Published online Dec 27, 2024. doi: 10.4254/wjh.v16.i12.1505
Revised: October 1, 2024
Accepted: October 31, 2024
Published online: December 27, 2024
Processing time: 192 Days and 3.1 Hours
The purpose of this case report is to describe a case of multiple intrahepatic artery aneurysms during treatment for IgG4-related sclerosing cholangitis (IgG4-SC) and to provide information for daily practice.
A 64-year-old Japanese woman was diagnosed with IgG4-SC five years prior and was receiving maintenance treatment with prednisolone 7.5-10 mg/day. She developed abdominal pain and a sudden onset of black stool and was admitted to our hospital. Abdominal contrast-enhanced computed tomography (CT) and ultra
Hepatic artery aneurysms should be considered poor prognostic complications of IgG4-SC.
Core Tip: Hepatic artery pseudoaneurysm is a disease with poor prognosis due to high rupture and mortality rates. IgG4-related sclerosing cholangitis (IgG4-SC) is a disease caused by an autoimmune mechanism. we report a case of multiple ruptured intrahepatic artery pseudoaneurysms that occurred during the treatment of IgG4-SC. We consider the cause is complex: IgG4-related vasculitis, severe acute obstructive cholangitis and arteriosclerosis. Hepatic artery aneurysms should be considered poor prognostic complications of IgG4-SC.
- Citation: Tamura H, Ozono Y, Uchida K, Uchiyama N, Hatada H, Ogawa S, Iwakiri H, Kawakami H. Multiple intrahepatic artery aneurysms during the treatment for IgG4-related sclerosing cholangitis: A case report. World J Hepatol 2024; 16(12): 1505-1514
- URL: https://www.wjgnet.com/1948-5182/full/v16/i12/1505.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i12.1505
Visceral artery aneurysms (VAAs) are rare and occur in the celiac, superior mesenteric, and inferior mesenteric branches. Hepatic artery aneurysms account for 10%-20% of these VAAs. Pseudoaneurysms can be iatrogenic or inflammatory and have a high probability of rupture[1,2]. IgG4-related sclerosing cholangitis (IgG4-SC) is a disease caused by an autoi
In this report, we describe a case of multiple ruptured intrahepatic artery pseudoaneurysms that occurred during the treatment of IgG4-SC. There have been a few reports of hepatic artery aneurysms with IgG4-related vasculopathy[6-9], there have been no reports of multiple lesions in more peripheral hepatic arteries and complications of IgG4-SC. Hepatic artery aneurysms should be considered poor prognostic complications of IgG4-SC and are reported here with a review of the literature.
A 64-year-old Japanese woman presented to gastroenterology hospital with abdominal pain and a sudden onset of black stool.
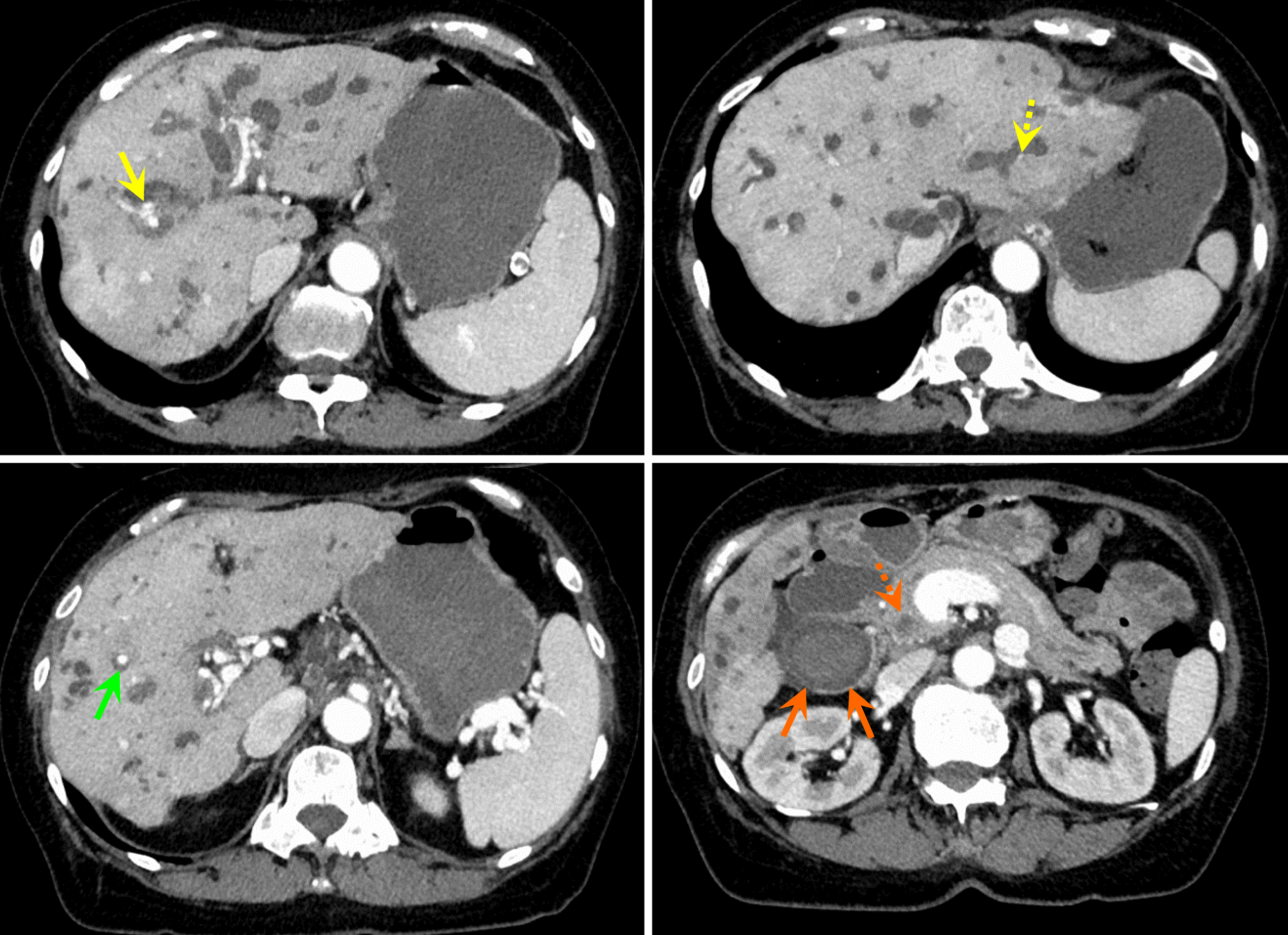
Symptoms developed suddenly during the treatment of IgG4-SC. Emergency contrast-enhanced computed tomography (CT) revealed an exacerbation of intrahepatic bile duct dilatation. In addition, biliary hemorrhage was suspected because of the high absorption from the gallbladder into the common bile duct. Contrast-enhanced areas within the intrahepatic bile duct appeared to be hepatic aneurysms; however, these areas were small and could not be identified at this point (Figure 1).
She was diagnosed with severe relapse of IgG4-SC and was treated with steroid pulse therapy (methylprednisolone sodium succinate 1 g/day, day 1-3). Prednisolone 15 mg/day was administered from day 4 as post-treatment. She was transferred to our department on day 9 for close examination and treatment.
Five years ago, the patient was diagnosed with IgG4-SC and retroperitoneal fibrosis. She was started on 30 mg/day of prednisolone (0.6 mg/kg), and the elevation of hepatobiliary enzymes improved. Prednisolone was tapered and continued at a maintenance dose of 7.5-10 mg/day.
The patient denied any family history.
On physical examination her height was 163.7 cm and her weight was 63.1 kg, and her vital signs were as follows: Blood pressure, 111/70 mmHg; pulse rate, 96 beats/min; temperature, 37.0 °C; and Glasgow coma score, E4V5M6. Furthermore, abdominal pain and persistent melena was found.
An elevation of blood bilirubin levels and hepatobiliary enzymes (aspartate aminotransferase, 41 U/L; alanine aminotransferase, 52 U/L; lactate dehydrogenase, 235 U/L; alkaline phosphatase, 195 U/L; γ-glutamyl transpeptidase, 122 U/L; total bilirubin, 4.6 