Published online Feb 27, 2024. doi: 10.4254/wjh.v16.i2.211
Peer-review started: December 1, 2023
First decision: December 12, 2023
Revised: December 31, 2023
Accepted: January 30, 2024
Article in press: January 30, 2024
Published online: February 27, 2024
Processing time: 88 Days and 3.3 Hours
Chronic liver disease (CLD) was associated with adverse clinical outcomes among people with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
To determine the effects of SARS-CoV-2 infection on the incidence and treatment strategy of hepatocellular carcinoma (HCC) among patients with CLD.
A retrospective, territory-wide cohort of CLD patients was identified from an electronic health database in Hong Kong. Patients with confirmed SARS-CoV-2 infection [coronavirus disease 2019 (COVID-19)+CLD] between January 1, 2020 and October 25, 2022 were identified and matched 1:1 by propensity-score with those without (COVID-19-CLD). Each patient was followed up until death, outcome event, or November 15, 2022. Primary outcome was incidence of HCC. Secondary outcomes included all-cause mortality, adverse hepatic outcomes, and different treatment strategies to HCC (curative, non-curative treatment, and palliative care). Analyses were further stratified by acute (within 20 d) and post-acute (21 d or beyond) phases of SARS-CoV-2 infection. Incidence rate ratios (IRRs) were estimated by Poisson regression models.
Of 193589 CLD patients (> 95% non-cirrhotic) in the cohort, 55163 patients with COVID-19+CLD and 55163 patients with COVID-19-CLD were included after 1:1 propensity-score matching. Upon 249-d median follow-up, COVID-19+CLD was not associated with increased risk of incident HCC (IRR: 1.19, 95%CI: 0.99-1.42, P = 0.06), but higher risks of receiving palliative care for HCC (IRR: 1.60, 95%CI: 1.46-1.75, P < 0.001), compared to COVID-19-CLD. In both acute and post-acute phases of infection, COVID-19+CLD were associated with increased risks of all-cause mortality (acute: IRR: 7.06, 95%CI: 5.78-8.63, P < 0.001; post-acute: IRR: 1.24, 95%CI: 1.14-1.36, P < 0.001) and adverse hepatic outcomes (acute: IRR: 1.98, 95%CI: 1.79-2.18, P < 0.001; post-acute: IRR: 1.24, 95%CI: 1.13-1.35, P < 0.001), compared to COVID-19-CLD.
Although CLD patients with SARS-CoV-2 infection were not associated with increased risk of HCC, they were more likely to receive palliative treatment than those without. The detrimental effects of SARS-CoV-2 infection persisted in post-acute phase.
Core Tip: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with chronic liver disease (CLD) leads to worse adverse clinical outcomes. In our study, we found that although CLD patients with SARS-CoV-2 infection did not have higher risk of developing liver cancer, they are more likely to receive palliative treatment for hepatocellular carcinoma, compared to CLD patients who did not have SARS-CoV-2 infection. Coronavirus disease 2019 also led to increased risks of all-cause mortality and adverse hepatic outcomes. These detrimental effects of SARS-CoV-2 infection were observed in both acute and post-acute phases among CLD patients.
- Citation: Mak LY, Chung MSH, Li X, Lai FTT, Wan EYF, Chui CSL, Cheng FWT, Chan EWY, Cheung CL, Au ICH, Xiong X, Seto WK, Yuen MF, Wong CKH, Wong ICK. Effects of SARS-CoV-2 infection on incidence and treatment strategies of hepatocellular carcinoma in people with chronic liver disease. World J Hepatol 2024; 16(2): 211-228
- URL: https://www.wjgnet.com/1948-5182/full/v16/i2/211.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i2.211
In the year 2020, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection led to coronavirus disease 2019 (COVID-19) pandemic across the globe. The disruption to the routines due to social distancing measures has affected all sectors of the society. Healthcare systems were particularly stretched by the enormous influx of SARS-CoV-2 infected patients, inevitably leading to change in clinical practice such as adoption of virtual consultations, suspension of healthcare services including cancellation of planned investigations, procedures and treatments. Colorectal cancer and lung cancer are examples of chronic conditions that were negatively influenced by the pandemic, with significant delays in screening, diagnosis and workup. In subjects with chronic liver disease (CLD), SARS-CoV-2 infection has been associated with an increased risk of short-term mortality, predominantly caused by respiratory failure and observed in cirrhotic patients[1-4]. In comparison, it remains controversial whether SARS-CoV-2 infection increases the risk of non-respiratory causes of death among non-cirrhotic CLD, a condition that affects 1.5 billion persons globally[5,6]. There is no data regarding how COVID-19 interplay with the risk of hepatocellular carcinoma (HCC) and subsequent treatment strategy, which not only depends on the general performance status of the subject, the liver reserve, and the tumor status[7], but also the relative resource allocation within the health care system in the event of the pandemic.
Much is unknown regarding precisely how COVID-19 affects prognosis and liver outcomes in CLD. In particular, the detrimental effects of SARS-CoV-2 infection seem to linger beyond the acute phase of infection and are associated with a number of conditions, collectively termed ‘post-acute sequelae of SARS-CoV-2 infection’ (PASC), also known as ‘long COVID’ or ‘post-COVID-19 syndrome’[8,9]. Among the numerous conditions associated with PASC (e.g., pulmonary, neuropsychiatric, gastrointestinal, endocrine, renal, etc.), hepatic effects of recent SARS-CoV-2 infection have not been well-characterized[10]. In addition, it was hypothesized that SARS-CoV-2 infection will lead to long-lasting impacts on the quality of cirrhosis care, resulting from the initial intense period of prioritization of healthcare services with delays in routine care, and subsequent return of backlog presentations of illness and protracted period of suboptimal outcomes[11]. Therefore, it is important to understand the immediate and long-term consequences of SARS-CoV-2 infection in patients with CLD, and how they affect the incidence and oncological treatment for HCC.
In this study, we determined the risk of incident HCC, all-cause mortality, adverse hepatic outcomes, and the impact on treatment strategies for patients with liver cancer in a large cohort of patients with CLD and laboratory proven SARS-CoV-2 infection, in comparison to a contemporaneous cohort of patients with CLD who did not have SARS-CoV-2 infection in Hong Kong.
Our data were extracted from territory-wide cohort of patients with anonymized electronic health records provided by the Hong Kong Hospital Authority (HA), and COVID-19 vaccination records were available from the Department of Health (DH), The Government of Hong Kong Special Administrative Region. Electronic medical records of patients with COVID-19 were retrieved from the HA, and included demographics, disease diagnoses, drug prescriptions, laboratory tests, hospital admissions, emergency departments, and inpatient procedures. The HA data were linked to the COVID-19 vaccination records provided by the DH using the unique identification numbers. This linked database has been used extensively for studies on COVID-19 vaccine safety[12-14] and PASC[15].
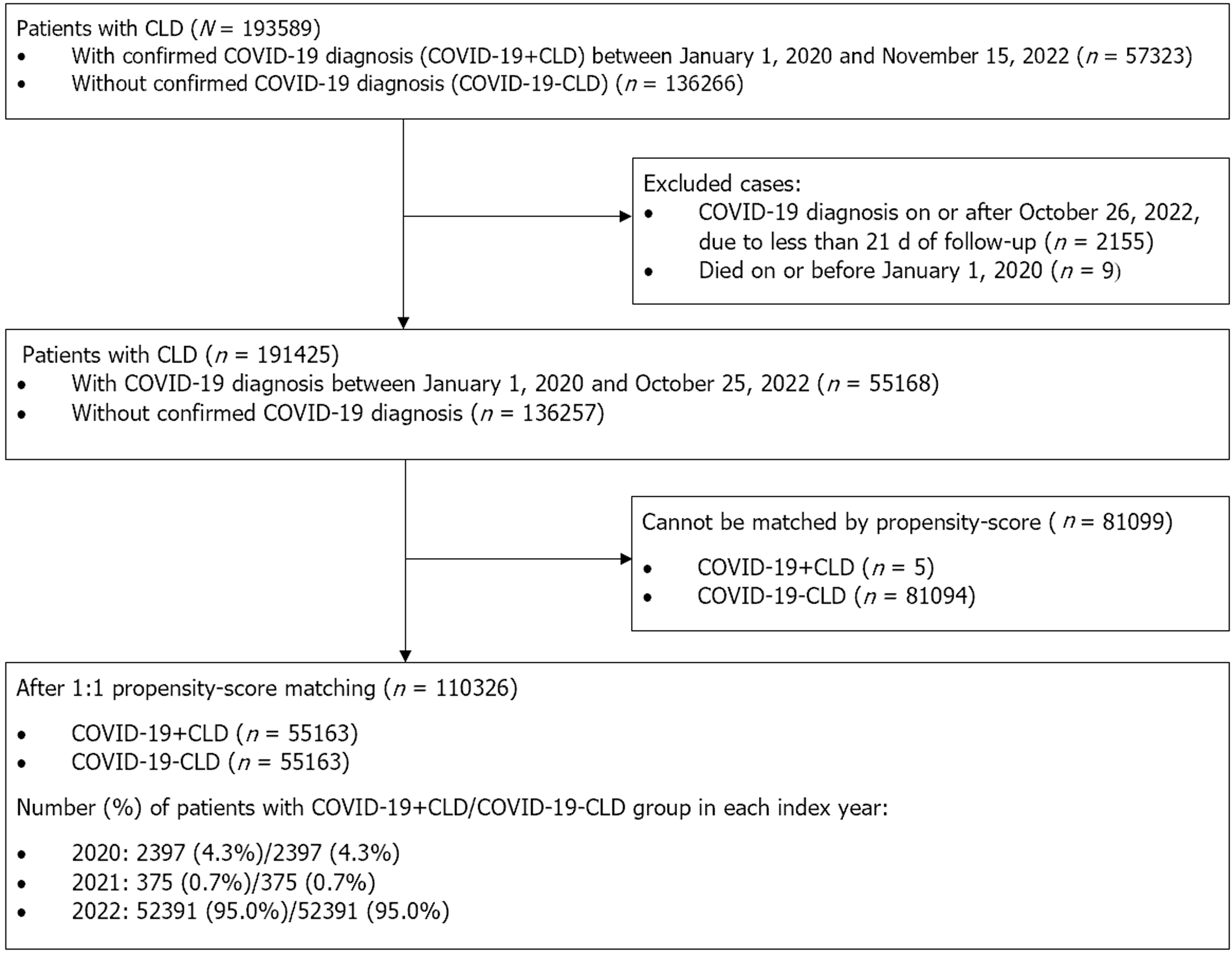
This study included patients with CLD between January 1, 2020 and November 15, 2022. CLD was defined as patients having any of the following diagnoses: (1) Viral hepatitis B (HBV) infection; (2) viral hepatitis C; (3) chronic hepatitis; (4) fatty liver disease; (5) alcoholic liver disease (ALD); (6) alcoholic hepatitis; (7) Wilson’s disease, (8) autoimmune hepatitis; and (9) primary biliary cholangitis and primary sclerosing cholangitis. Each of the above diseases was identified by International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis code, prescription of hepatitis antivirals, or positive hepatitis B surface antigen test (Supplementary Table 1). Patients who were identified by a positive result on the SARS-CoV-2 reverse transcription polymerase chain reaction test or rapid antigen test during the observational period were classified into the COVID-19+CLD group. The index date was set at the first date of SARS-CoV-2 infection for patients in the COVID-19 group (i.e. only the first infection was eligible for analysis). Patients who did not have confirmed SARS-CoV-2 infection during the observational period were classified into the control group, i.e. COVID-19-CLD. The pseudo-index date of COVID-19-CLD patients was set at the first date of the respective year (i.e., January 1, 2020, January 1, 2021, or January 1, 2022) to maintain a similar follow-up period between the COVID-19+CLD and COVID-19-CLD groups. Patients in the COVID-19-CLD group were matched 1:1 by propensity-score with patients in the COVID-19+CLD group of each index year sequentially starting from 2020 until 2022, and without replacement. Unmatched control patients were eligible for matching with COVID-19+CLD patients in the subsequent index year, with the baseline characteristics of COVID-19-CLD groups updated using the new pseudo-index date (i.e. January 1 of the following year). Each patient was observed from the index or pseudo-index date to the occurrence of outcomes, death, or the end of observational period (i.e., November 15, 2022), whichever occurred earlier.
Patients who died on or before the index date, or had less than 21 d of follow-up (i.e., patients with COVID-19 diagnosed on or after October 26, 2022) were further excluded[16].
The primary study outcome was HCC incidence. The secondary outcomes included: (1) All-cause mortality; (2) adverse hepatic outcomes cirrhosis, HCC, liver decompensation (composite outcome including hepatorenal syndrome, liver failure, hepatic coma/encephalopathy, ascites, and variceal bleeding); (3) curative treatment to HCC (hepatic resection, liver transplantation, radiofrequency ablation of liver); (4) non-curative treatment to HCC (transarterial chemoembolization, radiotherapy to liver, systemic chemotherapy or immunotherapy); and (5) palliative care.
Each of the above outcomes was identified by ICD-9-CM diagnosis and procedure code, prescription of antivirals for hepatitis, and fibrosis-4 index (FIB-4)[17] (Supplementary Table 1).
The risks of study outcomes during the acute and post-acute phase of SARS-CoV-2 infection were further stratified and analyzed among those with index date at 2022 amid the Omicron predominance period. The acute phase of infection was defined as the first 20 d after the index date[18] and the post-acute phase of infection was defined from 21 d of the index date onwards. For the analysis of the post-acute phase of infection when the index date was set to 21 d after COVID-19 diagnosis, patients who died within 20 d of the index date, or had less than 21 d of follow-up were excluded.
Baseline characteristics were captured based on ICD-9-CM diagnosis, procedure codes, and treatment records as follows: age, sex, pre-existing comorbidities [Charlson Comorbidity Index (CCI), cirrhosis, HCC, liver decompensation], oncological treatment received prior to the index date (curative treatment to HCC, non-curative treatment to HCC, palliative care), and COVID-19 vaccination status (Supplementary Table 1). The FIB-4[17] was also used to enhance case identification for cirrhosis. Fully vaccinated patients were defined as those with at least two doses of BNT162b2 (Comirnaty) or three doses of COVID-19 Vaccine (Vero Cell), Inactivated (CoronaVac)[19].
Descriptive statistics of baseline characteristics between the COVID-19 groups and matched control groups were presented as mean and standard deviation, or median and interquartile range (IQR) for continuous variables, and count and proportion for categorical variables.
We constructed propensity-score models conditional on age, sex, CCI, and COVID-19 vaccination status in a logistic regression model. We performed 1:1 propensity-score matching using a caliper width of 0.05. Standardized mean differences (SMDs) of each covariate between the groups after propensity-score matching were calculated, which was interpreted as balanced when the SMD was below the threshold of 0.1[20]. The incidence rate ratio (IRR) and corresponding 95% confidence intervals (CIs) were estimated using the Poisson regression model.
Subgroup analyses were performed on several patient groups, including age groups (≤ 50 vs > 50 years), causes of CLD (HBV vs other causes), the presence of cirrhosis, the presence of multiorgan dysfunction, COVID-19 vaccination status (fully vaccinated vs not fully vaccinated), respective years of COVID-19 diagnosis (year of 2020 vs 2021 vs 2022). Multiorgan dysfunction was defined as patients having any 3 or more organ system malfunctions in the following categories, including: (1) Neurological; (2) psychiatric; (3) respiratory; (4) cardiovascular; (5) hematologic; (6) endocrine; (7) nephrological; (8) hepatic; (9) gastrointestinal; and (10) dermatologic disorder. Each subgroup analysis was re-constructed with a new propensity-score model, and the pairs of patients with COVID-19 and their respective controls were rematched. Furthermore, subgroup analyses among COVID-19+CLD patients were performed on two patient groups, including hospitalization groups (hospitalized vs non-hospitalized) and receipt of antiviral medications for COVID-19 infection. COVID-19+CLD patients were identified as antiviral users if they received any of the following antiviral medications, including: (1) Molnupiravir; (2) nirmatrelvir/ritonavir; and (3) remdesivir. Each subgroup analysis among COVID-19+CLD patients was also re-constructed with a new propensity-score model, and rematched between hospitalized and non-hospitalized patients or antiviral users and non-users, respectively.
All statistical analyses were performed using Stata (version 17). The analyses were conducted by Chung MSH and analyzed independently by Xi X and Au ICH for quality assurance. All significance tests were two-tailed, where P values < 0.05 were considered statistically significant.
This work was supported by a research grant from Collaborative Research Fund, University Grants Committee, HKSAR Government (principal investigator, Wong ICK; reference no. C7154-20GF). Lai FTT, Wong CKH, and Wong ICK are partially supported by the Laboratory of Data Discovery for Health (D24H) funded by AIR@InnoHK administered by Innovation and Technology Commission. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
A total of 193589 CLD patients of whom 57323 patients had confirmed SARS-CoV-2 infection between January 1, 2020 and November 15, 2022 in Hong Kong, and 136266 CLD patients without SARS-CoV-2 infection were identified (Figure 1). After applying the exclusion criteria followed by 1:1 propensity-score matching, 55163 patients with COVID-19 and CLD (COVID-19+CLD) and 55163 matched controls (COVID-19-CLD) were included in the present study. The baseline characteristics are presented in Table 1. Baseline age (58.8 vs 58.7), gender (male gender: 51.2% vs 51.6%), medical comorbidities (CCI: 3.2 vs 3.1), and COVID-19 vaccination status (fully vaccinated: 50.1% vs 50.0%) were balanced between the two groups. Additionally, underlying cirrhosis (3.4% vs 3.1%), decompensated liver disease (2.8% vs 2.6%), HCC (2.2% vs 2.2%), and previous treatment for HCC (curative: 2.0% vs 1.9%; non-curative: 4.3% vs 3.9%; palliative care: 3.3% vs 3.0%) were matched between COVID-19 patients with CLD and controls with CLD (all SMD < 0.1). Of note, the majority (95.0%) of included subjects came from year 2022 when the omicron strain of SARS-CoV-2 was ubiquitous.
| Baseline characteristics | After matching | |||||||||||||||||||
| 2020-2022 | 2020 | 2021 | 2022 | |||||||||||||||||
| COVID-19 patients (n = 55163) | Control (n = 55163) | SMD | COVID-19 patients (n = 2397) | Control (n = 2397) | SMD | COVID-19 patients (n = 375) | Control (n = 375) | SMD | COVID-19 patients (n = 52391) | Control (n = 52391) | SMD | |||||||||
| n/Mean | %/SD | n/Mean | %/SD | n/Mean | %/SD | n/Mean | %/SD | n/Mean | %/SD | n/Mean | %/SD | n/Mean | %/SD | n/Mean | %/SD | |||||
| Age, yr1 | 58.8 | 14.7 | 58.7 | 13.9 | 0.01 | 54.8 | 17.2 | 54.5 | 16.2 | 0.02 | 57.6 | 15.6 | 57.5 | 14.2 | 0 | 59 | 14.5 | 58.9 | 13.8 | 0.01 |
| ≤ 50 | 15482 | -28.1 | 14659 | -26.6 | 0.03 | 878 | -36.6 | 906 | -37.8 | 0.02 | 127 | -33.9 | 110 | -29.3 | 0.1 | 14477 | -27.6 | 13643 | -26 | 0.04 |
| > 50 | 39681 | -71.9 | 40504 | -73.4 | 1519 | -63.4 | 1491 | -62.2 | 248 | -66.1 | 265 | -70.7 | 37914 | -72.4 | 38748 | -74 | ||||
| Sex | 0.01 | 0.03 | 0.05 | 0.01 | ||||||||||||||||
| Male | 28244 | -51.2 | 28484 | -51.6 | 1274 | -53.1 | 1244 | -51.9 | 192 | -51.2 | 183 | -48.8 | 26778 | -51.1 | 27057 | -51.6 | ||||
| Female | 26919 | -48.8 | 26679 | -48.4 | 1123 | -46.9 | 1153 | -48.1 | 183 | -48.8 | 192 | -51.2 | 25613 | -48.9 | 25334 | -48.4 | ||||
| Fully vaccinated | 27633 | -50.1 | 27576 | -50 | 0 | 0 | 0 | 0 | 0 | NA | 0 | 0 | 0 | 0 | NA | 27633 | -52.7 | 27576 | -52.6 | 0 |
| Pre-existing comorbidities | ||||||||||||||||||||
| Charlson's Comorbidity Index1,2 | 3.2 | 2.3 | 3.1 | 2.2 | 0.04 | 2.3 | 1.9 | 2.4 | 1.9 | 0 | 2.5 | 1.8 | 2.5 | 1.6 | 0 | 3.2 | 2.3 | 3.1 | 2.2 | 0.05 |
| 0-4 | 42972 | -77.9 | 44994 | -81.6 | 0.09 | 2153 | -89.8 | 2145 | -89.5 | 0.01 | 334 | -89.1 | 340 | -90.7 | 0.11 | 40485 | -77.3 | 42509 | -81.1 | 0.1 |
| 5-6 | 8338 | -15.1 | 7168 | -13 | 191 | -8 | 200 | -8.3 | 33 | -8.8 | 32 | -8.5 | 8114 | -15.5 | 6936 | -13.2 | ||||
| 7-16 | 3853 | -7 | 3001 | -5.4 | 53 | -2.2 | 52 | -2.2 | 8 | -2.1 | 3 | -0.8 | 3792 | -7.2 | 2946 | -5.6 | ||||
| Cirrhosis | 1898 | -3.4 | 1737 | -3.1 | 0.02 | 11 | -0.5 | 41 | -1.7 | 0.12 | 2 | -0.5 | 4 | -1.1 | 0.06 | 1885 | -3.6 | 1692 | -3.2 | 0.02 |
| HCC | 1199 | -2.2 | 1189 | -2.2 | 0 | 5 | -0.2 | 24 | -1 | 0.1 | 0 | 0 | 2 | -0.5 | 0.1 | 1194 | -2.3 | 1163 | -2.2 | 0 |
| Liver decompensation | 1563 | -2.8 | 1420 | -2.6 | 0.02 | 14 | -0.6 | 41 | -1.7 | 0.11 | 1 | -0.3 | 4 | -1.1 | 0.1 | 1548 | -3 | 1375 | -2.6 | 0.02 |
| Hepatorenal syndrome | 158 | -0.3 | 160 | -0.3 | 0 | 2 | -0.1 | 3 | -0.1 | 0.01 | 0 | 0 | 0 | 0 | 0.07 | 156 | -0.3 | 157 | -0.3 | 0 |
| Liver failure | 298 | -0.5 | 303 | -0.5 | 0 | 5 | -0.2 | 15 | -0.6 | 0.06 | 0 | 0 | 1 | -0.3 | 0.07 | 293 | -0.6 | 287 | -0.5 | 0 |
| Hepatic coma/encephalopathy | 218 | -0.4 | 159 | -0.3 | 0.02 | 0 | 0 | 0 | 0 | NA | 1 | -0.3 | 0 | 0 | 0.07 | 217 | -0.4 | 159 | -0.3 | 0.02 |
| Ascites | 394 | -0.7 | 330 | -0.6 | 0.01 | 5 | -0.2 | 5 | -0.2 | 0 | 0 | 0 | 0 | 0 | NA | 389 | -0.7 | 325 | -0.6 | 0.01 |
| Variceal bleeding | 973 | -1.8 | 842 | -1.5 | 0.02 | 6 | -0.3 | 24 | -1 | 0.1 | 0 | 0 | 3 | -0.8 | 0.13 | 967 | -1.8 | 815 | -1.6 | 0.02 |
| Treatment for HCC used before index date | ||||||||||||||||||||
| Curative treatment to HCC | 1111 | -2 | 1065 | -1.9 | 0.01 | 4 | -0.2 | 25 | -1 | 0.11 | 0 | 0 | 3 | -0.8 | 0.13 | 1107 | -2.1 | 1037 | -2 | 0.01 |
| Non-curative treatment to HCC | 2388 | -4.3 | 2125 | -3.9 | 0.02 | 19 | -0.8 | 53 | -2.2 | 0.12 | 0 | 0 | 4 | -1.1 | 0.15 | 2369 | -4.5 | 2068 | -3.9 | 0.03 |
| Palliative care | 1811 | -3.3 | 1675 | -3 | 0.01 | 20 | -0.8 | 43 | -1.8 | 0.08 | 1 | -0.3 | 8 | -2.1 | 0.17 | 1790 | -3.4 | 1624 | -3.1 | 0.02 |
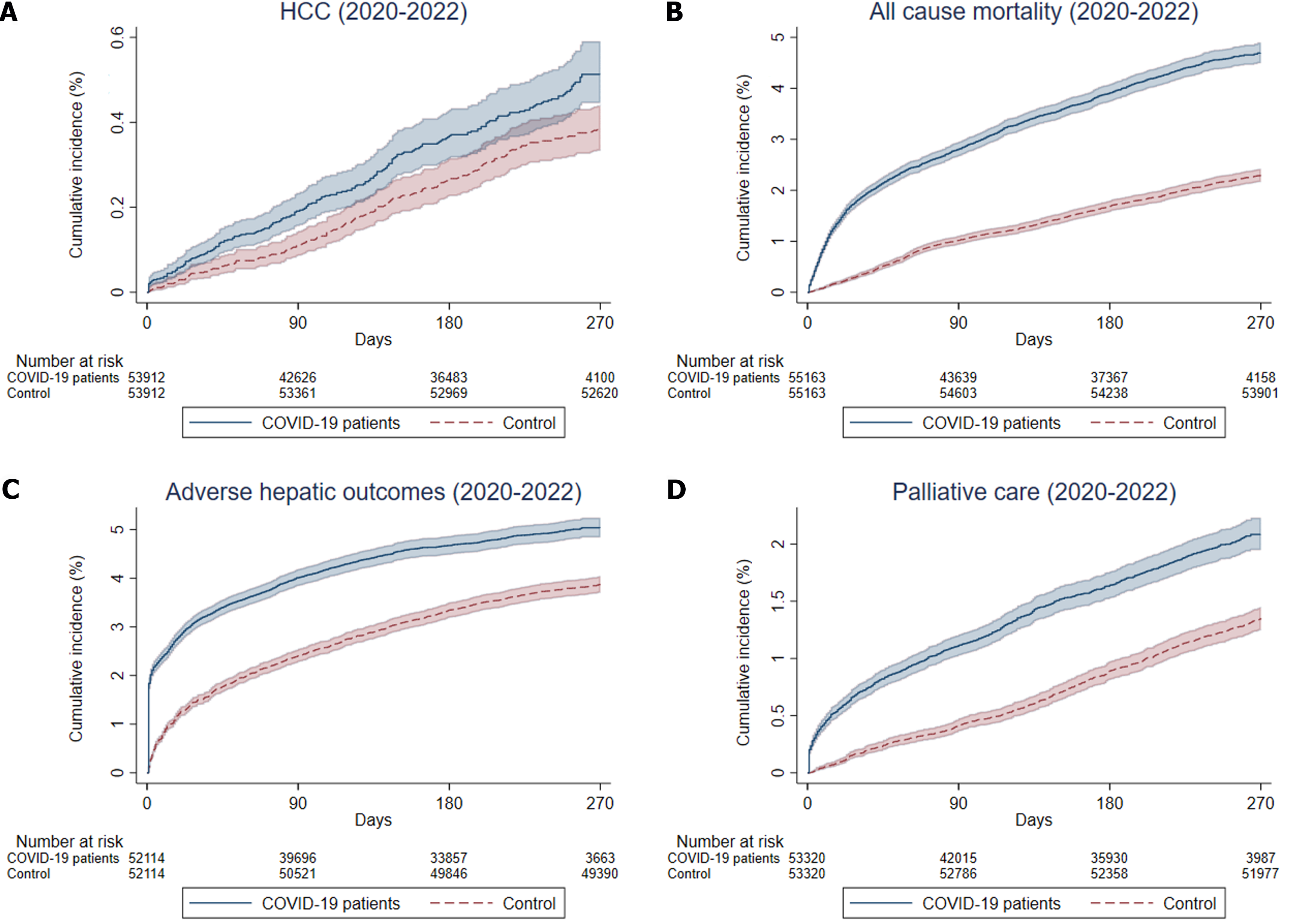
The median follow-up duration was 249 (IQR: 108-259) days in the COVID-19+CLD group and 318 (IQR: 318-318) days in the COVID-19-CLD group. The crude incidence rates of HCC were 64.2 and 54.0 events per 10000 person-years for COVID-19+CLD and COVID-19-CLD, respectively. There was a trend for increased risk of HCC among COVID-19+CLD group compared to COVID-19-CLD group (IRR: 1.19, 95%CI: 0.99-1.42, P = 0.06) but did not reach statistical significance. There were 2273 and 1600 events of all-cause mortality, for COVID-19+CLD group and COVID-19-CLD group, respectively. The crude incidence rates of all-cause mortality were 676.5 (95%CI: 648.9-704.9) events per 10000 person-years (2273 events/33601 person-years) for COVID-19+CLD group, and 306.2 (95%CI: 291.4-321.6) events per 10000 person-years (1600 events/52258 person-years) for COVID-19-CLD group. The cumulative incidence of HCC, all-cause mortality, adverse hepatic outcomes, and palliative care among COVID-19+CLD patients and COVID-19-CLD are shown in Figure 2. COVID-19+CLD patients were associated with significantly higher risks of all-cause mortality (IRR: 2.21, 95%CI: 2.07-2.36, P < 0.001), adverse hepatic outcomes (IRR: 1.74, 95%CI: 1.64-1.85, P < 0.001), which were predominantly contributed by incident cirrhosis (IRR: 1.79, 95%CI: 1.68-1.89, P < 0.001), followed by liver decompensation (IRR: 1.36, 95%CI: 1.17-1.57, P < 0.001), compared to the COVID-19-CLD (Table 2).
| Outcomes | 2020-2022 | ||||||||||||
| COVID-19 patients (n = 55163) | Control (n = 55163) | COVID-19 patients vs control | |||||||||||
| Cumulative incidence | Crude incidence rate | Cumulative incidence | Crude incidence rate | ||||||||||
| (Events/10000 person-yr) | (Events/10000 person-yr) | ||||||||||||
| New events | Rate, % | Estimate | 95%CI | Person-years | New events | Rate, % | Estimate | 95%CI | Person-years | IRR1 | 95% CI | P value | |
| HCC | 211 | 0.39 | 64.2 | (55.8, 73.5) | 32870 | 276 | 0.51 | 54 | (47.8, 60.8) | 51087 | 1.19 | (0.99, 1.42) | 0.06 |
| All-cause mortality | 2273 | 4.12 | 676.5 | (648.9, 704.9) | 33601 | 1600 | 2.9 | 306.2 | (291.4, 321.6) | 52258 | 2.21b | (2.07, 2.36) | < 0.001 |
| Adverse hepatic outcomes | 2407 | 4.62 | 789.3 | (758.1, 821.5) | 30495 | 2183 | 4.19 | 453.5 | (434.7, 473.0) | 48133 | 1.74b | (1.64, 1.85) | < 0.001 |
| Cirrhosis | 2493 | 4.68 | 803.8 | (772.6, 836.0) | 31014 | 2206 | 4.14 | 450.1 | (431.5, 469.2) | 49015 | 1.79b | (1.68, 1.89) | < 0.001 |
| Liver decompensation hepator | 88 | 0.16 | 26.3 | (21.1, 32.4) | 33468 | 106 | 0.19 | 20.4 | (16.7, 24.6) | 52070 | 1.29 | (0.97, 1.71) | 0.08 |
| Liver failure | 71 | 0.13 | 21.3 | (16.6, 26.8) | 33371 | 91 | 0.17 | 17.5 | (14.1, 21.5) | 51902 | 1.21 | (0.89, 1.66) | 0.22 |
| Hepatic coma/encephalopathy | 60 | 0.11 | 17.9 | (13.7, 23.1) | 33454 | 61 | 0.11 | 11.7 | (9.0, 15.1) | 52033 | 1.53a | (1.07, 2.18) | 0.02 |
| Ascites | 129 | 0.24 | 38.7 | (32.3, 46.0) | 33341 | 154 | 0.28 | 29.7 | (25.2, 34.8) | 51882 | 1.30a | (1.03, 1.65) | 0.03 |
| Variceal bleeding | 150 | 0.28 | 45.5 | (38.5, 53.4) | 32977 | 159 | 0.29 | 31 | (26.4, 36.2) | 51316 | 1.47b | (1.17, 1.84) | < 0.001 |
| Curative treatment to HCC | 130 | 0.24 | 39.5 | (33.0, 46.9) | 32892 | 174 | 0.32 | 34 | (29.1, 39.5) | 51156 | 1.16 | (0.93, 1.46) | 0.2 |
| Hepatic resection | 86 | 0.16 | 25.9 | (20.7, 32.0) | 33206 | 109 | 0.2 | 21.1 | (17.3, 25.5) | 51664 | 1.23 | (0.93, 1.63) | 0.16 |
| Liver transplantation | 13 | 0.02 | 3.9 | (2.1, 6.6) | 33442 | 20 | 0.04 | 3.8 | (2.3, 5.9) | 52028 | 1.01 | (0.50, 2.03) | 0.97 |
| Radiofrequency ablation of liver | 51 | 0.09 | 15.3 | (11.4, 20.1) | 33294 | 75 | 0.14 | 14.5 | (11.4, 18.2) | 51784 | 1.06 | (0.74, 1.51) | 0.76 |
| Non-curative treatment to HCC | 394 | 0.75 | 122.5 | (110.7, 135.3) | 32151 | 627 | 1.19 | 125.6 | (116.0, 135.8) | 49916 | 0.98 | (0.86, 1.11) | 0.7 |
| Transarterial chemoembolization | 83 | 0.15 | 25 | (19.9, 31.0) | 33239 | 125 | 0.23 | 24.2 | (20.1, 28.8) | 51691 | 1.03 | (0.78, 1.36) | 0.82 |
| Radiotherapy to liver | 3 | 0.01 | 0.9 | (0.2, 2.6) | 33543 | 3 | 0.01 | 0.6 | (0.1, 1.7) | 52189 | 1.56 | (0.31, 7.71) | 0.59 |
| Systemic chemotherapy or immunotherapy | 351 | 0.66 | 108.2 | (97.2, 120.2) | 32434 | 547 | 1.03 | 108.6 | (99.7, 118.1) | 50363 | 1 | (0.87, 1.14) | 0.96 |
| Palliative care | 924 | 1.73 | 285.8 | (267.7, 304.8) | 32332 | 904 | 1.7 | 179 | (167.5, 191.0) | 50510 | 1.60b | (1.46, 1.75) | < 0.001 |
Among patients with CLD, there were no significant differences in the risks of receiving curative (IRR: 1.16, 95%CI: 0.93-1.46, P = 0.20) or non-curative (IRR: 0.98, 95%CI: 0.86-1.11, P = 0.70) treatment to HCC. COVID-19+CLD patients were at higher chance of receiving palliative care (IRR: 1.60, 95%CI: 1.46-1.75, P < 0.001) compared to COVID-19-CLD patients (Table 2).
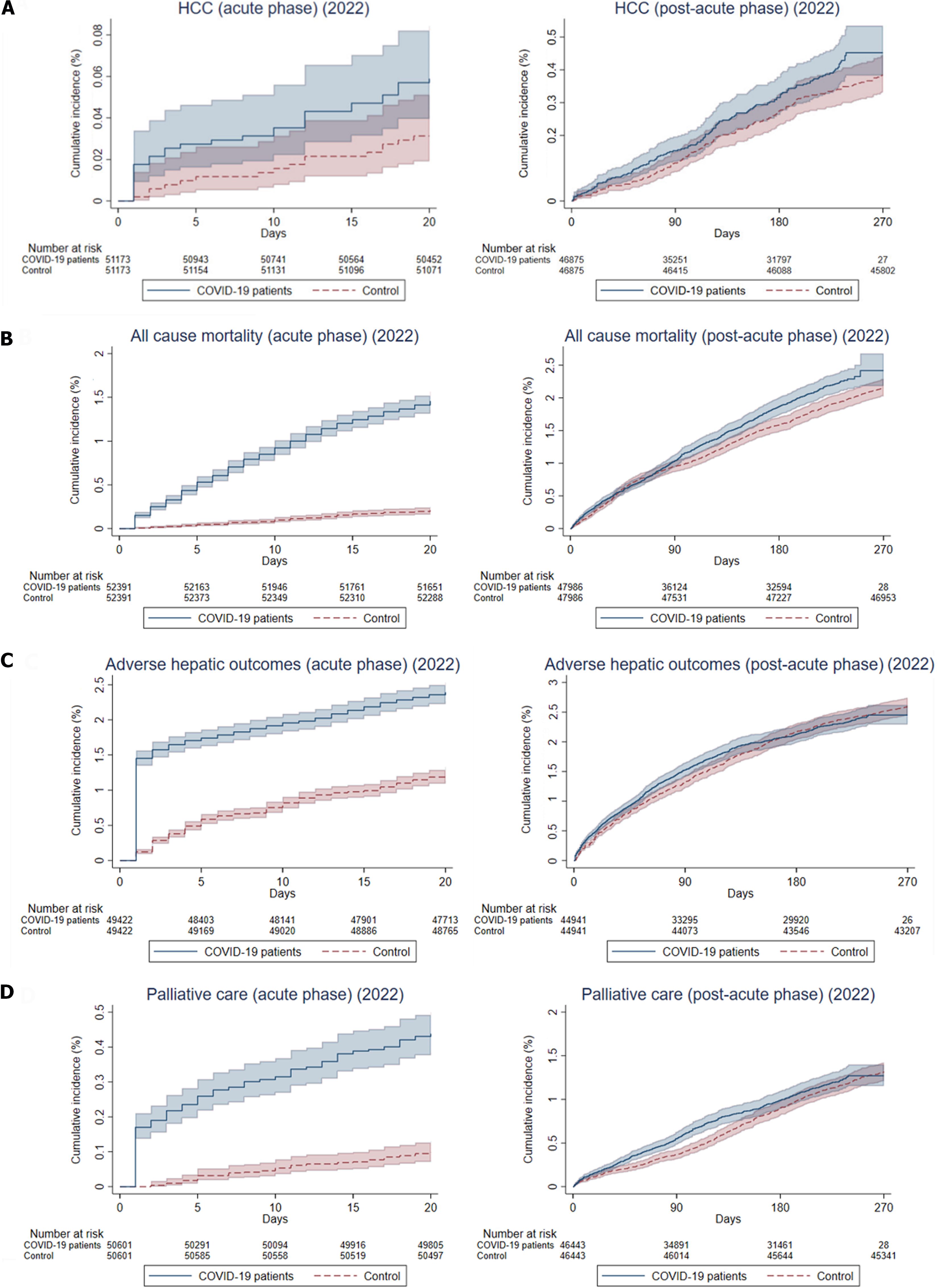
During the acute phase of infection, patients with CLD who had confirmed SARS-CoV-2 infection in 2022 were associated with significantly higher risks of HCC (IRR: 1.89, 95%CI: 1.03-3.47, P = 0.04) and all-cause mortality (IRR: 7.06, 95%CI: 5.78-8.63, P < 0.001). The risks of adverse hepatic outcomes were increased (IRR: 1.98, 95%CI: 1.79-2.18, P < 0.001), not only contributed by an increased risk of HCC, but also cirrhosis (IRR: 1.88, 95%CI: 1.71-2.06, P < 0.001) and liver decompensation (IRR: 2.85, 95%CI: 1.77-4.58, P < 0.001). There were no significant differences in the incidence of receiving curative treatment (IRR: 0.57, 95%CI: 0.25-1.28, P = 0.17) or non-curative treatment (IRR: 1.24, 95%CI: 0.77-2.01, P = 0.38), but a significantly higher chance of receiving palliative care (IRR: 4.46, 95%CI: 3.28-6.06, P < 0.001) for the COVID-19 patients, compared to the controls (Table 3).
| Outcomes | Acute phase of infection (within 21 d) | Post-acute phase of infection (beyond 21 d) | ||||||||||||||||
| COVID-19 patients in 2022 (n = 52391) | Control (n = 52391) | COVID-19 patients in 2022 vs control | COVID-19 patients in 2022 (n = 47986) | |||||||||||||||
| Cumulative incidence | Crude incidence rate (Events/10000 person-yr) | Cumulative incidence | Crude incidence rate (Events/10000 person-yr) | Cumulative incidence | Crude incidence rate (Events/10000 person-yr) | |||||||||||||
| New events | Rate | Estimate | 95%CI | Person-yr | New events | Rate | Estimate | 95%CI | Person-yr | IRR1 | 95%CI | P value | New events | Rate | Estimate | 95%CI | Person-yr | |
| HCC | 30 | 0.06 | 107.9 | (72.8, 154.0) | 2781 | 16 | 0.03 | 57.1 | (32.6, 92.8) | 2,801 | 1.89a | (1.03, 3.47) | 0.04 | 151 | 0.32 | 66.2 | (56.1, 77.7) | 22,797 |
| All-cause mortality | 764 | 1.46 | 2683.7 | (2496.7, 2880.9) | 2847 | 109 | 0.21 | 380.1 | (312.1, 458.5) | 2868 | 7.06b | (5.78, 8.63) | < 0.001 | 854 | 1.78 | 365.6 | (341.5, 391.0) | 23356 |
| Adverse hepatic outcomes | 1180 | 2.39 | 4469.6 | (4218.2, 4732.1) | 2640 | 607 | 1.23 | 2259.6 | (2083.4, 2446.7) | 2686 | 1.98b | (1.79, 2.18) | < 0.001 | 895 | 1.99 | 415 | (388.3, 443.1) | 21564 |
| Cirrhosis | 1260 | 2.49 | 4676.2 | (4421.6, 4941.8) | 2694 | 683 | 1.35 | 2490 | (2306.7, 2683.9) | 2743 | 1.88b | (1.71, 2.06) | < 0.001 | 900 | 1.96 | 409.6 | (383.2, 437.2) | 21974 |
| Liver decompensation hepatorenal syndrome | 20 | 0.04 | 70.5 | (43.1, 108.9) | 2837 | 6 | 0.01 | 21 | (7.7, 45.7) | 2859 | 3.36b | (1.35, 8.36) | 0.009 | 54 | 0.11 | 23.2 | (17.4, 30.3) | 23278 |
| Liver failure | 14 | 0.00% | 49.5 | (27.0, 83.0) | 2831 | 4 | 0.01 | 14 | (3.8, 35.9) | 2852 | 3.53a | (1.16, 10.71) | 0.03 | 43 | 0.09 | 18.5 | (13.4, 24.9) | 23217 |
| Hepatic coma/encephalopathy | 8 | 0.02 | 28.2 | (12.2, 55.6) | 2835 | 6 | 0.01 | 21 | (7.7, 45.7) | 2856 | 1.34 | (0.47, 3.87) | 0.59 | 38 | 0.08 | 16.3 | (11.6, 22.4) | 23261 |
| Ascites | 22 | 0.04 | 77.9 | (48.8, 117.9) | 2826 | 10 | 0.02 | 35.1 | (16.8, 64.6) | 2847 | 2.22a | (1.05, 4.68) | 0.04 | 82 | 0.17 | 35.4 | (28.1, 43.9) | 23176 |
| Variceal bleeding | 37 | 0.07 | 132.5 | (93.3, 182.6) | 2793 | 12 | 0.02 | 42.6 | (22.0, 74.5) | 2,815 | 3.11b | (1.62, 5.96) | < 0.001 | 85 | 0.18 | 37.1 | (29.7, 45.9) | 22898 |
| Curative treatment to HCC | 9 | 0.02 | 32.3 | (14.8, 61.3) | 2786 | 16 | 0.03 | 57 | (32.6, 92.6) | 2807 | 0.57 | (0.25, 1.28) | 0.17 | 93 | 0.2 | 40.7 | (32.9, 49.9) | 22,828 |
| Hepatic resection | 4 | 0.01 | 14.2 | (3.9, 36.4) | 2815 | 10 | 0.02 | 35.3 | (16.9, 64.8) | 2836 | 0.4 | (0.13, 1.28) | 0.12 | 64 | 0.13 | 27.7 | (21.4, 35.4) | 23072 |
| Liver transplantation | 3 | 0.01 | 10.6 | (2.2, 30.9) | 2835 | 1 | 0 | 3.5 | (0.1, 19.5) | 2856 | NA | NA | NA | 6 | 0.01 | 2.6 | (0.9, 5.6) | 23268 |
| Radiofrequency ablation of liver | 2 | 0 | 7.1 | (0.9, 25.6) | 2822 | 5 | 0.01 | 17.6 | (5.7, 41.0) | 2,843 | 0.4 | (0.08, 2.08) | 0.28 | 41 | 0.09 | 17.7 | (12.7, 24.0) | 23137 |
| Non-curative treatment to HCC | 37 | 0.07 | 136.1 | (95.8, 187.6) | 2719 | 30 | 0.06 | 109.6 | (73.9, 156.4) | 2738 | 1.24 | (0.77, 2.01) | 0.38 | 289 | 0.63 | 129.5 | (115.0, 145.4) | 22309 |
| Transarterial chemoembolization | 9 | 0.02 | 31.9 | (14.6, 60.6) | 2817 | 11 | 0.02 | 38.8 | (19.4, 69.4) | 2837 | 0.82 | (0.34, 1.99) | 0.67 | 64 | 0.13 | 27.7 | (21.3, 35.4) | 23096 |
| Radiotherapy to liver | 0 | 0 | 0 | NA | 2845 | 1 | 0 | 3.5 | (0.1, 19.4) | 2866 | NA | (0.00, 0.00) | NA | 3 | 0.01 | 1.3 | (0.3, 3.8) | 23338 |
| Systemic chemotherapy or immunotherapy | 28 | 0.06 | 102 | (67.8, 147.5) | 2744 | 21 | 0.04 | 76 | (47.0, 116.2) | 2763 | 1.34 | (0.76, 2.36) | 0.31 | 261 | 0.56 | 115.9 | (102.2, 130.8) | 22527 |
| Palliative care | 221 | 0.44 | 805 | (702.3, 918.4) | 2745 | 50 | 0.1 | 180.5 | (134.0, 238.0) | 2770 | 4.46b | (3.28, 6.06) | < 0.001 | 447 | 0.96 | 198.1 | (180.2, 217.3) | 22565 |
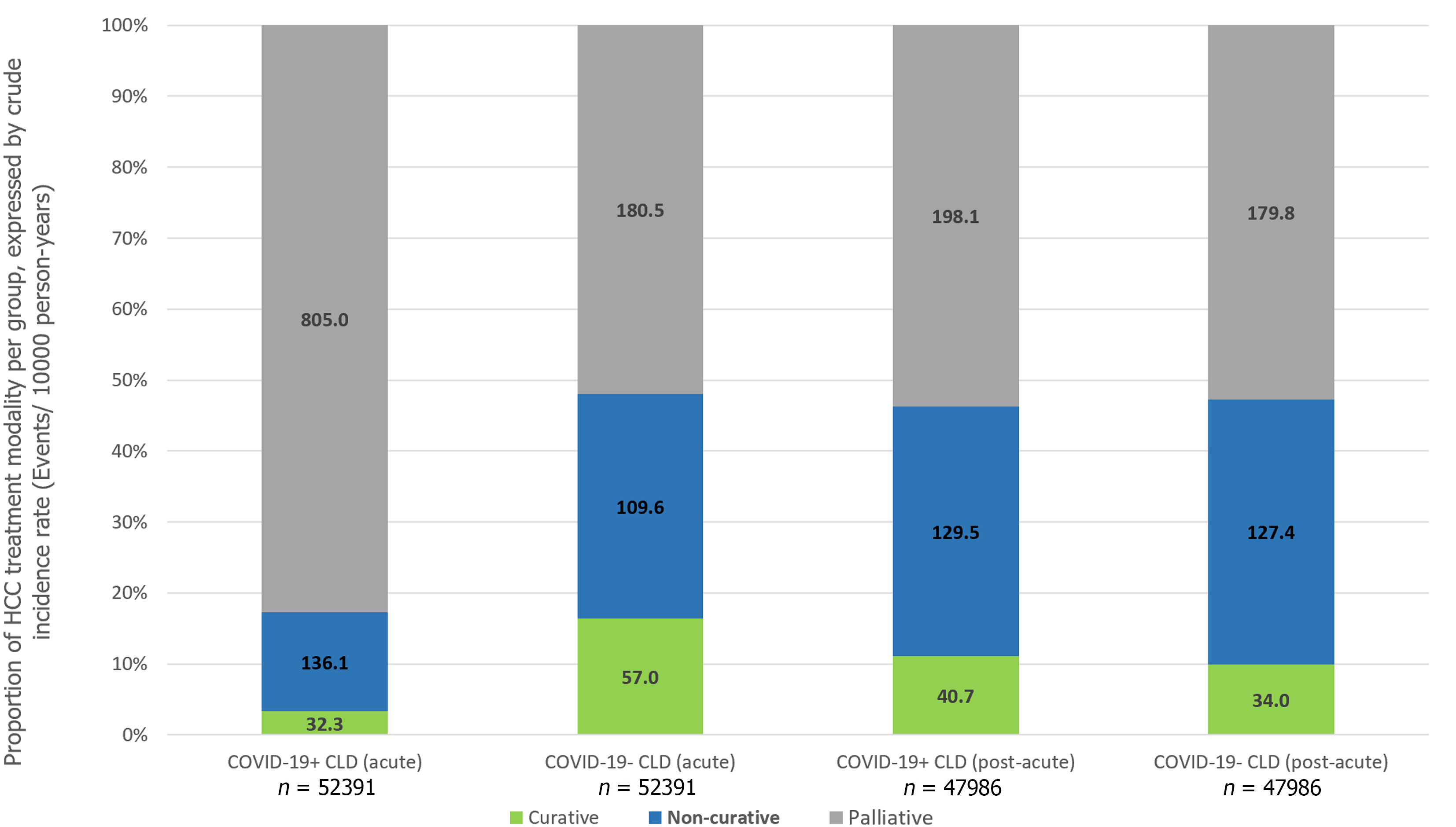
In the post-acute phase of infection, CLD patients with SARS-CoV-2 infection were still associated with significantly higher risks of HCC (IRR: 1.24, 95%CI: 1.00-1.53, P = 0.05), all-cause mortality (IRR: 1.24, 95%CI: 1.14-1.36, P < 0.001) and adverse hepatic outcomes (IRR: 1.24, 95%CI: 1.13-1.35, P < 0.001), but the risk ratios were numerically diminished compared to the acute phase. Risk of incident cirrhosis (IRR: 1.28, 95%CI: 1.17-1.39, P < 0.001) and liver decompensation (IRR: 1.26, 95%CI: 1.05-1.52, P = 0.01) in CLD patients with COVID-19 were maintained compared to controls. There were no significant differences in the incidence of receiving curative treatment (IRR: 1.20, 95%CI: 0.92-1.57, P = 0.18), non-curative treatment (IRR: 1.02, 95%CI: 0.88-1.18, P = 0.82), and palliative care (IRR: 1.10, 95%CI: 0.98-1.24, P = 0.11) for HCC (Table 3). Figure 3 shows the cumulative incidence of HCC, all-cause mortality, adverse hepatic outcomes, and palliative care in the acute and post-acute phases of SARS-CoV-2 infection and Figure 4 shows the proportion of treatment modalities of HCC stratified by the presence and phase of SARS-CoV-2 infection.
Results of HCC among most subgroups showed that there were no significant differences between the subgroups compared, which were generally consistent with the main results. Meanwhile, results of the subgroups of patients with cirrhosis, HBV, multi-organ dysfunction, and patients in the year 2022 showed that COVID-19+CLD was associated with significantly higher risks of HCC, while result of subgroup of patients in the year 2020 showed a significantly lower risk of HCC, compared to COVID-19-CLD. Results of all-cause mortality outcome among subgroups were mostly consistent with the main results, except for the subgroup of younger patients (age ≤ 50) and patients in the year 2020, 2021. The increased risks of adverse hepatic outcomes in CLD patients with SARS-CoV-2 infection were mostly consistent with the main results, regardless of causes of CLD, presence of multi-organ dysfunction, COVID-19 vaccination status, or the time period. For the observed heightened risks of liver decompensation in SARS-CoV-2 infected patients with CLD compared to uninfected patients with CLD, the results in the subgroups were also mostly consistent for older patients (age > 50), HBV causes of CLD, patients with multi-organ dysfunction, fully vaccinated individuals, and patients who were diagnosed with COVID-19 in the year 2022 (Supplementary Table 2). The observed higher risks of palliative care in all subgroups were consistent with the main results, regardless of cirrhosis, etiology of CLD, multi-organ dysfunction, and COVID-19 vaccination status (Supplementary Table 2). The results of subgroup analyses among COVID-19+CLD patients showed no significant differences in the incidence of HCC, all-cause mortality, adverse hepatic outcomes, and receiving palliative care in the hospitalization subgroup. Nevertheless, antiviral users were associated with significantly higher risk of adverse hepatic outcomes, compared to patients who did not receive any antiviral medications (Supplemen
In this large real-world cohort of patients with pre-existing CLD, we demonstrated that SARS-CoV-2 infection was significantly associated with an increased risk of all-cause mortality and adverse hepatic outcomes, which is consistent with the literature. We observed that while the overall risk of incident HCC was not increased, alterations in treatment strategies for HCC were inevitable following COVID-19 in patients with CLD, with an increased risk of receiving palliative care as the definitive treatment for HCC. The negative influence of SARS-CoV-2 infection on patients with CLD observed during the acute phase persisted through to the post-acute phase, albeit in a diminished manner. Our cohort is further distinguished from other published studies by the inclusion of mostly (> 95%) non-cirrhotic patients, whose underlying CLD was due to HBV in 40% of the cohort (Supplementary Table 2), in contrast to other published studies that investigated individuals with cirrhosis[1-3], with ALD as the predominant etiology[21]. Importantly, instead of uninfected healthy controls, historic cohorts or SARS-CoV-2 infected patients without CLD, we compared the risk against contemporaneous CLD patients without SARS-CoV-2 infection, after matching for age, gender, comorbidity, COVID-19 vaccination status, and observation period. In addition, we demonstrated that the increased risk of all-cause mortality in COVID-19+CLD was at least contributed by adverse hepatic outcomes, namely incident cirrhosis and liver decompensation (hepato-renal syndrome, liver failure, hepatic encephalopathy, ascites, and variceal bleeding). For the first time, the risk of adverse hepatic outcomes in the acute and post-acute phase of COVID-19 among patients with CLD was reported. We showed that the risk of incident cirrhosis persisted in the post-acute phase among COVID-19+CLD patients. Similarly, the risk of liver decompensation was most pronounced in the acute phase of SARS-CoV-2 infection, but was maintained in a diminished manner in the post-acute phase. Although the exact mechanisms are not known, one can postulate that SARS-CoV-2 infection and the resultant cytokine activation[22,23] and immune perturbations[24] resulted in further liver injury, and accelerated liver fibrogenesis due to activation of hepatic stellate cells responsible for fibrogenesis[25] in CLD subjects who are already predisposed to cirrhosis, leading to earlier onset of this complication. Even after the resolution of SARS-CoV-2 infection, which is a predominantly extra-hepatic acute illness, the risk of new-onset cirrhosis and liver decompensation remains exaggerated compared to uninfected controls. This finding carries potential implications on enhanced surveillance and monitoring of patients with CLD who have recovered from SARS-CoV-2 infection.
Although the risk of HCC was not found to be significantly increased among COVID-19+CLD patients in the overall cohort (IRR 1.19, 95%CI: 0.99-1.42, P = 0.06), there was an increased risk of HCC in both acute (IRR 1.89, 95%CI: 1.03-3.47, P = 0.04) and post-acute phase (IRR 1.24, 95%CI: 1.00-1.53, P = 0.05). This phenomenon cannot be explained by the higher risk of cirrhosis and liver decompensation following SARS-CoV-2 infection as the time window was too short for hepatocarcinogenesis. Although SARS-CoV-2 has been suggested to demonstrate liver tropism as confirmed by viral RNA and spike protein detection in autopsy liver specimens[26,27], it is not known to cause carcinogenic mutations or induce pro-oncogenic proteins like what hepatitis B virus does[28,29]. Therefore, non-biological mechanisms likely exist to account for the observed increased risk of HCC in CLD patients infected by SARS-CoV-2 infection. We hypothesized that it might be related to paradoxically earlier detection of tumors in patients with COVID-19 who are also known to have increased risk of acute liver injury[30,31], that triggered off imaging workups for abnormal liver enzymes. Importantly, the chance of receiving palliative care was markedly increased in the acute phase (IRR 4.46) but not in the post-acute phase of SARS-CoV-2 infection. Understandably, during the acute phase of infection, patients might be too sick to receive more aggressive treatment such as surgical resection, and the common association with abnormal liver enzymes would have precluded these subjects from medical oncological treatment such as immunotherapy or targeted therapy[7]. During the initial phase of COVID-19 pandemic, there was implementation of lockdown strategies and prioritization of healthcare services to prevention and management of SARS-CoV-2 in virtually all health care facilities[32]. It inevitably led to delays in routine care, such as patient follow-up[33], HCC surveillance and priority referrals to relevant disciplines to manage HCC.[11] Even for subjects with milder disease course of COVID-19 and preserved liver function, because of such disruption in the routine clinical service, essential abdominal imaging such as ultrasound scans and computed tomography scans[34,35] was not performed for CLD patients in a capacity similar to pre-COVID era[36]. This would inevitably lead to delays in HCC diagnosis, causing these patients to be diagnosed at a more advanced stage of cancer and eventually became ineligible for loco-regional oncological treatments for HCC even when their medical condition was otherwise stable[7]. This hypothesis is further supported by the fact that patients had a paradoxically ‘reduced’ risk of HCC during earlier period of COVID-19 (i.e., year 2020) coinciding with lockdown and suspension of services, but an increased risk of HCC towards the later stages of the COVID-19 pandemic (i.e. year 2022) when healthcare services gradually resumed (Supplementary Table 2). In the post-acute phase, when the infection resolved, regardless of whether there was COVID-19 induced abnormal liver biochemistry, these patients would be re-evaluated for eligibility to receive oncological treatment, thus contributing to the resolved risk of receiving palliative care.
In the subgroup analysis, we showed that the increased risks of all-cause mortality, liver decompensation, and palliative strategy for HCC were more pronounced among older subjects, cirrhotic patients, HBV-related CLD, presence of multi-organ dysfunction, and unvaccinated/non-fully vaccinated subgroups. We observed no increased risk for these adverse outcomes in year 2020 and 2021. Intriguingly, there was a significantly reduced risk of liver decompensation among COVID-19 subjects with CLD during year 2020 compared to uninfected CLD subjects. In the early stage of the pandemic, when vaccination and antiviral treatment were unavailable, intensive monitoring and supportive treatment were the only measures that could be taken. In addition, every confirmed case of COVID-19 infection was hospitalized regardless of severity. These practices might have paradoxically led to heightened vigilance, allowing opportunistic surveillance for laboratory abnormalities and optimization of the underlying CLD, which in turn lowered the risk of liver decompensation. Importantly, full vaccination was associated with a numerically lower risk of all-cause mortality and adverse hepatic outcomes in COVID-19 subjects with CLD (IRR 1.53 and 1.42, respectively) compared to non-fully vaccinated COVID-19 subjects with CLD (IRR 2.37 and 1.73, respectively). Similarly, full vaccination was associated with numerically lower chance of palliative care in COVID-19 subjects with CLD (IRR 1.41, 95%CI: 1.15-1.71, P < 0.001) compared to non-fully vaccinated COVID-19 subjects with CLD (IRR 1.68, 95%CI: 1.50-1.89, P < 0.001). Although the immediate threats of the COVID-19 pandemic is waning with the widespread adoption of vaccination and availability of antiviral therapies, the pandemic is not yet over[37] and vigilance should be maintained to protect vulnerable subjects from the adverse effects of COVID-19. Booster doses for COVID-19 vaccine for the general population are recommended[38], due to rapid emergence of variant strains and to maintain immunological memory. As COVID-19 vaccines have been proven safe to use without increasing risk of acute liver injury[12], the findings from our current study further supports the uptake of the COVID-19 vaccine among patients with CLD.
Our study has some limitations. Firstly, we did not adjust for the severity of SARS-CoV-2 infection during the acute phase, and further stratification of the risks of adverse outcomes based on disease severity was not possible. Disease severity might also have confounded the observed higher risk of adverse hepatic outcomes among antiviral users (Supplementary Table 3), compared to no antiviral use, because antivirals were mainly indicated among those with, or at risk of more severe COVID-19 infection[39]. Secondly, the diagnosis and outcomes were based on coding, and might have detected fewer events than expected due to non-coded conditions. This might have contributed to the small sample size in certain subgroups, leading to under-powered issue for the statistical analysis.
In conclusion, this large cohort consisting of 110,326 patients with CLD demonstrated SARS-CoV-2 infection was not associated with increased risk of HCC, but significantly higher risk of all-cause mortality, adverse hepatic outcomes, and with negative effect in treatment strategy for HCC. We found that although CLD patients with SARS-CoV-2 infection were not associated with increased risk of liver cancer, they are more likely to receive palliative treatment for HCC, compared to CLD patients who did not have SARS-CoV-2 infection. We showed for the first time that these detrimental effects of SARS-CoV-2 infection are observed in both the acute and post-acute phases among patients with CLD. Specifically, new-onset cirrhosis and liver decompensation are shown to be a type of clinical presentation of PASC, with a persisting risk of these hepatic PASCs even after the resolution of acute SARS-CoV-2 infection. These findings have important implications for monitoring and surveillance strategies for patients with CLD who have recovered from SARS-CoV-2 infection, and vaccination against SARS-CoV-2 infection should continue to be advocated among patients with CLD.
Chronic liver disease (CLD) was associated with adverse clinical outcomes among people with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
There is no data regarding how coronavirus disease 2019 (COVID-19) interplay with the risk of hepatocellular carcinoma (HCC) and subsequent treatment strategy. In addition, much is known about the immediate and long-term consequences of SARS-CoV-2 infection in CLD patients, and how they affect the incidence and oncological treatment for HCC.
We determined the effects of SARS-CoV-2 infection on the incidence and treatment strategy of HCC among patients with CLD.
A retrospective, territory-wide cohort of CLD patients was identified from an electronic health database in Hong Kong. Patients with confirmed SARS-CoV-2 infection (COVID-19+CLD) between January 1, 2020 and October 25, 2022 were identified and matched 1:1 by propensity-score with those without (COVID-19-CLD). Each patient was followed up until death, outcome event, or November 15, 2022. Primary outcome was incidence of HCC. Secondary outcomes included all-cause mortality, adverse hepatic outcomes, and different treatment strategies to HCC (curative, non-curative treatment, and palliative care). Analyses were further stratified by acute (within 20 d) and post-acute (21 d or beyond) phases of SARS-CoV-2 infection. Incidence rate ratios (IRRs) were estimated by Poisson regression models.
Of 193589 CLD patients (> 95% non-cirrhotic) in the cohort, 55163 patients with COVID-19+CLD and 55163 patients with COVID-19-CLD were included after 1:1 propensity-score matching. Upon 249-d median follow-up, COVID-19+CLD was not associated with increased risk of incident HCC (IRR: 1.19, 95%CI: 0.99-1.42, P = 0.06), but higher risks of receiving palliative care for HCC (IRR: 1.60, 95%CI: 1.46-1.75, P < 0.001), compared to COVID-19-CLD. In both acute and post-acute phases of infection, COVID-19+CLD were associated with increased risks of all-cause mortality (acute: IRR: 7.06, 95%CI: 5.78-8.63, P < 0.001; post-acute: IRR:1.24, 95%CI: 1.14-1.36, P < 0.001) and adverse hepatic outcomes (acute: IRR: 1.98, 95%CI: 1.79-2.18, P < 0.001; post-acute: IRR: 1.24, 95%CI: 1.13-1.35, P < 0.001), compared to COVID-19-CLD.
Although CLD patients with SARS-CoV-2 infection were not associated with increased risk of HCC, they were more likely to receive palliative treatment than those without. We showed for the first time that the detrimental effects of SARS-CoV-2 infection persisted in post-acute phase.
Our findings have important implications for strategies of monitoring and surveillance for patients with CLD who have recovered from SARS-CoV-2 infection, and vaccination against SARS-CoV-2 infection should continue to be advocated among CLD patients.
The authors thank the Department of Health and Hospital Authority for the generous provision of data for this study. Lai FTT, Wong CKH and Wong ICK’s post were partly funded by D24H. Hence this work was partly supported by AIR@InnoHK administered by Innovation and Technology Commission.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Elshimi E, Egypt S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
| 1. | Ioannou GN, Liang PS, Locke E, Green P, Berry K, O'Hare AM, Shah JA, Crothers K, Eastment MC, Fan VS, Dominitz JA. Cirrhosis and Severe Acute Respiratory Syndrome Coronavirus 2 Infection in US Veterans: Risk of Infection, Hospitalization, Ventilation, and Mortality. Hepatology. 2021;74:322-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 2. | Ge J, Pletcher MJ, Lai JC; N3C Consortium. Outcomes of SARS-CoV-2 Infection in Patients With Chronic Liver Disease and Cirrhosis: A National COVID Cohort Collaborative Study. Gastroenterology. 2021;161:1487-1501.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 3. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 4. | Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, Shaw J, Pearson M, Chew M, Fagan A, de la Rosa Rodriguez R, Worthington J, Olofson A, Weir V, Trisolini C, Dwyer S, Reddy KR. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70:531-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 5. | Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650-2666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 718] [Article Influence: 143.6] [Reference Citation Analysis (0)] |
| 6. | Cheemerla S, Balakrishnan M. Global Epidemiology of Chronic Liver Disease. Clin Liver Dis (Hoboken). 2021;17:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 317] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 7. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6038] [Article Influence: 862.6] [Reference Citation Analysis (3)] |
| 8. | Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. 2021;12:6571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 215] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 9. | Bowe B, Xie Y, Al-Aly Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med. 2022;28:2398-2405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 336] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 10. | Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW, Wan EY. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3262] [Cited by in RCA: 2992] [Article Influence: 748.0] [Reference Citation Analysis (0)] |
| 11. | Tapper EB, Asrani SK. The COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. J Hepatol. 2020;73:441-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 12. | Wong CKH, Mak LY, Au ICH, Lai FTT, Li X, Wan EYF, Chui CSL, Chan EWY, Cheng WY, Cheng FWT, Yuen MF, Wong ICK. Risk of acute liver injury following the mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccines. J Hepatol. 2022;77:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 13. | Lai FTT, Li X, Peng K, Huang L, Ip P, Tong X, Chui CSL, Wan EYF, Wong CKH, Chan EWY, Siu DCW, Wong ICK. Carditis After COVID-19 Vaccination With a Messenger RNA Vaccine and an Inactivated Virus Vaccine : A Case-Control Study. Ann Intern Med. 2022;175:362-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 14. | Li X, Tong X, Yeung WWY, Kuan P, Yum SHH, Chui CSL, Lai FTT, Wan EYF, Wong CKH, Chan EWY, Lau CS, Wong ICK. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2022;81:564-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 15. | Lam ICH, Wong CKH, Zhang R, Chui CSL, Lai FTT, Li X, Chan EWY, Luo H, Zhang Q, Man KKC, Cheung BMY, Tang SCW, Lau CS, Wan EYF, Wong ICK. Long-term post-acute sequelae of COVID-19 infection: a retrospective, multi-database cohort study in Hong Kong and the UK. EClinicalMedicine. 2023;60:102000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 16. | Byrne AW, McEvoy D, Collins AB, Hunt K, Casey M, Barber A, Butler F, Griffin J, Lane EA, McAloon C, O'Brien K, Wall P, Walsh KA, More SJ. Inferred duration of infectious period of SARS-CoV-2: rapid scoping review and analysis of available evidence for asymptomatic and symptomatic COVID-19 cases. BMJ Open. 2020;10:e039856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 17. | Ibáñez-Samaniego L, Bighelli F, Usón C, Caravaca C, Fernández Carrillo C, Romero M, Barreales M, Perelló C, Madejón A, Marcos AC, Albillos A, Fernández I, García-Samaniego J, Calleja JL, Bañares R. Elevation of Liver Fibrosis Index FIB-4 Is Associated With Poor Clinical Outcomes in Patients With COVID-19. J Infect Dis. 2020;222:726-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 18. | Cohen K, Ren S, Heath K, Dasmariñas MC, Jubilo KG, Guo Y, Lipsitch M, Daugherty SE. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2022;376:e068414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 113] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 19. | McMenamin ME, Nealon J, Lin Y, Wong JY, Cheung JK, Lau EHY, Wu P, Leung GM, Cowling BJ. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22:1435-1443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 230] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 20. | Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51:171-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 558] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 21. | Gao X, Lv F, He X, Zhao Y, Liu Y, Zu J, Henry L, Wang J, Yeo YH, Ji F, Nguyen MH. Impact of the COVID-19 pandemic on liver disease-related mortality rates in the United States. J Hepatol. 2023;78:16-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 22. | Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, Tarhriz V, Farjami A, Ghasemian Sorbeni F, Farahzadi R, Ghasemnejad T. COVID-19 infection: an overview on cytokine storm and related interventions. Virol J. 2022;19:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 414] [Cited by in RCA: 359] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 23. | Taneva G, Dimitrov D, Velikova T. Liver dysfunction as a cytokine storm manifestation and prognostic factor for severe COVID-19. World J Hepatol. 2021;13:2005-2012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Boettler T, Csernalabics B, Salié H, Luxenburger H, Wischer L, Salimi Alizei E, Zoldan K, Krimmel L, Bronsert P, Schwabenland M, Prinz M, Mogler C, Neumann-Haefelin C, Thimme R, Hofmann M, Bengsch B. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. J Hepatol. 2022;77:653-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 91] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 25. | Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, Schwabe RF. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 750] [Cited by in RCA: 1052] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 26. | Wanner N, Andrieux G, Badia-I-Mompel P, Edler C, Pfefferle S, Lindenmeyer MT, Schmidt-Lauber C, Czogalla J, Wong MN, Okabayashi Y, Braun F, Lütgehetmann M, Meister E, Lu S, Noriega MLM, Günther T, Grundhoff A, Fischer N, Bräuninger H, Lindner D, Westermann D, Haas F, Roedl K, Kluge S, Addo MM, Huber S, Lohse AW, Reiser J, Ondruschka B, Sperhake JP, Saez-Rodriguez J, Boerries M, Hayek SS, Aepfelbacher M, Scaturro P, Puelles VG, Huber TB. Molecular consequences of SARS-CoV-2 Liver tropism. Nat Metab. 2022;4:310-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 122] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 27. | Quarleri J, Delpino MV. Molecular mechanisms implicated in SARS-CoV-2 Liver tropism. World J Gastroenterol. 2022;28:6875-6887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Lupberger J, Hildt E. Hepatitis B virus-induced oncogenesis. World J Gastroenterol. 2007;13:74-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 181] [Cited by in RCA: 193] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Sivasudhan E, Blake N, Lu Z, Meng J, Rong R. Hepatitis B Viral Protein HBx and the Molecular Mechanisms Modulating the Hallmarks of Hepatocellular Carcinoma: A Comprehensive Review. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 30. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 271] [Article Influence: 54.2] [Reference Citation Analysis (2)] |
| 31. | Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72:1169-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 32. | Feng Y, Young CH, Lau SH, He ML. Outbreak control management: Lessons from SARS-CoV-2 infections in 2020-2022 in Hong Kong, an international municipality with high-frequency travelers. MedComm (2020). 2022;3:e158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Oubaya N, Pombet T, Delestrain C, Remus N, Douvry B, Grenet D, Corvol H, Thouvenin G, Prulière-Escabasse V, Mounir H, Argoud D, Fretigne C, Costes L, Mackiewicz MP, Jung C, Ahamada L, Lanone S, Maitre B, Bégot AC, Epaud R. Impact of the COVID-19 pandemic and associated lockdown measures on the management, health, and behavior of the cystic fibrosis population in France during 2020 (MUCONFIN). Front Public Health. 2022;10:978627. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3790] [Article Influence: 473.8] [Reference Citation Analysis (1)] |
| 35. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2834] [Article Influence: 404.9] [Reference Citation Analysis (0)] |
| 36. | Toyoda H, Huang DQ, Le MH, Nguyen MH. Liver Care and Surveillance: The Global Impact of the COVID-19 Pandemic. Hepatol Commun. 2020;4:1751-1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 37. | The Lancet. The COVID-19 pandemic in 2023: far from over. Lancet. 2023;401:79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 68] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 38. | Rosenblum HG, Wallace M, Godfrey M, Roper LE, Hall E, Fleming-Dutra KE, Link-Gelles R, Pilishvili T, Williams J, Moulia DL, Brooks O, Talbot HK, Lee GM, Bell BP, Daley MF, Meyer S, Oliver SE, Twentyman E. Interim Recommendations from the Advisory Committee on Immunization Practices for the Use of Bivalent Booster Doses of COVID-19 Vaccines - United States, October 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1436-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 113] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 39. | Interim Clinical Considerations for COVID-19 Treatment in Outpatients. 2023. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/outpatient-treatment-overview.html. |