Published online Dec 27, 2024. doi: 10.4254/wjh.v16.i12.1395
Revised: October 2, 2024
Accepted: October 29, 2024
Published online: December 27, 2024
Processing time: 103 Days and 0.8 Hours
The effect of nonalcoholic fatty liver disease (NAFLD) on the efficacy of nuc
To investigate the influence of NAFLD on virological response in CHB patients undergoing NAs treatment.
Logistic regression analysis was conducted on a cohort of 465 CHB patients from two hospitals to determine whether NAFLD was a risk factor for adverse rea
NAFLD was identified as an independent risk factor for partial virological re
Coexistence of NAFLD may diminish virological response among CHB patients receiving antiviral treatment with NAs.
Core Tip: The effect of nonalcoholic fatty liver disease (NAFLD) on the antiviral therapy with nucleoside analogues (NAs) in patients with chronic hepatitis B (CHB) is controversial. The aim of this study was to investigate the virological response to first-line NAs antiviral treatment in patients with NAFLD and CHB, through dynamically monitoring virology indicators for 96 weeks, to determine the influence of NAFLD on the efficacy of NAs anti- hepatitis B virus treatment. To our knowledge, this is the first grading study based on HBV baseline viral load that confirms a reduction in virological response to NAs antiviral treatment caused by NAFLD.
- Citation: Li HD, Liu YN, Wu S, Quan XF, Wang XY, Xiang TD, Li SM, Xu L, Wang T, Wang H, Zheng X. Influence of nonalcoholic fatty liver disease on the therapeutic effect of nucleoside (acid) analogs for hepatitis B virus. World J Hepatol 2024; 16(12): 1395-1406
- URL: https://www.wjgnet.com/1948-5182/full/v16/i12/1395.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i12.1395
Chronic liver diseases, such as chronic hepatitis B (CHB) and nonalcoholic fatty liver disease (NAFLD), are highly prevalent globally[1]. According to the World Health Organization, approximately 257 million individuals worldwide were affected by the hepatitis B virus (HBV) in 2015[2]. In the meantime, the prevalence of NAFLD has been rising over the past decade due to rapid socio-economic growth and improved quality of life[3]. Researches have interpreted that the overall incidence of NAFLD in CHB patients is about 14% to 70%[4,5]. Both HBV infection and NAFLD can induce chronic liver inflammation, aggravate liver injury, and an elevated likelihood of developing liver cirrhosis and hepatocellular carcinoma[6-8]. The implementation of antiviral therapy is crucial in inhibiting the progression of disease among CHB patients.
In recent years, nucleoside analogues (NAs) have been extensively utilized in antiviral treatment of CHB. Previous studies[9-11] have reported a decrease in HBV viral load in non-antiviral CHB patients comorbid with NAFLD, indicating that NAFLD may potentially inhibit HBV replication. However, the impact of NAFLD on the prognosis of antiviral therapy for CHB is a controversial issue. In a retrospective study of 555 CHB patients, Li et al[12] found that the presence of NAFLD does not exert any detrimental impact on the complete virological suppression achieved by NAs antiviral therapy, both in the short and long-term. Another retrospective study[13] of the CHB cohort treated with Entecavir (ETV) observed that the virology response rate of patients with NAFLD was similar to CHB patients. However, a separate retrospective study[14] on 524 CHB patients undergoing antiviral therapy showed a negative correlation between concomitant NAFLD and HBV DNA levels. Additionally, a clinical study[15] involving 267 CHB patients treated with ETV demonstrated a significant association between liver steatosis and the failure of antiviral treatment. Currently, there are no specific recommended guidelines for nucleotide antiviral treatment in patients with NAFLD complicated with CHB.
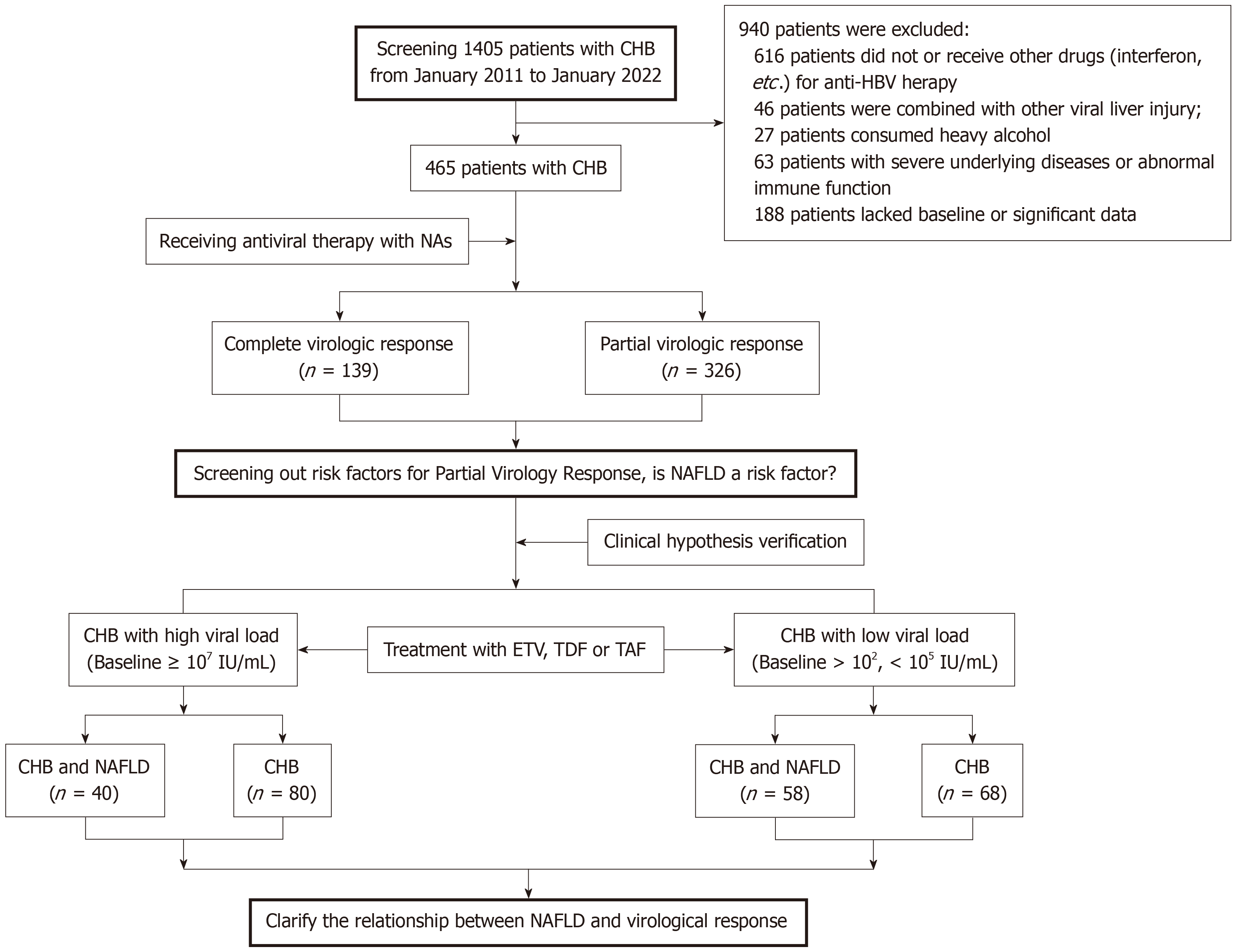
In view of this, we conducted a retrospective analysis on 465 CHB patients who underwent standard antiviral treatment with NAs at two hospitals, aiming to explore the association between NAFLD and virological response to antiviral treatment. Subsequently, these research findings were validated in both high- and low-viral-load CHB populations, while considering the impact of liver fibrosis during the course of antiviral treatment, aiming to ascertain the influence of NAFLD on virologic response to NAs-based antiviral therapy.
We retrospectively analyzed adult patients with CHB who received outpatient treatment at the Department of Infectious Diseases at Wuhan Jinyintan Hospital and Wuhan Union Hospital from January 2011 to January 2022. The demographics, clinical data, abdominal imaging, and laboratory information of the patients were recorded. Initially, we compared va
Subsequently, the high-viral-load (HBV DNA ≥ 107 IU/mL) and low-viral-load (1 × 102 IU/mL < HBV DNA < 1 × 105 IU/mL) CHB patients with and without of NAFLD were compared respectively, to determine the influence of NAFLD on the virological response to nucleotide analog ETV or tenofovir disoproxil fumarate (TDF) anti-HBV therapy (Figure 1).
Patients enrolled in this study were required to meet both the diagnostic criteria for CHB and NAFLD. According to the “Guidelines of prevention and treatment for chronic hepatitis B (2019 version)”[16], CHB was defined as hepatitis B surface antigen (HBsAg) or/and HBV DNA presence for over 6 months. The diagnosis of NAFLD was in accordance with AASLD's practice guide “Diagnosis and Management of Nonalcoholic Fatty liver Disease”[17], which was demonstrated by imaging or histology as evidence of hepatic steatosis, without any secondary causes such as excessive alcohol con
We excluded patients with the following conditions from this study: Liver disease caused by non-HBV infections or combined with other hepatitis virus infections; patients with immune deficiency diseases, autoimmune hepatitis, or those receiving systemic immune regulation treatment for related diseases; pregnant or lactating women; and individuals undergoing other anti-HBV therapies such as interferon.
Statistical analysis was conducted using Univariate and Multivariate Logistic regression tests, Student's t-test, and Pearson's χ2 test. Continuous variables were reported as median (interquartile range) or mean ± SD. while categorical variables were described by numbers and proportion (%). For the analysis of continuous variables that follow a normal distribution, we employed the student's t-test, while for categorical variables, Pearson's χ2 test was utilized. Mann-Whitney U tests were performed to analysis of nonnormally distributed variables with two independent samples. Kaplan-Meier method was used to determine the negative conversion rate (NCR) of HBV DNA, and the normalization rates of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). GraphPad Prism (Version 8.0, SanDiego, California, United States) and SPSS (version 25, IBM, United States) software were employed for charting and statistical analysis. A P-value of ≤ 0.05 was regarded as statistically significant.
A total of 1405 adults with CHB were assessed during the study period, and 465 individuals met the inclusion criteria. Among those who received antiviral therapy with NAs, 139 subjects obtained CVR, while 326 subjects experienced PVR, and the risk factors of PVR were observed. Subsequently, to verify the relationship between NAFLD coexisting with CHB and virological response, we conducted a dynamic analysis of viral response to NAs antiviral treatment in patients with high and low HBV DNA viral load levels (Figure 1).
Table 1 presents the baseline demographic data and clinical characteristics of all enrolled subjects, who were monitored for an average duration of 28 months. In the study population, men accounted for a higher proportion than women (68.8% vs 31.2%), with an average age was 35 years.
| Variable | Chronic hepatitis B | Complete virologic response | Partial virologic response | t/χ2 | P value |
| (n = 465) | (n = 139) | (n = 326) | |||
| Gender (n = 465) | χ2 = 0.006 | 0.940 | |||
| Male | 320 (68.8) | 96 (69.1) | 224 (68.7) | ||
| Female | 145 (31.2) | 43 (30.9) | 102 (31.3) | ||
| Age (years), n = 465 | 35.5 ± 10.5 | 38.1 ± 11.7 | 34.4 ± 9.7 | t = 3.514 | 0.0005 |
| HBV DNA baseline level, log10 (IU/mL), n = 465 | 6.1 ± 6.0 | 5.6 ± 1.9 | 6.4± 2.0 | t = 3.661 | 0.0003 |
| Virus shedding time (months), n = 428 | 9.8 ± 8.7 | 6.9 ± 5.8 | 11.2 ± 9.5 | t = 4.919 | < 0.0001 |
| HBsAg baseline level, log10 (IU/mL + 1), n = 461 | 3.4 ± 1.0 | 3.0 ± 0.9 | 3.6 ± 1.0 | t = 5.479 | < 0.0001 |
| HBeAg | χ2 = 14.57 | < 0.0001 | |||
| Positive | 298 (64.1) | 71 (51.1) | 227 (69.6) | ||
| Negative | 167 (35.9) | 68 (48.9) | 99 (30.4) | ||
| NAFLD | χ2 = 5.815 | 0.016 | |||
| Yes | 133 (28.6) | 29 (20.9) | 104 (31.9) | ||
| No | 332 (71.4) | 110 (79.1) | 222 (68.1) | ||
| Leucocytes (G/L), n = 396 | 5.5 ± 1.7 | 5.4 ± 1.8 | 5.6 ± 1.6 | t = 0.7417 | 0.4587 |
| Haemoglobin (g/L), n = 396 | 148.2 ± 18.2 | 148.6 ± 17.2 | 147.9 ± 18.7 | t = 0.3339 | 0.7386 |
| Neutrophils (G/L), n = 396 | 3.2 ± 1.4 | 3.2 ± 1.4 | 3.3 ± 1.4 | t = 0.6343 | 0.5263 |
| Lymphocytes (G/L), n = 396 | 1.8 ± 0.6 | 1.8 ± 0.6 | 1.8 ± 0.6 | t = 0.0444 | 0.9646 |
| Total bilirubin log10 (μmol/L), n = 464 | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | t = 0.8594 | 0.3906 |
| Alanine aminotransferase log10 (U/L), n = 464 | 1.7 ± 0.4 | 1.7 ± 0.4 | 1.7 ± 0.4 | t = 0.6270 | 0.5310 |
| Aspartate aminotransferase log10 (U/L), n = 464 | 1.6 ± 0.3 | 1.6 ± 0.3 | 1.6 ± 0.3 | t = 1.432 | 0.1527 |
| Albumin (g/L), n = 464 | 44.9 ± 3.3 | 44.9 ± 3.5 | 44.9 ± 3.2 | t = 0.0135 | 0.9892 |
| Uric acid (umol/L), n = 447 | 337.5 ± 92.8 | 327.7 ± 76.5 | 341.6 ± 98.8 | t = 1.454 | 0.1467 |
| Fasting glucose (mmol/L), n = 229 | 5.5 ± 1.1 | 5.6 ± 1.5 | 5.4 ± 0.9 | t = 1.283 | 0.2009 |
| Triglyceride (mmol/L), n = 264 | 1.4 ± 1.1 | 1.2 ± 0.6 | 1.4 ± 1.2 | t = 2.056 | 0.0408 |
| Total cholesterol (mmol/L), n = 264 | 4.5 ± 1.0 | 4.4 ± 1.0 | 4.9 ± 0.9 | t = 3.694 | 0.0003 |
| High-density lipoprotein (mmol/L), n = 264 | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.3 | t = 1.761 | 0.0794 |
| Low-density lipoprotein (mmol/L), n = 264 | 2.8 ± 0.9 | 2.82 ± 0.90 | 2.8 ± 0.9 | t = 0.1163 | 0.9075 |
| Treatment follow-up period (months), n = 465 | 28.3 ± 17.5 | 39.8 ± 16.9 | 22.9 ± 15.0 | t = 10.52 | < 0.0001 |
| aAPRI score, n = 396 | 0.42 (0.28-0.81) | 0.41 (0.27-0.90) | 0.43 (0.29-0.81) | Z = -0.571 | 0.568 |
| Low (< 0.5) | 226 (57.07) | 68 (58.62) | 158 (56.43) | t = 0.161 | 0.688 |
| Intermediate (0.5-1.5) | 128 (32.32) | 36 (31.03) | 92 (32.86) | t = 0.125 | 0.724 |
| High (> 1.5) | 42 (10.61) | 12 (10.35) | 30 (10.71) | t = 0.012 | 0.913 |
| bFibrosis-4 score, n = 395 | 0.93 (0.52-1.82) | 1.00 (0.50-1.94) | 0.89 (0.54-1.74) | Z = -0.232 | 0.816 |
| Low (< 1.45) | 266 (67.34) | 74 (63.79) | 192 (68.82) | t = 0.940 | 0.332 |
| Intermediate (1.45-3.25) | 82 (20.76) | 22 (18.97) | 60 (21.50) | t = 0.321 | 0.571 |
| High (> 3.25) | 47 (11.90) | 20 (17.24) | 27 (9.68) | t = 4.472 | 0.034 |
Based on the virological response outcome, patients were divided into two groups: The CVR group and the PVR group. The gender composition did not show any significant difference between these two groups (P = 0.94). However, it was observed that patients in the CVR group had a higher age compared to those in the PVR group (38.1 vs 34.4, P = 0.0005). Significant differences were found between the PVR and CVR groups regarding baseline levels of HBsAg and HBV DNA, duration of HBV shedding, and HBeAg positivity rate. These parameters were all higher in the PVR group than in the CVR group. Interestingly, our findings revealed a greater proportion of NAFLD patients in the PVR group when compared to the CVR group (31.9% vs 20.9%). Moreover, this particular group also exhibited higher baseline levels of triglyceride and total cholesterol (1.4 vs 1.2 for triglyceride; 4.9 vs 4.4 for total cholesterol), suggesting a potential association with NAFLD development. However, no significant differences were observed when comparing fibrosis-4 (FIB-4) scores and AST to platelet ratio index (APRI) scores reflecting liver fibrosis or cirrhosis between the CVR and PVR groups respectively (P > 0.05).
Subsequently, we conducted multivariate logistic regression analysis, and found that NAFLD, Virus shedding time, and HBsAg baseline level were independent predictors of PVR after antiviral therapy with NAs in CHB patients, respectively. Notably, the presence of NAFLD was positively associated with PVR following antiviral treatment, as CHB patients with NAFLD had a 1.792-fold higher likelihood of developing PVR compared to those without NAFLD. To assess the influence of liver fibrosis on the antiviral response to NAs, we established Univariate analysis and Multivariate analysis model II and III; however, no significant correlations were observed between FIB-4 or APRI scores and PVR outcomes (Table 2).
| Variable | Univariate analysis | Multivariate analysis model I | Multivariate analysis model II | Multivariate analysis model III | ||||||||
| β | OR (95%CI) | P value | β | OR (95%CI) | P value | β | OR (95%CI) | P value | β | OR (95%CI) | P value | |
| Age (years) | -0.033 | 0.968 (0.949-0.986) | 0.001 | -0.013 | 0.987 (0.965-1.009) | 0.253 | -0.004 | 0.996 (0.971-1.021) | 0.746 | -0.003 | 0.997 (0.973-1.023) | 0.842 |
| HBV DNA baseline level, log10 (IU/mL) | 0.183 | 1.201 (1.086-1.328) | < 0.001 | 0.017 | 1.017 (0.886-1.168) | 0.809 | 0.011 | 1.011 (0.87-1-176) | 0.884 | 0.008 | 1.008 (0.867-1.173) | 0.913 |
| Virus shedding time (months) | 0.063 | 1.065 (1.033-1.097) | < 0.001 | 0.033 | 1.033 (1.001-1.067) | 0.046 | 0.038 | 1.039 (1.002-1.078) | 0.039 | 0.039 | 1.04 (1.002-1.078) | 0.037 |
| HBeAg positive (vs negative) | 0.787 | 2.196 (1.461-3.302) | < 0.001 | -0.18 | 0.836 (0.468-1.492) | 0.544 | -0.06 | 0.942 (0.501-1.773) | 0.853 | -0.083 | 0.92 (0.488-1.736) | 0.797 |
| HBsAg baseline level, log10 (IU/mL+1) | 0.624 | 1.866 (1.5-2.322) | < 0.001 | 0.507 | 1.661 (1.254-2.2) | < 0.001 | 0.517 | 1.677 (1.237-2.273) | 0.001 | 0.52 | 1.682 (1.239-2.282) | 0.001 |
| NAFLD (vs non-NAFLD) | 0.575 | 1.777 (1.11-2.845) | 0.017 | 0.583 | 1.792 (1.068-3.006) | 0.027 | 0.92 | 2.508 (1.368-4.6) | 0.003 | 0.9 | 2.459 (1.34-4.514) | 0.004 |
| APRI score | -0.069 | 0.934 (0.836-1.043) | 0.224 | -0.052 | 0.949 (0.853-1.056) | 0.34 | ||||||
| FIB-4 score | -0.041 | 0.96 (0.92-1.002) | 0.061 | -0.023 | 0.977 (0.939-1.016) | 0.247 | ||||||
Previously, our findings indicated that NAFLD was associated with a reduced virological response to NAs antiviral treatment in CHB patients. Therefore, we enrolled CHB patients with elevated viral load to undergo a follow-up period exceeding 30 months after initiation of NAs antiviral therapy (33.8 vs 35.1 months, P = 0.7233). The results indicated that the male population in NAFLD patients was significantly higher compared to the non-NAFLD group (92.5% vs 66.3%, P = 0.004), while there was no noteworthy distinction in age between the two groups (29.5 vs 31.0, P = 0.2480). Regarding the utilization rate of antiviral drugs ETV and/or TDF, there were no notable disparities observed between the two groups, however, due to poor treatment efficacy, 60% of study subjects in the NAFLD group required switching or combination therapy with two NAs compared to 48.7% in the non-NAFLD group. We evaluated baseline levels of HBsAg and HBV DNA, along with the rates of seroconversion for HBsAg and HBeAg, but observed no disparities between the two cohorts. Surprisingly, virological shedding time was significantly prolonged in the NAFLD group compared to the non-NAFLD group (16.8 vs 13 months, P = 0.0033), and there was a significantly lower proportion of CVR compared to that observed in the non-NAFLD group (12.5% vs 38.8%, P = 0.003). In terms of biochemical markers, there was no significant difference in baseline ALT levels between the two groups (P = 0.0722). However, AST levels were observed to be lower in the NAFLD group compared to the non-NAFLD group (P = 0.0122) (Table 3).
| Characteristics | NAFLD | Non-NAFLD | t/χ2 | P value |
| (n = 40) | (n = 80) | |||
| Gender | χ2 = 8.450 | 0.004 | ||
| Male | 37 (92.5) | 53 (66.3) | ||
| Female | 3 (7.5) | 27 (33.7) | ||
| Age (years) | 29.5 ± 5.6 | 31.0 ± 7.1 | t = 1.161 | 0.2480 |
| HBV DNA baseline level, log10 (IU/mL) | 8.1 ± 0.5 | 8.0 ± 0.5 | t = 0.3877 | 0.6990 |
| Virus shedding time (months) | 16.8 ± 6.1 | 13.0 ± 6.8 | t = 3.003 | 0.0033 |
| Virology breakthrough time (months) | 6.8 ± 5.6 | 10.9 ± 11.5 | t = 1.378 | 0.1735 |
| HBsAg baseline level, log10 (IU/mL) | 4.4 ± 0.6 | 4.2 ± 0.7 | t = 1.252 | 0.2148 |
| Antiviral therapy | t = 1.985 | 0.371 | ||
| ETV | 4 (10.0) | 15 (18.8) | t = 1.532 | 0.216 |
| TDF | 12 (30.0) | 26 (32.5) | t = 0.077 | 0.781 |
| ETV and TDF | 24 (60.0) | 39 (48.7) | t = 1.353 | 0.245 |
| low-level viremia | 30 (75.0) | 49 (61.3) | t = 2.241 | 0.134 |
| Virological response | χ2 = 8.750 | 0.003 | ||
| Complete virologic response | 5 (12.5) | 31 (38.8) | ||
| Partial virologic response | 35 (87.5) | 49 (61.2) | ||
| Serological response (HBsAg) | χ2 = 0.0000 | 1.000 | ||
| Yes | 1 (2.5) | 2 (2.5) | ||
| No | 39 (97.5) | 78 (97.5) | ||
| Serological response (HBeAg) | χ2 = 3.116 | 0.078 | ||
| Yes | 1 (2.5) | 12 (15.0) | ||
| No | 39 (97.5) | 68 (85.0) | ||
| Leucocytes, (G/L) | 6.4 ± 2.1 | 5.5 ± 1.6 | t = 2.414 | 0.0175 |
| Erythrocyte, (T/L) | 5.2 ± 0.4 | 4.9 ± 0.5 | t = 3.308 | 0.0013 |
| Platelets, (G/L) | 228.5 ± 51.2 | 200.4 ± 54.2 | t = 2.489 | 0.0144 |
| Haemoglobin, (G/L) | 155.4 ± 16.6 | 146.5 ± 16.7 | t = 2.533 | 0.0128 |
| Neutrophils, (G/L) | 3.8 ± 1.8 | 3.1 ± 1.3 | t = 2.232 | 0.0278 |
| Lymphocytes, (G/L) | 2.0 ± 0.5 | 1.9 ± 0.5 | t = 1.277 | 0.2043 |
| Total bilirubin, log10 (μmol/L) | 1.2 ± 0.2 | 1.2 ± 0.2 | t = 0.2773 | 0.7820 |
| ≤ 19 | 29 (72.5) | 54 (67.5) | t = 0.313 | 0.576 |
| > 19 | 11 (27.5) | 26 (32.5) | ||
| Alanine aminotransferase, log10 (U/L) | 1.7 ± 0.3 | 1.9 ± 0.5 | t = 1.814 | 0.0722 |
| ≤ 35 | 6 (15.0) | 17 (21.3) | t = 0.672 | 0.412 |
| > 35 | 34 (85.0) | 63 (78.7) | ||
| Aspartate aminotransferase, log10 (U/L) | 1.5 ± 0.2 | 1.7 ± 0.4 | t = 2.545 | 0.0122 |
| ≤ 40 | 21 (52.5) | 29 (36.3) | t = 2.897 | 0.089 |
| > 40 | 19 (47.5) | 51 (63.7) | ||
| APRI score | 0.5 ± 0.4 | 0.8 ± 0.6 | t = 2.639 | 0.0096 |
| Fibrosis-4 score | 0.7 ± 0.4 | 1.0 ± 0.6 | t = 2.631 | 0.0098 |
| Total protein, (g/L) | 75.6 ± 5.8 | 74.3 ± 5.1 | t = 1.336 | 0.1841 |
| Albumin, (g/L) | 45.7 ± 2.4 | 43.7 ± 3.4 | t = 3.414 | 0.0009 |
| Globulin, (g/L) | 29.9 ± 5.2 | 30.6 ± 4.3 | t = 0.7613 | 0.4480 |
| Urea nitrogen, (mmol/L) | 4.6 ± 1.3 | 4.2 ± 1.2 | t = 1.599 | 0.1125 |
| Creatinine, (μmol/L) | 76.3 ± 7.5 | 71.1 ± 11.2 | t = 2.542 | 0.0124 |
| Uric acid, (μmol/L) | 413.7 ± 95.7 | 314.0 ± 86.8 | t = 5.546 | < 0.0001 |
| Fasting glucose, (mmol/L) | 5.3 ± 0.5 | 5.8 ± 2.4 | t = 0.8360 | 0.4074 |
| Triglyceride, (mmol/L) | 1.6 ± 1.6 | 1.0 ± 0.5 | t = 2.085 | 0.0418 |
| Total cholesterol, (mmol/L) | 4.5 ± 0.8 | 4.2 ± 0.9 | t = 1.328 | 0.1897 |
| High-density lipoprotein, (mmol/L) | 1.0 ± 0.2 | 1.2 ± 0.3 | t = 1.886 | 0.0647 |
| Low-density lipoprotein, (mmol/L) | 2.9 ± 0.7 | 2.5 ± 0.8 | t = 1.768 | 0.0827 |
| Treatment follow-up period, (months) | 33.8 ± 16.8 | 35.1 ± 17.8 | t = 0.3550 | 0.7233 |
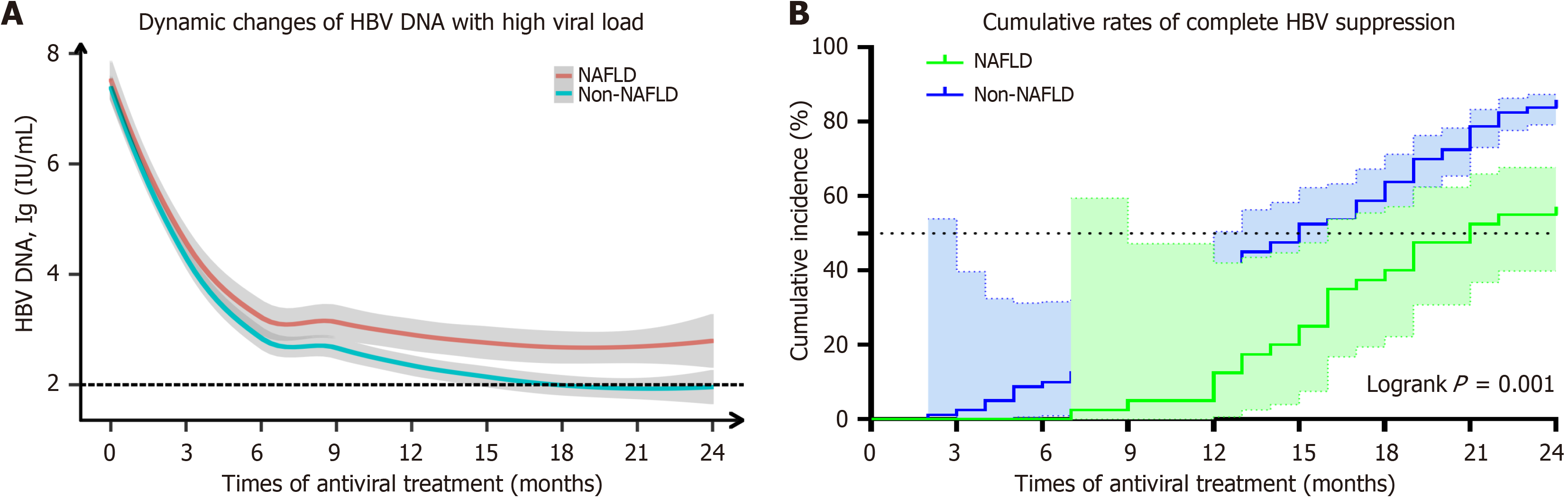
Patients with high viral load were dynamically observed for 24 months following initial antiviral therapy with ETV or TDF, and evaluations were conducted every 3 months to assess the HBV viral load levels and the cumulative rate of HBV DNA negativity. The findings demonstrated a notable disparity in the reduction rate of HBV viral load between the NAFLD and Non-NAFLD groups after 9 months of antiviral treatment. Specifically, at the 9th, 12th, 15th, and 24th months, the log10 levels of HBV viral load (in NAFLD vs non-NAFLD) were as follows: 3.1 vs 2.7, 2.9 vs 2.4, 2.8 vs 2.1, and finally at month twenty-four: 2.9 vs 2.0 (P < 0.05).
In contrast to the non-NAFLD group, where antiviral treatment led to a decrease in HBV DNA levels below detectable limits after fifteen months, no such reduction was observed in the NAFLD group (Figure 2A, Supplementary Table 1). Additionally, it was noted that at twenty-four months post-treatment, patients with NAFLD had a significantly lower NCR for HBV compared to those without NAFLD (62.5% vs 86.3%, P = 0.001) (Figure 2B, Supplementary Table 2).
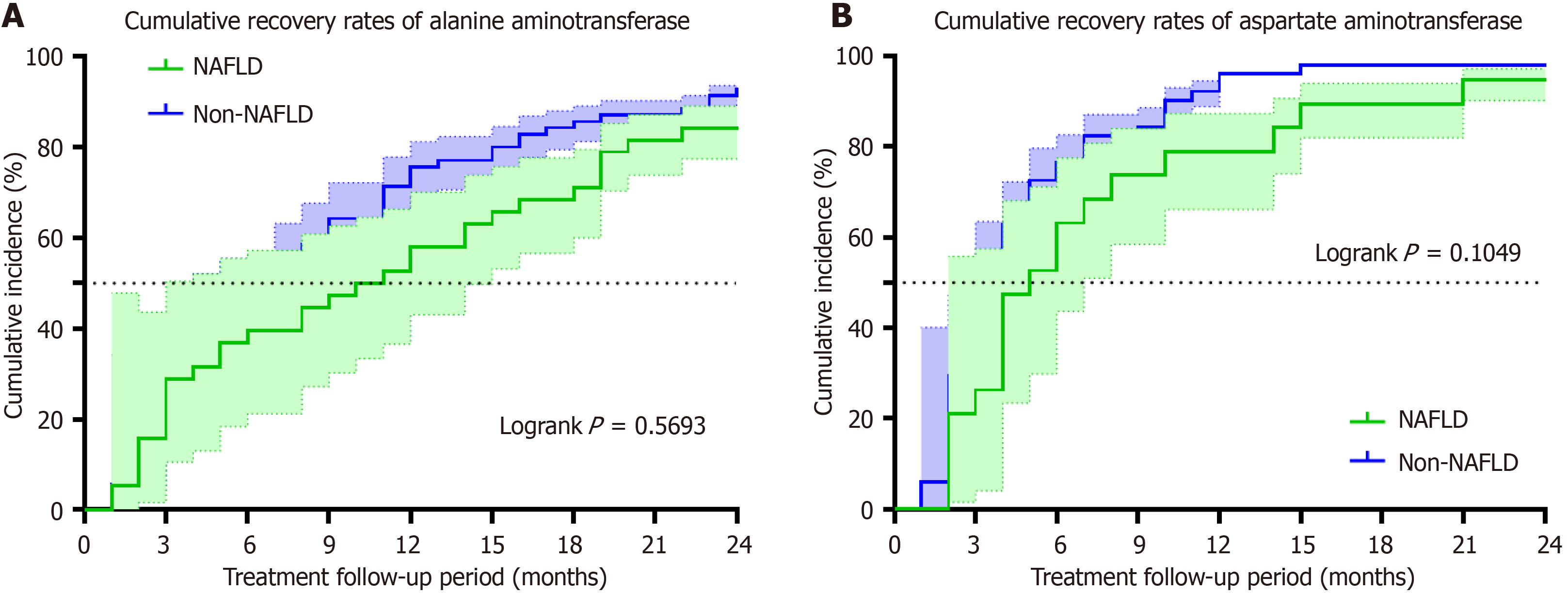
However, with regard to biochemical response, when comparing the normalization rates of ALT between NAFLD group and non-NAFLD group, we observed rates of 39.5% vs 44.3%, 57.9% vs 75.7%, 71.1% vs 85.7%, and 84.2% vs 92.9% at the 6th, 12th, 18th, and 24th months respectively; however, no significant difference was found at any stage (Figure 3A, Supplementary Table 3).
Similarly, the normalization rates of AST in both NAFLD and Non-NAFLD cohorts were recorded as 63.2% vs 76.5% in the 6th month, 79.0% vs 96.1% in the 12th month, 89.5% vs 98.0% in the 18th month, and 94.7% vs 98.0% in the 24th month respectively, there was no notable disparity observed between the two groups in terms of statistical significance (Fi
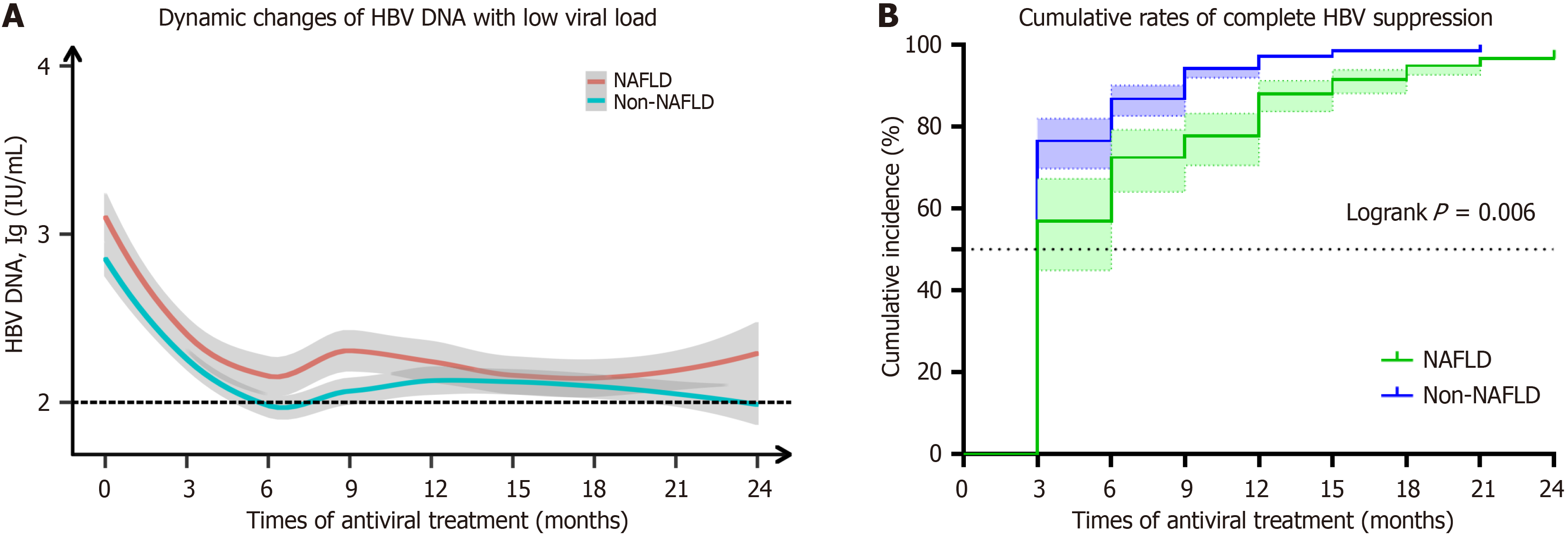
Similarly, patients with low viral load (1 × 102 IU/mL < HBV DNA < 1 × 105 IU/mL) were dynamically observed for 24 months after initial antiviral therapy with ETV or TDF, and evaluation was conducted every 3 months to monitor the level of HBV viral load level, HBV DNA NCR. The findings suggest that the rate of decline in HBV viral level was co
A notable disparity in the HBV NCR between the two groups emerged after twenty-four months of antiviral therapy (P = 0.006). Furthermore, we noted that during the initial three to nine months of antiviral treatment, the NAFLD group exhibited a significantly reduced cumulative conversion rate of HBV compared to the non-NAFLD group (P < 0.05). Nevertheless, this distinction did not persist beyond twelve months following initiation of antiviral treatment (Figure 4B, Supplementary Table 5).
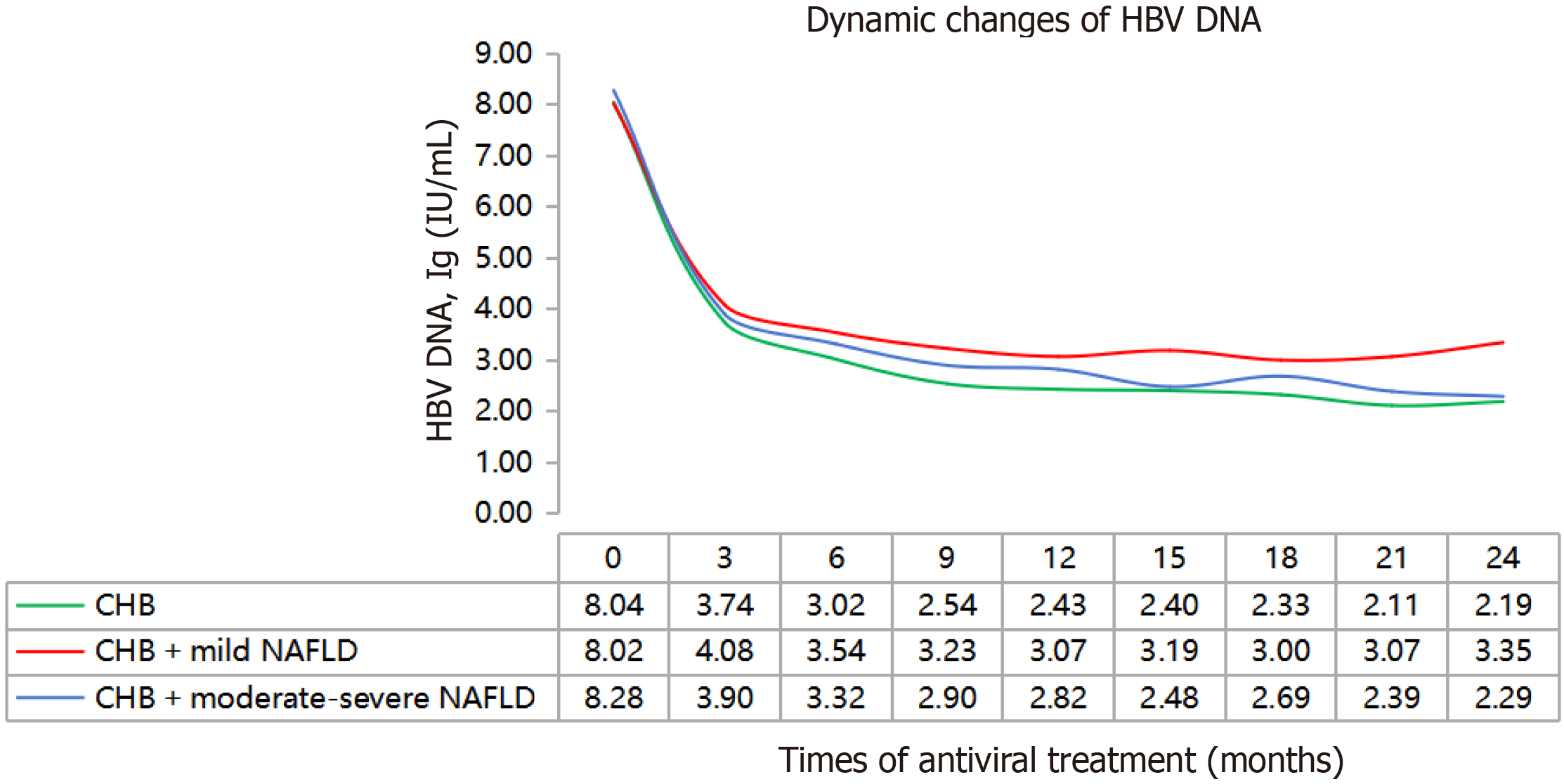
This study categorized CHB patients with high viral load and coexisting NAFLD into mild and moderate to severe fatty liver groups based on their abdominal ultrasound and control attenuation parameter (CAP). The control group consisted of CHB patients with high viral load. Initially, all participants were treated with first-line nucleotide analogues antiviral therapy, while monitoring HBV DNA levels every 3 months. The findings revealed that compared to patients with CHB, the decline in HBV DNA levels was slower in those with CHB and NAFLD, particularly those in the mild NAFLD subgroup (Figure 5).
In recent years, the prevalence of NAFLD has increased among CHB patients due to improvements in living standards, changes in dietary habits, and the rising rates of obesity and metabolic syndrome[18,19]. Concerns have emerged re
Furthermore, we stratified the previous study cohort based on viral load levels and HBeAg status. Patients with high viral load and positive HBeAg were selected for treatment with first-line antiviral drugs ETV and TDF. Subsequent dynamic observation and analysis further validated that coexistence of NAFLD delayed HBV clearance and reduced the rate of complete response to antiviral therapy. Our findings are consistent with prior studies[20,21], highlighting a ne
Meanwhile, we conducted a similar follow-up study to observe the dynamic changes in HBV DNA levels after initial antiviral therapy in CHB patients with low viral load at baseline. Our findings suggest that in the NAFLD group, there was a comparatively gradual decrease in HBV DNA levels and a lower cumulative rate of viral clearance compared to the non-NAFLD group, particularly during the first 9 months of antiviral treatment. These results indicate that coexistence of NAFLD also attenuates the early antiviral treatment response of NAs in individuals with low viral load at baseline.
Patients with CHB may experience liver tissue damage and reduced effectiveness of antiviral treatment due to the potential negative impact of NAFLD. Research indicates that leptin plays a role in modulating T cell function; when this regulation is disrupted, it can hinder the ability of CHB patients to clear the virus, complicating HBeAg seroconversion[23,24]. Insulin resistance serves as a pivotal factor in the pathogenesis of NAFLD, which can impair immune func
Recently, a parameter called the CAP has been used to assess the degree of hepatic steatosis. According to the research findings by Chen et al[27], CHB patients who were administered ETV as their initial antiviral therapy demonstrated a reduced rate of HBV DNA seroconversion in individuals with significantly elevated baseline levels of CAP, suggesting a limited response to antiviral treatment compared to those with normal CAP. However, another study[28] demonstrated a negative correlation between CAP value and the achievement of CVR. In this research, we performed a comparative examination of the response to antiviral therapy using NA in patients with varying degrees of NAFLD and CHB in
Hepatic fibrosis is a crucial stage in the progression of CHB and NAFLD. In our study, we evaluated the liver non-invasive detection indicators FIB-4 and APRI scores. Surprisingly, no notable disparity was detected in the FIB-4 and APRI scores when comparing the CVR and PVR groups. Furthermore, multivariate analysis revealed that these scores were not identified as risk factors for PVR, which aligns with previous research findings[12]. However, it is intriguing to note that among individuals with high viral load and positive HBeAg status, patients with NAFLD-associated CHB exhibited lower FIB-4 and APRI scores compared to those with simple CHB. Previous research has indicated that the coexistence of NAFLD does not contribute to an increased occurrence of liver fibrosis in CHB patients[18], according to the results of a recent study[30], the presence of NAFLD is considered to be an independent negative factor for significant and advanced liver fibrosis in patients with CHB, although further validation through larger sample sizes is warranted.
This study has several limitations. Firstly, this investigation constitutes a retrospective clinical cohort study, which may be subject to case selection bias. Consequently, the findings necessitate further validation through prospective studies. Secondly, this retrospective clinical study only includes a Chinese ethnic group population, which may not be representative of other ethnic groups worldwide. Additionally, the diagnosis of NAFLD primarily relies on liver imaging re
In conclusion, our study on the CHB patients with varying HBV viral loads, and initial standard 96 weeks of treatment with first-line antiviral drugs further verified that NAFLD can reduce the antiviral response of NAs, highlighting the need for increased attention towards these patients. Moreover, the findings from this retrospective study serve as a valuable point of reference for the development of standardized clinical guidelines, and further prospective studies are warranted to validate these results, provide more scientifically guided antiviral treatment strategies for patients with CHB complicated by NAFLD.
The authors would like to express their acknowledgments to the patients involved in this study.
| 1. | Cai J, Zhang XJ, Li H. Progress and challenges in the prevention and control of nonalcoholic fatty liver disease. Med Res Rev. 2019;39:328-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 2. | World Health Organization. Global hepatitis report 2017. Geneva: World Health Organization; 2017. Available from: http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. |
| 3. | Ding C, Fu X, Zhou Y, Liu X, Wu J, Huang C, Deng M, Li Y, Li L, Yang S. Disease burden of liver cancer in China from 1997 to 2016: an observational study based on the Global Burden of Diseases. BMJ Open. 2019;9:e025613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, Janssen HLA, Patel K. Nonalcoholic Steatohepatitis Is Associated With Liver-Related Outcomes and All-Cause Mortality in Chronic Hepatitis B. Hepatology. 2020;71:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 5. | Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in hepatitis B virus infected patients: meta-analysis of risk factors and comparison with hepatitis C infected patients. J Gastroenterol Hepatol. 2011;26:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Liu SY, Wang D, Liu J, Yang LP, Chen GY. Influence of nonalcoholic fatty liver disease on response to antiviral treatment in patients with chronic hepatitis B: A meta-analysis. World J Hepatol. 2024;16:465-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Rizzo GEM, Cabibbo G, Craxì A. Hepatitis B Virus-Associated Hepatocellular Carcinoma. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 8. | Mattos ÂZ, Debes JD, Dhanasekaran R, Benhammou JN, Arrese M, Patrício ALV, Zilio AC, Mattos AA. Hepatocellular carcinoma in nonalcoholic fatty liver disease: A growing challenge. World J Hepatol. 2021;13:1107-1121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Hui RWH, Seto WK, Cheung KS, Mak LY, Liu KSH, Fung J, Wong DK, Lai CL, Yuen MF. Inverse relationship between hepatic steatosis and hepatitis B viremia: Results of a large case-control study. J Viral Hepat. 2018;25:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | Zhang Z, Pan Q, Duan XY, Liu Q, Mo GY, Rao GR, Fan JG. Fatty liver reduces hepatitis B virus replication in a genotype B hepatitis B virus transgenic mice model. J Gastroenterol Hepatol. 2012;27:1858-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Hu D, Wang H, Wang H, Wang Y, Wan X, Yan W, Luo X, Ning Q. Non-alcoholic hepatic steatosis attenuates hepatitis B virus replication in an HBV-immunocompetent mouse model. Hepatol Int. 2018;12:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Li J, Le AK, Chaung KT, Henry L, Hoang JK, Cheung R, Nguyen MH. Fatty liver is not independently associated with the rates of complete response to oral antiviral therapy in chronic hepatitis B patients. Liver Int. 2020;40:1052-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Zhu LY, Wang YG, Wei LQ, Zhou J, Dai WJ, Zhang XY. The effects of the insulin resistance index on the virologic response to entecavir in patients with HBeAg-positive chronic hepatitis B and nonalcoholic fatty liver disease. Drug Des Devel Ther. 2016;10:2739-2744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Peleg N, Issachar A, Sneh Arbib O, Cohen-Naftaly M, Braun M, Leshno M, Barsheshet A, Shlomai A. Liver steatosis is a strong predictor of mortality and cancer in chronic hepatitis B regardless of viral load. JHEP Rep. 2019;1:9-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Jin X, Chen YP, Yang YD, Li YM, Zheng L, Xu CQ. Association between hepatic steatosis and entecavir treatment failure in Chinese patients with chronic hepatitis B. PLoS One. 2012;7:e34198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Chinese Society of Infectious Diseases; Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. [The guidelines of prevention and treatment for chronic hepatitis B (2019 version)]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:938-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 111] [Reference Citation Analysis (0)] |
| 17. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4935] [Article Influence: 705.0] [Reference Citation Analysis (9)] |
| 18. | Zheng Q, Zou B, Wu Y, Yeo Y, Wu H, Stave CD, Cheung RC, Nguyen MH. Systematic review with meta-analysis: prevalence of hepatic steatosis, fibrosis and associated factors in chronic hepatitis B. Aliment Pharmacol Ther. 2021;54:1100-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Charatcharoenwitthaya P, Pongpaibul A, Kaosombatwattana U, Bhanthumkomol P, Bandidniyamanon W, Pausawasdi N, Tanwandee T. The prevalence of steatohepatitis in chronic hepatitis B patients and its impact on disease severity and treatment response. Liver Int. 2017;37:542-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Zhu Y, Yang Q, Lv F, Yu Y. The Effect of Hepatosteatosis on Response to Antiviral Treatment in Patients with Chronic Hepatitis B: A Meta-Analysis. Gastroenterol Res Pract. 2017;2017:1096406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Chen Y, Liu Q, Han J, Gao S, Xiao Q, Huang X, Lu L, Zhou X. A Meta-Analysis of How Nonalcoholic Fatty Liver Disease Affect Antiviral Treatment of Patients with e Antigen-Positive Chronic Hepatitis B. Emerg Med Int. 2022;2022:4774195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 22. | Zhang S, Zhang X, Jin H, Dou Y, Li L, Yuan X, Dong C, Hou M, Nan YM, Shang J. Adverse Effect of Nonalcoholic Fatty Liver Disease on the Therapeutic Response in Patients with Chronic Hepatitis B. J Clin Transl Hepatol. 2023;11:67-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Selimoglu MA, Ertekin V. Is leptin a predictive factor in the end of therapy response in chronic hepatitis B? Pediatr Int. 2005;47:378-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Venken K, Seeuws S, Zabeau L, Jacques P, Decruy T, Coudenys J, Verheugen E, Windels F, Catteeuw D, Drennan M, Van Calenbergh S, Lambrecht BN, Yoshimura A, Tavernier J, Elewaut D. A bidirectional crosstalk between iNKT cells and adipocytes mediated by leptin modulates susceptibility for T cell mediated hepatitis. J Hepatol. 2014;60:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Ye J, Hu X, Wu T, Wu Y, Shao C, Li F, Lin Y, Feng S, Wang W, Zhong B. Insulin resistance exhibits varied metabolic abnormalities in nonalcoholic fatty liver disease, chronic hepatitis B and the combination of the two: a cross-sectional study. Diabetol Metab Syndr. 2019;11:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Wong YJ, Nguyen VH, Yang HI, Li J, Le MH, Wu WJ, Han NX, Fong KY, Chen E, Wong C, Rui F, Xu X, Xue Q, Hu XY, Leow WQ, Goh GB, Cheung R, Wong G, Wong VW, Yu MW, Nguyen MH. Impact of fatty liver on long-term outcomes in chronic hepatitis B: a systematic review and matched analysis of individual patient data meta-analysis. Clin Mol Hepatol. 2023;29:705-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 27. | Chen J, Wang ML, Long Q, Bai L, Tang H. High value of controlled attenuation parameter predicts a poor antiviral response in patients with chronic hepatits B. Hepatobiliary Pancreat Dis Int. 2017;16:370-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Kim DS, Jeon MY, Lee HW, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, Kim SU. Influence of hepatic steatosis on the outcomes of patients with chronic hepatitis B treated with entecavir and tenofovir. Clin Mol Hepatol. 2019;25:283-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Ceylan B, Arslan F, Batırel A, Fincancı M, Yardımcı C, Fersan E, Paşaoğlu E, Yılmaz M, Mert A. Impact of fatty liver on hepatitis B virus replication and virologic response to tenofovir and entecavir. Turk J Gastroenterol. 2016;27:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Yao R, Lu S, Xue R, Wang J, Qiu Y, Chen Y, Liu J, Zhu L, Zhan J, Jiang S, Yin S, Tong X, Ding W, Li J, Zhu C, Huang R, Wu C. NAFLD is associated with less severe liver fibrosis in chronic hepatitis B: A multi-center, retrospective study. Ann Hepatol. 2024;29:101155. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |