Published online Jan 27, 2024. doi: 10.4254/wjh.v16.i1.91
Peer-review started: October 20, 2023
First decision: November 16, 2023
Revised: November 21, 2023
Accepted: December 12, 2023
Article in press: December 12, 2023
Published online: January 27, 2024
Processing time: 95 Days and 7.5 Hours
Although the past decade has seen remarkable advances in treatment options for hepatocellular carcinoma (HCC), the dismal overall prognosis still envelops HCC patients. Several comparative trials have been conducted to study whether tran
To study the potential synergies and safety of sorafenib plus TACE vs sorafenib alone for treating advanced HCC, by performing a systematic review and meta-analysis.
This study was conducted following the PRISMA statement. A systematic literature search was conducted using the Cochrane Library, Embase, PubMed, and Web of Science databases. Data included in the present work were collected from patients diagnosed with advanced HCC receiving sorafenib plus TACE or sorafenib alone. Data synthesis and meta-analysis were conducted using Review Manager software.
The present study included 2780 patients from five comparative clinical trials (1 was randomized control trial and 4 were retrospective studies). It was found that patients receiving sorafenib plus TACE had better prognoses in terms of overall survival (OS), with a combined hazard ratio (HR) of 0.65 [95% confidence interval (95%CI): 0.46–0.93, P = 0.02, n = 2780]. Consistently, progression free survival (PFS) and time to progression (TTP) differed significantly between the sorafenib plus TACE arm and sorafenib arm (PFS: HR = 0.62, 95%CI: 0.40–0.96, P = 0.03, n = 443; TTP: HR = 0.73, 95%CI: 0.64-0.83, P < 0.00001, n = 2451). Disease control rate (DCR) was also significantly increased by combination therapy (risk ratio = 1.36, 95%CI: 1.02-1.81, P = 0.04, n = 641). Regarding safety, the incidence of any adverse event (AE) was increased due to the addition of TACE; however, no significant difference was found in grade ≥ 3 AEs.
The combination of sorafenib with TACE has superior efficacy to sorafenib monotherapy, as evidenced by prolonged OS, PFS, and TTP, as well as increased DCR. Additional high-quality trials are essential to further validate the clinical benefit of this combination in the treatment of advanced HCC.
Core Tip: No consensus is available in the literature about whether addition of transarterial chemoembolization (TACE) could improve survival in patients receiving sorafenib for advanced hepatocellular carcinoma. This is the first systematic review and meta-analysis comparing sorafenib/TACE combination therapy and sorafenib monotherapy for advanced hepatocellular carcinoma. We investigated these two treatments in terms of overall survival, progression free survival, time to progression, disease control rate, and adverse events.
- Citation: Yang HJ, Ye B, Liao JX, Lei L, Chen K. Sorafenib plus transarterial chemoembolization vs sorafenib alone for patients with advanced hepatocellular carcinoma: A systematic review and meta-analysis. World J Hepatol 2024; 16(1): 91-102
- URL: https://www.wjgnet.com/1948-5182/full/v16/i1/91.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i1.91
As a global health problem, liver cancer, including hepatocellular carcinoma (HCC), represents the sixth most frequent malignancy and the fourth cause of cancer-related death[1]. In particular, the incidence of HCC is rising, with an annual incidence of above 0.6 million patients at present, which is estimated to be > 1 million by 2025 worldwide[2]. There have been remarkable advances in treatment options for HCC, and several treatment options have been adopted as standard of care according to clinical practice guidelines[3-5]. In principle, potentially curative therapies (i.e., surgical resection, local ablation, and liver transplantation) are preferred for early-stage tumours, transarterial chemoembolization (TACE) is recommended for intermediate-stage tumours, and systemic drugs (i.e., sorafenib, and atezolizumab plus bevacizumab) are the mainstay of treatment for advanced tumors. All these therapies have contributed to a progressive improvement in life expectancy of HCC patients[4-7]. However, the dismal overall prognosis still envelops HCC patients primarily because of the late diagnosis and frequent relapse[8].
Because quite many HCCs are diagnosed at an advanced stage, namely, Barcelona Clinic Liver Cancer (BCLC) stage C[9], how to prolong the survival of patients with advanced HCC is more crucial than the treatments for early stage HCC. It has not achieved global consensus on the definition of advanced HCC, which is generally indicated in cases with portal vein infiltration, extrahepatic metastasis, or progression on curative treatments[10]. Sorafenib, an inhibitor of several tyrosine kinases, such as VEGFR-2 (vascular endothelial growth factor receptor-2), PDGFR-β (platelet-derived growth factor receptor-β), and Raf serine/threonine kinases, is the first molecular targeting drug approved for the treatment of advanced HCC, and yet the standard first-line therapy internationally[11,12]. However, the overall survival (OS) outcomes of most patients are still far from satisfactory, and further prolonging survival is challenging.
To augment the clinical benefit of sorafenib, several clinical studies have evaluated the effects of addition of other systemic/locoregional therapies to it[13-15], including TACE[16]. TACE is a vascular interventional surgery which can concentrate chemotherapeutic drugs at tumour site, thus blocking tumour feeding from the primary artery to delay tumor progression. As an effective therapy for unresectable HCC, TACE is recommended by most guidelines for HCC at intermediate stage or multifocal HCC[4,10,12]. Although TACE is preferred for HCC patients at BCLC stage B, in many countries, it is frequently performed across all disease stages as well, including advanced stage[17]. Treatment with TACE leads to VEGF upregulation and thus the increase of tumour angiogenesis, while sorafenib would be expected to strengthen the effectiveness of TACE by suppressing angiogenesis by inhibiting VEGF signaling. Several comparative trials worldwide have been conducted to study whether TACE could provide clinical benefit in patients receiving sorafenib for advanced HCC; however, the findings have been not consistent[18-23]. Hence, the efficacy of the combination therapy of sorafenib plus TACE in patients with advanced disease has not been thoroughly understood. This systematic review and meta-analysis aimed to assess the potential synergies and safety of sorafenib plus TACE as compared with sorafenib alone in the treatment of advanced HCC.
This systematic review and meta-analysis was conducted following the PRISMA statement. Four databases (PubMed, the Cochrane Library, EMbase, and Web of Science) were used in systematic search to capture relevant studies from inception to August 18, 2023. Two independent investigators (Yang HJ and Ye B) conducted this search. We used the combinations of the following keywords: Hepatocellular carcinoma/HCC, sorafenib /tyrosine kinase inhibitor/TKI/multikinase inhibitor/MKI, and transarterial chemoembolization/TACE/chemoembolization.
The criteria for including eligible studies into this meta-analysis were: (1) Study patients were diagnosed with advanced HCC, regardless of the kind of treatment that they have experienced before; (2) at least two intervention arms (TACE plus sorafenib vs sorafenib alone) were compared in the study; (3) one of the following outcomes must be included in study: OS, progression free survival (PFS), time to progression (TTP), or disease control rate (DCR). Studies published only as an abstract or those containing unobtainable/unusable data were excluded. Two independent investigators (Liao JX and Lei L) judged the records based on the title/abstract and then full-text. Any disagreement between the two investigators was discussed to reach a consensus.
Two investigators (Yang HJ and Ye B) independently extracted the data of baseline characteristics and outcome measures from eligible studies using a specially-designed standardized extraction form. Study data included first author, year of publication, study design, sample size, age, gender, Eastern Cooperative Oncology Group performance status (ECOG-PS), BCLC stage, Child-Pugh class, alpha-fetoprotein (AFP), portal vein tumor thrombus (PVTT), follow-up, description of interventions, and type of outcome measures. Efficacy outcome measures included OS, PFS, and TTP, described as hazard ratio (HR) with 95% confidence interval (95%CI), and DCR, defined as the percentage of patients whose response was complete response, partial response, or stable disease. Safety outcomes included any adverse event (AE) reported by patients, grade ≥ 3 AEs, and typical AEs. Any controversy between investigators was resolved by discussion.
The quality of the randomized controlled trials (RCT) was assessed using the Jadad scale, while the retrospective studies were assessed using the Newcastle-Ottawa scale.
Meta-analysis was conducted using Review Manager 5.4 (Cochrane Collaboration, Oxford, United Kingdom) using a random-effects model. Pooled continuous data are described as HR while pooled dichotomous data are described as risk ratio (RR), with 95%CI. Heterogeneity was assessed through χ2 test and I2 statistic, with values over 60% indicating substantial heterogeneity. Sensitivity analysis was conducted through the leave-one-out approach if needed. Publication bias was not assessed since the number of included studies was too small.
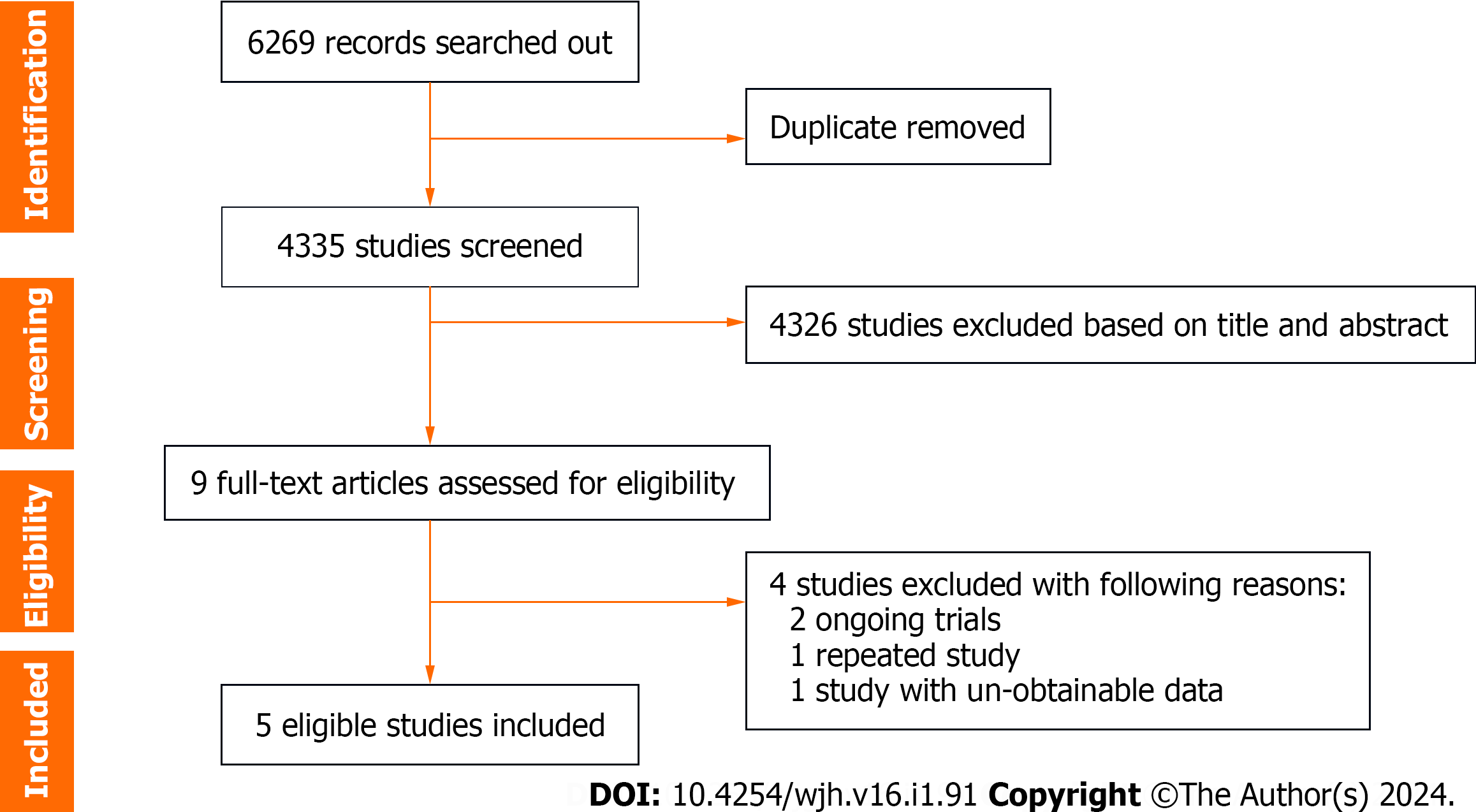
Overall, a total of 4335 unique studies were captured after removing duplicates, and then nine were retained as potentially eligible trials for full-text assessment. After deleting four ineligible studies (two ongoing trials, one repeated study, and one unobtainable data study), five studies were finally included for meta-analysis[19-23] (Figure 1).
The baseline characteristics of patients from the included studies are summarized in Table 1. The five studies consisted of four retrospective studies[19,21-23] and one RCT[20]. A total of 2780 patients with advanced HCC were included, of which 751 received sorafenib plus TACE and 2029 received sorafenib alone. The participants were at ages of 50 to 70 years mostly, with the majority being male. At baseline, all patients had an ECOG-PS of 0 or 1-2, and most patients had BCLC stage C, and Child-Pugh class A. Four of five trials reported the characteristics of AFP and PVTT in patients[19-22].
| Ref. | Study design | Group | Number of cases | Age1 (yr) | Male | ECOG-PS | BCLC stage | Child-Pugh class | AFP | PVTT | |||||
| 0 | 1-2 | A | B | C | A | B | |||||||||
| Koch et al[19], 2021 | Retrospective cohort study | Sorafenib + TACE | 54 | 64 (34-77) | 47 (87) | 16 (30) | 38 (70) | 0 (0) | 0 (0) | 54 (100) | 40 (74) | 14 (26) | < 400 ng/mL; 36 (66) | ≥ 400 ng/mL; 18 (34) | 18 (33) |
| Sorafenib alone | 82 | 66 (28-85) | 72 (88) | 37 (45) | 45 (55) | 0 (0) | 0 (0) | 82 (100) | 61 (74) | 21 (26) | 52 (64) | 30 (36) | 27 (33) | ||
| Park et al[20], 2019 | Multi-center RCT phase III | Sorafenib + TACE | 170 | 60 ± 10 | 136 (80) | 136 (80) | 34 (20) | 3 (2) | 39 (23) | 128 (75) | 148 (87) | 22 (13) | < 200 ng/mL; 79 (47) | ≥ 200 ng/mL; 91 (54) | 68 (40) |
| Sorafenib alone | 169 | 61 ± 10 | 147 (87) | 140 (83) | 29 (17) | 0 (0) | 44 (26) | 125 (74) | 147 (87) | 22 (13) | 76 (45) | 93 (55) | 63 (37) | ||
| Kok et al[23], 2019 | Retrospective cohort study | Sorafenib + TACE | 426 | 60 (51-69) | 355 (83) | NA | 0 (0) | 0 (0) | 426 (100) | 426 (100) | 0 (0) | NA | NA | ||
| Sorafenib alone | 1686 | 60 (52-68) | 1410 (84) | NA | 0 (0) | 0 (0) | 1686(100) | 1686 (100) | 0 (0) | NA | NA | ||||
| Wu et al[21], 2017 | Retrospective study | Sorafenib + TACE | 56 | 50 ± 12 | 48 (86) | NA | 0 (0) | 10 (18) | 46 (82) | 45 (80) | 11 (20) | < 400 ng/mL; 33 (59) | ≥ 400 ng/mL; 23 (41) | 32 (57) | |
| Sorafenib alone | 48 | 48 ± 13 | 46 (96) | NA | 0 (0) | 16 (33) | 32 (67) | 46 (96) | 2 (4) | 23 (49) | 24 (51) | 24 (50) | |||
| Zhang et al[22], 2015 | Retrospective study | Sorafenib + TACE | 45 | 50 ± 9 | 43 (96) | 45 (100) | NA | 34 (76) | 11 (24) | < 200 ng/mL; 3 (7) | ≥ 200 ng/mL; 42 (93) | 45 (100) | |||
| Sorafenib alone | 44 | 54 ± 10 | 41 (93) | 44 (100) | NA | 34 (77) | 10 (23) | 9 (20) | 35 (80) | 44 (100) | |||||
The details on intervention characteristics and outcome measures of the included trials are summarized in Table 2. Obvious differences were found in intervention program, namely, the sequence and interval between sorafenib administration and TACE operation in the sorafenib plus TACE arm across studies. Sorafenib treatment was started after TACE operation in two trials[19,22], while sorafenib administration was initiated prior to TACE in another three[20,21,23]. Generally, sorfenib was orally administrated at 400 mg twice daily[20,21]. Among trials reporting the median period of sorafenib administration, it ranged from the minimum 0.1 mo to maximum 48.4 mo across studies. Varied combinations of outcome measures from OS, PFS, TTP, and DCR, were adopted in different trials, with OS adopted in all trials.
| Ref. | Intervention | Patients | Follow-up (mo) | Sorafenib dose | Sorafenib duration (mo) | OS (mo) | TTP (mo) | PFS (mo) | DCR (%) | |||
| Median | HR (95%CI) | Median | HR (95%CI) | Median | HR (95%CI) | |||||||
| Koch et al[19], 2021 | TACE was usually initiated before sorafenib | 54 | NA | NA | NA | 16.5 | 0.34 (0.23-0.53) | 7.0 | NA | NA | NA | 28/53 (53) |
| Sorafenib alone | 82 | NA | NA | 8.4 | 4.1 | NA | 17/74 (23) | |||||
| Park et al[20], 2019 | Sorafenib initiated within 3 d of randomization, first TACE initiated between 7 and 21 d after randomization | 170 | 14 (4-27) | 200-400 mg twice daily, then 400 mg twice daily | 5.5 (0.1-41.6) | 12.8 | 0.91 (0.69-1.21) | 5.3 | 0.67 (0.53-0.85) | 5.2 | 0.73 (0.59-0.91) | 103/170 (61) |
| Sorafenib initiated within 3 d of randomization | 169 | 19 (2-27) | 4.3 (0.2-48.4) | 10.8 | 3.5 | 3.6 | 80/169 (47) | |||||
| Kok et al[23], 2019 | Sorafenib prior to TACE | 426 | 7.4 (4.7-11.5) | NA | 4.7 (4.2-5.3) | 12.5 | 0.74 (0.63-0.88) | 4.7 | 0.76 (0.65-0.89) | NA | NA | NA |
| Sorafenib alone | 1686 | 4.4 (2.3-8.4) | 2.8 (2.6-3.0) | 6.7 | 2.8 | NA | ||||||
| Wu et al[21], 2017 | Sorafenib prior to TACE | 56 | NA | 400 mg twice daily | NA | 22 | 0.50 (0.28-0.89) | NA | NA | 8 | 0.46 (0.27-0.78) | 27/48 (56)1 |
| Sorafenib alone | 48 | NA | NA | 18 | NA | 6 | 23/40 (58) | |||||
| Zhang et al[22], 2015 | Sorafenib started 1-3 d after TACE | 45 | 7.3 (2-18) | NA | 5.6 (1-18) | 7 | 1.17 (0.52-1.81) | 3 | NA | NA | NA | 24/43 (60) |
| Sorafenib alone | 44 | 5.4 (1-17) | 6 | 3 | NA | 18/44 (51) | ||||||
The quality of the data from four retrospective studies[19,21-23] was evaluated using the Newcastle-Ottawa scale. All retrospective studies received a score of 8, suggesting that the data were of good quality. The quality of the Park et al[20]'s study that was a RCT, was evaluated using the Jadad scale. The data were considered of high quality as it received a score of 3. All included studies may have detection bias as the outcome assessors in all trials were not blinded. The details of study quality assessment are summarized in Table 3.
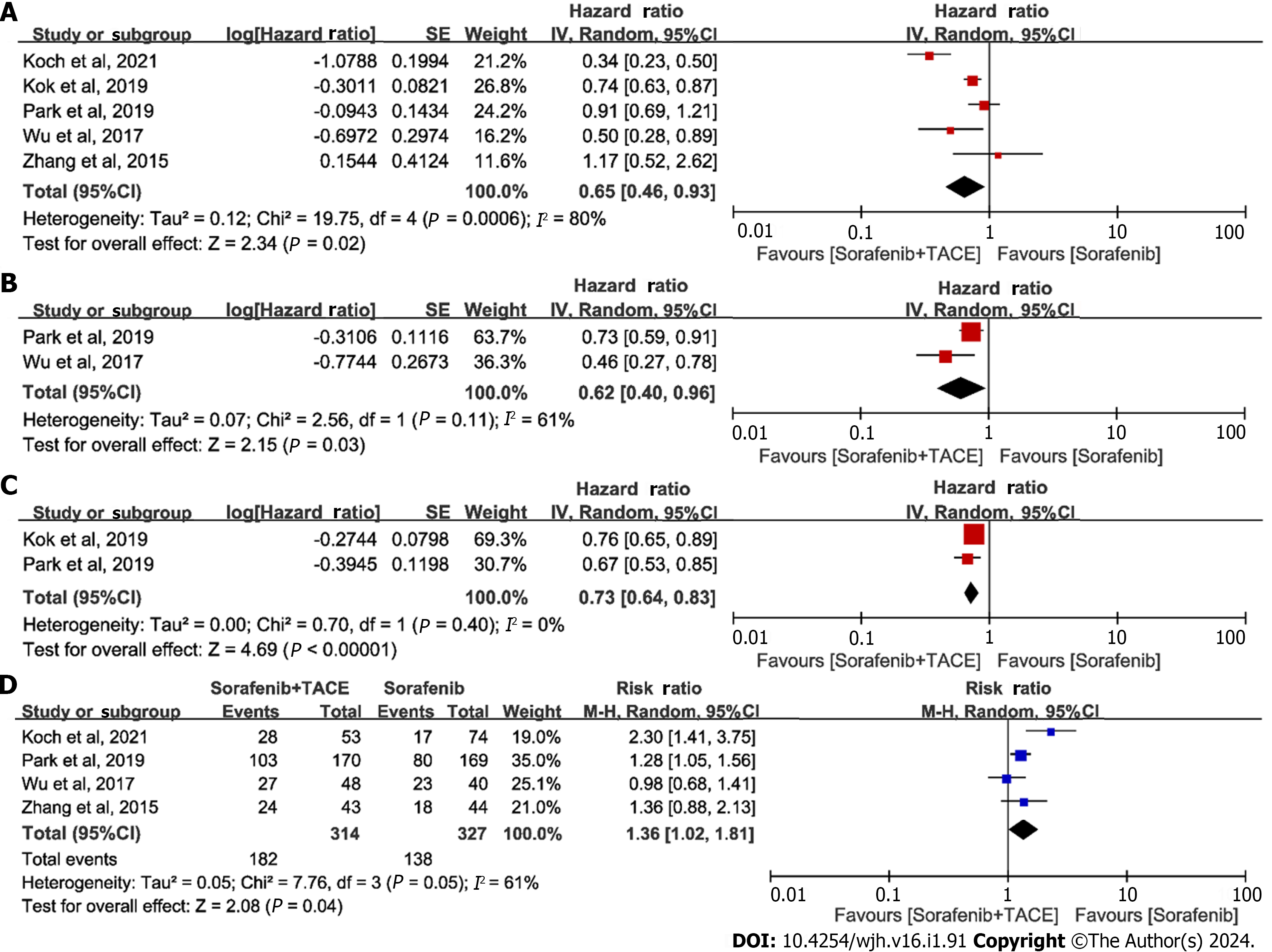
OS-primary outcome:OS is objective and clinically relevant, serving as the sole robust endpoint in the management of HCC, and all included trials reported OS as an endpoint. Thus, OS was chosen as the primary outcome in the present study. All five studies[19-23] provided point estimates and 95%CI for HR regarding OS; hence, all were included for the meta-analysis. The results suggested that patients treated with sorafenib plus TACE had better outcomes regarding OS compared to those treated with sorafenib alone: HRs ranged from 0.34 to 1.17, with a combined HR of 0.65 (95%CI: 0.46–0.93, P = 0.02, n = 2780; Figure 2A). Because of high heterogeneity across studies, a sensitivity analysis was conducted. Removal of the study of Koch et al[19] caused the heterogeneity to become non-significant, while the results of the pooled OS were almost identical.
Only two[20,21] of the five studies reported the point estimate (HR) and 95%CI for PFS. The combined HR showed that the PFS significantly differed between patients treated with sorafenib plus TACE and those treated with sorafenib alone (combined HR = 0.62, 95%CI: 0.40–0.96, P = 0.03, n = 443; Figure 2B).
Three studies were excluded from the meta-analysis without 95%CI for TTP; hence, only two[20,23] studies were used for the meta-analysis. The pooled result was positive, with an HR of 0.73 (95%CI: 0.64-0.83, P < 0.00001, n = 2451; Figure 2C), indicating better outcome regarding TTP achieved by sorafenib plus TACE as compared with sorafenib alone.
Four[19-22] of five studies were included in meta-analysis for DCR after excluding the study of Kok et al[23], which did not provide the relevant data. The meta-analysis yielded positive results for pooled DCR, with a combined RR of 1.36 (95%CI: 1.02-1.81, P = 0.04, n = 641, Figure 2D), revealing that patients receiving sorafenib plus TACE had better prognoses in terms of DCR, compared to those treated with sorafenib alone. Sensitivity analysis indicated that removal of the study of Koch et al[19] eliminated the heterogeneity, while the results of pooled DCR were almost identical.
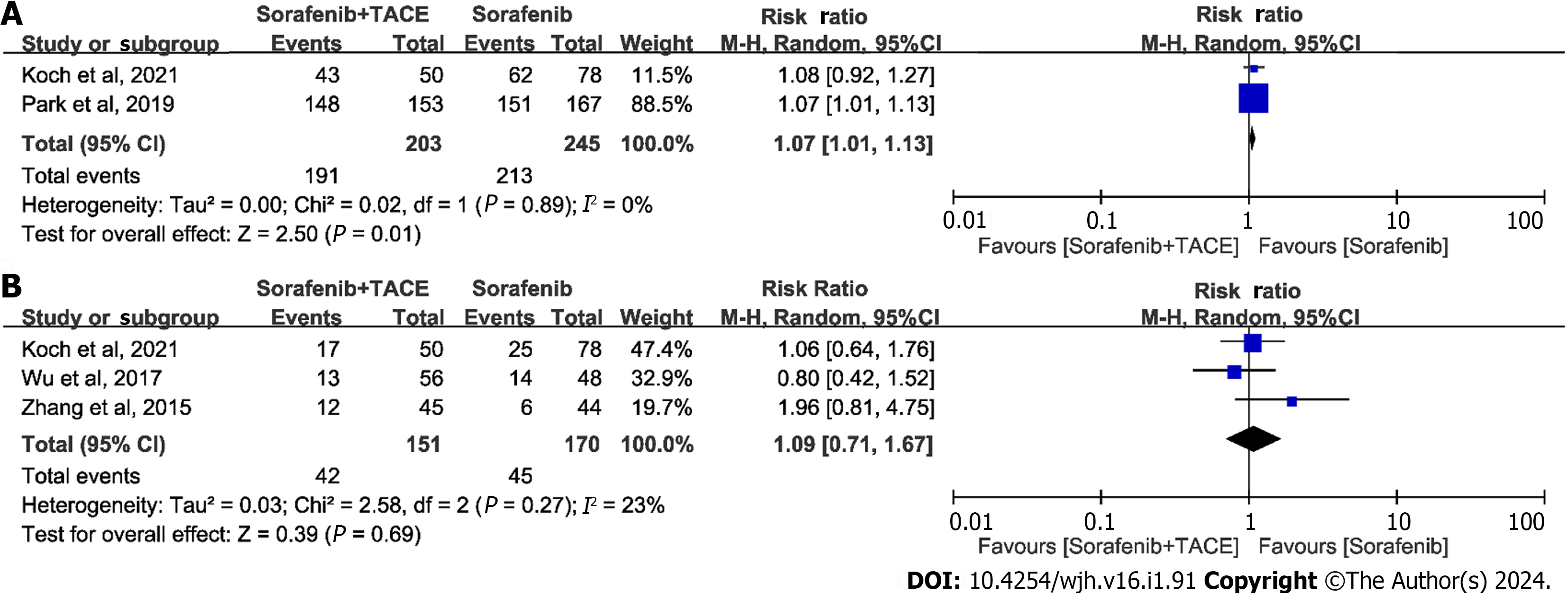
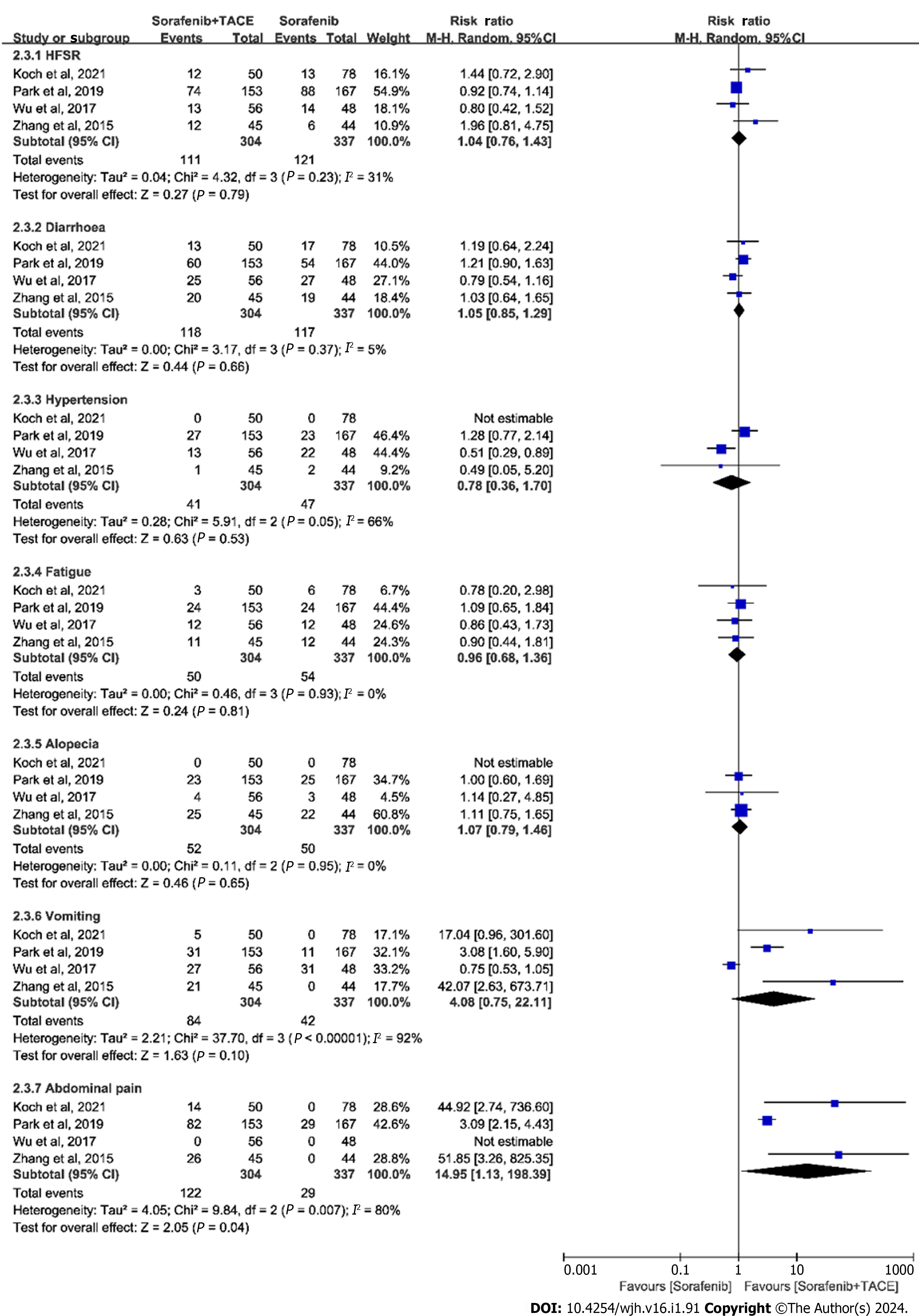
The summary of AEs is shown in Table 4. We classified the outcomes of AEs as any AE, grade ≥ 3 AEs, and typical AEs. The meta-analysis for any AE with inclusion of two studies demonstrated that the differences in the incidence of any AE was significant (RR = 1.07, 95%CI: 1.01-1.13, P = 0.01, n = 448; Figure 3A). Whereas, the incidence of grade ≥ 3 AEs was not statistically significant (RR = 1.09, 95%CI: 0.71-1.67, P = 0.69, n = 321; Figure 3B), as indicated by meta-analysis including the studies of Koch et al[19] and Wu et al[21]. The typical AEs across the trials related to sorafenib plus TACE treatment were hand-foot skin reactions (HFSR), diarrhea, hypertension, fatigue, alopecia, abdominal pain, and vomiting. The pooled results of typical AEs are presented in a forest plot in Figure 4. Among these AEs, only abdominal pain showed a significant difference between the sorafenib plus TACE group and sorafenib group (combined RR = 14.95, 95%CI: 1.13-198.39, P = 0.04, n = 641), while others demonstrated no significant difference (Figure 4).
| Ref. | Group | Patients | AE | ||||||||
| Any | Grade ≥ 3 | HFSR | Diarrhoea | Hypertension | Fatigue | Alopecia | Abdominal pain | Vomiting | |||
| Koch et al[19], 2021 | Sorafenib + TACE | 50 | 43 (86) | 17 (34) | 12 (24) | 13 (26) | NA | 3 (6) | NA | 14 (41) | 5 (14) |
| Sorafenib alone | 78 | 62 (80) | 25 (32) | 13 (17) | 17 (22) | NA | 6 (8) | NA | 0 (0) | 0 (0) | |
| Park et al[20], 2019 | Sorafenib + TACE | 153 | 148 (97) | NA | 74 (48) | 60 (39) | 27 (18) | 24 (16) | 23 (15) | 82 (54) | 31 (20) |
| Sorafenib alone | 167 | 151 (90) | NA | 88 (53) | 54 (32) | 23 (14) | 24 (14) | 25 (15) | 29 (17) | 11 (7) | |
| Kok et al[23], 2019 | Sorafenib + TACE | NA | |||||||||
| Sorafenib alone | NA | ||||||||||
| Wu et al[21], 2017 | Sorafenib + TACE | 56 | NA | 13 (23) | 30 (54) | 25 (45) | 13 (23) | 12 (21) | 4 (7) | NA | 31 (55) |
| Sorafenib alone | 48 | NA | 14 (29) | 36 (75) | 27 (56) | 22 (46) | 12 (25) | 3 (6) | NA | 27 (56) | |
| Zhang et al[22], 2015 | Sorafenib + TACE | 45 | NA | 12 (27) | 29 (64) | 20 (44) | 1 (2) | 11 (24) | 25 (56) | 26 (58) | 21 (47) |
| Sorafenib alone | 44 | NA | 6 (14) | 26 (59) | 19 (43) | 2 (5) | 12 (27) | 22 (50) | 0 (0) | 0 (0) | |
The present work presents the most comprehensive synthesis of data for currently available comparisons of the efficacy and safety of sorafenib plus TACE vs sorafenib alone in treating patients with advanced HCC. We identified data for meta-analysis from five studies that enrolled a total of 2780 patients[19-23]. We found that the addition of TACE to sorafenib improved OS, PFS, TTP, and DCR, compared to sorafenib alone. Besides, addition of TACE increased the incidence of any AE but not grade ≥ 3 AEs.
As a multi-kinase inhibitor, sorafenib was speculated to assist TACE in the management of HCC, as it can suppress angiogenesis in tumours by abolishing VEGF upregulation induced by TACE[24]. Therefore, numerous clinical studies have compared the efficacy and safety of sorafenib combined with TACE vs TACE alone; however, they yielded inconsistent results. Therefore, the potential synergies remain controversial in treating patients with unresectable HCC. Likewise, since TACE was also suggested as a treatment option for advanced HCC[25,26], investigators worldwide began to study whether TACE could improve the outcomes of patients treated with sorafenib for advanced HCC. Zhang et al[22] reported that the addition of TACE to sorafenib did not provide benefit regarding OS and PFS vs sorafenib monotherapy (OS: 7.0 mo vs 6.0 mo, P = 5.544; PFS: 3.0 mo vs 3.0 mo, P = 5.924). Whereas, the study of Wu et al[21] showed that TACE + sorafenib combination yielded better OS (HR = 0.498, 95%CI: 0.278-0.892, P = 0.019), based on multivariate Cox regression analysis. The only multi-center phase III trial[20] comprising 339 patients with advanced HCC reported that the addition of TACE to sorafenib did not improve OS (HR = 0.91; 90%CI: 0.69-1.21, P = 0.290), but improved PFS and TTP. On the contrary, two studies of Kok et al[23] and Koch et al[19] demonstrated that the combination therapy significantly prolong OS compared to sorafenib monotherapy (381 d vs 204 d, HR = 0.74, 95%CI: 0.63-0.88, P = 0.021[23]; 12.8 mo vs 10.8 mo, 16.5 mo vs 8.4 mo, HR = 0.34, 95%CI: 0.23-0.53, P < 0.001[19]). In agreement with the majority of these studies, our meta-analysis also revealed a significantly longer OS in patients receiving TACE + sorafenib than in those receiving sorafenib monotherapy. Besides, the outcomes of PFS, TTP, and DCR were also significantly improved by the addition of TACE. Regarding safety, the incidence of any AE was increased due to the addition of TACE; however, no significant difference was found in grade ≥ 3 AEs. Specifically, the most common AEs were HFSR, diarrhoea, and hypertension for sorafenib, while abdominal pain for TACE[27]. Our meta-analysis indicated that the addition of TACE did not seem to increase toxicity associated with sorafenib. Taken together, the presented data support using sorafenib/TACE combination therapy for the treatment of advanced HCC. However, these positive findings still need further confirmation by more high-quality multi-centre RCTs with large samples and reliable design.
The findings of our meta-analysis were limited by the small number of included studies (range, 2–5 comparative studies). Especially, the majority of included studies were not randomized, assessor-blinded trials. Our work was also limited by the obvious heterogeneity across studies used in the meta-analysis for several outcomes, which might originate from the differences in clinical characteristics of patients of different studies, such as ECOG-PS, BCLC stage, and Child-Pugh class. Finally, there were differences across included trials in the definition of tumour response. The phase III study defined tumour response using the RECIST 1.1 criteria[20], three studies used mRECIST criteria[19,21,22], and one study did not report the criteria used[23].
In summary, the combination of sorafenib with TACE has superior efficacy to sorafenib monotherapy, as evidenced by the prolonged OS, PFS, and TTP, as well as the increased DCR. The addition of TACE does not cause additional toxicity associated with sorafenib. Additional RCTs are required to further investigate the clinical benefit of this combination therapy in treatment of advanced HCC.
Hepatocellular carcinoma (HCC) is a rising global health problem which represents one of the leading causes of cancer-related mortality. Although remarkable advances in treatments have been achieved for HCC, the overall prognosis is still dismal in patients, especially those at advanced stage. Several trials have focused on combining sorafenib with other systemic therapies to augment its clinical benefit.
Recently, a number of comparative trials worldwide have been conducted to investigate whether sorafenib/transarterial chemoembolization (TACE) combination therapy could improve clinical outcomes in patients with advanced HCC, compared with sorafenib monotherapy. However, the obtained findings are conflicting.
To investigate the potential synergies and safety of sorafenib plus TACE vs sorafenib alone for treating advanced HCC.
This meta-analysis involved a large sample size to evaluate whether sorafenib plus TACE provides clinical benefit vs sorafenib monotherapy in patients with advanced HCC, in terms of overall survival (OS), progression free survival (PFS), time to progression (TTP), disease control rate (DCR), and adverse events (AEs).
It was found that patients treated with sorafenib plus TACE had better prognoses in terms of prolonged OS, PFS, and TTP, as well as increased DCR. Besides, the incidence of any AE was increased due to the addition of TACE; however, there was no significant effect on grade ≥ 3 AEs.
The combination of sorafenib with TACE has superior efficacy to sorafenib monotherapy, with an acceptable safety profile.
The addition of TACE to sorafenib is clinically feasible and safe in patients with advanced HCC. The positive findings of the present study might be beneficial to the management of advanced HCC. Additional randomized controlled studies are still necessary to further validating these clinical benefits.
The authors are grateful to the scholars who participated in this study for their contributions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gorrell MD, Australia S-Editor: Lin C L-Editor: Wang TQ P-Editor: Cai YX
| 1. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3161] [Article Influence: 526.8] [Reference Citation Analysis (37)] |
| 2. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3852] [Article Influence: 963.0] [Reference Citation Analysis (3)] |
| 3. | Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, Lencioni R, Greten TF, Kudo M, Mandrekar SJ, Zhu AX, Finn RS, Roberts LR; AASLD Panel of Experts on Trial Design in HCC. Trial Design and Endpoints in Hepatocellular Carcinoma: AASLD Consensus Conference. Hepatology. 2021;73 Suppl 1:158-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 277] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 4. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6038] [Article Influence: 862.6] [Reference Citation Analysis (3)] |
| 5. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3232] [Article Influence: 461.7] [Reference Citation Analysis (1)] |
| 6. | Greten TF, Lai CW, Li G, Staveley-O'Carroll KF. Targeted and Immune-Based Therapies for Hepatocellular Carcinoma. Gastroenterology. 2019;156:510-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 7. | Nault JC, Cheng AL, Sangro B, Llovet JM. Milestones in the pathogenesis and management of primary liver cancer. J Hepatol. 2020;72:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Vande Lune P, Abdel Aal AK, Klimkowski S, Zarzour JG, Gunn AJ. Hepatocellular Carcinoma: Diagnosis, Treatment Algorithms, and Imaging Appearance after Transarterial Chemoembolization. J Clin Transl Hepatol. 2018;6:175-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Xiang X, Zhong JH, Wang YY, You XM, Ma L, Xiang BD, Li LQ. Distribution of tumor stage and initial treatment modality in patients with primary hepatocellular carcinoma. Clin Transl Oncol. 2017;19:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3017] [Article Influence: 431.0] [Reference Citation Analysis (3)] |
| 11. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4648] [Article Influence: 273.4] [Reference Citation Analysis (0)] |
| 12. | Korean Liver Cancer Study Group (KLCSG); National Cancer Center, Korea (NCC). 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015;16:465-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 13. | Abou-Alfa GK, Shi Q, Knox JJ, Kaubisch A, Niedzwiecki D, Posey J, Tan BR Jr, Kavan P, Goel R, Lammers PE, Bekaii-Saab TS, Tam VC, Rajdev L, Kelley RK, El Dika I, Zemla T, Potaracke RI, Balletti J, El-Khoueiry AB, Harding JJ, Suga JM, Schwartz LH, Goldberg RM, Bertagnolli MM, Meyerhardt J, O'Reilly EM, Venook AP. Assessment of Treatment With Sorafenib Plus Doxorubicin vs Sorafenib Alone in Patients With Advanced Hepatocellular Carcinoma: Phase 3 CALGB 80802 Randomized Clinical Trial. JAMA Oncol. 2019;5:1582-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 14. | Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ, Bruix J, Qin S, Thuluvath PJ, Llovet JM, Leberre MA, Jensen M, Meinhardt G, Kang YK. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 429] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 15. | Chen SW, Lin LC, Kuo YC, Liang JA, Kuo CC, Chiou JF. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2014;88:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, Kaneko S, Tsubouchi H, Suh DJ, Furuse J, Okusaka T, Tanaka K, Matsui O, Wada M, Yamaguchi I, Ohya T, Meinhardt G, Okita K. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 458] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 17. | Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS, Sherman M. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155-2166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 939] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 18. | Lee WC, Hung HC, Lee JC, Wang YC, Cheng CH, Wu TH, Lee CF, Wu TJ, Chou HS, Chan KM. Treatment strategy of adding transcatheter arterial chemoembolization to sorafenib for advanced stage hepatocellular carcinoma. Cancer Rep (Hoboken). 2021;4:e1294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Koch C, Göller M, Schott E, Waidmann O, Op den Winkel M, Paprottka P, Zangos S, Vogl T, Bechstein WO, Zeuzem S, Kolligs FT, Trojan J. Combination of Sorafenib and Transarterial Chemoembolization in Selected Patients with Advanced-Stage Hepatocellular Carcinoma: A Retrospective Cohort Study at Three German Liver Centers. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Park JW, Kim YJ, Kim DY, Bae SH, Paik SW, Lee YJ, Kim HY, Lee HC, Han SY, Cheong JY, Kwon OS, Yeon JE, Kim BH, Hwang J. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: The phase III STAH trial. J Hepatol. 2019;70:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 21. | Wu FX, Chen J, Bai T, Zhu SL, Yang TB, Qi LN, Zou L, Li ZH, Ye JZ, Li LQ. The safety and efficacy of transarterial chemoembolization combined with sorafenib and sorafenib mono-therapy in patients with BCLC stage B/C hepatocellular carcinoma. BMC Cancer. 2017;17:645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Zhang Y, Fan W, Wang Y, Lu L, Fu S, Yang J, Huang Y, Yao W, Li J. Sorafenib With and Without Transarterial Chemoembolization for Advanced Hepatocellular Carcinoma With Main Portal Vein Tumor Thrombosis: A Retrospective Analysis. Oncologist. 2015;20:1417-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Kok VC, Chen YC, Chen YY, Su YC, Ku MC, Kuo JT, Yoshida GJ. Sorafenib with Transarterial Chemoembolization Achieves Improved Survival vs. Sorafenib Alone in Advanced Hepatocellular Carcinoma: A Nationwide Population-Based Cohort Study. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 500] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 25. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1987] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 26. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J; Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 27. | Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 419] [Article Influence: 59.9] [Reference Citation Analysis (0)] |