Published online Feb 27, 2024. doi: 10.4254/wjh.v16.i2.152
Peer-review started: December 6, 2023
First decision: December 18, 2023
Revised: December 26, 2023
Accepted: January 17, 2024
Article in press: January 17, 2024
Published online: February 27, 2024
Processing time: 83 Days and 4.8 Hours
The prevalence of metabolic-associated fatty liver disease (MAFLD) has increased substantially in recent years because of the global obesity pandemic. MAFLD, now recognized as the number one cause of chronic liver disease in the world, not only increases liver-related morbidity and mortality among sufferers but also worsens the complications associated with other comorbid conditions such as cardiovascular disease, type 2 diabetes mellitus, obstructive sleep apnoea, lipid disorders and sarcopenia. Understanding the interplay between MAFLD and these comorbidities is important to design optimal therapeutic strategies. Sar
Core Tip: Metabolic-associated fatty liver disease (MAFLD) is associated with sarcopenia in a significant proportion of individuals. Sarcopenia can be a consequence of the comorbidities associated with MAFLD (such as obesity or adiposity) or a direct result of advanced stages of MAFLD, such as fibrosis and cirrhosis. On the other hand, sarcopenia can worsen MAFLD due to reduced exercise capacity and the release of various myokines. Understanding the strong interlink between MAFLD and sarcopenia is important to plan appropriate therapeutic strategies. We discuss the pathobiological aspects of this interlink and the potential clinical and metabolic complications of the coexistence of MAFLD and sarcopenia in this comprehensive clinical update review.
- Citation: Viswanath A, Fouda S, Fernandez CJ, Pappachan JM. Metabolic-associated fatty liver disease and sarcopenia: A double whammy. World J Hepatol 2024; 16(2): 152-163
- URL: https://www.wjgnet.com/1948-5182/full/v16/i2/152.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i2.152
Metabolic-associated fatty liver disease (MAFLD) and sarcopenia are two chronic health conditions with profound adverse implications in modern society. MAFLD encompasses a spectrum of liver conditions, ranging from simple steatosis (fatty liver) to non-alcoholic steatohepatitis (NASH) which involves inflammation and liver cell damage, advanced liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). On the other hand, sarcopenia represents the progressive loss of muscle mass, strength, and function associated with aging, sedentarism, obesity, and conditions that cause reduced mobility. These two conditions have gained considerable attention due to their parallel rise in prevalence and potential interconnections. MAFLD is currently the most common liver disorder worldwide, affecting nearly one-third of the global population[1]. The definition and diagnostic criteria for sarcopenia have evolved in recent years. The European Working Group on Sarcopenia in Older People and the Asian Working Group for Sarcopenia have proposed consensus definitions that consider muscle mass, muscle strength, and physical performance measures[2,3]. Recent studies have highlighted the association between sarcopenia and various chronic diseases, including cardiovascular disease, diabetes mellitus, and liver disease, emphasizing the importance of early detection and intervention[4,5]. MAFLD and sarcopenia are two interrelated conditions that pose significant challenges in clinical practice.
The intricate link between MAFLD and sarcopenia involves shared mechanisms, such as chronic low-grade inflammation, oxidative stress, insulin resistance (IR), and alterations in adipokines and myokines[6,7]. MAFLD contributes to sarcopenia through negative impacts on muscle protein synthesis and metabolism, leading to wasting. Conversely, sarcopenia exacerbates MAFLD by influencing IR, dyslipidaemias, and systemic inflammation, and promoting liver fat accumulation through physical inactivity, weight gain, and central obesity[8,9]. The cumulative effect creates a cycle that worsens health outcomes and highlights the complex interplay between metabolic, inflammatory, and hormonal factors.
The clinical association between MAFLD and sarcopenia has important implications for patient management and outcomes. Understanding this association is crucial for identifying patients at high risk and implementing appropriate interventions to mitigate the disease progression. Recognizing and addressing these conditions early in clinical practice can help to improve patient prognosis and overall well-being. This review aims to explore the relationship between MAFLD and sarcopenia, shedding light on underlying mechanisms, common risk factors, potential consequences, and possible interventions.
Central to understanding mechanisms between MAFLD and sarcopenia is the concept of myosteatosis, which represents the infiltration of fat into skeletal muscle, contributing to functional decline as well as the loss of skeletal muscle mass seen in sarcopenia. Myosteatosis is seen in both NASH as well as MAFLD. It may be a superior indicator and predictor of hepatocellular deterioration compared to sarcopenia, especially in the early stages of NASH, since it precedes the onset of sarcopenia[10,11]. Additionally, myosteatosis has a strong association with liver stiffness in obese patients with MAFLD[12], highlighting its role in the dynamic relationship between sarcopenia and MAFLD.
Numerous shared risk factors augment the bidirectional relationship between MAFLD and sarcopenia, acting as a precipitator of metabolic dysregulation. Factors include the male gender, physical inactivity, metabolic syndrome, older age, and raised total cholesterol, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) chole
| Ref. | Study design | Study population | Size | Sarcopenic assessment | MAFLD assessment | Conclusions |
| Seo et al[17], 2022 | Longitudinal | Korean | 115568 | BIA | Non-invasive models | Increases in relative skeletal muscle mass over time may lead to benefits in prevention of development of NAFLD or the resolution of existing NAFLD |
| Zhai et al[18], 2018 | Cross-sectional | Chinese | 494 | DXA | US | NAFLD is not independently associated with sarcopenia |
| Wijarnpreecha et al[19], 2019 | Cross-sectional | American | 11325 | BIA | US | Sarcopenia was independently associated with increased odds of NAFLD and NAFLD-associated advanced fibrosis independent of well-defined risk factors |
| Hsieh et al[20], 2021 | Cross-sectional | Korean | 521 | CT | Liver biopsy | Patients with significant fibrosis had lower Skeletal muscle index and muscle attenuation than those without |
| Zhao et al[21], 2023 | Cross-sectional | American | 2065 | DXA | LUTE | Higher appendicular skeletal muscle mass was associated with a lower risk of MAFLD, while the risk of significant fibrosis in females was increased with the trunk skeletal muscle mass |
| Hsieh et al[22], 2023 | Longitudinal | Korean | 338 | CT | Liver biopsy | Severe myosteatosis is significantly associated with early NASH and fibrosis progression in early-stage MAFLD |
| Tanaka et al[23], 2020 | Cross-sectional | Japanese | 632 | CT | Non-invasive models | Both skeletal muscle index and skeletal muscle density are independently associated with the prevalence of MAFLD |
| Choe et al[24], 2023 | Cohort | Korean | 4038 | BIA | Non-invasive models | Both lower muscle mass index and genetic risk variants are important contributors to the development of MAFLD |
| Ref. | Study design | Study population | Size | Sarcopenic assessment | MAFLD assessment | Conclusions |
| Roh et al[25], 2022 | Longitudinal | Korean | 1595 | DXA | Non-invasive models | The presence of NAFLD may predict future risk of low muscle mass and low muscle strength, with a greater impact on LMS than on LMM |
| Sinn et al[26], 2022 | Cross-sectional | Korean | 52815 | BIA | US | Participants with NAFLD were at increased risk of sarcopenia, indicated by faster loss of skeletal muscle mass |
| Altajar et al[27], 2023 | Cross-sectional | Korean | 6414 | BIA | CAP | The presence of MAFLD is significantly associated with an increased risk of low muscle mass with varying risks according to the MAFLD subgroups |
The intricate relationship between MAFLD and sarcopenia is characterized by shared pathogenic mechanisms, comp
MAFLD is associated with low levels of growth hormone (GH) and insulin-like growth factor (IGF-1), contributing to increased hepatic IR and body adiposity. GH is crucial in increasing beta-oxidation of FFAs, potentially ameliorating hepatic lipid content, whilst IGF-1 exerts anti-inflammatory and anti-fibrotic effects in the liver[35]. Hormonal changes, particularly in post-menopausal women and individuals with altered testosterone levels, further complicate this relationship. The prevalence of sarcopenia is high amongst post-menopausal women[36], with menopausal hormonal therapy demonstrating a possible protective effect[37]. Testosterone is essential for muscle regeneration in males, and its deficiency contributes to reduced lean muscle mass in sarcopenia. Additionally, this deficiency may coincide with obesity and culminate in a pro-inflammatory state through the release of mediators such as IL-6 and TNF-α[38-40], thereby worsening sarcopenia and steatohepatitis.
In MAFLD, inflammation is further intensified by lipotoxicity, leading to increased reactive oxygen species and oxidative stress. This cascade results in intrahepatic cellular damage and higher circulating levels of FFAs within the cytosol[41,42]. Myokines, signalling molecules produced by skeletal muscle, play a role in energy metabolism. Sarcopenic MAFLD individuals show reduced levels of myokines, attributed to muscle loss and lack of physical activity[43]. Irisin, an exercise-induced myokine, demonstrates a protective effect against fatty liver[44] and shows a positive relationship with fibroblast growth factor 21 in animal studies, suggesting its potential therapeutic effects of reversing hepatic steatosis[45,46] (Figure 1).
The relationship between fatty liver disease (formerly non-alcoholic fatty liver disease (NAFLD), now MAFLD) and sarcopenic muscle degeneration has been extensively explored in numerous studies, though variations in selection criteria exist due to the recent redefinition of the disease. The shift to MAFLD has broadened the identification of individuals with liver disease.
Between 2018-19, four meta-analyses investigated the link between NAFLD and sarcopenia, with one study exploring the progression of fatty liver disease. One meta-analysis reported a significantly increased risk of NAFLD in patients with sarcopenia (pooled odds ratio of 1.54), emphasizing a substantial association despite there being statistical heterogeneity[47]. Another meta-analysis confirmed this increased risk of both NAFLD and a heightened risk of significant fibrosis[48]. In concordance with these results, a strong association was found between sarcopenia and advanced liver disease, with an odds ratio of 2.41[49]. Cai et al’s meta-analysis involving 19 studies, further reported higher risks of NAFLD, NASH, and significant fibrosis in individuals with reduced skeletal mass[50].
Diagnostic variation between studies is evident as there are no standardized diagnostic criteria to measure skeletal muscle mass and fat in the liver. Diagnostic computed tomography (CT)/magnetic resonance imaging (MRI) are superior modalities for skeletal mass measurement however, they pose a challenge in larger study settings. Dual-energy X-ray absorptiometry (DXA) is a preferred tool for its ease of use, but there are limitations in estimating lean mass and quan
Recent studies, focusing on MAFLD as opposed to NAFLD, provide a pragmatic clinical evaluation accounting for metabolically deranged individuals. A large study involving 8371 patients revealed increased risks of significant liver fibrosis and atherosclerotic cardiovascular disease in those with both MALFD and sarcopenia. Sarcopenic individuals with MAFLD exhibited higher odds ratios for significant fibrosis (Fibrosis 4 Index: odds ratio - 4.51, NAFLD fibrosis score: odds ratio - 5.72) and cardiovascular disease (odds ratio 4.08) compared to non-sarcopenic counterparts[52]. Another investigation involving 6424 subjects, using Fibro scan and bioimpedance analysis (BIA), found an association between MAFLD and increased risk of low muscle mass adjusted for weight and BMI, with the diabetic MAFLD subgroup showing the highest risk[53]. Notably a reverse relationship was found between appendicular skeletal muscle mass and the risk of MAFLD across both sexes, with appendicular skeletal mass of the highest quartile being associated with the least risk of MAFLD[54].
Lean MAFLD poses a challenge to clinicians as fatty liver disease presents in individuals with a lower BMI and adipose tissue and contradicts the criteria of MAFLD, which is usually associated with metabolic syndrome and obesity[55]. A United States-based population study found the prevalence of NAFLD to be 4 per 100000 and lean NAFLD to be 0.6 per 100000, where patients with lean NAFLD tended to be older, females, smokers, and of Asian race[56]. Global estimation of lean NAFLD was around 4.1%, with Asian populations having the highest prevalence (4.8%)[57]. Ha et al’s investigation revealed that lean MAFLD individuals faced a relative risk of 1.12 for cardiovascular mortality and 1.88 for liver-related mortality compared to non-lean individuals[58]. However, varied findings from a Chinese cohort study indicate that, while obese NAFLD individuals have a higher cardiovascular disease risk, lean NAFLD individuals still face elevated risks of all-cause death, digestive system cancers, and obesity-related cancers[59].
Despite having milder features of metabolic syndrome, lean individuals have been shown to have a higher prevalence of metabolic abnormalities such as dyslipidaemia, hypertension, IR, and diabetes mellitus[60]. Sarcopenic patients have shown upregulated serum levels of N-acetylneuraminyl-glycoproteins, lactic acid, LDL triglycerides, and VLDL5 Levels, along with reduced HDL4 levels[61]. A study by Nabi et al[62] confirmed patients with lean NAFLD were associated with advanced liver fibrosis (odds ratio = 1.26) compared to non-lean individuals, highlighting the necessity to re-evaluate assumptions about the health of lean individuals. The optimization in skeletal muscle mass, as opposed to the sole reduction in visceral adiposity, has been suggested for optimal management of lean MAFLD[63].
Sarcopenic obesity, being the co-existence of loss of muscle mass alongside increased body adiposity/fat mass, is a prevalent condition affecting 11% of older adults globally[64]. In males, identified risk factors include increased body mass index (BMI), waist circumference, and triglyceride levels, along with a decreased skeletal muscle mass index. For females, height, weight, BMI, waist circumference, and systolic blood pressure, as well as smoking status and fasting glucose are identified as significant risk factors, according to a large nationwide Korean study[65].
The pathophysiology of sarcopenic obesity can be attributed to a multitude of mechanisms such as aging, IR, low-grade inflammation, and hormonal changes[66]. Aging induces a progressive loss in muscle mass and an increase in adiposity due to a decline in basal metabolic rate, inactivity, and hormonal fluctuation. The increased visceral fat and net loss of muscle mass, coupled with aging can encourage a reduction in GH production[67], negatively impact protein synthesis, and exacerbate sarcopenia.
Diagnosis, as detailed by the European Society for Clinical Nutrition and Metabolism[68], involves screening for high BMI and waist circumference, using surrogate indicators for sarcopenia with diagnostic cut-offs based on ethnicity, age, and gender. Functional assessments, including muscle strength evaluation and body composition analysis based on DXA, BIA, and CT, are used to finalize the diagnosis. Advanced liver disease warrants the use of CT/MRI imaging for accuracy, due to its ability to effectively eliminate the confounding effects of fluid retention, particularly ascites, and oedema, associated with portal hypertension[69].
Sarcopenic obesity has been shown to increase the risk of all-cause mortality, and fragility fractures in elderly patients with type 2 diabetes mellitus[70,71]. Low muscle-to-fat ratio in older adults has been linked to impaired health outcomes and increased cardiometabolic and cardiovascular risk[72,73]. Sarcopenic obesity is also associated with a significant increase in the risk of coronary artery calcification[74]. In children, sarcopenic obesity is linked to the development of metabolic syndrome and worsened outcomes in type 2 diabetes mellitus[75].
Moreover, sarcopenic obesity also increases the risk of MAFLD development and fibrosis progression[76]. Increased visceral adiposity and IR, resulting in hepatic fat accumulation[77], contribute to worsened hepatic fibrosis, as demonstrated by Kim et al[78]. Understanding the relationship between sarcopenic obesity and these health outcomes is crucial for holistic patient care.
In MAFLD, the presence of sarcopenia worsens disease progression and outcomes. Reduced muscle mass leads to physical inactivity, which can exacerbate metabolic dysfunction, IR, and the accumulation of visceral adiposity[79]. Inflammation in the liver is further aggravated by the sarcopenia-associated cytokine and adipokine profiles. Conversely, MAFLD can contribute to the development of sarcopenia through chronic inflammation, leading to muscle breakdown.
IR, resulting from MAFLD, impairs glucose uptake into the muscle, and metabolic alterations in MAFLD can lead to increased oxidant stress in muscle tissue. IR, a common feature in MAFLD, sarcopenia, and diabetes mellitus, worsens glycemic control, inflammation, and metabolic dysfunction, thereby escalating the risk of cardiovascular disease. Notably, the presence of sarcopenia in diabetes mellitus increases the risk of all-cause mortality and cardiovascular mortality[80]. Studies, such as the post-hoc analysis of the ATTICA study, detailed that a lower skeletal muscle perc
A further study of 852 diabetic participants demonstrated a higher risk of carotid atherosclerotic progression over a 6-8-yr span in those with both sarcopenia and MAFLD (odds ratio 2.2)[84]. Albuminuria, a marker of renal dysfunction and cardiovascular risk, was also shown to have significantly higher rates in patients with both conditions[85].
Moreover, the progression of fibrosis in the presence of sarcopenia has been extensively studied. In a recent study of 2422, sarcopenic NAFLD demonstrated higher liver fibrosis rates than NAFLD alone. Rates of significant fibrosis were elevated (18.3% vs 3.2%) and a similar marked increase was seen in advanced fibrosis[86]. The interplay between the two conditions has been shown to worsen surgical outcomes and long-term post-operative survival of HCC patients with MAFLD[87]. Sarcopenia was identified as an independent risk factor for both recurrence-free survival and overall survival in sarcopenic MAFLD patients with HCC. Additionally, sarcopenia in MAFLD is associated with higher rates of depression and fatigue, along with a reduced quality of life[88].
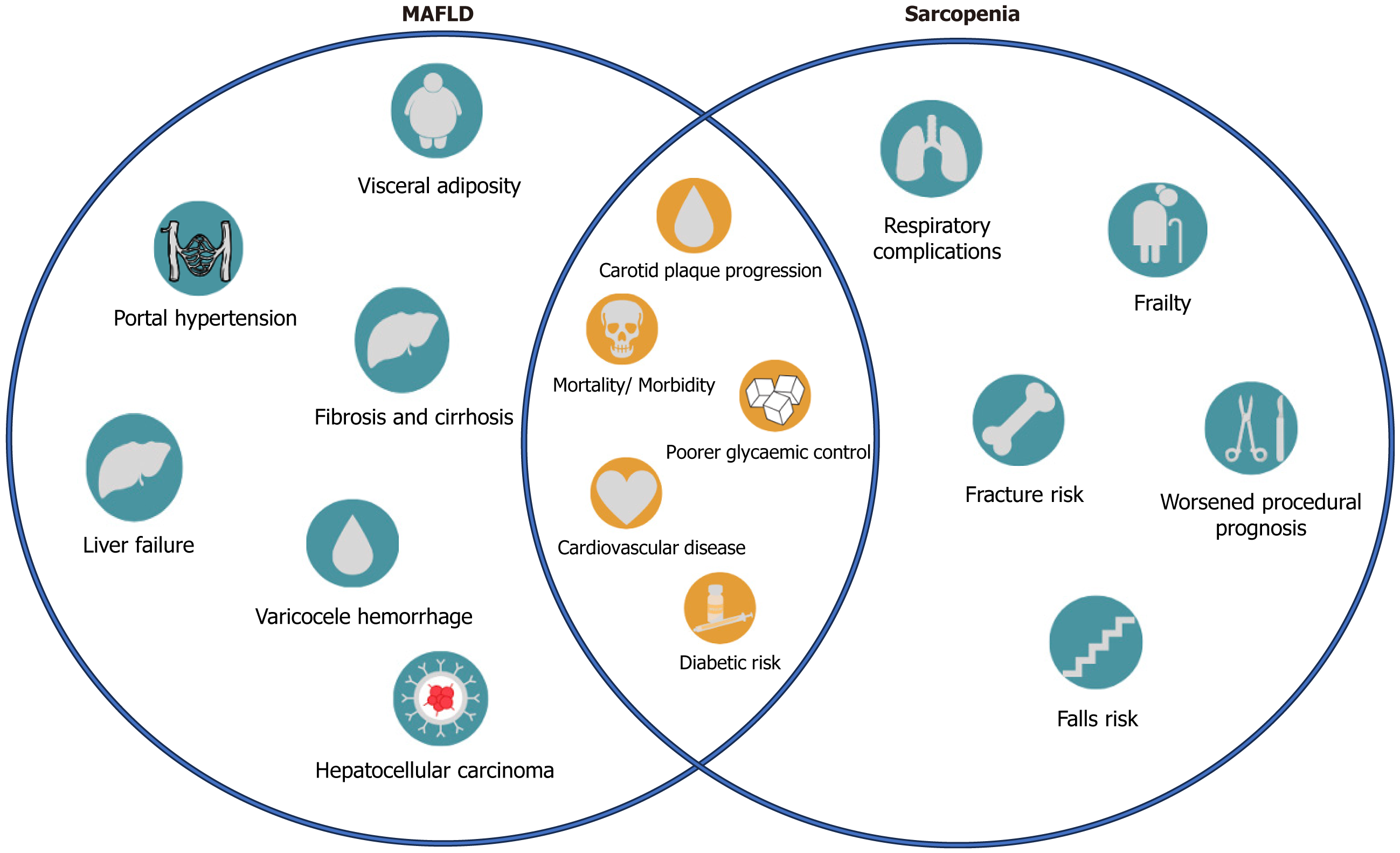
The synergy of both conditions leads to increased metabolic dysregulation, contributing to a more adverse clinical course of MAFLD, emphasizing the need for a comprehensive assessment to address the multiple implications of having both sarcopenia and MAFLD. Figure 2 illustrates the clinical implications of sarcopenia and MAFLD, as discussed prior.
A multifaceted approach must be used to address the combination of MAFLD and sarcopenia. Conservative management of MAFLD involves gradual weight loss and regular physical activity, both of which improve hepatic steatosis and quality of life[89,90], as well as improving liver stiffness[91]. Aerobic and resistance training, especially moderate resistance training, was found to be beneficial in ameliorating IR[92]. Bariatric surgery may be offered to individuals with BMI > 40 kg/m2 and obesity-related comorbidities, with the exclusion of those with decompensated cirrhosis and concomitant portal hypertension[93].
When managing sarcopenia, a meta-analysis highlights the effectiveness of nutritional supplementation and physical activity for outcomes such as muscle mass, strength, and physical performance[94]. Strength training induces muscle hypertrophy and mitigates the decline in lean muscle tissue[95]. Although physical activity has been shown protective effects against both conditions[96], its utility is limited due to the heightened frailty seen in the advanced stages of these diseases[97]. Evaluation of sarcopenia through imaging and muscle strength assessment is crucial, before commencing treatment. Whilst protein supplementation alone may not be useful for sarcopenia[98], branched-chain amino acids show promise, especially in sarcopenic patients with cirrhosis[99].
The implication of diet in sarcopenia and MAFLD remains an area for exploration, although it is a smaller prognostic factor in patients with sarcopenic MAFLD compared to physical activity[100]. Pharmacological treatment often targets pre-existing co-morbidities such as cardiovascular disease, diabetes mellitus, and lipid abnormality, focusing on regulating the patients’ metabolic status. For example, Vitamin E supplementation demonstrates significant improve
Emerging pharmacological interventions aim to target inflammatory and fibrotic pathways in fatty liver disease. A review by Rojas et al[106] outlines therapies in phase II/III clinical trials, which focus on reducing fatty acid accumulation and regressing fibrosis.
Novel mechanisms have been researched to explain the pathogenesis of both conditions, as well as to unravel their bidirectional nature. A recent study was done on the involvement of the phosphoenolpyruvate carboxykinase 1 (PCK1) enzyme in MAFLD and NASH progression[107]. This study aimed to investigate the role of PCK1, a gluconeogenic enzyme, in the promotion of MAFLD through the study of rodents. It was found that PCK1 was downregulated in NASH patients and rodents with MAFLD. A further study by Xu et al[108] investigated transcription pattern mapping to identify the core genes and possible therapeutic targets that regulate MAFLD and sarcopenia, revealing 8 shared genes with common pathways.
Another area of interest is the impact of aging and associated low-grade inflammation on metabolic-associated diseases. As opposed to metaflammation, which is the inflammation present under overnutrition and metabolic disease, Inflammaging is a relatively new term that describes a low-grade inflammation that arises through aging[109]. Its mech
Understanding the clinical association between MAFLD and sarcopenia is crucial for a comprehensive approach to patient care. By recognizing the bidirectional relationship, shared risk factors, and impact on various outcomes, healthcare providers can implement targeted interventions, promote early detection, and optimize treatment strategies for individuals affected by these conditions. Further research is needed to unravel the complex mechanisms, explore targeted interventions, and develop personalized treatment strategies for individuals with this complex clinical asso
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cossiga V, Italy S-Editor: Gao CC L-Editor: A P-Editor: Zhang YL
| 1. | Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, Ye Q, Huang DQ, Zhao C, Zhang J, Liu C, Chang N, Xing F, Yan S, Wan ZH, Tang NSY, Mayumi M, Liu X, Rui F, Yang H, Yang Y, Jin R, Le RHX, Xu Y, Le DM, Barnett S, Stave CD, Cheung R, Zhu Q, Nguyen MH. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:2809-2817.e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 401] [Article Influence: 133.7] [Reference Citation Analysis (2)] |
| 2. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6987] [Cited by in RCA: 8473] [Article Influence: 564.9] [Reference Citation Analysis (0)] |
| 3. | Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, Kojima T, Kuzuya M, Lee JSW, Lee SY, Lee WJ, Lee Y, Liang CK, Lim JY, Lim WS, Peng LN, Sugimoto K, Tanaka T, Won CW, Yamada M, Zhang T, Akishita M, Arai H. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21:300-307.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2739] [Cited by in RCA: 3798] [Article Influence: 759.6] [Reference Citation Analysis (0)] |
| 4. | Zuo X, Li X, Tang K, Zhao R, Wu M, Wang Y, Li T. Sarcopenia and cardiovascular diseases: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14:1183-1198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 77] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 5. | Bertolotti M, Lonardo A, Mussi C, Baldelli E, Pellegrini E, Ballestri S, Romagnoli D, Loria P. Nonalcoholic fatty liver disease and aging: epidemiology to management. World J Gastroenterol. 2014;20:14185-14204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 232] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (1)] |
| 6. | Chan WK, Chuah KH, Rajaram RB, Lim LL, Ratnasingam J, Vethakkan SR. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J Obes Metab Syndr. 2023;32:197-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 286] [Article Influence: 143.0] [Reference Citation Analysis (1)] |
| 7. | Kang S, Moon MK, Kim W, Koo BK. Association between muscle strength and advanced fibrosis in non-alcoholic fatty liver disease: a Korean nationwide survey. J Cachexia Sarcopenia Muscle. 2020;11:1232-1241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Lee I, Kim J, Kang H. Estimated Cardiorespiratory Fitness Attenuates the Impacts of Sarcopenia and Obesity on Non-Alcoholic Fatty Liver in Korean Adults. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Kim G, Lee SE, Lee YB, Jun JE, Ahn J, Bae JC, Jin SM, Hur KY, Jee JH, Lee MK, Kim JH. Relationship Between Relative Skeletal Muscle Mass and Nonalcoholic Fatty Liver Disease: A 7-Year Longitudinal Study. Hepatology. 2018;68:1755-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 10. | Nachit M, De Rudder M, Thissen JP, Schakman O, Bouzin C, Horsmans Y, Vande Velde G, Leclercq IA. Myosteatosis rather than sarcopenia associates with non-alcoholic steatohepatitis in non-alcoholic fatty liver disease preclinical models. J Cachexia Sarcopenia Muscle. 2021;12:144-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 11. | Nachit M, Kwanten WJ, Thissen JP, Op De Beeck B, Van Gaal L, Vonghia L, Verrijken A, Driessen A, Horsmans Y, Francque S, Leclercq IA. Muscle fat content is strongly associated with NASH: A longitudinal study in patients with morbid obesity. J Hepatol. 2021;75:292-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 12. | Nachit M, Lanthier N, Rodriguez J, Neyrinck AM, Cani PD, Bindels LB, Hiel S, Pachikian BD, Trefois P, Thissen JP, Delzenne NM. A dynamic association between myosteatosis and liver stiffness: Results from a prospective interventional study in obese patients. JHEP Rep. 2021;3:100323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | World Health Organization. WHO European regional obesity report 2022. World Health Organization. [cited 22 Sept 2023]. In: World Health Organization [Internet]. Available from: https://www.who.int/europe/publications/i/item/9789289057738. |
| 14. | Yuan Q, Wang H, Gao P, Chen W, Lv M, Bai S, Wu J. Prevalence and Risk Factors of Metabolic-Associated Fatty Liver Disease among 73,566 Individuals in Beijing, China. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 15. | Guan L, Zhang X, Tian H, Jin X, Fan H, Wang N, Sun J, Li D, Li J, Wang X, Zeng Z, Li Y. Prevalence and risk factors of metabolic-associated fatty liver disease during 2014-2018 from three cities of Liaoning Province: an epidemiological survey. BMJ Open. 2022;12:e047588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Kurose S, Nishikawa S, Nagaoka T, Kusaka M, Kawamura J, Nishioka Y, Sato S, Tsutsumi H, Kimura Y. Prevalence and risk factors of sarcopenia in community-dwelling older adults visiting regional medical institutions from the Kadoma Sarcopenia Study. Sci Rep. 2020;10:19129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Seo JY, Cho EJ, Kim MJ, Kwak MS, Yang JI, Chung SJ, Yim JY, Yoon JW, Chung GE. The relationship between metabolic dysfunction-associated fatty liver disease and low muscle mass in an asymptomatic Korean population. J Cachexia Sarcopenia Muscle. 2022;13:2953-2960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Zhai Y, Xiao Q, Miao J. The Relationship between NAFLD and Sarcopenia in Elderly Patients. Can J Gastroenterol Hepatol. 2018;2018:5016091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Wijarnpreecha K, Kim D, Raymond P, Scribani M, Ahmed A. Associations between sarcopenia and nonalcoholic fatty liver disease and advanced fibrosis in the USA. Eur J Gastroenterol Hepatol. 2019;31:1121-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Hsieh YC, Joo SK, Koo BK, Lin HC, Kim W. Muscle alterations are independently associated with significant fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2021;41:494-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Zhao Q, Yin Y, Deng Y. Metabolic associated fatty liver disease and sarcopenia additively increase mortality: a real-world study. Nutr Diabetes. 2023;13:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 22. | Hsieh YC, Joo SK, Koo BK, Lin HC, Lee DH, Chang MS, Park JH, So YH, Kim W; Innovative Target Exploration of NAFLD (ITEN) Consortium. Myosteatosis, but not Sarcopenia, Predisposes NAFLD Subjects to Early Steatohepatitis and Fibrosis Progression. Clin Gastroenterol Hepatol. 2023;21:388-397.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 23. | Tanaka M, Okada H, Hashimoto Y, Kumagai M, Nishimura H, Oda Y, Fukui M. Relationship between nonalcoholic fatty liver disease and muscle quality as well as quantity evaluated by computed tomography. Liver Int. 2020;40:120-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Choe HJ, Lee H, Lee D, Kwak SH, Koo BK. Different effects of low muscle mass on the risk of non-alcoholic fatty liver disease and hepatic fibrosis in a prospective cohort. J Cachexia Sarcopenia Muscle. 2023;14:260-269. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Roh E, Hwang SY, Yoo HJ, Baik SH, Lee JH, Son SJ, Kim HJ, Park YS, Lee SG, Cho BL, Jang HC, Kim BJ, Kim M, Won CW, Choi KM. Impact of non-alcoholic fatty liver disease on the risk of sarcopenia: a nationwide multicenter prospective study. Hepatol Int. 2022;16:545-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Sinn DH, Kang D, Kang M, Guallar E, Hong YS, Lee KH, Park J, Cho J, Gwak GY. Nonalcoholic fatty liver disease and accelerated loss of skeletal muscle mass: A longitudinal cohort study. Hepatology. 2022;76:1746-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 27. | Altajar S, Wang N, Rosenthaler MP, Murabito JM, Long MT. NAFLD Associates with Sarcopenia Defined by Muscle Mass and Slow Walking Speed: A Cross-Sectional Analysis from the Framingham Heart Study. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Joo SK, Kim W. Interaction between sarcopenia and nonalcoholic fatty liver disease. Clin Mol Hepatol. 2023;29:S68-S78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 29. | Fazakerley DJ, Krycer JR, Kearney AL, Hocking SL, James DE. Muscle and adipose tissue insulin resistance: malady without mechanism? J Lipid Res. 2019;60:1720-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 30. | Sylow L, Tokarz VL, Richter EA, Klip A. The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab. 2021;33:758-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 189] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 31. | de Proença ARG, Pereira KD, Meneguello L, Tamborlin L, Luchessi AD. Insulin action on protein synthesis and its association with eIF5A expression and hypusination. Mol Biol Rep. 2019;46:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Zhang J, Zhao Y, Xu C, Hong Y, Lu H, Wu J, Chen Y. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: a cross-sectional study. Sci Rep. 2014;4:5832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 33. | Sakurai Y, Kubota N, Yamauchi T, Kadowaki T. Role of Insulin Resistance in MAFLD. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 238] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 34. | Branković M, Jovanović I, Dukić M, Radonjić T, Oprić S, Klašnja S, Zdravković M. Lipotoxicity as the Leading Cause of Non-Alcoholic Steatohepatitis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 35. | Ma IL, Stanley TL. Growth hormone and nonalcoholic fatty liver disease. Immunometabolism (Cobham). 2023;5:e00030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 36. | Monterrosa-Castro A, Ortiz-Banquéz M, Mercado-Lara M. Prevalence of sarcopenia and associated factors in climacteric women of the Colombian Caribbean. Menopause. 2019;26:1038-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Kim SW, Kim R. The association between hormone therapy and sarcopenia in postmenopausal women: the Korea National Health and Nutrition Examination Survey, 2008-2011. Menopause. 2020;27:506-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Van de Velde F, Bekaert M, Hoorens A, Geerts A, T'Sjoen G, Fiers T, Kaufman JM, Van Nieuwenhove Y, Lapauw B. Histologically proven hepatic steatosis associates with lower testosterone levels in men with obesity. Asian J Androl. 2020;22:252-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Mohamad NV, Wong SK, Wan Hasan WN, Jolly JJ, Nur-Farhana MF, Ima-Nirwana S, Chin KY. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 202] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 40. | Bianchi VE. The Anti-Inflammatory Effects of Testosterone. J Endocr Soc. 2019;3:91-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 41. | Clare K, Dillon JF, Brennan PN. Reactive Oxygen Species and Oxidative Stress in the Pathogenesis of MAFLD. J Clin Transl Hepatol. 2022;10:939-946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 42. | Masarone M, Rosato V, Dallio M, Gravina AG, Aglitti A, Loguercio C, Federico A, Persico M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid Med Cell Longev. 2018;2018:9547613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 468] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 43. | Jo D, Yoon G, Kim OY, Song J. A new paradigm in sarcopenia: Cognitive impairment caused by imbalanced myokine secretion and vascular dysfunction. Biomed Pharmacother. 2022;147:112636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 44. | Ulualan G, Kiraz ZK, Kırel B. Relation of serum irisin levels to obesity and non-alcoholic fatty liver disease. Turk J Pediatr. 2022;64:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Jürimäe J, Remmel L, Tamm AL, Purge P, Maasalu K, Tillmann V. Associations of Circulating Irisin and Fibroblast Growth Factor-21 Levels with Measures of Energy Homeostasis in Highly Trained Adolescent Rhythmic Gymnasts. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 46. | Yano K, Yamaguchi K, Seko Y, Okishio S, Ishiba H, Tochiki N, Takahashi A, Kataoka S, Okuda K, Liu Y, Fujii H, Umemura A, Moriguchi M, Okanoue T, Itoh Y. Hepatocyte-specific fibroblast growth factor 21 overexpression ameliorates high-fat diet-induced obesity and liver steatosis in mice. Lab Invest. 2022;102:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 47. | Wijarnpreecha K, Panjawatanan P, Thongprayoon C, Jaruvongvanich V, Ungprasert P. Sarcopenia and risk of nonalcoholic fatty liver disease: A meta-analysis. Saudi J Gastroenterol. 2018;24:12-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 48. | Pan X, Han Y, Zou T, Zhu G, Xu K, Zheng J, Zheng M, Cheng X. Sarcopenia Contributes to the Progression of Nonalcoholic Fatty Liver Disease- Related Fibrosis: A Meta-Analysis. Dig Dis. 2018;36:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 49. | Yu R, Shi Q, Liu L, Chen L. Relationship of sarcopenia with steatohepatitis and advanced liver fibrosis in non-alcoholic fatty liver disease: a meta-analysis. BMC Gastroenterol. 2018;18:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 50. | Cai C, Song X, Chen Y, Chen X, Yu C. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Hepatol Int. 2020;14:115-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 51. | Chianca V, Albano D, Messina C, Gitto S, Ruffo G, Guarino S, Del Grande F, Sconfienza LM. Sarcopenia: imaging assessment and clinical application. Abdom Radiol (NY). 2022;47:3205-3216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 111] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 52. | Chun HS, Kim MN, Lee JS, Lee HW, Kim BK, Park JY, Kim DY, Ahn SH, Kim SU. Risk stratification using sarcopenia status among subjects with metabolic dysfunction-associated fatty liver disease. J Cachexia Sarcopenia Muscle. 2021;12:1168-1178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 53. | Zhou T, Ye J, Lin Y, Wang W, Feng S, Zhuo S, Zhong B. Impact of skeletal muscle mass evaluating methods on severity of metabolic associated fatty liver disease in non-elderly adults. Br J Nutr. 2023;130:1373-1384. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 54. | Xiao P, Liang P, Gao P, Wu J. Sex- and region-specific associations of skeletal muscle mass with metabolic dysfunction-associated fatty liver disease. Front Endocrinol (Lausanne). 2022;13:1057261. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 55. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2841] [Article Influence: 568.2] [Reference Citation Analysis (1)] |
| 56. | Almomani A, Kumar P, Onwuzo S, Boustany A, Krishtopaytis E, Hitawala A, Alshaikh D, Albakri A, Hussein L, Hussein E, Asaad I. Epidemiology and prevalence of lean nonalcoholic fatty liver disease and associated cirrhosis, hepatocellular carcinoma, and cardiovascular outcomes in the United States: a population-based study and review of literature. J Gastroenterol Hepatol. 2023;38:269-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 57. | Lu FB, Zheng KI, Rios RS, Targher G, Byrne CD, Zheng MH. Global epidemiology of lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2020;35:2041-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 58. | Ha J, Yim SY, Karagozian R. Mortality and Liver-Related Events in Lean Versus Non-Lean Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2023;21:2496-2507.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 44] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 59. | Lan Y, Lu Y, Li J, Hu S, Chen S, Wang Y, Yuan X, Liu H, Wang X, Wu S, Wang L. Outcomes of subjects who are lean, overweight or obese with nonalcoholic fatty liver disease: A cohort study in China. Hepatol Commun. 2022;6:3393-3405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 60. | Younes R, Bugianesi E. NASH in Lean Individuals. Semin Liver Dis. 2019;39:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 61. | Zhu X, Huang Q, Ma S, Chen L, Wu Q, Wu L, Ma H, Li X, Li Q, Aleteng Q, Hu Y, He W, Gao J, Lin H, Tang H, Gao X, Xia M. Presence of sarcopenia identifies a special group of lean NAFLD in middle-aged and older people. Hepatol Int. 2023;17:313-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Nabi O, Lapidus N, Boursier J, de Ledinghen V, Petit JM, Kab S, Renuy A, Zins M, Lacombe K, Serfaty L. Lean individuals with NAFLD have more severe liver disease and poorer clinical outcomes (NASH-CO Study). Hepatology. 2023;78:272-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 61] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 63. | Zhang X, He Z, Si Q, Hu X, Yang L, Gu X, Du L, Wang L, Pan L, Li Y, Li J, Yang B. The Association of Sarcopenia and Visceral Obesity with Lean Nonalcoholic Fatty Liver Disease in Chinese Patients with Type 2 Diabetes Mellitus. J Diabetes Res. 2022;2022:2229139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 64. | Gao Q, Mei F, Shang Y, Hu K, Chen F, Zhao L, Ma B. Global prevalence of sarcopenic obesity in older adults: A systematic review and meta-analysis. Clin Nutr. 2021;40:4633-4641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 65. | Hwang J, Park S. Gender-Specific Prevalence and Risk Factors of Sarcopenic Obesity in the Korean Elderly Population: A Nationwide Cross-Sectional Study. Int J Environ Res Public Health. 2023;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 66. | Wang M, Tan Y, Shi Y, Wang X, Liao Z, Wei P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front Endocrinol (Lausanne). 2020;11:568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 67. | Lewitt MS. The Role of the Growth Hormone/Insulin-Like Growth Factor System in Visceral Adiposity. Biochem Insights. 2017;10:1178626417703995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 68. | Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, Batsis JA, Bauer JM, Boirie Y, Cruz-Jentoft AJ, Dicker D, Frara S, Frühbeck G, Genton L, Gepner Y, Giustina A, Gonzalez MC, Han HS, Heymsfield SB, Higashiguchi T, Laviano A, Lenzi A, Nyulasi I, Parrinello E, Poggiogalle E, Prado CM, Salvador J, Rolland Y, Santini F, Serlie MJ, Shi H, Sieber CC, Siervo M, Vettor R, Villareal DT, Volkert D, Yu J, Zamboni M, Barazzoni R. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes Facts. 2022;15:321-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 404] [Article Influence: 134.7] [Reference Citation Analysis (0)] |
| 69. | Hui Y, Cui B, Wang X, Sun M, Li Y, Lang W, Guo G, Mao L, Yu Z, Fan X, Sun C. Sarcopenic obesity in liver disease: Handling both sides of the penny. Portal Hypertens Cirrhosis. 2022;1:42-46. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 70. | von Berens Å, Obling SR, Nydahl M, Koochek A, Lissner L, Skoog I, Frändin K, Skoglund E, Rothenberg E, Cederholm T. Sarcopenic obesity and associations with mortality in older women and men - a prospective observational study. BMC Geriatr. 2020;20:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 71. | Chuan F, Chen S, Ye X, Kang S, Mei M, Tian W, Liao K, Li Y, Gong L, Li R, Zhou B. Sarcopenic obesity predicts negative health outcomes among older patients with type 2 diabetes: The Ageing and Body Composition of Diabetes (ABCD) cohort study. Clin Nutr. 2022;41:2740-2748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 72. | Yu PC, Hsu CC, Lee WJ, Liang CK, Chou MY, Lin MH, Hsiao FY, Peng LN, Chen LK. Muscle-to-fat ratio identifies functional impairments and cardiometabolic risk and predicts outcomes: biomarkers of sarcopenic obesity. J Cachexia Sarcopenia Muscle. 2022;13:368-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 73. | Cho HW, Chung W, Moon S, Ryu OH, Kim MK, Kang JG. Effect of Sarcopenia and Body Shape on Cardiovascular Disease According to Obesity Phenotypes. Diabetes Metab J. 2021;45:209-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 74. | Chung GE, Park HE, Lee H, Kim MJ, Choi SY, Yim JY, Yoon JW. Sarcopenic Obesity Is Significantly Associated With Coronary Artery Calcification. Front Med (Lausanne). 2021;8:651961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 75. | Park H, Jun S, Lee HA, Kim HS, Hong YS, Park H. The Effect of Childhood Obesity or Sarcopenic Obesity on Metabolic Syndrome Risk in Adolescence: The Ewha Birth and Growth Study. Metabolites. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 76. | Song W, Yoo SH, Jang J, Baik SJ, Lee BK, Lee HW, Park JS. Association between Sarcopenic Obesity Status and Nonalcoholic Fatty Liver Disease and Fibrosis. Gut Liver. 2023;17:130-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 77. | Hsing JC, Nguyen MH, Yang B, Min Y, Han SS, Pung E, Winter SJ, Zhao X, Gan D, Hsing AW, Zhu S, Wang CJ. Associations Between Body Fat, Muscle Mass, and Nonalcoholic Fatty Liver Disease: A Population-Based Study. Hepatol Commun. 2019;3:1061-1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 78. | Kim TH, Jeong CW, Lee C, Noh S, Lim DW, Kim JW, Kim HJ, Kim YR. Association between Body Composition Contents and Hepatic Fibrosis in Sarcopenic Obesity. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 79. | Rezuş E, Burlui A, Cardoneanu A, Rezuş C, Codreanu C, Pârvu M, Rusu Zota G, Tamba BI. Inactivity and Skeletal Muscle Metabolism: A Vicious Cycle in Old Age. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 80. | Song E, Hwang SY, Park MJ, Jang A, Kim KJ, Yu JH, Kim NH, Yoo HJ, Seo JA, Kim SG, Baik SH, Choi KM. Additive impact of diabetes and sarcopenia on all-cause and cardiovascular mortality: A longitudinal nationwide population-based study. Metabolism. 2023;148:155678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 81. | Kouvari M, Panagiotakos DB, Yannakoulia M, Georgousopoulou E, Critselis E, Chrysohoou C, Tousoulis D, Pitsavos C; ATTICA Study Investigators. Transition from metabolically benign to metabolically unhealthy obesity and 10-year cardiovascular disease incidence: The ATTICA cohort study. Metabolism. 2019;93:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 82. | Kouvari M, Polyzos SA, Chrysohoou C, Skoumas J, Pitsavos CS, Panagiotakos DB, Mantzoros CS. Skeletal muscle mass and abdominal obesity are independent predictors of hepatic steatosis and interact to predict ten-year cardiovascular disease incidence: Data from the ATTICA cohort study. Clin Nutr. 2022;41:1281-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 83. | Kim D, Wijarnpreecha K, Sandhu KK, Cholankeril G, Ahmed A. Sarcopenia in nonalcoholic fatty liver disease and all-cause and cause-specific mortality in the United States. Liver Int. 2021;41:1832-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 84. | Cho Y, Park HS, Huh BW, Lee YH, Seo SH, Seo DH, Ahn SH, Hong S, Kim SH. Non-Alcoholic Fatty Liver Disease with Sarcopenia and Carotid Plaque Progression Risk in Patients with Type 2 Diabetes Mellitus. Diabetes Metab J. 2023;47:232-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 85. | Han E, Kim MK, Im SS, Jang BK, Kim HS. Non-alcoholic fatty liver disease and sarcopenia is associated with the risk of albuminuria independent of insulin resistance, and obesity. J Diabetes Complications. 2022;36:108253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 86. | Harring M, Golabi P, Paik JM, Shah D, Racila A, Cable R, Srishord M, Younossi ZM. Sarcopenia Among Patients With Nonalcoholic Fatty Liver Disease (NAFLD) Is Associated With Advanced Fibrosis. Clin Gastroenterol Hepatol. 2023;21:2876-2888.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 87. | Kong Q, Yi M, Teng F, Li H, Chen Z. Sarcopenia Imperils Postoperative Long-Term Survival in HCC Patients with Metabolic Dysfunction-Associated Fatty Liver Disease: A Propensity Score Matching Analysis. J Hepatocell Carcinoma. 2023;10:1367-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 88. | Sheptulina AF, Yafarova AA, Golubeva JA, Mamutova EM, Kiselev AR, Drapkina OM. Clinically Meaningful Fatigue and Depression Are Associated with Sarcopenia in Patients with Non-Alcoholic Fatty Liver Disease. J Pers Med. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 89. | Fouad Y, Esmat G, Elwakil R, Zakaria S, Yosry A, Waked I, El-Razky M, Doss W, El-Serafy M, Mostafa E, Anees M, Sakr MA, AbdelAty N, Omar A, Zaki S, Al-Zahaby A, Mahfouz H, Abdalla M, Albendary M, Hamed AK, Gomaa A, Hasan A, Abdel-Baky S, El Sahhar M, Shiha G, Attia D, Saeed E, Kamal E, Bazeed S, Mehrez M, Abdelaleem S, Gaber Y, Abdallah M, Salama A, Tawab DA, Nafady S. The egyptian clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Saudi J Gastroenterol. 2022;28:3-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 90. | Abdelbasset WK, Tantawy SA, Kamel DM, Alqahtani BA, Soliman GS. A randomized controlled trial on the effectiveness of 8-week high-intensity interval exercise on intrahepatic triglycerides, visceral lipids, and health-related quality of life in diabetic obese patients with nonalcoholic fatty liver disease. Medicine (Baltimore). 2019;98:e14918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 91. | Oh S, So R, Shida T, Matsuo T, Kim B, Akiyama K, Isobe T, Okamoto Y, Tanaka K, Shoda J. High-Intensity Aerobic Exercise Improves Both Hepatic Fat Content and Stiffness in Sedentary Obese Men with Nonalcoholic Fatty Liver Disease. Sci Rep. 2017;7:43029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 92. | Shamsoddini A, Sobhani V, Ghamar Chehreh ME, Alavian SM, Zaree A. Effect of Aerobic and Resistance Exercise Training on Liver Enzymes and Hepatic Fat in Iranian Men With Nonalcoholic Fatty Liver Disease. Hepat Mon. 2015;15:e31434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 93. | Ayres ABS, Carneiro CRG, Gestic MA, Utrini MP, Chaim FDM, Callejas-Neto F, Chaim EA, Cazzo E. Identification of Predictors of Non-alcoholic Steatohepatitis and Its Severity in Individuals Undergoing Bariatric Surgery. Obes Surg. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 94. | Negm AM, Lee J, Hamidian R, Jones CA, Khadaroo RG. Management of Sarcopenia: A Network Meta-Analysis of Randomized Controlled Trials. J Am Med Dir Assoc. 2022;23:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 95. | Wackerhage H, Schoenfeld BJ, Hamilton DL, Lehti M, Hulmi JJ. Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise. J Appl Physiol (1985). 2019;126:30-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 96. | Chun HS, Lee M, Lee HA, Oh SY, Baek HJ, Moon JW, Kim YJ, Lee J, Kim H, Kim HY, Yoo K, Kim TH, Kim SU. Association of Physical Activity With Risk of Liver Fibrosis, Sarcopenia, and Cardiovascular Disease in Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2023;21:358-369.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 97. | Bunchorntavakul C, Reddy KR. Review article: malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment Pharmacol Ther. 2020;51:64-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 98. | Mertz KH, Reitelseder S, Bechshoeft R, Bulow J, Højfeldt G, Jensen M, Schacht SR, Lind MV, Rasmussen MA, Mikkelsen UR, Tetens I, Engelsen SB, Nielsen DS, Jespersen AP, Holm L. The effect of daily protein supplementation, with or without resistance training for 1 year, on muscle size, strength, and function in healthy older adults: A randomized controlled trial. Am J Clin Nutr. 2021;113:790-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 99. | Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, Takai K, Shimizu M, Moriwaki H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 288] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 100. | Yi Y, Wang C, Ding Y, He J, Lv Y, Chang Y. Diet was less significant than physical activity in the prognosis of people with sarcopenia and metabolic dysfunction-associated fatty liver diseases: Analysis of the National Health and Nutrition Examination Survey III. Front Endocrinol (Lausanne). 2023;14:1101892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 101. | Wang MY, Prabahar K, Găman MA, Zhang JL. Vitamin E supplementation in the treatment on nonalcoholic fatty liver disease (NAFLD): Evidence from an umbrella review of meta-analysis on randomized controlled trials. J Dig Dis. 2023;24:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 102. | Ghosal S, Datta D, Sinha B. A meta-analysis of the effects of glucagon-like-peptide 1 receptor agonist (GLP1-RA) in nonalcoholic fatty liver disease (NAFLD) with type 2 diabetes (T2D). Sci Rep. 2021;11:22063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 103. | Park MJ, Kim H, Kim MG, Kim K. Comparison of glucagon-like peptide-1 receptor agonists and thiazolidinediones on treating nonalcoholic fatty liver disease: A network meta-analysis. Clin Mol Hepatol. 2023;29:693-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 104. | Hameed I, Hayat J, Marsia S, Samad SA, Khan R, Siddiqui OM, Khan MO, Malik S, Fatima K, Fudim M, Krasuski RA. Comparison of sodium-glucose cotransporter-2 inhibitors and thiazolidinediones for management of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2023;47:102111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 105. | Ayada I, van Kleef LA, Zhang H, Liu K, Li P, Abozaid YJ, Lavrijsen M, Janssen HLA, van der Laan LJW, Ghanbari M, Peppelenbosch MP, Zheng MH, de Knegt RJ, Pan Q. Dissecting the multifaceted impact of statin use on fatty liver disease: a multidimensional study. EBioMedicine. 2023;87:104392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 106. | Rojas Á, Lara-Romero C, Muñoz-Hernández R, Gato S, Ampuero J, Romero-Gómez M. Emerging pharmacological treatment options for MAFLD. Ther Adv Endocrinol Metab. 2022;13:20420188221142452. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 107. | Ye Q, Liu Y, Zhang G, Deng H, Wang X, Tuo L, Chen C, Pan X, Wu K, Fan J, Pan Q, Wang K, Huang A, Tang N. Deficiency of gluconeogenic enzyme PCK1 promotes metabolic-associated fatty liver disease through PI3K/AKT/PDGF axis activation in male mice. Nat Commun. 2023;14:1402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 108. | Xu Z, Yu Z, Li S, Tian Z, Yuan J, You F. Exploration of the core gene signatures and mechanisms between NAFLD and sarcopenia through transcriptomic level. Front Endocrinol (Lausanne). 2023;14:1140804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 109. | Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1183] [Cited by in RCA: 1877] [Article Influence: 268.1] [Reference Citation Analysis (0)] |