Published online Dec 27, 2024. doi: 10.4254/wjh.v16.i12.1429
Revised: July 23, 2024
Accepted: July 30, 2024
Published online: December 27, 2024
Processing time: 189 Days and 23.3 Hours
A new nomenclature of metabolic associated steatotic liver disease (MASLD) was proposed in 2023, thus expanding the diagnostic name of “MASLD combined with other etiologies”.
To investigate the clinical profiles of patients with concurrent MASLD and ch
This study included participants from the Taiwan Bio-bank. The diagnostic cri
In a total of 18980 participants (mean age, 55.18 ± 10.35 years; males, 30.42%), there were 7654 (40.3%) MASLD patients and 2128 (11.2%) HBV carriers. After propensity score matching for age and gender, HBV carriers had a lower percentage of MASLD than healthy controls. Those with dual etiology had higher aspartate aminotransferase, alanine aminotransferase (ALT), and FIB-4 levels, but lower gamma glutamyl transferase (GGT) levels than MASLD patients. In contrast, those with dual etiology had higher ALT and GGT levels, but lower FIB-4 than “HBV alone” patients. The risk of atherosclerosis was similar among these three groups.
MASLD-HBV patients have worse liver fibrosis severity than MASLD patients, but better liver fibrosis stage than “HBV alone” patients, suggesting a complex interaction between MASLD and chronic HBV infection.
Core Tip: Patients with concurrent metabolic associated steatotic liver disease (MASLD) and hepatitis B virus (HBV) in
- Citation: Wang SW, Chang YW, Wang C, Cheng YM, Hsieh TH, Wang CC, Kao JH. Clinical profiles and their interaction of concurrent metabolic associated steatotic liver disease and hepatitis B virus infection. World J Hepatol 2024; 16(12): 1429-1440
- URL: https://www.wjgnet.com/1948-5182/full/v16/i12/1429.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i12.1429
The diagnostic name of nonalcoholic fatty liver disease (NAFLD) has been continuously evolving[1]. In 2020, it was transitioned to metabolic dysfunction-associated fatty liver disease (MAFLD), signifying a heightened emphasis on the pathogenesis and criteria of metabolic dysfunction[2]. Subsequently, in 2023, the American Association for the Study of Liver Diseases proposed Metabolic Associated Steatotic Liver Disease (MASLD) for avoiding stigmatizing effect of “fat”[3]. Apart from the change in nomenclature, the disease's diagnostic criteria also underwent modifications. MASLD is defined by having hepatic steatosis plus meeting any one of cardiometabolic criteria, including the following conditions: Body mass index (BMI) or waist circumference (WC), blood glucose, blood pressure, and blood lipid profile [including triglycerides (TG) and high-density lipoprotein (HDL)]. For this newly defined MASLD, details regarding its disease progression, complications, prognosis, and clinical outcomes remain unknown, and there is currently insufficient relevant research.
Hepatitis B virus (HBV) infection is a major cause of chronic liver diseases, as studies have confirmed that HBV can lead to hepatic inflammation, fibrosis, cirrhosis or the development of hepatocellular carcinoma (HCC)[4]. Despite the emergence of vaccines and new antiviral treatments, HBV infection continues to pose a significant threat to global health[5]. Previous studies revealed that hepatic steatosis can inhibit HBV replication and seems to have no impact on the fibrosis progression among patients with chronic HBV infection[6-8]. In contrast, concurrent chronic HBV infection increases the risk of liver disease progression in MAFLD patients[9]. However, the interaction between MASLD and chronic HBV infection remains unknown.
As for “Steatotic Liver Disease” with metabolic dysfunction, it is further subdivided into three categories. One category is MASLD, referring to no combination with other etiologies. Another category is "MetALD," involving a combination of MASLD and increased alcohol intake. The last category includes MASLD combined with other etiologies such as HBV, hepatitis C virus (HCV), autoimmune hepatitis, etc. In our study, we aim to investigate the impact of MASLD combined with chronic HBV infection (MASLD-HBV) on disease progression, clinical outcomes and prognosis compared to “MASLD alone” or “chronic HBV infection alone” group. This can enhance our understanding of the prognosis and disease progression for such patients in clinical practice.
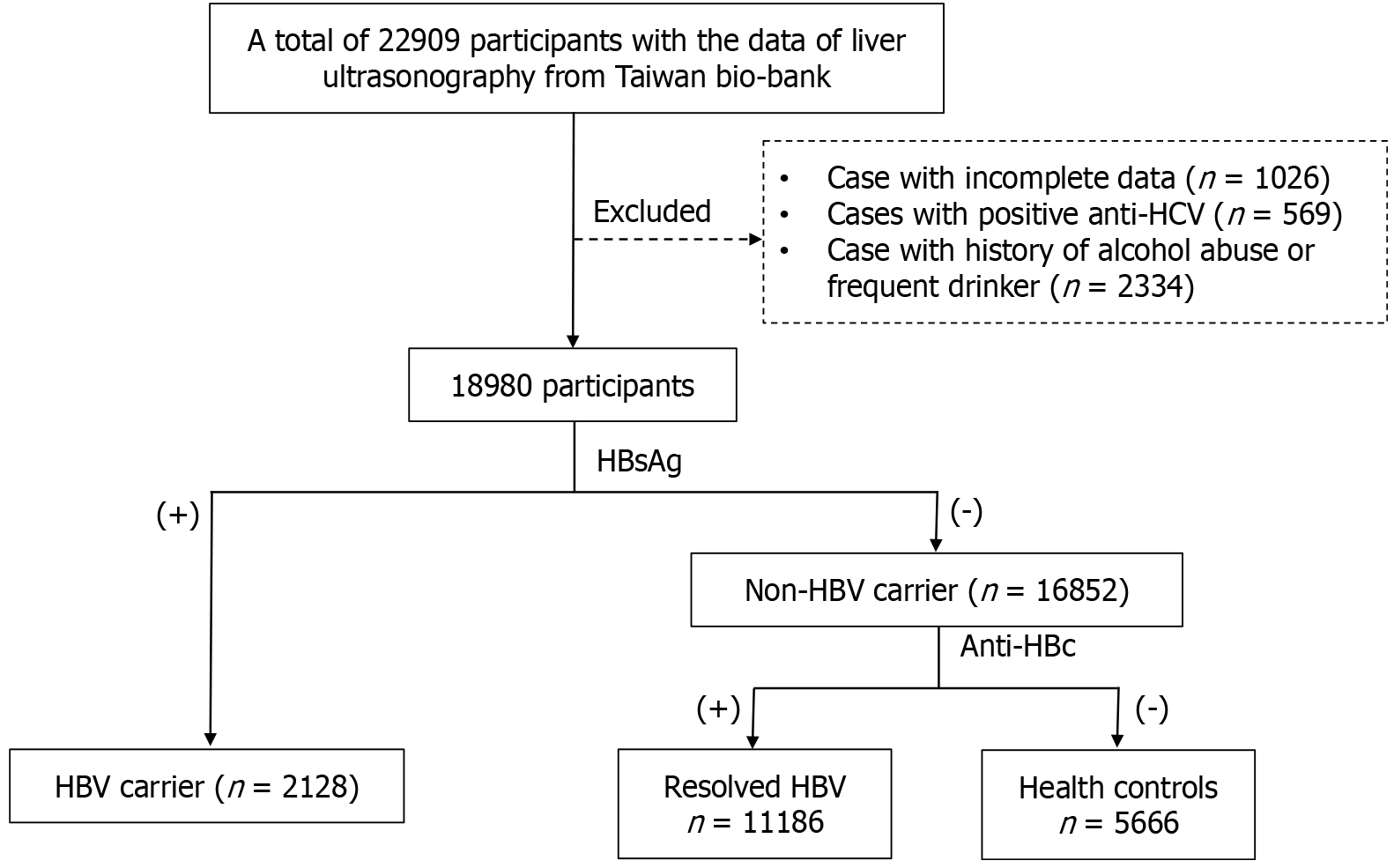
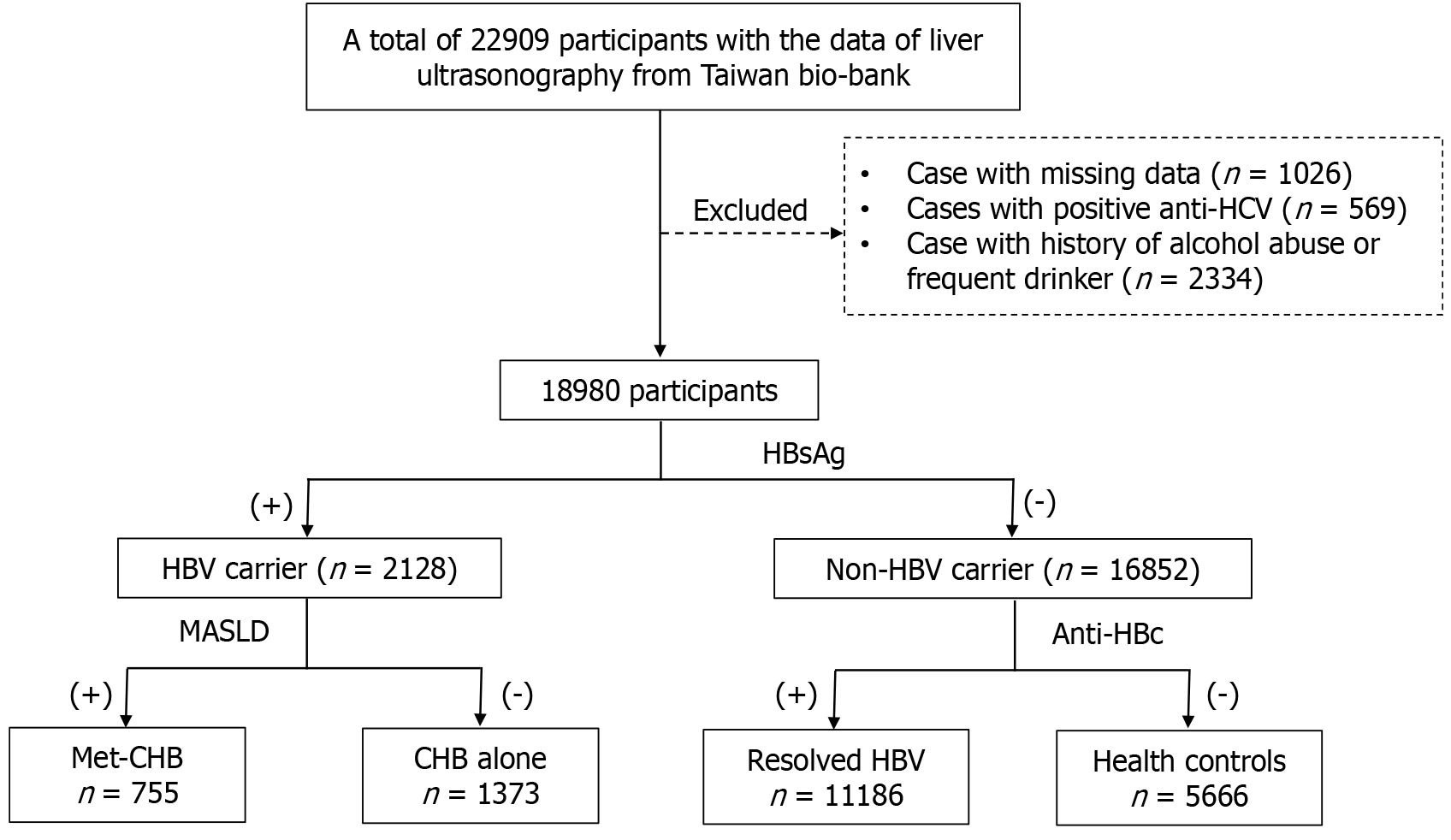
This study is a retrospective cross-sectional analysis utilizing data sourced from the Taiwan Bio-bank. Initially, submission of the research proposal is required. Upon approval by the Taiwan Bio-bank, a designated account with a link will be provided. Through this approved account, we could access the necessary data. This study includes the data of participants who have undergone liver ultrasonography examinations. Participants with incomplete data were excluded. The linked data comprise basic information, questionnaire responses, and the results of blood tests. Additional data include electrocardiograms, abdominal ultrasounds, carotid duplex ultrasounds, and bone density test results. The diagnostic criteria of MASLD include hepatic steatosis and metabolic dysfunction. Hepatic steatosis on ultrasonography is based on increased liver brightness, contrast between liver and kidney, deep echo attenuation, and poor visualization of portal vein walls. Metabolic dysfunction was defined as having any one of the cardiometabolic criteria. As this study aimed to investigate MASLD concurrent with chronic HBV infection and its impact on the severity of liver diseases, participants with positive anti-HCV antibody were excluded to avoid interference from other causes of chronic liver diseases. The potential influence of moderate alcohol consumption on the severity of MASLD remains unclear. To minimize the interference of alcohol consumption on the severity of liver diseases, only participants with no drinking habit or occasional consumption of alcohol are included. Patients with a history of alcohol abuse who have quit or those who have been continuously drinking for more than three months were excluded to ensure the accuracy of the study. The final set of patients were included in the statistical analysis (Figure 1). Fibrosis-4 score (FIB-4) was used to determine the severity of liver fibrosis because it was validated not only in NAFLD patients, but also in those with chronic HBV infection. A previous meta-analysis with 8274 individuals showed the diagnostic value of FIB-4 was modest in detecting liver fibrosis for those with chronic hepatitis B. The area under the curve of 0.9 was achieved if using the cut-off value of FIB-4 > 2 for detecting liver fibrosis[10].
This study consisted of three steps. In the first step, the study population was divided into three subgroups based on the results of hepatitis B surface antigen (HBsAg) and anti-hepatitis B core antibody (anti-HBc): HBV carrier, resolved HBV, and healthy control groups. A comparison was made between the HBV carrier and the healthy control group. Su
The Taiwan Bio-bank is a government-supported research initiative primarily aimed at establishing a database based on the Taiwanese population. The main objective is to facilitate medical research and understand the characteristics of diseases in the Taiwanese population, ultimately improving the health and treatment of individuals in Taiwan. Study participants are voluntary and over the age of 20. The enrollment began in 2008, and to date, data from over 202959 participants have been collected. The enrollment process involves obtaining participants' consent, followed by a questionnaire that covers basic information, medical history, smoking, alcohol consumption, dietary habits, and exercise routines. Regarding alcohol consumption history, participants were categorized into three groups: Non-drinkers or social drinkers, individuals who used to have a history of alcohol abuse but have quit, and those who have been consistently drinking for over three months. The type, quantity, and frequency of alcohol consumed were also recorded, allowing the calculation of weekly alcohol intake. Blood tests included hematological and biochemical analyses, as well as hepatitis virus testing. Participants may consent to the storage of blood, urine, and DNA samples for future analysis. Participants were recommended to undergo follow-up assessments every two to five years. During the first follow-up, in addition to basic examinations, abdominal ultrasound, carotid duplex, and dual-energy X-ray absorptiometry were performed[12-14].
The following criteria were applied: BMI ≥ 25 (23 Asia) or WC > 94 cm (men) and 80 cm (women) or ethnicity adjusted; fasting serum glucose ≥ 100 mg/dL or 2-hour post-load glucose level ≥ 140 mg/dL or glycated hemoglobin (HbA1c) ≥ 5.7% or type 2 diabetes or treatment for type 2 diabetes; blood pressure ≥ 130/85 mg/dL or specific antihypertensive drug treatment; blood TG ≥ 150 mg/dL or lipid lowering treatment; and plasma HDL-cholesterol ≤ 40 mg/dL in men and ≤ 50 mg/dL in women or lipid lowering treatment.
This study was performed in accordance with the principles of the 1975 Declaration of Helsinki and approved with waived informed consent by the Research Ethics Committee of Taipei Tzu Chi Hospital; Buddhist Tzu Chi Medical Foundation (Approval Numbers: 10-XD-055 and 11-X-074) and the Ethics and Governance Council of the Taiwan Bio-bank (Approval Numbers: TWBR11102-03).
The data were expressed as mean ± SD for continuous variables and number (percentage) for categorical variables. Statistical analysis was performed using SPSS version 26.0 (IBM Corp., Armonk, NY, United States). The clinical characteristics and outcomes were compared between HBV carriers and healthy controls; between resolved HBV and healthy controls group; between dual etiology and HBV alone; and between dual etiology and MASLD alone. Propensity score matching (PSM) was performed if age and/or sex are unmatched. These data were analyzed by χ2 test and student’s t-test. Univariate and multivariate logistic regression analyses were used to evaluate the factors of significant liver fibrosis in MASLD patients. A P value less than 0.05 was considered statistically significant.
This study initially involved 22909 participants, of which 1026 with incomplete data were excluded. There were a total of 569 individuals with positive anti-HCV antibody. Based on alcohol consumption habits, the participants who had history of alcohol abuse but quit or those who had been consistently drinking in the last three months were excluded. To mi
According to the status of HBsAg, the study population was divided into two groups: HBV carriers and non-HBV carriers. Among the non-HBV carriers, based on the status of anti-HBc in serum, two subgroups were formed: Resolved HBV and healthy control group. Compared to healthy control group, the resolved HBV group had a higher average age, a higher proportion of males, and a higher prevalence of MASLD. Using age and gender-matched PSM analysis, no significant difference was found in the prevalence of MASLD between these two groups (Table 1). Compared to the healthy control group, the HBV carrier group was older and had a higher proportion of males. The prevalence of MASLD was lower in the HBV carrier group. Using age and gender-matched PSM analysis, it was found that the HBV carrier group had higher levels of ALT, FIB-4, and a higher percentage of FIB-4 > 2. Their levels of TG, cholesterol, and low-density lipoprotein cholesterol (LDL) were lower, along with lower values for FLI and a lower prevalence of MASLD (Table 2).
| Characteristic | Before PSM | After PSM | ||||
| Resolved HBV, n = 11186 | Healthy controls, n = 5666 | P value | Resolved HBV, n = 5015 | Healthy controls, n = 5015 | P value | |
| Age, year | 57.41 ± 9.31 | 51.29 ± 11.25 | < 0.001 | 53.39 ± 10.23 | 53.32 ± 10.32 | 0.729 |
| Male | 3445 (30.80) | 1553 (27.41) | < 0.001 | 1252 (24.97) | 1331 (26.54) | 0.071 |
| BMI, kg/m2 | 24.20 ± 3.60 | 24.13 ± 3.88 | 0.266 | 24.12 ± 3.83 | 24.16 ± 3.82 | 0.619 |
| Body fat % | 29.41 ± 7.46 | 29.52 ± 7.44 | 0.375 | 29.87 ± 7.45 | 29.71 ± 7.42 | 0.309 |
| WC, cm | 83.48 ± 9.78 | 82.82 ± 10.32 | < 0.001 | 82.66 ± 10.10 | 83.02 ± 10.15 | 0.076 |
| Metabolic parameters | ||||||
| MASLD | 4670 (41.75) | 2229 (39.34) | 0.003 | 2007 (40.02) | 2033 (40.54) | 0.597 |
| Diabetes | 1383 (12.36) | 474 (8.37) | < 0.001 | 503 (10.03) | 463 (9.23) | 0.176 |
| Hypertension | 2072 (18.52) | 724 (12.78) | < 0.001 | 731 (14.58) | 711 (14.18) | 0.569 |
| Hyperlipidemia | 1431 (12.79) | 548 (9.67) | < 0.001 | 499 (9.95) | 539 (10.75) | 0.190 |
| Glucose, mg/dL | 98 (21.71) | 95 (19.00) | < 0.001 | 97 (21.48) | 96 (19.25) | 0.166 |
| HbA1c, % | 5.93 ± 0.84 | 5.80 ± 0.75 | < 0.001 | 5.86 ± 0.84 | 5.83 ± 0.77 | 0.106 |
| TG, mg/dL | 120.91 ± 99.13 | 115.42 ± 77.93 | < 0.001 | 118.04 ± 116.37 | 117.85 ± 78.99 | 0.924 |
| CHO, mg/dL | 199.78 ± 36.71 | 197.94 ± 36.28 | 0.002 | 198.72 ± 36.05 | 199.22 ± 36.07 | 0.482 |
| HDL, mg/dL | 55.26 ± 13.62 | 55.89 ± 13.74 | 0.005 | 55.80 ± 13.57 | 55.96 ± 13.74 | 0.568 |
| LDL, mg/dL | 122.75 ± 32.30 | 121.78 ± 32.15 | 0.065 | 122.19 ± 31.92 | 122.44 ± 31.91 | 0.695 |
| Liver parameters | ||||||
| AST, U/L | 25.11 ± 11.28 | 24.35 ± 12.12 | < 0.001 | 24.31 ± 12.71 | 24.56 ± 12.29 | 0.325 |
| ALT, U/L | 23.05 ± 20.82 | 23.10 ± 22.66 | 0.885 | 22.48 ± 24.76 | 23.24 ± 23.11 | 0.113 |
| GGT, U/L | 22.74 ± 25.62 | 22.21 ± 20.17 | 0.138 | 21.79 ± 25.47 | 22.47 ± 20.53 | 0.143 |
| Fatty liver index | 25.73 ± 23.03 | 24.85 ± 24.05 | 0.023 | 24.48 ± 23.63 | 25.25 ± 23.87 | 0.103 |
| FIB-4 | 1.46 ± 0.74 | 1.23 ± 0.61 | < 0.001 | 1.30 ± 0.61 | 1.30 ± 0.62 | 0.550 |
| FIB-4 > 2 | 1739 (15.55) | 578(10.20) | < 0.0001 | 558 (11.13) | 576 (11.49) | 0.5703 |
| Other parameters | ||||||
| Carotid plaque | 3684 (32.93) | 1266 (22.34) | < 0.001 | 1253 (24.99) | 1250 (24.93) | 0.945 |
| Characteristic | Before PSM | After PSM | ||||
| HBV carrier, n = 2128 | Healthy controls, n = 5666 | P value | HBV carrier, n = 2128 | Healthy controls, n = 2128 | P value | |
| Age, year | 53.85 ± 9.68 | 51.29 ± 11.25 | < 0.001 | 53.85 ± 9.68 | 53.88 ± 9.71 | 0.926 |
| Male | 775 (36.42) | 1553 (27.41) | < 0.001 | 775 (36.42) | 772 (36.28) | 0.924 |
| BMI, kg/m2 | 24.16 ± 3.65 | 24.13 ± 3.88 | 0.778 | 24.16 ± 3.65 | 24.32 ± 3.80 | 0.151 |
| Body fat % | 28.66 ± 7.67 | 29.52 ± 7.44 | < 0.001 | 28.66 ± 7.67 | 28.76 ± 7.62 | 0.670 |
| WC, cm | 83.27 ± 10.29 | 82.82 ± 10.32 | 0.086 | 83.27 ± 10.29 | 83.83 ± 10.14 | 0.072 |
| Metabolic parameters | ||||||
| MASLD | 755 (35.48) | 2229 (39.34) | 0.002 | 755 (35.48) | 925 (43.47) | < 0.001 |
| Diabetes | 204 (9.59) | 474 (8.37) | 0.088 | 204 (9.59) | 206 (9.68) | 0.917 |
| Hypertension | 293 (13.77) | 724 (12.78) | 0.247 | 293 (13.77) | 330 (15.51) | 0.109 |
| Hyperlipidemia | 182 (8.55) | 548 (9.67) | 0.131 | 182 (8.55) | 244 (11.47) | 0.002 |
| Glucose, mg/dL | 96 (21.23) | 95 (19.00) | 0.036 | 96 (21.23) | 97 (21.66) | 0.325 |
| HbA1c, % | 5.83 ± 0.83 | 5.80 ± 0.75 | 0.110 | 5.83 ± 0.83 | 5.86 ± 0.84 | 0.205 |
| TG, mg/dL | 107.73 ± 73.91 | 115.42 ± 77.93 | < 0.001 | 107.73 ± 73.91 | 121.34 ± 80.35 | < 0.001 |
| CHO, mg/dL | 194.28 ± 35.76 | 197.94 ± 36.28 | < 0.001 | 194.28 ± 35.76 | 198.76 ± 35.46 | < 0.001 |
| HDL, mg/dL | 55.23 ± 13.62 | 55.89 ± 13.74 | 0.059 | 55.23 ± 13.62 | 54.74 ± 13.70 | 0.242 |
| LDL, mg/dL | 118.93 ± 31.26 | 121.78 ± 32.15 | < 0.001 | 118.93 ± 31.26 | 122.86 ± 31.63 | < 0.001 |
| Liver parameters | ||||||
| AST, U/L | 27.97 ± 15.57 | 24.35 ± 12.12 | < 0.001 | 27.97 ± 15.57 | 24.71 ± 10.15 | < 0.001 |
| ALT, U/L | 28.57 ± 29.68 | 23.10 ± 22.66 | < 0.001 | 28.57 ± 29.68 | 23.63 ± 17.79 | < 0.001 |
| GGT, U/L | 20.03 ± 19.47 | 22.21 ± 20.17 | < 0.001 | 20.03 ± 19.47 | 23.47 ± 22.09 | < 0.001 |
| Fatty liver index | 23.12 ± 21.76 | 24.85 ± 24.05 | 0.004 | 23.12 ± 21.76 | 26.89 ± 24.28 | < 0.001 |
| FIB-4 | 1.49 ± 0.75 | 1.23 ± 0.61 | < 0.001 | 1.49 ± 0.75 | 1.31 ± 0.59 | < 0.001 |
| FIB-4 > 2 | 407 (19.13) | 578 (10.20) | < 0.0001 | 407 (19.13) | 260 (12.22) | < 0.0001 |
| Other parameter | ||||||
| Carotid plaque | 533 (25.05) | 1266 (22.34) | 0.012 | 533 (25.05) | 566 (26.60) | 0.248 |
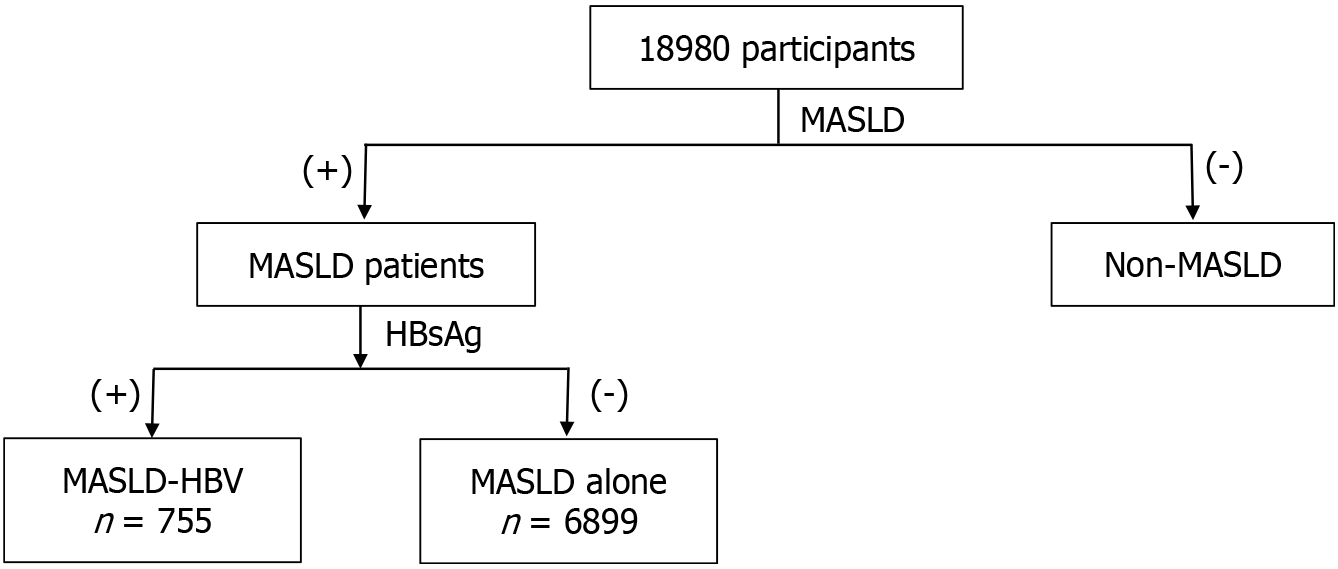
In the second analysis step of this study, participants with MASLD were selected from the study population. They were then divided into two groups based on the status of serum HBsAg. One group was MASLD combined with chronic HBV infection, while the other group was “MASLD alone”. Compared to the “MASLD alone” group, the dual etiology group was younger with a higher proportion of males. Using age and gender-matched PSM analysis, it was found that the dual etiology group had lower levels of TG, cholesterol, and LDL. Additionally, they exhibited higher levels of AST, ALT, FIB-4, and higher percentage of FIB-4 > 2; but low levels of GGT and FLI (Table 3).
| Characteristic | Before PSM | After PSM | ||||
| Dual etiology, n = 755 | MASLD alone, n = 6899 | P value | Dual etiology, n = 755 | MASLD alone, n = 755 | P value | |
| Age, year | 53.83 ± 9.62 | 56.09 ± 10.08 | < 0.001 | 53.83 ± 9.62 | 53.83 ± 9.62 | > 0.999 |
| Male | 324 (42.91) | 2536 (36.76) | 0.001 | 324 (42.91) | 324 (42.91) | > 0.999 |
| BMI, kg/m2 | 26.25 ± 3.69 | 26.13 ± 3.66 | 0.387 | 26.25 ± 3.69 | 26.25 ± 3.69 | 0.988 |
| Body fat % | 31.32 ± 7.66 | 31.87 ± 7.52 | 0.058 | 31.32 ± 7.66 | 31.20 ± 7.88 | 0.757 |
| WC, cm | 88.76 ± 10.41 | 88.44 ± 9.37 | 0.369 | 88.76 ± 10.41 | 89.01 ± 9.62 | 0.628 |
| Metabolic parameters | ||||||
| Diabetes | 135 (17.88) | 1275 (18.48) | 0.686 | 135 (17.88) | 144 (19.07) | 0.551 |
| Hypertension | 153 (20.26) | 1,591 (23.06) | 0.082 | 153 (20.26) | 154 (20.40) | 0.949 |
| Hyperlipidemia | 93 (12.32) | 1091 (15.81) | 0.012 | 93 (12.32) | 103 (13.64) | 0.444 |
| Glucose, mg/dL | 102 (26.32) | 102 (25.71) | 0.961 | 102 (26.32) | 104 (31.15) | 0.197 |
| HbA1c, % | 6.11 ± 1.06 | 6.12 ± 0.98 | 0.716 | 6.11 ± 1.06 | 6.17 ± 1.20 | 0.261 |
| TG, mg/dL | 139.82 ± 96.00 | 154.43 ± 120.06 | < 0.001 | 139.82 ± 96.00 | 165.52 ± 230.03 | 0.005 |
| CHO, mg/dL | 196.49 ± 38.11 | 200.82 ± 37.26 | 0.003 | 196.49 ± 38.11 | 203.09 ± 36.60 | 0.001 |
| HDL, mg/dL | 49.71 ± 11.55 | 49.75 ± 11.44 | 0.931 | 49.71 ± 11.55 | 48.94 ± 11.41 | 0.191 |
| LDL, mg/dL | 123.25 ± 33.16 | 125.89 ± 33.29 | 0.038 | 123.25 ± 33.16 | 128.21 ± 33.23 | 0.004 |
| Liver parameters | ||||||
| AST, U/L | 28.70 ± 13.27 | 26.72 ± 14.20 | < 0.001 | 28.70 ± 13.27 | 26.03 ± 9.82 | < 0.001 |
| ALT, U/L | 33.40 ± 26.16 | 29.62 ± 26.51 | < 0.001 | 33.40 ± 26.16 | 29.10 ± 18.42 | < 0.001 |
| GGT, U/L | 24.21 ± 16.74 | 27.96 ± 28.63 | < 0.001 | 24.21 ± 16.74 | 28.91 ± 24.95 | < 0.001 |
| Fatty liver index | 37.72 ± 23.66 | 39.99 ± 24.69 | 0.016 | 37.72 ± 23.66 | 41.90 ± 25.45 | 0.001 |
| FIB-4 | 1.35 ± 0.64 | 1.30 ± 0.66 | 0.064 | 1.35 ± 0.64 | 1.22 ± 0.57 | < 0.001 |
| FIB-4 > 2 | 100 (13.25) | 699 (10.13) | 0.0079 | 100 (13.25) | 59 (7.81) | 0.0006 |
| Other parameters | ||||||
| Carotid plaque | 198 (26.23) | 2307 (33.44) | < 0.001 | 198 (26.23) | 208 (27.55) | 0.562 |
The patients with positive HBsAg were categorized into two groups based on the presence of MASLD. One group was dual etiology group, while the other group was “HBV alone”. Compared to the “HBV alone” group, the dual etiology group had a higher proportion of males. Using gender-matched PSM analysis, it was observed that patients in the dual etiology group exhibited poor glucose and lipid profiles. In terms of liver function, the levels of ALT and GGT were higher in the dual etiology group, but the FIB-4 index and the percentage of FIB-4 > 2 were lower than HBV alone group. There were no significant differences between these two groups in terms of atherosclerosis (Table 4).
| Characteristic | Before PSM | After PSM | ||||
| Dual etiology, n = 755 | HBV alone, n = 1373 | P value | Dual etiology, n = 755 | HBV alone, n = 755 | P value | |
| Age, year | 53.83 ± 9.62 | 53.87 ± 9.72 | 0.932 | 53.83 ± 9.62 | 53.80 ± 9.58 | 0.957 |
| Male | 324 (42.91) | 451 (32.85) | < 0.001 | 324 (42.91) | 324 (42.91) | > 0.999 |
| BMI, kg/m2 | 26.25 ± 3.69 | 23.00 ± 3.07 | < 0.001 | 26.25 ± 3.69 | 23.05 ± 3.09 | < 0.001 |
| Body fat % | 31.32 ± 7.66 | 27.20 ± 7.27 | < 0.001 | 31.32 ± 7.66 | 26.04 ± 7.60 | < 0.001 |
| WC, cm | 88.76 ± 10.41 | 80.24 ± 8.88 | < 0.001 | 88.76 ± 10.41 | 80.45 ± 8.87 | < 0.001 |
| Metabolic parameters | ||||||
| Diabetes | 135 (17.88) | 69 (5.03) | < 0.001 | 135 (17.88) | 41 (5.43) | < 0.001 |
| Hypertension | 153 (20.26) | 140 (10.20) | < 0.001 | 153 (20.26) | 81 (10.73) | < 0.001 |
| Hyperlipidemia | 93 (12.32) | 89 (6.48) | < 0.001 | 93 (12.32) | 58 (7.68) | 0.003 |
| Glucose, mg/dL | 102 (26.32) | 93 (16.97) | < 0.001 | 102 (26.32) | 94 (20.10) | < 0.001 |
| HbA1c, % | 6.11 ± 1.06 | 5.68 ± 0.62 | < 0.001 | 6.11 ± 1.06 | 5.69 ± 0.70 | < 0.001 |
| TG, mg/dL | 139.82 ± 96.00 | 90.08 ± 50.25 | < 0.001 | 139.82 ± 96.00 | 91.52 ± 51.42 | < 0.001 |
| CHO, mg/dL | 196.49 ± 38.11 | 193.06 ± 34.35 | 0.040 | 196.49 ± 38.11 | 193.44 ± 34.53 | 0.104 |
| HDL, mg/dL | 49.71 ± 11.55 | 58.27 ± 13.73 | < 0.001 | 49.71 ± 11.55 | 57.96 ± 13.89 | < 0.001 |
| LDL, mg/dL | 123.25 ± 33.16 | 116.55 ± 29.91 | < 0.001 | 123.25 ± 33.16 | 117.00 ± 30.02 | < 0.001 |
| Liver parameters | ||||||
| AST, U/L | 28.70 ± 13.27 | 27.56 ± 16.69 | 0.084 | 28.70 ± 13.27 | 28.10 ± 15.93 | 0.426 |
| ALT, U/L | 33.40 ± 26.16 | 25.91 ± 31.13 | < 0.001 | 33.40 ± 26.16 | 27.29 ± 34.92 | < 0.001 |
| GGT, U/L | 24.21 ± 16.74 | 17.73 ± 20.46 | < 0.001 | 24.21 ± 16.74 | 18.88 ± 23.99 | < 0.001 |
| Fatty liver index | 37.72 ± 23.66 | 15.09 ± 15.64 | < 0.001 | 37.72 ± 23.66 | 15.85 ± 16.07 | < 0.001 |
| FIB-4 | 1.35 ± 0.64 | 1.57 ± 0.80 | < 0.001 | 1.35 ± 0.64 | 1.61 ± 0.78 | < 0.001 |
| FIB-4 > 2 | 100 (13.25) | 307 (22.36) | < 0.0001 | 100 (13.25) | 185 (24.50) | < 0.001 |
| Other parameters | ||||||
| Carotid plaque | 198 (26.23) | 335 (24.40) | 0.352 | 198 (26.23) | 194 (25.70) | 0.814 |
Using univariate analysis, older age, diabetes mellitus, hypertension, dyslipidemia, glucose, HbA1c, cholesterol, LDL, AST, ALT, GGT, and HBV were factors associated with advanced liver fibrosis. All significant variables in the univariate analysis were further evaluated by multivariate analysis, except AST and ALT due to the components of FIB-4. In multivariate analysis, older age, GGT, and HBV were independent risk factors for advanced liver fibrosis. The odds ratio of chronic HBV infection for advanced liver fibrosis is 2.15 in MASLD patients (Table 5).
| Factor | Univariate | Multivariate | ||||
| OR | 95%CI | P value | AOR | 95%CI | P value | |
| Age, year | 1.147 | (1.123-1.172) | < 0.001 | 1.151 | (1.126-1.177) | < 0.001 |
| Male | 0.986 | (0.749-1.299) | 0.922 | |||
| BMI, kg/m2 | 0.975 | (0.939-1.013) | 0.189 | |||
| Body fat, % | 0.989 | (0.971-1.007) | 0.217 | |||
| WC, cm | 1.006 | (0.992-1.020) | 0.406 | |||
| Diabetes mellitus | 1.767 | (1.313-2.379) | < 0.001 | 0.919 | (0.593-1.424) | 0.706 |
| Hypertension | 1.563 | (1.172-2.084) | 0.002 | 0.734 | (0.536-1.005) | 0.054 |
| Dyslipidemia | 1.511 | (1.091-2.092) | 0.013 | 1.009 | (0.715-1.422) | 0.961 |
| Glucose, mg/dL | 1.006 | (1.002-1.009) | 0.004 | 0.999 | (0.991-1.007) | 0.791 |
| HbA1c, % | 1.191 | (1.077-1.316) | 0.001 | 1.149 | (0.903-1.462) | 0.259 |
| Triglyceride, mg/dL | 1.000 | (0.999-1.001) | 0.869 | |||
| Cholesterol, mg/dL | 0.993 | (0.989-0.997) | < 0.001 | 1.000 | (0.992-1.008) | 0.998 |
| HDL, mg/dL | 1.007 | (0.996-1.018) | 0.219 | |||
| LDL, mg/dL | 0.990 | (0.985-0.994) | < 0.001 | 0.993 | (0.984-1.001) | 0.089 |
| AST, U/L | 1.050 | (1.043-1.056) | < 0.001 | |||
| ALT, U/L | 1.013 | (1.009-1.017) | < 0.001 | |||
| GGT, U/L | 1.004 | (1.001-1.006) | 0.006 | 1.004 | (1.001-1.007) | 0.011 |
| FLI | 1.001 | (0.996-1.007) | 0.615 | |||
| HBV | 1.546 | (1.055-2.265) | 0.025 | 2.154 | (1.446-3.209) | < 0.001 |
In this study of 18980 participants from Taiwan Bio-bank, the prevalence of HBV carriers and MASLD was 11.2% and 40.3%, respectively. After PSM for age and gender, the HBV carriers had lower lipid profiles, FLI, and lower percentage of MASLD, but higher ALT and FIB-4 scores than healthy controls. There was no significant difference in the clinical characteristics between those with resolved HBV and healthy controls. Compared with “MASLD alone” group, dual etiology group had lower lipid profiles and FLI, but higher ALT and FIB-4 scores. Conversely, the dual etiology group had higher metabolic profiles, FLI, GGT, and ALT levels, but lower FIB-4 scores compared to “HBV alone” patients. These findings suggest that chronic HBV infection worsens liver inflammation and fibrosis in MASLD patients. Although MASLD worsens liver inflammation, it protects liver fibrosis progression in patients with chronic HBV infection. Age, GGT, and chronic HBV infection were factors associated with advanced liver fibrosis in MASLD patients.
Current research indicates that patients with chronic HBV infection have a lower incidence of hepatic steatosis and hyperlipidemia in the general population. A large-scale community-based study on 56336 residents in Taiwan found that HBV carriers had a lower prevalence of hypertriglyceridemia and hypercholesterolemia than non-HBV carriers[15]. One case-control study showed that HBV patients had a significantly higher serum adiponectin level but lower serum triglyceride than healthy controls. A large series study involving 33439 participants from the general population revealed that HBV carriers actually had a lower incidence of hepatic steatosis[16]. A study conducted in Hong Kong with 1013 participants who underwent proton magnetic resonance spectroscopy showed a significantly lower incidence of hepatic steatosis among HBV carriers compared to healthy controls. Multivariate analysis demonstrated that even after adjusting for confounding factors, HBV infection remained an independent factor associated with a reduced risk of hepatic steatosis[17]. Our previous systemic review concludes that chronic HBV infection has an association with better lipid profiles and appears to have a protective effect against hepatic steatosis according to large-scale studies or studies utilizing more precise instruments for detecting hepatic steatosis[18]. The current study shows that according to the new diagnostic criteria and definition of metabolic dysfunction, the better lipid profiles and lower incidence of MASLD are found in populations with chronic HBV infection compared to healthy control groups. The effects not only exist in the general population, but also in MASLD patients. Regarding the comparison between individuals who have recovered from HBV infection and healthy controls, no difference was found in the prevalence of MAFLD and lipid profiles.
In accordance with the diagnostic criteria for MAFLD, proposed in 2020, our previous studies have shown that compared to patients with pure MAFLD, those with concurrent MAFLD and chronic HBV infection have a higher risk of liver fibrosis but a lower risk of atherosclerosis[19]. However, MAFLD does not exclude other causes of liver diseases, and has different diagnostic criteria for metabolic dysfunction compared to MASLD. Based on the diagnostic criteria for MASLD proposed in 2023, this is the first study to compare those with MASLD combined with chronic HBV infection to MASLD patients. We found that the former group had higher indices of liver inflammation and fibrosis, while there was no difference in the risk of atherosclerosis between these two groups.
MASLD and chronic HBV infection are both significant causes of chronic liver disease. Currently, the impact of MASLD on chronic HBV infection is unknown. Further research is needed to understand the clinical outcomes and prognosis of patients with dual etiology. MASLD itself can lead to chronic liver inflammation, fibrosis, and even progression to cirrhosis and HCC. Previous studies have shown that in patients with chronic HBV infection, histological evidence of hepatic steatosis correlates more with host metabolic factors than with viral factors, particularly with host BMI and serum TG levels[20]. A meta-analysis of 47 studies involving 4100 patients with chronic HBV infection who underwent liver biopsy found an inverse relationship between HBV viral load and hepatic steatosis[21]. NAFLD is associated with lower HBV viral load and antiviral response in pediatric population[22]. Hepatic steatosis can attenuate HBV replication in mouse models[23]. Additionally, sporadic studies suggest that among patients with chronic HBV infection, those with hepatic steatosis have a higher rate of HBsAg clearance and less severe liver fibrosis[24,25]. However, the impact on overall survival and the occurrence of liver cirrhosis and HCC is currently unclear. The definition of MASLD includes additional diagnostic criteria for metabolic dysfunction beyond hepatic steatosis. To my knowledge, this study is the first to investigate the impact of MASLD on patients with chronic HBV infection. This large-scale study demonstrates that among patients with chronic HBV infection, those combined with MASLD exhibit higher liver inflammation indices, but lower fibrosis indices. The inverse effects of these two conditions, though consistent with previous research, warrant further investigation into the underlying mechanisms.
This study has several strengths. Firstly, the coexistence of MASLD and chronic HBV infection represents a novel diagnostic entity, and its clinical manifestations are not well understood. To the best of my knowledge, this is the first study to investigate this condition. Secondly, the Taiwan Bio-bank provides a large database, and Taiwan is also an endemic area for HBV infection. These advantages can be helpful realizing the disease entity. Thirdly, carotid duplex is a clinical method for directly diagnosing the presence of atherosclerosis in large and medium-sized blood vessels. This dataset contains such data, enabling the simultaneous assessment of the risk of atherosclerosis in this population. However, this study indeed has some limitations. Firstly, although ultrasound is the most commonly used method for diagnosing hepatic steatosis in clinical practice, it is typically used to diagnose moderate to severe cases, with lower accuracy for mild fatty liver. Secondly, liver biopsy is the gold standard for assessing the severity of liver fibrosis, but it is not suitable for large population studies. Additionally, methods such as magnetic resonance elastography or liver stiffness measurement are expensive and not available in all hospitals. In this study, we utilized FIB-4 to predict the severity of liver fibrosis, which has been validated for use in MASLD or chronic hepatitis B infection. This marker is most suitable for predicting the severity of liver fibrosis in populations with dual etiology. Thirdly, this study is cross-sectional and cannot realize the long-term risk of clinical events, such as the incidence of liver cirrhosis and HCC or overall survival. However, it is well established that the severity of liver fibrosis can accurately predict the prognosis of liver disease. Furthermore, the data of HBV genotypes, viral load, and anti-viral treatment are unavailable. The further studies including the data of long-term follow-up, liver biopsy, and multicenter, multi-ethnic study design are needed to provide more accurate and personalized health care services for the global scope of patients.
Taiwan is an endemic area for chronic hepatitis B infection. This study utilized data from the Taiwan Bio-bank, where the prevalence of chronic HBV infection accounted for 11%. It was found that individuals who had recovered from hepatitis B infection showed no clinical differences compared to the healthy control group. However, patients with chronic HBV infection exhibited better lipid profiles and lower percentage of MASLD, but a higher severity of liver inflammation and fibrosis indices compared to the healthy control group. Among patients with MASLD, those combined with chronic HBV infection displayed a higher liver inflammation and fibrosis indices compared to those with MASLD alone. However, among patients with chronic HBV infection, those with concurrent MASLD had higher liver inflammation but lower fibrosis indices compared to those with chronic HBV infection alone. Further research is needed to confirm the impact on the occurrence of liver cirrhosis and hepatocellular carcinoma in MASLD patients with chronic HBV infection.
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7517] [Article Influence: 835.2] [Reference Citation Analysis (0)] |
| 2. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2199] [Article Influence: 439.8] [Reference Citation Analysis (1)] |
| 3. | Chan WK, Chuah KH, Rajaram RB, Lim LL, Ratnasingam J, Vethakkan SR. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J Obes Metab Syndr. 2023;32:197-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 273] [Article Influence: 136.5] [Reference Citation Analysis (1)] |
| 4. | Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 992] [Article Influence: 62.0] [Reference Citation Analysis (1)] |
| 5. | Lin CL, Kao JH. Perspectives and control of hepatitis B virus infection in Taiwan. J Formos Med Assoc. 2015;114:901-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Hanif H, Khan MM, Ali MJ, Shah PA, Satiya J, Lau DTY, Aslam A. A New Endemic of Concomitant Nonalcoholic Fatty Liver Disease and Chronic Hepatitis B. Microorganisms. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Jiang D, Chen C, Liu X, Huang C, Yan D, Zhang X, Zhou Y, Lin Y, Zhou Y, Guan Z, Ding C, Lan L, Zhu C, Wu J, Li L, Yang S. Concurrence and impact of hepatic steatosis on chronic hepatitis B patients: a systematic review and meta-analysis. Ann Transl Med. 2021;9:1718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 8. | Fernandez CJ, Alkhalifah M, Afsar H, Pappachan JM. Metabolic Dysfunction-Associated Fatty Liver Disease and Chronic Viral Hepatitis: The Interlink. Pathogens. 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Cheng YM, Wang CC. Achieving global uniformity for the new name and diagnostic criteria of non-alcoholic fatty liver disease. J Formos Med Assoc. 2024;123:636-637. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Yin Z, Zou J, Li Q, Chen L. Diagnostic value of FIB-4 for liver fibrosis in patients with hepatitis B: a meta-analysis of diagnostic test. Oncotarget. 2017;8:22944-22953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Zhu G, Hom J, Li Y, Jiang B, Rodriguez F, Fleischmann D, Saloner D, Porcu M, Zhang Y, Saba L, Wintermark M. Carotid plaque imaging and the risk of atherosclerotic cardiovascular disease. Cardiovasc Diagn Ther. 2020;10:1048-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Lin JC, Fan CT, Liao CC, Chen YS. Taiwan Biobank: making cross-database convergence possible in the Big Data era. Gigascience. 2018;7:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Cheng YM, Wang CC, Kao JH. Metabolic associated fatty liver disease better identifying patients at risk of liver and cardiovascular complications. Hepatol Int. 2023;17:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 14. | Cheng KL, Wang SW, Cheng YM, Hsieh TH, Wang CC, Kao JH. Prevalence and clinical outcomes in subtypes of metabolic associated fatty liver disease. J Formos Med Assoc. 2024;123:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Chen JY, Wang JH, Lin CY, Chen PF, Tseng PL, Chen CH, Chang KC, Tsai LS, Chen SC, Lu SN. Lower prevalence of hypercholesterolemia and hyperglyceridemia found in subjects with seropositivity for both hepatitis B and C strains independently. J Gastroenterol Hepatol. 2010;25:1763-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Cheng YL, Wang YJ, Kao WY, Chen PH, Huo TI, Huang YH, Lan KH, Su CW, Chan WL, Lin HC, Lee FY, Wu JC. Inverse association between hepatitis B virus infection and fatty liver disease: a large-scale study in populations seeking for check-up. PLoS One. 2013;8:e72049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Wong VW, Wong GL, Chu WC, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Chan HY, Woo J, Chan FK, Chan HL. Hepatitis B virus infection and fatty liver in the general population. J Hepatol. 2012;56:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 18. | Wang CC, Cheng PN, Kao JH. Systematic review: chronic viral hepatitis and metabolic derangement. Aliment Pharmacol Ther. 2020;51:216-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 19. | Cheng YM, Hsieh TH, Wang CC, Kao JH. Impact of HBV infection on clinical outcomes in patients with metabolic dysfunction-associated fatty liver disease. JHEP Rep. 2023;5:100836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 20. | Altlparmak E, Koklu S, Yalinkilic M, Yuksel O, Cicek B, Kayacetin E, Sahin T. Viral and host causes of fatty liver in chronic hepatitis B. World J Gastroenterol. 2005;11:3056-3059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in hepatitis B virus infected patients: meta-analysis of risk factors and comparison with hepatitis C infected patients. J Gastroenterol Hepatol. 2011;26:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Wang L, Wang Y, Liu S, Zhai X, Zhou G, Lu F, Zhao J. Nonalcoholic fatty liver disease is associated with lower hepatitis B viral load and antiviral response in pediatric population. J Gastroenterol. 2019;54:1096-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Hu D, Wang H, Wang H, Wang Y, Wan X, Yan W, Luo X, Ning Q. Non-alcoholic hepatic steatosis attenuates hepatitis B virus replication in an HBV-immunocompetent mouse model. Hepatol Int. 2018;12:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 24. | Mak LY, Hui RW, Fung J, Liu F, Wong DK, Cheung KS, Yuen MF, Seto WK. Diverse effects of hepatic steatosis on fibrosis progression and functional cure in virologically quiescent chronic hepatitis B. J Hepatol. 2020;73:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 25. | Liu L, Li H, Zhang Y, Zhang J, Cao Z. Hepatitis B virus infection combined with nonalcoholic fatty liver disease: Interaction and prognosis. Heliyon. 2023;9:e13113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |