INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most prevalent and lethal malignancies globally, particularly in regions with high rates of hepatitis B virus and hepatitis C virus infections, making it a significant public health concern. According to global cancer statistics, HCC accounts for 75%-85% of all primary liver cancers worldwide, with particularly high incidence in China, largely due to the widespread prevalence of viral hepatitis[1]. Despite recent advances in antiviral therapy and cancer screening techniques, treatment options for advanced HCC remain limited, with poor overall prognosis.
The development and progression of HCC is a complex, multi-step process driven by various factors, including chronic inflammation, fibrosis, cirrhosis, immune evasion, and angiogenesis. In recent years, the hypoxic microenvironment within tumors and its associated molecular pathways have emerged as focal points of HCC research[2]. Hypoxia-inducible factor-1α (HIF-1α), a key transcription factor regulating cellular responses to hypoxia, promotes tumor cell proliferation, invasion, and metastasis by regulating the expression of genes related to angiogenesis, metabolism, and survival[3,4]. Angiogenesis is one of the crucial processes for tumor growth and metastasis. Angiopoietin-2 (Ang-2), a key regulator of angiogenesis, has recently been shown to play a significant role in the development and progression of HCC[5]. By binding to its tyrosine kinase (Tie-2) receptor, Ang-2 regulates vascular stability and promotes new blood vessel formation, especially under hypoxic conditions where its expression is significantly upregulated. Studies have shown a complex regulatory relationship between HIF-1α and Ang-2, with HIF-1α directly or indirectly regulating Ang-2 expression, further promoting angiogenesis and tumor progression in HCC.
Therefore, a detailed investigation into the interaction between HIF-1α and Ang-2, and the elucidation of their specific roles in HCC initiation, progression, and metastasis, has significant clinical implications. Understanding the HIF-1α/Ang-2 axis could provide new therapeutic avenues for anti-angiogenic treatment and multidrug resistance (MDR) management in HCC and offer theoretical and experimental support for targeted therapeutic strategies against liver cancer.
MOLECULAR MECHANISMS OF HYPOXIA IN HCC
Hypoxia is a defining feature of the tumor microenvironment in HCC, triggering a series of molecular events that promote cell survival, proliferation, and angiogenesis. In the HCC tumor microenvironment, the rapid growth of tumor tissue outpaces the oxygen supply by the vasculature, leading to hypoxia. Hypoxia induces various adaptive responses in tumor cells, especially through HIF-1α-mediated pathways[6,7]. HIF-1α is highly sensitive to oxygen concentration, and its stability increases under low-oxygen conditions, activating the transcription of multiple downstream genes involved in angiogenesis, metabolic adaptation, and tumor survival.
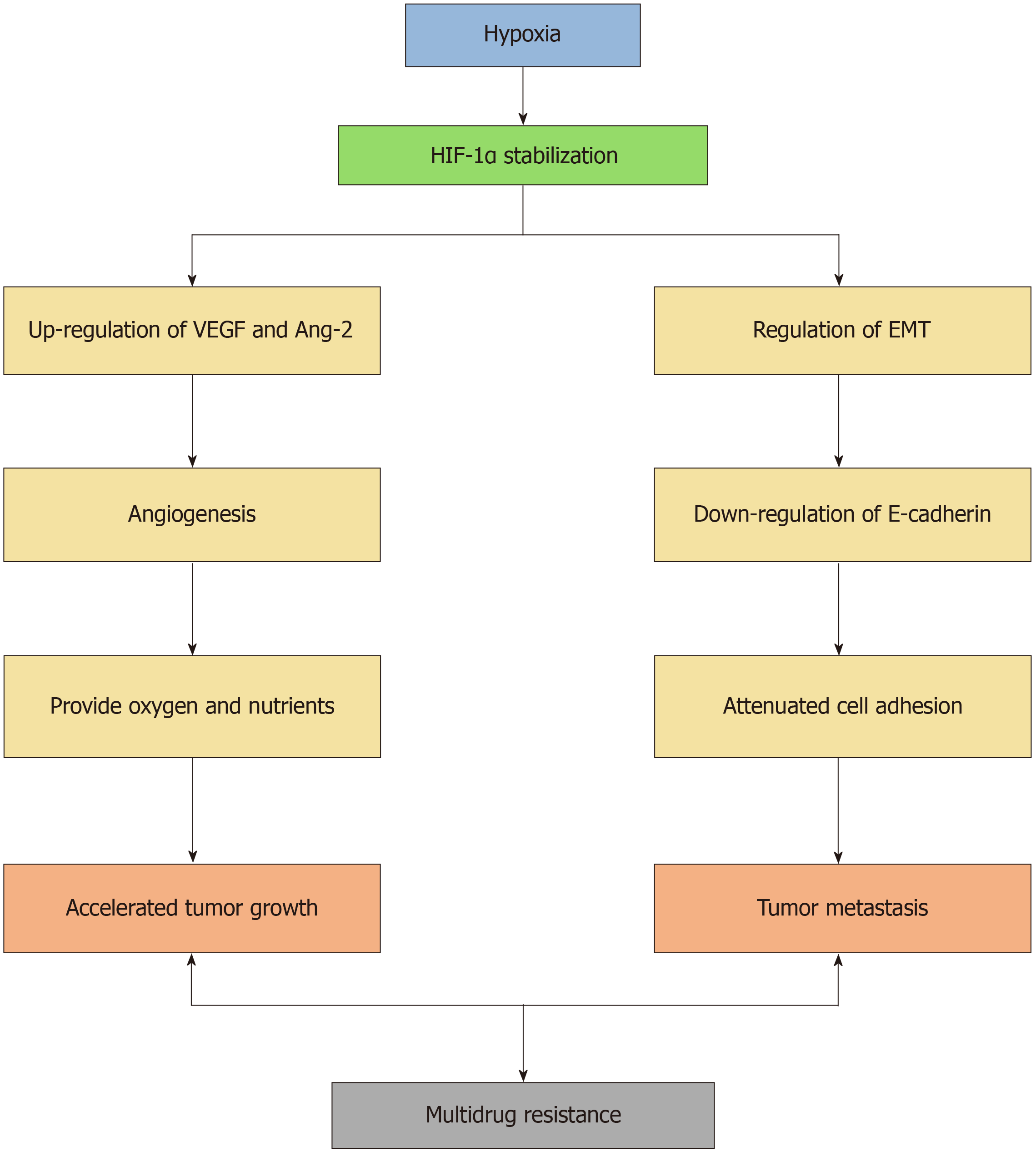
Specifically, HIF-1α upregulates angiogenesis-related factors such as vascular endothelial growth factor (VEGF) and Ang-2, promoting the formation of new blood vessels to supply oxygen and nutrients to cancer cells, thus accelerating tumor progression. Moreover, under hypoxic conditions, HIF-1α regulates the epithelial-mesenchymal transition (EMT) process, enabling tumor cells to acquire enhanced migratory and invasive capabilities. This phenomenon is particularly important in HCC, where metastatic potential is closely associated with prognosis. Studies have shown that HIF-1α promotes HCC metastasis by regulating key transcription factors involved in EMT, such as Twist, Snail, and Vimentin, while downregulating E-cadherin, which weakens cell adhesion (Figure 1)[8,9].
Figure 1 Hypoxia-inducible factor-1α/angiopoietin-2 pathway in hepatocellular carcinoma progression.
HIF-1α: Hypoxia-inducible factor-1α; VEGF: Vascular endothelial growth factor; Ang-2: Angiopoietin-2; EMT: Epithelial-mesenchymal transition.
THE ROLE OF ANG-2 IN HCC PROGRESSION
Ang-2 is closely associated with angiogenesis, primarily functioning to destabilize mature blood vessels under pathological conditions, facilitating new blood vessel formation. Studies have shown that Ang-2 plays a crucial role in the progression of HCC, particularly during the transition from benign to malignant stages of liver disease[10]. In HCC models, Ang-2 expression is significantly correlated with HIF-1α, and higher malignancy levels coincide with increased Ang-2 expression.
Yang et al[11] illustrated this relationship using both animal models and clinical samples, highlighting the connection between Ang-2 and HCC metastasis. Moreover, the synergistic interaction between Ang-2 and VEGF is recognized as a key mechanism driving both angiogenesis and HCC progression. This interaction between Ang-2 and VEGF makes Ang-2 not only a central driver of angiogenesis in HCC but also a promising biomarker for early detection of metastasis. Elevated serum levels of Ang-2 in patients have been correlated with advanced tumor stages and poorer clinical outcomes. Recent studies suggest that inhibiting Ang-2 represents a novel strategy to suppress tumor angiogenesis, particularly when combined with anti-VEGF therapies in multi-target treatment protocols[12]. This approach may offer improved outcomes for HCC patients. Dual inhibition of Ang-2 and VEGF could effectively target multiple pathways involved in tumor vascularization, potentially improving outcomes for patients with aggressive and highly vascularized HCC[13]. Ang-2 has emerged as a therapeutic target for a variety of diseases. For example, anti-Ang-2 antibodies have shown efficacy in diabetic macular edema, and combined use of anti-Ang-2, anti-VEGF, and immune checkpoint inhibitors (ICIs) has proven beneficial in unresectable HCC cases[14].
Beyond serving as a biomarker for tumor progression, Ang-2 levels could also guide therapeutic decisions. Patients with elevated Ang-2 levels might benefit from more aggressive treatment strategies, such as anti-angiogenic therapies targeting the HIF-1α/Ang-2 axis[15]. Targeting Ang-2 with specific inhibitors has demonstrated efficacy in reducing tumor vascularization, thereby suppressing tumor growth and metastasis. This strategy shows particular promise when combined with other therapies, such as immune ICIs, which reprogram the tumor microenvironment to enhance the body’s anti-tumor immune response[16]. Recently, peptidomes like L1-10 have been shown to inhibit Ang-2/Tie-2 interactions, enhancing vascular morphology, mitigating endothelial dysfunction, and improving liver fibrosis. Ang-2 inhibition in a nonalcoholic steatohepatitis model of diabetes-related fibrosis also reversed nonalcoholic steatohepatitis and prevented HCC development. These findings suggest that targeting the HIF-1α/Ang-2 axis could significantly improve outcomes for HCC patients and reduce liver injury[17].
REGULATION OF ANG-2 BY HIF-1Α
HIF-1α is a heterodimer composed of oxygen-regulated α and β subunits. Under normoxic conditions, HIF-1α is rapidly degraded via the ubiquitin-proteasome pathway. However, under hypoxic conditions, HIF-1α stabilizes and translocates to the nucleus, where it binds to hypoxia response elements in the promoters of target genes, including Ang-2. Yang et al[11] provided strong evidence for the direct regulation of Ang-2 transcription in HCC cells by HIF-1α. Inhibition of HIF-1α through specific microRNAs significantly downregulated Ang-2 expression, along with reduced cell proliferation, invasion, and migration. Moreover, HIF-1α silencing also affected the EMT process, which is crucial for HCC cell metastasis. These findings highlight the central role of the HIF-1α/Ang-2 axis in HCC progression, suggesting that targeting this pathway could be an effective therapeutic strategy. Recent studies further emphasize the potential of HIF-1α and Ang-2 in HCC therapy. For instance, HIF-1α not only regulates angiogenesis but also influences multiple key biological processes, including tumor stem cell maintenance, immune evasion, and lipid metabolism[18]. These diverse functions position HIF-1α as a promising therapeutic target for HCC, and combination therapies targeting this molecule are currently under early clinical evaluation.
THERAPEUTIC POTENTIAL OF TARGETING HIF-1Α AND ANG-2 IN HCC
In patients with advanced HCC, current treatment options such as sorafenib and multi-target Tie-2 inhibitors show limited efficacy due to the hypoxic tumor microenvironment and associated MDR. The upregulation of angiogenic factors like Ang-2 and VEGF under hypoxic conditions is a major cause of treatment resistance[19,20]. Ang-2 functions as a chemoattractant by recruiting pro-angiogenic myeloid cells, particularly Tie-2-expressing macrophages, which further enhance angiogenesis and immunosuppression in the tumor microenvironment. This mechanism may explain resistance to antiangiogenic therapy and poor survival in HCC patients with elevated Ang-2 expression[21].
Targeting HIF-1α and its downstream effectors, including Ang-2, provides a novel alternative therapeutic strategy. Inhibiting HIF-1α not only downregulates Ang-2 but also impedes EMT, a critical process in tumor invasion and metastasis. Furthermore, combining HIF-1α inhibitors with conventional anti-cancer therapies may enhance therapeutic efficacy by overcoming MDR and reducing tumor angiogenesis. For example, pairing HIF-1α inhibitors with anti-angiogenic agents like bevacizumab provides a synergistic effect by targeting multiple angiogenic pathways[22].
More effective angiogenesis-targeted therapies are under investigation. Among these, the Ang-2/Tie-2 axis has emerged as a viable alternative to VEGF-based therapies. Both Ang-1 and Ang-2 are key regulators of angiogenesis, with Ang-2 showing particular potential in tumor treatment. Multiple clinical trials are currently evaluating the dual inhibition of VEGF and Ang-2. Antibodies such as trebananib, CVX 060, and nesvacumab have shown promise in inhibiting Ang-2, although resistance and efficacy issues, particularly related to tumor endothelial cells, remain challenges[23]. Additionally, microRNA-based therapies targeting HIF-1α and Ang-2 in HCC cells have demonstrated significant reductions in tumor growth and metastasis in preclinical studies[24,25]. Combining HIF-1α inhibitors with standard anti-cancer treatments has shown increased efficacy by overcoming MDR and inhibiting angiogenesis.
Recent preclinical and early-stage clinical trials have demonstrated the potential of combining HIF-1α inhibitors with immune ICIs, such as programmed death 1 (PD-1)/programmed death-ligand 1 and cytotoxic-T-lymphocyte-associated antigen 4 blockers, to overcome the immunosuppressive effects of tumor hypoxia. Hypoxia-induced HIF-1α promotes the survival of immunosuppressive cells, including myeloid-derived suppressor cells and tumor-associated macrophages, which inhibit the infiltration and function of cytotoxic T lymphocytes and natural killer cells. By inhibiting HIF-1α, these suppressive effects are mitigated, leading to enhanced cytotoxic T lymphocyte and natural killer cells infiltration, improved immune-mediated tumor rejection, and reduced immune evasion, particularly in aggressive, highly angiogenic liver cancer subtypes[15]. Studies have further shown that combining HIF-1α inhibitors with PD-1 inhibitors significantly enhances tumor rejection in preclinical models by reprogramming the tumor microenvironment to become less immunosuppressive and more responsive to immune-based therapies[13]. Moreover, dual blockade strategies with PD-1/programmed death-ligand 1 and cytotoxic T lymphocyte associated antigen 4 inhibitors activate T cells at multiple stages of the immune response, offering long-term therapeutic benefits by reshaping immune memory and preventing tumor recurrence[15]. These findings suggest that targeting both hypoxia pathways and immune checkpoints may offer a promising approach to improving survival outcomes in patients with advanced HCC.
CONCLUSION
Hypoxia-induced upregulation of Ang-2 plays a critical role in HCC angiogenesis, tumor progression, and metastasis. The study by Yang et al[11] emphasizes the potential of Ang-2 as an early biomarker for hepatocarcinogenesis and suggests that targeting the HIF-1α/Ang-2 axis could provide a novel therapeutic approach for patients with advanced HCC. Future research should focus on further elucidating the molecular mechanisms underlying the HIF-1α/Ang-2 interaction and evaluating the feasibility of combining HIF-1α or Ang-2 inhibitors with existing treatments to improve patient outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade C, Grade C
Novelty: Grade B, Grade B, Grade B
Creativity or Innovation: Grade B, Grade B, Grade B
Scientific Significance: Grade B, Grade B, Grade B
P-Reviewer: Tchilikidi KY; Wu ZH; Yaqub M S-Editor: Bai Y L-Editor: A P-Editor: Xu ZH