mg/dL; direct bilirubin, 2.2 mg/dL), as well as elevated inflammatory markers (white blood cell, 9200/μL; C-reactive protein, 5.22 mg/dL), subacute anemia (hemoglobin, 9.1 g/dL), mild blood clotting disorder (prothrombin time, 18.6 seconds), hypoalbuminemia (albumin, 2.54 g/dL). No abnormality was found in IgG and IgG4 measurement (Table 1).
| Lab investigation | Value |
| Hematologic test | |
| White blood cells (/μL) | 9200 |
| Neutrophil (%) | 91.7 |
| Lymphocyte (%) | 4.3 |
| Monocyte (%) | 3.7 |
| Eosinophil (%) | 0.2 |
| Basophil (%) | 0.1 |
| Red blood cells (/μL) | 279 × 104 |
| MCV (fL) | 105.7 |
| MCH (pg) | 32.6 |
| MCHC (g/dL) | 30.8 |
| Hemoglobin (g/dL) | 9.1 |
| Platelet count (/μL) | 21.2 × 104 |
| Coagulation | |
| PT (second) | 18.6 |
| PT (%) | 51.3 |
| PT-INR | 1.53 |
| APTT | 35.6 |
| Chemistry | |
| AST (U/L) | 41 |
| ALT (U/L) | 52 |
| LD (U/L) | 235 |
| ALP (U/L) | 195 |
| γ-GT (U/L) | 122 |
| AMY (U/L) | 89 |
| Lipase (U/L) | 17.2 |
| Total bilirubin (mg/dL) | 4.6 |
| Direct bilirubin (mg/dL) | 2.2 |
| Total protein (g/dL) | 5.95 |
| Albumin (g/dL) | 2.54 |
| BUN (mg/dL) | 11.3 |
| Creatinine (mg/dL) | 0.83 |
| Sodium (mmol/L) | 136 |
| Potassium (mmol/L) | 4.1 |
| Chloride (mmol/L) | 93 |
| CRP (mg/dL) | 5.22 |
| Glucose (mg/dL) | 158 |
| P-ANCA (EU) | 1.3 |
| C-ANCA | 1.0 |
| IgG (mg/dL) | 1685 |
| IgG4 (mg/dL) | 44.4 |
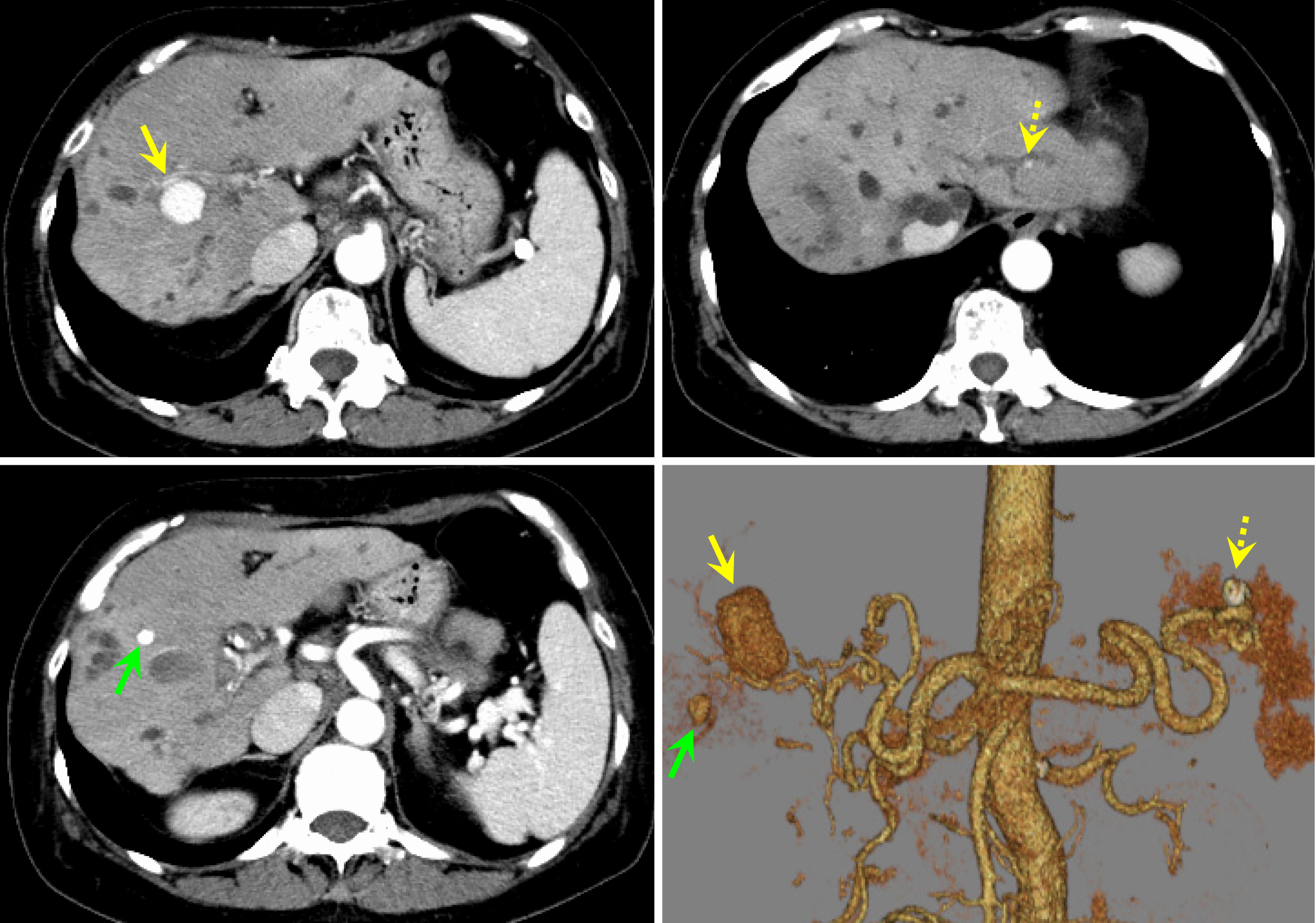
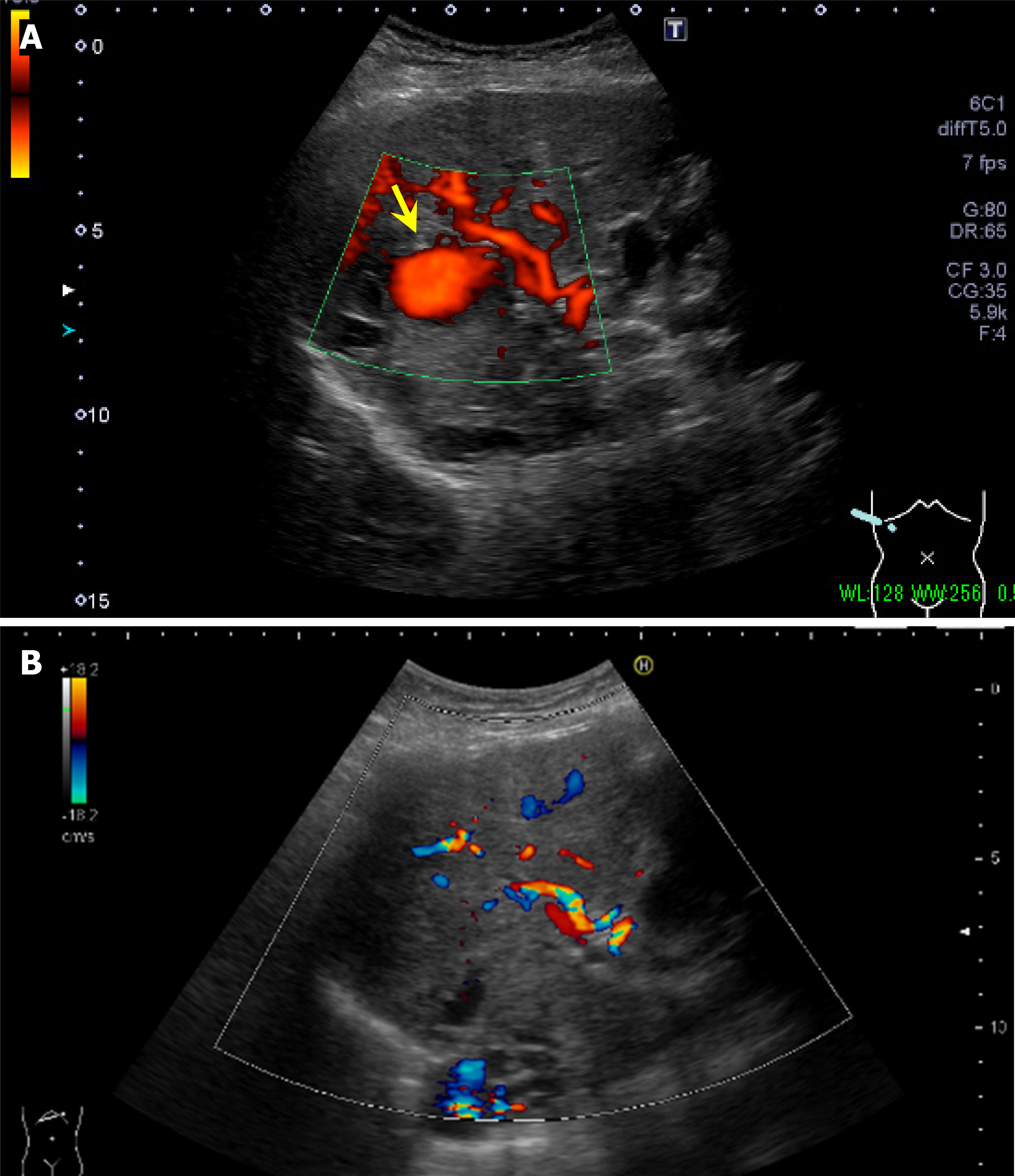
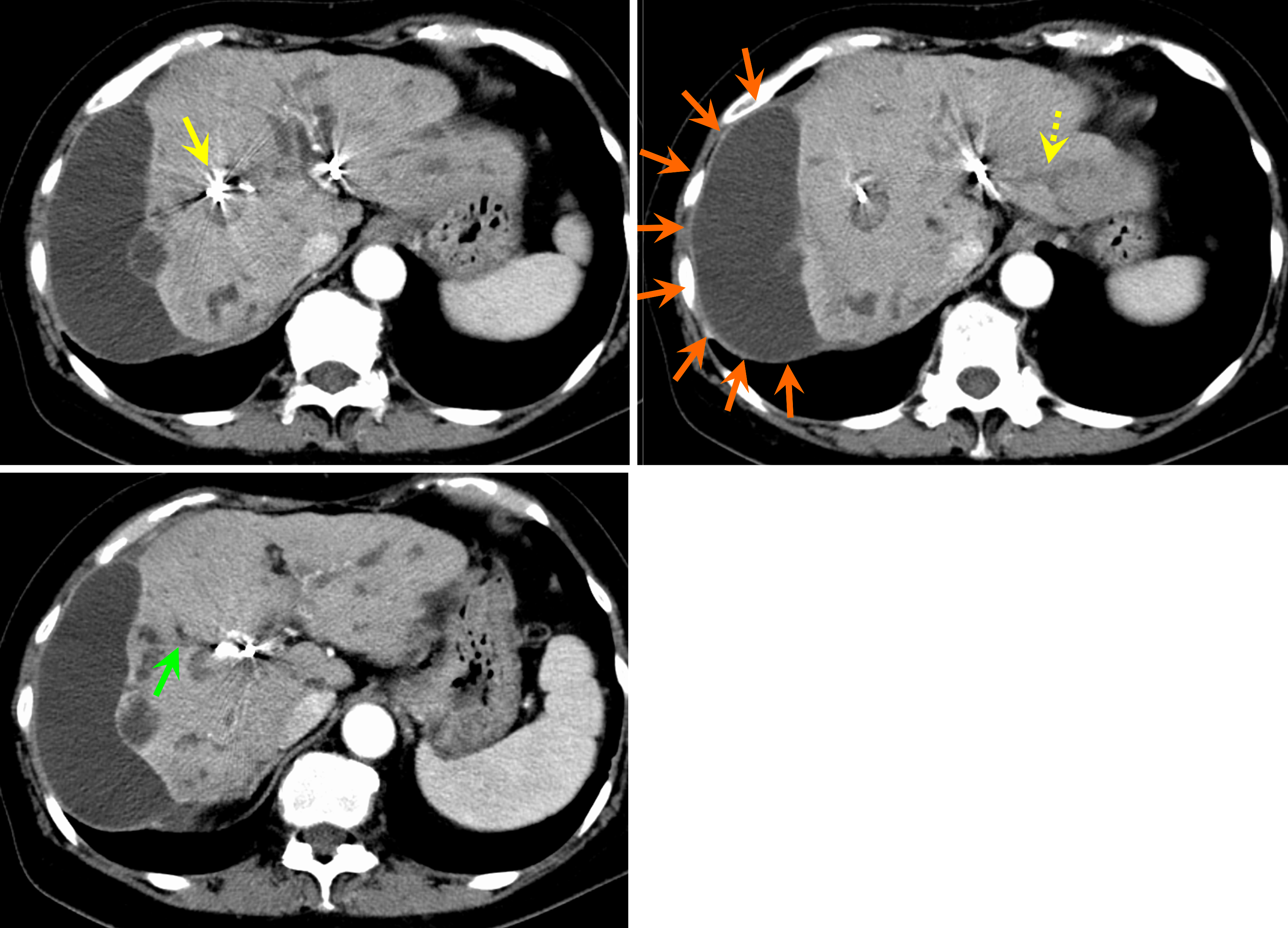
Contrast-enhanced CT scan of the abdomen revealed nodular contrast-enhanced areas approximately 20 mm in size appeared in hepatic S 7/8, 7 mm in size in S5, and 4 mm in size in the lateral segment (Figure 2). No findings suggested mucosal abnormalities or bleeding in the esophagus, stomach, or duodenum. Abdominal ultrasonography revealed a mass lesion at hepatic S 7/8 showing pulsating blood flow (Figure 3A).
Combined with the patient’s medical history, the final diagnosis was ruptured intrahepatic artery pseudoaneurysms and biliary hemorrhage.
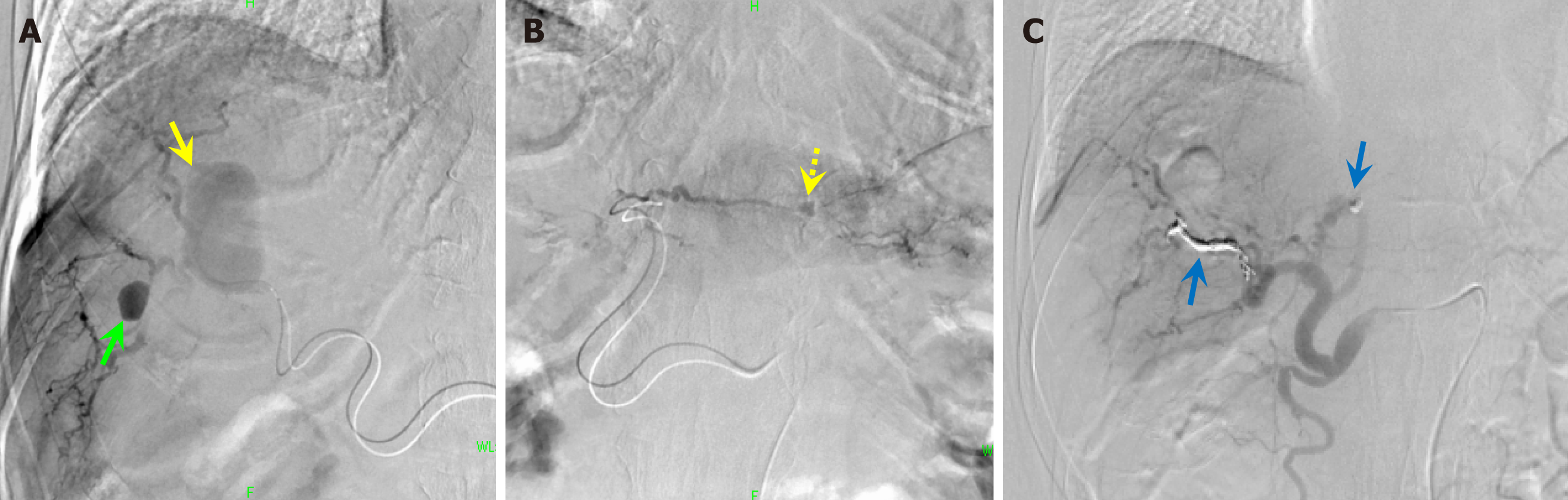
Embolization was considered. Angiography revealed that the lesions were located in the right and left lobes of the liver at A7 and A2, respectively. Since peripheral catheter insertion was difficult, we injected a gel-like intravascular embolization prosthetic agent (Cerescue® Astellas Pharma Inc., Tokyo, Japan.) in the periphery and then embolized the central part with microcoils (Figure 4).
On the day after embolization, the nonechogenic mass lesions with blood flow signals observed before embolization disappeared (Figure 3B). On abdominal contrast-enhanced CT, the day after embolization, the hepatic artery aneurysms had disappeared. A fluid collection appeared on the surface of the right lobe of the liver, which was thought to be a biloma caused by rupture of the peripheral bile duct (Figure 5).
Thereafter, the progression of anemia ceased and the melena resolved. Serum aspartate aminotransferase and alanine aminotransferase levels increased from the day after treatment, reaching 298/311 U/L on the third day after treatment but improved from the fourth day. She had acute cholangitis and continued to receive cefoperazone/sulbactam (2 g/day) at the previous hospital. However, she developed a fever on the second day after treatment, and the antibiotic was changed to meropenem (3 g/day), assuming multidrug-resistant bacteria. The blood bilirubin level decreased slowly after treatment but stopped falling at 3-4 mg/mL. Endoscopic bile duct drainage was considered but was not performed because it was predicted to be ineffective due to extensive bile duct stenosis from the hilar region to the intrahepatic bile ducts and peripheral bile duct dilatation.
The patient was transferred to a local hospital and underwent percutaneous transhepatic bile duct drainage; however, there was little improvement, leading to liver failure.
VAAs are rare entities involving the celiac, superior mesenteric, and inferior mesenteric arteries, and their branches, with a prevalence of 0.1%-2%[1]. Hepatic artery aneurysms were first described by the English anatomist Wilson in 1819 and account for 10%-20% of all VAAs[1,2]. Aneurysms are classified as true aneurysms, in which all three layers of the artery form the aneurysm, and pseudoaneurysms, in which the outer membrane layer forms the aneurysm due to the failure of the inner membrane layer. The causes of the former include congenital diseases, such as Ehlers-Danlos syndrome, infectious diseases, such as infective endocarditis, and atherosclerosis. Causes of the latter include medical causes such as surgery and procedures, infectious diseases, and inflammatory diseases[2]. The rupture rate of pseudoaneurysms is up to 80%, and the mortality rate is reported to be 20%-40%[1,10]. When a hepatic artery aneurysm ruptures, bleeding occurs in the bile duct, resulting in the Quincke's triad (biliary colic, melena, and obstructive jaundice)[1]. The localization of hepatic artery aneurysms has been reported to be 63% in the common hepatic artery, 28% in the right hepatic artery, 20% in the intrahepatic artery, and 5% in the left hepatic artery[11]. In a study of 33 patients with hepatic artery aneurysms, multiple hepatic aneurysms were present in three patients (8%)[10]. The present case was characterized by multiple simultaneous pseudoaneurysms in the intrahepatic artery.
IgG4 vasculopathy most commonly affects the aorta and presents as aortic wall thickening with or without the formation of aneurysms. Aortitis and periaortitis are found in nearly 41% of cases of IgG4-related disease[12], but the presentation of vasculitis of mediumsized vessels as aneurysms is quite rare[6]. There have been four cases from three reports of hepatic artery aneurysms with IgG4-related disease[6-9](Table 2). Kasa et al[7] reported the pathologic examination of the resected hepatic aneurysmal wall confirmed the presence of a significant number of IgG4-positive plasma cells and storiform fibrosis. In our case, IgG4-related aneurysms is also suspected. However, our case is characterized by more peripheral intrahepatic artery lesions and cholangitis.
| Ref. | Age | Sex | Arteriosclerosis exacerbating factors | IgG4-related lesions in other organs | Localization of aneurysm | Size of aneurysm | Treatment | Outcome |
| Vlachou et al[9], 2011 | 46 | Female | ND | ND | Common hepatic artery | ND | ND | ND |
| Vlachou et al[9], 2011 | ND | ND | ND | ND | Hepatic artery | ND | ND | ND |
| Yadav et al[6], 2021 | 55 | Male | Hypertension | Paravertebral soft- tissue thickening | Left anterior descending artery Right intercostal artery Common hepatic artery Inferior pancreaticoduodenal artery Superior mesenteric artery | ND | Surgery | Aneurysms were cured. The patient was put on corticosteroids therapy |
| Kasa et al[7], 2024 | 49 | Male | Current smoker Hypertension Dyslipidemia | None | Common hepatic artery | 30 mm | Surgery | Aneurysm were cured, but recurred at a left internal iliac artery and right renal artery |
| Present case | 64 | Female | High doses of glucocorticoids for an extended period | Cholangitis retroperitoneal fibrosis | Intrahepatic arteries | 20 mm, 7 mm, and 4 mm | Transarterial embolization | Aneurysms were cured, but liver failure developed |
To date, there have been five reports of hepatic artery aneurysms triggered by cholangitis, excluding cases caused by physical irritation from endoscopic retrograde cholangiopancreatography or bile duct stents[13-17]; however, there have been no case reports of IgG4-SC as the cause of cholangitis. Takeda et al[13] considered that cholangitis causes hepatic artery aneurysms because severe inflammation of the intrahepatic bile ducts spreads to the hepatic parenchyma and causes erosion of the surrounding hepatic artery wall. In the present case, the bile duct stenosis in the hilar region and peripheral bile duct dilation worsened over five years after the onset of the disease. The aneurysms were not located in the hilar region, the main site of IgG4-SC, but were peripherally distributed. This suggests that severe acute obstructive cholangitis associated with hilar bile duct stenosis due to IgG4-SC is a direct cause of hepatic artery aneurysm formation.
Takeda et al[13] revealed the presence of severe atherosclerosis of the hepatic artery in a histopathological study of a patient who underwent hepatic resection after embolization of a hepatic artery aneurysm and pointed out that this may have contributed to the formation of the aneurysm. She did not have hypertension, diabetes mellitus, or dyslipidemia, which are the main causes of atherosclerosis but had been receiving glucocorticoids for a long time. Several reports suggest that glucocorticoids accelerate systemic atherosclerosis, suggesting a direct effect of glucocorticoids and the indirect effect of the metabolic abnormalities described above[18-20]. Hepatic atherosclerosis is a possible factor in this case because the patient was treated with high doses of glucocorticoids for an extended period.
In a case report of an inflammatory hepatic artery aneurysm triggered by cholecystitis, England et al[21] reported the rarity of pseudoaneurysm formation in the cystic artery despite the high incidence of cholecystitis, which may reflect early thrombosis of the cystic artery as a response to adjacent inflammation. In our case, prolonged PT due to liver cirrhosis observed before the onset of hepatic artery aneurysms may have contributed to the simultaneous formation of multiple hepatic artery aneurysms.
In IgG4-SC, the improvement rate of bile duct stenosis with glucocorticoid treatment is as high as approximately 90%[3], but the relapse rate during dose reduction or maintenance treatment is high, ranging from 31% to 50%[5,22]. Ghazale et al[23] reported a high relapse rate in patients with stenosis in the proximal extrahepatic bile duct. In our case, serum IgG4 levels quickly normalized after the induction of prednisolone, and blood bilirubin levels and hepatobiliary enzymes improved, but the patient relapsed several times when prednisolone was tapered off. Because the total lifetime dose of prednisolone was > 15 g, the risk of infection and osteoporosis would have made it difficult to increase the dose further. We believe that this case can be replicated in the future when the number of steroid-dependent, steroid-resistant, and long-term management cases of IgG4-SC increases. Endoscopic biliary tract drainage at an appropriate time is important for prevention. For refractory cases, azathioprine or rituximab (a monoclonal antibody consisting of an anti-human CD20 human-mouse chimeric antibody) has been reported to be effective[24], but further studies are warranted.
The Society of Vascular Surgery clinical practice guidelines for managing VAAs[25] recommend treating pseudoaneurysms regardless of their size or location. These treatments include ligation, resection and reconstruction, arterial transplantation, hepatectomy, and endovascular therapy; however, endovascular treatment methods are increasingly being performed to avoid invasive open surgery[26,27]. However, when the aneurysm is located peripherally in the liver, as in this case, it is difficult to guide the catheter, and selective coil embolization may not be possible. The liver failure was thought to be caused by hepatic ischemia due to hepatic artery embolism and severe acute obstructive cholangitis. It is necessary to explain to the patient that, in such cases, the extent of inhibition must be increased, and depending on the hepatic reserve capacity of the background liver and portal/arterial blood flow ratio, there is a possibility of decreased hepatic function after the procedure.
Hepatic artery aneurysms should be considered poor prognostic complications of IgG4-SC.
| 1. | Hemp JH, Sabri SS. Endovascular management of visceral arterial aneurysms. Tech Vasc Interv Radiol. 2015;18:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Pitton MB, Dappa E, Jungmann F, Kloeckner R, Schotten S, Wirth GM, Mittler J, Lang H, Mildenberger P, Kreitner KF, Oberholzer K, Dueber C. Visceral artery aneurysms: Incidence, management, and outcome analysis in a tertiary care center over one decade. Eur Radiol. 2015;25:2004-2014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 3. | Tanaka A, Tazuma S, Okazaki K, Nakazawa T, Inui K, Chiba T, Takikawa H. Clinical Features, Response to Treatment, and Outcomes of IgG4-Related Sclerosing Cholangitis. Clin Gastroenterol Hepatol. 2017;15:920-926.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Hart PA, Kamisawa T, Brugge WR, Chung JB, Culver EL, Czakó L, Frulloni L, Go VL, Gress TM, Kim MH, Kawa S, Lee KT, Lerch MM, Liao WC, Löhr M, Okazaki K, Ryu JK, Schleinitz N, Shimizu K, Shimosegawa T, Soetikno R, Webster G, Yadav D, Zen Y, Chari ST. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut. 2013;62:1771-1776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 461] [Cited by in RCA: 373] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 5. | Huggett MT, Culver EL, Kumar M, Hurst JM, Rodriguez-Justo M, Chapman MH, Johnson GJ, Pereira SP, Chapman RW, Webster GJM, Barnes E. Type 1 autoimmune pancreatitis and IgG4-related sclerosing cholangitis is associated with extrapancreatic organ failure, malignancy, and mortality in a prospective UK cohort. Am J Gastroenterol. 2014;109:1675-1683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Yadav A, Godasu G, Buxi TBS, Sheth S. Multiple Artery Aneurysms: Unusual Presentation of IgG4 Vasculopathy. J Clin Imaging Sci. 2021;11:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Kasa K, Ohki T, Ito E, Fukasawa N, Shukuzawa K, Shimoda M. Immunoglobulin G4-related hepatic artery aneurysm. J Vasc Surg Cases Innov Tech. 2024;10:101377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Rossi M, Virgilio E, Laurino F, Orgera G, Menè P, Pirozzi N, Ziparo V, Cavallini M. Giant Hepatic Artery Aneurysm Associated with Immunoglobulin G4-Related Disease Successfully Treated Using a Liquid Embolic Agent. Korean J Radiol. 2015;16:953-954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Vlachou PA, Khalili K, Jang HJ, Fischer S, Hirschfield GM, Kim TK. IgG4-related sclerosing disease: autoimmune pancreatitis and extrapancreatic manifestations. Radiographics. 2011;31:1379-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Abbas MA, Fowl RJ, Stone WM, Panneton JM, Oldenburg WA, Bower TC, Cherry KJ, Gloviczki P. Hepatic artery aneurysm: factors that predict complications. J Vasc Surg. 2003;38:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 224] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 11. | Arneson MA, Smith RS. Ruptured hepatic artery aneurysm: case report and review of literature. Ann Vasc Surg. 2005;19:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Yabusaki S, Oyama-Manabe N, Manabe O, Hirata K, Kato F, Miyamoto N, Matsuno Y, Kudo K, Tamaki N, Shirato H. Characteristics of immunoglobulin G4-related aortitis/periaortitis and periarteritis on fluorodeoxyglucose positron emission tomography/computed tomography co-registered with contrast-enhanced computed tomography. EJNMMI Res. 2017;7:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Takeda K, Tanaka K, Endo I, Togo S, Shimada H. Two-stage treatment of an unusual haemobilia caused by intrahepatic pseudoaneurysm. World J Hepatol. 2010;2:52-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Goyal A, Madhusudhan KS, Gamanagatti S, Baruah B, Shalimar, Sharma R. Radiological management of multiple hepatic artery pseudoaneurysms associated with cholangitic abscesses. Indian J Radiol Imaging. 2016;26:99-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Fernández Conesa M, Milena Muñoz A, Valero González MÁ. Active bleeding due to a hepatic arterial pseudoaneurysm that occurred after acute cholangitis. Rev Esp Enferm Dig. 2020;112:240. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Cheung PL, Lee YS, Tan CB, Lau HY, Siu CW, Chan CX, Chan WT, Ho CH. Endovascular Management of Hepatic Artery Pseudoaneurysms: A Case Series. Vasc Specialist Int. 2023;39:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | An JY, Lee JS, Kim DR, Jang JY, Jung HY, Park JH, Jin SS. Coil embolization of ruptured intrahepatic pseudoaneurysm through percutaneous transhepatic biliary drainage. Yeungnam Univ J Med. 2018;35:109-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med. 2004;141:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 532] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 19. | Varas-Lorenzo C, Rodriguez LA, Maguire A, Castellsague J, Perez-Gutthann S. Use of oral corticosteroids and the risk of acute myocardial infarction. Atherosclerosis. 2007;192:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Tihista M, Gu A, Wei C, Weinreb JH, Rao RD. The impact of long-term corticosteroid use on acute postoperative complications following lumbar decompression surgery. J Clin Orthop Trauma. 2020;11:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | England RE, Marsh PJ, Ashleigh R, Martin DF. Case report: pseudoaneurysm of the cystic artery: a rare cause of haemobilia. Clin Radiol. 1998;53:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Hart PA, Topazian MD, Witzig TE, Clain JE, Gleeson FC, Klebig RR, Levy MJ, Pearson RK, Petersen BT, Smyrk TC, Sugumar A, Takahashi N, Vege SS, Chari ST. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo Clinic experience. Gut. 2013;62:1607-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 23. | Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, Clain JE, Pearson RK, Petersen BT, Vege SS, Lindor K, Farnell MB. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 24. | Kamisawa T, Nakazawa T, Tazuma S, Zen Y, Tanaka A, Ohara H, Muraki T, Inui K, Inoue D, Nishino T, Naitoh I, Itoi T, Notohara K, Kanno A, Kubota K, Hirano K, Isayama H, Shimizu K, Tsuyuguchi T, Shimosegawa T, Kawa S, Chiba T, Okazaki K, Takikawa H, Kimura W, Unno M, Yoshida M. Clinical practice guidelines for IgG4-related sclerosing cholangitis. J Hepatobiliary Pancreat Sci. 2019;26:9-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 25. | Chaer RA, Abularrage CJ, Coleman DM, Eslami MH, Kashyap VS, Rockman C, Murad MH. The Society for Vascular Surgery clinical practice guidelines on the management of visceral aneurysms. J Vasc Surg. 2020;72:3S-39S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 315] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 26. | Graham I, Kanitra J, Berg R, Haouilou J. Management of a common and proper hepatic artery aneurysm. J Vasc Surg Cases Innov Tech. 2021;7:283-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Grotemeyer D, Duran M, Park EJ, Hoffmann N, Blondin D, Iskandar F, Balzer KM, Sandmann W. Visceral artery aneurysms--follow-up of 23 patients with 31 aneurysms after surgical or interventional therapy. Langenbecks Arch Surg. 2009;394:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |