Published online Oct 27, 2024. doi: 10.4254/wjh.v16.i10.1177
Revised: August 21, 2024
Accepted: September 6, 2024
Published online: October 27, 2024
Processing time: 156 Days and 18.7 Hours
Liver cirrhosis is the end stage of progressive liver fibrosis as a consequence of chronic liver inflammation, wherein the standard hepatic architecture is replaced by regenerative hepatic nodules, which eventually lead to liver failure. Cirrhosis without any symptoms is referred to as compensated cirrhosis. Complications such as ascites, variceal bleeding, and hepatic encephalopathy indicate the onset of decompensated cirrhosis. Gastroesophageal varices are the hallmark of clini
To determine the accuracy of the platelet count-to-spleen diameter (PC/SD) ratio to evaluate esophageal varices (EV) in patients with cirrhosis.
This retrospective observational study was conducted at Tikur Anbessa Specia
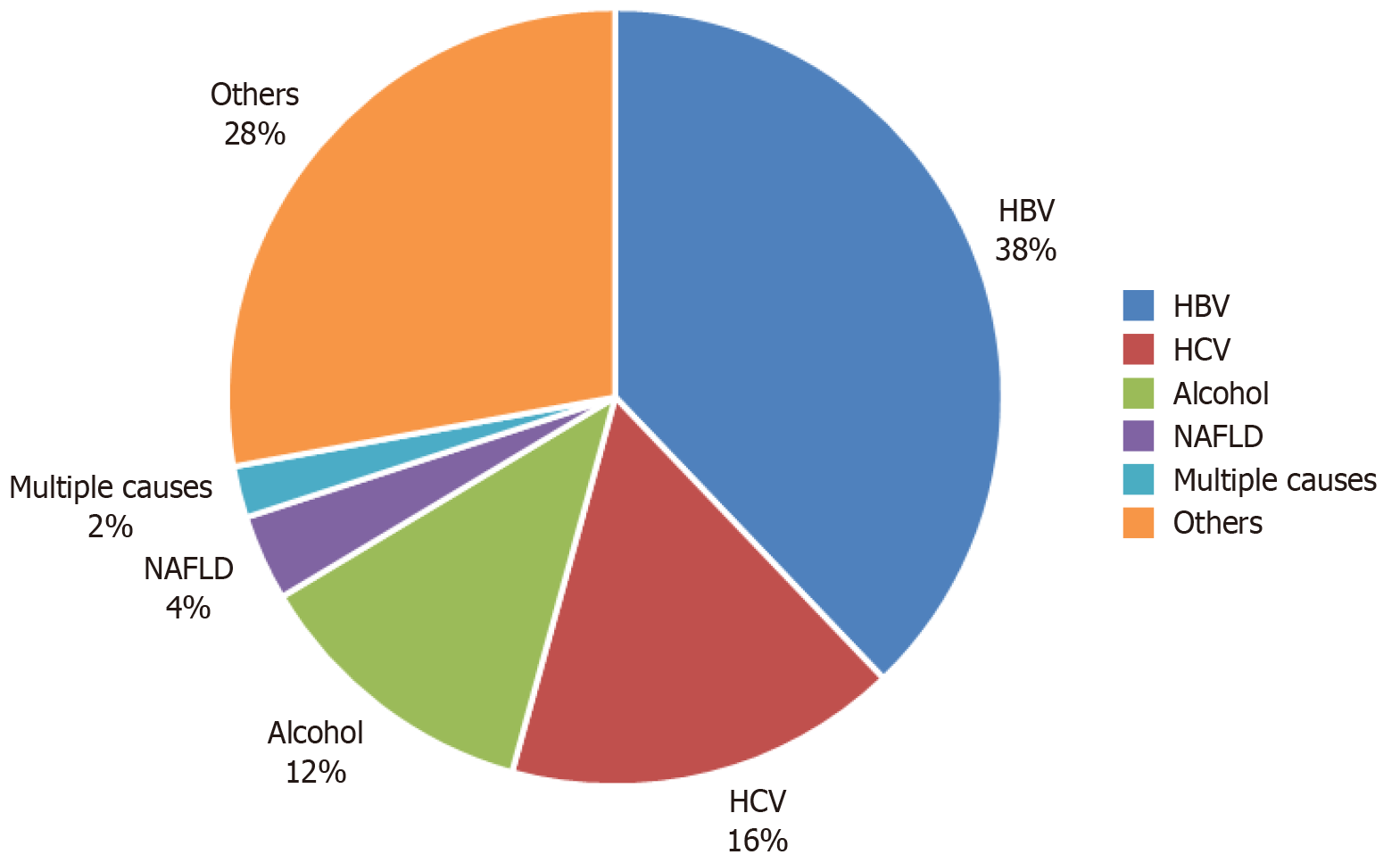
Of the 140 participants, 67% were men. Hepatitis B (38%) was the most common cause of cirrhosis, followed by cryptogenic cirrhosis (28%) and hepatitis C (16%). Approximately 83.6% of the participants had endoscopic evidence of EV, whereas 51.1% had gastric varices. Decompensated cirrhosis and PC were associated with the presence of EV with adjusted odds ratios of 12.63 (95%CI: 3.16-67.58, P = 0.001) and 0.14 (95%CI: 0.037-0.52, P = 0.004), respectively. A PC/SD ratio < 1119 had a sensitivity of 86.32% and specificity of 70% with area under the curve of 0.835 (95%CI: 0.736-0.934, P < 0.001).
A PC/SD ratio < 1119 predicts EV in patients with cirrhosis. It is a valuable, noninvasive tool for EV risk assess
Core Tip: Esophageal varices are a serious complication of liver cirrhosis. This study evaluated the platelet count-to-spleen diameter ratio as a non-invasive predictor of these varices in Ethiopian patients. We found a ratio below 1119 accurately identified at-risk patients, outperforming platelet count and spleen diameter alone. This ratio could be a valuable screening tool in resource-limited settings, but further research is needed to confirm its effectiveness.
- Citation: Mossie GY, Nur AM, Ayalew ZS, Azibte GT, Berhane KA. Platelet counts to spleen diameter ratio: A promising noninvasive tool for predicting esophageal varices in cirrhosis patients. World J Hepatol 2024; 16(10): 1177-1187
- URL: https://www.wjgnet.com/1948-5182/full/v16/i10/1177.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i10.1177
Liver cirrhosis is the end stage of liver fibrosis that results in hepatic architecture distortion[1]. It is a consequence of a long period of inflammation that results in the replacement of liver parenchyma by diffuse hepatic fibrosis with regenerative nodules, leading to portal hypertension[2]. The presence of complications, such as ascites, variceal bleeding, or hepatic encephalopathy, indicates decompensated cirrhosis. Contrarily, the absence of these symptoms suggests compensated cirrhosis[3]. The Child-Pugh score further stratifies patients into three classes. Class A predominantly comprises compensated patients, whereas classes B and C encompass a majority of decompensated patients. Gastroesophageal varices, which is a key indicator of clinically significant portal hypertension, form a compensatory mechanism to alleviate the elevated pressure within the portal venous system and divert blood flow to the systemic circulation[3]. The prevalence of gastroesophageal varices among patients with cirrhosis ranges from 25% to 35% and increases to 40% and 85% in patients with compensated and decompensated cirrhosis, respectively. Despite standard treatment, variceal bleeding is associated with high mortality rate, with 10%-15% experiencing treatment failure, 21% rebleeding, and 24% mortality within the first 6 weeks[4].
A significant public health challenge looms large on the global stage, i.e., liver disease. It is estimated to cause 2 million deaths annually, making it a substantial contributor to global mortality. Cirrhosis, viral hepatitis, and hepatocellular carcinoma (HCC) each exact a heavy toll, with roughly 1 million deaths attributed to each annually, making them the leading causes of death. Collectively, these liver-related conditions account for a staggering 3.5% of all deaths worldwide. Several factors fuel this substantial disease burden. High levels of alcohol consumption, with over 75 million individuals diagnosed with alcohol use disorders worldwide, significantly increase the risk of alcohol-associated liver disease. Overweight and obesity (affecting 2 billion adults) as well as diabetes (over 400 million cases) is a significant contributor to nonalcoholic fatty liver disease and HCC. Despite ongoing efforts, the global persistence of viral hepatitis remains a cause for concern. Furthermore, drug-induced liver injury is emerging as a growing threat, posing a substantial risk factor for cases of acute hepatitis[5].
In Ethiopia, cirrhosis is the 7th leading cause of death, accounting for approximately 24 deaths per 100000 individuals in 2019. A systematic review conducted by Tesfaye et al[6] to assess the etiologic spectrum of chronic liver disease revealed that hepatitis B virus, alcohol, and hepatitis C virus (HCV) were the most typical causes, accounting for pooled estimates of 40.0%, 17.0%, and 15.0%, respectively, and the overall hospital mortality rate of chronic liver disease patients was 25.0%[6]. Furthermore, a cross-sectional study by Mengistie[7] that investigated patients with gastrointestinal bleeding found that varices were the most common cause of upper gastrointestinal bleeding, accounting for 46.1% of the cases.
A study conducted at the University of Gondar, Northwest Ethiopia, in April 2023 reported that the prevalence of gastroesophageal varices was 52%. This study also demonstrated that patients with cirrhosis with a longer illness duration and a platelet count (PC) < 50000 have higher odds of bleeding[8].
Despite the higher prevalence of esophageal varices (EV) in Ethiopia, advanced diagnostic and screening modalities, such as upper gastrointestinal endoscopy, are only available in limited areas. Therefore, noninvasive tools such as PC-to-spleen diameter (SD) ratio would be a good alternative for screening EV.
The American Association for the Study of Liver Diseases (AASLD) guidelines recommend esophagogastroduodenoscopy (EGD) for variceal screening in patients with cirrhosis, except those who meet the following criteria: Liver stiffness measurement < 20 kPa and PC > 150000/mm³. In addition, the guidelines advise repeating EGD at 1-2-year intervals based on the variceal grade, stage of cirrhosis, and presence of associated risk factors[9]. This strategy creates significant challenges in developing countries where the prevalence of liver cirrhosis is high and the availability of endoscopy is limited to a few centers due to cost constraints. In the past two decades, extensive research has been conducted on the predictive value of various noninvasive predictors of EV[10]. Several investigations have shown that PC, splenomegaly, PC/SD ratio, advanced Child-Pugh class, serum albumin level, and high portal vein diameter are useful noninvasive predictors of EV in patients with cirrhosis[11]. Owing to their simplicity, noninvasiveness, affordability, and ease of use in EV prediction, with reasonable accuracy in some cases, these markers are valuable in clinical settings[10]. Noninvasive prediction of esophageal variceal grade during patient registration can guide the need for prophylactic beta-blockers or endoscopic variceal ligation in patients with cirrhosis having portal hypertension[12]. These predictive markers may exhibit geographical variability due to disparities in the underlying causes and severity of liver disease across different populations.
Because upper gastrointestinal endoscopy is not readily available in resource-limited settings, the PC/SD ratio would be an alternative noninvasive tool for screening EV in patients with cirrhosis. This tool may facilitate early identification of EV, enabling patients to undergo upper gastrointestinal endoscopy to confirm the presence of varices.
General objective: To evaluate the diagnostic accuracy of the PC/SD ratio for the prediction of EV in patients with liver cirrhosis.
Specific objectives: (1) To assess the sensitivity and specificity of the PC/SD ratio compared with endoscopy for the detection of EV; (2) To assess the sensitivity and specificity of the PC/SD ratio for the prediction of large varices; and (3) To assess the effect of etiology and stage of cirrhosis on the value of PC/SD in relation to the presence of EV.
A hospital-based retrospective cross-sectional analytical study was conducted at Tikur Anbessa Specialized Hospital and Adera Medical Center from May 2023 to January 2024. The study included all patients aged > 18 years who were diagnosed with liver cirrhosis; underwent PC measurement, ultrasonography examination, and EGD within 3 months; and were not taking chemotropic or other medications that can affect PC. Patients with the following conditions were excluded from the study.
Diagnosis of HCC (this condition can significantly impact liver function and spleen size, confounding the association between PC and SD).
Use of medications for the primary prevention of variceal bleeding (these medications directly influence variceal formation and size, affecting the study outcome).
History of esophageal variceal bleeding (this indicates a different disease progression and could bias the results).
Excessive alcohol consumption during the study period (alcohol can affect liver function and PC, potentially influencing the findings of the study).
History of variceal ligation, sclerotherapy, and portal hypertension surgery (these interventions alter the natural course of the disease and may interfere with the relationship between PC and SD).
Presence of chronic malaria, liver abscess, abdominal tuberculosis, hematologic malignancies, and sickle cell anemia (these conditions can independently affect PC and spleen size, thereby confounding the results).
Presence of comorbidities affecting spleen size or PC, such as lymphoproliferative disorders, metastatic malignancies, and visceral leishmaniasis.
Hemodynamic instability (this condition can affect multiple physiological parameters, including PC, making it difficult to interpret the results).
Sample-size determination: All eligible patients with complete data were included in the study. Therefore, sample-size calculation and sampling were not required.
Sampling procedure: The participants were selected using a nonprobability convenience sampling method. Individuals who met the inclusion criteria were selected from the endoscopy registry and health management information system monthly audit report. Those who met any of the exclusion criteria were excluded from the study. Their charts and care data were reviewed, and follow-up interviews were conducted using a structured questionnaire at the clinic. As the convenience sampling method may cause sampling bias and reduce the generalizability of the study, we conducted a pilot study to test the appropriateness of the questionnaire before starting data collection.
Data were collected from the study population by trained medical professionals using structured questionnaires and chart reviews using the KoboToolbox. The questionnaire has four sections: Sociodemographic factors, physical findings, laboratory results, clinical conditions (Supplementary Table 1). An abdominal ultrasound performed by a senior resident/senior radiologist had documentation of the longest bipolar diameter of the spleen taken. EGD screening was performed by a senior gastroenterologist, and the presence and grading of EV and gastric varices were documented. The authors checked for the completeness and appropriateness of the collected data every day. Constructive comments were given to each data collector.
Data were collected using a structured questionnaire administered using the KoboToolbox. After exporting data to the SPSS software, the data were cleaned and analyzed. Continuous variables were expressed using medians with interquartile ranges, whereas categorical variables were summarized using frequencies and percentages. As the data were nonparametric, we used the receiver operating characteristic (ROC) curve to obtain the area under the curve (AUC) and the Youden index to obtain the specific cutoff point at which the value has better sensitivity and specificity. The ROC curve helps determine the optimal cutoff point for the PC/SD ratio that best discriminates between patients with and without EV. The presence or absence of EV is a binary outcome, making it ideal for ROC curve analysis.
The ROC curve and Youden index can help determine the best threshold for classifying patients as having or not having EV based on the PC/SD ratio.
The ROC curves were plotted for SD, PC, and PC/SD ratio. Cutoff values were determined using the Youden index. Sensitivity, specificity, positive predictive value (PPV), negative predictive value, positive likelihood ratio (LR+), and negative likelihood ratio (LR-) were calculated using the MedCalc statistical software.
The diagnosis of liver cirrhosis was based on the presence of two or all three of the following.
Clinical signs of chronic liver disease (clubbing, palmar erythema, spider naevi, gynecomastia, distended abdominal veins, female pubic hair pattern, encephalopathy, splenomegaly, or ascites).
Impaired liver function test consistent with cirrhosis [elevated international normalized ratio (INR) and low serum albumin].
Ultrasound diagnosis of cirrhosis (shrunken or enlarged nodular liver with increased echotexture, blunt edge, and distorted architecture, with or without a dilated portal vein, thickened gallbladder wall, splenomegaly, or ascites).
Grade I: Varicose veins that disappear on insufflations.
Grade II: Varicose veins that are not confluent and do not disappear on insufflation.
Grade III: Varicose veins that are not confluent and do not disappear on insufflation.
Grade III is considered to indicate large EV, whereas grades I and II is considered to indicate small varicose veins.
Thrombocytopenia is defined as PC < 150000/mm3. PC < 100000/mm3 is considered to be severe.
Hepatitis B virus is defined as positivity for Hepatitis B surface antigen; HCV is defined as positivity for anti-HCV Ab and HCV-RNA.
Grade I: Mild ascites that is only detectable by ultrasound.
Grade II: Moderate ascites evident by moderate symmetrical distension of the abdomen.
Grade III: Large or gross ascites with marked abdominal distension.
Of the 140 participants, 67.9% were men. In the majority of the participants (66.2%), there was no history of jaundice, and alcohol use was relatively low (15%). A significant proportion of patients had ascites (37.9%) and decompensated cirrhosis (50%). Hepatitis B was the most common etiology of cirrhosis (40%), followed by hepatitis C (16.4%). Furthermore, the majority of participants (83.6%) had endoscopic evidence of EV with varying degrees of severity. Approximately one-third of the patients exhibited stigmata of bleeding (Table 1 and Figure 1).
| Variables | Frequency | Percentage (%) | Median with IQR | ||
| Age (years) | - | - | 40.5 (31-54) | ||
| Sex | Male | 95 | 67.9 | - | |
| Female | 45 | 32.1 | - | ||
| History of jaundice | Yes | 47 | 33.6 | - | |
| No | 93 | 66.2 | - | ||
| History of alcohol use | Yes | 21 | 15 | - | |
| No | 119 | 85 | - | ||
| Ascites | Yes | 53 | 37.9 | - | |
| No | 87 | 62.1 | - | ||
| Cirrhosis classification | Compensated | 70 | 50 | - | |
| Decompensated | 70 | 50 | - | ||
| HBsAg | Reactive | 56 | 40 | - | |
| Non-reactive | 84 | 60 | - | ||
| HCV Ab | Reactive | 23 | 16.4 | - | |
| Non-reactive | 117 | 83.6 | - | ||
| Ascites on ultrasounds | Yes | 60 | 42.8 | - | |
| No | 79 | 56.4 | - | ||
| Not reported on ultrasound | 1 | 0.7 | - | ||
| EV on endoscopy | Yes | Grade 1 | 32 | 27.4 | - |
| Grade 2 | 47 | 40.2 | - | ||
| Grade 3 | 38 | 32.5 | - | ||
| No | 23 | 16.4 | - | ||
| Stigmata of bleeding | Yes | 43 | 30.7 | - | |
| No | 81 | 57.8 | - | ||
| Not reported on the endoscopy | 16 | 11.4 | - | ||
Complete blood count, splenic diameter, liver size, and PC/SD ratio were determined in all the participants. Liver enzymes were done for the majority of the participants (97.1%). Portal vein diameter and INR were measured in 37.8% of the individuals (Table 2).
| Variables | Percentage of participants for whom the laboratory tests were done (%) | Median with IQR | |
| AST (IU) | 97.1 | 66 (41-119) | |
| ALT (IU) | 97.1 | 48.5 (32-79.9) | |
| Total bilirubin (mg/dL) | 86.4 | 1.5 (0.89-2.62) | |
| Albumin (g/dL) | 60.7 | 3.78 (3-4.15) | |
| INR | 37.8 | 1.45 (1.25-1.81) | |
| Hemoglobin (g/dL) | 100 | 13.65 (11.6-15.57) | |
| WBC count (× 10³/mL) | 100 | 5.0 (3.8-6.575) | |
| Platelet count (× 10³/mL) | 100 | 104 (73-139) | |
| Creatinine (mg/dL) | 90 | 0.8 (0.6-0.92) | |
| Ultrasound findings | SD (mm) | 100 | 140 (123-161.75) |
| Liver size (mm) | 100 | 140 (130-148) | |
| Portal vein diameter (mm) | 37.8 | 12 (10-14.9) | |
| PC/SD | 100 | 750.88 (452.2-1099) | |
Regarding sex, distribution was similar between the groups (P = 0.243). No significant difference was observed in terms of age (P = 0.889). Patients with varices had significantly lower aspartate aminotransferase (P = 0.035) and hemoglobin (P < 0.001) levels than those without varices. alanine aminotransferase and white blood cell count did not exhibit statistically significant differences. The PC was significantly lower in patients with varices (P < 0.001). The SD was significantly larger in patients with varices (P = 0.001), whereas the liver size showed no significant difference (P = 0.832). The PC/SD ratio was significantly lower in patients with varices (P < 0.001), indicating its potential as a predictor of the presence of varices (Table 3, Figure 2).
| Variables | Cirrhosis with varices | Cirrhosis without varices | P value | |
| Sex | Male | 65.8% | 78.3% | 0.2431 |
| Female | 64.2% | 21.7% | - | |
| Age | 41 (31-53) | 39 (30-55) | 0.889 | |
| AST | 67 (43.7-121.5) | 41.5 (31-85.25) | 0.035 | |
| ALT | 47 (32-79.8) | 51 (27.5-83) | 0.96 | |
| WBC count | 5 (3.7-6.55) | 5.6 (4.5-6.8) | 0.386 | |
| Hemoglobin | 13.3 (11.25-15.1) | 15.9 (14-16.4) | 0.001a | |
| Platelet count | 94 (68-132) | 159 (128-207) | 0.001a | |
| Ultrasound features | SD (mm) | 141 (124-168) | 125 (115-141) | 0.001a |
| Liver size (mm) | 141 (130-148) | 140 (130-147) | 0.832 | |
| PC/SD | 693.8 (423.6-1053) | 1360 (920-1886.9) | 0.001a | |
PC/SD: A lower PC/SD ratio was significantly associated with EV in both crude and adjusted analyses (P < 0.001).
Hemoglobin: Lower hemoglobin levels were associated with varices in the crude analysis (P = 0.002), but this association was not significant after adjustment for other factors.
PC: Lower PC was associated with varices in the crude analysis (P = 0.002), and this association remained significant after adjustment (P = 0.004).
SD: Larger SD was associated with varices in the crude analysis (P = 0.002), but this association was lost after adjustment.
Ascites on ultrasound: The presence of ascites on ultrasound was associated with varices in the crude analysis (P = 0.003), but this association did not persist on the adjusted analysis.
Stage of cirrhosis: A higher stage of cirrhosis was significantly associated with varices in both crude and adjusted analyses (P < 0.001).
These findings suggest that PC/SD, PC, and cirrhosis stage are independent predictors of EV in patients with liver cirrhosis (Table 4).
| Variable | Crude OR (95%CI) | P value | AOR (95%CI) | P value |
| PC/SD | 0.069 (0.025-0.195) | < 0.001 | - | - |
| Hemoglobin | 0.695 (0.551-0.875) | 0.002 | - | - |
| Platelet count | 0.129 (0.036-0.56) | 0.002 | 0.140 (0.037-0.526) | 0.004a |
| SD | 1.037 (10.13-1.061) | 0.002 | - | - |
| Ascites on ultrasound | 0.044 (0.006-0.337) | 0.003 | - | - |
| Stage of cirrhosis | 14.571 (3.264-65.055) | < 0.001 | 12.623 (3.164-67-586) | 0.001a |
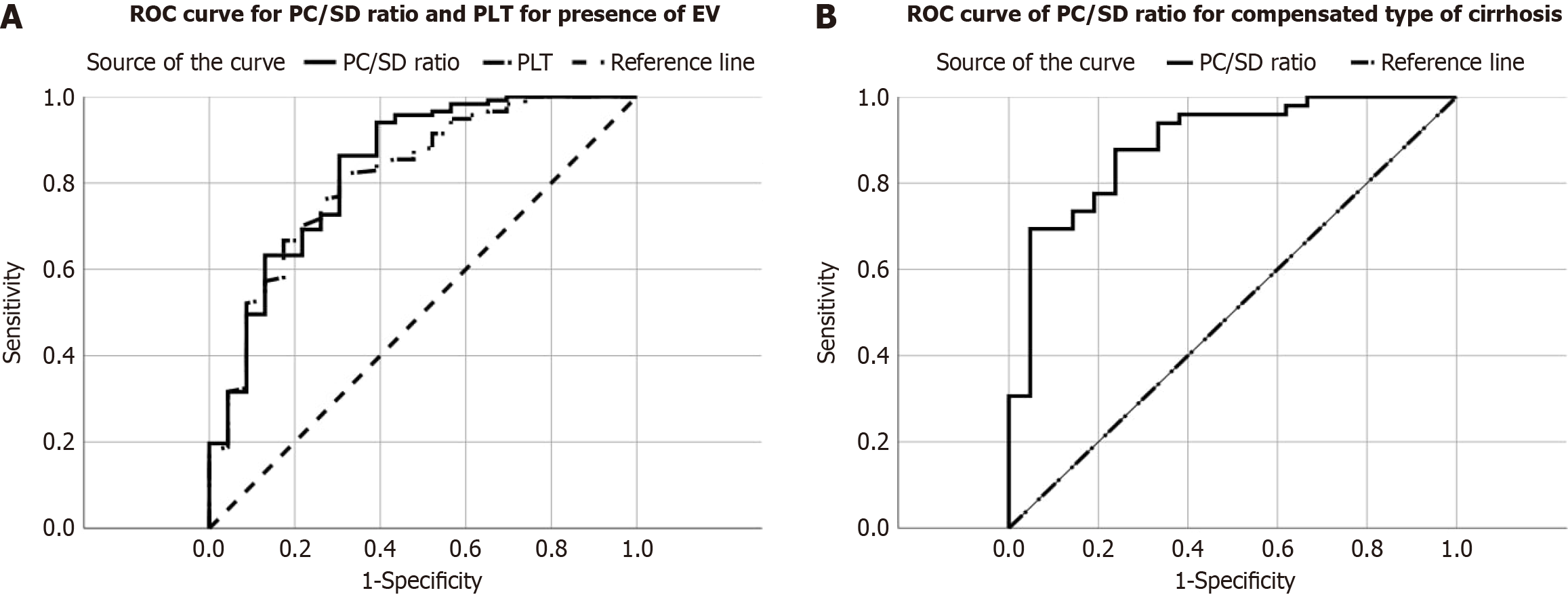
The PC/SD ratio demonstrated the highest diagnostic accuracy, with an AUC of 0.835, suggesting good discrimination between patients with and without varices. While both PC and SD exhibited a moderate diagnostic performance, the PC/SD ratio appeared to be a more promising noninvasive tool for identifying patients at risk of EV (Table 5). In summary, (1) PC/SD had the highest diagnostic accuracy; (2) All three markers exhibited moderate to good sensitivity; (3) Specificity was relatively low for all markers; and (4) PC/SD exhibited the highest PPV.
| Non-invasive markers | Cut-off value | Sensitivity (%) | Specificity (%) | AUROC (95%CI) | P value | PPV (%) | NPV (%) | Accuracy (%) |
| Platelet count | 138000/mL | 82.05 | 69.57 | 0.815 (0.718-0.913) | < 0.001 | 93.20 | 43.24 | 80 |
| Spleen diameter | 120.5 mm | 83.76 | 47.83 | 0.712 (0.605-0.820) | 0.001 | 89.09 | 36.67 | 77.86 |
| PC/SD | 1118.74 | 86.32 | 69.57 | 0.835 (0.736-0.934) | < 0.001 | 93.52 | 50 | 83.57 |
PC/SD could be a valuable tool for the initial screening of EV in patients with cirrhosis, particularly in settings where endoscopic evaluation is limited.
PC/SD demonstrated the highest diagnostic accuracy, as indicated by the highest area under the ROC (AUROC) curve of 0.889. This suggests that PC/SD can best discriminate between patients with and without EV in compensated cirrhosis. PC showed good sensitivity and specificity, with a moderate AUC of 0.872. SD had lower sensitivity and specificity than the other two markers, and its AUC was notably lower at 0.713 (Table 6).
| Non-invasive marker | Cut-off value | Sensitivity (%) | Specificity (%) | AUROC (95%CI) | P value | PPV (%) | NPV (%) | Accuracy (%) |
| Platelet count | 119500/mL | 73.47 | 90.48 | 0.872 (0.785-0.959) | 0.001a | 94.74 | 59.38 | 78.57 |
| Spleen diameter | 133.5 mm | 59.18 | 76.19 | 0.713 (0.591-0.836) | 0.005 | 85.29 | 44.44 | 64.29 |
| PC/SD | 830.3 | 69.39 | 95.24 | 0.889 (0.805-0.973) | 0.001a | 97.14 | 57.14 | 77.14 |
Both markers demonstrated moderate diagnostic performance. SD had a slightly higher sensitivity (78.95%) than PC/SD ratio (63.16%), indicating better ability to identify patients with large EV. However, PC/SD exhibited higher specificity (55.7%) than SD (60.76%), demonstrating better ability to correctly identify patients without large EV. The AUROC for both markers was below 0.7, indicating suboptimal diagnostic accuracy. These findings suggest that neither SD nor PC/SD alone is a highly reliable predictor of large EV (Table 7).
| Non-invasive marker | Cut-off value | Sensitivity (%) | Specificity (%) | AUROC (95%CI) | P value | PPV (%) | NPV (%) | Accuracy (%) |
| Spleen diameter | 140.5 mm | 78.95 | 60.76 | 0.695 (0.595-0.795) | 0.001a | 49.18 | 85.71 | 66.67 |
| PC/SD | 696.5 | 63.16 | 55.7 | 0.595 (0.488-0.702) | 0.095 | 40.68 | 75.86 | 58.12 |
Gastroesophageal variceal bleeding is a potential complication observed in 25%-35% of patients with cirrhosis. It carries a significant mortality risk, with a 6-week mortality rate of 15% to 25%[13]. Several society guidelines, including the Baveno VII consensus and the AASLD, recommend EGD for EV diagnosis and risk stratification[14,15]. However, a limited number of noninvasive tests are available to predict EV. Among these, the PC/SD ratio is a promising option. It offers several advantages, such as ease of use, wide availability, and good predictive value in identifying high-risk patients.
Several studies have reported that PC/SD ratio > 909 offers high reliability in predicting EV[10,16-18]. A meta-analysis of 20 studies revealed that a PC/SD ratio cutoff of 909 exhibits a sensitivity of 92%, specificity of 87%, and a hierarchical summary ROC of 0.95 for EV prediction[19].Our study identified a higher cutoff value of 1119, with a sensitivity of 86% and a specificity of 70%. This finding is consistent with the observations of Jamil et al[20], who reported a cutoff value of 1077 (sensitivity: 89%, specificity: 81%) in their study. Similarly, Patil et al[21] proposed a PC/SD ratio < 1400 (sensitivity: 90%, specificity: 82%) for predicting EV in an Indian population. A meta-analysis by Chawla et al[22], encompassing eight studies that emphasized the concept of population-specific PC/SD cutoff values for optimal prediction. Another study conducted in Africa has also yielded comparable results[23].
Our study found that an SD > 120.5 mm has a good sensitivity (83.76%) with low specificity (47.83%) for the presence of EV. This cutoff value is higher than that in a study by Okon et al[23], where an SD > 102 mm predicted 75% of EV cases with an accuracy of 85%. Previous studies have reported that splenomegaly can be a good indicator of EV. Ashraf et al[24] reported that a spleen size > 130 mm yielded a sensitivity of 87.7% and a specificity of 83.3% for EV prediction, which is comparable to our findings as well as to other studies[25].
Furthermore, a PC < 138000 exhibited a higher sensitivity (82.05%) but a lower specificity (69.57%) with a better AUC for EV detection.
This study reported an association between SD and the presence of large EV. An SD > 142.5 mm exhibited a sensitivity of 61% and a specificity of 81%, with an acceptable accuracy (AUC = 0.763). Contrarily, the PC/SD ratio failed to achieve similar performance for predicting large EVs. These findings are consistent with the observations of Duah et al[26], who reported no significant association between the PC/SD ratio and large EV prediction. However, a study by Barrera et al[27] yielded contrasting results. A PC/SD ratio < 830.8 yielded a sensitivity of 76.9% and a specificity of 74.2% (ROC curve area: 0.78), demonstrating better accuracy than our study despite similar sensitivity[27,28].
This study aimed to evaluate the sensitivity and specificity of the PC/SD ratio in identifying EV among patients with cirrhosis in Ethiopia. Our findings suggest that the PC/SD ratio offers superior accuracy to those of PC and SD. The cutoff value identified in our study diverges from a similar survey conducted by Gebregziabiher et al[28] in Gondar, Ethiopia. Their study reported lower cutoff values: 818 for the PC/SD ratio, 121000 for PC, and 145 mm for SD. While the sensitivity, specificity, and AUC values of PC and PC/SD in our study are comparable to their findings, the sensitivity of SD in our study is lower. Interestingly, our analysis revealed that these noninvasive parameters exhibited better predictive value in patients with compensated cirrhosis than those with decompensated cirrhosis. This observation contradicts the above findings by Gebregziabiher et al[28].
Due to the retrospective nature of the study, we cannot control for confounding variables that can be identified with appropriate patient selection and proper randomization. Confounding variables, such as cirrhosis duration, etiology, and severity, could affect the PC, spleen size, and time to develop EV. Replication of this study by controlling these confounding variables in a prospective study is recommended.
The PC/SD ratio is a promising non-invasive tool for identifying EV in cirrhotic patients, especially those with compensated cirrhosis. These findings are expected to guide physicians in resource-limited settings in screening for EV. However, further multicentered studies with a prospective design and larger sample size should be done to increase the generalizability of this study.
| 1. |
|
| 2. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 862] [Article Influence: 215.5] [Reference Citation Analysis (1)] |
| 3. | Guo XZ, Qi XS. Variceal Bleeding in Liver Cirrhosis. Singapore: Springer, 2021. [DOI] [Full Text] |
| 4. | Baiges A, Hernández-Gea V. Management of Liver Decompensation in Advanced Chronic Liver Disease: Ascites, Hyponatremia, and Gastroesophageal Variceal Bleeding. Clin Drug Investig. 2022;42:25-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 5. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2297] [Article Influence: 382.8] [Reference Citation Analysis (0)] |
| 6. | Tesfaye BT, Feyissa TM, Workneh AB, Gudina EK, Yizengaw MA. Chronic Liver Disease in Ethiopia with a Particular Focus on the Etiological Spectrums: A Systematic Review and Meta-Analysis of Observational Studies. Can J Gastroenterol Hepatol. 2021;2021:8740157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Mengistie YC. The pattern and outcome of upper gastrointestinal bleeding at St. Paul's Millenium Medical College, Addis Ababa, Ethiopia. Ethiop Med J. 2020;58:323-327. |
| 8. | Baye ML, Abay Z, Tesfaye T, Ahmed E, Arage G, Zewude EA, Anley DT. Gastroesophageal variceal hemorrhage in patients with chronic liver diseases attending university of Gondar Specialized comprehensive hospital in Ethiopia: Institutional based cross-sectional study. Heliyon. 2023;9:e15133. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1441] [Article Influence: 180.1] [Reference Citation Analysis (3)] |
| 10. | Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, Mele MR, Testa E, Mansi C, Savarino V, Testa R. Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut. 2003;52:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 299] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 11. | Hong WD, Zhu QH, Huang ZM, Chen XR, Jiang ZC, Xu SH, Jin K. Predictors of esophageal varices in patients with HBV-related cirrhosis: a retrospective study. BMC Gastroenterol. 2009;9:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Cherian JV, Deepak N, Ponnusamy RP, Somasundaram A, Jayanthi V. Non-invasive predictors of esophageal varices. Saudi J Gastroenterol. 2011;17:64-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Liu YB, Chen MK. Epidemiology of liver cirrhosis and associated complications: Current knowledge and future directions. World J Gastroenterol. 2022;28:5910-5930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (21)] |
| 14. | de Franchis R, editor. Portal Hypertension VII. Switzerland AG: Springer Nature, 2022. [DOI] [Full Text] |
| 15. | Kaplan DE, Ripoll C, Thiele M, Fortune BE, Simonetto DA, Garcia-Tsao G, Bosch J. AASLD Practice Guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2024;79:1180-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 140] [Article Influence: 140.0] [Reference Citation Analysis (1)] |
| 16. | Schwarzenberger E, Meyer T, Golla V, Sahdala NP, Min AD. Utilization of platelet count spleen diameter ratio in predicting the presence of esophageal varices in patients with cirrhosis. J Clin Gastroenterol. 2010;44:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Giannini EG, Botta F, Borro P, Dulbecco P, Testa E, Mansi C, Savarino V, Testa R. Application of the platelet count/spleen diameter ratio to rule out the presence of oesophageal varices in patients with cirrhosis: a validation study based on follow-up. Dig Liver Dis. 2005;37:779-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Agha A, Anwar E, Bashir K, Savarino V, Giannini EG. External validation of the platelet count/spleen diameter ratio for the diagnosis of esophageal varices in hepatitis C virus-related cirrhosis. Dig Dis Sci. 2009;54:654-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Ying L, Lin X, Xie ZL, Hu YP, Shi KQ. Performance of platelet count/spleen diameter ratio for diagnosis of esophageal varices in cirrhosis: a meta-analysis. Dig Dis Sci. 2012;57:1672-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Jamil Z, Malik M, Durrani AA. Platelet count to splenic diameter ratio and other noninvasive markers as predictors of esophageal varices in patients with liver cirrhosis. Turk J Gastroenterol. 2017;28:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Patil S, Patnaik SK, Kanungo M, Uthansingh K, Narayan J, Pradhan S, Mishra D, Sahu MK, Pati GK. Platelet Count/Spleen Diameter Ratio as a Non-Invasive Predictor of Esophageal Varices in Cirrhotic Patients: A Single-Center Experience. Gastroenterol Insights. 2024;15:98-106. [DOI] [Full Text] |
| 22. | Chawla S, Katz A, Attar BM, Gupta A, Sandhu DS, Agarwal R. Platelet count/spleen diameter ratio to predict the presence of esophageal varices in patients with cirrhosis: a systematic review. Eur J Gastroenterol Hepatol. 2012;24:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Okon JB, Ake F, Diakite M, Koffi OK, Kone A. Predictive Values of Platelets Count and Spleen Diameter in the Diagnosis of Esophageal Varices in Black African Cirrhotic Patients. OJGas. 2020;10:317-328. [DOI] [Full Text] |
| 24. | Ashraf DG, El-sayed I. Esophageal varices predictive score in liver cirrhosis. Egypt J Intern Med. 2018;30:72-77. [RCA] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 25. | González-Ojeda A, Cervantes-Guevara G, Chávez-Sánchez M, Dávalos-Cobián C, Ornelas-Cázares S, Macías-Amezcua MD, Chávez-Tostado M, Ramírez-Campos KM, Ramírez-Arce Adel R, Fuentes-Orozco C. Platelet count/spleen diameter ratio to predict esophageal varices in Mexican patients with hepatic cirrhosis. World J Gastroenterol. 2014;20:2079-2084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Duah A, Nkrumah KN, Tachi K. Non-invasive markers as predictors of oesophageal varices in cirrhotic patient in a teaching hospital in Ghana. Ghana Med J. 2019;53:142-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Barrera F, Riquelme A, Soza A, Contreras A, Barrios G, Padilla O, Viviani P, Pérez-Ayuso RM. Platelet count/spleen diameter ratio for non-invasive prediction of high risk esophageal varices in cirrhotic patients. Ann Hepatol. 2009;8:325-330. [PubMed] [DOI] [Full Text] |
| 28. | Gebregziabiher HT, Hailu W, Abay Z, Bizuneh S, Meshesha MD. Accuracy of non-invasive diagnosis of esophageal varices among cirrhotic patients in a low-income setting. Heliyon. 2023;9:e23229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |