Published online Nov 27, 2024. doi: 10.4254/wjh.v16.i11.1265
Revised: September 9, 2024
Accepted: September 25, 2024
Published online: November 27, 2024
Processing time: 139 Days and 18.4 Hours
Spontaneous bacterial peritonitis (SBP) is one of the most important complications of patients with liver cirrhosis entailing high morbidity and mortality. Making an accurate early diagnosis of this infection is key in the outcome of these patients. The current definition of SBP is based on studies performed more than 40 years ago using a manual technique to count the number of polymorphs in ascitic fluid (AF). There is a lack of data comparing the traditional cell count method with a current automated cell counter. Moreover, current international guidelines do not mention the type of cell count method to be employed and around half of the centers still rely on the traditional manual method.
To compare the accuracy of polymorph count on AF to diagnose SBP between the traditional manual cell count method and a modern automated cell counter against SBP cases fulfilling gold standard criteria: Positive AF culture and signs/symptoms of peritonitis.
Retrospective analysis including two cohorts: Cross-sectional (cohort 1) and case-control (cohort 2), of patients with decompensated cirrhosis and ascites. Both cell count methods were conducted simultaneously. Positive SBP cases had a pathogenic bacteria isolated on AF and signs/symptoms of peritonitis.
A total of 137 cases with 5 positive-SBP, and 85 cases with 33 positive-SBP were included in cohort 1 and 2, respectively. Positive-SBP cases had worse liver function in both cohorts. The automated method showed higher sensitivity than the manual cell count: 80% vs 52%, P = 0.02, in cohort 2. Both methods showed very good specificity (> 95%). The best cutoff using the automated cell counter was polymorph ≥ 0.2 cells × 109/L (equivalent to 200 cells/mm3) in AF as it has the higher sensitivity keeping a good specificity.
The automated cell count method should be preferred over the manual method to diagnose SBP because of its higher sensitivity. SBP definition, using the automated method, as polymorph cell count ≥ 0.2 cells × 109/L in AF would need to be considered in patients admitted with decompensated cirrhosis.
Core Tip: The traditional cutoff recommended by international guidelines (250 polymorphs × 106/L in ascitic fluid) to diagnose spontaneous bacterial peritonitis (SBP) was set when automated cell counters were not available. There are no data comparing the manual and the automated cell count in patients with cirrhosis against gold standard SBP cases and employing the cell analyzers currently available. This study compares both cells count methods against SBP cases fulfilling gold standard criteria and shows that the automated method has better sensitivity: 80% vs 52%, P = 0.02 and has a good specificity (96%). It also shows that the most accurate cutoff to diagnose SBP is 0.2 polymorphs × 109/L. Guidelines should recommend the use of one of the modern automated cell counters instead of the manual method.
- Citation: Acevedo-Haro JG, Mohamed W, Moodley P, Bendall O, Bennett K, Keelty N, Chan S, Waddy S, Hosking J, Thomas W, Tilley R. Sensitivity of diagnosis of spontaneous bacterial peritonitis is higher with the automated cell count method. World J Hepatol 2024; 16(11): 1265-1281
- URL: https://www.wjgnet.com/1948-5182/full/v16/i11/1265.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i11.1265
Infections constitute one of the most important complications in patients with cirrhosis because of their high frequency and severity[1]. Infections can trigger development of acute-on-chronic liver failure (ACLF)[2,3] and increase mortality 4-fold[4]. SBP is a frequent infection in patients with cirrhosis and ascites; it exacerbates the degree of portal hypertension leading to further decompensation. Almost 10% of patients with ACLF have SBP at or during their admission; mortality in these patients remains high, up to 46% at 28 days[3]. In order to standardize the diagnosis of SBP, international experts met in 2000[5] and recommended diagnosing SBP when the polymorph cell count (PMN) ≥ 250 cells per mm3 of ascitic fluid (AF), as this cut-off had the greatest sensitivity according to studies available at the time; of note, those studies were carried out between the 70’s and early 90’s and they all employed the manual cell count method[5]. This definition has been widely accepted and remains the current standard of care[6,7].
The accuracy of the tests employed to diagnose SBP is crucial as it is key to avoid delays in treatment, which could have a devastating impact on the outcome. Delayed paracentesis is associated with double the in-hospital mortality rate[8], and mortality in patients with cirrhosis and septic shock increases 10% every hour of delay in starting antibiotics[9]. At present, four studies have assessed only the correlation between the manual and the automated cell count methods in the diagnosis of SBP[10-13] which showed to be good but did not compare the accuracy of these methods independently against the gold standard which is defined as a combination of clinical and microbiological characteristics[5]. Moreover, the automated cell counters employed on those four studies either no longer exist (ADVIA®; Technicon System H1, Bayer) or have been superseded by new models (Cell-Dyn 3700, Abbott; UF-100®, Sysmex). More recently, two other reports showed the correlation of the cell count between currently available automated cell counters (Sysmex XE-500 and LH750 Beckman Coulter) and the manual method was good, but the methods were again not compared independently against the gold standard and more importantly, there were no clinical data collected, thus the studies included cases with a variety of causes of ascites, not only cirrhosis and portal hypertension[14,15]. In addition, a survey we conducted among members of the British Association for the Study of the Liver in 2022 showed that 7/14 (50%) centers who responded used either only the manual method or a combination of both methods (unpublished). Therefore, there is a need to assess the accuracy of the manual method and a current model of automated cell counter to diagnose SBP in patients with cirrhosis and portal hypertension related (PHT)-ascites, comparing both methods independently against the gold standard SBP diagnosis. This is the aim of this 5-year long retrospective study.
We assessed the accuracy of manual and automated cell count methods on two cohorts of cases with cirrhosis and ascites admitted to University Hospitals Plymouth (UHP) as part of a local audit. All patients with cirrhosis and ascites have AF cell count at admission, and when there is suspicion of infection or undergoing paracentesis[6,7]. All AF samples are sent to Microbiology for culture.
Cohort 1 had a cross-sectional design[16,17] and was composed of all consecutive cases admitted to UHP between December 2017 and February 2019 due to decompensation of cirrhosis. Consecutive cases were identified through AF samples from the Microbiology electronic database. After exclusion criteria were applied, all day-cases having elective large volume paracentesis (LVP) were also excluded.
Cohort 2 had a case-control design[16,17]-specifically, a two-gate design with representative sampling[18]. The cases were identified through AF samples with positive culture, from the Microbiology electronic database, collected from December 2017 to November 2022. After exclusion criteria, those fulfilling positive-SBP criteria were included. The controls were identified on all consecutive AF samples from patients attending for elective LVP between December 2021 and November 2022 from the Day-Case Unit register. After exclusion criteria, those fulfilling strict negative-SBP criteria were included.
The exclusion criteria: Absence of liver cirrhosis, ascites not caused by portal hypertension, presence of permanent ascitic drain, secondary peritonitis, liver transplant, advanced hepatocellular carcinoma (Barcelona Clinic Liver Cancer-C/D), no cell count performed either by manual or automated method, no clinical information available, and fluid sample different from ascites. Repeat samples were excluded as follows: When both cell count methods gave a negative result, only samples taken the same day were excluded; when at least one cell count method gave a positive result, subsequent samples during the same admission were excluded as they would be difficult to interpret.
Positive-SBP criteria: It was the same in both cohorts: (1) Isolation of a pathogenic bacterium on AF; and (2) The presence of at least one clinical sign/symptom of peritonitis, i.e. alteration in gastrointestinal motility (vomiting, diarrhoea, or ileus), rebound tenderness, abdominal pain, temperature ≥ 37.5 °C, or shock[5]. Signs/symptoms of peritonitis not documented on the clinical notes were considered absent. The classification of bacteria according to pathogenicity is listed in Table 1. The culture result was not available when the index tests were performed.
| Bacteria | |
| Pathogenic | |
| Staphylococcus aureus | |
| Enterococcus faecalis | |
| Enterococcus faecium | |
| Escherichia coli | |
| Klebsiella pneumoniae | |
| Pseudomona aeruginosa | |
| Anaerobes | |
| Clostridium innocuum | |
| Streptococcus gallolyticus | |
| Streptococcus agalactie | |
| Indeterminate | |
| Sphingomonas paucimobilis | |
| Acinetobacter sp. | |
| Streptococcus sanguinis | |
| Streptococcus parasanguinis | |
| Streptococcus salivarius | |
| Non-pathogenic | |
| Staphylococcus epidermidis | |
| Staphylococcus capitis | |
| Staphylococcus haemolyticus | |
| Staphylococcus hominis | |
| Staphylococcus saprophyticus | |
| Staphylococcus pasteuri | |
| Staphylococcus pettenkoferi | |
| Staphylococcus warneri | |
| Staphylococcus saccharolyticus | |
| Corynebacterium striatum | |
| Corynebacterium jeikeium | |
| Micrococcus luteus | |
| Dermacoccus nishinomiyaensis | |
| Kocuria rhizophilia |
Negative-SBP criteria: In cohort 1: All remaining cases not fulfilling positive-SBP criteria. In cohort 2: All the following strict criteria had to be fulfilled (to make SBP exclusion as reliable as possible): (1) No admission to hospital within 4 weeks after inclusion- except for another day-case elective paracentesis; (2) Negative AF culture result; (3) No record of any sign/symptom of peritonitis on the medical notes-as described in the definition of positive-SBP; and (4) No antibiotic prescribed -except SBP prophylaxis or rifaximin for HE.
Manual cell count method: It was performed in the Microbiology Department by a technician employing a modified Fuchs-Rosenthal disposable double-sided cell count chamber, C-Chip DHC-F01. The interobserver coefficient of variability is assessed regularly and its average on white cell count was 0.116 at the time of the study. The result is given in cells × 106/L which is equivalent to cells/mm3. The result was positive when AF PMN ≥ 250 cells × 106/L. The result was negative when AF PMN < 250 cells × 106/L. There were no indeterminate results.
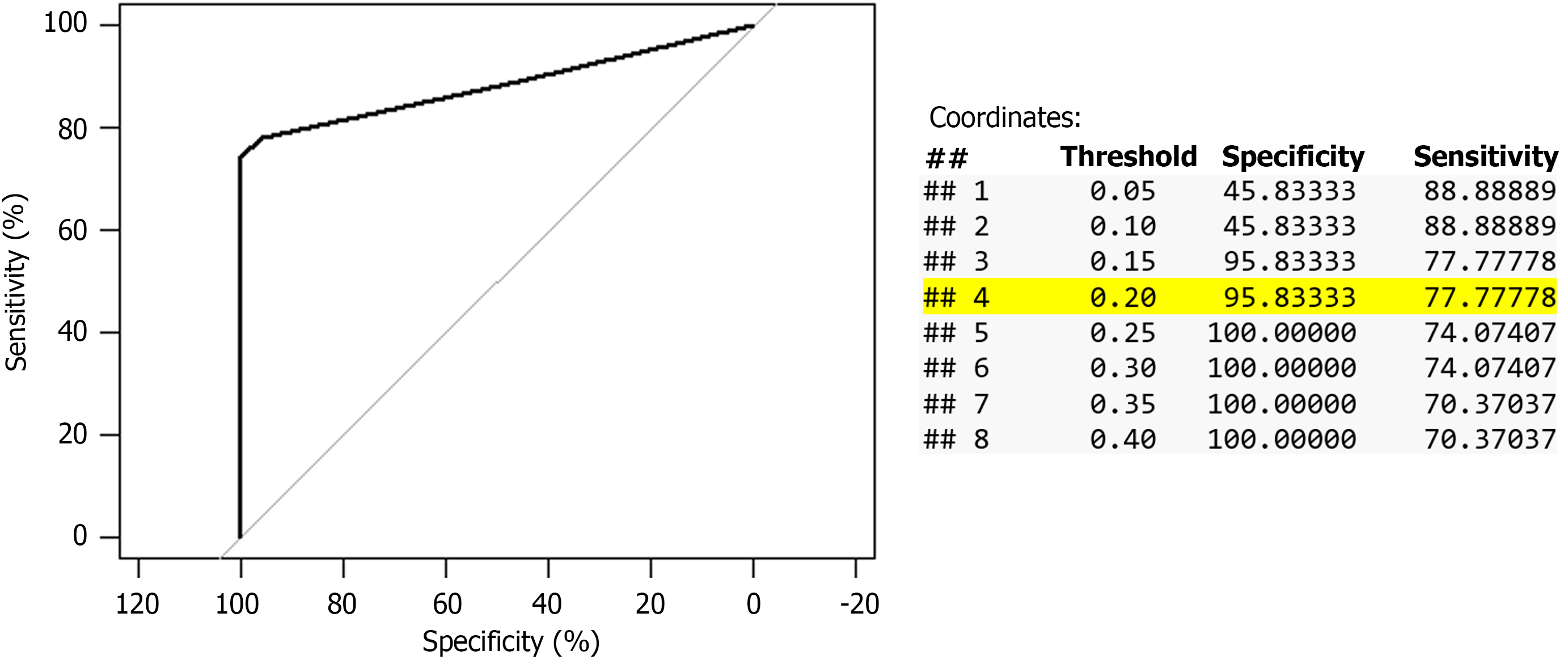
Automated cell count method: It was performed in the Central Laboratory using the Abbott Alinity HQ high-throughput full blood count analyzer, which uses optical scatter and fluorescence for differential cell count. The Alinity cell counter express the results in cells × 109/L. Therefore, 250 cells/mm3 would be equivalent to 0.25 cells × 109/L, however the results are expressed with only one decimal, e.g. 0.1, 0.2, 0.3, etc. To identify the best cutoff to diagnose positive-SBP, receiver operating characteristic (ROC) analysis and 4 × 4 tables showing the accuracy with different cutoffs were obtained; PMN ≥ 0.2 cells × 109/L (equivalent to 200 cells/mm3) showed the best accuracy, Figure 1 and Table 2. The result was negative when the AF PMN ≤ 0.1 cells × 109/L, or when no differential cell count was given and the total white cell count (WCC) ≤ 0.1 cells × 109/L, the latter cases were included in the 4 × 4 tables but not in the ROC analysis. The result was indeterminate when there was no differential cell count and the total WCC ≥ 0.2 cells × 109/L, these cases were included in the analysis as negative result to avoid spectrum bias[16].
| | SBP | Total | |
| Positive | Negative | ||
| Manual method cell count | |||
| Result cutoff ≥ 200 cells × 106 | |||
| Positive | 19 (61) | 0 | 19 |
| Negative | 12 | 50 (100) | 62 |
| Total | 31 | 50 | 81 |
| Result cutoff ≥ 250 cells × 106 | |||
| Positive | 18 (58) | 0 | 18 |
| Negative | 13 | 50 (100) | 63 |
| Total | 31 | 50 | 81 |
| Result cutoff ≥ 300 cells × 106 | |||
| Positive | 18 (58) | 0 | 18 |
| Negative | 13 | 50 (100) | 63 |
| Total | 31 | 50 | 81 |
| Automated method cell count | |||
| Result cutoff ≥ 0.1 cells × 109/L | |||
| Positive | 24 (89) | 26 | 50 |
| Negative | 3 | 25 (49) | 28 |
| Total | 27 | 51 | 78 |
| Result cutoff ≥ 0.2 cells × 109/L | |||
| Positive | 21 (78) | 2 | 50 |
| Negative | 6 | 49 (96) | 28 |
| Total | 27 | 51 | 78 |
| Result cutoff ≥ 0.3 cells × 109/L | |||
| Positive | 20 (74) | 0 | 20 |
| Negative | 7 | 51 (100) | 58 |
| Total | 27 | 51 | 78 |
| Result cutoff ≥ 0.4 cells × 109/L | |||
| Positive | 19 (70) | 0 | 20 |
| Negative | 8 | 51 (100) | 58 |
| Total | 27 | 51 | 78 |
Variables are summarized as frequencies and percentages, mean ± SD or median and IQR, as appropriate. For comparison of demographic and clinical variables between groups (positive-SBP vs negative-SBP) unpaired Student t tests for continuous variables with parametric distribution, Mann-Whitney’s U test for those with non-parametric distribution, and χ² or Fisher’s exact test for categorical variables was used. In cohort 2, ROC analysis was used to determine optimum cutoffs for the automatic and manual cell count methods. Statistical analyses were performed using IBM SPSS v28.0 and R version 4.2.2. STARD diagrams displayed in Figure 2 and Figure 3.
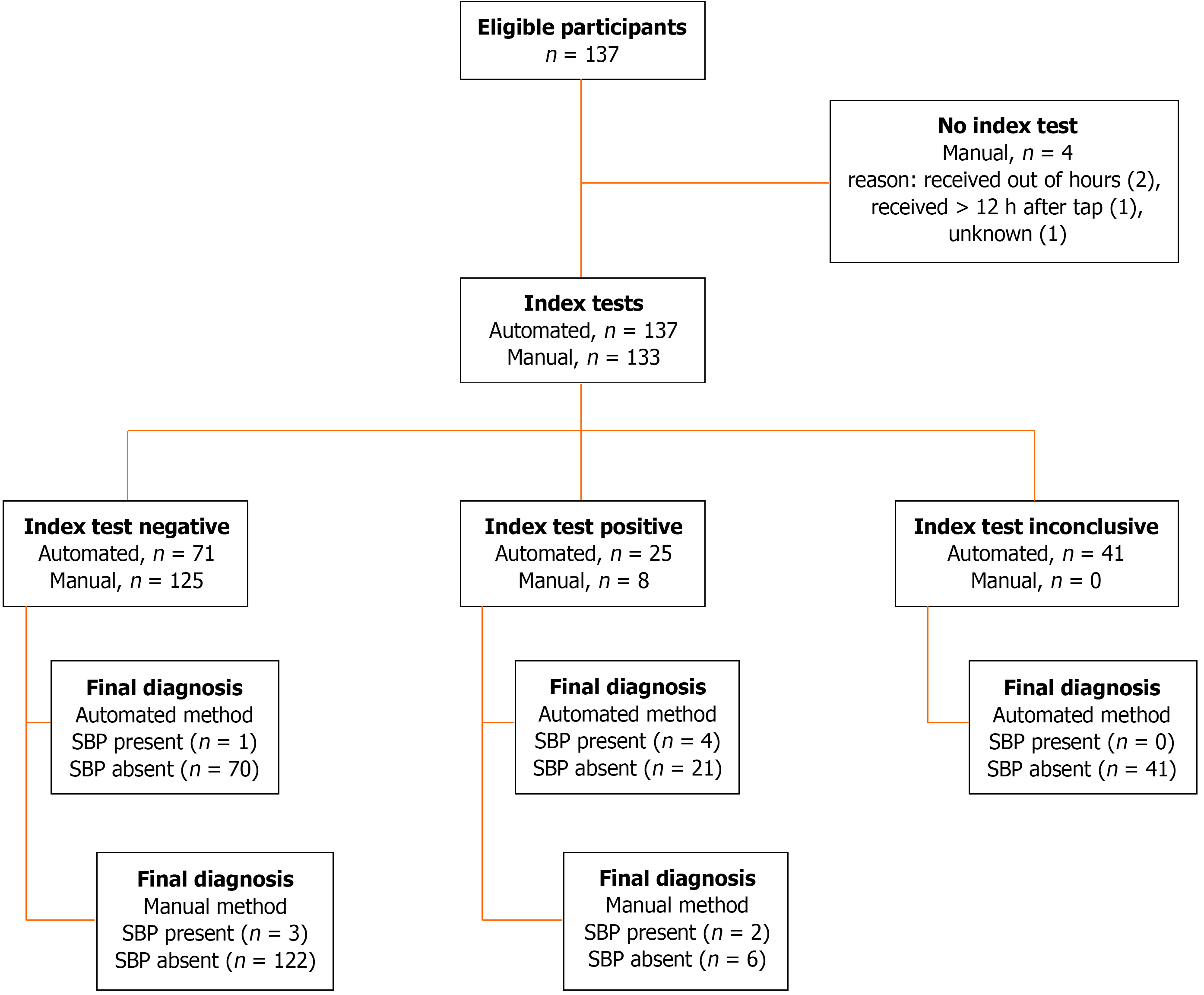
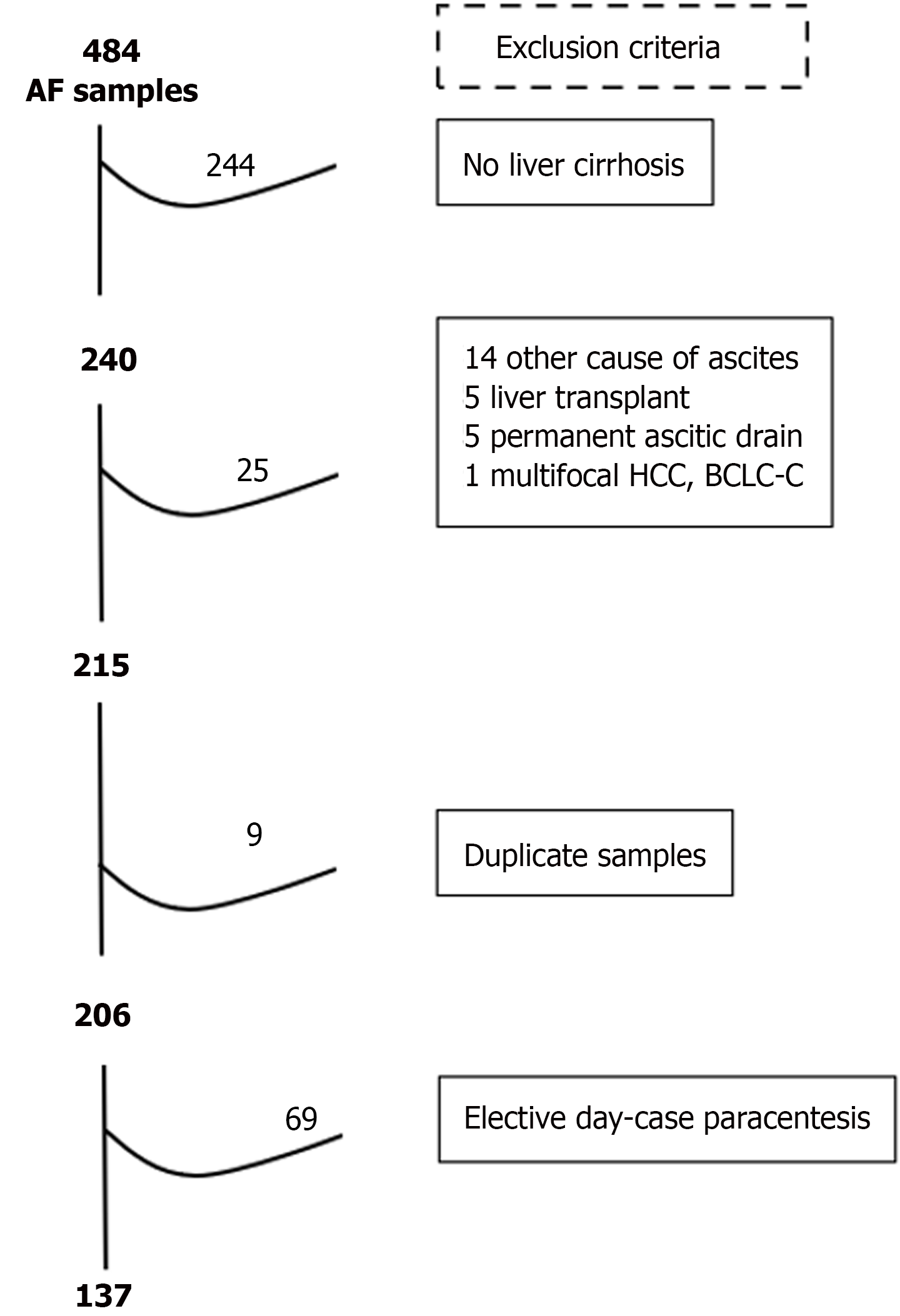
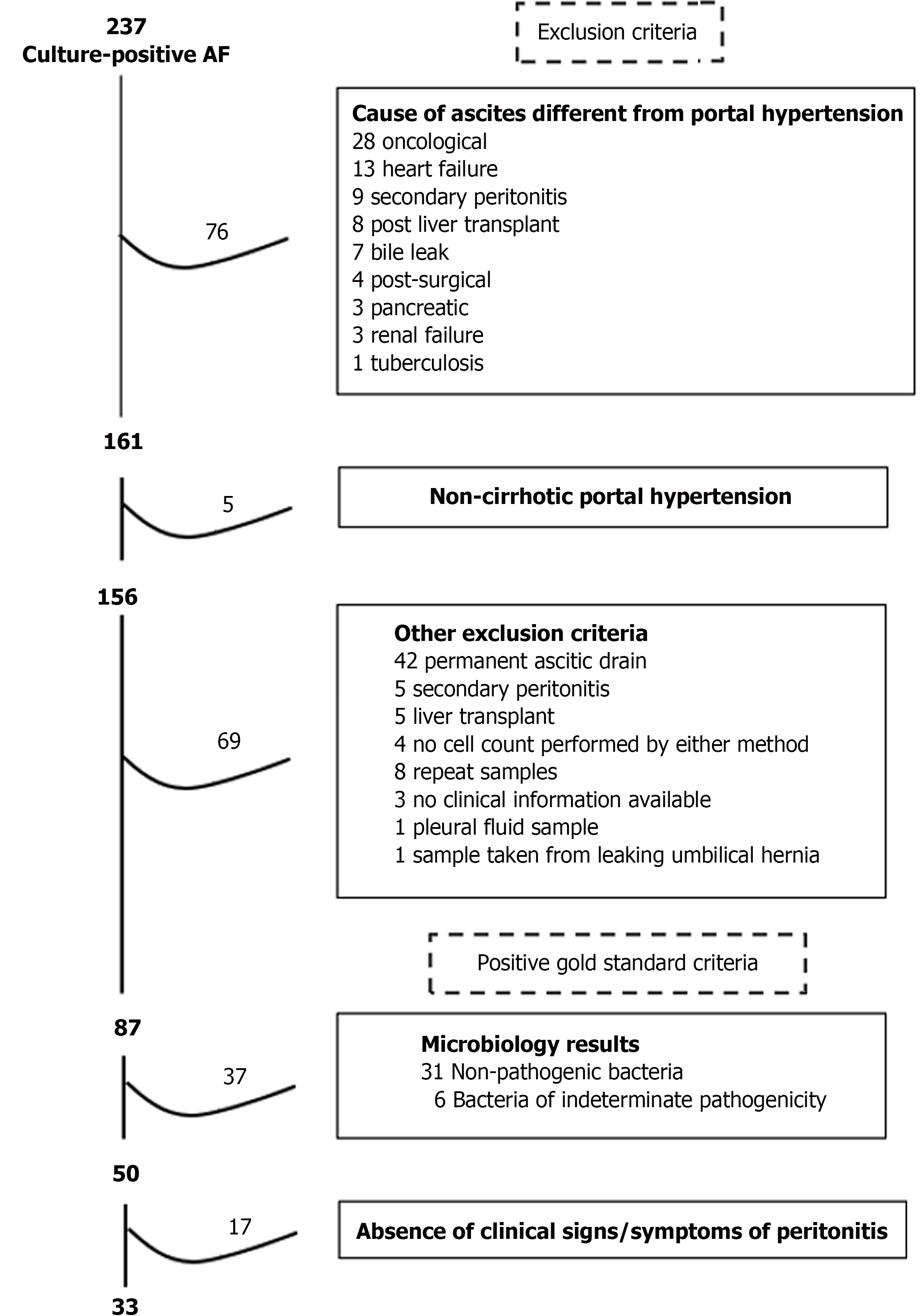
Cohort 1 (cross-sectional) was composed of 137 cases from 74 patients admitted with decompensated cirrhosis: 5 positive-SBP cases in 5 patients, and 132 negative-SBP cases in 69 patients. Initially, 484 cases were identified. After applying the exclusion criteria, 215 cases were selected. The most common cause of exclusion was absence of liver cirrhosis (n = 244) followed by ascites not related to portal hypertension (n = 14). Moreover, 9 duplicate cases and 69 cases who had elective LVP were excluded, leaving 137 cases for analysis, Figure 4. There were only 5 SBP cases in this cohort and thus, the study was expanded to a case-control cohort.
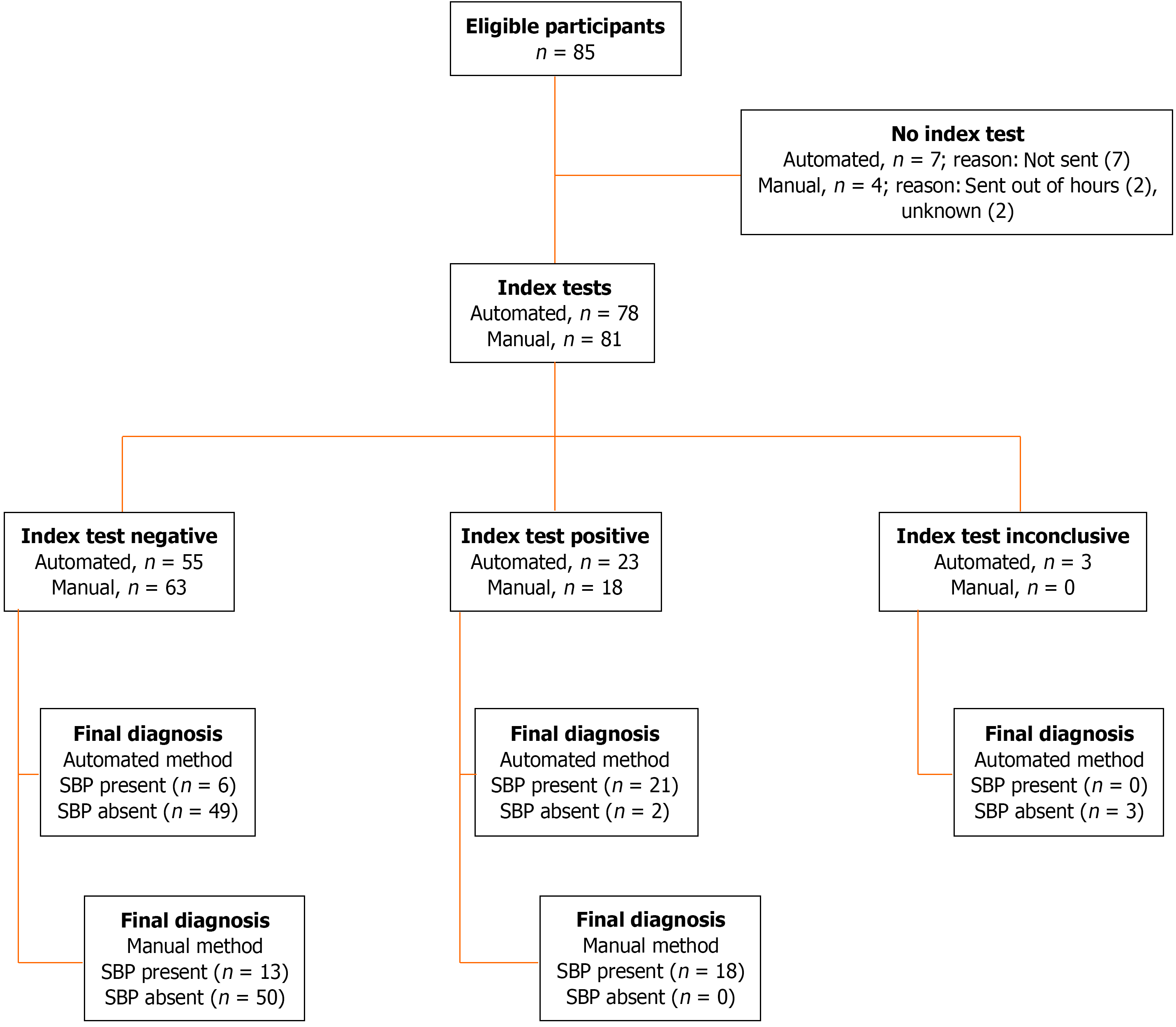
Cohort 2 (case-control) was composed of 85 cases from 46 patients: 33 cases in 32 patients in the positive-SBP group, and 52 cases in 16 patients in the negative-SBP group. Two patients were cases in both groups.
The positive-SBP cases were selected as follows: 237 cases with AF positive culture were identified during the 5-year period. After applying the exclusion criteria, 87 cases were selected. The most common cause of exclusion was the presence of permanent ascitic drain (n = 42) followed by oncological ascites (n = 28). 50/87 cases had a pathogenic bacteria isolated in the AF. Finally, 33/50 cases having at least one clinical sign/symptom of peritonitis were included, Figure 5. Only one excluded case growing an indeterminate bacterium had positive sign/symptom of peritonitis.
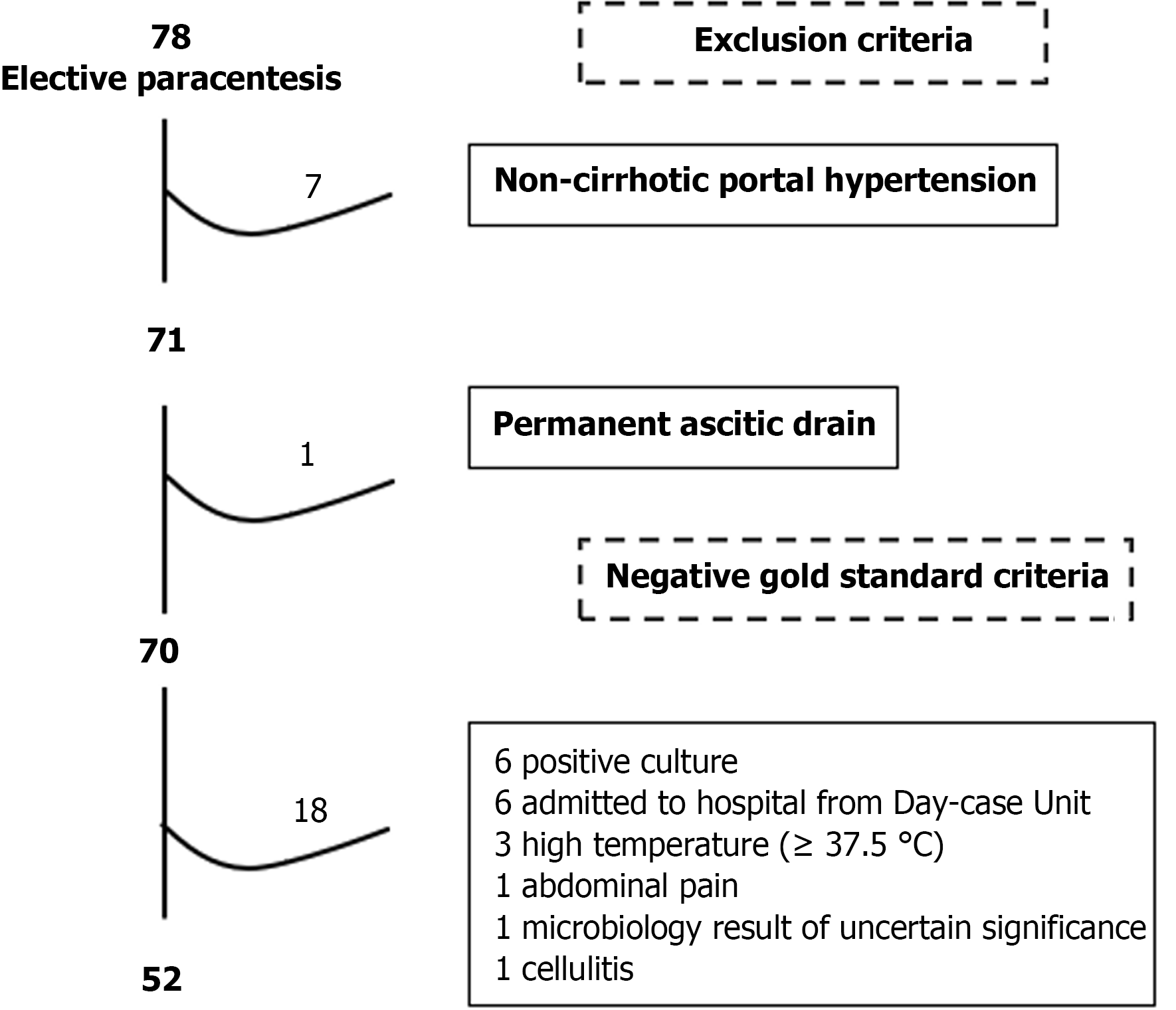
The negative-SBP cases were selected as follows: 78 cases with PHT related ascites attending elective LVP were identified during the 1-year period. After the exclusion criteria were applied, 70 cases were selected. When negative-SBP criteria were applied, 52 cases were finally included (Figure 6).
There was no difference between both groups in relation to sex, age, etiology of cirrhosis, presence of diabetes, use of betablockers, immunosuppression, SBP prophylaxis or rifaximin, main cause of admission or renal function. Cases with SBP had worse liver function: More prolonged international normalized ratio (INR) (2.0 ± 0.5 vs 1.5 ± 0.4, P = 0.01), higher Child-Pugh score (12.0 ± 1.0 vs 10.1 ± 1.7 points, P = 0.02), model for end-stage liver disease (MELD) score (25 ± 7 vs 19 ± 7, P = 0.06), and CLIF-C ACLF score (53.5 ± 7.3 vs 43.3 ± 8.4, P = 0.02), Table 3.
| Negative-SBP (n = 132) | Positive-SBP (n = 5) | P value | |
| Demographic and clinical characteristics | |||
| Male sex | 73 (55) | 3 (60) | 1.0 |
| Age (years) | 62.1 ± 12.9 | 71.8 ± 14.2 | 0.10 |
| Diabetes | 23 (21) | 2 (40) | 0.30 |
| Betablockers | 40 (37) | 3 (60) | 0.61 |
| Immunosuppression | 2 (2) | 1 (20) | 0.13 |
| SBP prophylaxis | 26 (24) | 0 | 0.59 |
| Rifaximin | 6 (6) | 0 | 1.0 |
| Alcohol-related cirrhosis | 94 (71) | 3 (60) | 0.51 |
| Main cause of admission | |||
| Infection | 34 (26) | 2 (40) | 0.35 |
| Ascites | 62 (47) | 2 (40) | |
| Jaundice | 9 (7) | 1 (20) | |
| Encephalopathy | 9 (7) | 0 | |
| UGIB | 11 (8) | 0 | |
| AKI | 2 (1) | 0 | |
| Other1 | 5 (4) | 0 | |
| Admission in ITU | 5 (4) | 0 | 1.0 |
| Shock | 2 (2) | 0 | 1.0 |
| Hospital mortality | 9/85 (11) | 2/5 (40) | 0.11 |
| Liver and renal function | |||
| Bilirubin (µmol/L) | 99 ± 115 | 188 ± 208 | 0.10 |
| Albumin (g/L) | 28 ± 5 | 29 ± 4 | 0.91 |
| INR | 1.5 ± 0.4 | 2.0 ± 0.5 | 0.01 |
| Ascites-Grade 32 | 84 (64) | 2 (40) | 0.36 |
| Encephalopathy | |||
| Grade 0-1 | 91 (69) | 3 (60) | 0.53 |
| Grade 2-3 | 41 (31) | 2 (40) | |
| Creatinine (µmol/L) | 84 ± 47 | 104 ± 56 | 0.36 |
| Sodium (mmol/L) | 132 ± 6 | 133 ± 7 | 0.99 |
| Child-Pugh (score) | 10.1 ± 1.7 | 12.0 ± 1.0 | 0.02 |
| MELD score (points) | 19 ± 7 | 25 ± 7 | 0.06 |
| UKELD score (points) | 58 ± 5 | 61 ± 2 | 0.25 |
| CLIF-C ACLF score (points) | 43.3 ± 8.4 | 53.5 ± 7.3 | 0.02 |
| ACLF grade | |||
| No ACLF | 68 (57) | 3 (60) | 0.66 |
| ACLF grade 1 | 40 (33) | 1 (20) | |
| ACLF grade 2-3 | 12 (10) | 1 (20) | |
Inflammation markers were similar between both groups: C-reactive protein (CRP) levels (59 ± 26 vs 42 ± 40 mg/L, P = 0.33), serum leukocyte levels (7.5 ± 2.3 vs 10.2 ± 6.6 cells × 10/9L, P = 0.36), and presence of Systemic Inflammatory Response Syndrome (SIRS) criteria (0 vs 31%, P = 0.17). Both cells count methods showed significantly higher ascites polymorph count in positive-SBP cases than negative-SBP cases: 4.76 ± 3.86 vs 0.47 ± 1.89 cells × 109/L, P < 0.001, using the automated method; and 1226 ± 1706 vs 72 ± 259 cells × 106/L, P < 0.001, using the manual method, Table 4.
| Negative-SBP (n = 132) | Positive-SBP (n = 5) | P value | |
| Markers of inflammation | |||
| CRP (mg/L) | 42 ± 40 | 59 ± 26 | 0.33 |
| CRP > 5 mg/L | 106 (89) | 5 (100) | 1.0 |
| Leukocyte count in serum (× 109/L) | 10.2 ± 6.6 | 7.5 ± 2.3 | 0.36 |
| Neutrophil count in serum (× 109/L) | 7.6 ± 5.7 | 5.8 ± 1.9 | 0.50 |
| Temperature (°C) | 37.0 ± 0.7 | 37.5 ± 0.3 | 0.14 |
| Heart rate (beats per minute) | 91 ± 15 | 92 ± 19 | 0.87 |
| Respiratory rate (breaths per minute) | 18 ± 4 | 19 ± 1 | 0.74 |
| Presence of SIRS | 33/108 (31) | 0/5 | 0.17 |
| MAP (mmHg) | 79 ± 16 | 89 ± 10 | 0.19 |
| Characteristics of the ascitic fluid | |||
| Albumin (g/L) | 7.7 ± 4.9 | 8.5 ± 2.1 | 0.74 |
| Auto. polymorph count (cells × 109/L) | 0.47 ± 1.89 | 4.76 ± 3.86 | < 0.001 |
| Manual polymorph count (cells × 106/L) | 72 ± 259 | 1226 ± 1706 | < 0.001 |
The manual method was performed in 133 (97%) cases and gave differential cell count in all cases. Its sensitivity was 40%, it gave a positive result in 2/5 positive-SBP cases. Its specificity was 95%, it gave a negative result in 122/128 negative-SBP cases. Its positive predictive value (PPV) was 25% (2/8), and its negative predictive value (NPV) was 98% (122/125), Table 5.
| | Positive-SBP | Negative-SBP | Total |
| Manual method cell count | |||
| Test result | |||
| Positive | 2 (40) | 6 | 8 |
| Negative | 3 | 122 (95) | 125 |
| Total | 5 | 128 | 133 |
| Automated method cell count | |||
| Test result | |||
| Positive | 4 (80) | 21 | 25 |
| Negative | 1 | 111 (84) | 112 |
| Total | 5 | 132 | 137 |
The automated method was performed in all 137 (100%) cases, but gave the differential cell count on only 75 (55%) samples; 21 cases without differential cell count had total WCC ≤ 0.1 cells × 109/L and thus gave a negative result. Therefore, indeterminate results were obtained in 41 (30%) cases, which were considered negative for the analysis, Figure 4. Its sensitivity was 80%, it gave a positive result in 4/5 positive-SBP cases. Its specificity was 84%, it gave a negative result in 111/132 negative-SBP cases. Its PPV was 16% (4/25), and its NPV was 99% (111/112), Table 5.
Statistical comparison between the manual and automated cell count methods could not be performed due to the scarcity of positive-SBP cases.
There was no difference between both groups in relation to sex, age, etiology of cirrhosis, presence of diabetes and use of betablockers or immunosuppression. However, positive-SBP cases used rifaximin more frequently (25% vs 2%, P = 0.002) and SBP prophylaxis less frequently (16% vs 46%, P = 0.005) than negative-SBP cases. The main cause of admission in the positive-SBP cases was infection (52%) followed by ascites (21%) and jaundice (15%), while it was elective paracentesis in all negative-SBP cases. Positive-SBP cases had worse liver function: Higher bilirubin levels (158 ± 164 vs 42 ± 27 μmol/L, P < 0.001), more prolonged INR (1.8 ± 0.7 vs 1.3 ± 0.2, P = 0.005), clinical encephalopathy (48% vs 0%, P < 0.001) and higher Child-Pugh score (11.3 ± 1.9 vs 9.3 ± 1.3 points, P < 0.001); worse renal function: Higher creatinine levels (140 ± 112 vs 69 ± 18 μmol/L, P < 0.001) and MELD score (21.9 ± 10.1 vs 12.2 ± 4.0 points, P < 0.001); and were more critically ill: Higher rate of ACLF grade 2-3 (29% vs 0%, P < 0.001), lower mean arterial pressure (74 ± 17 vs 84 ± 12 mmHg, P = 0.01), shock (22% vs 0%, P < 0.001), admission to ITU (21% vs 0%, P < 0.001), and in-hospital mortality (42% vs 0%, P < 0.001) compared to negative-SBP cases. The cause of death in the positive-SBP cases was ACLF in 9/14 and septic shock in 5/14 cases. More negative-SBP cases had ascites grade-3 because they all attended for LVP (94% vs 61%, P < 0.001), Table 6.
| | Negative-SBP (n = 52) | Positive-SBP (n = 33) | P value |
| Demographics and clinical characteristics | |||
| Male sex | 21 (40) | 17 (52) | 0.37 |
| Age (years) | 55.0 ± 13.5 | 60.4 ± 14.8 | 0.08 |
| Alcohol-related cirrhosis | 45 (87) | 29 (88) | 0.16 |
| Diabetes | 6 (12) | 7 (22) | 0.23 |
| Betablockers | 16 (31) | 13 (41) | 0.48 |
| Immunosuppression | 0 | 1 (3) | 0.38 |
| SBP prophylaxis | 24 (46) | 5 (16) | 0.005 |
| Rifaximin | 1 (2) | 8 (25) | 0.002 |
| Main cause of admission | |||
| Elective LVP | 52 (100) | ||
| Infection | 17 (52) | ||
| Ascites | 7 (21) | ||
| Jaundice | 5 (15) | ||
| Encephalopathy | 1 (3) | ||
| UGIB | 1 (3) | ||
| AKI | 2 (6) | ||
| Admission in ITU | 0 | 7 (21) | < 0.001 |
| Shock | 0 | 7 (22) | < 0.001 |
| Hospital mortality | 0/52 | 14/33 (42) | < 0.001 |
| Liver and renal function | |||
| Bilirubin (µmol/L) | 42 ± 27 | 158 ± 164 | < 0.001 |
| Albumin (g/L) | 30 ± 4 | 29 ± 5 | 0.23 |
| INR | 1.3 ± 0.2 | 1.8 ± 0.7 | < 0.001 |
| Ascites-Grade 31 | 49 (94) | 20 (61) | < 0.001 |
| Encephalopathy | |||
| Grade 0-1 | 52 (100) | 17 (52) | < 0.001 |
| Grade 2-3 | 0 | 16 (48%) | |
Positive-SBP cases showed higher degree of inflammation: Higher CRP levels (57 ± 47 vs 22 ± 17 mg/L, P < 0.001), serum leukocyte count (11.1 ± 5.1 vs 7.3 ± 2.1 cells × 10/9L, P < 0.001) and higher prevalence of SIRS (50% vs 13%, P = 0.004) than negative-SBP cases. Both cells count methods showed higher AF polymorph count in positive-SBP cases than negative-SBP cases: 3.27 ± 3.68 vs 0.06 ± 0.06 cells × 109/L, P < 0.001 on the automated method; and 2157 ± 4105 vs 20 ± 22 cells × 106/L, P < 0.001, on the manual method, Table 7.
| Negative-SBP (n = 52) | Positive-SBP (n = 33) | P value | |
| Markers of inflammation | |||
| CRP (mg/L) | 22 ± 17 | 57 ± 47 | < 0.001 |
| CRP > 5 mg/L | 24 (89) | 30 (91) | 1.00 |
| Leukocyte count in serum (× 109/L) | 7.3 ± 2.1 | 11.1 ± 5.1 | < 0.001 |
| Neutrophil count in serum (× 109/L) | 5.0 ± 1.8 | 8.6 ± 4.4 | < 0.001 |
| Temperature (°C) | 36.9 ± 0.3 | 37.1 ± 1.0 | 0.22 |
| Heart rate (beats per minute) | 102 ± 16 | 104 ± 20 | 0.75 |
| Respiratory rate (breaths per minute) | 18 ± 3 | 21 ± 5 | 0.02 |
| Presence of SIRS | 4 (13) | 14 (50) | 0.004 |
| MAP (mmHg) | 84 ± 12 | 74 ± 17 | 0.01 |
| Characteristics of the ascitic fluid | |||
| Albumin (g/L) | 8.0 ± 4.1 | 7.5 ± 5.8 | 0.61 |
| Automated polymorph count (cells × 109/L) | 0.06 ± 0.06 | 3.27 ± 3.68 | < 0.001 |
| Manual polymorph count (cells × 106/L) | 20 ± 22 | 2157 ± 4105 | < 0.001 |
The manual method was performed in 81/85 (95%) samples and gave differential cell count in all of them. The PMN count ranged from 0 to 21280 cells × 106/L. Its sensitivity was 58%, it gave positive result in 18/31 positive-SBP cases. Its specificity was 100%, it gave negative result in all 50 negative-SBP cases. Its PPV was 100% (18/18), and its NPV was 79% (50/63), Table 8.
| | Positive-SNP | Negative-SNP | Total |
| Manual method cell count | |||
| Test result | |||
| Positive | 18 (58) | 0 | 18 |
| Negative | 13 | 50 (100) | 63 |
| Total | 31 | 50 | 81 |
| Automated method cell count | |||
| Test result | |||
| Positive | 21 (78) | 2 | 23 |
| Negative | 6 | 49 (96) | 55 |
| Total | 27 | 51 | 78 |
The automated method was performed in 78/85 (92%) samples. It gave the differential cell count on 75 (88%) samples. Three (4%) samples were indeterminate and considered negative result for the analysis. The PMN count ranged from 0.0 to 11.5 cells x109. Its sensitivity was 78%, it gave a positive result in 21/27 positive-SBP cases. Its specificity was 96%, it gave a negative result in 49/55 negative-SBP cases. Its PPV was 91% (21/23), and its NPV was 89% (49/55), Table 9.
| | Automated method | Total | |
| Positive | Negative | ||
| Positive-SBP cases | |||
| Manual method | |||
| Positive | 13 | 0 | 13 (52) |
| Negative | 7 | 5 | 12 |
| Total | 20 (80) | 5 | 25 |
| Negative-SBP cases | |||
| Manual method | |||
| Positive | 0 | 0 | 0 |
| Negative | 2 | 47 | 49 (100) |
| Total | 0 | 47 (96) | 49 |
To compare the sensitivity and specificity of the manual and automated cell count methods we analyzed only paired samples: 74/85 cases having both methods performed. The sensitivity of the automated method was superior to the manual method, 80% (20/25) vs 52% (13/25), P = 0.02. The specificity was 100% (49/49) and 96% (47/49) in the manual and automated method, respectively, Table 9.
To address the potential bias that the automated method was more sensitive because it used a lower cutoff, we compared it with the manual method using the same cutoff ≥ 200 PMN × 106/L. The sensitivity of the automated method was still superior to the manual method, 80% (20/25) vs 56% (14/25), P = 0.03. The specificity remained the same, Table 10.
| Automated method | Total | |||||
| Positive | Negative | |||||
| Positive-SBP cases | ||||||
| Manual method | ||||||
| Positive | 14 | 0 | 14 (56) | |||
| Negative | 6 | 5 | 11 | |||
| Total | 20 (80) | 5 | 25 | |||
| Negative-SBP cases | ||||||
| Manual method | ||||||
| Positive | 0 | 0 | 0 | |||
| Negative | 2 | 47 | 49 (100) | |||
| Total | 2 | 47 (96) | 49 | |||
There were 33 positive-SBP cases in total. The 5 cases in cohort 1 are included in cohort 2. The most common origin was community-acquired, present in 18 (55%), followed by healthcare-associated in 11 (33%) and nosocomial in 4 (12%) cases. Blood or urine cultures were taken in 22 (67%) cases, 8 of them (24%) showed the same bacterium isolated in the AF culture. The most frequent bacterium isolated was Escherichia coli in 18 (55%) cases, followed by Enterococcus faecium in 8 (24%). With regards to empirical antibiotic therapy (EAT), the most frequently employed was piperacillin/tazobactam in 15 (46%) cases, followed by meropenem in 6 (18%), Table 11. EAT was started before the ascitic tap in 16 cases, and after the ascitic tap in 15 cases, the time to administration was not available in 2 cases. In the latter 15 cases, the EAT was started between 0.8 and 48.6 hours after the ascitic tap was performed. There was a tendency to undergo increased delay in EAT when the ascitic tap gave a (false) negative result: 26.2 ± 19.7 vs 12.5 ± 12.8 hours, P = 0.16 in the cases with negative (n = 3) and with positive result (n = 11), respectively, using the automated method; and 27.5 ± 12.2 vs 11.1 ± 14.0 hours, P = 0.055 in the cases with negative (n = 5) and with positive result (n = 8), respectively, using the manual method.
| Positive-SBP (n = 33) | |
| Origin of infection | |
| Community-acquired | 18 (55) |
| Healthcare-associated | 11 (33) |
| Nosocomial | 4 (12) |
| Cultures | |
| Blood or urine culture taken | 22 (67) |
| Blood or urine culture, same bacteria | 8 (24) |
| Bacteria isolated | |
| E. coli | 18 (55) |
| Enterococcus faecium | 8 (24) |
| Enterococcus faecalis | 3 (9) |
| Klebsiella pneumoniae | 1 (3) |
| S aureus | 1 (3) |
| Streptococcus agalactie | 1 (3) |
| Clostridium innocuum | 1 (3) |
| Empirical antibiotic used | |
| Piperacillin/tazobactam | 15 (46) |
| meropenem | 6 (18) |
| Levofloxacin | 4 (12) |
| Co-amoxiclav | 4 (12) |
| Teicoplanin | 2 (6) |
| Tigecycline | 1 (3) |
| Flucoxacillin | 1 (3) |
This is the first study comparing the diagnostic accuracy of both types of cells count methods currently employed to diagnose SBP in patients with cirrhosis and ascites: The traditional manual method and a contemporary automated method, against SBP cases defined by gold standard criteria. It shows that the automated method is more sensitive than the manual method, which is clinically very important as increased sensitivity will reduce the number of missed SBP cases and delays in treatment, as suggested by the delay in starting antibiotics seen in cases with false negative cell count results which more than doubles the delay in cases with positive result using the manual method. This difference was less evident with the automated method because running both methods at the same time may have led doctors to prefer the result of the manual over the automated method because it looks falsely more accurate as it is expressed with more decimal figures and in similar units as the traditional definition of SBP. The main limitations of this study are its retrospective design and the small number of positive cases in the cross-sectional study (cohort 1). Thus, the number of positive-SBP cases was only 5 cases (4%) due to the strict criteria used to diagnose SBP and thus, no statistical comparison was possible. In this sense, studies have reported low rates of positive culture in SBP cohorts, around 6%[19] and even in septic shock it is only 36%[20]. We compensated this limitation by subsequently conducting a case-control study (cohort 2) which confirmed the difference in sensitivity was statistically significant.
The sensitivity is assessed in the positive-SBP cases which, in our study, were clinically more unwell, 25% were on rifaximin due to previous hepatic encephalopathy, they showed higher prevalence of septic shock (22%) and hospital mortality (42%), compared to other studies reporting shock in 8%, and 30-day mortality in 26% of SBP cases[19,21]. This difference is explained because all other studies defined SBP according to the cell count result and not to the gold standard criteria and thus, most SBP cases included were culture negative. Moreover, positive AF and blood cultures have been reported as independent predictors of mortality[19,21]. Along these lines, all SBP cases in our cohort grew a pathogenic bacterium on their AF which was also isolated in either blood or urine cultures in 24% of cases. Therefore, our SBP cases had higher bacterial burden and bloodstream circulation than other cohorts. This can also account for the worse liver function positive-SBP cases had, even when compared to cases admitted with other decompensations, including other types of infections.
The drawback of using the gold standard SBP criteria is that the sensitivity of the cell count methods (index tests) would be higher than when applied to cohorts including the whole spectrum of the disease, i.e. culture negative SBP[16]. However, not using the gold standard SBP criteria would only allow assessment of the correlation between both cell count methods, but no significant comparison of their accuracy could be possible. Furthermore, a “surrogate” gold standard would be needed which would include the cell count result as part of its definition and thus, the sensitivity would be falsely increased due to disease verification bias[17]. Hence, SBP gold standard criteria were required despite the potential bias of selecting the most unwell cases. It can also be argued that this subgroup (culture-positive SBP) is the most in need of diagnostic tests with increased sensitivity.
The specificity is assessed in the negative cases and it was very good in both cohorts, although slightly lower in cohort 1, probably because the negative-SBP cases were inpatients with decompensated cirrhosis and ascites who did not fulfil SBP gold standard criteria and thus, they comprised some actual SBP cases such as those with negative culture, asymptomatic (around 14% of SBP are asymptomatic[22]), or when data on signs/symptoms of peritonitis was missing on clinical records. The specificity was higher in cohort 2, likely because negative-SBP cases were strictly selected.
Other disadvantages of the manual cell count method, in addition to its lower sensitivity, are its wide interobserver variability, it is labour intensive/time consuming, could be challenging to get out of hours (contacting technician on-call) and it is more expensive: £7.50 vs £1.04 per sample. Moreover, the automated cell counter has the advantage that it is already in place in the laboratory to process blood samples and we used the same mode and working channel to process the AF samples even though the automated analyzer has a specific channel for body fluids, however it could not be employed because there is no External Quality Assessment (EQA) scheme for body fluids in the United Kingdom currently.
Before using the automated cell counter, we ran our own internal quality assessment with repeat testing using the standard blood channel and blood mode. AF generally contains low white cell burden; the lower the cell count the greater the imprecision (deviation index) of the test becomes. We know from full blood count EQA returns that for low white cell count, the error of the mean is reported to one decimal place which means the test cannot be more precise than down to one decimal place. Based on analysis of different cutoffs points, we propose a new definition of SBP based on the results of the automated cell counter, i.e., PMN ≥ 0.2 cells × 109/L instead of the traditional definition based on the manual cell count method as it has the best sensitivity (80%) keeping a very good specificity (96%).
The automated cell counter performance giving differential cell count was good in cohort 2, composed by culture-positive cases, leaving only 2/79 (3%) samples with indeterminate result. However, when employed in cohort 1, composed by cases with wider spectrum of disease, 22 (16%) cases had indeterminate results, probably because there was not a clear interface between the cell populations due to the presence of debris or apoptozed cells that the analyzer may struggle to identify. This problem may be reduced by employing a body fluid mode which may provide the best fluid/reagent characteristic, thus it would merit further investigation.
Finally, it is important not only to employ an automated cell counter, but a recent model should be selected because the accuracy varies. In a previous audit[23], our group assessed the Sysmex XE2100 model automated cell counter on a cross-sectional cohort of patients with cirrhosis and ascites including 224 cases admitted from November 2015 to June 2016. Automated cell count was performed in 196 (88%) cases, but it gave differential cell count in only 5 (3%) samples, which had total white cell count > 1.0 cells × 109/L. That model has been superseded by the XN generation of Sysmex analyzers.
In conclusion, although this is a retrospective study, the data found on the case-control cohort supports the automated cell count method, using one of the latest models available, as the preferred cell count method over the manual method to diagnose SBP because of its higher sensitivity which is key when dealing with this potentially lethal complication in patients with advanced cirrhosis and ascites. Moreover, SBP could be defined as PMN ≥ 0.2 cells × 109/L in AF in patients admitted with decompensated cirrhosis.
to the study according to the local policy for audits which only involves standard of
care treatment and collection of pseudonymized data.
| 1. | Jalan R, Fernández J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R, Albillos A, Lammert F, Wilmer A, Mookerjee R, Vila J, Garcia-Martinez R, Wendon J, Such J, Cordoba J, Sanyal A, Garcia-Tsao G, Arroyo V, Burroughs A, Ginès P. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 644] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 2. | Trebicka J, Fernández J, Papp M, Caraceni P, Laleman W, Gambino C, Giovo I, Uschner FE, Jimenez C, Mookerjee R, Gustot T, Albillos A, Bañares R, Janicko M, Steib C, Reiberger T, Acevedo J, Gatti P, Bernal W, Zeuzem S, Zipprich A, Piano S, Berg T, Bruns T, Bendtsen F, Coenraad M, Merli M, Stauber R, Zoller H, Ramos JP, Solè C, Soriano G, de Gottardi A, Gronbaek H, Saliba F, Trautwein C, Özdogan OC, Francque S, Ryder S, Nahon P, Romero-Gomez M, Van Vlierberghe H, Francoz C, Manns M, Garcia E, Tufoni M, Amoros A, Pavesi M, Sanchez C, Curto A, Pitarch C, Putignano A, Moreno E, Shawcross D, Aguilar F, Clària J, Ponzo P, Jansen C, Vitalis Z, Zaccherini G, Balogh B, Vargas V, Montagnese S, Alessandria C, Bernardi M, Ginès P, Jalan R, Moreau R, Angeli P, Arroyo V; PREDICT STUDY group of the EASL-CLIF Consortium. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73:842-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 345] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 3. | Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, Reverter E, Martínez J, Saliba F, Jalan R, Welzel T, Pavesi M, Hernández-Tejero M, Ginès P, Arroyo V; European Foundation for the Study of Chronic Liver Failure. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67:1870-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 400] [Article Influence: 57.1] [Reference Citation Analysis (2)] |
| 4. | Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246-1256, 1256.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 838] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 5. | Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32:142-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 613] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 6. | European Association for the Study of the Liver. ; European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1822] [Article Influence: 260.3] [Reference Citation Analysis (2)] |
| 7. | Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, Wong F, Kim WR. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 462] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 8. | Kim JJ, Tsukamoto MM, Mathur AK, Ghomri YM, Hou LA, Sheibani S, Runyon BA. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109:1436-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 9. | Arabi YM, Dara SI, Memish Z, Al Abdulkareem A, Tamim HM, Al-Shirawi N, Parrillo JE, Dodek P, Lapinsky S, Feinstein D, Wood G, Dial S, Zanotti S, Kumar A; Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Antimicrobial therapeutic determinants of outcomes from septic shock among patients with cirrhosis. Hepatology. 2012;56:2305-2315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Angeloni S, Nicolini G, Merli M, Nicolao F, Pinto G, Aronne T, Attili AF, Riggio O. Validation of automated blood cell counter for the determination of polymorphonuclear cell count in the ascitic fluid of cirrhotic patients with or without spontaneous bacterial peritonitis. Am J Gastroenterol. 2003;98:1844-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Cereto F, Genescà J, Segura R. Validation of automated blood cell counters for the diagnosis of spontaneous bacterial peritonitis. Am J Gastroenterol. 2004;99:1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Riggio O, Angeloni S, Parente A, Leboffe C, Pinto G, Aronne T, Merli M. Accuracy of the automated cell counters for management of spontaneous bacterial peritonitis. World J Gastroenterol. 2008;14:5689-5694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Rerknimitr R, Limmathurotsakul D, Bhokaisawan N, Kongkam P, Treeprasertsuk S, Kullavanijaya P. A comparison of diagnostic efficacies among different reagent strips and automated cell count in spontaneous bacterial peritonitis. J Gastroenterol Hepatol. 2010;25:946-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Lippi G, Cattabiani C, Benegiamo A, Gennari D, Pavesi F, Caleffi A, Pipitone S. Evaluation of the fully automated hematological analyzer Sysmex XE-5000 for flow cytometric analysis of peritoneal fluid. J Lab Autom. 2013;18:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Fleming C, Brouwer R, van Alphen A, Lindemans J, de Jonge R. UF-1000i: validation of the body fluid mode for counting cells in body fluids. Clin Chem Lab Med. 2014;52:1781-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Hall MK, Kea B, Wang R. Recognising Bias in Studies of Diagnostic Tests Part 1: Patient Selection. Emerg Med J. 2019;36:431-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 17. | Kea B, Hall MK, Wang R. Recognising bias in studies of diagnostic tests part 2: interpreting and verifying the index test. Emerg Med J. 2019;36:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Rutjes AW, Reitsma JB, Vandenbroucke JP, Glas AS, Bossuyt PM. Case-control and two-gate designs in diagnostic accuracy studies. Clin Chem. 2005;51:1335-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 378] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 19. | Sanglodkar U, Jain M, Venkataraman J. Predictors of immediate and short-term mortality in spontaneous bacterial peritonitis. Indian J Gastroenterol. 2020;39:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Meersseman P, Hernández-tejero M, Diaz JM, Wauters J, Hermans G, Aziz F, Ceunen H, Prado Gonzalez VE, Arteaga M, Haro JA, Valdivieso M, Sanchez C, Arroyo V, Wilmer A, Fernandez J. THU-049 Supplemental low-dose hydrocortisone in cirrhotic patients with septic shock: a double-blind, randomised, placebo-controlled, multicentre trial; the SCotCH study. J Hepatol. 2024;80:S157. [DOI] [Full Text] |
| 21. | Ubhi N, Mourad A, Tausan M, Lewis D, Smethurst J, Wenlock R, Gouda M, Bremner S, Verma S. Outcomes after hospitalisation with spontaneous bacterial peritonitis over a 13-year period: a retrospective cohort study. Eur J Gastroenterol Hepatol. 2023;35:384-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Samonakis DN, Gatselis N, Bellou A, Sifaki-Pistolla D, Mela M, Demetriou G, Thalassinos E, Rigopoulou EI, Kevrekidou P, Tziortziotis I, Azariadi K, Kavousanaki M, Digenakis E, Vassiliadis T, Kouroumalis EA, Dalekos GN. Spontaneous bacterial peritonitis: a prospective Greek multicenter study of its epidemiology, microbiology, and outcomes. Ann Gastroenterol. 2022;35:80-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Waddah M, Bendall O, Moodley P, Bennett K, Chan S, Keelty N, Steer J, Siewruk J, Thomas W, Tilley R, Acevedo-Haro JG. P71 Comparison between manual and automated cell count methods in the diagnosis of SBP. Poster presentations. 2023. [DOI] [Full Text] |