (78.28) | 69 (36.13) | 91 (47.64) | 31 (16.23) | 403 (78.40) | |
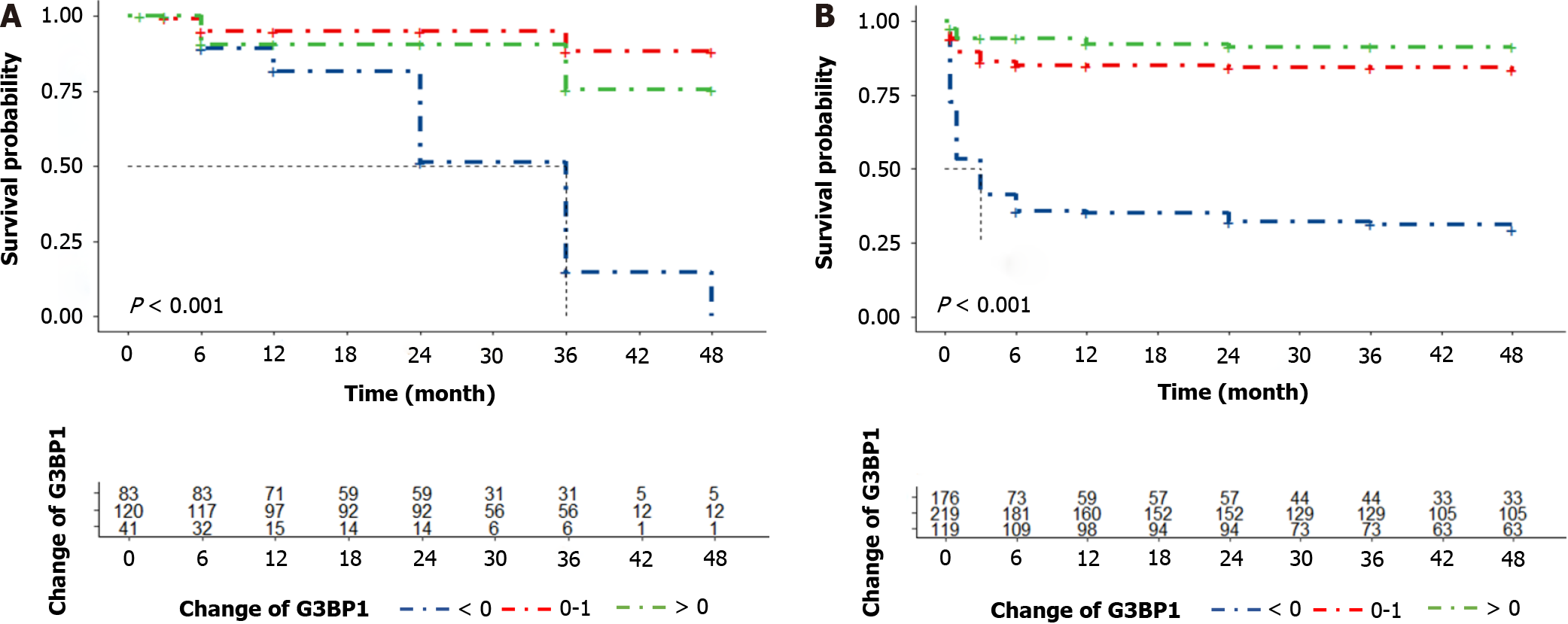
As shown in Figure 3, in the Kaplan-Meier analysis, difG3BP1 levels in the T1, T2 and T3 groups were associated with survival in ALF and ACLF patients with ALSS in both the training and validation cohorts.
As shown in Figure 4A, according to the diagram of the relationship between model error and the number of decision trees, the error rate decreased rapidly in the initial stage with the increase of the number of decision trees. When the number of decision trees was 50-200, the error rate was basically maintained at a certain level. The 23 variables including the patient’s age, treatment method, family history of liver disease, AFP, WBC, N%, PTA, PT, INR, TBIL, LDH, PLT, SAA, CRP, PCT, ALB, ALT, AST and GFR were used to construct a random forest prediction model. As shown in Figure 4B, the importance evaluation index of model prediction effect was ranked in descending order. The top two variables were LDH and AFP.
Subsequently, LDH and AFP levels were grouped into Cox regression models to establish model 1. As shown in Table 2, difG3BP1 ≥ 0 and < 0 as cut were divided into two groups, namely T2 + T3 G3BP1 group (n = 161) and T1 G3BP1 group (n = 83). The unadjusted HR model only included the G3BP1 grouping variable. Before adjustment, the number of endpoints was respectively 14 (8.7%) and 64 (77.1%) for difG3BP1 ≥ 0 and < 0, and the unadjusted HR was 4.5811.70 (95%CI: 6.50-21.04). The subgroup analysis was based on model for end-stage liver disease (MELD) score, and the subgroups were divided into high-risk group (n = 154) and medium-low risk group (n = 90). In the high-risk group, the number of T2 + T3 G3BP1 and T1 G3BP1 groups was 94 and 60, respectively. Before adjustment, the number of endpoints was respectively 13 (13.8%) and 53 (88.3%) for difG3BP1 ≥ 0 and < 0, with an unadjusted HR of 8.01 (95%CI: 4.33-14.81). In the low-intermediate risk group, the number of T2 + T3 G3BP1 group and T1 G3BP1 group were respectively 67 and 23. Before adjustment, the number of progressions was respectively 1 (1.5%) and 11 (47.8%) for difG3BP1 ≥ 0 and < 0, with an unadjusted HR of 39.46 (95%CI: 5.05-308.3).
| G3BP1 cut points | Progression (%)1 | Unadjusted HR (95%CI) | P value | Model 13 | Adjusted HR (95%CI) P value | |
| Total cohort (n = 244) | ||||||
| T1 + T2 G3BP1 (n = 161) | ≥ 0 | 14 (8.7) | 1.0 (Reference) | 1.0 (Reference) | ||
| T3 G3BP1 (n = 83) | < 0 | 64 (77.1) | 11.70 (6.50-21.04) | < 0.001 | 9.23 (5.08-16.75) | < 0.001 |
| Subgroup with MELD2 high risk (n = 154) | ||||||
| T2 + T3 G3BP1 (n = 94) | ≥ 0 | 13 (13.8) | 1.0 (Reference) | 1.0 (Reference) | ||
| T1 G3BP1 (n = 60) | < 0 | 53 (88.3) | 8.01 (4.33-14.81) | < 0.001 | 5.71 (3.04-10.74) | < 0.001 |
| Subgroup with MELD low-medium risk (n = 90) | ||||||
| T2 + T3 G3BP1 (n = 67) | ≥ 0 | 1 (1.5) | 1.0 (Reference) | 1.0 (Reference) | ||
| T1 G3BP1 (n = 23) | < 0 | 11 (47.8) | 39.46 (5.05-308.3) | < 0.001 | 23.80 (2.96-191.3) | < 0.001 |
After adjusting the linear variables LDH and AFP, the adjusted HR for G3BP1 in overall population, high-risk group, and low-intermediate risk group were respectively 9.23 (95%CI: 5.08-16.75), 5.71 (95%CI: 3.04-10.74), and 23.80 (95%CI: 2.96-191.3). In the overall G3BP1 population, the difG3BP1 < 0 group had a nearly 10-fold increased risk compared with the ≥ 0 group. For the subgroup analysis, patients with decreased G3BP1 (difG3BP1 < 0) had a higher risk of MELD progression (P < 0.01). This indicated that G3BP1 had a good value for prognostic evaluation.
In this study, the inflammatory markers IL-1β, IL-18 and TNF-α were included to evaluate the prognosis of patients. As shown in Table 3, the cut-off points of IL-1β, IL-18, and TNF-α were taken as the median levels at admission, which were respectively 168.0 ng/mL, 13.425 ng/mL, and 5.465 ng/mL. Before adjustment, when IL-1β ≤ 168.0 ng/mL and > 168.0 ng/mL, the numbers of endpoints were respectively 18 (14.8%) and 60 (49.2%). When IL-18 ≤ 13.425 ng/mL and > 13.425 ng/mL, the numbers of endpoints were respectively 41 (33.6%) and 37 (30.3%). When TNF-α ≤ 5.465 ng/mL and > 5.465 ng/mL, the numbers of endpoints were respectively 48 (39.3%) and 30 (24.6%). The unadjusted HRs were respectively 4.58 (95%CI: 2.69-7.80), 0.91 (95%CI: 0.58-1.43), and 0.53 (95%CI: 0.34-0.84).
| Biomarker | G3BP1 cut points | Progression (%) | Unadjusted HR (95%CI) | P value | Model 11 | Adjusted HR (95%CI) P value |
| IL-1β (pg/mL) | ||||||
| T1 IL-1β (n = 122) | ≤ 168.0 | 15 (12.3) | 1.0 (Reference) | 1.0 (Reference) | ||
| T2 IL-1β (n = 122) | > 168.0 | 58 (47.5) | 5.38 (3.03-9.54) | < 0.01 | 4.27 (2.37-7.67) | < 0.01 |
| IL-18 (pg/mL) | ||||||
| T1 IL-18 (n = 122) | ≤ 13.425 | 38 (31.1) | 1.0 (Reference) | 1.0 (Reference) | ||
| T2 IL-18 (n = 122) | > 13.425 | 35 (28.7) | 0.94 (0.59-1.49) | 0.78 | 0.95 (0.59-1.52) | 0.83 |
| TNF-α (pg/mL) | ||||||
| T1 TNF-α (n = 122) | ≤ 5.465 | 46 (37.7) | 1.0 (Reference) | 1.0 (Reference) | ||
| T2 TNF-α (n = 122) | > 5.465 | 27 (22.1) | 0.50 (0.31-0.80) | < 0.01 | 0.55 (0.34-0.90) | 0.02 |
Therefore, IL-1β (P < 0.01) and TNF-α (P < 0.01) were associated with the prognosis of the disease before adjustment. The adjusted HRs were respectively 3.82 (95%CI: 2.22-6.57), 0.93 (95%CI: 0.59-1.46), and 0.57 (95%CI: 0.36-0.91). Similarly, IL-1β (P < 0.01) and TNF-α (P = 0.02) were also closely associated with the prognosis of the disease after adjustment. Further comparison in Table 2 was showed that the risk coefficients of IL-1β and TNF-α were not as high as those of G3BP1 before and after adjustment.
After multivariate regression analysis was used to screen out the independent risk factors for death in ALF and ACLF patients, four types of risk prediction models were established for patients after ALSS treatment. As shown in Table 4, the first category was the univariate model of biomarkers, which included G3BP1, IL-1β, IL-18, and TNF-α. The C-index in the test set was respectively 0.75 (95%CI: 0.68-0.81), 0.68 (95%CI: 0.63-0.74), 0.54 (95%CI: 0.48 0.63), 0.57 (95%CI: 0.50-0.64). Therefore, G3BP1 had better discrimination power and higher consistency than other indicators in the univariate model. Furthermore, the CI of G3BP1 in the validation cohort was 0.75 (95%CI: 0.71-0.78), indicating that G3BP1 was stable in both the test cohort and the validation cohort. The source and diagnosis of validation group of 514 patients were consistent with the training set. The baseline data for patients in the validation group was shown in Table 1.
| Biomarkers | C statistic (95%CI)1 | |
| Training cohort (n = 244) | Validation cohort (n = 514)2 | |
| Univariable models of biomarkers | ||
| G3BP1 | 0.75 (0.68-0.81) | 0.75 (0.71-0.78) |
| IL-1β | 0.68 (0.63-0.74) | |
| IL-18 | 0.54 (0.48-0.63) | |
| TNF-α | 0.57 (0.50-0.64) | |
| Clinical models | ||
| Clinical data | 0.78 (0.72-0.84) | 0.71 (0.66-0.76) |
| Models containing clinical data and biomarkers | ||
| Clinical data + G3BP1 | 0.84 (0.77-0.89) | 0.80 (0.76-0.84) |
| Clinical data + IL-1β | 0.80 (0.75-0.85) | |
| Clinical data + IL-18 | 0.78 (0.72-0.84) | |
| Clinical data + TNF-α | 0.78 (0.72-0.84) | |
| Model containing clinical data and multiple biomarkers | ||
| Clinical data + G3BP1 + IL-1β + IL-18 + TNF-α3 | 0.84 (0.79-0.90) | |
The second type was a clinical model, in which LDH and AFP were selected, with a C-index of 0.78 (95%CI: 0.72-0.84) in the test set. In the validation set, the C-index was 0.71 (95%CI: 0.66-0.76). The third category was the composite model of clinical data (LDH and AFP) combined with biomarkers, including clinical data + G3BP1, clinical data + IL-1β, clinical data + IL-18, and clinical data + TNF-α. The C-index in the test set was respectively 0.84 (95%CI: 0.77-0.89), 0.80 (95%CI: 0.75-0.85), 0.78 (95%CI: 0.72-0.84), 0.78 (95%CI: 0.72-0.84). Therefore, the predictive power of clinical data + G3BP1 was stronger in the composite model in which clinical data were combined with biomarkers. Further study found that the C-index of clinical data + G3BP1 in the validation set was 0.80 (95%CI: 0.76-0.84).
Among the four indicators of G3BP1, IL-1β, IL-18 and TNF-α, G3BP1 and IL-1β have stronger predictive ability. In the fourth category, a composite model with clinical data (LDH and AFP) combined with multiple biomarkers, only G3BP1 and IL-1β were selected. Specifically, the prediction model of clinical data + G3BP1 + IL-1β had a C-index of 0.84 (95%CI: 0.79-0.90). Therefore, the clinical data (AFP + LDH) + G3BP1 was used to establish the model, the C index reached 0.84, and reached 0.8 in the validation set, which could better assist clinical practice. When IL-1β was added, the C index was still 0.84, which was indicated that the AFP + LDH + G3BP1 model could predict the endpoint well with only 3 indicators.
Accurate prediction of the prognosis for ALF and ACLF patients is helpful for clinical decision-making and treatment selection[21]. ALSS is a treatment based on symptomatic support, which mainly removes toxic substances from patients through plasma separation equipment[11,22]. Therefore, ALSS is considered to create conditions for hepatocyte regeneration and liver function recovery and be also used as a bridge before liver transplantation[17,21]. Recent studies have shown that ALSS can significantly improve the short-term prognosis of liver failure[23,24]. However, there are also some patients after receiving ALSS treatment, hepatocytes still cannot effectively self-repair and regeneration, leading to the poor treatment effect and short-term prognosis. Therefore, early and accurate judgment for the short-term prognosis could provide guidance for the implementation of timely and effective treatment.
G3BP1 could interact with viral protein to regulate viral replication by regulating the assembly of SGs[13], which is the potential target for inflammation and infectious diseases[13]. Recent studies have shown that G3BP1 plays an important role in immune and inflammatory responses. The embryonic fibroblasts from G3BP1 knockout mouse shows impaired apoptosis and proliferation[12]. G3BP1 is also a positive regulator of innate immune responses including retinoic acid-inducible gene I mediated cellular antiviral pathway and cyclic GMP-AMP synthase-stimulator of interferon genes pathway[25]. G3BP1 interacts with IL-33 and promotes the transfer of IL-33 from the nucleus to the cytoplasm, where IL-33 cannot be directly released to the outside of the cell[26]. G3BP1 antagonizes the activation of protein kinase R, thereby inhibiting the inflammatory response[27].
In this study, the baseline plots of different difG3BP1 levels in the training set were firstly analyzed. The age, gender, diagnosis, and treatment methods were not statistically significant. The value of difG3BP1 was then cut into two groups with 0 as the cut-off point. Group 0 had a nearly 10-fold increased risk compared to group ≥ 0. According to the Kaplan-Meier curve, survival rate in the 0-test set was lower than in the difG3BP1 ≥ 0 group (P < 0.001), which was also similarly in the validation set. For the subgroup analysis, group 0 presented a very high risk of progression to the disease regardless of MELD scores in high-risk or low-risk groups. This indicated that G3BP1 was good for prognostic assess
To further compare the predictive efficacy of G3BP1, three inflammatory factors (TNF-α, IL-1β and IL-18) closely related to liver failure were selected to predict this disease. The results showed that both TNF-α and IL-1β were closely related to the prognosis of liver failure before and after adjustment. However, its risk coefficient was lower when compared with G3BP1, suggesting that G3BP1 was more effective in predicting liver failure than the inflammatory factors. For the random forest prediction model, the most importance evaluation index were LDH and AFP. It was consistent with the previous studies, elevated AFP levels can be used as evidence for liver regeneration[28,29], increased LDH reflected the hepatocyte injury[30]. AFP is a glycoprotein with a molecular weight of 70 kDa produced by fetal liver and yolk sac during embryonic development, which was detected by Bergstrand et al[31] in human fetal serum in 1956. AFP can promote the proliferation of parenchymal cells and prevent cell apoptosis induced by various factors. When under the liver injury, it will continuously proliferate and grow[32,33]. LDH is a cytoplasmic enzyme widely expressed in tissues. When starved of oxygen, this enzyme converts pyruvate into lactic acid[34]. Clinical studies have shown that multi-system diseases, such as hepatic encephalopathy[35], kidney injury[36], lung injury[37] and myocardial injury[38], can be accompanied by elevated serum LDH levels. The level and activity of LDH in the ALF mice liver are increased, and the LDH inhibitors can reduce liver damage and improve the survival rate of ALF mice[39]. Compared with other indicators, G3BP1 had the highest C index value and better consistency. It was also more stable in the validation set. Modeling with AFP + LDH + G3BP1, the C index in the test set and validation set were respectively 0.84 and 0.8, which could better assist clinical practice. Therefore, the combination of G3BP1, AFP and LDH modeling ability index could well predict the endpoint of ALF and ACLF patients treated with ALSS.
In conclusion, this study retrospectively analyzed the clinical characteristics and laboratory indicators of ALF and ACLF patients treated with ALSS. It was found that G3BP1, AFP, LDH, TNF-α and IL-1β were independent risk factors. G3BP1 was the most effective predictor of liver failure. The model established by G3BP1, AFP and LDH had high predictive value. The selected laboratory indexes are objective, and easy to detect, which has low cost and was simple to calculate. This model could help clinicians effectively identify the risk of death in ALF and ACLF patients treated ALSS. However, there are still limitations in this study. For example, the study population is Chinese, and further research is needed considering regional differences. Due to the limited sample size, more in-depth exploration of multi-center prospective large sample studies is still needed in the future study. Therefore, G3BP1 could effectively predict liver failure, which has great value to timely provide liver transplantation opportunity for patients who have failed for drug and ALSS treatment. It can effectively reduce the fatality rate and improve the prognosis of patients.
Acute liver failure (ALF) and acute on chronic liver failure (ACLF) are the most common causes of liver disease death. As an effective treatment, Artificial liver support system (ALSS) can reduce bilirubin in a short term, improve inflammatory storm, regulate immunity, which has been widely used in the treatment of ALF and ACLF. However, there is no clinical predict model could evaluate the prognosis of patients with ALF and ACLF treated with ALSS. G3BP1 could inhibit inflammatory response, and the increased expression of G3BP1 was positively correlated with the prognosis of liver failure. However, there is no correlation between G3BP1 and the prognosis for the liver failure patients treated by ALSS.
The significant characteristics of ALF and ACLF are rapid progression of the disease, which can lead to rapid occurrence of extrahepatic organ failure, manifested as liver coma or hepatorenal syndrome, leading to high mortality rate and extremely poor prognosis. Therefore, early judgment of prognosis and timely intervention during the progression of ALF and ACLF are of great significance to improving survival rate.
The purpose of this study was to verify the predictive efficacy of G3BP1 in patients with ALF and ACLF treated with ALSS, and to provide reference for the clinical development of a new prediction model. Based on the above studies, we investigated whether G3BP1 can predict the prognosis of patients with ALF and ACLF, and provide a basis for clinical decision-making programs and timing.
A total of 244 patients with ALF and ACLF were enrolled in this study. The levels of G3BP1 on admission and at discharge were detected. The validation set of 514 patients was collected to verify the predicted effect of G3BP1 and the viability of prognosis. Univariate and multivariate Cox proportional hazards models were used to detect G3BP1, clinical biomarkers [lactate dehydrogenase (LDH), alpha-fetoprotein (AFP) and prothrombin time (PT), etc.], and inflammatory factors [tumor necrosis factor-α (TNF-α), interlenkin (IL)-1β and IL-18] for the risk of progression after ALSS treatment. We treated biomarkers as continuous traits (log-transformed) or as 3-level categorical variables (low, medium, and high) defined according to the tertials of the biomarker level. We developed a reference Cox proportional hazards model for progression after ALSS treatment of liver failure in the training cohort and tested whether the inclusion of biomarker levels further improved risk prediction. The random forest success rate curve model of R software was used to select the survival prognosis factors of patients in the training set. Stepwise Cox regression was used to evaluate the number of variables with the concordance index (C-index) value to obtain the best C-index value with fewer prognostic factors.
The value of difference of G3BP1 between the value of discharge and admission (difG3BP1) was then cut into two groups with 0 as the cut-off point. Group 0 had a nearly 10-fold increased risk compared to group ≥ 0. According to the Kaplan-Meier curve, survival rate in the 0-test set was lower than in the difG3BP1 ≥ 0 group (P < 0.001), which was also similarly in the validation set. For the subgroup analysis, group 0 presented a very high risk of progression to the disease regardless of model for end-stage liver disease scores in high-risk or low-risk groups. This indicated that G3BP1 was good for prognostic assessment. Moreover, TNF-α and IL-1β were closely related to the prognosis of liver failure before and after adjustment. However, its risk coefficient was lower when compared with G3BP1, suggesting that G3BP1 was more effective in predicting liver failure than the inflammatory factors. For the random forest prediction model, the most importance evaluation index were LDH and AFP. Compared with other indicators, G3BP1 had the highest C index value and better consistency. It was also more stable in the validation set. Modeling with AFP + LDH + G3BP1, the C index in the test set and validation set were respectively 0.84 and 0.8, which could better assist clinical practice. Therefore, the combination of G3BP1, AFP and LDH modeling ability index could well predict the endpoint of ALF and ACLF patients treated with ALSS.
For the clinical characteristics and laboratory indicators of ALF and ACLF patients treated with ALSS, G3BP1, AFP, LDH, TNF-α and IL-1β were independent risk factors. G3BP1 was the most effective predictor of liver failure. The model established by G3BP1, AFP and LDH had high predictive value. The selected laboratory indexes are objective, and easy to detect, which has low cost and was simple to calculate. This model could help clinicians effectively identify the risk of death in ALF and ACLF patients treated ALSS.
G3BP1 could effectively predict liver failure, which has great value to timely provide liver transplantation opportunity for patients who have failed for drug and ALSS treatment. The combination of G3BP1, AFP and LDH could accurately evaluate the disease condition and predict the clinical endpoint of patients. Based on the predicted role of G3BP1, it can effectively reduce the fatality rate and improve the prognosis of patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar R, India S-Editor: Zhang H L-Editor: A P-Editor: Yuan YY
| 1. | Maiwall R, Sarin SK. Plasma Exchange in Acute and Acute on Chronic Liver Failure. Semin Liver Dis. 2021;41:476-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Maiwall R, Bajpai M, Choudhury AK, Kumar A, Sharma MK, Duan Z, Yu C, Hu J, Ghazinian H, Ning Q, Ma K, Lee GH, Lim SG, Shah S, Kalal C, Dokmeci A, Kumar G, Jain P, Rao Pasupuleti SS, Paulson I, Kumar V, Sarin SK; AARC working Party. Therapeutic plasma-exchange improves systemic inflammation and survival in acute-on-chronic liver failure: A propensity-score matched study from AARC. Liver Int. 2021;41:1083-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Vasques F, Cavazza A, Bernal W. Acute liver failure. Curr Opin Crit Care. 2022;28:198-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Ngu NLY, Flanagan E, Bell S, Le ST. Acute-on-chronic liver failure: Controversies and consensus. World J Gastroenterol. 2023;29:232-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Siddiqui MS, Stravitz RT. Intensive care unit management of patients with liver failure. Clin Liver Dis. 2014;18:957-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Tujios S, Stravitz RT, Lee WM. Management of Acute Liver Failure: Update 2022. Semin Liver Dis. 2022;42:362-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 7. | Olivo R, Guarrera JV, Pyrsopoulos NT. Liver Transplantation for Acute Liver Failure. Clin Liver Dis. 2018;22:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, Shi D, Jiang J, Sun S, Jin L, Ye P, Yang L, Lu Y, Li T, Huang J, Xu X, Chen J, Hao S, Chen Y, Xin S, Gao Z, Duan Z, Han T, Wang Y, Gan J, Feng T, Pan C, Li H, Huang Y, Xie Q, Lin S, Li L; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67:2181-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 298] [Article Influence: 42.6] [Reference Citation Analysis (2)] |
| 9. | Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P, Hopf C, Alessandria C, Rodriguez E, Solis-Muñoz P, Laleman W, Trebicka J, Zeuzem S, Gustot T, Mookerjee R, Elkrief L, Soriano G, Cordoba J, Morando F, Gerbes A, Agarwal B, Samuel D, Bernardi M, Arroyo V; CANONIC study investigators of the EASL-CLIF Consortium. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 735] [Article Influence: 66.8] [Reference Citation Analysis (1)] |
| 10. | Choudhury A, Jindal A, Maiwall R, Sharma MK, Sharma BC, Pamecha V, Mahtab M, Rahman S, Chawla YK, Taneja S, Tan SS, Devarbhavi H, Duan Z, Yu C, Ning Q, Jia JD, Amarapurkar D, Eapen CE, Goel A, Hamid SS, Butt AS, Jafri W, Kim DJ, Ghazinian H, Lee GH, Sood A, Lesmana LA, Abbas Z, Shiha G, Payawal DA, Dokmeci AK, Sollano JD, Carpio G, Lau GK, Karim F, Rao PN, Moreau R, Jain P, Bhatia P, Kumar G, Sarin SK; APASL ACLF Working Party. Liver failure determines the outcome in patients of acute-on-chronic liver failure (ACLF): comparison of APASL ACLF research consortium (AARC) and CLIF-SOFA models. Hepatol Int. 2017;11:461-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 11. | Larsen FS. Artificial liver support in acute and acute-on-chronic liver failure. Curr Opin Crit Care. 2019;25:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Omer A, Barrera MC, Moran JL, Lian XJ, Di Marco S, Beausejour C, Gallouzi IE. G3BP1 controls the senescence-associated secretome and its impact on cancer progression. Nat Commun. 2020;11:4979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 13. | Li W, Wang Y. Stress granules: potential therapeutic targets for infectious and inflammatory diseases. Front Immunol. 2023;14:1145346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 14. | Paget M, Cadena C, Ahmad S, Wang HT, Jordan TX, Kim E, Koo B, Lyons SM, Ivanov P, tenOever B, Mu X, Hur S. Stress granules are shock absorbers that prevent excessive innate immune responses to dsRNA. Mol Cell. 2023;83:1180-1196.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 71] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 15. | Li W, Li W, Li X, Wang L, Wang Y. Effect of P53 nuclear localization mediated by G3BP1 on ferroptosis in acute liver failure. Apoptosis. 2023;28:1226-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Li WY, Yang F, Li X, Wang LW, Wang Y. Stress granules inhibit endoplasmic reticulum stress-mediated apoptosis during hypoxia-induced injury in acute liver failure. World J Gastroenterol. 2023;29:1315-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 17. | Gan JH, Zhou XQ, Qin AL, Luo EP, Zhao WF, Yu H, Xu J. Hybrid artificial liver support system for treatment of severe liver failure. World J Gastroenterol. 2005;11: 890-894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Wang Y, Chen Q, Jiao F, Shi C, Pei M, Wang L, Gong Z. Histone deacetylase 2 regulates ULK1 mediated pyroptosis during acute liver failure by the K68 acetylation site. Cell Death Dis. 2021;12:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Wang Y, Li X, Chen Q, Jiao F, Shi C, Pei M, Wang L, Gong Z. The relationship between liver pathological inflammation degree and pyroptosis in chronic hepatitis B patients. J Med Virol. 2021;93:6229-6235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Zhang H, Chen Q, Jiao F, Shi C, Pei M, Lv J, Wang L, Gong Z. TNF-α/HMGB1 inflammation signalling pathway regulates pyroptosis during liver failure and acute kidney injury. Cell Prolif. 2020;53:e12829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 21. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 590] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 22. | García Martínez JJ, Bendjelid K. Artificial liver support systems: what is new over the last decade? Ann Intensive Care. 2018;8:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 23. | Alshamsi F, Alshammari K, Belley-Cote E, Dionne J, Albrahim T, Albudoor B, Ismail M, Al-Judaibi B, Baw B, Subramanian RM, Steadman R, Galusca D, Huang DT, Nanchal R, Al Quraini M, Yuan Y, Alhazzani W; GUIDE Group. Extracorporeal liver support in patients with liver failure: a systematic review and meta-analysis of randomized trials. Intensive Care Med. 2020;46:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Liu H, Zhang Q, Liu L, Cao Y, Ye Q, Liu F, Liang J, Wen J, Li Y, Han T. Effect of artificial liver support system on short-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure. Artif Organs. 2020;44:E434-E447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Wang B, Zhang L, Dai T, Qin Z, Lu H, Zhou F. Liquid-liquid phase separation in human health and diseases. Signal Transduct Target Ther. 2021;6:290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 489] [Cited by in RCA: 355] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 26. | Chen W, Chen S, Yan C, Zhang Y, Zhang R, Chen M, Zhong S, Fan W, Zhu S, Zhang D, Lu X, Zhang J, Huang Y, Zhu L, Li X, Lv D, Fu Y, Iv H, Ling Z, Ma L, Jiang H, Long G, Zhu J, Wu D, Wu B, Sun B. Allergen protease-activated stress granule assembly and gasdermin D fragmentation control interleukin-33 secretion. Nat Immunol. 2022;23:1021-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 27. | Gao P, Liu Y, Wang H, Chai Y, Weng W, Zhang Y, Zhou L, Ge X, Guo X, Han J, Yang H. Viral evasion of PKR restriction by reprogramming cellular stress granules. Proc Natl Acad Sci U S A. 2022;119:e2201169119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 28. | Schiødt FV, Ostapowicz G, Murray N, Satyanarana R, Zaman A, Munoz S, Lee WM. Alpha-fetoprotein and prognosis in acute liver failure. Liver Transpl. 2006;12:1776-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Kakisaka K, Kataoka K, Onodera M, Suzuki A, Endo K, Tatemichi Y, Kuroda H, Ishida K, Takikawa Y. Alpha-fetoprotein: A biomarker for the recruitment of progenitor cells in the liver in patients with acute liver injury or failure. Hepatol Res. 2015;45:E12-E20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Akbari G. Role of Zinc Supplementation on Ischemia/Reperfusion Injury in Various Organs. Biol Trace Elem Res. 2020;196:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Bergstrand CG, Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest. 1956;8:174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 319] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Mizejewski GJ. The alpha-fetoprotein third domain receptor binding fragment: in search of scavenger and associated receptor targets. J Drug Target. 2015;23:538-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Hayashi S, Nagaoka K, Tanaka Y. Blood-Based Biomarkers in Hepatitis B Virus-Related Hepatocellular Carcinoma, Including the Viral Genome and Glycosylated Proteins. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Laganá G, Barreca D, Calderaro A, Bellocco E. Lactate Dehydrogenase Inhibition: Biochemical Relevance and Therapeutical Potential. Curr Med Chem. 2019;26:3242-3252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 35. | Bosoi CR, Rose CF. Elevated cerebral lactate: Implications in the pathogenesis of hepatic encephalopathy. Metab Brain Dis. 2014;29:919-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Zager RA, Johnson AC, Becker K. Renal Cortical Lactate Dehydrogenase: A Useful, Accurate, Quantitative Marker of In Vivo Tubular Injury and Acute Renal Failure. PLoS One. 2013;8:e66776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | Drent M, Cobben NA, Henderson RF, Wouters EF, van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9:1736-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 365] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 38. | Martins JT, Li DJ, Baskin LB, Jialal I, Keffer JH. Comparison of cardiac troponin I and lactate dehydrogenase isoenzymes for the late diagnosis of myocardial injury. Am J Clin Pathol. 1996;106:705-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Ferriero R, Nusco E, De Cegli R, Carissimo A, Manco G, Brunetti-Pierri N. Pyruvate dehydrogenase complex and lactate dehydrogenase are targets for therapy of acute liver failure. J Hepatol. 2018;69:325-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |